Abstract
Small conductance Ca2+-activated K+ channels (SK) regulate action potential (AP) firing properties and excitability in many central neurons. However, the functional roles of SK channels of parasympathetic cardiac motoneurons (PCMNs) in the nucleus ambiguus have not yet been well characterized. In this study, the tracer X-rhodamine-5 (and 6)-isothiocyanate (XRITC) was injected into the pericardial sac to retrogradely label PCMNs in FVB mice at postnatal days 7–9. Two days later, XRITC-labeled PCMNs in brain stem slices were identified. With the use of whole cell current clamp, single APs and spike trains of different frequencies were evoked by current injections. We found that 1) PCMNs have two different firing patterns: the majority of PCMNs (90%) exhibited spike frequency adaptation (SFA) and the rest (10%) showed less or no adaptation; 2) application of the specific SK channel blocker apamin significantly increased spike half-width in single APs and trains and reduced the spike frequency-dependent AP broadening in trains; 3) SK channel blockade suppressed afterhyperpolarization (AHP) amplitude following single APs and trains and abolished spike-frequency dependence of AHP in trains; and 4) SK channel blockade increased the spike frequency but did not alter the pattern of SFA. Using whole cell voltage clamp, we measured outward currents and afterhyperpolarization current (IAHP). SK channel blockade revealed that SK-mediated outward currents had both transient and persistent components. After bath application of apamin and Ca2+-free solution, we found that apamin-sensitive and Ca2+-sensitive IAHP were comparable, confirming that SK channels may contribute to a major portion of Ca2+-activated K+ channel-mediated IAHP. These results suggest that PCMNs have SK channels that significantly regulate AP repolarization, AHP, and spike frequency but do not affect SFA. We conclude that activation of SK channels underlies one of the mechanisms for negative control of PCMN excitability.
Keywords: repolarization, afterhyperpolarization, transient outward currents, afterhyperpolarization currents
parasympathetic preganglionic cardiac motoneurons (PCMNs) in the nucleus ambiguus (NA) regulate cardiac functions (negative control of chronotropic, dromotropic, and inotropic actions) (40). Previously, we have demonstrated that the NA projects strongly to cardiac ganglia (8, 9). Lesions of the NA almost completely abolish the baroreflex control of heart rate (10). Therefore, the NA plays a key role in arterial baroreflex control of heart rate (baroreflex sensitivity). In many cardiovascular diseases (e.g., diabetes and chronic intermittent hypoxia as a model for sleep apnea and aging), baroreflex sensitivity is reduced (23, 24, 35, 37, 68, 69). Impairment of baroreflex has been used as an indicator of potential life-threatening arrhythmia and heart failure (14, 30). Therefore, it is very important to study the action potential (AP) firing properties and excitability of PCMNs, as well their underlying mechanisms under normal conditions. These studies will serve as a foundation for the investigation of disease-related changes in PCMNs.
Previously, it has been demonstrated that PCMNs in the NA of rats do not possess pacemaker-like firing activities and are intrinsically silent (43). The synaptic innervation of PCMNs is therefore critical for the tonic and reflex evoked changes in cardiac vagal activity that control cardiac functions. The most important pathway innervating PCMNs is from neurons in the nucleus of the solitary tract (NTS) (66). Stimulation of NTS axons activates NA PCMNs through glutamatergic transmission, which in turn regulates cardiac functions (44).
Small conductance (SK) Ca2+-activated K+ channels are intracellular Ca2+-sensitive, voltage-insensitive channels. SK channels can be selectively blocked by the bee toxin apamin (5) and UCL1684 (6, 13). In many central neurons, the functional roles of SK channels have been extensively characterized (47 59). SK channels mainly contribute to afterhyperpolarization potential (AHP) following APs and exert a negative or “brake” effect on the firing rate (spike frequency) of neurons (59). In an early pioneering work, Mendelowitz (43) found that NA cardiac motoneurons were intrinsically silent and did not exhibit significant spike frequency adaptation (SFA; a time-dependent decrease in spike frequency in response to a sustained suprathreshold input). In addition, apamin application increased excitability of these cardiac motoneurons. Recently, Chen and Toney (7) characterized the role of SK channels in regulating excitability of presympathetic neurons in the paraventricular nucleus (PVN) that project directly to the rostral ventrolateral medulla (RVLM) of rats. This study by Chen and Toney (7) provides important information on the role of ion channels in regulating excitability of presympathetic PVN neurons (3). However, physiological roles of SK channels in regulating the AP firing properties and excitability of PCMNs in the NA, which project to cardiac ganglia, have not yet been well characterized. In this study, we examined the effects of SK channels on the AP firing properties and excitability of PCMNs. In addition, we studied the apamin-sensitive and UCL1684-sensitive outward currents and AHP currents (IAHP), which may provide underlying mechanisms for AP firing properties and the negative control of PCMN excitability.
MATERIALS AND METHODS
Female (n = 12) and male FVB (n = 4) (or FVB/N in full name) mice were obtained from Jax Laboratory at age of 2–3 mo. The FVB strain has been frequently used for producing transgenic mice because it is easy to inject pronuclei at the single-cell stage in this strain, and it is a healthy, fertile breeder. Previously, Epstein et al. (15) used FVB mice to produce OVE26 transgenic line, which develops type 1 diabetes because of β-cell-specific damage due to a calmodulin transgene regulated by the insulin promoter. FVB (normal control) and OVE26 diabetic mice have been previously used to examine diabetes-induced heart and kidney complications (23, 24, 32, 33, 37, 69, 71). In each cage, one male mouse was housed with three female mice. Mice were maintained on a 12-h light/dark cycle and received food and water ad libitum. All animals were then maintained in the transgenic animal facility at the University of Central Florida. When females became pregnant, they were transferred into individual cages. Procedures were approved by the University of Central Florida Animal Care and Use Committee and followed the guidelines established by National Institutes of Health. Efforts were made to reduce the number of animals used and their suffering.
Fluorescent retrograde labeling of PCMNs and medullary slice preparation.
On postnatal days 7–9 (P7–9; n = 68), FVB neonatal mice were anesthetized with 3% isoflurane (Abbott Laboratories, North Chicago, IL) and cooled to ∼4°C to decrease heart rate. After a right thoracotomy was performed, the retrograde fluorescent tracer X-rhodamine-5 (and 6)-isothiocyanate (XRITC, 2%, 4 μl, Molecular Probes, Eugene, OR) was injected into the pericardial sac at the base of the heart. After a recovery period of at least 48 h, neonatal mice were deeply anesthetized with 4% isoflurane, and their hindbrains were rapidly removed. The brain stem including PCMNs were sliced in serial sections (250 μm) using a vibrating blade microslicer (DTK-1000, Kyoto, Japan) and maintained in an interface chamber filled with artificial cerebral spinal fluid (aCSF) containing (in mM) 126 NaCl, 2.5 KCl, 2 CaCl2, 26 NaHCO3, 1.25 NaH2PO4, 2 MgSO4, and 10 dextrose, equilibrated with 95% O2-5% CO2, and pH adjusted to 7.4. Slices were transferred to a recording chamber maintained at room temperature (22–25°C). In brain stem slices, PCMNs were identified in the NA by the presence of the retrograde fluorescent tracer XRITC. These slices were viewed (Fig. 1A) with infrared illumination and differential interference optics (Carl Zeiss, Göttingen, Germany) and under fluorescent illumination (Fig. 1B) with a near-infrared sensitive, cooled charged-coupled device camera (AxioCom MRm, Carl Zeiss). XRITC-labeled PCMNs were identified by superimposing the fluorescent and infrared images (Fig. 1C).
Fig. 1.
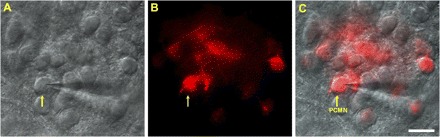
Patch-clamp recording of tracer X-rhodamine-5 isothiocyanite (XRITC)-labeled parasympathetic cardiac motoneurons (PCMNs) in the nucleus ambiguus (NA) in brain stem slices. A: DIC imaging of PCMNs. B: fluorescent imaging shows XRITC-labeled PCMNs in the NA that were labeled by injection of the fluorescent tracer XRITC (2% solution, 4 ml, Molecular Probes) into pericardial sac 2 days before experiment. C: merged image of A and B. Arrow indicates the recorded PCMN in C. Scale bar: 40 μm.
Whole cell current clamp recording.
Spike firing properties of PCMNs were examined. Within the recording chamber, slices were held in a stable position using a nylon net stretched over a flattened U-shaped platinum wire and were continuously superfused at room temperature with aCSF. For the Ca2+-free bath solution, 2 mM CaCl2 was replaced by 3 mM MgCl2. Patch electrodes were fabricated from borosilicate glass (1.5 mm outer diameter; World Precision Instruments, Sarasota, FL) with a Flaming Brown horizontal puller (P-97, Sutter Instruments, Novato, CA). Electrodes were heat polished to a final tip resistance of 3–5 MΩ and filled with pipette solution containing (in mM) 120 potassium-gluconate, 20 KCl, 2 MgCl2, 10 HEPES, 0.1 EGTA, 2 ATP, and 0.25 GTP, with pH adjusted to 7.3 with KOH. Note that a relatively low concentration of EGTA (0.1 mM) was used to allow intracellular free Ca2+ to accumulate and activate SK channels during membrane potential depolarization (4). This low EGTA loading in intracellular recording solution was previously used to study the role of Ca2+-activated K+ channels in regulating excitability in central neurons (e.g., hippocampus dentate gyrus and PVN neurons) (4, 7).
To generate a single AP, a 10-ms depolarizing current pulse of sufficient intensity (40–100 pA) to trigger a single AP was applied. We used a 10-ms duration because this stimulus duration has been commonly used to evoke single APs in other central neurons (12, 18, 25). To standardize AP recordings, neurons were depolarized to a holding potential of −60 mV by DC application through the recording electrode. AP was recorded within minutes if the following criteria were met: A resting membrane potential of less than −60 mV and an AP peak amplitude of >70 mV.
AP half-width was measured as the spike width at the half-maximal voltage using Clampfit 9.2 (Axon instruments, Foster City, CA). Since AHP reached the peak at about 10 ms and returned to the baseline after 100 ms following single APs, AHP amplitudes were only measured at 10 and 50 ms. AHPs following a spike train were elicited by a 1-s, 400 pA current step from a holding potential of −60 mV. Since AHP reached the peak at about 100 ms and returned to the baseline after 1,000 ms following trains, AHPs were measured at 50, 100, 500, and 1,000 ms after the end of the depolarizing current injection. To investigate the firing frequency of PCMNs, current injection steps were applied from 20 to 200 pA in 20-pA increments. Instantaneous firing rate was measured as the inverse of the interspike intervals. Input resistance was determined by Ohm's law using a linear regression within the linear range (generally ±10 mV from the resting potential) of the voltage-current (I–V) relationship that had been established by plotting the steady-state voltage change in response to a series of depolarizing and hyperpolarizing current injections. To determine the AP threshold, AP trains were evoked by a 1.5-s, 0.2 pA/ms ramp current from a holding potential of −60 mV in control aCSF, and the threshold was measured at the beginning of the first AP.
Whole cell voltage clamp recording.
Electrodes were heat polished to a final tip resistance of 2–3 MΩ and filled with pipette solution containing (in mM) 110 K-gluconate, 10 KCl, 5 NaCl, 2 MgCl2, 10 HEPES, 0.5 EGTA, 1 ATP, 0.2 GTP, and 0.1 leupeptin, with pH adjusted to 7.3 with KOH. To record SK currents, cells were superfused with an oxygenated HEPES solution that contained (in mM) 140 NaCl, 3 KCl, 2 CaCl2, 2 MgCl2, 10 HEPES, 1 tetraethylammonium (TEA), 0.0005 tetrodotoxin (TTX), 0.005 glybenclamide, and 10 glucose, pH adjusted to 7.4 with NaOH. TEA, TTX, and glybenclamide were included routinely in the HEPES solution unless otherwise indicated. A low concentration of TEA (1 mM) was added to suppress the large-conductance Ca2+-activated K+ channels (BK) and voltage-dependent K+ currents (26). For the Ca2+-free bath solution, 2 mM CaCl2 was replaced by 3 mM MgCl2 to give a HEPES solution containing 5 mM Mg2+. Experiments were conducted at room temperature (22–25°C).
Outward K+ currents were evoked by a series of 250-ms depolarizing steps from −70 to +40 mV with +10 mV increments. I–V relationships were generated by measuring the peak of the transient outward current. The peak value of the transient outward current was plotted against membrane potential and was fitted by the Boltzmann equation (55). To study IAHP, afterhyperpolarization currents were first evoked by a 100-ms depolarizing voltage step of +10 mV and immediately followed by a return to −50 mV for 1.5 s. To avoid possible interference between responses, depolarizing voltage steps were delivered every 5 s. The peak amplitude of IAHP was measured following the end of the −50-mV voltage step.
Single-channel recording.
Cells were superfused with an oxygenated HEPES solution as described above. Single-channel currents were recorded in an outside-out patch configuration. The electrode was heat polished to a final tip resistance of 2–3 MΩ, and the pipette was filled with a standard internal solution containing (in mM) 110 K-gluconate, 10 KCl, 5 NaCl, 2 MgCl2, 10 HEPES, 0.5 EGTA, 1 ATP, 0.2 GTP, and 0.1 leupeptin, with pH adjusted to 7.3 with KOH. After a good seal (>5 GΩ) was made, the pipette was withdrawn from the soma and the outside-out configuration was made. The extracellular face of the channel was perfused with aCSF containing 2 mM Ca2+, and the cytoplasmic face was in the pipette internal solution with 0 mM Ca2+. When multiple channel types were observed in a patch, SK currents were distinguished from others by the following characteristics: 1) outward currents, 2) voltage independence, 3) amplitudes of <10 pA, and 4) apamin sensitivity. In single channel recordings, currents were low-pass filtered at 1 kHz and acquired at a sampling rate of 10 kHz using the Clampex acquisition program of pCLAMP. Data were collected for 1–3 min. To determine channel opening probability, the number of opening events was obtained using the half-amplitude threshold criterion (11). The number of opening events-current amplitude histogram was fitted using a Gaussian function. Duration of opening times was measured at half-amplitude threshold. Open-time curves were fitted by an exponential function.
The series resistance was in the range of 5–10 MΩ (typically about 5 MΩ) and was compensated by 60% on-line. Membrane potential measurement was not corrected for the liquid junction potential (about 15 mV). The holding potentials were not corrected for liquid junction potentials. Leak currents were subtracted using a standard P/4 protocol (2). Before seals (5 GΩ) were made on cells, offset potentials were nulled. Capacitance subtraction was used in all recordings.
Drugs and chemicals.
All channel blockers used in the present study were purchased from Sigma-Aldrich (St. Louis, MO). The blockers were applied by directly adding them to the superfusate from stock solutions.
Data acquisition and analysis.
Data acquisition was controlled using the ClampEx program in the pClamp 9 software package (Axon Instruments, Foster City, CA). Signals were recorded using a MultiClamp 700B patch-clamp amplifier (Axon instruments). Responses were low-pass filtered at 3 kHz and digitized at 50 kHz with a 16-bit analog-to-digital data acquisition systems (Digidata 1322A, Axon instruments).
Data were presented as means ± SE. Student's t-test, one-way or two-way analysis of variance (ANOVA) with repeated measures followed by a Tukey-Kramer post hoc test were used. P < 0.05 was considered as significant.
RESULTS
A total of 298 PCMNs from 68 mice meeting the criteria, as mentioned in materials and methods, were recorded and analyzed in the different experiments. The primary parameters of passive membrane and AP were averaged in 25 randomly selected cells. The resting membrane potential was −67.4 ± 2.0 mV, input resistance was 246.0 ± 11.2 MΩ, AP amplitude was 83.6 ± 1.4 mV, membrane time constant was 727.2 ± 3.2 μs, and membrane capacitance was 62.7 ± 1.4 pF.
AHP, firing rate, and SFA in trains.
Spike trains of 4, 7, and 10 Hz were elicited by adjusting intensities of 1 s depolarizing current from a holding membrane potential of −60 mV (Fig. 2A). Noticeably, the peak amplitudes of AHP were obviously reduced as the spike number increased in all trains of different frequencies (Figs. 2A and 4G).
Fig. 2.
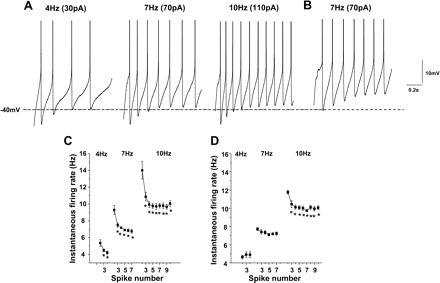
Spike adaptation in PCMNs. A: PCMN with early spike adaptation. Action potential (AP) trains of 4 (left), 7 (middle), and 10 (right) Hz were evoked by 1 s current injection at different intensities. Early spike adaptation was observed in these trains. B: PCMN without obvious spike adaptation. A representative voltage trace showing repetitive spike discharges with relatively steady spike frequency during a current pulse injection. No obvious spike adaptation was observed. C: instantaneous firing rate for 4-, 7-, and 10-Hz AP trains was reduced as the spike number increased (*P < 0.05). D: group data from PCMNs without obvious spike adaptation. Instantaneous firing rate for 4 and 7 AP trains did not significantly change as the spike number increased (P > 0.05). In 10-Hz trains, instantaneous firing rate decreased in the second to third spikes (*P < 0.05) but did did not show any further change following the third spike (P > 0.05).
Fig. 4.
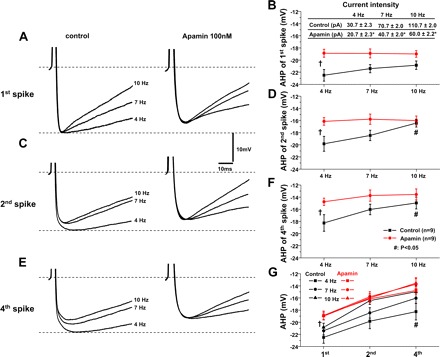
SK channels regulate spike frequency-dependent AHP in AP trains. A: in 4-, 7-, and 10-Hz AP trains, the AHP peak amplitude of the first spike in these trains was comparable (A, left, B). After apamin application, AHP peak amplitude of the first spike was reduced and comparable in these trains (A, right, B). C: in 4-, 7-, and 10-Hz AP trains, the AHP peak amplitude of the second spike significantly decreased in the 10-Hz train compared with that in the 4-Hz train (C, left, D). Apamin reduced the AHP amplitude of the second spike in 4- and 7-Hz trains. In the 10-Hz train, the AHP peak amplitude of the second spike was comparable in control and apamin groups. After apamin, the AHP peak amplitude in all these trains was comparable (C, right, D) (P > 0.05). E: similarly, the AHP amplitude of the fourth spike significantly decreased in the 10-Hz compared with that in the 4-Hz train (C, left, D). Apamin reduced the AHP amplitude of the fourth spike in 4- and 7-Hz trains. In the 10-Hz train, the AHP amplitude of the fourth spike was comparable in control and apamin-treated groups. After apamin, the AHP amplitude in all these trains was comparable. The AHP amplitude of the fourth spike was significantly decreased in the 10-Hz compared with that in the 4-Hz train (E, left, F). G: apamin abolished the frequency dependency of AHP peak amplitude of individual spikes in spike trains. Inset in B, *P < 0.05 apamin vs. control. †ANOVA, P < 0.05 apamin vs. control; #P < 0.05 10 Hz vs. 4 Hz in control.
Two types of trains were found according to SFA. SFA is a significant decrease in instantaneous spike frequency as the spike number increases. The majority (90%) of all analyzed 298 PCMNs exhibited significant SFA. Figure 2A shows representative SFA in trains of 4, 7, and 10 Hz. In each train, significant SFA was observed between the first and fourth spikes, which is confirmed in Fig. 2C (P < 0.05; one-way ANOVA). The remaining 10% of PCMNs showed small or insignificant SFA, i.e., Fig. 2B shows an example of a spike train of 7 Hz from such a neuron. Figure 2D shows that there was no significant SFA in 4- and 7-Hz trains, but there was a small adaptation between the first and second spikes in the 10-Hz train (P < 0.05; one-way ANOVA). In the following study, we only included SFA neurons.
SK channels regulate AHP.
To study the effect of SK channels on AHP following single APs and spike trains, apamin (100 nM) and UCL1684 (100 nM) were applied to the bath solution. Single APs were evoked by injecting a 10-ms depolarizing current pulse with an intensity sufficient to generate only one AP (Fig. 3A). When compared with control, apamin and UCL1684 application similarly decreased AHP (Fig. 3C; P < 0.05). As with apamin and UCL1684 application, bath application of Ca2+-free solution also reduced AHP (Fig. 3D), further indicating that AHP was dependent on Ca2+-activated K+ channels. SK channel blockade reduced AHP by a similar amount at 50 ms as does the prevention of Ca2+ influx by Ca2+-free solution, although Ca2+-free solution induced a greater reduction of AHP than SK channel blockade at 10 ms (P < 0.05).
Fig. 3.
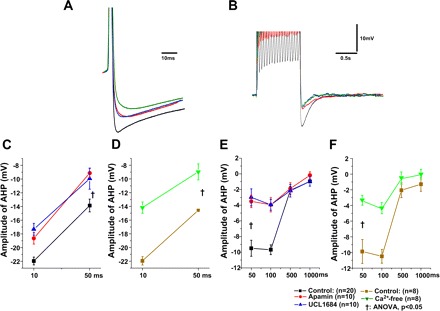
Small conductance Ca2+-activated K+ channels (SK)-mediated AHP following single AP and spike trains in PCMNs. A: applications of apamin (100 nM), UCL1684 (100 nM), and Ca2+-free solution reduced the AHP amplitude of single APs. B: applications of apamin (100 nM), UCL1684 (100 nM), and Ca2+-free solution reduced the AHP amplitude after a train of APs evoked by a 1-s depolarizing current injection (400 pA). C: application of apamin and UCL1684 significantly depressed the AHP amplitude of single APs at both 10 and 50 ms (P < 0.05). D: application of Ca2+-free solution significantly depressed the AHP amplitude of single APs at both 10 and 50 ms. E: application of apamin and UCL1684 significantly depressed the AHP amplitude following a train of APs at 50 and 100 ms after the end of the depolarizing current injection. F: application of Ca2+-free solution significantly depressed the AHP amplitude following a train of APs at 50 and 100 ms after the end of the depolarizing current injection. †One-way ANOVA, P < 0.05.
Spike trains were evoked by a 1 s, 400-pA current injection from a holding potential of −60 mV (Fig. 3B). When compared with controls, bath application of apamin and UCL1684 (Fig. 3E) and Ca2+ free (Fig. 3F) significantly reduced AHP at 50 and 100 ms (P < 0.05, two-way ANOVA). Noticeably, the reduction of AHP at 50 and 100 ms was comparable after bath application of apamin and UCL1684 (Fig. 3E) and Ca2+ free (Fig. 3F).
SK channels contribute to spike-frequency dependency of AHP in trains.
Spike trains with frequencies of 4, 7, and 10 Hz were elicited using the same protocol as in Fig. 2A. The role of SK channels in regulating the AHP following the first, second, and fourth APs in 4-, 7-, and 10-Hz trains was examined. Figure 4 shows representative AHP of individual spikes following spike trains in control aCSF and during apamin application. As shown in the left panel of Fig. 4A, C, and E, the peak amplitudes of AHP following the second and fourth spikes progressively decreased from 4 to 10 Hz in control aCSF (black lines in Fig. 4, D and F, P < 0.05; one-way ANOVA). No statistical difference in peak AHP of the first spike was found in these trains (Fig. 4A, left; black line in Fig. 4B).
Application of 100 nM apamin significantly reduced the AHP peak amplitudes for the first, second, and fourth spikes in 4-, 7-, and 10-Hz trains and abolished frequency-dependent reduction of the AHP peak amplitudes for the second or fourth spikes in 4-, 7-, and 10-Hz trains (right column of Fig. 4, C and E, and the red lines in Fig. 4, D and F, P < 0.05, two-way ANOVA). In control aCSF and apamin, AHP peak amplitude decreased as the spike number increased within each train (Fig. 4G, black lines; P < 0.05; one-way ANOVA). In addition, AHP peak amplitudes had a trend to decrease as the spike frequency increased across trains. AHP peak amplitudes significantly differed between 4- and 10-Hz trains (Fig. 4G; P < 0.05; two-way ANOVA).
SK channels regulate repolarization of single APs and spikes within trains.
In many central neurons, it was reported that SK channels do not contribute to AP repolarization (e.g., 16). To examine whether SK channels contribute to repolarization of single APs and spikes within trains, we tested the effects of SK channel blockade with apamin and/or UCL1684 on the half-width of the single APs and individual spikes within the 10-Hz train. Single APs and 10-Hz trains were elicited as in Fig. 2 and Fig. 3A. When compared with control, application of apamin and UCL1684 significantly increased the single AP half-width (Fig. 5, A and D, Fig. 3A; P < 0.05). In the 10-Hz train, the AP half-width increased as the spike number increased in both control and during apamin application (Fig. 5B, C, F; P < 0.05, one-way ANOVA). When compared with the spikes in control, apamin significantly increased the half-width of corresponding spikes in the 10-Hz train (Fig. 5F, P < 0.05, two-way ANOVA). Relative to the half-width of the first spike, the half-width of the fifth and tenth spikes increased by 20.8 ± 1.9% and 25.6 ± 2.3%, respectively (Fig. 5G). In contrast, during apamin application the half-width of the fifth and tenth spikes increased by 14.8 ± 1.5% and 17.6 ± 2.0% from the first spike, respectively (Fig. 5G).
Fig. 5.
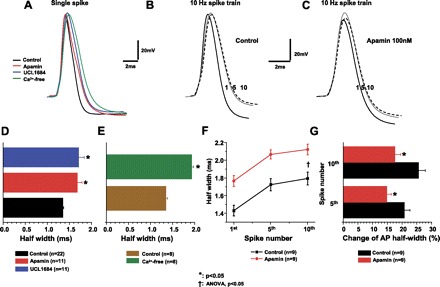
SK channels regulate repolarization of single APs and frequency-dependent spike broadening in the AP trains. Single spike and 10-Hz AP trains were generated as shown in Figs. 2 and 3. A: single APs were recorded in control artifical cerebral spinal fluid (aCSF) or Ca2+-free solution or during apamin or UCL1684 application. B: representative AP repolarization of the first, fifth, and tenth spikes in a 10-Hz train in control aCSF. C: representative AP repolarization of the first, fifth, and tenth spikes in a 10-Hz train during apamin application. D: apamin and UCL1684 significantly increased the half-width of single spikes. E: Ca2+-free significantly increased the half-width of single spikes. F: half-width increased as the spike number increased in control and during apamin. Apamin significantly increased the half-width of each AP in the 10-Hz train (P < 0.05). G: in the 10-Hz train, apamin induced a significantly smaller spike broadening in the fifth and tenth spike relative to the first spike than that in control (P < 0.05).
Since SK channels are Ca2+ dependent, we reasoned that blockade of Ca2+ may yield a similar effect on AP repolarization. Therefore, we prevented Ca2+ influx using a Ca2+-free solution. As with apamin application, Ca2+-free solution indeed increased the half-width of APs (Fig. 5E). When compared with SK blockade, application of Ca2+-free solution induced a larger half-width of APs (Ca2+ free: 1.93 ± 0.04 ms, apamin: 1.68 ± 0.11 ms; P < 0.05).
SK channels regulate excitability of PCMNs but do not affect SFA.
To evaluate the effect of SK channel blockade on excitability, spike trains were evoked by 1-s depolarizing currents (40–180 pA in 20-pA increments) from a holding potential of −60 mV in control aCSF (Fig. 6A) and bath application of apamin (Fig. 6B). Application of apamin significantly increased the mean firing rate (Fig. 6C; P < 0.05). The effect of SK channel blockade on SFA was also examined. As shown in Fig. 6D, although apamin significantly increased the mean instantaneous firing rate of individual spikes compared with that in control aCSF, the pattern of SFA was quite similar in control and apamin.
Fig. 6.
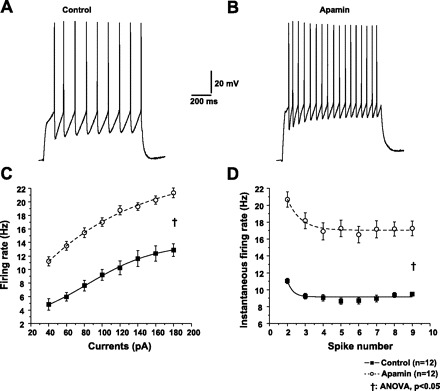
SK channels regulate spike firing frequency of PCMNs. A and B: representative AP trains were evoked by 1-s depolarizing current (100 pA) injection in control aCSF or during apamin. C: after apamin application, spike firing frequency significantly increased compared with that in control aCSF (ANOVA, †P < 0.05). D: apamin significantly increased instantaneous firing rate compared with that in control aCSF (ANOVA, †P < 0.05). Similar to control, apamin significantly reduced the interspike interval. The pattern of spike frequency adaptation (SFA) during apamin was similar to that in control.
SK channel blockade increased depolarizing input resistance (Rinput) and AP voltage threshold but decreased current intensity required to evoke APs.
To determine whether SK channels contribute to AP voltage threshold and depolarizing input resistance (Rinput) in PCMNs, ramp currents of 0–300 pA (0.2 pA/ms for 1,500 ms) were injected to induce gradual membrane potential depolarization and trigger APs at threshold (Fig. 7). Spike trains were elicited before and during apamin application. As shown in Fig. 7, A and B, instantaneous firing rate was progressively increased during depolarizing ramp current in control aCSF and apamin application (Fig. 7C). When compared with control aCSF, apamin application greatly increased instantaneous firing rate (P < 0.05, two-way ANOVA), which is consistent with Fig. 6. Blockade of SK channels with apamin noticeably decreased the current intensity which was required to evoke the first AP (Fig. 7, A, B, D; P < 0.05) and increased depolarizing Rinput (Fig. 7E, P < 0.01). Although the threshold was higher (Fig. 7F, P < 0.05), the rate of subthreshold membrane potential increase was higher after apamin application such that the membrane potential reached the AP threshold earlier compared with that in control aCSF (Fig. 7, A and B).
Fig. 7.
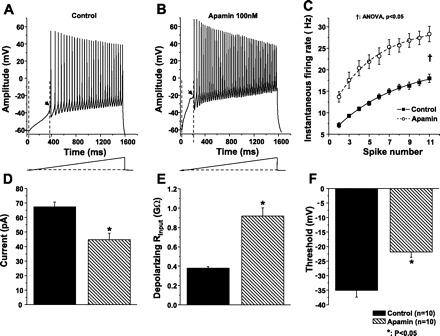
SK channels altered depolarizing input resistance and AP voltage threshold. A and B: AP trains were evoked by a 1.5-s, 0.2 pA/ms ramp current from a holding potential of −60 mV in control aCSF and during apamin application. C: instantaneous firing rate increased as the spike number increased in control aCSF and during apamin application. When compared with control aCSF, apamin induced a greater increase of instantaneous firing frequency (†P < 0.05). D: apamin significantly decreased the intensity of current injection which was required to evoke the first AP (*P < 0.05). E: depolarizing input resistance (Rinput) was defined as the ratio of membrane potential change (ΔVm) relative to membrane potential baseline and current injection increase (ΔI) at the beginning of the first AP. Apamin significantly increased Rinput compared with that in control (n = 10, *P < 0.05). F: AP threshold was measured at the beginning of the first AP as denoted by an arrow in A and B. Apamin increased the threshold (n = 10, *P < 0.05).
Identification of SK-mediated currents.
It has been established that transient outward K+ currents mainly contribute to AP repolarization (19, 41) and IAHP contribute to AHP (16). Therefore, we assumed that SK channels significantly contribute to transient outward currents and IAHP. To study SK outward currents, we used TTX (0.5 μM) and TEA (1 mM) to block Na+ and reduce voltage-dependent K+ and BK channels (sensitive to low concentrations of TEA) (20). In the following experiments, TTX and TEA were routinely added in the bath solution. Under these conditions, outward currents and IAHP were obtained.
First, we studied SK channel-mediated IAHP. IAHP was evoked by a 100-ms depolarizing voltage pulse from a holding potential of −70 to +10 mV followed by a 1.5-s −50 mV voltage pulse. As the first step, we tested whether SK channels mediate IAHP by using SK channel blockers apamin and UCL1684. Apamin (100 nM) and UCL1684 (100 nM) applications similarly attenuated IAHP (Fig. 8, A and B). With the subtraction of the currents before and after apamin- and UCL1684, apamin- and UCL1684-sensitive IAHP were obtained (Fig. 8, A′ and B′). The mean peak amplitudes of IAHP were 93.9 ± 15.4 pA (apamin sensitive) and 82.4 ± 18.1 pA (UCL1684 sensitive) (Fig. 8E). The decays of apamin- and UCL1684-sensitive IAHP were fitted by a single exponential equation (Fig. 8, A′ and B′, white curve). The time constants were 54.1 ± 8.8 ms (apamin sensitive) and 52.2 ± 10.7 ms (UCL1684 sensitive) (Fig. 8F), respectively. To further test whether IAHP was Ca2+ dependent, we used a Ca2+-free solution. As shown in Fig. 8C, Ca2+-free application markedly reduced the amplitude of IAHP. Ca2+-sensitive IAHP was determined by subtracting IAHP before and after Ca2+-free application (Fig. 8C′). The averaged peak amplitude was 100.0 ± 21.7 pA (Fig. 8E). The decay of Ca2+-sensitive IAHP in Fig. 8C′ was fitted by a single exponential equation (white curve), and the mean time constant was 83.8 ± 12.3 ms (Fig. 8F). When compared with apamin-sensitive IAHP, Ca2+-sensitive IAHP had similar peak amplitudes and time decay constants. To show the stability of the recorded cells during experiments, we ran a time control experiment. In eight cells, IAHP was first recorded and after 15 min, it was recorded again. The amplitude of IAHP was not different between the two recordings (Fig. 8D). Time control IAHP (the subtraction of the second from the first recordings) was 0.5 ± 7.4 pA, which was not different from 0 (Fig. 8, D, D′ and E).
Fig. 8.
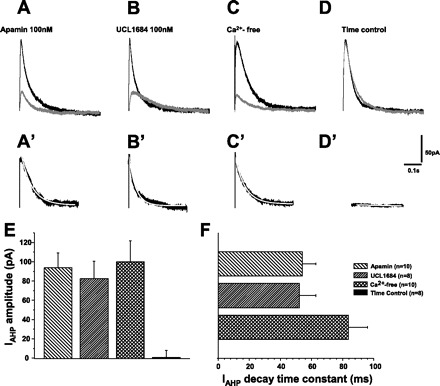
The properties of SK channel-mediated AHP current (IAHP). In A–C, black traces represent IAHP in control and gray traces show IAHP during apamin, UCL1684, and Ca2+-free application. A: apamin largely depressed IAHP. A′: apamin-sensitive IAHP (subtraction of the black and gray traces in A). B: similar to apamin, UCL1684 largely depressed IAHP. B′: UCL1684-sensitive IAHP (subtraction of the black and gray traces in B). C: similarly, Ca2+-free solution largely depressed IAHP (gray trace). C′: Ca2+-sensitive IAHP (subtraction of the black and gray traces in C). D: time control. IAHP (black trace) was first recorded. 15 min later, IAHP (gray trace) was recorded again. The amplitude of IAHP was not changed during this 15-min process. D′: time control IAHP (subtraction of the black and gray traces in D). E: apamin-sensitive, UCL1684-senstive, and Ca2+-sensitive IAHP were comparable (P > 0.05). Time control IAHP were not different from zero. F: decay time constant of apamin-sensitive, UCL1684-senstive, and Ca2+-sensitive IAHP was fitted by a single exponential equation (white curve superposed on the traces of A′, B′, and C′). The decay time constants of apamin-sensitive, UCL1684-senstive, and Ca2+-sensitive IAHP were not significantly different.
To identify and characterize the SK channels, we used an outside-out patch. As shown in Fig. 9A1 channel activities were recorded at holding potentials of −20 mV (top trace) and then +20 mV (middle trace). After apamin application, the channel activity completely disappeared (Fig. 9A1, bottom trace). Figure 9A2 shows the histogram of the number of opening events of the channel versus current amplitude at the holding potential of +20 mV from a PCMN. The average peak current amplitude was 6.4 ± 1.0 pA (n = 4). Figure 9A3 shows the open-time distribution using the events that had an opening time of >0.5 ms at the holding potential of +20 mV from the same PCMN as in Fig. 9A2. The average time constant of the exponential curve was 3.0 ± 1.3 ms (n = 4). The average channel open probability was 0.34 ± 0.09.
Fig. 9.
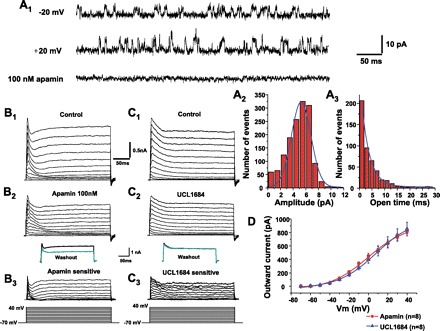
SK currents. A1: representative single channel currents recorded from an excised, outside-out patch held at −20 mV (top) and +20 mV (middle). Bottom shows an example after apamin application at a holding potential of +20 mV. After apamin application, the channel activity completely disappeared. A2: number of events vs. current amplitude relationship (all-point histogram) was fitted using a Gaussian function from the data at the holding potential of +20 mV for 2 s. A3: the open-time histogram was constructed from the data at a holding potential of +20 mV for 2 s, and the superimposed curve was fitted using single-exponential function. B1 and C1: family of outward currents were evoked by voltage steps from −70 to +40 mV for 250 ms with 10-mV increments every 5 s from the holding membrane potential of −70 mV as shown in the bottom of the traces. B2: outward currents evoked by voltage steps during application of apamin. B3: apamin-sensitive currents (subtraction of B1 and B2). Inset in B3 indicates that outward currents were irreversible after washout of apamin with control aCSF (67). C2: outward currents evoked by voltage steps in superfusing solution during UCL1684 application. C3: UCL1684-sensitive current (subtraction of C1 and C2). Inset in C3 indicates that outward currents were completely reversible after washout of UCL1684 with control HEPES solution. D: apamin-sensitive and UCL1684-sensitive currents were comparable, which were significantly activated by the membrane potentials above -40 mV.
As shown in Fig. 9, B and C, a family of outward currents were evoked by 250-ms voltage steps ranging from −70 to +40 mV in +10-mV increments from a holding potential of −70 mV in control HEPES solution. The outward currents were composed of a rapidly ascending and inactivating transient component and a persistent component. To examine whether these outward currents include the SK-mediated currents, we tested the effects of SK channel blockade on the transient outward currents. Apamin-sensitive transient outward currents (Fig. 9B3) were obtained by the difference of the currents before (Fig. 9B1) and after (Fig. 9B2) apamin application. Likewise, the UCL1684-sensitive transient outward currents (Fig. 9C3) were obtained by the difference of the outward currents before (Fig. 9C1) and after (Fig. 9C2) UCL1684 application. Apamin-sensitive and UCL1684-sensitive transient outward currents were comparable (Fig. 9D).
Reversal potential of apamin-sensitive IAHP.
To determine whether apamin-sensitive IAHP was mediated by K+ current, the reversal potential was estimated and compared with equilibrium potential of K+ (Ek). A family of IAHPs were evoked by a 100-ms, +10 mV voltage pulse from a holding potential of −70 mV followed by 1.5-s voltage steps of −120 mV to −10 mV with 10-mV increments. Figure 10A shows the representative apamin-sensitive IAHP. To determine the reversal potential, the peak amplitudes of apamin-sensitive IAHP were first plotted versus the membrane potential (Fig. 10B), and the reversal potential was defined as the potential when IAHP is equal to 0, which was −78 mV. After subtracting the liquid junction potential of 15 mV in PCMNs in our study, we estimated that the reversal potential of PCMNs was −93 mV, which is similar to the equilibrium potential Ek in the other central neurons as estimated by the Nernst equation.
Fig. 10.
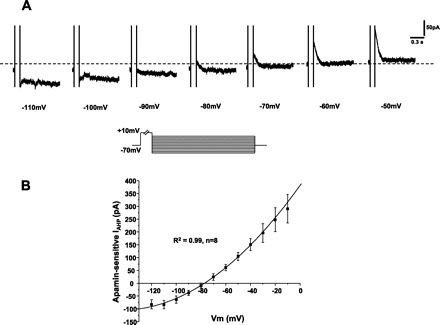
The reversal potential of apamin-sensitive IAHP. Apamin-sensitive IAHPs were evoked by a 100-ms, +10-mV depolarizing pulse followed by a family of 1.5-s voltage steps ranging from −110 to −10 mV. A: representative recordings of apamin-sensitive IAHPs in response to −110- to −50-mV steps with 10-mV increaments. B: IAHP curve versus voltage steps. The reversal potential of apamin-sensitive IAHP was estimated to be −78 mV.
DISCUSSION
We have examined the contributions of SK channels to AP properties (repolarization and afterhyperpolarization) in single APs and spike trains of different frequencies, and we characterized apamin-sensitive outward and IAHP. The present study has demonstrated that SK channels control the excitability of PCMNs by regulating their repolarization and AHP.
SFA.
Many neurons exhibit a time-dependent decrease in AP discharge rate in response to a sustained suprathreshold input. This phenomenon is termed SFA. SFA can be typically divided into two phases: 1) early, occurring over the first hundreds of milliseconds of firing; and 2) late occurring over tens of seconds or even minutes (4). The shorter initial interspike intervals associated with early adaptation may help to increase the speed of force generation in muscle fibers that can be sustained with lower frequencies (56). Later phases of adaptation may contribute to central fatigue during sustained muscular contractions (27). In the present study, we found that PCMNs exhibited an early SFA without a later phase of adaptation. After this initial phase SFA, PCMNs discharged at a steady discharge rate over the time of the current injection. Consistent with Miles et al. (45), our data showed that SK channels did not contribute to this early SFA of PCMNs. The mechanism underlying the early SFA in PCMNs is not clear, but it is likely, as shown in mammalian spinal motoneurons, that inactivation of the fast, inactivating sodium conductance may be a contributing factor to early SFA. It is possible that the PCMNs in NA, which receive glutaminergic synaptic drive from NTS-barosensitive neurons, generate the firing pattern of very little delay and an early SFA with repetitive longer subsequent intervals to effectively activate cardiac ganglionic neurons, which receive innervation from NA PCMNs (1, 9, 36) and thus regulating cardiac functions such as heart rate, as seen in the relationship between SFA and muscle contraction (56).
SK channels regulate the fast and medium AHPs.
The peak AHP amplitudes in PCMNs were significantly larger following single APs compared with APs following trains. This was consistent with the observations of other neurons (51, 61). SK channel blocker apamin suppressed amplitudes of AHPs by 30% for single APs and by 58% for trains at 50 ms.
In many neurons, AHP has multiple components, including fast, medium, and slow components (fAHP, mAHP, and sAHP, respectively). The fAHP that follows a single AP lasts a few to 10 ms (51, 61). The mAHP and sAHP have much longer durations (mAHP: from tens to several hundred ms; sAHP: from several hundred milliseconds to seconds) (16, 52). The mAHP largely contributes to spike frequency, and the sAHP mainly contributes to SFA (16). Unlike some other central neurons (31, 48, 49, 57), there is no clear boundary among fAHP, mAHP, and sAHP traces in PCMNs. In this study, we measured AHP at 10 and 50 ms following single APs and 50–1,000 ms following trains. Approximately 10 ms is in the fast or early medium duration range of AHP, and 50 ms is in the range of the medium duration of AHP. Since AHP almost returned to the resting membrane potentials at 100 ms following single APs and 500–1,000 ms following trains, PCMNs might not have a significant sAHP. This may explain why PCMNs only have SFA during the first few spikes, and no significant SFA was observed in the remaining part of the spike trains.
In the present study, the blockade of SK channels reduced the AHP amplitude at 10 and 50 ms following single APs. This suggests that SK channels in PCMNs not only contribute to mAHP but also likely the fAHP. Additionally, the blockade of SK channels reduced the AHP amplitude at 50 and 100 ms but did not affect AHP after 500 ms in trains. This strongly suggests that SK channels contribute to some of the fAHP, mostly to the mAHP, but not to the sAHP. We believe that PCMNs do not have a significant sAHP, which is consistent with the finding that PCMNs only have early frequency adaptation. Using voltage clamp, we found that PCMNs have significant outward currents and IAHP, which rapidly reach the peak in several milliseconds and decay exponentially with a time constant of about 50 ms. This latter kinetic property is associated with the timing of mAHP. This finding is consistent with other central neurons where the apamin-sensitive IAHP showed an exponential decay with a time constant of about 50 to 100 ms (57, 67). In contrast, the current underlying the slow IAHP (sIAHP) in other neurons shows a distinct rising phase, peaks between 400 and 700 ms after a train of APs, and decays with a time constant of ∼2,000 ms (28). Activation of such sIAHP limits spike firing frequency and is responsible for generating the SFA (52, 64). Because apamin-sensitive IAHP is mainly mIAHP, SK channels play little role in SFA.
Application of apamin and Ca2+-free bath solution revealed that apamin-sensitive IAHP was similar to Ca2+-sensitive IAHP in peak amplitude and decay time constant, indicating that apamin-sensitive IAHP may be Ca2+ dependent and mainly contribute to Ca2+-influx-mediated IAHP. These data are consistent with the recent data obtained from the PVN-RVLM presympathetic neurons where apamin-sensitive and Ca2+-sensitive IAHP are similar (7). With the use of current clamp, Ca2+-sensitive mAHP is similar to apamin-sensitive mAHP at 50 ms following single APs and at 50 and 100 ms following spike trains, but Ca2+-sensitive fAHP is significantly larger than apamin-sensitive fAHP (Fig. 3) at 10 ms following single APs, indicating that there may be some other Ca2+-activated K+ channels that may contribute to Ca2+-sensitive fAHP. According to our recent preliminary data (38), the BK channels may contribute to Ca2+-sensitive fAHP but not to Ca2+-sensitive mAHP. Therefore, we conclude that SK channel-mediated currents contribute to both mAHP and fAHP.
SK channels contribute to repolarization.
In many neurons of the central nervous system, influx of Ca2+ during APs activates K+ channels including BK channels and SK channels. BK channels are selectively blocked by paxilline (54, 62) and the currents mainly contribute to AP repolarization and the fAHP (a few ms; 17) that immediately follows it (28, 29, 50, 60). In contrast to BK channels, SK channels contribute minimally to the AP repolarization (16, 46).
In our study, we also examined the effect of blockade of SK channels on repolarization. Apamin and UCL1684 application to PCMNs significantly broadened the width of single APs and individual spikes in trains. During repetitive spike firing activity, the AP repolarization became slower and the spikes became broadened with subsequent spikes. Blockade of SK channels significantly increased the spike half-width of single APs as well as all spikes in the trains.
To further test whether inactivation of SK channels would contribute to spike broadening, we also used Ca2+-free solution to prevent Ca2+ influx in single AP experiments. Analogous to apamin application, Ca2+-free solution increased the AP half-width. Noticeably, AP broadening in Ca2+-free solution is larger than that seen with apamin application (Fig. 5), indicating that some other Ca2+-activated K+ may also contribute to AP broadening. According to our recent data, BK channels also contribute to AP broadening (38).
SK channels regulate neuronal excitability.
The role of SK channels in the regulation of neuronal and dendritic excitability has been established in many other central neurons (39, 42, 63). Calcium entry during APs activates SK currents that underlie AHP. SK currents regulate the firing rate and patterns of the APs (39, 42, 63). Though the SK channel proteins are expressed in NA neurons (53, 58), the physiological roles of SK channels in PCMNs have not yet been well characterized. Consistent with other central neurons, our study demonstrated that SK channels regulate AP properties and excitability of PCMNs. Blockade of SK channels with apamin and UCL1684 significantly increased spike firing rate, indicating that SK channels exert a powerful negative control over the firing rate of PCMNs. Two possible mechanisms may contribute to apamin-induced increase of firing rate. After apamin application, the depolarizing input resistance was increased such that the AP subthreshold depolarizing rate of membrane potentials was increased. In addition, the blocking mAHP of PCMNs may significantly increase the firing rate.
SK-channel function and neuropathology.
The normal functions of SK channels have been studied in many CNS neurons. The role of SK channels in the modulation of intrinsic firing patterns and excitability has implications on their possible involvement in neuronal dysfunction, either as part of the causal mechanism or as potential therapeutic targets (see Ref. 47 for a review). In various in vitro models, a downregulation of the SK-mediated IAHP paralleled the emergence of epileptiform activity, and SK channel inhibitors were shown to shape the duration and increase epileptiform bursting activity in the CA3 region. Conversely, SK channel enhancers (i.e., 1-EBIO) led to a cessation of spontaneous oscillatory activity in hyperexcitable neuronal networks and epileptiform activity in hippocampal slices. Similarly, in episodic ataxia, upregulating SK channel activity with enhancers that increase their Ca2+ sensitivity and spike timing precision may compensate for the disruption in the pacemaking precision of Purkinje cells. Chronic in vivo activation of cerebellar SK channels improves motor performance in ataxic phenotype of mice (65). In these models, upregulation of SK channels is needed to reduce hyperactivity in epilepsy and improve cerebellar-dependent motor coordination in ataxia. Similarly, it was reviewed that due to interrelations of altered Ca2+-activated K+ channels and cardiovascular disease states (such as hypertension, diabetes, dyslipidemia, and atherosclerosis), Ca2+-activated K+ channel openers may have the therapeutic potential as novel types of blood pressure-lowering drugs (21). Recently, we have studied the effect of maternal diabetes on the PCMNs in neonatal mice. We found that in contrast to other models mentioned above, SK outward currents and tail currents of PNCMs are increased in neonates from diabetic mothers and are associated with reduced excitability of PNCMs (34). Previously, we have found that chronic intermittent hypoxia and diabetes may impair baroreflex function, induce NA motoneuron death, and alter NA control over the heart rate (22, 23, 32, 36, 37, 68–70). Therefore, it will be critically important to study the alteration of the firing patterns and excitability of NA PCMNs and SK channels in these pathological diseases.
To summarize, in this study, we demonstrated that SK channels in PCMNs play a significant role in regulation of spike firing frequency but do not affect the firing adaptation. SK channels mediate the IAHP that contributes to not only the mAHP but also likely to the fAHP. We also found that SK channels in PCMNs contribute to repolarization. Although our study provides important information on the role of SK channels in regulating AP properties and excitability of PCMNs in the NA, further studies are needed to examine whether SK channels are the major type of Ca2+-activated K+ channels in regulating the excitability of NA PCMNs and how various disease states will change the regulation of SK channels on the firing pattern and excitability of PCMNs.
GRANTS
This work was supported by National Institutes of Health Grants HL-79636 and AG-021020 (to Z. J. Cheng).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Ai J, Epstein PN, Gozal D, Yang B, Wurster R, Cheng Z. Morphology and topography of nucleus ambiguus projections to cardiac ganglia in rats and mice. Neuroscience 149: 845–860, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Armstrong CM, Bezanilla F. Inactivation of the sodium channel. II. Gating current experiments. J Gen Physiol 70: 567–590, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barman SM. Hyped up about the hypothalamus. J Physiol 587: 4129–4130, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brenner R, Chen QH, Vilaythong A, Toney GM, Noebels JL, Aldrich RW. BK channel beta4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nature Neurosci 8: 1752–1759, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Castle NA, Haylett DG, Jenkinson DH. Toxins in the characterization of potassium channels. Trends Neurosci 12: 59–65, 1989 [DOI] [PubMed] [Google Scholar]
- 6. Campos Rosa J, Galanakis D, Ganellin CR, Dunn PM, Jenkinson DH. Bis-quinolinium cyclophanes: 6,10-diaza-3(1,3),8(1,4)-dibenzena-1,5(1,4)-diquinolinacyclodecaphane (UCL 1684), the first nanomolar, non-peptidic blocker of the apamin-sensitive Ca2+-activated K+ channel. J Med Chem 41: 2–5, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Chen QH, Toney GM. Excitability of paraventricular nucleus neurones that project to the rostral ventrolateral medulla is regulated by small-conductance Ca2+-activated K+ channels. J Physiol 587: 4235–4247, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheng Z, Powley TL. Nucleus ambiguus projections to cardiac ganglia of rat atria: an anterograde tracing study. J Comp Neurol 424: 588–606, 2000 [PubMed] [Google Scholar]
- 9. Cheng Z, Zhang H, Guo SZ, Wurster R, Gozal D. Differential control over vagal efferent postganglionic neurons in rat intrinsic cardiac ganglia by neurons in the nucleus ambiguus and the dorsal motor nucleus of the vagus: anatomical evidence. Am J Physiol Regul Integr Comp Physiol 286: R625–R633, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Cheng Z, Zhang H, Yu J, Wurster R, Gozal D. Attenuation of baroreflex sensitivity following domoic acid lesion of the nucleus ambiguus of rats. J Appl Physiol 96: 1137–1145, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Colquhoun D, Sigworth FJ. Fitting and statistical analysis of single channel records. In: Single Channel Recording (2nd ed), edited by Sakmann B, Neher E. New York: Plenum, 1995, p. 483–587 [Google Scholar]
- 12. Davies PJ, Ireland DR, McLachlan EM. Sources of Ca2+ for different 12. Ca2+-activated K+ conductances in neurones of the rat superior cervical ganglion. J Physiol 495: 353–366, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dunn PM. UCL1684: a potent blocker of Ca2+-activated K+ channels in rat adrenal chromaffin cells in culture. Eur J Pharmacol 368: 119–123, 1999 [DOI] [PubMed] [Google Scholar]
- 14. El-Menyar AA. Dysrhythmia and electrocardiographic changes in diabetes mellitus: pathophysiology and impact on the incidence of sudden cardiac death. J Cardiovasc Med (Hagerstown) 7: 580–855, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Epstein PN, Overbeek PA, Means AR. Calmodulin-induced early-onset diabetes in transgenic mice. Cell 58: 1067–1073, 1989. [DOI] [PubMed] [Google Scholar]
- 16. Faber ESL, Sah P. Physiological role of calcium-activated potassium currents in the rat lateral amygdala. J Neurosci 22: 1618–1628, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Faber ES, Sah P. Functions of SK channels in central neurons. Clin Exp Pharmacol Physiol 34: 1077–1083, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Foehring RC, Zhang XF, Lee JC, Callaway JC. Endogenous calcium buffering capacity of substantia nigral dopamine neurons. J Neurophysiol 102: 2326–2333, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Giese KP, Storm JF, Reuter D, Fedorov NB, Shao LR, Leicher T, Pongs O, Silva AJ. Reduced K+ channel inactivation, spike broadening, and after-hyperpolarization in Kvbeta1.1-deficient mice with impaired learning. Learn Mem 5: 257–273, 1998 [PMC free article] [PubMed] [Google Scholar]
- 20. Greffrath W, Magerl W, Disque-Kaiser U, Martin E, Reuss S, Boehmer G. Contribution of Ca2+-activated K+ channels to hyperpolarizing after-potentials and discharge pattern in rat supraoptic neurones. J Neuroendocrinol 16: 577–588, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Grgic I, Kaistha BP, Hoyer J, Köhler R. Endothelial Ca2+-activated K+ channels in normal and impaired EDHF-dilator responses–relevance to cardiovascular pathologies and drug discovery. Br J Pharmacol 157: 509–526, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gu H, Lin M, Liu J, Gozal D, Scrogin KE, Wurster R, Chapleau MW, Ma X, Cheng ZJ. Selective impairment of central mediation of baroreflex in anesthetized young adult Fischer 344 rats after chronic intermittent hypoxia. Am J Physiol Heart Circ Physiol 293: H2809–H2818, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Gu H, Epstein PN, Li L, Wurster RD, Cheng ZJ. Functional changes in baroreceptor afferent, central and efferent components of the baroreflex circuitry in type 1 diabetic mice (OVE26). Neuroscience 152: 741–752, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Gu H, Zhang ZH, Epstein PN, Li L, Harden SW, Wurster RD, Cheng ZJ. Impaired baroreflex control of renal sympathetic nerve activity in type 1 diabetic mice (OVE26). Neuroscience 161: 78–85, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Hallworth NE, Wilson CJ, Bevan MD. Apamin-sensitive small conductance calcium-activated potassium channels, through their selective coupling to voltage-gated calcium channels, are critical determinants of the precision, pace, and pattern of action potential generation in rat subthalamic nucleus neurons in vitro. J Neurosci 23: 7525–7542, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaczorowski GJ, Garcia ML. Pharmacology of voltage-gated and calcium-activated potassium channels. Curr Opin Chem Biol 3: 448–458, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Kernell D, Monster AW. Motoneuron properties and motor fatigue. An intracellular study of gastrocnemius motoneurons of the cat. Exp Brain Res 46: 197–204, 1982 [DOI] [PubMed] [Google Scholar]
- 28. Lancaster B, Adams PR. Calcium-dependent current generating the afterhyperpolarization of hippocampal neurons. J Neurophysiol 55: 1268–1282, 1986 [DOI] [PubMed] [Google Scholar]
- 29. Lancaster B, Nicoll RA. Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. J Physiol 389: 187–204, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lawrence IG, Weston PJ, Bennett MA, McNally PG, Burden DC, Thurston H. Is impaired baroreflex sensitivity a predictor or cause of sudden death in insulin-dependent diabetes mellitus? Diabet Med 14: 82–85, 1997. [DOI] [PubMed] [Google Scholar]
- 31. Li X, Bennett DJ. Apamin-sensitive calcium-activated potassium currents (SK) are activated by persistent calcium currents in rat motoneurons. J Neurophysiol 97: 3314–3330, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li L, Huang C, Ai J, Yan B, Gu H, Ma Z, Li AY, Xinyan S, Harden SW, Hatcher JT, Wurster RD, Cheng ZJ. Structural remodeling of vagal afferent innervation of aortic arch and nucleus ambiguus (NA) projections to cardiac ganglia in a transgenic mouse model of type 1 diabetes (OVE26). J Comp Neurol 518: 2771–2793, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Liang Q, Carlson EC, Donthi RV, Kralik PM, Shen X, Epstein PN. Overexpression of metallothionein reduces diabetic cardiomyopathy. Diabetes 51: 174–181, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Lin M, Chen QH, Wurster RD, Hatcher JT, Liu YQ, Li LH, Harden SW, Cheng ZJ. Maternal diabetes increases SK currents which alter action potential properties and excitability of cardiac motoneurones in the nucleus ambiguus. J Neurophysiol 104: 2125–2138, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lin M, Liu R, Gozal D, Wead WB, Chapleau MW, Wurster RD, Cheng Z. Chronic intermittent hypoxia impairs baroreflex control of heart rate but enhances heart rate responses to vagal efferent stimulation in anesthetized mice. Am J Physiol Heart Circ Physiol 293: H997–H1006, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Lin M, Ai J, Li L, Huang C, Chapleau MW, Liu R, Gozal D, Wead WB, Wurster RD, Cheng Z. Structural remodeling of nucleus ambiguus projections to cardiac ganglia following chronic intermittent hypoxia in C57BL/6J mice. J Comp Neurol 509: 103–117, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Lin M, Harden SW, Li L, Wurster RD, Cheng ZJ. Impairment of baroreflex control of heart rate in conscious transgenic mice of type 1 diabetes (OVE26). Auton Neurosci 152: 67–74, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Lin M, Chen QH, Li L, Wurster RD, Liu YQ, Cheng Z. Maternal diabetes (MD) increases large conductance Ca2+-activated K+ (BK) currents which alter action potential (AP) properties but do not affect excitability of parasympathetic cardiac motoneurons (PCMNs) in the nucleus ambiguus (NA) of neonatal mice. EXPER BIOL Abstract Number: 7205, Anaheim, CA 92802, 2010 [Google Scholar]
- 39. Liu X, Herbison AE. Small-conductance calcium-activated potassium channels control excitability and firing dynamics in gonadotropin-releasing hormone (GnRH) neurons. Endocrinology 149: 3598–3604, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Loewy AD, Spyer KM. Central Regulation of Autonomic Functions. New York: Oxford, 1990 [Google Scholar]
- 41. Ma M, Koester J. The role of K+ currents in frequency-dependent spike broadening in Aplysia R20 neurons: a dynamic-clamp analysis. J Neurosci 16: 4089–4101, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Maher BJ, Westbrook GL. SK channel regulation of dendritic excitability and dendrodendritic inhibition in the olfactory bulb. J Neurophysiol 94: 3743–3750, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Mendelowitz D. Firing properties of identified parasympathetic cardiac neurons in the nucleus ambiguus. Am J Physiol Heart Circ Physiol 271: H2609–H2614, 1996 [DOI] [PubMed] [Google Scholar]
- 44. Mendelowitz D. Advances in parasympathetic control of heart rate and cardiac function. News Physiol Sci 14: 155–161, 1999 [DOI] [PubMed] [Google Scholar]
- 45. Miles GB, Dai Y, Brownstone RM. Mechanisms underlying the early phase of spike frequency adaptation in mouse spinal motoneurones. J Physiol 566: 519–532, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pedarzani P, Kulik A, Muller M, Ballanyi K, Stocker M. Molecular determinants of Ca2+-dependent K+ channel function in rat dorsal vagal neurones. J Physiol 527: 283–290, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pedarzani P, Stocker M. Molecular and cellular basis of small- and intermediate-conductance, calcium-activated potassium channel function in the brain. Cell Mol Life Sci 65: 3196–3217, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Saar D, Grossman Y, Barkai E. Long lasting cholinergic modulation underlies rule learning in rats. J Neuorsci 21: 1385–1392, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Saar D, Barkai E. Long-term modifications in intrinsic neuronal properties and rule learning in rats. Eur J Neurosci 17: 2727–2734, 2003 [DOI] [PubMed] [Google Scholar]
- 50. Sah P, McLachlan EM. Potassium currents contributing to action potential repolarization and the afterhyperpolarization in rat vagal motoneurons. J Neurophysiol 68: 1834–1841, 1992 [DOI] [PubMed] [Google Scholar]
- 51. Sah P. Ca2+-activated K+ currents in neurons: types, physiological roles and modulation. Trends Neurosci 19: 150–154, 1996 [DOI] [PubMed] [Google Scholar]
- 52. Sah P, Faber ES. Channels underlying neuronal calcium-activated potassium currents. Prog Neurobiol 66: 345–353, 2002 [DOI] [PubMed] [Google Scholar]
- 53. Sailer CA, Kaufmann WA, Marksteiner J, Knaus HG. Comparative immunohistochemical distribution of three small-conductance Ca2+-activated potassium channel subunits, SK1, SK2, and SK3 in mouse brain. Mol Cell Neurosci 26: 458–469, 2004 [DOI] [PubMed] [Google Scholar]
- 54. Sanchez M, McManus OB. Paxilline inhibition of the alpha-subunit of the high-conductance calcium-activated potassium channel. Neuropharmacology 35: 963–968, 1996 [DOI] [PubMed] [Google Scholar]
- 55. Spray DC, Harris AL, Bennett MV. Equilibrium properties of a voltage-dependent junctional conductance. J Gen Physiol 77: 77–93, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Stein RB, Parmiggiani F. Optimal motor patterns for activating mammalian muscle. Brain Res 175: 372–376, 1979 [DOI] [PubMed] [Google Scholar]
- 57. Stocker M, Krause M, Pedarzani P. An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons. Proc Natl Acad Sci USA 96: 4662–4667, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stocker M, Pedarzani P. Differential distribution of three Ca2+-activated K+ channel subunits, SK1, SK2, and SK3, in the adult rat central nervous system. Mol Cell Neurosci 15, 476–493, 2000 [DOI] [PubMed] [Google Scholar]
- 59. Stocker M. Ca2+-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci 5: 758–770, 2004 [DOI] [PubMed] [Google Scholar]
- 60. Storm JF. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol 385: 733–759, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Storm JF. An after-hyperpolarization of medium duration in rat hippocampal pyramidal cells. J Physiol 409: 171–190, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Strøbaek D, Christophersen P, Holm NR, Moldt P, Ahring PK, Johansen TE, Olesen SP. Modulation of the Ca2+-dependent K+ channel, hslo, by the substituted diphenylurea NS 1608, paxilline and internal Ca2+. Neuropharmacology 35: 903–914, 1996 [DOI] [PubMed] [Google Scholar]
- 63. Teshima K, Kim SH, Allen CN. Characterization of an apamin-sensitive potassium current in suprachiasmatic nucleus neurons. Neuroscience 120: 65–73, 2003 [DOI] [PubMed] [Google Scholar]
- 64. Vergara C, Latorre R, Marrion NV, Adelman JP. Calcium-activated potassium channels. Curr Opin Neurobiol 8: 321–329, 1998 [DOI] [PubMed] [Google Scholar]
- 65. Walter JT, Alviña K, Womack MD, Chevez C, Khodakhah K. Decreases in the precision of Purkinje cell pacemaking cause cerebellar dysfunction and ataxia. Nat Neurosci 9: 389–397, 2006 [DOI] [PubMed] [Google Scholar]
- 66. Wang J, Irnaten M, Neff RA, Venkatesan P, Evans C, Loewy AD, Mettenleiter TC, Mendelowitz D. Synaptic and neurotransmitter activation of cardiac vagal neurons in the nucleus ambiguus. Ann NY Acad Sci 940: 237–246, 2001 [DOI] [PubMed] [Google Scholar]
- 67. Wolfart J, Neuhoff H, Franz O, Roeper J. Differential expression of the small-conductance, calcium-activated potassium channel SK3 Is critical for pacemaker control in dopaminergic midbrain neurons. J Neurosci 21: 3443–3456, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yan B, Soukhova-O'Hare GK, Li L, Lin Y, Gozal D, Wead WB, Wurster RD, Cheng Z. Attenuation of heart rate control and neural degeneration in nucleus ambiguus following chronic intermittent hypoxia in young adult Fischer 344 rats. Neuroscience 153: 709–720, 2008 [DOI] [PubMed] [Google Scholar]
- 69. Yan B, Li L, Harden SW, Gozal D, Lin Y, Wead WB, Wurster RD, Cheng Z. Chronic intermittent hypoxia impairs heart rate responses to AMPA and NMDA and induces loss of glutamate receptor neurons in nucleus ambiguous of F344 rats. Am J Physiol Regul Integr Comp Physiol 296: R299–R308, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yan B, Li L, Harden SW, Epstein PN, Wurster RD, Cheng Z. Diabetes induces neural degeneration in nucleus ambiguus (NA) and attenuates heart rate control in OVE26 mice. Exp Neurol 220: 34–43, 2009 [DOI] [PubMed] [Google Scholar]
- 71. Zheng S, Noonan WT, Metreveli NS, Coventry S, Kralik PM, Carlson EC, Epstein PN. Development of late-stage diabetic nephropathy in OVE26 diabetic mice. Diabetes 53: 3248–3257, 2004 [DOI] [PubMed] [Google Scholar]


