Abstract
A previously beach-stranded, juvenile, male, bottlenose dolphin (Tursiops truncatus) was diagnosed with vertebral osteomyelitis of unknown etiology. Antemortem serological testing suggested past or current Brucella sp. infection; however, this could not be confirmed prior to death despite multiple isolation attempts from aspirates, blood, and biopsies. Systemic antibiotics were administered for over a year to control the suspected infection; however, the animal succumbed peracutely to a highly pathogenic, enterotoxin-secreting Staphylococcus sp. Gross necropsy findings included a fistulous tract leading to locally extensive osteomyelitis of a coccygeal vertebra with sequestra and osteophytes from which a Brucella species was isolated. Histopathological examination of intestine revealed pseudomembranous enteritis with a uniform population of intraluminal Gram-positive cocci. Staphylococcus aureus was isolated in pure culture from the intestine and tested positive for the staphylococcal enterotoxin A gene by polymerase chain reaction analysis. Serum taken shortly before death had endotoxin and elevated antibody titers to staphylococcal enterotoxin A when compared to samples collected during a period of apparent good health eighteen months earlier. The isolation of a pyrogenic toxin superantigen-producing staphylococcal isolate, clinical signs, and diagnostic findings in this animal resembled some of those noted in human toxic shock syndrome. The present case highlights the clinical challenges of treating chronic illnesses, complications of long-term antibiotic use, and promotion of pathogenic strains in cases of prolonged rehabilitation of marine mammals.
Keywords: Brucella, marine mammal medicine, necrotizing enteritis, Staphylococcus aureus, staphylococcal enterotoxin A, staphylococcal exotoxin, vertebral osteomyelitis
A male bottlenose dolphin (Tursiops truncatus) estimated to be unweaned and 1.5 years of age stranded on the Texas coast in September 1998. Significant clinical signs on presentation included an unhealed shark bite approximately 9 × 15 cm on the left dorsolateral abdomen caudal to the dorsal fin, loss of part of the dorsal fin, dehydration, and weight loss. A small area of swelling was noted on the animal's right peduncle close to where the trunk merges with the flukes. There were no breaks in the skin at the site, and the lesion appeared unrelated to the shark bite. After six months of rehabilitation at a Texas facility, the animal was transferred to Mystic Aquarium (Mystic, CT) for continued rehabilitation.
Initial radiographs of the peduncular lesion revealed sclerosis of the affected vertebra with calcification in the adjacent soft tissues but normal intervertebral spacing (Figure 1a). The lesion was interpreted as inactive and no etiology was established. Throughout the next eight months serial blood sampling revealed that the animal's white blood cell levels remained within the high end of the reference interval for the species 38 with occasional, mild elevations of neutrophils and fibrinogen. On four occasions during this period, oral administration of enrofloxacin (∼6.0mg/kg) and clindamycin (∼5.5mg/kg) for 7-10 days or just enrofloxacin was associated with a rapid return to normal white cell differential and total cell count. Minor subtle changes in size and palpable temperature were noted in the peduncular lump, but these did not consistently correlate with hematological or behavioral changes. Bacterial isolation was attempted on superficial punch biopsies of the skin and blubber overlying the affected vertebra, as well as a discharge from a fistulous track that developed from the lesion, but no organisms were recovered. However, blood samples taken at this time were seropositive for Brucella antibodies on the card, buffered acid plate agglutination (BAPA), and rivanol tests developed for use with domestic animals (Texas Veterinary Medical Diagnostic Laboratory, 1 Sippel Road, College Station, TX).
Figure 1.
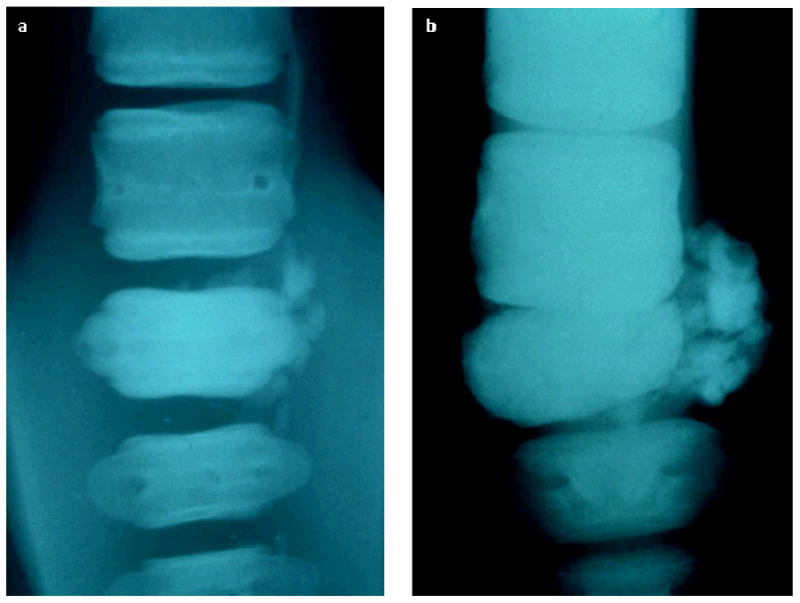
Radiographic imagings of the caudal vertebra taken at two different times, both images are a dorsoventral view with the more anterior vertebrae positioned at the top of the image. (a) Image taken shortly after arriving at Mystic Aquarium, showing sclerosis of the affected vertebra with soft-tissue calcification in the adjacent tissues but normal intervertebral spacing. (b) Image taken postmortem, 19 months later, showing further progression of the sclerosis of the affected vertebra and soft-tissue calcification as well as intervertebral chips, widening of the nutrient foramen, and collapse of the intervertebral space apparently allowing contact of the affected vertebra with the next most anterior vertebra
Nine months after transfer to Mystic, the animal acutely became anorexic. Concurrently, the peduncular lesion was noted to be warm to the touch and was thought to have increased in size since the animal's last examination one month previously. A complete blood count showed marked neutrophilia to 8650 cells/mm3 from a baseline average for this individual (compiled from multiple samplings over several months during times of normal behavior and apparent good health) of 4680 cells/mm3 and an elevated serum fibrinogen level (577 mg/dL from baseline 228 mg/dL). Radiographs of the vertebral lesion revealed further progression of the changes seen in the initial radiographs. These included roughening of vertebral margins, soft-tissue calcification and intervertebral bone chips, sclerosis of the affected vertebra, and widening of the nutrient foramen. Intervertebral spacing remained normal. A computed tomographic (CT) scan of the involved peduncular vertebra revealed a slab fracture involving nearly one-third of the vertebral body with evidence of devascularization and sequestrum formation (Figure 2a). Additional culture-based attempts to isolate a causative agent, including utilization of Brucella-specific culture methods on blood, aspirates, and deep biopsies, were unsuccessful. Despite an inability to isolate a causative agent, treatment was based on an assumption of Brucella sp. infection of the affected vertebra, which subsequently had sustained a pathological fracture. At various times during the year, systemic antibiotics, (e.g., doxycyclinea [400mg orally twice a day for 10d] ceftiofurb [200mg intramuscularly every 36h for 60d], clindamycinc, enrofloxacind as previously employed, and amoxicillin-clavulanatee [2188 mg orally twice a day for 14d]) were administered. Intralesional antibiotics included 200mg ceftiofur and one application of sustained release doxycycline-impregnated biodegradable microspheres 16. Aspirinf (650mg orally twice a day) and hydrocodoneg (5-10mg orally twice a day) were administered to control pain.
Figure 2.
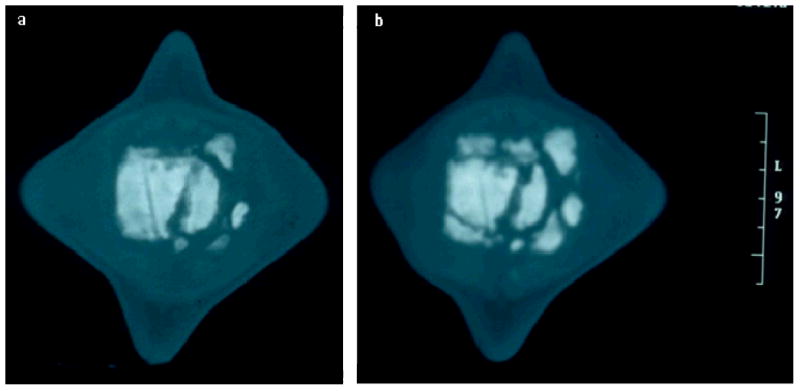
Computed tomography (CT) images of the affected vertebra taken at two different times. Both images are posterior –anterior views of sagittal sections approximately midway through the vertebral body with the animal's right on the image's right, a centimeter scale is on the right. Note: CT images shown here are parts of a series, viewing multiple adjacent images is required to fully visualize the findings. (a) Image taken during an acute episode of pain and mobility impairment showing a slab fracture involving nearly one-third of the right side of the vertebral body with evidence of devascularization and sequestrum formation. (b) Follow-up image taken eleven months later showing a severe progression of osteolysis and osteoproliferation with multiple bony fragments adjacent to the vertebra and in the joint space both anterior and posterior to the vertebra
The animal's general health continued to deteriorate over the next six months with intermittent decreases in food consumption and decreased activity. An acute decrease in the animal's voluntary use of its flukes and an increase in sensitivity when the flukes were manipulated were noted. A second CT examination one year after the first revealed severe progression of osteolysis and osteoproliferation with multiple bony fragments adjacent to the vertebra and in the joint space both anterior and posterior to the affected vertebra (Figure 2b).
Two months after the CT procedure, the animal experienced acute episodes of vomiting and diarrhea and became lethargic and anorexic with severe panleukopenia and mild dehydration. The animal was force-fed fish and freshwater, and ceftiofurb was administered intramuscularly. The following morning the animal displayed marked depression and disorientation. Blood collection from the vessels of the flukes and caudal peduncle was obtained with uncharacteristic difficulty suggesting decreased peripheral blood flow. Hematology showed persistence of panleukopenia and severe hemoconcentration. Serum chemistry values reflected electrolyte disturbances (hypochloremia, hyperphosphatemia, hyperkalemia, and hypernatremia), liver dysfunction (elevated alanine aminotransferase and aspartate aminotransferase), azotemia, and a profound hypoglycemia (Table 1). By this time, the animal was moribund and it died following intravenous administration of prednisolone sodium succinate, doxapram and epinephrine.
Table 1.
Hematology and serum chemistry: normal reference and peri-mortem values.
| Parameter | Reference intervala | 15 Jan 01 7:00 PM | 16 Jan 01 8:00 AM |
|---|---|---|---|
| WBC (cells/μL) | 6,447 - 11,405 | 2200 | 3100 |
| Neutrophils (cells/μL) | 3,750 - 7,234 | 1364 | 2015 |
| Lymphocytes (cells/μL) | 646 - 2,874 | 462 | 496 |
| Hematocrit % | 40 - 46 | 49 | 81 |
| Platelets | 41 - 55 | 128 | 50 |
| Glucose (mg/dL) | 80 - 136 | 101 | 15 |
| BUN (mg/dL) | 41 - 55 | 49 | 58 |
| Creatinine (mg/dL) | 0.9 - 1.6 | 1.4 | 2.8 |
| Chloride (mEq/L) | 115 - 125 | 120 | 113 |
| Phosphorus (mg/dL) | 4.4 - 6.4 | 6.6 | 12.7 |
| Potassium (mEq/L) | 3.4 - 4.0 | 5.4 | 8.3 |
| Sodium (mEq/L) | 152 - 158 | 168 | 164 |
| ALT (U/L) | 14 - 55 | 36 | 156 |
| AST (U/L) | 138 - 338 | 209 | 548 |
| Total Bilirubin (mg/dL) | 0.3 | 0.4 | |
| CK (mU/mL) | 84 - 268 | 255 | 339 |
Reference Ranges from appropriate age group (1-5 years) and sex.1
Major findings of the necropsy conducted on the day of the animal's death as well as subsequent analyses included gross, histopathologic, serologic, and microbiologic evidence of Brucella sp.-induced vertebral osteomyelitis as well as isolation of an enterotoxin- producing Staphylococcus sp. associated with necrotizing enteritis.
While antemortem tests developed for domestic species indicated the animal had antibodies to Brucella, an active infection was not confirmed until a Brucella sp. was isolated from samples obtained during necropsy, which were taken deeply along the vertebral fascia at the margin of the vertebral body lesion. Samples were inoculated onto trypticase soy agar plates containing 5% bovine serum, 7.5 U/ml bacitracin, 30 μg/ml cyclohexamide, 1.8 U/ml polymixin B. Cultures were incubated at 37° C in 10% carbon dioxide for a minimum of three weeks. The culture isolate was submitted to the National Veterinary Services Laboratory, Brucella Reference Laboratory (Ames, Iowa) to confirm identification 24. The isolate had different growth characteristics and a different dominant antigen from the Brucella sp. isolated from the then only previously documented clinical case of brucellosis in a captive cetacean 13, 28 but further speciation was not attempted. Post-mortem radiographs revealed progression of osteolysis and osteoproliferation involving the affected vertebra and further collapse of the intervertebral joint allowing still more contact between the affected vertebra and the adjacent more anterior vertebra (Figure 1b). The presence of endotoxin in the animal's serum was confirmed using a Limulus assayh9.
Tissue samples were collected from adrenal gland, blubber, brain, diaphragm, esophagus, heart, intestine, kidney, liver, lung, lymph nodes, pancreas, skin, spleen, stomach, testis, tongue, thymus, thyroid gland, and vertebrae and paravertebral tissue at the site of the lesion. Tissue samples were fixed by immersion in 10 % neutral buffered formalin, trimmed to fit plastic cassettes, processed routinely for paraffin embedment, sectioned at 4 μm width and stained with hematoxylin and eosin. Additional tissue sections of liver were stained using the Masson trichrome and Verhoeff-Van Gieson techniques, additional sections of intestine were stained using the Brown and Brenn Tissue Gram stain, additional sections of intestine were stained using the Brown and Brenn tissue Gram staing, and additional sections of the vertebral lesion were stained using the Grocott methenamine silver, Brown and Brenn tissue Gram, and Ziehl-Neelsen acid-fast techniques. Significant histopathologic findings were observed in the vertebral lesion, intestine, liver and lymphoid organs.
Microscopic examination of histologic sections of the affected coccygeal vertebra confirmed a locally extensive osteomyelitis containing sequestra, granulomas, fistulae, and osteophytes although no etiologic agent was identified in tissue sections stained using hematoxylin and eosin or in tissue sections stained using the Grocott methenamine silver, Brown and Brenn tissue Gram, and Ziehl-Neelsen acid-fast techniques. Histopathologic findings in sections of small intestine revealed necrotizing enteritis with reduced or absent villi, pseudomembranous aggregates of sloughed villous enterocytes along the mucosal surface and numerous intraluminal Gram-positive, coccoid bacteria, consistent with staphylococci. A coagulase-positive Staphylococcus sp. isolated from the anus, feces, blowhole, intestine, forestomach, and heart was confirmed to be Staphlococcus aureus using the standard coagulase test, colony pigmentation, and trehalose fermentation properties. Staphylococcal genomic DNA was extracted from cultures, purified, and subjected to a multiplex polymerase chain reaction (PCR) technique performed using primers specific for genes encoding nine staphylococcal superantigens (Figure 3) 29. Isolates were positive for the gene encoding staphylococcal enterotoxin A. Staphylococcal toxin antibody testing indicated a rising titer 3, 34.
Figure 3.
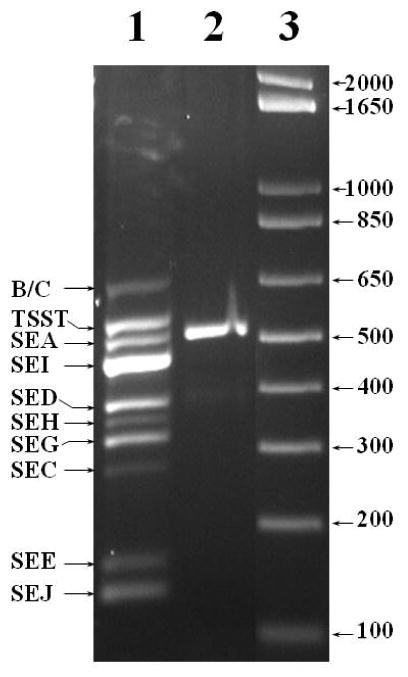
Agarose gel electrophoresis of the 520 bp multiplex PCR product from DNA isolated from the S. aureus isolate (designated ST-10) (lane 2) obtained from the dolphin described in this study. Lane 1 shows multiplex PCR products for the nine SE and TSST-1 superantigen controls designated in the left margin (B/C represents SEB and SEC). Lane 3 shows migration of size controls in a commercial product (1Kb Plus) obtained from Life Technologies.
Additional histopathologic findings included diffuse lymphocyte depletion in the spleen and other lymphoreticular organs. Multiple and marked lesions were noted in portal zones of the liver and included arteriolar sclerosis and reduplication, fibrosis, biliary hyperplasia, few portal venular ramifications and lymphohistiocytic portal hepatitis. These changes could be attributed as sequelae to the enteritis.
The initial phase of the current case epitomizes the difficulties encountered during treatment of bacterial disease when isolation attempts are not successful. Diagnosis by isolation may be difficult for any Brucella spp., requiring specialized media, unique culture conditions, and sometimes lengthy incubation. The availability of a number of PCR-based diagnostic tests has mitigated many of these problems. Serologic diagnosis in marine mammals has been complicated by the apparent lack of specificity and sensitivity of some techniques developed for detection of exposure in domestic animals 12,23,30. As such many institutions perform consensus testing where a number of different serological assays are performed and a seropositive diagnosis is only applied to samples testing positive by multiple means. Retrospectively, serum samples from the subject animal of the present case assayed as having amongst the highest levels of competitive inhibition of samples submitted from thousands of animals when utilizing a new competitive enzyme-linked immunosorbent assay (C-ELISA) that employed a marine isolate 27. Notwithstanding these shortcomings, both isolation of Brucella spp. and serologic evidence of infection or exposure have been documented in a variety of wild cetaceans and wild pinnipeds 1, 12, 30, and a case of Brucella abortion has been described in a captive bottlenose dolphin28. Several Brucella strains have been isolated from marine mammals that are distinct from recognized terrestrial Brucella species 4,17,19 and have a unique insertion sequence in the PCR product of the bp26 gene 7. Recently, marine Brucella isolates have been divided into two strains and named for the preferred host, B. ceti and B. pinnipedialis14. While the role of Brucella spp. in osteomyelitis and diskospondylitis has been documented in other species 37, including a case of presumed marine mammal-associated Brucella spinal osteomyelitis in a human patient 25, the case reported here is the first account in a marine mammal. Osteomyelitis is a relatively common diagnosis in cetaceans. Other documented cases of vertebral body osteomyelitis have been associated with Nocardia33, Staphylococcus sp 2, or have had undetermined etiologies 35. The relatively recent discovery of marine origin Brucella and difficulties in isolating Brucella spp. when culture efforts are not specifically aimed at Brucella12 may have resulted in a failure to determine the etiology in some earlier cases.
Despite a firm suspicion of a vertebral brucellosis in the present case, an organism was not isolated antemortem, and consequently, antibiotic sensitivity testing to determine the most appropriate antibiotic was not possible. Antibiotics were used that might have efficacy against Brucella spp., (e.g., doxycycline), but were ultimately not efficacious. The initial administration of doxycycline used an experimental protocol involving the local administration of sustained release antibiotic impregnated biodegradable microspheres 16. While this delivery vehicle has been used successfully for soft tissue infections in laboratory animals, the successful administration of the microspheres in the present case was hampered by the difficulty encountered in injecting the suspended particles into the rigid, affected tissues. These factors may have contributed to a suboptimal delivery of the drug to the focus of infection. When the animal's condition continued to deteriorate, this protocol was followed with oral doxycycline which may not have reached adequate systemic levels due to the large amounts of dietary calcium, in the form of fish bones within the animal's gastrointestinal tract, that could act to chelate the drug and reduce its absorption 36. Finally, the sequestra found at the site of osteomyelitis would have had poor exposure to antibiotics, even if appropriate drug levels were achieved systemically, and may have served as persistent niduses of infection.
Most significant in the current case are the effects of chronic illness and of long-term antibiotics on the patient, particularly on commensal and nontargeted bacteria. Chronic infections have been shown to make animals more susceptible to infection with other organisms. Exposure to Brucella antigen has been specifically shown to down-regulate the immune system by reducing lymphocyte blast transformation and Th1-type lymphokine production in mice 32. Additionally, long term antibiotic therapy can alter both the resistance patterns and the type of flora present. Increased resistance is observed and the growth of commensal bacteria is suppressed, allowing the emergence of pathogenic strains and their proliferation 18,20,31. The continued antibiotic therapy in this case probably resulted in selecting the toxigenic Staphylococcus that was isolated in pure culture from multiple sites postmortem.
The cause of death in the animal in the current study was likely acute systemic organ failure induced by the toxin-producing enteric staphylococci independently or in combination with concurrent endotoxemia from the Brucella sp.osteomyelitis. Staphylococcus aureus exotoxins can immunomodulate hosts, dramatically enhancing host susceptibility to endotoxin and causing shock 10,15. One could speculate that the presence of exotoxin and the concurrent release of endotoxin from the Brucella sp. infection resulted in a potentiation of the lethality of both toxins. Interestingly, a 2007 report cites the cases of two additional bottlenose dolphins that died with intercurrent Staphylococcal and Brucella spp. infections 26. The precise mechanism in the present case could be septic, enterotoxic, or endotoxic shock. The case reported here manifested both systemic involvement and isolation of an enterotoxin-producing S. aureus, but lacked the demonstration of fever, rash, desquamation, or hypotension included in the clinical definition of toxic shock syndrome 10. However, it is well documented that some classical symptoms in humans may not be replicated in other animal species. The missing parameters either were not measured or were difficult to assess and interpret in a marine mammal. In this case antemortem indications of systemic involvement included serum chemistry changes consistent with acute deleterious changes in renal and hepatic function (Table 1), vomiting and diarrhea indicating gastrointestinal involvement, and disorientation suggesting central nervous system impairment. Varying degrees of congestion were noted in multiple organs, (e.g., lung, liver, spleen, stomach and intestine), together with focal hemorrhage in the superficial gastric lamina propria. The combination of these clinical signs, serum biochemical abnormalities, gross and histopathologic findings in the intestine and results of serological analyses suggest S. aureus enteritis and toxemia due to enterotoxin A or possibly other superantigen toxins. Isolation of coagulase-positive Staphylococcus spp. from skin and body orifices of clinically normal dolphins is not unusual, and bacteria of this genus have been isolated in cases of respiratory disease 5,6,11,22, renal disease 21, a cerebral abscess 8, and skin lesions 39. The current case highlights the need to avoid complacency with staphylococcal infections and to maintain vigilance to the potential serious complications of isolates capable of toxin production, especially in otherwise compromised animals.
Acknowledgments
This report is a posthumous publication for both David St. Aubin and John Buck. The clinical work up and assessment could not have been accomplished without their earlier contributions. We would like to acknowledge the efforts of the Texas Marine Mammal Stranding Network in the rescue and initial rehabilitation of the animal discussed in this case report. We thank the administration and staff of the Westerly Hospital, Westerly, RI for providing computerized tomographic scanning services, the Department of Chemical Engineering, University of Colorado and the School of Pharmacy, University of Colorado Health Sciences Center for providing doxycycline impregnated microspheres for treating the osteomyelitis, and Pfizer Global Research and Development of Groton CT for serum chemistry analyses. We appreciate Gayle Sirpenski's assistance in record keeping, shipping, editing, photo-documentation, and other support. The suggestions of two anonymous reviewers assisted greatly in improving this manuscript. This work was supported by USDA grant 99-35201-8581 (GAB), PHS grants AI28401, P20-RR15587, and P20 RR016454 (GAB), and the Idaho Agricultural Experiment Station. This work is contribution #134 from the Sea Research Foundation.
Sources and Manufacturers
Vibramycin, Pfizer Inc, New York, NY
Naxcel, Pfizer Inc, New York, NY
Cleocin HCl Pfizer Inc, New York, NY
Baytril, Bayer Healthcare LLC, Animal Health Division, Shawnee Mission, KS
Augmentin, GlaxoSmithKline, Philadelphia, PA
Bayer aspirin, Bayer Healthcare LLC, Morristown NJ
Hycodan, Endo Pharmaceuticals Inc. Chadds Ford, PA
Limulus Amebocyte Lysate Gel-Clot assay, Charles River Laboratories International, Inc., Wilmington, MA 01887
1 kb Plus, Life Technologies Corp, Carlsbad, CA
References
- 1.Aguirre AA, Keefe TJ, Reif JS, et al. Infectious disease monitoring of the endangered Hawaiian monk seal. J Wildl Dis. 2007;43:229–241. doi: 10.7589/0090-3558-43.2.229. [DOI] [PubMed] [Google Scholar]
- 2.Alexander JW, Solangi MA, Riegel LS. Vertebral osteomyelitis and suspected diskospondylitis in an Atlantic bottlenose dolphin (Tursiops truncatus) J Wildl Dis. 1989;25:118–121. doi: 10.7589/0090-3558-25.1.118. [DOI] [PubMed] [Google Scholar]
- 3.Blomster-Hautamaa DA, Schlievert PM. Purification of toxic-shock syndrome toxin-1. Meth Enzymol. 1988;165:37–43. doi: 10.1016/s0076-6879(88)65009-9. [DOI] [PubMed] [Google Scholar]
- 4.Bricker BJ, Ewalt DR, Macmillan AP, et al. Molecular characterization of Brucella strains isolated from marine mammals. J Clin Microbiol. 2000;38:1258–1262. doi: 10.1128/jcm.38.3.1258-1262.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buck JD, Spotte S. Microbiology of captive white-beaked dolphins (Lagenorhynchus albirostris) with comments on epizootics. Zoo Biol. 1986;5:321–329. [Google Scholar]
- 6.Buck JD, Wells RS, Rhinehart HL, et al. Aerobic microorganisms associated with free-ranging bottlenose dolphins in coastal Gulf of Mexico and Atlantic Ocean waters. J Wildl Dis. 2006;42:536–544. doi: 10.7589/0090-3558-42.3.536. [DOI] [PubMed] [Google Scholar]
- 7.Cloeckaert A, Grayson M, Grepinet O. An IS711 element downstream by the bp26 gene is a specific marker of Brucella spp isolated from marine mammals. Clin Diag Lab Immunol. 2000;7:835–839. doi: 10.1128/cdli.7.5.835-839.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colgrove GS, Migaki G. Cerebral abscess associated with stranding in a dolphin. J Wildl Dis. 1976;12:271–274. doi: 10.7589/0090-3558-12.2.271. [DOI] [PubMed] [Google Scholar]
- 9.Cooper JF, Pearson SM. Detection of endotoxin in biological products by the limulus test. Dev Biol Stand. 1977;34:7–13. [PubMed] [Google Scholar]
- 10.Dinges MM, Orwin PM, Schlievert PM. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev. 2000;13:16–34. doi: 10.1128/cmr.13.1.16-34.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunn JL. Bacterial and mycotic diseases of cetaceans and pinnipeds. In: Dierauf LA, editor. CRC handbook of marine mammal health, disease, and rehabilitation. Boca Raton, FL: CRC Press; 1990. pp. 73–87. [Google Scholar]
- 12.Dunn JL, Buck JD, Robeck TR. Bacterial diseases of cetaceans and pinnipeds. In: Dierauf LA, Gulland FMD, editors. CRC handbook of marine mammal medicine. 2nd. Boca Raton, FL: CRC Press; 2001. pp. 309–355. [Google Scholar]
- 13.Ewalt DR, Payeur JB, Martin BM, et al. Characteristics of a Brucella species from a bottlenose dolphin (Tursiops truncatus) J Vet Diag Invest. 1994;6:448–452. doi: 10.1177/104063879400600408. [DOI] [PubMed] [Google Scholar]
- 14.Foster G, Osterman BS, Godfroid J, et al. Brucella ceti sp. nov. and Brucella pinnipedialis sp. nov. for Brucella strains with cetaceans and seals as their preferred host. International J Syst Evol Microbiol. 2007;57:2688–2693. doi: 10.1099/ijs.0.65269-0. [DOI] [PubMed] [Google Scholar]
- 15.Fujikawa H, Igarashi H, Usami H, et al. Clearance of endotoxin from blood of rabbits injected with staphylococcal toxic shock toxin-1. Infect Immunol. 1986;52:134–137. doi: 10.1128/iai.52.1.134-137.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goertz CEC, Dunn JL, Manning MC, et al. Vertebral osteomyelitis in a bottlenose dolphin (Tursiops truncatus): a novel treatment using sustained release antibiotic impregnated, biodegradable microspores. Proce Joint Conf Amer Assoc Zoo Vet and Int Assoc Aquat Anim Med. 2000;31:380–381. [Google Scholar]
- 17.Groussaud P, Shankster SJ, Koylass MS, et al. Molecular typing divides marine mammal strains of Brucella into at least three groups with distinct host preferences. J Med Microbiol. 2007;56:1512–1518. doi: 10.1099/jmm.0.47330-0. [DOI] [PubMed] [Google Scholar]
- 18.Gustafsson I, Sjölund M, Torell E, et al. Bacteria with increased mutation frequency and antibiotic resistance are enriched in the commensal flora of patients with high antibiotic usage. J Antimicrob Chemother. 2003;52:645–650. doi: 10.1093/jac/dkg427. [DOI] [PubMed] [Google Scholar]
- 19.Jensen AE, Cheville NF, Thoen CO, et al. Genomic fingerprinting and development of a dendrogram for Brucella spp. isolated from seals, porpoises, and dolphins. J Vet Diag Invest. 1999;11:152–157. doi: 10.1177/104063879901100208. [DOI] [PubMed] [Google Scholar]
- 20.Kaatz GW, Barriere SL, Schaberg DR, et al. The emergence of resistance to ciprofloxacin during treatment of experimental Staphylococcus aureus endocarditis. J Antimicrob Chemother. 1987;20:753–758. doi: 10.1093/jac/20.5.753. [DOI] [PubMed] [Google Scholar]
- 21.Ketterer PJ, Rosenfeld LE. Septic embolic nephritis in a dolphin caused by Staphylococcus aureus. Austral Vet J. 1974;50:123. doi: 10.1111/j.1751-0813.1974.tb05275.x. [DOI] [PubMed] [Google Scholar]
- 22.Kinoshita R, Brook F, Vedros N, et al. Staphylococcal isolations and clinical cases of Staphylococcus aureus in bottlenose dolphins at Ocean Park, Hong Kong. Proc Thirty-fifth Ann Int Assoc Aquat Anim Med Conf. 1994;35:159. [Google Scholar]
- 23.Maratea J, Ewalt DR, Frasca S, Jr, et al. Evidence of Brucella sp. infection in marine mammals stranded along the coast of southern New England. J Zoo Wildl Med. 2003;34:256–261. doi: 10.1638/02-053. [DOI] [PubMed] [Google Scholar]
- 24.Mayfield JE, Bantle JA, Ewalt DR, et al. Detection of Brucella cells and components. In: Neilsen K, Duncan JR, editors. Animal Brucellosis. Boca Raton, FL: CRC Press; 1990. pp. 97–120. [Google Scholar]
- 25.McDonald WL, Jamaludin R, Mackereth G, et al. Characterization of a Brucella sp. strain as a marine mammal type despite isolation from a patient with spinal osteomyelitis in New Zealand. J Clin Microbiol. 2006;44:4363–4370. doi: 10.1128/JCM.00680-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meegan J, Smith CR, Wong SK, et al. Int Assoc Aquat Anim Med Ann. FL: 2007. (Abstract) Brucella sp. infected bottlenose dolphin (Tursiops truncatus) cases in two populations: serologic and clinical evaluations. [Google Scholar]
- 27.Meegan J, Field C, Sidor I, et al. Development, validation, and utilization of a competitive enzyme-linked immunosorbent assay for the detection of antibodies against Brucella species in marine mammals. J Vet Diagn Invest. 2010;22:856–862. doi: 10.1177/104063871002200603. [DOI] [PubMed] [Google Scholar]
- 28.Miller WG, Adams G, Ficht TA, et al. Brucella-induced abortions and infection in bottlenose dolphins (Tursiops truncatus) J Zoo Wildl Med. 1999;30:100–110. [PubMed] [Google Scholar]
- 29.Monday SR, Bohach GA. Use of a multiplex PCR to detect classical and newly described pyrogenic toxin genes in staphylococcal isolates. J Clin Microbiol. 1999;37:3411–3414. doi: 10.1128/jcm.37.10.3411-3414.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nielsen O, Stewart RE, Nielsen K, et al. Serologic survey of Brucella spp. antibodies in some marine mammals of North America. J Wildl Dis. 2001;37:89–100. doi: 10.7589/0090-3558-37.1.89. [DOI] [PubMed] [Google Scholar]
- 31.Nord CE, Edlund C. Impact of antimicrobial agents on human intestinal microflora. J Antimicrob Chemother. 1990;2:218–237. doi: 10.1080/1120009x.1990.11739021. [DOI] [PubMed] [Google Scholar]
- 32.Onate A, Andrews E, Beltran A, et al. Frequent exposure of mice to crude Brucella abortus proteins down-regulates immune response. J Vet Med B Infect Dis Vet Public Health. 2000;47:677–682. doi: 10.1046/j.1439-0450.2000.00402.x. [DOI] [PubMed] [Google Scholar]
- 33.Robeck TR, Dalton LM, Young WG. Nocardia spp. induced chronic suppurative osteomyelitis in a beluga whale. Proc Twenty-sixth Ann Int Assoc Aquat Anim Med Conf. 1995;26:28. [Google Scholar]
- 34.Schlievert PM. Immunochemical assays for toxic-shock syndrome toxin-1. Meth Enzymol. 1988;165:339–344. doi: 10.1016/s0076-6879(88)65050-6. [DOI] [PubMed] [Google Scholar]
- 35.Stevens R, Hopkins T. Osteomyelitis in the spine of a bottlenose dolphin (Tursiops truncatus) Proc Twenty-first Ann Int Assoc Aquat Anim Med Conf. 1990;21:162. [Google Scholar]
- 36.Stoskopf MK, Willens S, McBain JF. Pharmaceuticals and formularies. In: Dierauf LA, Gulland FMD, editors. CRC handbook of marine mammal medicine. 2nd. Boca Raton, FL: CRC Press; 2001. pp. 703–727. [Google Scholar]
- 37.Thompson K. Bones and joints. In: Maxie MG, editor. Jubb, Kennedy and Palmer's Pathology of Domestic Animals. 5th. Vol. 1. San Diego, CA: Academic Press; 2007. pp. 1–184. [Google Scholar]
- 38.Venn-Watson S, Jensen ED, Ridgway SH. Effects of age and sex on clinicopathologic reference ranges in a healthy managed Atlantic bottlenose dolphin population. J Amer Vet Med Assoc. 2007;231:596–601. doi: 10.2460/javma.231.4.596. [DOI] [PubMed] [Google Scholar]
- 39.Veraldo PE, Kilpper-Balz R, Biavasco F, et al. Staphylococcus delphini sp. nov., a coagulase positive species isolated from dolphins. Int J Syst Bateriol. 1988;38:436–439. [Google Scholar]


