Abstract
Muscarinic receptors are expressed in the adrenal medullary (AM) cells of various mammals, but their physiological roles are controversial. Therefore, the ionic mechanism for muscarinic receptor-mediated depolarization and the role of muscarinic receptors in neuronal transmission were investigated in dissociated guinea-pig AM cells and in the perfused guinea-pig adrenal gland. Bath application of muscarine induced an inward current at −60 mV. This inward current was partially suppressed by quinine with an IC50 of 6.1 μM. The quinine-insensitive component of muscarine-induced currents changed the polarity at −78 mV and was inhibited by bupivacaine, a TWIK-related acid-sensitive K+ (TASK) channel inhibitor. Conversely, the current-voltage relationship for the bupivacaine-insensitive component of muscarine currents showed a reversal potential of −5 mV and a negative slope below −40 mV. External application of La3+ had a double action on muscarine currents of both enhancement and suppression. Immunoblotting and immunocytochemistry revealed expression of TASK1 channels and cononical transient receptor potential channels 1, 4, 5, and 7 in guinea-pig AM cells. Retrograde application of atropine reversibly suppressed transsynaptically evoked catecholamine secretion from the adrenal gland. The results indicate that muscarinic receptor stimulation in guinea-pig AM cells induces depolarization through inhibition of TASK channels and activation of nonselective cation channels and that muscarinic receptors are involved in neuronal transmission from the splanchnic nerve.
Keywords: TWIK-related acid-sensitive K+ channel, cononical transient receptor potential channel, neuronal transmission, acetylcholine, depolarization
adrenal medullary (am) cells secrete catecholamines in response to acetylcholine (ACh) from the splanchnic nerve terminal. This ACh-mediated signal is thought to be received mainly by nicotinic ACh receptors, and the role of muscarinic receptors in neurotransmission is controversial (1, 20–22, 30, 38, 39, 42, 52). Muscarinic receptors, however, have biochemically and/or functionally been detected in guinea-pig (22, 26), cat (2), bovine (2, 14), porcine (17), chicken (32), rat (23, 38, 39), and human (43) AM cells. In rat AM cells, stimulation of the M5 muscarinic receptors has been suggested to induce inhibition of TWIK-related acid-sensitive K+ channel 1 (TASK1) activity with the consequent secretion of catecholamine (20, 23). This muscarinic signaling was involved in synaptic transmission from the preganglionic nerve fiber only under conditions where the degradation of ACh by cholinesterase was impaired (20). In contrast, catecholamine secretion evoked by electrical stimulation of nerve fibers was partially suppressed by application of atropine, a muscarinic antagonist, in isolated perfused adrenal glands of spontaneously hypertensive rats (SHRs) but not normotensive Wistar-Kyoto (WKY) rats (39), suggesting plasticity of the muscarinic signaling in AM cells.
We have reported that muscarinic receptors in guinea-pig AM cells are coupled with nonselective cation (NS) channels (24), which are equally permeable to Na+ and K+ (26). This notion, however, might be modified in light of the recent finding that muscarinic receptor stimulation in rat AM cells induced an inhibition of TASK channel activity. TASK channels, which form a subfamily in the two-pore-domain background K+ channel family (3, 16), are characteristically suppressed by external H+, and pH sensitivity of the TASK1 channel, which was found to be expressed in rat AM cells (23), is in the physiological range (16). Thus TASK1 channels could be thought to detect acidosis, which may occur during severe exercise (36). The inhibition of TASK1 channels by H+ induces a depolarization with the consequent activation of voltage-dependent Ca2+ channels, resulting in facilitation of catecholamine secretion (23), which may help recovery from muscle fatigue (10). Based on this property, TASK1 channels are thought to play an essential role in the endocrine function in AM cells and thus are expected to be expressed in a wide range of mammalian AM cells. The first aim of the present study was to reexamine the ionic mechanism for muscarinic receptor-mediated depolarization in guinea-pig AM cells. The second aim was to explore whether muscarinic signaling participates in neuronal transmission or not. The results obtained indicated that muscarinic receptor stimulation is coupled with not only activation of NS channels but also inhibition of TASK channels. This multiplicity of signaling may allow for the muscarinic receptor to mediate neuronal transmission in guinea-pig AM cells.
MATERIALS AND METHODS
Male Hartley guinea pigs weighing 250–400 g (n = 57) were used. All experiment procedures involving animals were approved by the Institutional Animal Care and Use Committee of the University of Occupational and Environmental Health.
Immunoblotting.
The animals were killed by cervical dislocation, and the brain and adrenal glands were excised and immediately put into ice-cold Ca2+-deficient saline in which 1.8 mM CaCl2 was simply omitted from standard saline. The standard saline contained 137 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.5 mM MgCl2, 0.53 mM NaHPO4, 5 mM d-glucose, 5 mM HEPES, and 4 mM NaOH (pH 7.4). The adrenal cortex was removed from the adrenal gland using microscissors and forceps under stereoscopic observations. The preparations were minced and homogenized with a Potter-Elvehjem homogenizer in 10 vol of a solution containing 10 mM Tris·HCl (pH 7.4), 150 mM NaCl, and a protease inhibitor cocktail (set 1: Calbiochem, San Diego, CA). Homogenates were centrifuged at 500 g for 10 min at 4°C, and the subsequent postnucleus supernatants were mixed with equal volumes of a SDS buffer containing 25 mM Tris·HCl (pH 6.8), 4% SDS, and 20% glycerol. The protein concentrations of samples were determined using a BSA protein assay kit (Pierce, Rockford, IL). After addition of 2-mercaptoethanol [final content, 5% (vol/vol)] and bromophenol blue [0.05% (vol/vol)] to the sample, proteins were separated by 10% (w/v) SDS-PAGE and then transferred to a PVDF membrane. The membrane was blocked with 5% (wt/vol) fat-free powdered milk dissolved in PBS-T solution, which contained 2 mM NaH2PO4, 8 mM Na2HPO4, and 145 mM NaCl and 0.1% Tween 20. The PVDF membrane was incubated with rabbit anti-TASK1 antibody (Ab; APC-024: Alomone, Jerusalem, Israel), mouse anti-STIM1 monoclonal Ab (mAb; 610954; BD Bioscience, San Jose, CA), mouse anti-actin mAb (MAB1501R; Chemicon, Temecula, CA), mouse anti-cononical transient receptor potential (TRPC)4 mAb (75–119; Antibodies, Davis, CA), mouse anti-TRPC5 mAb (75–104; Antibodies), or mouse anti-TRPC7 mAb (73–123; Antibodies). The immunoreaction was detected by incubating the membrane with the respective secondary Ab linked to horseradish peroxidase (Amersham, Buckinghamshire, UK) and then with ECL-Plus (Amersham). Immunoblotting was repeated at least three times for each Ab. The neutralization of an Ab with its antigen was performed according to the manufacturer's instructions.
The immunoprecipitation assay was performed in a manner similar to that described elsewhere (34). Briefly, postnuclear supernatants were solubilized in an immunoprecipitation buffer [10 μM deoxycholate, 150 mM NaCl, 10 mM Tris·HCl (pH 7.4), and the protease inhibitor cocktail] to bring the final protein concentration to 1 to 2 μg/μl. The sample was centrifuged at 12,000 g for 3 min at 4°C to pellet insoluble materials. The supernatant was incubated with rabbit anti-TRPC1 Ab (SC-20110: Santa Cruz Biotechnology, Santa Cruz, CA) or rabbit IgG and then with protein G-Sepharose at 4°C for 3 h. The mixture was washed three times in buffer-I (1% NP-40, 150 mM NaCl, 20 mM Tris, and 2 mM EDTA, pH 7.5). Immunoprecipitated proteins were dissociated from beads by incubation in Laemmli sample buffer for 30 min at 37°C and then subjected to immunoblotting with anti-TRPC1 Ab (ACC-010: Alomone).
Whole cell recording.
The whole cell current was recorded in an isolated guinea-pig AM cell using the nystatin perforated patch method, as described elsewhere (23, 24). Briefly, adrenal medullae were treated with collagenase for 30 min and AM cells were dissociated mechanically with fine needles. The standard pipette solution contained 120 mM potassium isethionate, 20 mM KCl, 10 mM NaCl, 10 mM HEPES, and 2.6 mM KOH (pH 7.2). On the day of the experiment, nystatin dissolved in dimethyl sulfoxide (5 mg in 100 μl) was added to the pipette solution at a final concentration of 100 μg/ml. All chemicals were bath applied. The membrane potential was corrected for a liquid junction potential of −3 mV between the standard pipette solution and saline. For the analysis of the dose-dependent inhibition of muscarine-induced current (I) by quinine, Sigma plot (10.0: SPSS, Chicago, IL) was used to fit a peak value of I to the logistic equation I = (Imax × χa)/(IC50a + χa) + b, where I in the presence of quinine is expressed as a fraction of that in the absence, Imax represents the maximum value of quinine-sensitive I, χ is the concentration of quinine, IC50 is the concentration of quinine responsible for half the inhibition, a is a slope factor, and b is a constant, a component of I which is insensitive to quinine. Experiments were carried out at 26 ± 2°C. Data in cells (n, number of cells examined), which were obtained from at least two animals, are expressed as the means ± SE, and statistical significance was determined using Student's paired or unpaired t-test.
Perfusion experiments.
The adrenal glands were removed from rats under pentobarbital (60 mg/kg ip) anesthesia and then perfused retrogradely via the adrenal vein with saline at a rate of 0.15 ml/min, as reported previously (54, 55). The adrenal gland was placed between one pair of silver circles for electrical stimulation, and then the gland was transferred to a chamber. Catecholamines secreted from AM cells were measured using the amperometric method (54).
Immunocytochemistry.
Immunostaining of dissociated AM cells was performed, as described previously (23). For indirect immunofluorescence studies, cells were treated overnight with anti-TASK1 Ab, anti-TRPC1 Ab (Santa Cruz), anti-TRPC4 mAb, anti-TRPC5 mAb, anti-TRPC7 mAb, or anti-STIM1 mAb. After incubation, the cells were washed three times in PBS and then treated with a respective secondary Ab conjugated with Alexa 488 or 546 (Molecular Probe, Eugene, OR). The fluorescence was observed using a laser scanning confocal microscope (LSM5Pascal: Carl Zeiss, Tokyo, Japan). The objective lens was an oil-immersion lens with a magnification of ×63, and fluorescence was observed with appropriate laser lines and filter sets.
RT-PCR.
Poly(A)+RNA was isolated from rat brain, adrenal medulla, and adrenal cortex using the Micro-fast track kit (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Oligo dT primer was utilized for the RT reaction to obtain cDNAs. PCR reactions were carried out with 1.25 μl of DNA template, 4 pmol of primers (20), 2 mM of dNTPs, 0.5 U of rTaq (Takara, Otsu, Japan), and PCR buffers supplied with the kit in a final volume of 20 μl. The PCR protocol used started with an initial 3-min denaturation step at 94°C, followed by 30 to 40 cycles of the profile consisting of 30 s of denaturation at 94°C, 30 s of annealing at 54°C to 60°C, and 30 s of extension at 72°C. To obtain the maximum fidelity, a hot-start procedure was used. In each PCR reaction, an 198-bp PCR product of β-actin mRNA was coamplified and used as an internal standard. The PCR products were separated by 1.5% agarose gel electrophoresis and stained with ethidium bromide.
Source of reagents.
Muscarine chloride, methoxyverapamil hydrochloride (D-600), flufenamic acid, and bupivacaine hydrochloride were obtained from Sigma-Aldrich (St. Louis, MO); quinine was from Nacalai (Kyoto, Japan); collagenase was from Yakult (Tokyo, Japan).
RESULTS
Involvement of K+ channel.
Since TASK channels are expected to be expressed in various mammalian AM cells, muscarinic receptors in guinea-pig AM cells may be coupled with not only NS channels but also K+ channels. Therefore, we searched for pharmacological tools to selectively inhibit the NS channels involved without altering baseline (Fig. 1). Flufenamic acid (FFA) is known to enhance TRPC6 channel activity and to inhibit other TRPC channel subtypes (9, 27, 28), which are a candidate for receptor-regulated NS channels (31). Application of 100 μM FFA resulted in the gradual development of an inward current at the holding potential of −70 mV and produced an inhibition of muscarine-induced currents of 66% (Fig. 1, A and D). After washout, muscarine-induced currents were restored to the original level. Application of 100 μM Cd2+, a divalent cation that suppresses NS channels including TRPC5 (56) and voltage-dependent Ca2+ channels, resulted in production of outward currents and induced 37% inhibition of the muscarine current (Fig. 1, B and D). In contrast to these reagents, 10 μM D-600, an inhibitor of the Ca2+ channels, reversibly produced 41% inhibition of the muscarine current without any effect on the baseline (Fig. 1, C and D). Similar to D-600, 100 μM quinine, a chemical that is known to suppress NS channels (7, 49), reversibly produced an inhibition of the muscarine-induced current with no marked effect on the baseline (Fig. 2A). This quinine-induced inhibition was dose dependent with an IC50 of 6.1 μM and was not complete. The quinine-sensitive and -insensitive components were estimated to constitute 60 and 40% of the 10 μM muscarine-induced currents, respectively (Fig. 2B).
Fig. 1.
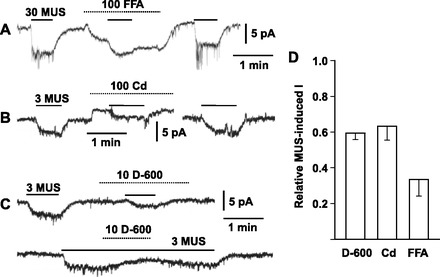
Pharmacological properties of muscarine-induced currents in dissociated guinea-pig adrenal medullary cells. A–C: reversible suppression of muscarine (MUS)-induced inward currents by 100 μM fulfenamic acid (FFA), 100 μM Cd2+, and 10 μM D-600, respectively. Whole cell current was recorded using the nystatin method in dissociated guinea-pig adrenal medullary (AM) cells. Holding potential was −70 mV (A), −65 mV (B), and −60 mV (C). MUS at 3 or 30 μM and other chemicals were added to saline during the indicated periods (bars and interrupted line for MUS and other chemicals, respectively). D: summary of 3 (D600 and Cd) or 30 μM (FFA) MUS-induced currents in the presence of 10 μM D-600, 100 μM Cd2+, or 100 μM FFA. MUS-induced currents in the presence of inhibitors were expressed as fractions of those in the absence. Data represent means ± SE (n = 7 for D-600; n = 6 for Cd2+; n = 4 for FFA).
Fig. 2.
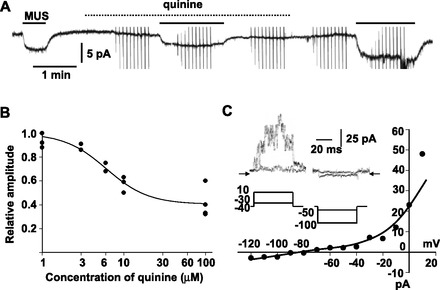
Quinine-insensitive component in MUS-induced current in guinea-pig AM cells. A: reversible suppression of 10 μM MUS-induced current by 100 μM quinine. Whole cell current was recorded at −60 mV with the nystatin method. Chemicals were added to saline during the indicated periods (bars for MUS; dotted line for quinine). B: summary of inhibition of 10 μM muscarine-induced currents by quinine of various concentrations in 4 cells. Peak amplitudes of muscarine-induced currents in the presence of quinine were expressed relative to averaged amplitudes of the currents before and after addition of quinine. These relative values were plotted against concentrations of quinine. Line represents y = 0.4 + 0.6/[1 + (x/6.1)1.7] (see materials and methods). C: current-voltage (I-V) relationship for muscarine-induced current in the presence of 100 μM quinine; 50-ms pulses were applied from the holding potential of −40 mV in 10-mV steps before, during, and after addition of muscarine to 100 μM quinine-containing saline. Muscarine-sensitive currents were obtained by subtracting current responses during addition of muscarine from averages of those before and after it. Current level at the end of pulse was measured and plotted against the command potential. Inset: muscarine-sensitive currents (top) obtained with the command pulses (bottom) in the presence of 100 μM quinine. Arrows indicate the zero current level.
To explore the ionic mechanism for the quinine-insensitive component, its current-voltage (I-V) relationship was examined with application of 50-ms pulses in 10-mV steps. As is evident in Fig. 2C, the muscarine-sensitive currents obtained by subtraction of the current responses in the presence of muscarine from those in the absence changed instantaneously with negative pulses; reversed its direction at −78.0 ± 2.1 mV (n = 5), a value that approximates the equilibrium potential (−83.6 mV) for K+; and revealed outward rectification. These properties of the muscarine current in the presence of quinine were consistent with those of TASK channels in rat AM cells (23). Thus we examined effects of bupivacaine, a TASK channel inhibitor (5), on 10 μM muscarine-induced currents. Application of 100 μM bupivacaine produced an inward current at −60 mV and inhibited the muscarine current by 36.2 ± 3.8% (n = 4; Fig. 3A). A decrease in external pH to 6.5 also elicited an inward current with the reversal potential of −77.4 ± 2.4 mV (n = 5) and abolished a further development of inward current in response to bupivacaine (Fig. 3B). These observations supported the notion that the bupivacaine-induced inward current is due to inhibition of TASK channels and the bupivacaine-sensitive component of muscarine-induced currents represents inhibition of TASK channel activity. In the presence of bupivacaine, 10 μM muscarine-induced currents were markedly suppressed by 100 μM quinine (95.3 ± 4.7%, n = 3; Fig. 3C). Taken together, these results suggested that the muscarine-induced current comprises two components that are sensitive to quinine and bupivacaine, respectively.
Fig. 3.
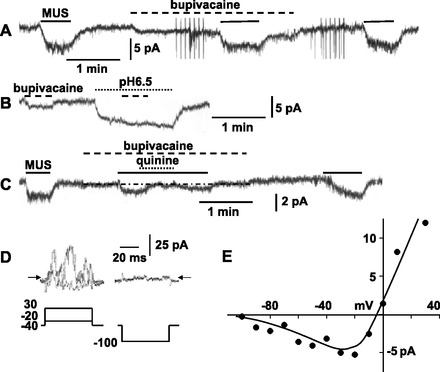
Involvement of NS channels in MUS-induced currents in guinea-pig AM cells. A: application of 100 μM bupivacaine partially suppresses 10 μM muscarine-induced currents. Whole cell current was recorded using the nystatin method. B: bupivacaine fails to induce a further inward current during exposure to saline with a pH of 6.5. C: 10 μM MUS-induced current in the presence of 100 μM bupivacaine is nearly abolished by 100 μM quinine. MUS, bupivacaine, and other chemicals were added to saline during the indicated periods (bars for MUS; interrupted lines for bupivacaine; dotted lines for pH of 6.5 or quinine). D and E: 10 μM MUS-sensitive currents in response to command pulses in 100 μM bupivacaine and I-V relationship, respectively. MUS-sensitive currents were obtained by subtracting averages of current responses before and after application of muscarine from those during it. Amplitude of current evoked by negative pulses was measured at the end of the pulse, whereas all the values of current in response to 50-ms positive pulses were measured and averaged. Arrows indicate the zero current level.
Involvement of NS channels.
To explore ion channels involved in the bupivacaine-insensitive component, the membrane potential was held at −40 mV to inactivate voltage-dependent Ca2+ channels and 50-ms pulses were applied before, during, and after administration of muscarine in the presence of bupivacaine. Muscarine-sensitive currents obtained under such conditions instantaneously changed in amplitude with negative pulses (Fig. 3D). The I-V relationship (Fig. 3E) showed that the reversal potential was about −5 mV (−5.4 ± 3.2 mV; n = 7) and a negative slope was present below −40 mV. This characteristic I-V relationship is reminiscent of that of heteromers of TRPC1 and TRPC4 or TRPC5 (8, 48).
Dual effect of La3+.
The involvement of TRPC4 or TRPC5 was explored by examining the effects of La3+. Trivalent cations, such as La3+ and Gd3+, are known to produce not only facilitatory but also inhibitory effects on TRPC4 and TRPC5 channels (29, 46), whereas they produce only an inhibitory effect on many NS channels, including other TRPC channel subtypes (9). As shown in Fig. 4A, application of 600 μM La3+ ions produced an inward current with a marked decrease in current noise level in some of the AM cells. This La3+-induced inward current had a reversal potential of −83 mV (n = 2) and markedly diminished with a decrease in external pH to 6.5 (Fig. 4B). These results suggested that the La3+-induced inward current was in part ascribed to inhibition of TASK channel activity. This notion was consistent with the failure of 10 μM muscarine to induce an inward current in the presence of 600 μM La3+ and 100 μM quinine (n = 4; Fig. 4C). In the presence of 600 μM La3+, a current response to 10 μM muscarine rapidly developed with the marked increase in peak values, and then the current gradually decayed to a plateau level with a large fluctuation after reaching a peak. This transient enhancement of the muscarine-induced current successively diminished with repetitive applications of muscarine. A third or fourth application of muscarine resulted in the production of only a plateau current with a diminished amplitude. In Fig. 4D, peak amplitudes of inward currents evoked by the first application of muscarine in the presence of La3+ were plotted against the concentration of La3+. It is evident that the enhancement occurred at concentrations ≥10 μM. On the other hand, the plateau amplitude of muscarine currents that were evoked by the third application significantly diminished in the presence of 3 μM La3+. This inhibition of the muscarine current became greater with an increase in La3+ concentration to 100 μM. However, the extent of inhibition by 600 μM La3+ exhibited a marked variability in the AM cells examined and averaged at 67% (Fig. 4E).
Fig. 4.
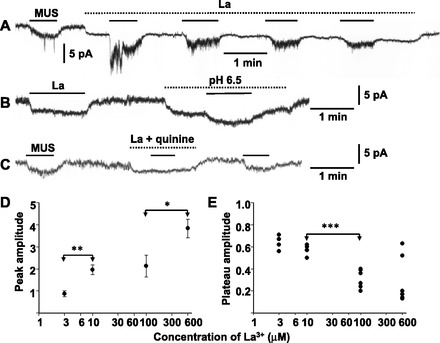
Dual action of La3+ on muscarine-induced current in AM cells. A: effects of 600 μM La3+ on 10 μM MUS-induced current. B: La3+-induced inward current is markedly suppressed by a decrease in external pH to 6.5. C: MUS fails to induce an inward current in the presence of 600 μM La3+ and 100 μM quinine. Whole cell current was recorded at −60 mV using the nystatin method in three different guinea-pig AM cells. MUS and other chemicals were added to saline during the indicated periods (bars for MUS; dotted lines for other chemicals). D: peak amplitudes of inward currents induced by first application of 10 μM MUS in the presence of La3+ are plotted against concentrations of La3+. Peak amplitude was expressed relative to that of the MUS-induced current before application of La3+ in the same cells. Data represent means ± SE of 3–4 cells. E: plateau levels of inward currents induced by third or fourth application of 10 μM MUS in the presence of La3+ are plotted against concentrations of La3+. Plateau level was expressed relative to that of the MUS-induced current before application of La3+. Peak and plateau levels were determined at the middle of current fluctuation. *P < 0.05, **P < 0.01, and ***P < 0.001, statistically significant difference.
Expression of TASK1 channel.
The aforementioned pharmacological and electrophysiological findings raised the possibility that guinea-pig AM cells express TASK channels, possibly TASK1. This possibility was examined with an anti-TASK1 Ab, the specificity of which had been confirmed (23). As shown in Fig. 5A, bands with molecular masses of 53 and 100 kDa were recognized in immunoblotting of homogenates of the guinea-pig adrenal medulla and adrenal cortex but not of the brain. These two bands were abolished by preabsorption of the Ab with its antigen, suggesting that the 100-kDa band represents a dimer of TASK1 proteins. Immunostaining for TASK1 channels reveals that TASK1-like immuoreactive materials were confined to the cell periphery in dissociated guinea-pig AM cells (Fig. 5C; n = 9). Taken together with the electrophysiological findings, the immunostaining indicated that TASK1 channel activity contributes to the resting membrane potential in guinea-pig AM cells.
Fig. 5.
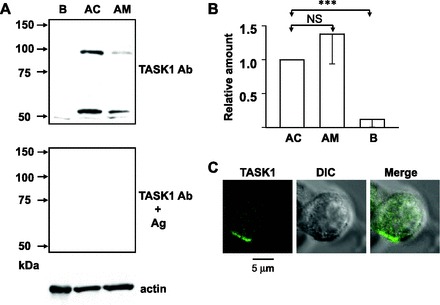
Expression of TWIK-related acid-sensitive K+ channel (TASK)1 channels in guinea-pig AM cells. A: immunoblots for TASK1 with and without preabsorption of anti-TASK1 Ab by antigen (Ag) and for actin. B, AC, and AM stand for guinea-pig brain, adrenal cortex, and adrenal medulla. Same amount of proteins (6 μg) was loaded for each lane. B: summary of expression levels of TASK1 proteins in guinea-pig AC (n = 5), AM (n = 5), and B (n = 5). Amounts of TASK1 in AM and B were expressed relative to that in AC. NS, no statistically significant difference. ***P < 0.001, statistically significant difference. C: immunocytochemical staining for TASK1 in a dissociated guinea-pig AM cell. AM cells were treated overnight with anti-TASK1 Ab diluted at 1:50. Left: confocal image for TASK1-like immunoreactivities (TASK1); middle: direct interference contrast (DIC) image; right: merge of left and middle.
Expression of TRPC channels.
Which subtypes of TRPC channels were expressed in guinea-pig AM cells was explored at the mRNA and protein levels. RT-PCR analysis of nucleotides extracted from adrenal medulla gave clear bands for TRPC1, TRPC3, TRPC6, and TRPC7 and faint bands for TRPC4 and TRPC5, whereas for the adrenal cortex nucleotide bands for TRPC1, TRPC4, TRPC5, and TRPC7 were detected. Immunoblotting of homogenates of the brain, adrenal cortex, and adrenal medulla revealed TRPC4 proteins of 110 kDa and TRPC5 of 106 kDa and TRPC7 of about 96 kDa. TRPC1 proteins, however, could not be detected in immunoblotting of any homogenates (35). Thus immunoprecipitation and immunoblotting were combined to detect TRPC1 channels. Figure 6C shows that this combination resulted in the recognition of TRPC1 channels of 86 kDa in brain and adrenal medulla. These findings led us to immunocytochemically examine their localization in dissociated AM cells (Fig. 7). TRPC1-like immunoreactivities were distributed in a reticular pattern in the cytoplasm (n = 16), as has been noted in rat AM cells (35). TRPC4-like (n = 8) and TRPC7-like immunoreactivities (n = 12) exhibited a punctate distribution. In contrast, TRPC5-like immunoreactivities were predominantly located at the cell periphery, probably at the cell membrane (n = 8).
Fig. 6.
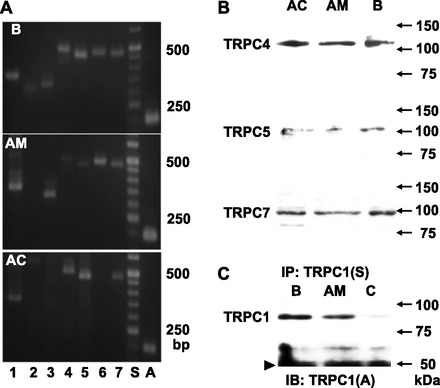
Analysis of cononical transient receptor potential (TRPC) channel expression at the mRNA and protein levels. A: PCR analysis of guinea-pig B, AC, and AM cDNAs for TRPC1–TRPC7 and β-actin. PCR products (TRPC1, 367 bp; TRPC2, 317 bp; TRPC3, 322 bp; TRPC4, 498 bp; TRPC5, 471 bp; TRPC6, 472 bp; TRPC7, 475 bp; β-actin, 198 bp) for each of the 7 TRPC subtypes and for β-actin were detected in brain cDNAs. Those for all the TRPC subtypes except TRPC2 were detected in AM cDNAs, whereas those for TRPCs 1, 4, 5, and 7 were recognized in AC cDNAs. B: immunoblotting of homogenates of guinea-pig AC, AM, and B for TRPCs 4, 5, and 7. C: detection of TRPC1 in guinea-pig B and AM. Homogenates of B and AM were subjected to immunoprecipitation (IP) with Santa Cruz-made anti-TRPC1 Ab (S) and rabbit IgG (C) and then to immunoblotting (IB) with Alomone-made anti-TRPC1 Ab (A). Arrowhead represents bands of heavy chain of IgG.
Fig. 7.
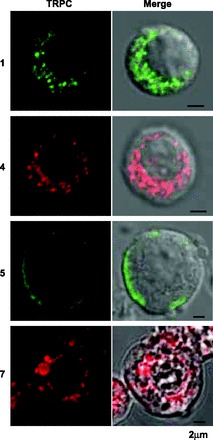
Immunocytochemical staining for TRPC channels in dissociated guinea-pig AM cells. Left: confocal images (TRPC) of TRPC1-, TRPC4-, TRPC5-, and TRPC7-like immunoreactivities. Right: merges of confocal and DIC images. Dissociated AM cells were treated overnight with anti-TRPC1 Ab (Santa Cruz) diluted at 1:100 (1), anti-TRPC4 mAb at 1:200 (4), anti-TRPC5 mAb at 1:200 (5), or anti-TRPC7 mAb at 1:50 (7). Green and red represent FITC-like and rhodamine-like fluorescence, respectively (see materials and methods).
Expression of STIM1.
Since STIM1 has been proposed to be indispensable for receptor-mediated regulation of TRPC channel activity (58; but also see 12), whether STIM1 proteins were expressed in AM cells or not was explored immunologically. Immunoblotting revealed that the amount of 90-kDa STIM1 in the guinea-pig adrenal medulla was one-third of that in the adrenal cortex (Fig. 8, A and B). The expression of STIM1 proteins in guinea-pig AM cells was also immunocytochemically confirmed (Fig. 8, C and D). Its immunostaining exhibited a reticular pattern in the cytoplasm, and the amount of the immunoreactive materials in the AM cells was also one-third of that in the adrenal cortical cells.
Fig. 8.
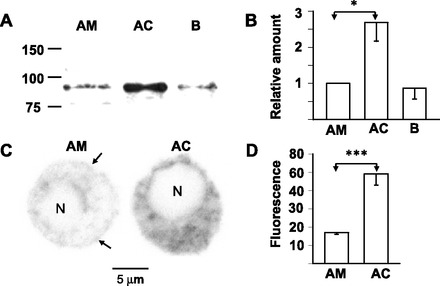
Expression of STIM1 in guinea-pig AM cells. A: immunoblot for STIM1 of homogenates of AM, AC, and B. B: summary of expression levels of STIM1 in AM (n = 6), AC (n = 6), and B (n = 6). C: confocal images of STIM1-like immunoreactivities in dissociated guinea-pig AM and AC cells. Cells were treated overnight with anti-STIM1 Ab diluted at 1:100. Arrows indicate STIM1-like immunoreactivities at the cell periphery. D: summary of amounts of STIM1-like immunoreactive materials in AM (n = 12) and AC (n = 12) cells. Amounts of immunoreactivities in the cytoplasm and nucleus (N) were individually measured using ImageJ software and expressed in arbitrary units. Amount in the nucleus, which was regarded to be nonspecific, was subtracted from that in the cytoplasm. *P < 0.05 and ***P < 0.001, statistically significant difference.
Physiological role of muscarinic receptors.
Whether muscarinic receptors were involved in synaptic transmission in AM cells or not was examined in isolated adrenal glands that were retrogradely perfused through the adrenal vein. The transmural stimulation of the adrenal gland with 1.5-ms pulses of 50 V for 30 s resulted in a reproducible increase in catecholamine secretion in a frequency-dependent manner (Fig. 9, A and B). Application of 3 μM atropine produced 20 and 32% reduction in secretion evoked by electrical stimulation at 5 and 10 Hz (Fig. 9, A, B, and D), respectively. The remaining secretion in the presence of atropine was almost completely abolished by a further addition of 0.5 mM hexamethonium (94.8 ± 3.1% reduction in four adrenal glands). Since 3 μM atropine might have suppressed nicotinic ACh receptors (40), the effects of 1 μM atropine were also examined. As shown in Fig. 9C, application of 1 μM atropine also suppressed electrically evoked secretion. The results suggested that muscarinic receptors were involved in part of the neuronal transmission in AM cells.
Fig. 9.
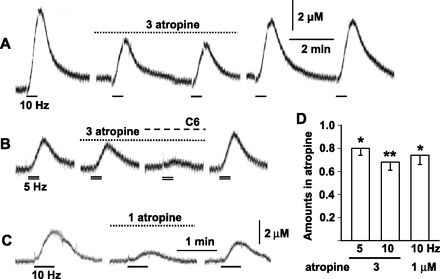
Involvement of muscarinic receptors in neuronal transmission in guinea-pig AM. A–C: amperometric records of catecholamine secretion in response to electrical stimulation. A and B were obtained from the same adrenal gland. Guinea-pig adrenal gland was retrogradely perfused through the adrenal vein with saline. Perfusate was exchanged with saline containing 1 or 3 μM atropine or 3 μM atropine and 500 μM hexamethonium (C6). The nerve fibers in the adrenal gland were electrically stimulated with 50-V pulses of 1.5-ms duration for 30 s at 5 (double lines) and 10 Hz (bars). Catecholamines were measured using the amperometric method. Records were interrupted for 6 min. D: summary of effects of 1 and 3 μM atropine on catecholamine secretion in response to electrical stimulation at 10 Hz (9 adrenal glands) and at 5 (6 adrenal glands) and 10 Hz (6 adrenal glands), respectively. Amounts of secreted catecholamines during application of atropine were expressed relative to averages of those before and after it. *P < 0.05 and **P < 0.01, statistically significant decrease.
DISCUSSION
Ionic mechanisms.
The major findings in the present experiment are that muscarinic receptor-induced depolarization in guinea-pig AM cells is ascribed to not only activation of NS channels but also inhibition of TASK channels and that TASK1 channels may be expressed as a homodimer. The bath application of quinine produced an inhibition of the muscarine-induced current in a dose-dependent manner, which is similar to that noted in the inhibition of muscarinic receptor-mediated NS channel activity in guinea-pig ileal smooth muscles (7). In the AM cells, the inhibition of muscarinic currents by quinine was incomplete, and the remaining quinine-insensitive component reversed its polarity at the membrane potential near the equilibrium potential for K+. This quinine-insensitive component was suppressed by bupivacaine, a TASK channel blocker, and did not exhibit time-dependent properties. The suppression of the whole-cell current by bupivacaine was masked by a decrease in external pH to 6.5 that had been found to inhibit TASK channel activity in rat AM cells (23). Based on these results, the quinine-sensitive component of the muscarinic current is concluded to be due to the inhibition of TASK channels and not of M-type K+ channels, which has been shown to be suppressed by histamine in cultured bovine AM cells (53). This notion is consistent with the finding that TASK channels are not sensitive to quinine at micromolar concentrations (13) and supported by the immunological detection of TASK1 channels at the cell periphery of guinea-pig AM cells. Immunoblotting of lysates of the guinea-pig adrenal medulla revealed two bands of 53 and 100 kDa, and both were abolished by the reabsorption of the Ab with its antigen. The result suggests that TASK1 channels in guinea-pig AM cells exist as a homodimer, in contrast to certain brain regions where TASK1 and TASK3 channels have been reported to form a heterodimer (4).
The bupivacaine-insensitive component of the muscarinic current was suppressed by quinine, and it reversed at about −5 mV. In addition, its I-V relationship had a negative slope region, as was originally noted for muscarine-induced currents in guinea-pig AM cells (26). These results indicate that the muscarinic current in guinea-pig AM cells comprises the bupivacaine-sensitive and quinine-sensitive components. The former and the latter may represent ∼40 and 60% of the 10-μM muscarine-induced current and are mediated by the inhibition of TASK channels and the activation of NS channels, respectively. This proportion may change with muscarinic agonists and their concentrations used. In rat AM cells, the EC50 for muscarine in inhibiting TASK channel activity was ∼10 μM and oxotremorine, which is a partial agonist for the M1 subgroup of muscarinic receptors (20, 45), was much less efficient than muscarine (23). In contrast, muscarine at five times lower concentrations induced inward currents in guinea-pig AM cells, and oxotremorine was so efficient that 1-μM oxotremorine-induced currents were three-fourths of 1-μM muscarine-induced currents (24). Therefore, the failure to detect the TASK channel component in our previous experiments (26) may be ascribed at least in part to differences in experimental conditions, especially the low concentrations of muscarine used.
The bath application of La3+ produced a double action, facilitation and inhibition, on the muscarinic current. Inhibition by divalent cations has been noted in a variety of cation channels including NS channels (9, 19), but the enhancement is a landmark of TRPC4, TRPC5, and TRPV1 channels (29, 51). Since TRPV1 channels are thermosensitive and active at temperatures above 42°C, but not at 26°C (50) where the present experiment was performed, the involvement of TRPV1 channels in the muscarinic current can be excluded. The single channel analysis of recombinant TRPC4 and TRPC5 channels revealed the external application of lanthanides, such as La3+ or Gd3+, results in an increase in open possibility with a reduction of single channel conductance (29, 46), and the former occurred at micromolar concentrations. Consistent with this result, application of 10 μM La3+ produced a significant enhancement of muscarine-induced currents. It would be interesting to note that the plateau level of the muscarinic current was very variable in cells examined in the presence of 600 μM La3+. This variability might be related to the finding that peak amplitudes of the muscarinic current were markedly enhanced by 600 μM La3+. The finding that muscarine failed to induce any current in the presence of La3+ and quinine suggests that La3+ inhibited not only the quinine-sensitive component but also the bupivacaine-sensitive component. TASK channels may have been suppressed by lanthanides, as was reported with TRAAK channels (33) belonging to the TREK subfamily of the two-pore-domain K+ channel family (3). This notion was supported by the findings that the La3+-induced inward current reversed its polarity near the equilibrium potential for K+ and a decrease in external pH to 6.5 resulted in diminution of La3+-induced inward currents.
The I-V relationship for the bupivacaine-insensitive component of muscarinic currents had a negative slope region, which is similar to that noted with heteromers of TRPC1 and TRPC4 or TRPC5 channels (48). TRPC5 channels were immunologically detected at the cell periphery, probably at the cell membrane, and TRPC5-GFP channels exogenously expressed in PC12 cells were also located at the cell periphery (35). On the other hand, TRPC4 channels and exogenously expressed TRPC4-GFP channels were distributed in a punctate pattern in the cytoplasm of guinea-pig AM and PC12 cells (unpublished observations by K. Harada), respectively. As noted in rat AM cells (35), the majority of TRPC1 channels exhibited a reticular distribution in the cytoplasm, probably being present in the enodplasmic reticulum. However, some of the TRPC1 channels were present in the vicinity of the cell membrane. If the heteromer of TRPC1 and TRPC5 channels is involved, TRPC1 channels present at the cell periphery might form a heteromer with TRPC5. This possibility, however, might not be feasible. TRPC5 channel activity measured by Ca2+ imaging in HEK293 cells was not suppressed by 10 μM D-600 (57), whereas in the present experiment D-600 at the same concentration reversibly inhibited muscarine-induced currents by 40% without altering the baseline. As another possibility, the heteromer of TRPC1 and TRPC4 present in intracellular vesicles might be translocated to the cell membrane in response to receptor stimulation, as was reported for TRPC3 (18, 47) and TRPC6 channels (6). A further study will be required to identify the ion channels involved in the quinine-sensitive component of muscarinic currents.
In contrast to guinea-pig AM cells, muscarinic stimulation in rat AM cells did not result in activation of NS channels, although several types of TRPC channels including TRPC5 have been detected at the protein and/or mRNA levels (35). The mechanism for the lack of NS channel activity in response to muscarine remains to be explored. It would be interesting to note that STIM1 was expressed in guinea-pig, but not rat (35), AM cells. STIM1 has been reported to be obligatory for receptor-mediated activation of TRPC channels exogenously expressed in HEK293 cells (58). In addition, knockdown of STIM1 with a small interfering RNA method resulted in a marked suppression of arachidonic-acid-regulated Ca2+-selective channels in HEK293 cells (37).
Synaptic transmission.
Muscarinic receptors have been shown to be expressed biochemically and/or functionally in AM cells of various mammals including humans (43). However, the physiological role of muscarinic receptors has not yet been elucidated. In the perfused adrenal medullae of rats, muscarinic receptors were found to be involved in synaptic transmission only under conditions where ACh degradation was suppressed (20). In the present experiments of guinea-pig adrenal glands, application of 1 μM atropine reversibly induced a significant decrease in catecholamine secretion in response to electrical stimulation. This decrease in secretion in the presence of atropine may be accounted for by the suppression of muscarinic receptors or the channel block of nicotinic ACh receptor complexes. Atropine has been shown to suppress neuronal nicotinic ACh receptor complexes endogenously present in AM cells (25, 40) and exogenously expressed in oocytes (41). This suppression of nicotinic ACh receptors was voltage dependent, increasing with hyperpolarization (41). When neuronal nicotinic ACh receptor complexes containing α3- or α4-subunits were exogenously expressed in oocytes, 1 μM atropine induced ∼10 or 20% inhibition of nicotine-induced currents, respectively. This efficient concentration of atropine for inhibiting nicotinic currents is much lower than that required for the suppression of the cholinergic excitatory postsynaptic current in rat submandibular ganglion cells (44). Moreover, the peak amplitude of excitatory postsynaptic currents in bullfrog sympathetic ganglion neurons was suppressed by atropine at 25 μM but not lower (11). Based on these results, the suppression of catecholamine secretion by 1 μM atropine may not be due to the channel block of nicotinic ACh receptor complexes. This notion is consistent with the finding that the channel block of nicotine-induced currents did not occur with atropine at concentrations of 2 μM or less in bovine AM cells (40). Furthermore, 3 μM atropine had no effect on transsynaptically evoked increases in excitability in rat AM cells (20). The present findings that catecholamine secretion evoked transsynaptically was reversibly suppressed by 1 μM atropine strongly indicate that muscarinic receptors are involved in the neuronal transmission in guinea-pig AM cells.
Finally, it would be worth considering why the muscarinic receptor participates in synaptic transmission in guinea-pig, but not rat, AM cells. As shown previously (20), M4 and M5 muscarinic receptors were predominantly expressed in rat AM cells, and the same subtypes are major receptors in guinea-pig AM cells (15; unpublished observations by K. Harada and M. Inoue). What is conspicuously different between rat and guinea-pig AM cells is the multiplicity of muscarinic signaling in the latter. The muscarinic receptor-induced depolarization in guinea-pig AM cells is due to the inhibition of TASK channel activity and the activation of NS channels, whereas that in rat AM cells is ascribed to the former alone. This multiplicity of muscarinic signaling in guinea-pig AM cells would enhance efficiency of muscarinic receptor-mediated synaptic transmission.
In conclusion, muscarinic receptor stimulation in guinea-pig AM cells induces depolarization through inhibition of TASK channels and activation of NS channels, which may include TRPC4 or TRPC5 channels, and muscarinic receptors are involved in neuronal transmission from the splanchnic nerve.
GRANTS
This work was supported in part by MEXT KAKENHI (21026029 to M. Inoue) and JSPS KAKENHI (21500360 to M. Inoue and 22790222 to H. Matsuoka). The anti-TRPC4, anti-TRPC5, and anti-TRPC7 mAbs were obtained from the University of California, Davis/National Institute of Neurological Disorders and Stroke/National Institute of Mental Health NeuroMab Facility, supported by National Institutes of Health Grant U24NS050606 and maintained by the Section of Neurobiology, Physiology and Behavior, College of Biological Sciences, University of California, Davis.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.I., K.H., H.M., and A.W. performed experiments; M.I., K.H., and H.M analyzed data; M.I., K.H., and A.W. wrote the manuscript; M.I., J.N., and A.W. were involved in conception and design of research.
ACKNOWLEDGMENTS
We are grateful to T. Hatama for technical assistance.
REFERENCES
- 1. Alamo L, García AG, Borges R. Electrically-evoked catecholamine release from cat adrenals. Role of cholinergic receptors. Biochem Pharmacol 42: 973–978, 1991 [DOI] [PubMed] [Google Scholar]
- 2. Ballesta JJ, Borges R, García AG, Hidalgo MJ. Secretory and radioligand binding studies on muscarinic receptors in bovine and feline chromaffin cells. J Physiol 418: 411–426,1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bayliss DA, Barrett PQ. Emerging roles for two-pore-domain potassium channels and their potential therapeutic impact. Trends Pharmacol Sci 29: 566–574, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berg AP, Talley EM, Manger JP, Bayliss DA. Motoneurons express heteromeric TWIK-related acid-sensitive K+(TASK) channels containing TASK-1 (KCNK3) and TASK-3(KCNK9) subunits. J Neurosci 24: 6693–6702, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buckler KJ, Williams BA, Honore E. An oxygen-, acid-and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J Physiol 525: 135–142, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cayouette S, Lussier MP, Mathieu EL, Bousquet SM, Boulay G. Exocytotic insertion of TRPC6 channel into the plasma membrane upon Gq protein-coupled receptor activation. J Biol Chem 279: 7241–7246, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Chen S, Inoue R, Ito Y. Pharmacological characterization of muscarinic receptor-activated cation channels in guinea-pig ileum. Br J Pharmacol 109: 793–801, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clapham DE. TRP channels as cellular sensors. Nature 426: 517–524, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Clapham DE, Julius D, Montell C, Schultz G. International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol Rev 57: 427–450, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Clausen T. Na+-K+ pump regulation and skeletal muscle contractility. Physiol Rev 83: 1269–1324, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Connor EA, Levy SM, Parsons RL. Kinetic analysis of atropine-induced alterations in bullfrog ganglionic fast synaptic currents. J Physiol 337: 137–158, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dehaven WI, Jones BF, Petranka JG, Smyth JT, Tomita T, Bird GS, Putney JW., Jr TRPC channels function independently of STIM1 and Orai1. J Physiol 587: 2275–2298, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Duprat F, Lesage F, Fink M, Reyes R, Heurteaux C, Lazdunski M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J 16: 5464–5471, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eberhard DA, Holz RW. Cholinergic stimulation of inositol phosphate formation in bovine adrenal chromaffin cells: distinct nicotinic and muscarinic mechanisms. J Neurochem 49: 1634–1643, 1987 [DOI] [PubMed] [Google Scholar]
- 15. Endo Y, Harada K, Fujishiro N, Imanaga I, Ogawa K, Inoue M. Localization of muscarinic receptor and cation channel in guinea-pig adrenal chromaffin cells. Acta Histochem Cytochem 38: 273–282, 2005 [Google Scholar]
- 16. Enyedi P, Czirjak G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev 90: 559–605, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Forsberg EJ, Li Q, Xu Y. Cation channel activated by muscarinic agonists on porcine adrenal chromaffin cells. Am J Physiol Endocrinol Metab 269: E43–E52, 1995 [DOI] [PubMed] [Google Scholar]
- 18. Goel M, Sinkins WG, Zuo CD, Hopfer U, Schilling WP. Vasopressin-induced membrane trafficking of TRPC3 and AQP2 channels in cells of the rat renal collecting duct. Am J Physiol Renal Physiol 293: F1476–F1488, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Hamill OP. Twenty odd years of stretch-sensitive channels. Pflügers Arch 453: 333–351, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Harada K, Matsuoka H, Sata T, Warashina A, Inoue M. Identification and role of muscarinic receptor subtypes expressed in rat adrenal medullary cells. J Pharmacol Sci 117: 253–264, 2011 [DOI] [PubMed] [Google Scholar]
- 21. Holman ME, Coleman HA, Tonta MA, Parkington HC. Synaptic transmission from splanchnic nerves to the adrenal medulla of guinea-pigs. J Physiol 478: 115–124, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Holman ME, Tonta MA, Coleman HA, Parkington HC. Muscarinic receptor activation in guinea-pig chromaffin cells causes decreased membrane conductance and depolarization. J Auton Nerv Syst 68: 140–144, 1998 [DOI] [PubMed] [Google Scholar]
- 23. Inoue M, Harada K, Matsuoka H, Sata T, Warashina A. Inhibition of TASK1-like channels by muscarinic receptor stimulation in rat adrenal medullary cells. J Neurochem 106: 1804–1814, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Inoue M, Imanaga I. Mechanism of activation of nonselective cation channels by putative M4 muscarinic receptor in guinea-pig chromaffin cells. Br J Pharmacol 114: 419–427, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Inoue M, Kuriyama H. Properties of the nicotinic-receptor-activated current in adrenal chromaffin cells of the guinea-pig. Pflügers Arch 419: 13–20, 1991 [DOI] [PubMed] [Google Scholar]
- 26. Inoue M, Kuriyama H. Muscarinic receptor is coupled with a cation channel through a GTP-binding protein in guinea-pig chromaffin cells. J Physiol 436: 511–529, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, Mori Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular α1-adrenoceptor-activated Ca2+-permeable cation channel. Circ Res 88: 325–332, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Jiang H, Zeng B, Chen GL, Bot D, Eastmond S, Elsenussi SE, Atkin SL, Boa AN, Xu SZ. Effect of non-steroidal anti-inflammatory drugs and new fenamate analogues on TRPC4 and TRPC5 channels. Biochem Pharmacol 83: 923–931, 2012 [DOI] [PubMed] [Google Scholar]
- 29. Jung S, Mühle A, Schaefer M, Strotmann R, Schultz G, Plant TD. Lanthanides potentiate TRPC5 currents by an action at extracellular sites close to the pore mouth. J Biol Chem 278: 3562–3571, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Kayaalp SO, Mclsaac RJ. Muscarinic component of splanchnic-adrenal transmission in the dog. Br J Pharmacol 36: 286–293, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim SJ, Kim YS, Yuan JP, Petralla RS, Worley PF, Linden DJ. Activation of the TRPC1 cation channel by metabotropic glutamate receptor mGluR1. Nature 426: 285–291, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Knight DE, Baker PF. Observations on the muscarinic activation of catecholamine secretion in the chicken adrenal. Neuroscience 19: 357–366, 1986 [DOI] [PubMed] [Google Scholar]
- 33. Maingret F, Fosset M, Lesage F, Lazdunski M, Honoré E. TRAAK is a mammalian neuronal mechano-gated K+ channel. J Biol Chem 274: 1381–1387, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Matsuoka H, Harada K, Endo Y, Warashina A, Doi Y, Nakamura J, Inoue M. Molecular mechanisms supporting a paracrine role of GABA in rat adrenal medullary cells. J Physiol 586: 4825–4842, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Matsuoka H, Harada K, Ikeda T, Uetsuki K, Sata T, Warashina A, Inoue M. Ca2+ pathway involve in the refilling of store sites in rat adrenal medullary cells. Am J Physiol Cell Physiol 296: C889–C899, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Medbø JI, Sejersted OM. Plasma potassium changes with high intensity exercise. J Physiol 421: 105–122, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mignen O, Thompson JL, Shuttleworth TJ. STIM1 regulates Ca2+ entry via arachidonate-regulated Ca2+-selective (ARC) channels without store depletion or translocation to the plasma membrane. J Physiol 579: 703–715, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nagayama T, Matsumoto T, Kuwakubo F, Fukushima Y, Yoshida M, Suzuki-Kusaba M, Hisa H, Kimura T, Satoh S. Role of calcium channels in catecholamine secretion in the rat adrenal gland. J Physiol 520: 503–512, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nagayama T, Matsumoto T, Yoshida M, Suzuki-Kusaba M, Hisa H, Kimura T, Satoh S. Role of cholinergic receptors in adrenal catecholamine secretion in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 277: R1057–R1062, 1999 [DOI] [PubMed] [Google Scholar]
- 40. Nooney JM, Peters JA, Lambert JJ. A patch clamp study of the nicotinic acetylcholine receptor of bovine adrenomedullary chromaffin cells in culture. J Physiol 455: 503–527, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Parker JC, Sarkar D, Quick MW, Lester RAJ. Interactions of atropine with heterologously expressed and native α3 subunit-containing nicotinic acetylcholine receptors. Br J Pharmacol 138: 801–810, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pelto-Huikko M, Dagerlind A, Kononen J, Lundberg JM, Villar M, Koistinaho J, Bravo R, Hökfelt T. Neuronal regulation of c-fos, c-jun, and junB immediate-early genes in rat adrenal medulla. J Neurosci 15: 1854–1868, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pérez-Alvarez A, Albillos A. Key role of the nicotinic receptor in neurotransmitter exocytosis in human chromaffin cells. J Neurochem 103: 2281–2290, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Rang HP. The action of ganglionic blocking drugs on the synaptic responses of rat submandibular ganglion cells. Br J Pharmacol 75: 151–168, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Richards MH, van Giersbergen PL. Human muscarinic receptors expressed in A9L and CHO cells: activation by full and partial agonists. Br J Pharmacol 114: 1241–1249, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Semtner M, Schaefer M, Pinkenburg O, Plant T. Potentiation of TRPC5 by protons. J Biochem 282: 33868–33878, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Singh BB, Lockwich TP, Bandyopadhyay BC, Liu X, Bollimuntha S, Brazer SC, Combs C, Das S, Leenders AGM, Sheng ZH, Knepper MA, Ambudkar SV, Ambudkar IS. VAMP2-dependent exocytosis regulates plasma membrane insertion of TRPC3 channels and contributes to agonist-stimulated Ca2+ influx. Mol Cell 15: 635–646, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Strübing C, Krapivinsky G, Krapivinsky L, Clapham DE. TRPC1 and TRPC5 from a novel cation channel in mammalian brain. Neuron 29: 645–655, 2001 [DOI] [PubMed] [Google Scholar]
- 49. Talavera K, Yasumatsu K, Yoshida R, Margolskee RF, Voets T, Ninomiya Y, Nilius B. The taste transduction channel TRPM5 is a locus for bitter-sweet taste interactions. FASEB J 22: 1343–1355, 2008 [DOI] [PubMed] [Google Scholar]
- 50. Tominaga M, Tominaga T. Structure and function of TRPV1. Pflügers Arch 451: 143–150, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Tousova K, Vyklicky L, Susankova K, Benedikt J, Vlachova V. Gadolinium activates and sensitizes the vanilloid receptor TRPV1 through the external protonation sites. Mol Cell Neurosci 30: 207–217, 2005 [DOI] [PubMed] [Google Scholar]
- 52. Wakade AR, Wakade TD. Contribution of nicotinic and muscarinic receptors in the secretion of catecholamines evoked by endogenous and exogenous acetylcholine. Neuroscience 10: 973–978, 1983 [DOI] [PubMed] [Google Scholar]
- 53. Wallace DJ, Chen C, Marley PD. Histamine promotes excitability in bovine adrenal chromaffin cells by inhibiting an M-current. J Physiol 540: 921–939, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Warashina A, Inoue M. Ca2+ imaging in perfused adrenal medullae. J UOEH 34: 163–173, 2012 [DOI] [PubMed] [Google Scholar]
- 55. Warashina A, Satoh Y. Modes of secretagogue-induced [Ca2+]i responses in individual chromaffin cells of the perfused rat adrenal medulla. Cell Calcium 30: 395–401, 2001 [DOI] [PubMed] [Google Scholar]
- 56. Xu SZ, Sukumar P, Zeng F, Li J, Jairaman A, English A, Naylor J, Ciurtin C, Majeed Y, Milligan CJ, Bahnasi YM, Al-Shawaf E, Porter KE, Jiang LH, Emery P, Sivaprasadarao A, Beech DJ. TRPC channel activation by extracellular thioredoxin. Nature 451: 69–72, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Xu SZ, Zeng F, Boulay G, Grimm C, Harteneck C, Beech DJ. Block of TRPC5 channels by 2-aminoethoxydiphenyl borate: a differential, extracellular and voltage-dependent effect. Br J Pharmacol 145: 405–414, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol 9: 636–645, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]


