Abstract
Objectives
The goals of this study were: 1) to determine if high-fat diet (HFD) feeding in female mice would negatively impact biomechanical and histologic consequences on the Achilles tendon and quadriceps muscle; and 2) to investigate whether exercise and branched-chain amino acid (BCAA) supplementation would affect these parameters or attenuate any negative consequences resulting from HFD consumption.
Methods
We examined the effects of 16 weeks of 60% HFD feeding, voluntary exercise (free choice wheel running) and BCAA administration in female C57BL/6 mice. The Achilles tendons and quadriceps muscles were removed at the end of the experiment and assessed histologically and biomechanically.
Results
HFD feeding significantly decreased the Achilles tendon modulus without histological alterations. BCAA administration significantly decreased the stiffness of Achilles tendons in the exercised normal diet mice. Exercise partially ameliorated both the weight gain and glucose levels in the HFD-fed mice, led to a significant decrease in the maximum load of the Achilles tendon, and an increase in the average fibril diameter of the quadriceps femoris muscle. There were significant correlations between body weight and several biomechanical properties, demonstrating the importance of controlling obesity for maintaining healthy tendon properties.
Conclusions
In summary, this study showed a significant impact of obesity and body weight on tendon biomechanical properties with limited effects of exercise and BCAAs.
Cite this article: Bone Joint Res 2013;2:186–92.
Keywords: Mouse, Voluntary exercise, Obesity, BCAA supplement, Achilles tendon
Article focus
We studied the effects of high-fat diet (HFD), exercise (free choice wheel running) and branched-chain amino acid (BCAA) administration in C57BL/6 mice for 16 weeks
We hypothesised that a HFD would have negative biomechanical and histological consequences on the Achilles tendon and quadriceps muscle
We hypothesised that exercise and BCAAs would attenuate these changes
Key messages
HFD feeding significantly decreased the Achilles tendon modulus
BCAA administration significantly decreased the stiffness of Achilles tendons in the exercised control diet mice
Exercise partially ameliorated both the weight gain and glucose levels in the HFD-fed mice, led to a significant decrease in the maximum load of the Achilles tendon, and led to an increase in the average fibril diameter of the quadriceps femoris muscle
Strengths and limitations
This soft-tissue property study included cohorts for multiple testing paradigms
The study was conducted in female mice
Introduction
Obesity is a well-known risk factor for many diseases and can negatively impact the musculoskeletal system.1 Few studies directly examine the effects of obesity on the biomechanical properties of tendon. A positive association in humans between increased adiposity and tendon injury was identified in one systematic review of the literature.2 Another study showed links between tendon pathology and central fat distribution in men, peripheral fat distribution in women, and age in both genders.3 Speculation of the cause of the tendon injuries included both systemic and mechanical alterations, but further investigation of the mechanisms is necessary in order to reach any conclusion. These studies also failed to control for other disease conditions including diabetes, which is associated with tendinopathies.4 Furthermore, there is increased tendinopathy with age,5 which may have impacted the study.2,3 Certainly additional studies into the effects of obesity on tendinopathy are warranted.
Animal studies on obesity and tendon alterations are also limited. Zucker rats are hyperphagic, suffer from both hyperinsulinaemia and hyperlipidaemia, and present a model of spontaneous genetic obesity. Increases in maximum displacement and strain and a decrease in average collagen fibril diameter were found in the deep digital flexor tendons of five-month-old male obese Zucker rats compared with lean rats.6 The authors speculated that the changes were due to lethargy and obesity, but could have been genetic in origin.
Exercise is prescribed to assist in weight management of obese patients. However, the impact of exercise on tendon properties of obese patients is poorly understood as most studies have been conducted in athletes and young adults. Exercise increases tendon cross-sectional area and stiffness in the athletic population.7-9 Studies of exercise effects on muscle properties are more thorough. However, there is conflicting evidence in obese patients regarding the effect of exercise on muscle fibre diameter. Resistance training in one study resulted in significant hypertrophy of muscle fibres in patients with metabolic syndrome.10 Other studies showed no difference with resistance training in older women11 or continuous endurance-type exercise in obese patients with type 2 diabetes.12 Many athletic trainers and others advocate the use of branched-chain amino acid (BCAA) supplementation during exercise training. BCAA supplementation decreases muscle damage associated with resistance training.13-16 This may occur through attenuation of blood lactate dehydrogenase accumulation, alterations of the immune system, and/or maintenance of a net anabolic hormonal profile.13-16Information on how BCAAs may affect tendons or muscles of obese patients is absent.
In this study we examined the effects of a high-fat diet (HFD), exercise (free choice wheel running) and BCAA administration for 16 weeks in C57BL/6 mice. We hypothesised that a HFD would have negative biomechanical and histological consequences on the Achilles tendon and quadriceps muscle, and that exercise and BCAAs would attenuate these changes.
Materials and Methods
Animal specimens
All experimental procedures were approved by the University of Kentucky Institutional Animal Care and Use Committee. A total of 80 female C57BL/6J mice (nine-weeks-old) were purchased from Jackson Laboratories (Bar Harbor, Maine). All mice were singly housed in shoebox cages (435 cm2) with wood chips (Teklad 7115 Coarse Sani-Chip bedding; Harlan Laboratories Inc., Indianapolis, Indiana) at 20° to 23°C room temperatures with a 14:10 hour light:dark cycle. The animals had food and water access ad libitum. After acclimatisation of one week, mice were treated for 16 weeks after being randomly assigned to one of eight groups (n = 10 per group) based on food type, exercise and BCAA administration, ensuring no systematic initial body weight differences between groups. The group size of ten was determined by power analysis of previous unpublished biomechanical results in our laboratory. Mice were fed a 10 kcal % fat control diet (D12450B; Research Diets, Inc., New Brunswick, New Jersey) or a 60 kcal % lard-based HFD (D12492; Research Diets, Inc.). Both diets contain 20 kcal % protein, but the control diet has 3.85 kcal/g compared with 5.24 kcal/g for the HFD. Exercise mice were placed in cages containing a voluntary running wheel (Phenome Technologies, Inc., Lincolnshire, Illinois). Sedentary mice were similarly housed in shoebox cages without wheels. Mice were given regular water or BCAAs in the drinking water to make a 2% solution (20 g/l). The BCAA concentration of 2% was chosen because of its similarity to other supplementation studies that noted beneficial effects with either BCAAs or leucine.17-20 BCAA supplementation (NutraBio BCAA 5000; NutraBio.com Inc., Middlesex, New Jersey) comprised a 2:1:1 ratio of leucine:valine:isoleucine. The mice had 24 hour access to running wheels and/or BCAA water except for brief periods of metabolic (glucose tolerance) testing and a three-hour fasting period before death by CO2 overdose.
Glucose tolerance testing
A glucose tolerance test (GTT) was performed during the tenth week of intervention on all 80 mice. Glucose levels were measured after a three-hour fast in tail blood using a Bayer Breeze2 glucose metre (Bayer HealthCare LLC, Tarrytown, New York). Mice then received a 2 g/kg body weight D-glucose intraperitoneal injection (Sigma-Aldrich, St. Louis, Missouri). Blood glucose readings were repeated 15, 30, 60, and 120 minutes post-injection. Area under the curve (AUC) for blood glucose levels was calculated using the ‘Area Below Curves’ function in a statistical software package (SigmaPlot v.11.0; Systat Software Inc., San Jose, California).
Biomechanical testing
Mice were weighed immediately after death. The right Achilles tendon complex was isolated with gastrocnemius muscle and foot attachments and stored in saline soaked gauze in a -80°C freezer until testing. Specimens were thawed to room temperature before testing. Tendon width and thickness were measured using a laser micrometer (LS7030-MT; Keyence, Elmwood Park, New Jersey) and initial length was measured using digital callipers. Following an established procedure,21 a custom grip was used to secure the calcaneus and the intramuscular tendon fibres were clamped in a grip secured to a 50 N load cell on a materials testing system (ElectroPuls E3000; Instron, Norwood, Massachusetts). The tendon was manually preloaded to 0.5 N and then loaded in tension at a rate of 10% tendon length/sec until tendon failure (defined as a 60% decrease in maximum load). Maximum load, displacement, stiffness, tensile stress, tensile strain and modulus were calculated from the load-displacement data. Stiffness and elastic modulus were calculated as the slope of the linear portion of the force-displacement and stress-strain curves, respectively. Points for the slope calculations were selected at 20% and 80% of the absolute peak in the load-displacement or strain-stress curves. Ten mice were examined for each group except for the control diet, sedentary group with BCAAs, which had only nine samples as a result of the loss of one sample during collection.
Histology
The left Achilles tendon and quadriceps muscle were harvested, fixed in 10% neutral buffered formalin, dehydrated through a gradient of alcohols and xylene, embedded in paraffin, sectioned at 5 µm, and stained with eosin and haematoxylin. The tendon mid-substance and insertion were examined for alterations in cell density, inflammatory cell infiltration, and extracellular matrix organization (n = 6 to 9 per group). Quadriceps fibre diameter was quantified on twenty muscle fibres (n = 4 to 9 per group) from cross-sections using ImageJ software from the National Institutes of Health (NIH). All analyses were performed blinded to treatment.
Statistical analysis
Three-way analysis of variance (ANOVA), implemented in v9.3 SAS software (SAS Institute, Cary, North Carolina) with observations weighted based on within-group variances, assessed how outcomes related to diet, exercise, and BCAA; post hoc comparisons were Bonferroni-adjusted. Pearson correlations were computed using Sigma Plot software (SigmaPlot v.11.0; Systat Software Inc.) to relate body weight and AUC to tendon/quadriceps properties. Statistical significance was defined by p < 0.05.
Results
Physiology
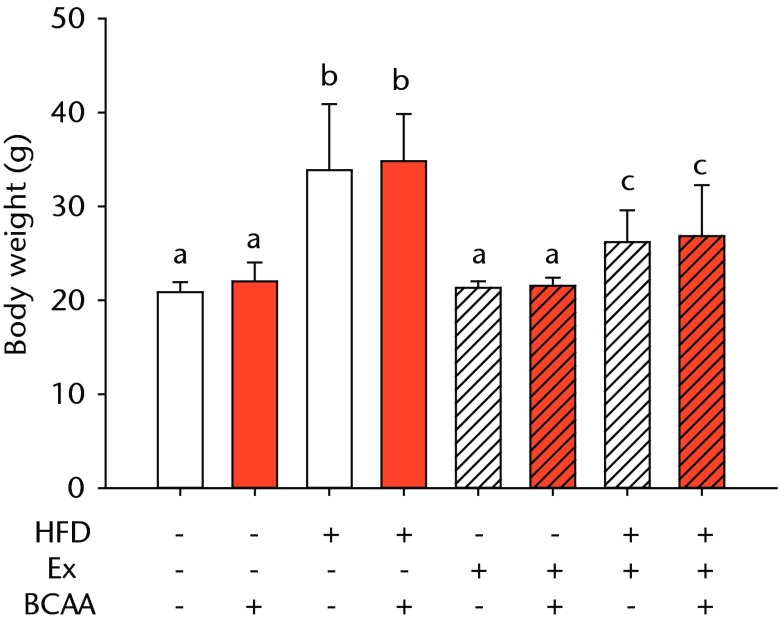
There was a significant main effect of both HFD feeding and exercise on body weight (p < 0.001) (Fig. 1a). There was also a significant interaction between the two interventions (p < 0.001) (Fig. 1a). With or without BCAAs, sedentary (no exercise) mice fed a HFD weighed a mean of 60% more than mice on a control diet (p < 0.001). Voluntary running led to a mean decrease in body weight of 23% in the HFD-fed mice compared with sedentary HFD-fed animals (p < 0.001). No significant difference was observed in the sedentary and exercised mice fed the control diet. BCAAs had no significant impact on body weight in any diet/exercise combination.
Figs. 1a - 1b.
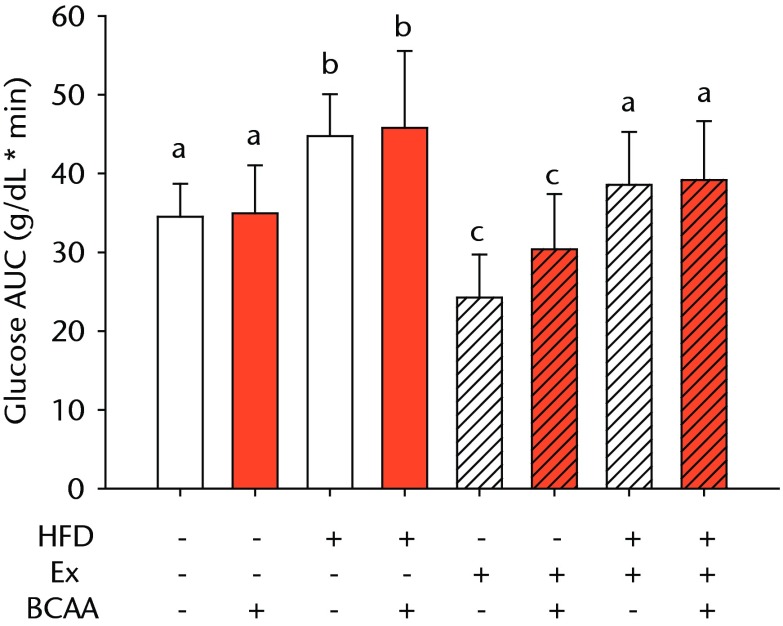
Bar charts showing a) the mean body weight (measured after 16 weeks of intervention, immediately after death) and b) the mean glucose tolerance for each group. During the tenth week of treatment, animals were fasted for three hours before recording fasting blood glucose. Blood glucose reading was repeated 15, 30, 60, and 120 minutes after glucose injection, and the area under the curve (AUC) for glucose disposal summarises the time course. In each chart, groups not sharing a letter (‘a’, ‘b’, or ‘c’) are significantly different, based on post-hoc comparisons following a significant two-way interaction between high-fat diet (HFD) and exercise (Ex) (p < 0.05). Error bars indicate standard deviation (BCAA, branched-chain amino acid).
A glucose tolerance test (GTT) was performed during the tenth week of treatment to measure glucose disposal after a glucose challenge. Examination of the AUC revealed a main effect of diet and exercise (p < 0.001 for both comparisons), but BCAAs did not significantly influence glucose tolerance (Fig. 1b). Sedentary HFD-fed animals exhibited impaired glucose tolerance compared with sedentary control diet-fed mice. Exercise significantly attenuated the glucose tolerance impairment caused by the HFD such that the AUC was not significantly different from the sedentary controls (p = 0.080). Further, exercise significantly improved glucose disposal in the control diet fed mice suggesting that exercise effects were largely independent of diet.
Tendon biomechanics
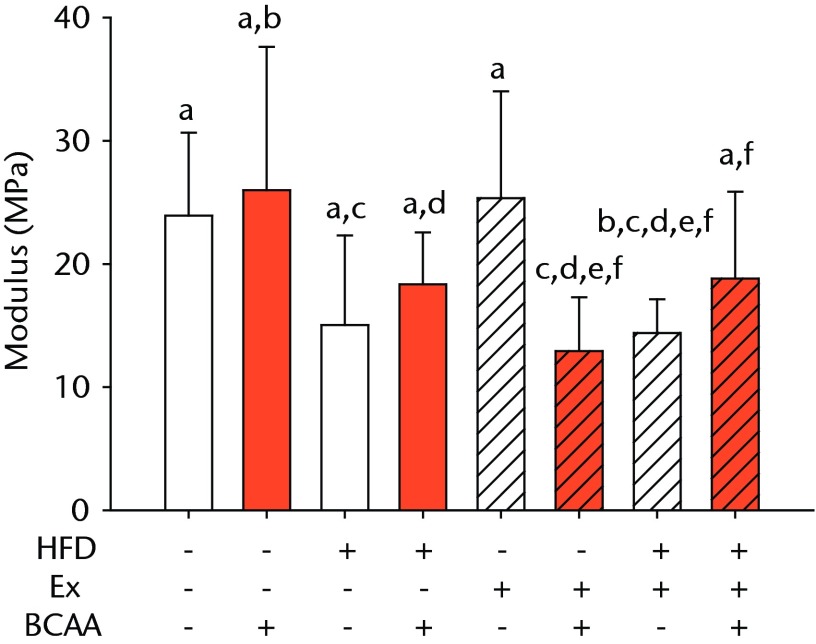
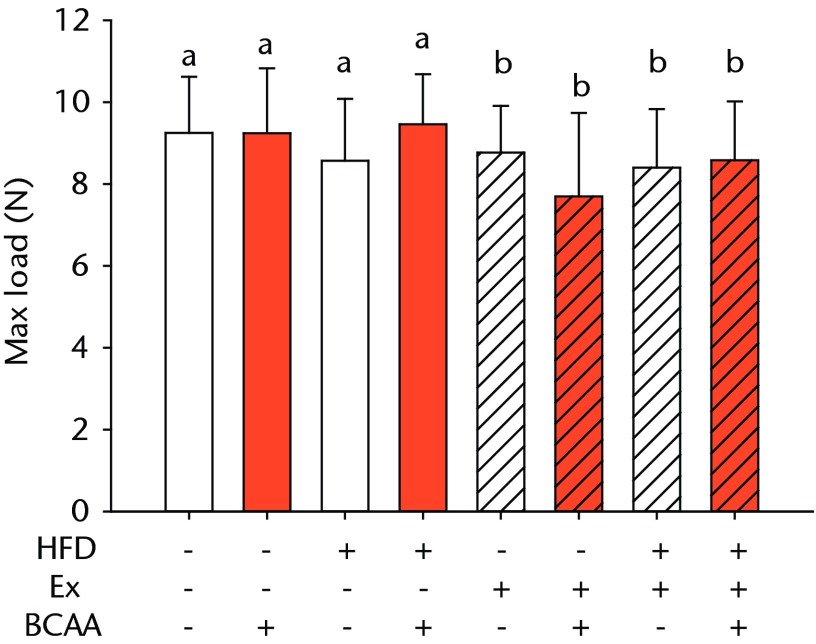
The biomechanical parameters of the Achilles tendon were analysed to determine whether HFD, exercise and BCAAs affected the tissue properties (Fig. 2, Table I). There was a main effect of diet in two comparisons. A significant increase in tendon cross-sectional area (p < 0.001) and a significant decrease in modulus (p = 0.001) were observed in mice on the HFD compared with the standard diet (Figs 2a and 2b, respectively). However, there were also significant two- and three-way interactions for modulus, such that exercised mice receiving BCAAs actually had increased modulus (though not significantly so) on the HFD compared with the standard diet. Exercise significantly reduced maximum load in the Achilles tendons (p = 0.025) (Fig. 2c).
Figs. 2a - 2d.
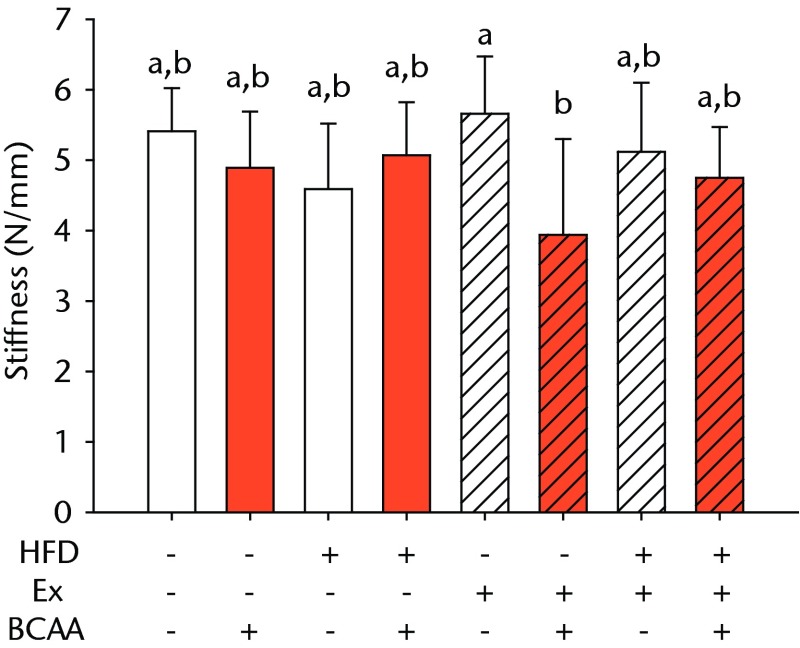
Bar charts of biomechanical parameters, showing the mean a) tendon cross-section area, b) modulus, c) maximum load and d) stiffness. Biomechanical parameters with significant changes. For tendon cross-section (a), groups not sharing a letter (‘a’ or ‘b’) are significantly different, based on a significant main effect for high fat diet (HFD) and no other significant main effects or interactions. For modulus (b), groups not sharing a letter (‘a’, ‘b’, ‘c’, ‘d’, ‘e’, or ‘f’) are significantly different, based on post-hoc comparisons following a significant three-way interaction between HFD, exercise (Ex), and branched-chain amino acid (BCAA). For maximum load (c), groups not sharing a letter (‘a’ or ‘b’) are significantly different, based on a significant main effect for exercise and no other significant main effects or interactions. For stiffness (d), groups not sharing a letter (‘a’ or ‘b’) are significantly different, based on post-hoc comparisons following significant two-way interactions between HFD and BCAA as well as between exercise and BCAA. Error bars indicate standard deviation.
Table I.
Biomechanical parameters of Achilles tendon specimens (BCAA, branched-chain amino acids)
| Treatment group | ||||||
|---|---|---|---|---|---|---|
| Diet | Activity | Supplement | Specimens (n) | Mean (sd) displacement (mm) | Mean (sd) strain (mm/mm) | Mean (sd) stress (MPa) |
| Control | Sedentary | Control | 10 | 2.44 (0.46) | 0.98 (0.27) | 13.28 (5.25) |
| Control | Sedentary | BCAA | 9 | 2.40 (0.40) | 0.88 (0.33) | 13.52 (2.91) |
| High-fat diet | Sedentary | Control | 10 | 2.73 (0.89) | 1.20 (0.64) | 11.13 (2.99) |
| High-fat diet | Sedentary | BCAA | 10 | 2.69 (0.79) | 1.08 (0.40) | 13.51 (3.60) |
| Control | Exercise | Control | 10 | 2.34 (0.41) | 0.88 (0.23) | 12.11 (3.75) |
| Control | Exercise | BCAA | 10 | 2.47 (0.90) | 1.04 (0.45) | 12.57 (3.72) |
| High-fat diet | Exercise | Control | 10 | 2.29 (0.45) | 1.01 (0.27) | 12.64 (4.84) |
| High-fat diet | Exercise | BCAA | 10 | 2.57 (0.39) | 0.93 (0.22) | 14.20 (3.35) |
BCAA supplementation caused a significant decrease in stiffness (p = 0.010) (Fig. 2d). However, there were also significant two-way interactions for stiffness, such that sedentary mice on the HFD actually had a trend toward increased stiffness (not significant) with BCAAs compared with regular water. Displacement, stress, and strain were not significantly altered by any of the treatments (Table I).
Histological analysis
This was performed on longitudinal sections of the Achilles tendons. Due to complications encountered in the sectioning of specimens, between six and nine tendons were analysed per group (58 tendons in total). Of these, 55 (95%) were normal in appearance and there were no appreciable differences between treatment groups. Figures 3a and 3b are representative sections from the groups that, a priori, might have been suspected to be most different (if any one of the treatments had an effect): namely, the overall control group (standard diet fed, sedentary, and regular water) and the high fat fed, exercise, and BCAA water group. Yet, even for these “extreme” groups, tendon appearances were similar (tendon appearances were similar for the other groups as well, but are not shown due to space limitations). Three tendons (5%) had mild alterations including mild inflammation with moderate increased cellularity at the Achilles tendon origin (HFD, sedentary and normal water), mild increased cellularity with cartilaginous differentiation in the tendon mid-substance (HFD, sedentary and BCAAs), and mild increased cellularity in the tendon mid-substance (HFD, exercise and normal water). Despite HFD leading to a significantly increased cross-sectional area, there was no histological evidence of change in fibrils on the longitudinal axis. It is interesting to note though that all three mice with histological abnormalities were on HFD.
Figs. 3a - 3c.
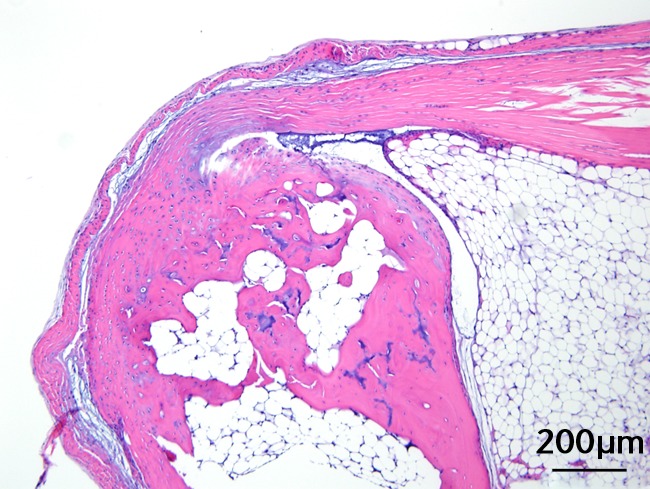
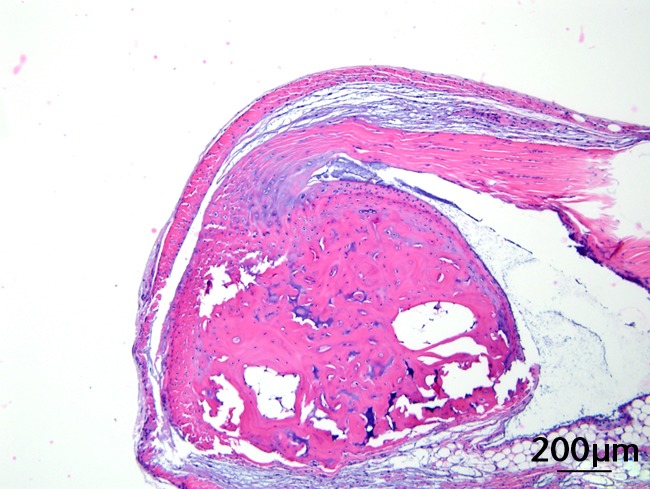
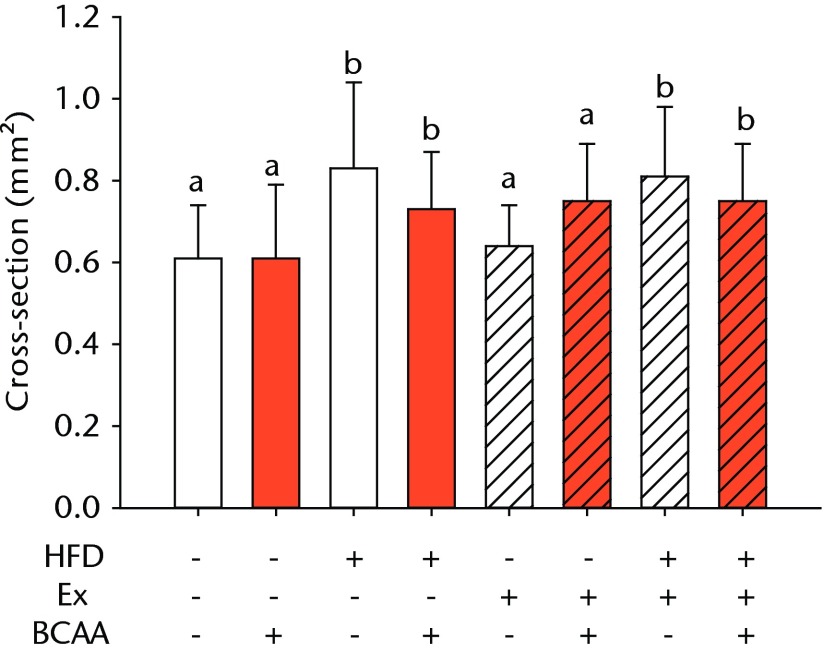
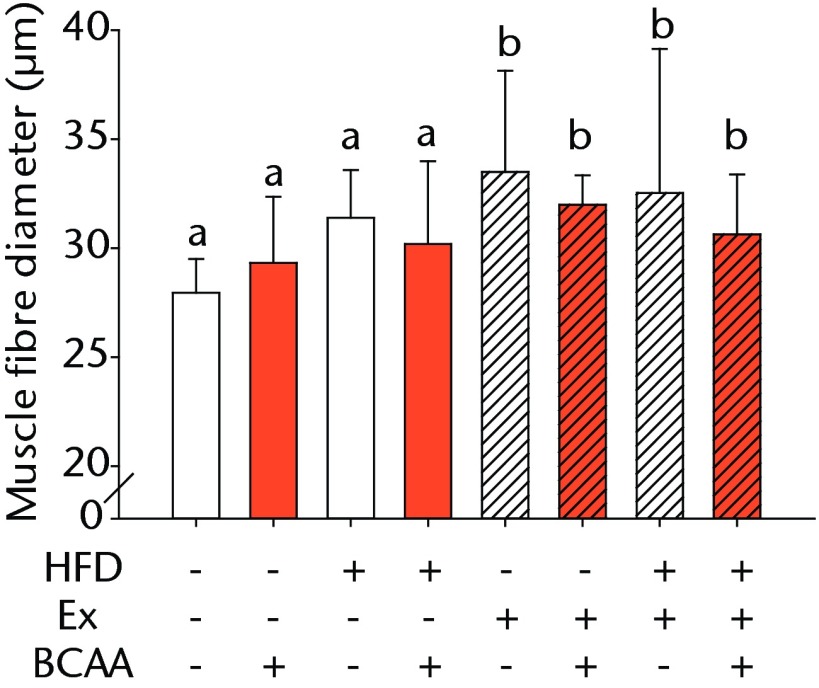
Figures 3a and 3b – histological images of the Achilles tendon at insertion in a) a sedentary, control-diet animal (neither high-fat diet (HFD) nor branched-chain amino acid (BCAA)), and b) in an exercise (Ex), HFD and BCAA treated mouse (both haematoxylin & eosin, bar 200 µm). Figure 3c – bar chart showing the mean quadriceps muscle fibre diameter measured following tissue collection. Groups not sharing a letter (‘a’ or ‘b’) are significantly different, based on a significant main effect for exercise and no other significant main effects or interactions. Error bars indicate standard deviation.
Morphometric analysis of the mid-substance of the quadriceps femoris muscle complex was performed (n = 4 to 9 per group) (Fig. 3c). Average fibril diameter in mice that exercised was significantly increased compared to sedentary mice (p = 0.017). Neither HFD feeding nor BCAA supplementation significantly affected muscle fibril diameter.
Body weight and tendon properties
We also examined how body weight and glucose tolerance related to the Achilles tendon biomechanical properties and quadriceps muscle morphometry (Table II). Values for each of the mice were analysed independent of group. There were significant positive correlations between body weight and cross-sectional area (p = 0.003), maximum load (p = 0.039), displacement (p = 0.002) and strain (p < 0.001), while body weight showed a negative relationship with modulus (p = 0.008) (Table II). There was also a significant positive correlation between body weight and AUC (p < 0.001). However, there was no significant correlation of AUC with any of the Achilles parameters (p = 0.061 to 0.941). Muscle fibre diameter did not significantly correlate with body weight or glucose tolerance (p = 0.819 and p = 0.836, respectively) (Table II).
Table II.
Correlation coefficients (r-values) and p-values showing the relationship of body weight and glucose tolerance with Achilles tendon parameters and quadriceps fibre diameter. All values from the mice (group designation was ignored) were included in the comparisons; n = 79 to 80 for each comparison, except n = 50 for fibre diameter
| Body weight | Area under curve | ||||
|---|---|---|---|---|---|
| Parameter | r-value | p-value | r-value | p-value | |
| Body weight | - | - | 0.602 | < 0.001 | |
| Area under curve | 0.602 | < 0.001 | - | - | |
| Cross-section | 0.328 | 0.003 | 0.136 | 0.235 | |
| Maximum load | 0.233 | 0.039 | 0.213 | 0.061 | |
| Displacement | 0.347 | 0.002 | 0.159 | 0.166 | |
| Stiffness | -0.001 | 0.995 | -0.014 | 0.902 | |
| Strain | 0.402 | < 0.001 | 0.025 | 0.826 | |
| Stress | -0.044 | 0.699 | 0.020 | 0.861 | |
| Modulus | -0.295 | 0.008 | 0.008 | 0.941 | |
| Fibre diameter | -0.033 | 0.819 | -0.030 | 0.836 | |
Discussion
Tendons experience stretch and extension in a wide range of strain rates and their mechanical properties make them well suited to act as biological springs.22 An understanding of their biomechanical properties is critical to assess their capability for maintaining normal body motility while preventing injury.22,23 In this study we examined the effects of HFD (and its resulting obesity), voluntary exercise, and BCAA treatment on the Achilles tendon of female C57BL/6 mice. Following the dietary change, obesity led to increased tendon cross-sectional area and decreased modulus. It is speculated that the tendon retains its ability to transmit load despite the decreased material property (modulus) by an increase in cross-sectional area, indicating that while the tendon increased in size, its capacity for load transmission was reduced. Increased tendon cross-sectional area can be caused by fatty infiltration and increased collagen deposition, but since there was no histologic evidence of fatty infiltration, increased extracellular matrix deposition was a plausible aetiology. There is speculation that increased fibril diameter, increased collagen cross-links, or both are involved in increased tendon stiffness.24-26 This would imply that a decrease in collagen cross-links and fibril diameter may decrease stiffness and there may have been a compensatory increase in collagen deposition and thus cross-section area. It is not known why a HFD would lead to altered collagen structure or cross-linking. In a related study, ultrastructural analysis of the deep digital flexor in Zucker rats showed an increased number of disorganized collagen fibril bundles and decreased mass average fibril diameter but no difference in the number of lipid droplets.6,27 Disorganisation of the fibrils and smaller fibrils could lead to a decreased modulus as the fibres would not be working in unison and not be able to transmit as much force. Future studies on the ultrastructural characteristics as well as collagen cross-linking in obese mice will elucidate the pathogenesis of the change.
Studies on the musculoskeletal tissues of obese females are underrepresented in the literature. However, in one study there was increased stiffness in the triceps-surae musculotendon unit of post-menopausal obese women compared with normal weight women.28 The authors speculated that increased adiposity in the skeletal muscle and decreased range of movement contributed to the change. Interestingly, our results differed as we found a decreased modulus and no significant effect on stiffness in the obese mice. Differences in the two studies may have arisen because the human studies were performed in aging women, which may have been complicated by other factors such as increases in cross-linking and advanced glycation end products.29 In addition, that study did not isolate the tendon and muscular changes, thus changes in the tendon were not specifically examined.
Exercise, besides significantly lowering body weight in the HFD mice, significantly lowered the maximum load at tendon failure. Since there was a significant correlation between body weight and maximum load, this observation may be partially explained by the body weight reduction and a reduced requirement on the tendon for maintaining strength. A potential limitation of this study was the use of female mice. Human females are less susceptible than males to tendon mechanical alterations following exercise.28 In female athletes there is no change in tendon stiffness following training, but increased stiffness is evident in male athletes.30 The gender difference is believed to be due to hormonal influences since elevated levels of oestradiol reduce collagen synthesis in ligaments.30-32 Thus, collagen remodelling may be muted in females because of the hormonal inhibition of oestradiol and could partially explain why exercise had limited impact on mouse tendons. Despite the limitation it is important to perform the study in female mice to determine their response, and it is recommended that a subsequent study be performed in male mice.
Many athletes take BCAA supplements (or other protein and/or amino acid supplements) to increase muscle mass and decrease muscle breakdown. While BCAAs did alter tendon stiffness and there were significant interactions between BCAAs and both diet and exercise for modulus, other effects were small to non-existent. Further experiments should be conducted in male and female mice with additional doses of BCAAs and for different lengths of time. In addition, experiments in humans should investigate the possible influences of amino acid supplementation in a fit population. Data from this study suggest that BCAA supplementation may not favourably influence most parameters, but adverse outcomes are not anticipated.
Morphometric analysis of the quadriceps muscle showed an increase in fibre diameter with exercise. Surprisingly there was no effect of HFD or BCAA supplementation, which contrasts with a previous study of male C57BL/6 mice with voluntary running wheel access for eight weeks. Pellegrino et al33 found alterations in both fibre size and myosin heavy chain isoform distribution as a result of amino acid supplementation. The different results might be attributable to the gender of the mice, the selected muscle, and/or type of amino acid supplement.
Besides using only female mice, a further limitation of our study is that only one time point was examined. Collagen remodelling is a slow process so a longer intervention may cause more significant alterations in tendon properties. Finally, voluntary exercise was the chosen intervention in this study and is not highly stressful for the mice. Another alternative would entail resistance training in mice to mimic the activities of common human physical training.
In conclusion, HFD feeding affected some of the biomechanical properties of tendons in C57BL/6J female mice without histologic abnormalities. BCAAs significantly decreased the stiffness of Achilles tendons, though this effect was moderated by diet and exercise. Despite exercise partially ameliorating both the weight gain and glucose levels in the 60% HFD group, the biomechanical impact of exercise was fairly limited; there was a significant decrease in the maximum load, a significant increase in the average fibril diameter of the quadriceps femoris muscle, and a significant interaction with BCAAs for stiffness and modulus. The correlations between body weight and biomechanical alterations demonstrated the importance of controlling obesity for maintaining healthy tendon properties. Because of the enormous consequences of obesity, further study on soft-tissue alterations is imperative.
Funding Statement
This study was supported by US NIH grants (National Center for Research Resources, 5P20 RR021954-05, the National Institute of General Medical Sciences, 8P20 GM103527-05, and The National Institute of Diabetes and Digestive and Kidney Diseases, R01 DK090460). KMP was supported by an NIH training grant (DK07778).
Footnotes
Author contributions:G. P. Boivin: Experimental design, Data collection, Data analysis, Histological analysis, Manuscript writing & review
K. M. Platt: Experimental design, In vivo animal handling, In vivo data collection, Data analysis, Manuscript writing & review
J. Corbett: Data collection, Mechanical testing, Manuscript editing
J. Reeves: Data collection, Mechanical testing, Manuscript editing
A. L. Hardy: Data collection, Mechanical testing, Manuscript editing
E. Y. Elenes: Biomechanical testing, Data collection, Data analysis, Manuscript writing & review
R. J. Charnigo: Statistical analysis, Manuscript editing
S. A. Hunter: Experiment design, Biomechanical testing, Data analysis, Manuscript writing & review
K. J. Pearson: Experiment design, Data analysis, Manuscript writing & review
ICMJE Conflict of Interest:None declared
References
- 1.Wearing SC, Hennig EM, Byrne NM, Steele JR, Hills AP. Musculoskeletal disorders associated with obesity: a biomechanical perspective. Obes Rev 2006;7:239–250 [DOI] [PubMed] [Google Scholar]
- 2.Gaida JE, Ashe MC, Bass SL, Cook JL. Is adiposity an under-recognized risk factor for tendinopathy?: a systematic review. Arthritis Rheum 2009;61:840–849 [DOI] [PubMed] [Google Scholar]
- 3.Gaida JE, Alfredson H, Kiss ZS, Bass SL, Cook JL. Asymptomatic Achilles tendon pathology is associated with a central fat distribution in men and a peripheral fat distribution in women: a cross sectional study of 298 individuals. BMC Musculoskelet Disord 2010;11:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramirez LC, Raskin P. Diabetic foot tendinopathy: abnormalities in the flexor plantar tendons in patients with diabetes mellitus. J Diabetes Complications 1998;12:337–339 [DOI] [PubMed] [Google Scholar]
- 5.Hess GW. Achilles tendon rupture: a review of etiology, population, anatomy, risk factors, and injury prevention. Foot Ankle Spec 2010;3:29–32 [DOI] [PubMed] [Google Scholar]
- 6.Biancalana A, Veloso LA, Gomes L. Obesity affects collagen fibril diameter and mechanical properties of tendons in Zucker rats. Connect Tissue Res 2010;51:171–178 [DOI] [PubMed] [Google Scholar]
- 7.Arampatzis A, Peper A, Bierbaum S, Albracht K. Plasticity of human Achilles tendon mechanical and morphological properties in response to cyclic strain. J Biomech 2010;43:3073–3079 [DOI] [PubMed] [Google Scholar]
- 8.Heinemeier KM, Kjaer M. In vivo investigation of tendon responses to mechanical loading. J Musculoskelet Neuronal Interact 2011;11:115–123 [PubMed] [Google Scholar]
- 9.Kubo K, Kanehisa H, Ito M, Fukunaga T. Effects of isometric training on the elasticity of human tendon structures in vivo. J Appl Physiol 2001;91:26–32 [DOI] [PubMed] [Google Scholar]
- 10.Geisler S, Brinkmann C, Schiffer T, et al. The influence of resistance training on patients with metabolic syndrome: significance of changes in muscle fiber size and muscle fiber distribution. J Strength Cond Res 2011;25:2598–2604 [DOI] [PubMed] [Google Scholar]
- 11.Campbell WW, Joseph LJO, Anderson RA, et al. Effects of resistive training and chromium picolinate on body composition and skeletal muscle size in older women. Int J Sport Nutr Exerc Metab 2002;12:125–135 [DOI] [PubMed] [Google Scholar]
- 12.Snijders T, Verdijk LB, Hansen D, Dendale P, van Loon LJ. Continuous endurance-type exercise training does not modulate satellite cell content in obese type 2 diabetes patients. Muscle Nerve 2011;43:393–401 [DOI] [PubMed] [Google Scholar]
- 13.Howatson G, Hoad M, Goodall S, et al. Exercise-induced muscle damage is reduced in resistance-trained males by branched chain amino acids: a randomized, double-blind, placebo controlled study. J Int Soc Sports Nutr 2012;9:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koba T, Hamada K, Sakurai M, et al. Branched-chain amino acids supplementation attenuates the accumulation of blood lactate dehydrogenase during distance running. J Sports Med Phys Fitness 2007;47:316–322 [PubMed] [Google Scholar]
- 15.Negro M, Giardina S, Marzani B, Marzatico F. Branched-chain amino acid supplementation does not enhance athletic performance but affects muscle recovery and the immune system. J Sports Med Phys Fitness 2008;48:347–351 [PubMed] [Google Scholar]
- 16.Nicastro H, da Luz CR, Chaves DF, et al. does branched-chain amino acids supplementation modulate skeletal muscle remodeling through inflammation modulation?: possible mechanisms of action. J Nutr Metab 2012;2012:136937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arakawa M, Masaki T, Nishimura J, Seike M, Yoshimatsu H. The effects of branched-chain amino acid granules on the accumulation of tissue triglycerides and uncoupling proteins in diet-induced obese mice. Endocr J 2011;58:161–170 [DOI] [PubMed] [Google Scholar]
- 18.Macotela Y, Emanuelli B, Bång AM, et al. Dietary leucine: an environmental modifier of insulin resistance acting on multiple levels of metabolism. PLoS One 2011;6:2118–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo K, Yu YH, Hou J, Zhang Y. Chronic leucine supplementation improves glycemic control in etiologically distinct mouse models of obesity and diabetes mellitus. Nutr Metab (Lond) 2010;7:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Guo K, LeBlanc RE, et al. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes 2007;56:1647–1654 [DOI] [PubMed] [Google Scholar]
- 21.Probst A, Palmes D, Freise H, et al. A new clamping technique for biomechanical testing of tendons in small animals. J Invest Surg 2000;13:313–318 [DOI] [PubMed] [Google Scholar]
- 22.Shadwick RE. Elastic energy storage in tendons: mechanical differences related to function and age. J Appl Physiol 1990;68:1033–1040 [DOI] [PubMed] [Google Scholar]
- 23.Kubo K, Kanehisa H, Fukunaga T. Gender differences in the viscoelastic properties of tendon structures. Eur J Appl Physiol 2003;88:520–526 [DOI] [PubMed] [Google Scholar]
- 24.Avery NC, Bailey AJ. Enzymic and non-enzymic cross-linking mechanisms in relation to turnover of collagen: relevance to aging and exercise. Scand J Med Sci Sports 2005;15:231–240 [DOI] [PubMed] [Google Scholar]
- 25.Birch HL. Tendon matrix composition and turnover in relation to functional requirements. Int J Exp Pathol 2007;88:241–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parry DA. The molecular and fibrillar structure of collagen and its relationship to the mechanical properties of connective tissue. Biophys Chem 1988;29:195–209 [DOI] [PubMed] [Google Scholar]
- 27.Biancalana A, Velloso LA, Taboga SR, Gomes L. Implications of obesity for tendon structure, ultrastructure and biochemistry: a study on Zucker rats. Micron 2012;43:463–469 [DOI] [PubMed] [Google Scholar]
- 28.Faria A, Gabriel R, Abrantes J, Brás R, Moreira H. Triceps-surae musculotendinous stiffness: relative differences between obese and non-obese postmenopausal women. Clin Biomech (Bristol, Avon) 2009;24:866–871 [DOI] [PubMed] [Google Scholar]
- 29.Abate M, Schiavone C, Pelotti P, Salini V. Limited joint mobility (LJM) in elderly subjects with type II diabetes mellitus. Arch Gerontol Geriatr 2011;53:135–140 [DOI] [PubMed] [Google Scholar]
- 30.Westh E, Kongsgaard M, Bojsen-Moller J, et al. Effect of habitual exercise on the structural and mechanical properties of human tendon, in vivo, in men and women. Scand J Med Sci Sports 2008;18:23–30 [DOI] [PubMed] [Google Scholar]
- 31.Liu SH, Al-Shaikh RA, Panossian V, Finerman GA, Lane JM. Estrogen affects the cellular metabolism of the anterior cruciate ligament: a potential explanation for female athletic injury. Am J Sports Med 1997;25:704–709 [DOI] [PubMed] [Google Scholar]
- 32.Yu WD, Panossian V, Hatch JD, Liu SH, Finerman GA. Combined effects of estrogen and progesterone on the anterior cruciate ligament. Clin Orthop Relat Res 2001;383:268–281 [DOI] [PubMed] [Google Scholar]
- 33.Pellegrino MA, Brocca L, Dioguardi FS, Bottinelli R, D’Antona G. Effects of voluntary wheel running and amino acid supplementation on skeletal muscle of mice. Eur J Appl Physiol 2005;93:655–664 [DOI] [PubMed] [Google Scholar]