Abstract
BACKGROUND
Children with food allergy have been shown to have increased small intestinal permeability (IP) following ingestion of the offending food as well as during elimination diets. We investigated IP in asymptomatic food-allergic children during an elimination diet to identify clinical characteristics associated with altered IP.
METHODS
Urinary recovery ratios of lactulose and mannitol (L/M) were determined five hours following ingestion of 7.5 g of lactulose and 2 g of mannitol in 131 cow’s milk- and egg-allergic children. An L/M ratio of ≥0.025 was considered abnormal based upon previously established laboratory internal references. A chart review was conducted to assess the clinical characteristics of these patients.
RESULTS
A total of 50 (38%) of the 131 children (median 6.7, range 4.8 – 8.9 years); 66.2% male) with food allergy had elevated IP while asymptomatic on strict elimination diets. Age and height negatively correlated with IP. However, in the regression model analysis, abnormal IP was associated with shorter stature independently of age. Otherwise, food allergic patients with increased IP were comparable in gender, nutritional status, age of onset of food allergy, history of reactions, atopic diseases and family history of food allergies to those with normal IP.
CONCLUSIONS
Elevated IP was found in about one-third of asymptomatic food-allergic children on elimination diets and was associated with shorter stature. Our results suggest that increased IP may be an intrinsic trait in a subset of food allergic children. However, large, prospective studies are necessary to determine the role of impaired intestinal barrier in food allergy.
Keywords: food allergy (hypersensitivity), egg allergy, CM allergy, intestinal permeability, lactulose/mannitol ratio
INTRODUCTION
The physiologic gut barrier regulates the entry of molecules and microorganisms into the circulation, through the paracellular space, and thus affects their ability to trigger a systemic immune response. Under normal conditions, the small intestine differentially allows the absorption of small molecules while excluding larger ones. The selective permeability function relies on both immunologic and non-immunologic factors, including mucin, the microvilli, immunoglobulins, and intraepithelial lymphocytes (1, 2). Alteration of intestinal permeability (IP) is hypothesized to play a key role in the pathophysiologic mechanisms of celiac disease, cystic fibrosis, inflammatory bowel disease, infectious gastroenteritis, malnutrition, necrotizing enterocolitis, diabetes mellitus type I, atopic dermatitis (AD), and food allergy (1-4).
In IgE-mediated food allergy, IgE-sensitization and recruitment of mast cells commonly occurs in the gastrointestinal mucosa. These pre-sensitized mast cells can then be triggered by allergens in the lamina propria. During an allergic reaction, a series of inflammatory mediators are released that impair IP leading to increases in transepithelial allergen transport, thereby perpetuating the inflammatory reaction. Furthermore, IgE-CD23-mediated allergen transport across the mucosa may play an important role in the perpetuation of sensitization (5).
In vivo methods to assess IP rely primarily on the oral ingestion of non-metabolized macromolecular probes and subsequent measurement of their urinary excretion. These probes include differently sized polyethylene glycol (PEG) molecules, radioactive 51Cr-EDTA and 14C-mannitol, and various combinations of sugar molecules (lactulose, mannitol, rhamnose, and cellobiose) (1, 6-10). Urinary levels of lactulose (L) and mannitol (M) and the L/M ratio is one of the most commonly used quantitative assessments of IP (1). Mannitol is a small monosaccharide (MW 182 g/mol) that can diffuse through abundant aqueous pores along the intestinal crypt-villus axis. Lactulose, in contrast, is a larger disaccharide (MW 342 g/mol) whose movement is restricted to larger pores at the base of the villi or through areas of damaged mucosa.(3, 6, 11) With increased IP (e.g. damaged intestinal mucosa), an elevated L/M ratio is expected (12). Determination of the ratio, rather than the rate of movement of any one probe, minimizes confounding factors such as defects in collection, gastric retention, transit time and renal clearance.
Elevated IP has been reported in food hypersensitivity in adults and children (3, 7, 8, 10, 13-15). It is unclear whether elevated IP is a consequence of an allergic insult to the gut mucosa (i.e. damage) or whether it predisposes certain individuals for development of food allergy. In order to address this question the current study was designed to measure IP in asymptomatic food-allergic children treated with a specific allergen elimination diet. In the absence of the dietary exposure to the offending allergen and symptoms of disease, these children were assumed to have intestinal function reflecting their usual state. The study also included measures to identify clinical characteristics associated with altered IP.
METHODS
Patient population
This prospective study included 131 children (ages 3-17 years) with IgE-mediated cow’s milk (CM, n=56, 42.7%) and/or egg allergy (n=75, 57.3%) confirmed by a supervised oral food challenge (OFC) (16-17), who had results of IP testing available for analysis. Children of all ethnic backgrounds were evaluated at the pediatric allergy clinic at the Mount Sinai Hospital, New York, between 2004 and 2007 (16-17). All children observed a strict avoidance diet at the time of the baseline assessment and completed a 5-day diet record including the type and amount of food consumed to analyze for traces of CM or egg and other foods the subject was allergic to. None of subjects reported any chronic gastrointestinal symptoms, including loose stools, diarrhea or abdominal pain. Children were challenged with the baked forms of the food they were allergic to; if tolerated, an OFC to concentrated unheated CM or egg followed (16-17, Fig. 1). If the baked CM or egg OFC was negative, but unheated CM or egg OFC was positive, 1-3 servings of baked CM or egg was recommended to be included in the child’s diet daily. Compliance was assessed by self report. Seven age-matched healthy children with no personal or family history of food allergies served as controls. The study was approved by the Institutional Review Board at the Mount Sinai School of Medicine in New York, NY. Informed consent was obtained from the parents.
Figure 1.
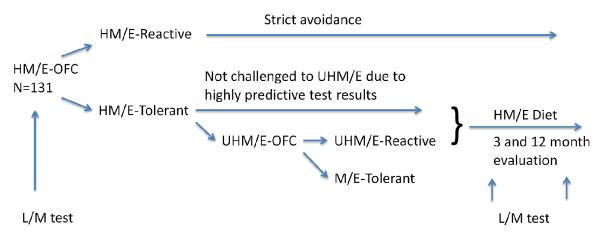
Flow chart of the study design. H=heated, M=milk, E=egg, OFC=oral food challenge, UH=unheated, L/M=lactulose/mannose.
A chart review collected information regarding demographics, weight and height, family history of atopic or inflammatory gastrointestinal diseases, history of atopic diseases, gastrointestinal manifestations of allergy, environmental allergen sensitizations to house dust mites (HDM), tree, grass and ragweed pollen, serum allergen-specific and total IgE measured by ImmunoCAP® (Phadia, currently ThermoFisher Scientific, Portage, MI, USA). Weight and height percentiles for age and z scores were calculated with Nutchildren (Epi Info 3.4.1; Centers for Disease Control and Prevention, Atlanta, Ga). Anaphylaxis severity during OFC was classified utilizing the scoring system by Sampson (18). Briefly, mild symptoms were characterized as grade 1, moderate as 2 and 3, and severe, near-fatal/fatal symptoms as 4-5.
Intestinal permeability test
IP was assessed by the direct measurement of urinary clearance of two non-metabolized sugar molecules, lactulose (L) and mannitol (M), in a manner similar to methods described previously (19). This test is safe, non-invasive, and reproducible (10). Patients and their parents were given written and verbal instructions. A pre-measured amount of lactulose (7.5 g) and mannitol (2.0 g) with Kool Aid for taste (1.5 g) was dissolved in 20 ml of water to administer before bedtime, at least 2 hours after the last meal or drink. A fixed amount of probe was used in this study as the patients were older than we had studied before. Since the primary outcome measure is a ratio of the two probes, the test is not sensitive to small variations in the amount of the probes given. Urine was collected in a clean container over the subsequent 5-10 hours overnight and was delivered to the hospital within 24 hours, in the majority of cases, in the morning following an overnight collection. IP was measured the night before the initial CM or egg OFC for all recruited children; and at 3 and 12 months in a random sample of children who added baked egg or CM to the diet and provided repeated urine samples. Upon arrival, a 15 ml aliquot of urine was mixed with 5 ml of 10% thymol and frozen at −70°C. Samples were analyzed by High-Performance Liquid Chromatography (see below). A measured L/M ratio ≥0.025 was considered elevated based on previously established internal laboratory reference values for children, similar to those established in laboratories from around the world.
High-Performance Liquid Chromatography (HPLC)
Fractional excretion of lactulose and mannitol were calculated from urinary concentrations determined by HPLC. Samples of urine (10 ml) were obtained for analysis. Briefly, cellobiose was added as an internal standard. Samples were deionized by adding 1 g of a 1:1.5 (weight: weight) mixture of Amberlite IR-120 and IRA-400 resin (BDH chemicals, Toronto, Ontario, Canada). The supernatant was then filtered through a 45 μm millipore filter (Millipore, Bedford, Massachusetts, USA). Samples were separated on a Dionex Carbopac MA-1 anion exchange column (Dionex, Ontario, Canada) in a Dionex HPLC using 520 mM NAOH as the isocratic mobile phase. Peak identification was performed using pulsed amperometric electrochemical detection on a gold electrode. Quantitation was performed using known standards at multiple concentrations, with linear interpolations between concentrations. The fractional excretion of L and M was calculated from urinary concentrations of these sugars; the L/M ratio is reported.
Statistical analysis
The distribution of the variables of interest was assessed with the Shapiro-Wilk test. Descriptive statistics are presented as median (interquartile range) for the continuous variables. Associations between categorical data were assessed using the Chi-square and the Fisher’s exact test. The Spearman’s rho correlation coefficient was calculated for non-normally distributed variables. The Wilcoxon rank-sum test or the Student’s t-test was used to compare continuous variables between children with elevated and normal IP groups. Regression and logistic regression analysis was performed to examine the association of IP as a continuous and categorical (normal vs abnormal) variable, respectively, with each of the independent variables of interest. The models were adjusted for potential effect modifiers or confounders like age and type of sensitization. All reported p-values were based on 2-sided tests and compared with a significance level of 5%. Stata 9.1 for Windows (Stata Corp LP, College Station, TX) and SigmaStat (Version 2.03, SPSS, IL, USA) were used for all statistical calculations and plots.
RESULTS
The study included 131 food-allergic subjects, with a median age of 6.7 years (IQR, 4.8 – 8.9); 100 (66.2%) male. The weight-for-age and height-for-age z scores were slightly below the median for age (median −0.09 (IQR, −1 – 0.5) and −0.3 (−1 – 0.4), respectively. The diet record showed no intake of CM or egg-containing foods within 5 days preceding OFCs, neither did it show exposure to other relevant food allergens for the individual subjects. The median age of healthy non-atopic controls was 6.7 (3.5 - 13.5) years, with 57% males.
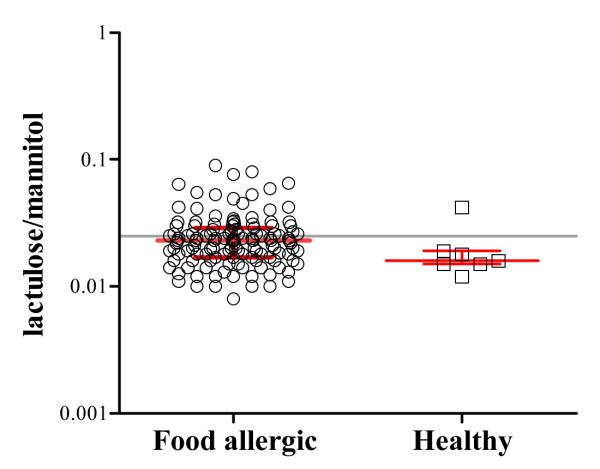
Of the 131 subjects, 50 patients (38%) had an elevated (≥0.025) and 81 had a normal IP (<0.025) (Fig. 2). The median IP was 0.031 (0.026-0.042) in those with elevated IP and 0.018 (0.015-0.021) in those with normal IP. Among the 7 healthy controls, 6 had completely normal IP (Fig. 2).
Figure 2.
Intestinal permeability (IP) in food allergic patients (n=131) and healthy controls (n=7) with no history of family history of food allergy as measured by urinary lactulose/ mannitol (L/M) ratio. The grey horizontal line represents an intestinal permeability of 0.025 (the upper limit of the normal reference values). Shown is the median with IQR.
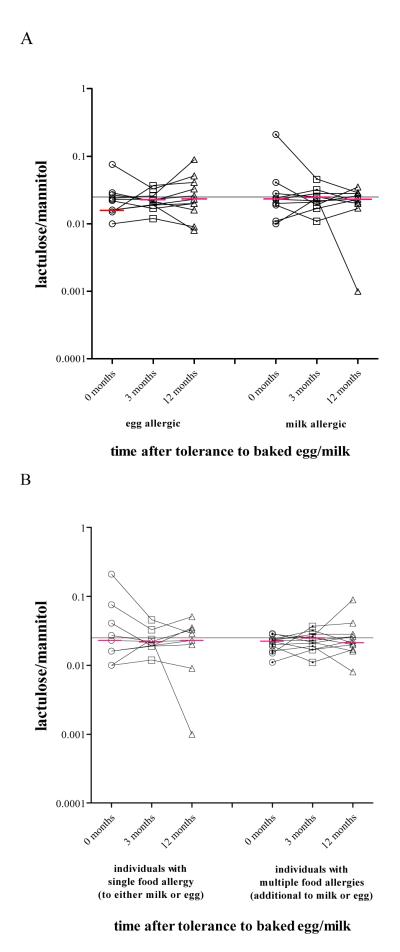
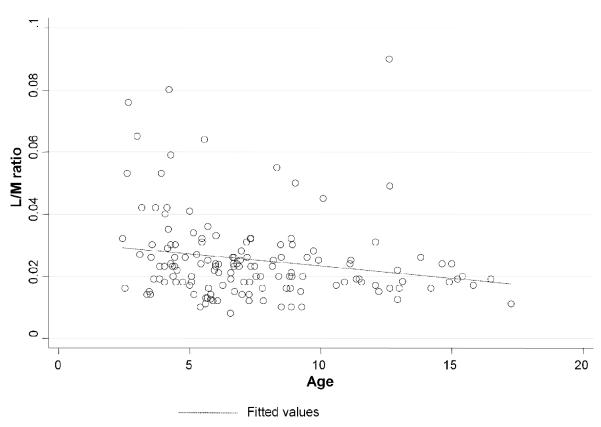
When IP values were longitudinally assessed, there was no significant change in IP values from baseline to either 3 or 12 months after baked CM/egg were added to the diet (Fig. 3A). Presence of additional food allergies had no effect on IP (Fig. 3B). Subjects with an elevated IP were younger (p=0.015, Fig. 4), and had shorter duration of the elimination diet than those with normal IP, although several years in duration in both groups (median 5.1, range 3.5 – 7.8 in those with elevated IP and median 6.5, range 5 - 9.9 in those with normal IP, p=0.005, Table 1). IP was weakly, although statistically significantly, negatively correlated with age, duration of the elimination diet, height and weight (Spearman’s rho coefficients: rhoage=−0.251, p=0.005, rhoelimination=−0.159, p=0.029, rhoheight=−0.294, p=0.001 and rhoweight=−0.241, p=0.006, respectively). The subjects with elevated IP less often had a lifetime history of asthma (p=0.020) and were less commonly sensitized to grass pollen (p=0.027), but not to other pollens or HDM than those with normal IP (Table 1). However, logistic regression analysis (see below, Table 2) showed those not to be significantly related to IP. In pollen-sensitized subjects, abnormal IP values were reported as commonly during and outside relevant pollen season (p=0.160). Food allergic patients with increased IP did not differ from those with normal IP in any of the other atopic parameters and characteristics, including GI symptoms (Table 1). The levels of specific IgE and IgG4 against egg white, ovalbumin, ovomucoid, casein and β-lactoglobulin were comparable between the groups (please see supplemental e-Table 1).
Figure 3.
Intestinal permeability changes in individuals allergic to egg (n=13) or CM (n=14) three and twelve months after they became tolerant to either baked CM or baked egg. (A) Intestinal permeability changes in children allergic to CM or egg with or without known concomitant food allergies after they became tolerant to either baked CM or baked egg. (B) All between and within (longitudinal) group comparisons are non-significant. The grey horizontal line represents an intestinal permeability of 0.025 (the upper limit of the normal reference values).
Figure 4.
Intestinal permeability (IP) as measured by urinary lactulose/ mannitol (L/M) ratio as a function of age. There was a significant negative correlation between age and IP (p=0.015). For this reason age was treated as a potential confounder and all inferences were adjusted for age in future analyses.
Table 1.
Clinical characteristics of CM or egg-allergic subjects with normal vs. elevated intestinal permeability measured by urinary lactulose/mannitol (L/M) ratio.
| Intestinal permeability | ||||
|---|---|---|---|---|
| Elevated (n=50) Lac/Man ≥0.025 |
Normal (n=81) Lac/Man<0.025 |
p-value | ||
| Demographics | ||||
| Age (years) | 5.7 (4.2 – 8.5) | 7 (5.6 – 9.3) | 0.007 * | |
| Height (cm) | 112.9 (104.3 - 130) | 120.8 (111.5 – 139) | 0.011 * | |
| height-to-age z-score | −0.44 (−1.04 – 0.25) | −0.15 (−0.91 – 0.42) | 0.254* | |
| Weight (kg) | 19.4 (16.6 – 27.8) | 22.3 (18.7 – 37.8) | 0.011 * | |
| Weight-to-age z-score | −0.44 (−1.09 – 0.52) | −0.04 (−0.83 – 0.55) | 0.277* | |
|
| ||||
| Atopic features | ||||
|
| ||||
| Age of first reaction (months) | 6.5 (2 – 12) | 6 (2 – 10) | 0.520* | |
| Multiple food allergies | 82.7% | 90.1% | 0.287 | |
| Asthma | 56.6% | 74.4% | 0.031 | |
| Atopic Eczema | 82% | 92.6% | 0.800 | |
| GI manifestation of food allergy ever | 21.4% | 23.9% | 0.735 | |
| Anaphylaxis ever | 53.1% | 53.2% | 0.991 | |
| Duration of elimination diet (years) | 5.1 (3.5 – 7.8) | 6.5 (5 - 9.9) | 0.005 * | |
| Family history of food allergy | 50% | 50.8% | 0.936 | |
| Total IgE (IU/L) | 262(139 – 742) | 437(155 – 1022) | 0.197* | |
|
| ||||
| Symptoms at OFC | ||||
|
| ||||
| Anaphylaxis grade in food challenge1 |
1 | 11 | 24 | |
| 2 | 21 | 16 | 0.116 | |
| 3 | 1 | 4 | ||
| 4 | 5 | 6 | ||
| Urticaria | 14 | 19 | 0.508 | |
| Sneezing | 3 | 4 | >0.999 | |
| Rhinitis | 2 | 3 | >0.999 | |
| Cough | 11 | 6 | 0.063 | |
| Wheeze | 1 | 4 | 0.380 | |
| GI symptoms | 18 | 14 | 0.119 | |
|
| ||||
| Environmental sensitizations2 | 0.936 | |||
|
| ||||
| Tree pollen (birch, oak) | 59.6% | 66.7% | 0.405 | |
| Grass pollen | 26.5% | 46.8% | 0.023 | |
| Common ragweed pollen | 42% | 54.9% | 0.151 | |
| House dust mite | 54.6% | 54% | 0.959 | |
All values in median (IQR)
OFC, oral food challenge.
Wilcoxon rank-sum test.
Sampson 2003, ref 18 (There were no subjects with a grade of 5).
Environmental sensitizations were defined as a detectable specific IgE antibody in serum (≥0.35 kUA/L, UniCAP) and or positive skin prick test, wheal diameter ≥3 mm greater than saline control.
Table 2.
Intestinal permeability as a continuous (A) and binary (B) variable: multivariate analysis after adjusting for age, gender and duration of elimination diet. Asthma is additionally adjusted for type of aeroallergen sensitization.
| A | |||
|---|---|---|---|
| Parameters | coefficient | SE | p-value |
|
| |||
| Height (cm) | −0.0003 | 0.0001 | 0.004 |
|
| |||
| Asthma | −0.0049 | 0.0027 | 0.07 |
|
| |||
| Asthma | −0.0034 | 0.0027 | 0.21 |
| Grass pollen sensitization | −0.0054 | 0.0025 | 0.03 |
|
| |||
| Asthma | −0.0044 | 0.0027 | 0.10 |
| Tree pollen sensitization | −0.0021 | 0.0025 | 0.40 |
|
| |||
| Asthma | −0.0049 | 0.0028 | 0.08 |
| Ragweed pollen sensitization | −0.0013 | 0.0026 | 0.62 |
|
| |||
| Asthma | −0.0006 | 0.0026 | 0.83 |
| HDM sensitization | −0.0021 | 0.0025 | 0.40 |
| B | |||
|---|---|---|---|
| Parameters | Odds Ratio | 95% CI | p-value |
|
| |||
| Height (cm) | 0.9 | 0.9 – 0.9 | 0.041 |
|
| |||
| Asthma | 0.7 | 0.3 – 1.6 | 0.397 |
|
| |||
| Asthma | 0.8 | 0.3 – 1.8 | 0.528 |
| Grass sensitization | 0.5 | 0.2 – 1.2 | 0.120 |
|
| |||
| Asthma | 0.7 | 0.31– 1.6 | 0.430 |
| Tree sensitization | 0.9 | 0.4 – 2.2 | 0.972 |
|
| |||
| Asthma | 0.6 | 0.3– 1.5 | 0.295 |
| Ragweed sensitization | 0.9 | 0.4 – 2.2 | 0.929 |
|
| |||
| Asthma | 0.9 | 0.4 – 2.4 | 0.943 |
| HDM sensitization | 1.3 | 0.6 – 3.3 | 0.515 |
Height and age were significantly correlated, as expected (rho=0.79, p <0.001), however, in the regression model with IP as the continuous dependent variable, height remained significantly correlated with IP after adjusting for age, duration of elimination diet and gender (p=0.004, Table 2A). Grass-sensitized subjects had higher median IP than the grass non-sensitized subjects (0.023 vs 0.021, respectively), whereas non-asthmatic patients had higher median IP values than asthmatics (0.025 vs 0.021) (Table 2A). To account for these findings and approach the association of IP impairment based on the reference threshold of 0.025, we applied a logistic regression model, with IP as the binary outcome of interest (normal vs increased). In this model, asthma adjusted for each of the four different types of sensitizations (grass, ragweed, trees and HDM) was non-significant as was grass sensitization. Height remained significant after adjusting for gender, age and elimination period (p=0.041, Table 2B). After adjusting for the same parameters, the subjects with normal IP were taller by a mean value of 5.4±2.5 cm than the subjects with increased IP (p=0.037) in CM or egg allergy.
DISCUSSION
We report increased IP during strict elimination diets in a large subset (38%) of children with CM or egg allergy. IP was inversely correlated with height independently of age, but it was not correlated with any other clinical parameter, including family history, other atopic manifestations, environmental sensitizations nor symptoms during OFC. Since the subjects were asymptomatic on long-term elimination diets, these data argue against increased IP as a consequence of an effector phase of an allergic reaction unless IP reflects long-lasting effect of a past allergic insult or an ongoing insult due to poor compliance or unknowing ingestion of small amounts of allergen on the gut mucosa. Alternatively, these data suggest that elevated IP may be an intrinsic trait in a subset of patients with food allergy.
Three studies have reported increased IP in food hypersensitivity during a food elimination diet (13-15). Increased IP was reported in 60 subjects with IgE-mediated allergy to CM, celery, and seafood at baseline (age and duration of elimination diet unknown) and further increased during OFC (14). Increased IP was also found in 41 OFC-proven food-allergic patients (14 to 52 years) after 6-month elimination diet, half of them with an IgE-mediated allergy to nuts and half with non-IgE mediated allergy to CM and egg (15). Lastly, Dupont et al. (13) reported elevated IP during an elimination diet in 3 of 12 (25%) young children (6 months to 2 years) with CM-sensitive enteropathy and in 6 of 28 (21%) children (6 months to 15 years) with food-associated AD (although statistically elevated only in the latter group). IP increased following a positive OFC in both groups (13).
The fundamental question remains whether elevated IP seen in a subset of food allergic children is a primary defect predisposing them to food allergy or a consequence of the ongoing (symptomatic or asymptomatic) exposure to allergic triggers. Our subjects were asymptomatic and had been on elimination diets for a long time without any recent OFCs. However, we cannot exclude the possibility of accidental exposures to foods without apparent symptoms increasing IP. However, introduction of large amounts of baked goods into their diets did not alter IP. IP was not affected by presence of additional food allergies. Therefore, IP appears to be independent of the type or number of food allergies or tolerance of baked foods that precedes tolerance of the unheated CM and egg (20, 21). This suggests that increased IP is a primary defect that may be relevant for development of but not for maintenance of food allergy, at least in a subset of individuals with IgE-mediated CM and egg allergy. Since small amounts of luminal antigen can activate mast cells leading to increased IP perpetuating an allergic response (1, 22, 23), it is also possible that persistently elevated IP represents an asymptomatic response to low level or innocuous allergen exposure, which may require a long time to resolve. In celiac disease, histologic improvement is typically seen within 9-12 months of gluten-free diet although persistent abnormalities not due to ingestion of trace amounts of gluten exist in some patients.(24)
We found that intestinal permeability was negatively associated with height even after adjusting for age, gender and duration of elimination diet. This was not the case for weight and BMI. Therefore, it is unlikely that this growth impairment results from poor dietary intake, malabsorption or maldigestion, as reported in rural Gambian non-allergic children with intestinal mucosal enteropathy (25). Short stature may be associated with although not caused by increased IP. Interestingly, Hijazi et al. (26) who found increased IP in asthmatic children also reported height (and weight) to be significantly higher in healthy controls with normal IP, consistent with our findings.
The limitations to the present study include the fact that we did not assess IP in subjects before food allergies developed to dissect out the chicken and egg paradigm. Also, assessment of family members could clarify the issue of familial predisposition to elevated IP, although it would not explain why certain individuals develop food allergy and others do not. It would have been valuable but not practical to obtain detailed food diaries or recordings of viral infections months preceding the IP measurement to assess whether such insults might have a long-lasting affect on IP. However, repeated measures showing stable IP over time suggest an intrinsic problem rather than a defect secondary to an allergen insult. Even with these limitations, this is the largest cohort of food-allergic children with IP measurements.
In conclusion, elevated IP is found in about one-third of asymptomatic food-allergic children on a food allergen restricted diet. Prospective studies are necessary to determine if elevated IP is a primary defect or a consequence of a long-lasting allergic insult on the intestinal mucosa and to clarify the relationship between elevated IP and shorter stature.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ramon Bencharitiwong, PhD for technical assistance.
Supported in part by grants from National Institute of Allergy and Infectious Diseases: K08-AI091655 (KMJ), AI-44236 (HAS), AI 059318 (ANW) and the CTSA ULI RR 029887 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH); RR026134.
REFERENCES
- 1.Farhadi A, Banan A, Fields J, Keshavarzian A. Intestinal barrier: An interface between health and disease. J Gastroenterol Hepatol. 2003;18:479–97. doi: 10.1046/j.1440-1746.2003.03032.x. [DOI] [PubMed] [Google Scholar]
- 2.van Elburg RM, Uil JJ, de Monchy JG, Heymans HS. Intestinal permeability in pediatric gastroenterology. Scand J Gastroenterol Suppl. 1992;194:19–24. doi: 10.3109/00365529209096021. [DOI] [PubMed] [Google Scholar]
- 3.Kalach N, Rocchiccioli F, de Boissieu D, Benhamou PH, Dupont C. Intestinal permeability in children: Variation with age and reliability in the diagnosis of cow’s CM allergy. Acta Paediatr. 2001;90:499–504. [PubMed] [Google Scholar]
- 4.Meddings J. The significance of gut barrier in disease. Gut. 2008;57:438–40. doi: 10.1136/gut.2007.143172. [DOI] [PubMed] [Google Scholar]
- 5.Perrier C, Corthesy B. Gut permeability and food allergies. Clin Exp Allergy. 2011;41:20–8. doi: 10.1111/j.1365-2222.2010.03639.x. [DOI] [PubMed] [Google Scholar]
- 6.van Elburg RM, Uil JJ, Kokke FT, Mulder AM, van de Broek WG, Mulder CJ, et al. Repeatability of the sugar-absorption test, using lactulose and mannitol, for measuring intestinal permeability for sugars. J Pediatr Gastroenterol Nutr. 1995;20:184–8. doi: 10.1097/00005176-199502000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Jackson PG, Lessof MH, Baker RW, Ferrett J, MacDonald DM. Intestinal permeability in patients with eczema and food allergy. Lancet. 1981;1:1285–6. doi: 10.1016/s0140-6736(81)92459-4. [DOI] [PubMed] [Google Scholar]
- 8.Falth-Magnusson K, Kjellman NI, Magnusson KE, Sundqvist T. Intestinal permeability in healthy and allergic children before and after sodium-cromoglycate treatment assessed with different-sized polyethyleneglycols (PEG 400 and PEG 1000) Clin Allergy. 1984;14:277–86. doi: 10.1111/j.1365-2222.1984.tb02207.x. [DOI] [PubMed] [Google Scholar]
- 9.Oman H, Blomquist L, Henriksson AE, Johansson SG. Comparison of polysucrose 15000, 51Cr-labelled ethylenediaminetetraacetic acid, and 14C-mannitol as markers of intestinal permeability in man. Scand J Gastroenterol. 1995;30:1172–7. doi: 10.3109/00365529509101627. [DOI] [PubMed] [Google Scholar]
- 10.Troncone R, Caputo N, Florio G, Finelli E. Increased intestinal sugar permeability after challenge in children with cow’s CM allergy or intolerance. Allergy. 1994;49:142–6. doi: 10.1111/j.1398-9995.1994.tb00816.x. [DOI] [PubMed] [Google Scholar]
- 11.Goto K, Chew F, Torun B, Peerson JM, Brown KH. Epidemiology of altered intestinal permeability to lactulose and mannitol in guatemalan infants. J Pediatr Gastroenterol Nutr. 1999;28:282–90. doi: 10.1097/00005176-199903000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Lunn PG, Northrop CA, Northrop AJ. Automated enzymatic assays for the determination of intestinal permeability probes in urine. 2. mannitol. Clin Chim Acta. 1989;183(2):163–70. doi: 10.1016/0009-8981(89)90332-x. [DOI] [PubMed] [Google Scholar]
- 13.Dupont C, Barau E, Molkhou P, Raynaud F, Barbet JP, Dehennin L. Food-induced alterations of intestinal permeability in children with cow’s CM-sensitive enteropathy and atopic dermatitis. J Pediatr Gastroenterol Nutr. 1989;8:459–65. doi: 10.1097/00005176-198905000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Andre C, Andre F, Colin L, Cavagna S. Measurement of intestinal permeability to mannitol and lactulose as a means of diagnosing food allergy and evaluating therapeutic effectiveness of disodium cromoglycate. Ann Allergy. 1987;59:127–30. [PubMed] [Google Scholar]
- 15.Ventura MT, Polimeno L, Amoruso AC, Gatti F, Annoscia E, Marinaro M, et al. Intestinal permeability in patients with adverse reactions to food. Dig Liver Dis. 2006;38:732–6. doi: 10.1016/j.dld.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Nowak-Wegrzyn A, Bloom KA, Sicherer SH, Shreffler WG, Noone S, Wanich N, Sampson HA. Tolerance to extensively heated CM in children with cow’s CM allergy. J Allergy Clin Immunol. 2008;122(2):342–7. 347.e1–2. doi: 10.1016/j.jaci.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 17.Lemon-Mulé H, Sampson HA, Sicherer SH, Shreffler WG, Noone S, Nowak-Wegrzyn A. Immunologic changes in children with egg allergy ingesting extensively heated egg. J Allergy Clin Immunol. 2008;122(5):977–983.e1. doi: 10.1016/j.jaci.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Sampson HA. Anaphylaxis and emergency treatment. Pediatrics. 2003;111:1601–8. [PubMed] [Google Scholar]
- 19.Zamora SA, Hilsden RJ, Meddings JB, Butzner JD, Scott RB, Sutherland LR. Intestinal permeability before and after ibuprofen in families of children with Crohn’s disease. Can J Gastroenterol. 1999;13:31–6. doi: 10.1155/1999/457315. [DOI] [PubMed] [Google Scholar]
- 20.Leonard SA, Sampson HA, Sicherer SH, Noone S, Moshier EL, Godbold J, Nowak-Węgrzyn A. Dietary baked egg accelerates resolution of egg allergy in children. J Allergy Clin Immunol. 2012;130(2):473–80.e1. doi: 10.1016/j.jaci.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JS, Nowak-Węgrzyn A, Sicherer SH, Noone S, Moshier EL, Sampson HA. Dietary baked CM accelerates the resolution of cow’s CM allergy in children. J Allergy Clin Immunol. 2011;128(1):125–131.e2. doi: 10.1016/j.jaci.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berin MC, Kiliaan AJ, Yang PC, Groot JA, Kitamura Y, Perdue MH. The influence of mast cells on pathways of transepithelial antigen transport in rat intestine. J Immunol. 1998;161:2561–6. [PubMed] [Google Scholar]
- 23.Bischoff SC, Kramer S. Human mast cells, bacteria, and intestinal immunity. Immunol Rev. 2007;217:329–37. doi: 10.1111/j.1600-065X.2007.00523.x. [DOI] [PubMed] [Google Scholar]
- 24.Selby WS, Painter D, Collins A, Faulkner-Hogg KB, Loblay RH. Persistent mucosal abnormalities in coeliac disease are not related to the ingestion of trace amounts of gluten. Scand J Gastroenterol. 1999;34:909–14. doi: 10.1080/003655299750025390. [DOI] [PubMed] [Google Scholar]
- 25.Campbell DI, Lunn PG, Elia M. Age-related association of small intestinal mucosal enteropathy with nutritional status in rural gambian children. Br J Nutr. 2002;88:499–505. doi: 10.1079/BJN2002697. [DOI] [PubMed] [Google Scholar]
- 26.Hijazi Z, Molla AM, Al-Habashi H, Muawad WM, Molla AM, Sharma PN. Intestinal permeability is increased in bronchial asthma. Arch Dis Child. 2004;89:227–9. doi: 10.1136/adc.2003.027680. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.