Abstract
The mechanisms underlying tolerance to noninherited maternal Ags (NIMA) are not fully understood. In this study, we designed a double-transgenic model in which all the offspring’s CD8+ T cells corresponded to a single clone recognizing the Kb MHC class I protein. In contrast, the mother and the father of the offspring differed by the expression of a single Ag, Kb, that served as NIMA. We investigated the influence of NIMA exposure on the offspring thymic T cell selection during ontogeny and on its peripheral T cell response during adulthood. We observed that anti-Kb thymocytes were exposed to NIMA and became activated during fetal life but were not deleted. Strikingly, adult mice exposed to NIMA accepted permanently Kb+ heart allografts despite the presence of normal levels of anti-Kb TCR transgenic T cells. Transplant tolerance was associated with a lack of a proinflammatory alloreactive T cell response and an activation/expansion of T cells producing IL-4 and IL-10. In addition, we observed that tolerance to NIMA Kb was abrogated via depletion of CD4+ but not CD8+ T cells and could be transferred to naive nonexposed mice via adoptive transfer of CD4+CD25high T cell expressing Foxp3 isolated from NIMA mice.
Transplantation tolerance, defined as the lack of donor-specific inflammatory immunity associated with long-term allograft survival, was initially described in recipients that had been exposed to alloantigens during development. In 1945, seminal studies by Owen et al. (1) showed that fetal exposure to alloantigens via vascular anastomoses led to indefinite survival of allotransplants in bovine twins. A few years later, Billingham et al. (2) described the first experimental model of neonatal tolerance induction in rodents. It was reported that adult mice injected with fully allogeneic splenocytes during fetal or neonatal periods of life were rendered tolerant to skin grafts from the same donor. These seminal studies demonstrated that exposure to Ags during fetal/neonatal period of life impacts dramatically the future offspring’s immune system.
Maternal cells and molecules, as well as microbes, traffic regularly from the mother to the fetus/neonate during pregnancy and breast-feeding (3). This phenomenon has been implicated in the offspring’s susceptibility to autoimmune diseases and infections, as well as its ability to reject allogeneic transplants (4). The most compelling evidence of the maternal influence on the offspring’s immune system has been provided by studies evaluating the role of noninherited maternal Ags (NIMA) in transplantation (4). It is now well established that the transmission of NIMA during fetal and neonatal periods of life has a long-term impact on the alloimmune response and subsequent allotransplant rejection in adult individuals. Van Rood et al. (4) provided initial evidence showing the influence of NIMA exposure on humoral and cellular alloimmunity in humans. It was observed that a large portion of patients who produced anti-donor Abs after blood transfusion did not form Abs to NIMA. In contrast, the same subjects consistently mounted vigorous humoral responses to noninherited paternal Ags (5). Later, Bean et al. (6) reported the absence of MLRs after maternal transfusions but not paternal ones. Subsequent observations of both prolonged survival of kidney transplants from sibling or cadaver donors and suppression of graft-versus-host (GVH) reactions after bone marrow transplantation further confirmed the tolerogenic effects of NIMA (7).
Although much evidence has been accumulated for the influence of NIMA in transplant patients, few studies have addressed this effect in experimental models. Iványi and Démant (8) showed prolonged survival of maternal skin grafts in newborn rabbits. Similarly, Zhang and Miller (9) reported some tolerogenic effects of NIMA on semiallogeneic maternal skin transplants in mice. In this model, both pregnancy and breast-feeding were required to achieve long-term graft survival. In collaboration with Burlingham’s group (10), we previously investigated the effects of NIMA on polyclonal T and B cell alloresponses and allotransplant rejection in mice. We reported that the majority of H-2b/b offspring of semiallogeneic (H-2b/d) mothers accept fully allogeneic DBA/2 heart grafts (graft survival >180 d) (10). Strikingly, no signs of intimal thickening and fibrosis, which are characteristic features of chronic rejection, were detected in heart transplants collected from NIMA-exposed mice. In this model, long-term survival of heart transplants expressing NIMA was observed exclusively in offspring that had been both carried and breast-fed by a mother expressing NIMA (10). We also demonstrated a specific influence of NIMA on the development of offspring’s B lymphocytes in a BCR transgenic (Tg) model, distinct from the fate of self-reactive B cells in the same model (11, 12). Collectively, these studies underscore the potent tolerogenic effects of NIMA in allotransplantation. In contrast, Molitor-Dart et al. (13) have recently reported that, under certain circumstances, the presentation of NIMA can result in offspring’s sensitization rather than tolerization. However, the mechanisms by which NIMA actually drive the immune system toward transplant tolerance or rejection remain unclear. Elucidation of this question is likely to pave the way for the design of novel tolerance protocols in clinical transplantation.
In this study, we used a model in which a single NIMA is the MHC class I H-2 Kb molecule in a Kb-Tg mouse and the offspring express an anti-Kb TCR transgene on CD8+ T cells. We observed that the fetus’s anti-Kb TCR Tg thymic T cells were exposed and activated to NIMA during pregnancy and neonatal life up to 3 wk of age, leading to the deletion of half of T cells during this period. The adult offspring displayed long-term survival of NIMA Kb-expressing heart allotransplants. Tolerance to NIMA was mediated via the suppression of the proinflammatory response by anti-Kb CD8+ T cells and the activation/expansion of CD4+CD25high Foxp3+ regulatory T cells (Tregs) recognizing the Kb alloantigen. The implications of these findings for the design of tolerance protocols in allotransplantation are discussed.
Materials and Methods
Mice and transplantations
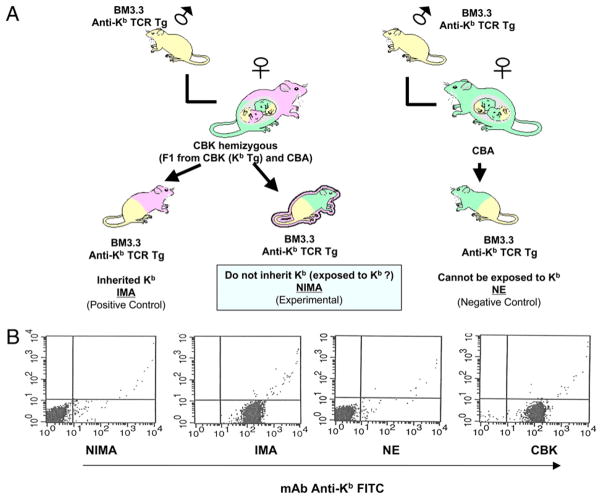
Mice were bred and maintained at Massachusetts General Hospital and Institut Jacques Monod’s animal facilities under specific pathogen-free conditions. All animal care and handling were performed according to institutional guidelines. The day of the vaginal plug was considered as day 0.5 of gestation. CBK Tg (CBA/ca mice [H-2k] expressing a Kb MHC class I transgene) were used as donors in heart transplants (14). Offspring of BM3.3 anti-Kb TCR Tg male mice (15) and F1 (CBA/ca × CBK) females were used as NIMA (offspring that do not inherit Kb) and IMA (offspring that inherit Kb maternal Ag) recipient mice (Fig. 1). To separate NIMA and IMA offspring, we stained blood from orbital sinus with an anti-Kb FITC mAb. No cells from NIMA were Kb+, whereas all the cells were found to express Kb in IMA mice. Offspring of BM3.3 anti-Kb TCR Tg male and CBK female mice were used as positive control animals for tolerance to Kb (Kb inherited as self: IMA). Offspring of BM3.3 anti-Kb TCR Tg male and wild type CBA female mice (never exposed to Kb: NE) were used as negative control animals (i.e., lack of tolerance to Kb). NIMA, IMA, and NE mice were transplanted in the peritoneal cavity with a vascularized CBK (Kb-Tg) heart using the microsurgical technique previously described by Corry et al. (16). Graft rejection was monitored by daily palpation of heart and confirmed by histological techniques. In some experiments, CD4+ or CD8+ T cells were depleted from recipient mice with anti-CD4 (GK1.5) and anti-CD8 (53.6.72) mAbs (1 mg given i.p. at days −3 and −1 pretransplant), respectively.
FIGURE 1.
The model. A, To obtain NIMA and IMA mice, we mated Bm3.3 anti-Kb TCR Tg male mice with (CBK [CBA, Kb Tg] × CBA) F1 female mice. The offspring, which inherited both the anti-Kb TCR transgene and the Kb transgene, were referred to as IMA mice. The offspring, which inherited the anti-Kb TCR transgene but not the Kb trans-gene, were referred to as NIMA mice. NE control offspring were obtained by mating BM3.3 anti-Kb TCR Tg female mice with CBA male mice (these mice could never be exposed to Kb). B, The expression of MHC class I glycoprotein Kb was assessed in positive control mice IMA and CBK, negative control mice NE, and experimental NIMA mice. Representative FACS profiles obtained with spleen cells are shown.
Cell suspensions
Cells were isolated from thymus and spleen of individual fetuses at 18.5 d postcoitum (pc) and neonates at 3.5 d postpartum (pp). Organs were gently pressed through a sieve using a syringe plunger and suspended in PBS containing 4% FCS and 0.1% sodium azide (PBS/FCS/NaN3). Viable cells were counted by trypan blue exclusion.
Immunofluorescence staining and flow cytometric analyses
Aliquots of 5 × 105 nucleated cells were incubated for 40 min at 4°C with an optimal amount of the following mAbs: anti-CD8 coupled to FITC, anti-CD4 coupled to PE, anti-CD25 coupled to FITC, anti-CD44 coupled to PE, anti-TCR β-chain coupled to PE (all purchased from Pharmingen), and anti-BM3.3 clonotype Ti98 prepared according to conventional techniques and coupled to biotin. Cells were washed twice in PBS/FCS/NaN3. Biotinylated Ab was revealed by incubating cells for 20 min at 4°C with streptavidin-PE or streptavidin-allophycocyanin. After washing, cells were analyzed on a CyAn LX flow cytometer (DakoCytomation) equipped with 488- and 635-nm lasers. The cell populations analyzed were gated on the viable lymphoid cell population on the basis of forward and side scatter criteria. When possible, at least 104 Ti98+ cells were analyzed from each sample.
T cell assays
The deletion of anti-Kb TCR Tg T cells was monitored with an anti-clonotypic mAb (Ti.98) using FACS analysis. The frequencies of type 1 and 2 cytokine-producing T cells responding to Kb via the direct allor-ecognition pathway were determined using an ELISPOT method as previously described (4).
Morphology analyses
Cardiac transplants were fixed in 10% buffered formalin, embedded in paraffin, coronally sectioned, and stained with H&E for evaluation of cellular infiltrates and myocyte damage (acute rejection) by light microscopy. For assessment of chronic rejection, cardiac grafts were stained with Verhoeff’s elastin (vessel arteriosclerosis scoring) or Mason’s trichrome (evaluation of fibrosis). Arteriosclerosis was assessed by light microscopy, and the percentage of luminal occlusion and intimal thickening was determined using a scoring system, as previously described (17). Only vessels that display a clear internal elastic lamina were included in mor-phometric analysis (five to seven vessels per section). All arteries were scored by at least two examiners in a blinded fashion.
Statistical analyses
Statistical analyses were performed using STATView software (Abacus Concepts, Berkeley, CA). The p values were calculated using paired t test. A p value <0.05 was considered statistically significant.
Results
In this study, we used a mouse Tg model in which the NIMA is an MHC class I transgene, Kb, and all the offspring’s T cells express an anti-Kb TCR transgene (BM3.3 Tg mice; Fig. 1). The TCR from CD8+ T cells of BM3.3 mice recognize intact Kb MHC class I molecules bound to an 8-mer peptide (INFDFNTI) called BM1 (direct allorecognition). Both CBK (Kb Tg) and Bm3.3 (anti-Kb TCR Tg) mice used as parents were engineered in CBA/Ca (H-2k) mice. To study the NIMA effect, we mated heterozygous female mice (CBA × CBK) F1 with homozygous Bm3.3 TCR Tg anti-Kb male mice. In this setting, all the offspring inherit the TCR Tg from their father and express anti-Kb TCR on their CD8+ T cells. Half the offspring are expected to inherit the Kb transgene from their mother and are referred to as IMA mice. The other half of the offspring should not inherit Kb from their mother; these mice are called NIMA mice. In addition, non-Tg CBA females were mated with Bm3.3 anti-Kb TCR Tg males. In the absence of a Kb transgene in the mother, the resulting offspring do not inherit Kb and are, therefore, never exposed to it and are referred to as NE mice. The design of NIMA, IMA, and NE mice is depicted in Fig. 1A. The phenotype of NE, NIMA, and IMA offspring was ascertained by staining splenocytes using anti-Kb Abs. As expected, the splenocytes of CBK and IMA mice expressed MHC class I Kb molecules, whereas the spleen cells from NE and NIMA mice did not display Kb on their surface (Fig. 1B).
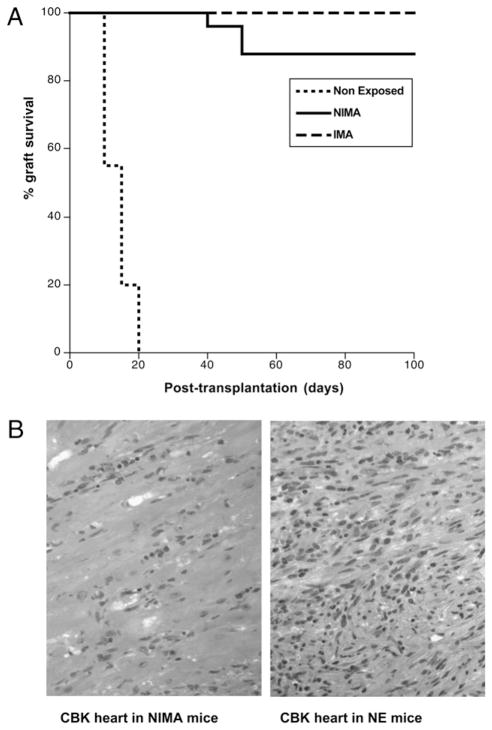
Next, we investigated the influence of NIMA on allotransplant rejection by the offspring. To test this, we transplanted NIMA mice with a CBK (Kb Tg) allogeneic heart. Acute and chronic rejections of the cardiac allografts were monitored by palpation and his-tological techniques. In these experiments, NE mice, which are never exposed to Kb, and IMA mice that inherit Kb from their mothers were used as control recipients for rejection and tolerance, respectively. As expected, NE mice rejected CBK heart transplants in an acute fashion (12 ± 4 d; Fig. 2A), whereas IMA mice accepted their transplants indefinitely (Fig. 2A). Twenty-two of the 25 NIMA mice tested (>80%) accepted CBK heart transplants permanently. Three mice rejected their transplants, although in a markedly delayed fashion (40–60 d). As shown in Fig. 2B, massive infiltration and tissue damage were detected in the heart transplants of control NE mice tested 12 d after grafting. In contrast, in NIMA mice, histological examination of cardiac transplants performed 50 d after allograft placement revealed no inflammatory cell infiltrates and a well-preserved tissue architecture (Fig. 2B). Therefore, NIMA mice are tolerant to Kb+ allo-geneic CBK heart transplants. This suggests that although NIMA mice did not inherit Kb MHC class I Ag from their mothers, they had been exposed to this allo-MHC Ag during their development.
FIGURE 2.
NIMA mice accept Kb+ allogeneic CBK heart transplants. NE, IMA (express Kb), and NIMA (experimental group) mice underwent het-erotopic vascularized transplantation with a heart derived from a CBK (Kb Tg) mouse. Transplant rejection was monitored for 100 d by palpation (heartbeat) and histological methods. A, Percentages of graft survival at different time points after transplantation. Graft survival was analyzed using the Kaplan–Meier method, and survival curves were compared using the log-rank test. B, Histology of BALB/c heart transplants from control rejecting NE and tolerant NIMA mice. Microphotographs (H&E, original magnification ×40) are representative of four NE and NIMA mice tested individually.
It was possible that tolerance to Kb allografts in NIMA mice was due to the deletion of anti-Kb TCR Tg T cells during thymic selection. To test this, we assessed the presence of TCR Tg T cells in the peripheral blood of adult mice by FACS using an anti-clonotypic mAb, Ti98. Control non-TCR Tg CBA (NE) and CBK mice displayed a normal polyclonal population of CD8+ T cells, which did not express the Ti98 clonotype (Fig. 3A), whereas virtually all the CD8+ T cells found in Bm3.3 TCR Tg mice were Ti98+ (Fig. 3B). Strikingly, no Ti98+ were detected in Bm3.3 mice, which had inherited Kb from their mothers (IMA) (Fig. 3C). Therefore, NE mice did not delete their anti-Kb TCR Tg T cells, whereas IMA mice in which Kb represents a self-antigen deleted their anti-Kb TCR Tg Ti98+ T cells. Most important, normal levels of Ti98+ T cells similar to those observed in control BM3.3 NE mice were found in NIMA mice (Fig. 3D). Therefore, deletion of anti-Kb TCR Tg CD8+ T cells is not responsible for Kb-specific tolerance in adult NIMA mice.
FIGURE 3.
Frequencies of T cells expressing the anti-Kb TCR protein in IMA and NIMA mice. The expression of the anti-Kb TCR Tg protein on the surface of peripheral blood CD3+CD8+ T cells was assessed by FACS analysis using the anti-clonotypic mAb, Ti98. Representative FACS pro-files obtained with control non-Tg CBA mice (A), control anti-Kb TCR Tg Bm3.3 mice (B), IMA offspring that inherited both the anti-Kb TCR and the Kb protein (C), and NIMA mice that inherited the anti-Kb TCR transgene but not the Kb transgene (D). The results are representative of >100 mice in each group.
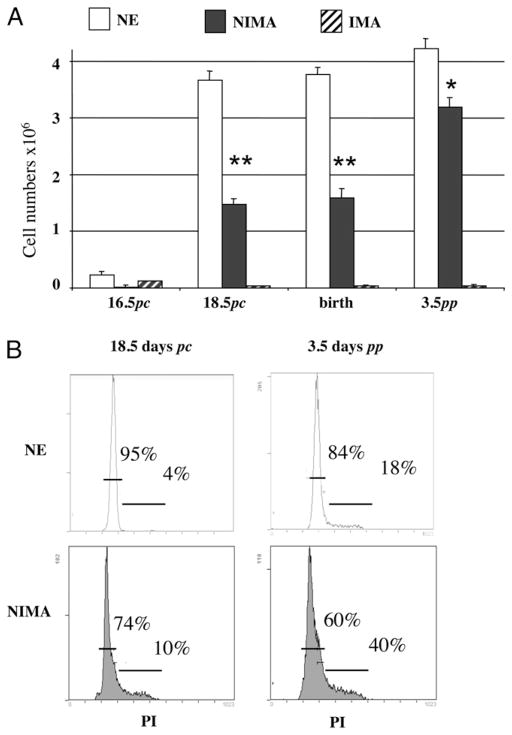
Next, we examined whether T cells from NIMA mice are exposed to Kb alloantigen during thymic development. The thymi of NE, IMA, and NIMA mice were collected during fetal life at days 16.5 and 18.5 postcoitum (pc) (fetuses), at birth time, and 3.5 d pp (neonates). As shown in Fig. 4A, a few T cells were detected at day 16.5 pc in all mouse groups, but numbers were already reproducibly decreased in NIMA fetuses. At day 18.5 pc and birth time, control NE mice displayed high numbers of Kb-specific thymocytes, whereas none was found in IMA mice. This is consistent with a model in which BM3.3 T cells expanded in NE mice (positive selection), whereas they were deleted in IMA mice (negative selection). At this time point, TCR Tg T cells were detected in NIMA mice, although at half the frequency found in control NE mice. In turn, at day 3.5 pp, the number of anti-Kb T cells had doubled in NIMA mice but remained significantly lower than that observed in NE mice (Fig. 4A), and the same observation could be made at 3 wk pp (data not shown). Virtually no anti-Kb TCR Tg T cells were found at each of these time points in IMA mice, a result consistent with their clonal deletion (Fig. 4A). Altogether, these results indicate that NIMA mice are exposed to and affected by NIMA Kb allo-MHC class I Ag during fetal life. To confirm this, thymocytes from NE and NIMA mice were permeabilized and tested at day 18.5 pc and 3.5 pp for their proliferation rate using propidium iodide. The FACS profiles presented in Fig. 4B show the frequencies of T cells in G0-G1 phase (left peak) and in S-M/G2 phase (right peak). The results show a marked increase in the proliferation rates in NIMA mice as compared with NE mice in both fetuses (4 versus 10%; p,<0.05) and neonates (18 versus 40%; p,<0.05). This observation further supports the view that, in NIMA mice, T cells are exposed to NIMA and activated to proliferate during fetal thymic development. This phenomenon occurred up to weaning age at the end of the transfer of maternal cells through suckling.
FIGURE 4.
Fetal and neonatal NIMA-exposed T cells are partially deleted from the thymus. Thymus T cells from NE or NIMA mice were analyzed by flow cytometry, using the Ti98 mAb specific for the BM3.3 Tg TCR. A, Compilation of cell numbers (± SEM) of BM3.3 Tg thymic T cells from NE control, NIMA, or IMA animals as 16.5- and 18.5-d pc fetuses, newborns, or 3.5-d-old neonates; at least seven animals were studied in each group in three separate experiments. *p,<0.05; **p,<0.01. B, Flow cytometry profiles obtained from NE or NIMA BM3.3 Tg thymic T cells from 18.5-d pc fetuses and 3.5-d-old neonates, after per-meabilization and staining with propidium iodide (PI). Percentages of cells in G0/G1 or S/G2/M phases of the cell cycle are given in each graph. One experiment representative of at least three for each group is shown.
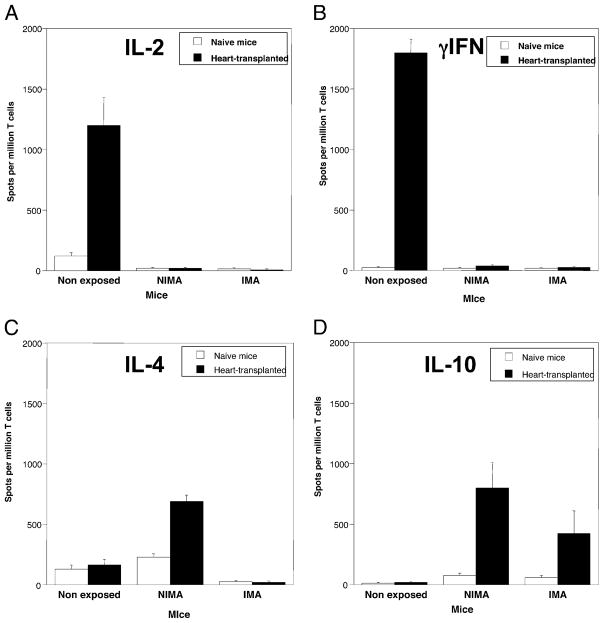
In another set of experiments, we compared anti-Kb T cell-mediated alloresponses in adult NE, IMA, and NIMA mice (Fig. 5). T cells from the spleen of naive mice and mice transplanted with a CBK heart were isolated and placed in culture with allo-geneic irradiated CBK stimulator cells (MLR), a test that is traditionally used to detect direct alloreactivity. The frequencies of anti-Kb alloreactive T cells producing type 1 (IL-2 and IFN-γ) and type 2 (IL-4 and IL-10) cytokines were measured using an ELI-SPOT assay as previously described (18). In naive control NE mice, ~200 activated T cells per million T cells were found to produce IL-2, IFN-γ, and IL-4, but no IL-10, which is consistent with the frequencies previously reported for a primary MLR. As expected, these frequencies were much greater (>1000 spots/million) in NE mice that had been transplanted with a CBK heart, whereas no T cells producing IL-10 were detected in these mice. In contrast, no activated T cells producing IL-2, IFN-γ, and IL-4 were detected in both naive and transplanted IMA mice. Interestingly, however, a few T cells producing IL-10 were found in naive and transplanted IMA mice. In NIMA mice, although no alloreactive T cells producing IL-2 and IFN-γ were found, some T cells producing IL-4 were detected. In addition, IL-10–secreting anti-Kb T cells were detected in naive NIMA mice and particularly in NIMA mice that had received a cardiac allograft. These cyto-kines are traditionally secreted by type 2 (TH2/CT2) cells and Tregs. Our results also imply that, although most Ti98+ TCR Tg T cells had been deleted in developing IMA mice, some anti-Kb T cells producing IL-10 had escaped negative selection and could become activated in adults after exposure to Kb alloantigen.
FIGURE 5.
Frequencies of cytokine-producing T cells in naive and transplanted mice. The frequencies of alloreactive anti-Kb T cells secreting proinflammatory type 1 cytokines IL-2 (A), IFN-γ (B), and type 2 “regulatory” cytokines IL-4 (C) and IL-10 (D) were measured by ELISPOT. Spleen cells from nontransplanted (naive, white bars) and mice recipient of a CBK heart transplant (10 d post-transplant, solid bars) were collected and stimulated in vitro with irradiated CBK Kb Tg splenocytes (MLR). The results are presented as cytokine-producing spots per million T cells ± SD. The results are representative of four experiments each including two to three mice tested individually.
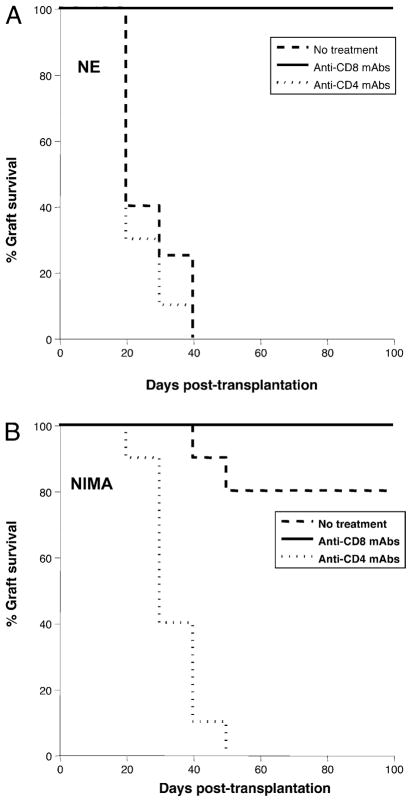
Next, we investigated the mechanisms underlying transplantation tolerance in adult NIMA mice. First, NE, NIMA, and IMA mice were treated with depleting anti-CD4 or anti-CD8 Abs starting 3 d before transplantation with an allogeneic Kb+ CBK heart. This resulted in the near-complete depletion of CD4+ and CD8+ T cell subsets for more than 2 wk after Ab administration (data not shown). As shown in Fig. 6A, the depletion of CD8+ T cells resulted in long-term survival of Kb+ heart transplants in NE mice, whereas the anti-CD4 mAb treatment had no effect. This is consistent with the fact that CBK allografts placed in these BM3.3 Tg mice are rejected primarily by CD8+ TCR Tg anti-Kb T cells. Although some CD4+ T cells can be found in these mice, they apparently do not contribute to the rejection of Kb+ allografts. The majority (>80%) of nontreated NIMA mice either accepted Kb+ allotransplants or exhibited marked delayed rejection (>60 d post-transplantation; Fig. 6B). All NIMA mice treated with anti-CD8 Abs retained CBK cardiac transplants in-definitely, a result that is consistent with the observations made in NE mice. Most important, the majority of NIMA mice treated with anti-CD4 mAbs rejected CBK hearts between 10 and 30 d post-transplantation (Fig. 6B). Histological examination of the rejected transplants revealed massive inflammatory infiltrates and tissue damage typical of acute cellular rejection (data not shown). Therefore, depletion of CD4+ T cells in NIMA mice had abolished tolerance to Kb alloantigen. Surprisingly, we observed that depletion of CD4+ T cells in IMA mice induced the rejection of CBK heart transplants in >50% of the mice. Therefore, tolerance to Kb can be broken in IMA mice, a result suggesting that clonal deletion of anti-Kb T cells is not the sole mechanism underlying tolerance induction and/or maintenance in these mice (data not shown).
FIGURE 6.
Effects of CD4+ or CD8+ T cell depletion on tolerance to Kb cardiac allotransplants. NE (A) or NIMA (B) mice were treated with depleting anti-CD4 (GK1.5) or anti-CD8 (53.6.72) mAbs administered i.p. 5 d before transplantation with a heart derived from a Kb Tg CBK mouse. Control mice that received no Abs (no treatment) were also transplanted. Acute rejection of allografts was monitored for 100 d. The results are expressed as percentages of graft survival obtained with 5–12 mice in each group. Graft survival was analyzed using the Kaplan–Meier method, and survival curves were compared using the log-rank test.
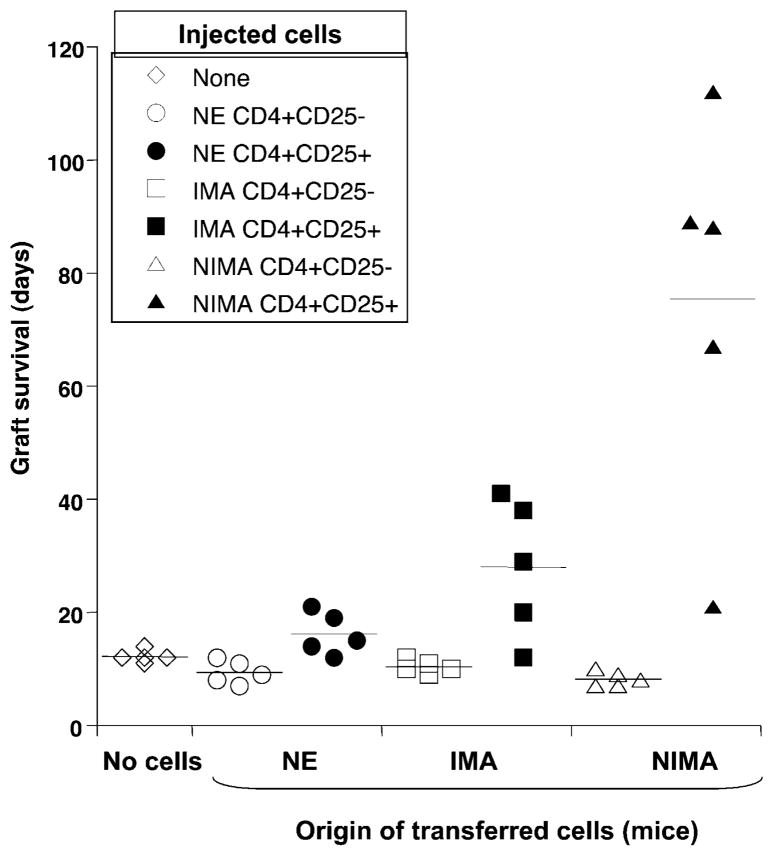
The results obtained in NIMA mice with anti-CD4 mAbs prompted us to test whether these mice display CD4+ Tregs responsible for inducing or maintaining tolerance to Kb alloantigen. To address this question, we isolated CD4+CD25high and CD4+ CD25− T cells from the spleens of NIMA mice by FACS sorting (>91% purity). More than 92% of CD4+CD25high T cells were Foxp3+, whereas the CD4+CD25− T cells did not express significant Foxp3 levels (data not shown). Each subpopulation was adoptively transferred (5 × 105 cells given i.v.) into CBA naive mice 5 d before their transplantation with a CBK heart. As shown in Fig. 7, the mice administered with CD4+CD25− T cells from NIMA mice rejected CBK cardiac allografts in an acute fashion (mean survival time [MST]: 12 ± 2 d). In contrast, adoptive transfer of five CBA mice with CD4+CD25high T cells collected from NIMA mice resulted in a significant increase (p,<0.02) of allograft survival in four of five mice (MST: 89 ± 18 d). Histo-logical examination of the transplanted hearts revealed no signs of chronic allograft vasculopathy in these mice (data not shown). It is noteworthy that these adoptively transferred mice rejected acutely third-party BALB/c (H-2d) cardiac allografts (data not shown). In contrast, adoptive transfer of CD4+CD25− T cells, as well as CD4+CD25high T cells from NE mice, had no effect on graft rejection. Therefore, the tolerance to Kb can be adoptively transferred to CBA NE mice using CD4+CD25high T cells derived from the spleens of NIMA mice. In NIMA mice, anti-Kb TCR Tg T cells are not deleted and some Kb-specific CD4+ Tregs may be selected, which can ensure tolerance to Kb allografts. Interestingly, some modest but significant prolongation of allograft survival was also observed on transfer of CD4+CD25high from IMA mice (MST: 28 ± 5 d; p = 0.04). This result further supports the view that, in IMA mice, although CD8+ anti-Kb T cells are eliminated in the developing thymus, some CD4+ Tregs escape negative selection and can confer some protection against rejection of Kb+ allografts following adoptive transfer in naive CBA mice.
FIGURE 7.
CD4+CD25+ Foxp3+ T cells from NIMA mice can transfer tolerance to Kb+ allografts. CD4+CD25− and CD4+CD25+ T cells were purified from either NE (circles), IMA (squares), or NIMA (triangles) mice. Each T cell subset (5 × 105 cells) was separately injected i.v. into naive CBA mice, which received a CBK heart transplant 3 d later. Control mice, which received no T cells, were also tested (diamonds). The effects of adoptive transfer of CD4+CD25− (open symbols) and CD4+CD25+ (solid symbols) T cells on graft survival were monitored. Each point corresponds to the survival data from a single mouse. Horizontal bars represent the MST (days).
Discussion
Transplantation tolerance, defined broadly as long-term allograft survival in the absence of immunosuppressive treatment, is regularly achieved in nature during pregnancy. Mammalian pregnancy and subsequent nursing of the newborn appears to have a profound influence on the neonate’s developing immune system that is retained in adulthood. In animal models, the passage of maternal cells and Ags during gestation and breast-feeding is thought to imprint long-term unresponsiveness of NIMA-specific inflam-matory T cells in offspring. This phenomenon is clinically relevant as exemplified by the beneficial effects of matching donors and recipients for NIMA in human recipients of blood transfusion and kidney allotransplants (19). In addition, there is a body of evidence suggesting that the presence of NIMA also influences the adult’s susceptibility to autoimmune disorders (4, 20–22). Altogether, these observations indicate that NIMA play a critical role in the establishment and regulation of the entire immune system. However, the mechanisms underlying the induction of a NIMA effect in the fetus and neonates and its maintenance in adults are not fully understood. Gaining insights into this question will set the path for the design of novel strategies for manipulating the immune system in health and disease.
The elucidation of the mechanisms underlying the NIMA effect has been difficult because of the fact that the precise nature of the NIMA and the T cell clones recognizing these maternal Ags are unknown. To overcome this, we designed a double-Tg model in which the mother’s NIMA and offspring’s anti-NIMA T cells were well defined. In this model, all the offspring’s CD8+ T cells corresponded to a single clone recognizing the Kb MHC class I protein. In contrast, the mother and the father of the offspring differed by the expression of a single Ag, Kb, that served as NIMA. This allowed us to study the influence of NIMA exposure on the offspring T cell repertoire selection during ontogeny and on its T cell response during adulthood. First, we showed that adult NIMA mice were tolerant to Kb as they accepted Kb+ heart allo-transplants permanently. This implies that these mice have been exposed to NIMA Kb presumably during pregnancy or breast-feeding, or both. It is not clear whether this results from the transplacental passage of Kb+ maternal cells or soluble Kb molecules, or both. Several studies have documented the passage of hematopoietic maternal cells from the mother to the fetus (3, 23). Among them, T lymphocytes are regularly detected in umbilical cord blood samples from neonates. The presence of maternal T cells is commonly observed in SCID patients (24–33). A study by Kobayashi et al. (34) has documented that maternal CD4+ T cells are present in various tissues of a male infant with a SCID phenotype resulting from Artemis gene mutation. In a murine model system, involving the transfer of LacZ-, scid/scid, or wild type (+/+) blastocysts to pseudopregnant female animals, Piotrowski et al. (35) have demonstrated that in 90% of scid/scid fetuses, nucleated maternal cells were present in at least one lymphoid organ. In another study using GFP Tg female mice, Zhou et al. (36) have reported the presence of GFP+ maternal cells in fetal organs including the thymus, spleen, and liver. In addition, a recent study by Dutta et al. (37) demonstrates the presence of maternal hemato-poietic microchimerism in lymphoid but also nonlymphoid organs, with predominance in the heart.
In this study, we showed that anti-Kb TCR Tg CD8+ T cells present in the fetal and neonatal thymi of NIMA offspring display an activated phenotype. In addition, NIMA exposure is associated with a lower frequency of Kb-specific T cells in the developing thymus of NIMA mice compared with control CBA (NE) mice. This suggests that from day 16.5 pc through the time of birth and up to 3 wk pp, the presence of NIMA was associated with either the deletion of some developing anti-Kb T cells or an inefficient positive selection of these T cells. Unexpectedly, our results also show that thymocytes from the NIMA fetuses display a greater rate of proliferation while they are present at a lower frequency than their NE counterparts. The observation that TCR Tg developing thymic T cells from NIMA mice display a greater proliferation rate than those of NE mice support a partial deletional model rather than a lack of positive selection. Most important, the presence of normal frequencies of anti-Kb T cells in adult NIMA mice demonstrates that tolerance to Kb is not ensured only via deletion of anti-Kb T cells during thymic development.
Functional analysis of anti-Kb alloreactive T cells in NIMA mice revealed the absence of proinflammatory T cells producing IL-2 and IFN-γ. In turn, although these mice were tolerant to CBK allografts, they displayed some T cells producing IL-4 and high numbers of IL-10–producing T cells when challenged with Kb+ allostimulators, that is, through the direct allorecognition pathway. Therefore, exposure of fetuses or neonates, or both, to NIMA resulted in the selection of T cells producing type 2 cytokines. Notably, the majority of the T cells producing IL-4 and IL-10 on stimulation with Kb+ allogeneic cells displayed a CD4+ phenotype. Indeed, the BM3.3 Tg mice used in this study were not bred on a RAG knockout background and displayed low but significant numbers of CD4+ T cells that were not Tg T cells (Ti98−). This suggested that, in NIMA mice, anti-Kb CD8+ Tg T cells could not reject CBK allografts because they were suppressed by CD4+ T cells. This was confirmed by the observation that depletion of CD4+ T cells in NIMA mice restored their ability to reject CBK cardiac allografts. The presence of allospecific, IL-4–producing, CD4+ T cells in NIMA is consistent with the concept that neonatal tolerance is associated with activation of Th2 cells. In support of this, Fortshuber et al. have previously reported that neonatal tolerance is mediated via the positive selection of Th2 cells during development (38). Alternatively, it is possible that Tregs ensured tolerance to NIMA. Indeed, we showed that tolerance to Kb could be transferred to control NE mice by injection of CD4+CD25+ Foxp3+ T cells collected from the spleens of NIMA mice. The majority of these tolerogenic CD4+ T cells secreted IL-10 and was donor-specific in that they did not suppress the rejection of third-party allografts (data not shown). Therefore, these Tregs are likely to correspond to inducible regulatory Tr1 cells rather than natural Tregs (39). This conclusion corroborates the results reported by others showing the presence of CD4+CD25+Foxp3+ Tregs secreting IL-10 and TGF-β in the lymph nodes of mice transplanted with a NIMA+ allograft (40–42). Further supporting this view, Mold et al. (43) have recently reported the presence of such Tregs in human fetal lymph nodes. Our study using adoptive transfer experiments demonstrates that these regulatory cells can be isolated and mediate allotransplant tolerance in vivo. This does not, however, exclude that some other cells, including CD8+ Tregs, and/or mechanisms can contribute to NIMA tolerance in this and other models.
The revelation of a powerful and beneficial NIMA effect in our Tg transplant model fully confirms and extends the original report of Owen et al. (44) regarding a tolerogenic effect of alloantigen pre-exposure on humoral immunity in adults. It is not clear why maternal chimerism and subsequent T cell tolerance to NIMA has been selected through evolution in mammals. It can be speculated that this process prevents the fetus’s immune system from attacking the mother as observed in GVH reactions. However, it seems unlikely that a few fetal T cells that are hyporesponsive to allostimulation could induce a life-threatening GVH-like disease in the mother. Alternatively, the passage of maternal leukocytes might be useful to protect the fetus against pathogens and/or contribute to its proper immune development and maturation. There are implications of the NIMA effect in pediatric transplantation where a child receives a kidney from his or her mother. In this setting, pretransplant maternal transfusion may reactivate and expand NIMA-specific Tregs, thereby amplifying the NIMA tol-erogenic effect and ensuring tolerance to the transplant. The implications of the NIMA effect for a variety of other applications are numerous and include cord blood stem cell transplantation (45) and cadaveric organ transplantation, as well as nontransplant fields such as autoimmunity and development of antitumor vaccination approaches using “self” antigenic peptides, both of which may benefit from an understanding of the basic mechanisms of NIMA tolerance.
Acknowledgments
This work was supported by the National Institute of Child Health and Human Development, National Institutes of Health (Grants RO1HD050484 and KO2AI53103 to G.B.), the French Ministry of Education and Research (to S.M.C. and C.V.), the Fondation pour la Recherche Médicale and Ligue contre le Cancer (to S.M.C.), and a North Atlantic Treaty Organization Science Program collaborative grant (to G.B. and C.K.-L.).
We thank Dr A. Guimezanes (Centre d’Immunologie Marseille-Luminy, Marseille, France) for providing CBK and BM3.3 Tg mice and Ti98 mAb.
Abbreviations used in this article
- GVH
graft-versus-host
- IMA
inherited maternal Ag
- MST
mean survival time
- NE
never exposed to Kb
- NIMA
noninherited maternal Ag
- pc
postcoitum
- pp
postpartum
- Tg
transgenic
- Treg
regulatory T cell
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Owen RD. Immunogenetic consequences of vascular anastomoses between bovine twins. Science. 1945;102:400–401. doi: 10.1126/science.102.2651.400. [DOI] [PubMed] [Google Scholar]
- 2.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 3.Vernochet C, Caucheteux SM, Kanellopoulos-Langevin C. Bidirectional cell trafficking between mother and fetus in mouse placenta. Placenta. 2007;28:639–649. doi: 10.1016/j.placenta.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 4.van Rood JJ, Claas F. Both self and non-inherited maternal HLA antigens influence the immune response. Immunol Today. 2000;21:269–273. doi: 10.1016/s0167-5699(00)01628-5. [DOI] [PubMed] [Google Scholar]
- 5.Claas FH, Gijbels Y, van der Velden-de Munck J, van Rood JJ. Induction of B cell unresponsiveness to noninherited maternal HLA antigens during fetal life. Science. 1988;241:1815–1817. doi: 10.1126/science.3051377. [DOI] [PubMed] [Google Scholar]
- 6.Bean MA, Mickelson E, Yanagida J, Ishioka S, Brannen GE, Hansen JA. Suppressed antidonor MLC responses in renal transplant candidates conditioned with donor-specific transfusions that carry the recipient’s noninherited maternal HLA haplotype. Transplantation. 1990;49:382–386. doi: 10.1097/00007890-199002000-00031. [DOI] [PubMed] [Google Scholar]
- 7.van Rood JJ, Loberiza FR, Jr, Zhang MJ, Oudshoorn M, Claas F, Cairo MS, Champlin RE, Gale RP, Ringdén O, Hows JM, Horowitz MH. Effect of tolerance to noninherited maternal antigens on the occurrence of graft-versus-host disease after bone marrow transplantation from a parent or an HLA-haploidentical sibling. Blood. 2002;99:1572–1577. doi: 10.1182/blood.v99.5.1572. [DOI] [PubMed] [Google Scholar]
- 8.Iványi P, Démant P. Prolonged survival of maternal skin grafts in newborn rabbits. Folia Biol (Praha) 1965;11:321–323. [PubMed] [Google Scholar]
- 9.Zhang L, Miller RG. The correlation of prolonged survival of maternal skin grafts with the presence of naturally transferred maternal T cells. Transplantation. 1993;56:918–921. doi: 10.1097/00007890-199310000-00027. [DOI] [PubMed] [Google Scholar]
- 10.Andrassy J, Kusaka S, Jankowska-Gan E, Torrealba JR, Haynes LD, Marthaler BR, Tam RC, Illigens BM, Anosova N, Benichou G, Burlingham WJ. Tolerance to noninherited maternal MHC antigens in mice. J Immunol. 2003;171:5554–5561. doi: 10.4049/jimmunol.171.10.5554. [DOI] [PubMed] [Google Scholar]
- 11.Caucheteux SM, Vernochet C, Wantyghem J, Gendron MC, Kanellopoulos-Langevin C. Tolerance induction to self-MHC antigens in fetal and neonatal mouse B cells. Int Immunol. 2008;20:11–20. doi: 10.1093/intimm/dxm116. [DOI] [PubMed] [Google Scholar]
- 12.Vernochet C, Caucheteux SM, Gendron MC, Wantyghem J, Kanellopoulos-Langevin C. Affinity-dependent alterations of mouse B cell development by noninherited maternal antigen. Biol Reprod. 2005;72:460–469. doi: 10.1095/biolreprod.104.035048. [DOI] [PubMed] [Google Scholar]
- 13.Molitor-Dart ML, Andrassy J, Haynes LD, Burlingham WJ. Tolerance induction or sensitization in mice exposed to noninherited maternal antigens (NIMA) Am J Transplant. 2008;8:2307–2315. doi: 10.1111/j.1600-6143.2008.02417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarazona R, Sponaas AM, Mavria G, Zhou M, Schulz R, Tomlinson P, Antoniou J, Mellor AL. Effects of different antigenic micro-environments on the course of CD8+ T cell responses in vivo. Int Immunol. 1996;8:351–358. doi: 10.1093/intimm/8.3.351. [DOI] [PubMed] [Google Scholar]
- 15.Auphan N, Curnow J, Guimezanes A, Langlet C, Malissen B, Mellor A, Schmitt-Verhulst AM. The degree of CD8 dependence of cytolytic T cell precursors is determined by the nature of the T cell receptor (TCR) and influ-ences negative selection in TCR-transgenic mice. Eur J Immunol. 1994;24:1572–1577. doi: 10.1002/eji.1830240718. [DOI] [PubMed] [Google Scholar]
- 16.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16:343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Russell ME, Hancock WW, Akalin E, Wallace AF, Glysing-Jensen T, Willett TA, Sayegh MH. Chronic cardiac rejection in the LEW to F344 rat model. Blockade of CD28-B7 costimulation by CTLA4Ig modulates T cell and macrophage activation and attenuates arteriosclerosis. J Clin Invest. 1996;97:833–838. doi: 10.1172/JCI118483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benichou G, Valujskikh A, Heeger PS. Contributions of direct and indirect T cell alloreactivity during allograft rejection in mice. J Immunol. 1999;162:352–358. [PubMed] [Google Scholar]
- 19.Burlingham WJ, Grailer AP, Heisey DM, Claas FHJ, Norman D, Mohanakumar T, Brennan DC, de Fijter H, van Gelder T, Pirsch JD, et al. The effect of tolerance to noninherited maternal HLA antigens on the survival of renal transplants from sibling donors. N Engl J Med. 1998;339:1657–1664. doi: 10.1056/NEJM199812033392302. [DOI] [PubMed] [Google Scholar]
- 20.Bianchi DW. Fetal cells in the mother: from genetic diagnosis to diseases associated with fetal cell microchimerism. Eur J Obstet Gynecol Reprod Biol. 2000;92:103–108. doi: 10.1016/s0301-2115(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 21.Nelson JL. Pregnancy immunology and autoimmune disease. J Reprod Med. 1998;43:335–340. [PubMed] [Google Scholar]
- 22.Nelson JL. Microchimerism in human health and disease. Autoimmunity. 2003;36:5–9. doi: 10.1080/0891693031000067304. [DOI] [PubMed] [Google Scholar]
- 23.Maurel MC, Kanellopoulos-Langevin C. Heredity—venturing beyond genetics. Biol Reprod. 2008;79:2–8. doi: 10.1095/biolreprod.107.065607. [DOI] [PubMed] [Google Scholar]
- 24.O’Reilly RJ, Patterson JH, Bach FH, Bach ML, Hong R, Kissmeyer-Nielsen F, Therkelsen AJ. Chimerism detected by HL-A typing. Transplantation. 1973;15:505–507. [PubMed] [Google Scholar]
- 25.Pollack MS, Kapoor N, Sorell M, Kim SJ, Christiansen FT, Silver DM, Dupont B, O’Reilly RJ. DR-positive maternal engrafted T cells in a severe combined immunodeficiency patient without graft-versus-host disease. Transplantation. 1980;30:331–334. doi: 10.1097/00007890-198011000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Pollack MS, Kirkpatrick D, Kapoor N, Dupont B, O’Reilly RJ. Identification by HLA typing of intrauterine-derived maternal T cells in four patients with severe combined immunodeficiency. N Engl J Med. 1982;307:662–666. doi: 10.1056/NEJM198209093071106. [DOI] [PubMed] [Google Scholar]
- 27.Flomenberg N, Dupont B, O’Reilly RJ, Hayward A, Pollack MS. The use of T cell culture techniques to establish the presence of an intrauterine-derived maternal T cell graft in a patient with severe combined immunodeficiency (SCID) Transplantation. 1983;36:733–735. [PubMed] [Google Scholar]
- 28.Geha RS, Reinherz E. Identification of circulating maternal T and B lymphocytes in uncomplicated severe combined immunodeficiency by HLA typing of subpopulations of T cells separated by the fluorescence-activated cell sorter and of Epstein Barr virus-derived B cell lines. J Immunol. 1983;130:2493–2495. [PubMed] [Google Scholar]
- 29.Thompson LF, O’Connor RD, Bastian JF. Phenotype and function of engrafted maternal T cells in patients with severe combined im-munodeficiency. J Immunol. 1984;133:2513–2517. [PubMed] [Google Scholar]
- 30.Le Deist F, Raffoux C, Griscelli C, Fischer A. Graft vs graft reaction resulting in the elimination of maternal cells in a SCID patient with maternofetal GVHd after an HLA identical bone marrow transplantation. J Immunol. 1987;138:423–427. [PubMed] [Google Scholar]
- 31.Barrett MJ, Buckley RH, Schiff SE, Kidd PC, Ward FE. Accelerated development of immunity following transplantation of maternal marrow stem cells into infants with severe combined immunodeficiency and transplacentally acquired lymphoid chimerism. Clin Exp Immunol. 1988;72:118–123. [PMC free article] [PubMed] [Google Scholar]
- 32.Wahn V, Yokota S, Meyer KL, Janssen JW, Hansen-Hagge TE, Knobloch C, Koletzko S, Stein H, Friedrich W, Bartram CR. Expansion of a maternally derived monoclonal T cell population with CD3 +/CD8+/T cell receptor-gamma/delta+ phenotype in a child with severe combined immunodeficiency. J Immunol. 1991;147:2934–2941. [PubMed] [Google Scholar]
- 33.Müller SM, Ege M, Pottharst A, Schulz AS, Schwarz K, Friedrich W. Transplacentally acquired maternal T lymphocytes in severe combined immunodeficiency: a study of 121 patients. Blood. 2001;98:1847–1851. doi: 10.1182/blood.v98.6.1847. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi N, Agematsu K, Nagumo H, Yasui K, Katsuyama Y, Yoshizawa K, Ota M, Yachie A, Komiyama A. Expansion of clonotype-restricted HLA-identical maternal CD4+ T cells in a patient with severe combined immunodeficiency and a homozygous mutation in the Artemis gene. Clin Immunol. 2003;108:159–166. doi: 10.1016/s1521-6616(03)00095-0. [DOI] [PubMed] [Google Scholar]
- 35.Piotrowski P, Croy BA. Maternal cells are widely distributed in murine fetuses in utero. Biol Reprod. 1996;54:1103–1110. doi: 10.1095/biolreprod54.5.1103. [DOI] [PubMed] [Google Scholar]
- 36.Zhou L, Yoshimura Y, Huang Y, Suzuki R, Yokoyama M, Okabe M, Shimamura M. Two independent pathways of maternal cell transmission to offspring: through placenta during pregnancy and by breast-feeding after birth. Immunology. 2000;101:570–580. doi: 10.1046/j.1365-2567.2000.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dutta P, Molitor-Dart M, Bobadilla JL, Roenneburg DA, Yan Z, Torrealba JR, Burlingham WJ. Microchimerism is strongly correlated with tolerance to noninherited maternal antigens in mice. Blood. 2009;114:3578–3587. doi: 10.1182/blood-2009-03-213561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forsthuber T, Yip HC, Lehmann PV. Induction of TH1 and TH2 immunity in neonatal mice [see comments] Science. 1996;271:1728–1730. doi: 10.1126/science.271.5256.1728. [DOI] [PubMed] [Google Scholar]
- 39.Bettini M, Vignali DA. Regulatory T cells and inhibitory cytokines in autoimmunity. Curr Opin Immunol. 2009;21:612–618. doi: 10.1016/j.coi.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molitor-Dart ML, Andrassy J, Kwun J, Kayaoglu HA, Roenneburg DA, Haynes LD, Torrealba JR, Bobadilla JL, Sollinger HW, Knechtle SJ, Burlingham WJ. Developmental exposure to noninherited maternal antigens induces CD4+ T regulatory cells: relevance to mechanism of heart al-lograft tolerance. J Immunol. 2007;179:6749–6761. doi: 10.4049/jimmunol.179.10.6749. [DOI] [PubMed] [Google Scholar]
- 41.Aoyama K, Koyama M, Matsuoka K, Hashimoto D, Ichinohe T, Harada M, Akashi K, Tanimoto M, Teshima T. Improved outcome of allogeneic bone marrow transplantation due to breastfeeding-induced tolerance to maternal antigens. Blood. 2009;113:1829–1833. doi: 10.1182/blood-2008-05-155283. [DOI] [PubMed] [Google Scholar]
- 42.Matsuoka K, Ichinohe T, Hashimoto D, Asakura S, Tanimoto M, Teshima T. Fetal tolerance to maternal antigens improves the outcome of allogeneic bone marrow transplantation by a CD4+ CD25+ T-cell-dependent mechanism. Blood. 2006;107:404–409. doi: 10.1182/blood-2005-07-3045. [DOI] [PubMed] [Google Scholar]
- 43.Mold JE, Michaëlsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, Lee TH, Nixon DF, McCune JM. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Owen RD, Wood HR, Foord AG, Sturgeon P, Baldwin LG. Evidence for actively acquired tolerance to Rh antigens. Proc Natl Acad Sci USA. 1954;40:420–424. doi: 10.1073/pnas.40.6.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Rood JJ, Stevens CE, Smits J, Carrier C, Carpenter C, Scaradavou A. Reexposure of cord blood to noninherited maternal HLA antigens improves transplant outcome in hematological malignancies. Proc Natl Acad Sci USA. 2009;106:19952–19957. doi: 10.1073/pnas.0910310106. [DOI] [PMC free article] [PubMed] [Google Scholar]