Abstract
Although it is known that obesity increases the risk of endometrial cancer and is linked to higher mortality rates in the general population, the association between obesity and mortality among endometrial cancer survivors is unclear. We performed a medline search using exploded Mesh keywords ‘endometrial neoplasms/’ and (‘body mass index/’ or ‘obesity/’) and (‘survival analysis/’ or ‘mortality/’ or (survivor* or survival*).mp.). We also inspected bibliographies of relevant papers to identify related publications. Our search criteria yielded 74 studies, 12 of which met inclusion criteria. Four of the included studies reported a statistically or marginally significant association between obesity and higher all cause mortality among endometrial cancer survivors after multivariate adjustment. The suggestive association between body mass index and higher all cause mortality among women with endometrial cancer was comparable to the magnitude of association reported in prospective studies of healthy women. Of the five studies that examined progression-free survival and the two studies reporting on disease-specific mortality, none reported an association with obesity. Future studies are needed to understand disease-specific mortality, the importance of obesity-onset timing and whether mechanisms of obesity-related mortality in this population of women differ from those of the general population.
Keywords: endometrial neoplasms, survivorship, mortality, body mass index
INTRODUCTION
It is estimated that in the United States 47 130 women will be diagnosed with endometrial (uterine corpus) cancer in 2012, making it the fourth most common incident cancer among women.1 An estimated 8120 deaths from endometrial cancer were expected in 2011, making it the second most common cause of gynecologic cancer death.2 Uterine cancer is a broad term that encompasses all cancers that develop in the uterus, the most common of which is endometrial cancer, or cancers arising in the lining of the uterus.1 Previous publications show that parity, body mass index (BMI), physical activity and diet may explain up to 80% of the risk of endometrial cancer.3 Most (80–90%) endometrial cancers are type 1(ref. 4) and are normally hormone sensitive, early stage, and have good prognosis, while type 2 tumors tend to be higher grade and are more likely to recur.5 Endometrial cancer staging includes a lymph node evaluation from the pelvic and paraaortic regions, and treatment for endometrial cancer is surgical removal of the uterus, fallopian tubes and ovaries.6 Fewer women with endometrial cancer benefit from adjuvant radiotherapy, hormone therapy, or chemotherapy.5 Despite relatively high 5-year survival rates of 95.5% for localized disease, overall survival is around 80%, and the American Cancer Society’s annual report on cancer statistics shows a statistically significant decrease in 5-year endometrial cancer survival rates from 1975–2005.5,7
Rationale for review
Endometrial cancer survival has been closely linked to tumor characteristics of stage, myometrial invasion, histological type and differentiation.5 Although we know that obesity is strongly associated with endometrial cancer risk,8 the role of obesity in endometrial cancer survival is unclear. Previous studies on BMI and cancer-specific mortality among women who were healthy at baseline report the strongest association between BMI and endometrial cancer-specific mortality out of all cancer sites, with hazard ratios (HRs) up to 6.25 (95% confidence interval (CI): 3.75–10.42), comparing women with a BMI ≥40 kg m2− to those who were normal weight.9,10 This magnitude of risk is greater than the approximate twofold risk of all cause mortality for similar aged women with a BMI ≥40 kg m2− observed in the Cancer Prevention Study-II11 or in other large prospective cohorts such as NIH-AARP.12 However, these associations between BMI and mortality were observed among women who were healthy at baseline, rather than among women diagnosed with endometrial cancer.
Factors that influence cancer survival are of increasing importance as the lifestyle-related mortality risk factors for this population may differ from those of the general population. However, studies on the association between obesity and survival specifically among women diagnosed with endometrial cancer provide conflicting evidence and previous review papers on obesity and cancer mortality either do not comprehensively report on endometrial cancer13 or do not specifically focus on the association between obesity and survival.8 Still, cross-sectional surveys of endometrial cancer survivors highlight the prevalence of obesity, low physical activity and poor quality diet. A mailed questionnaire to 200 endometrial cancer survivors from the MD Anderson Cancer Center tumor registry showed that 66% of the women were overweight or obese after diagnosis.14 Many consider cancer diagnosis to be a ‘teachable moment’ to encourage lifestyle risk factor changes. If obesity is associated with higher mortality risk than in the general population, this high percentage of overweight endometrial cancer survivors may be an appropriate targeted population for such teachable moments.
The purpose of this review is to summarize published studies and relevant mechanisms, and to highlight gaps in the literature relating obesity to overall and disease-specific survival among women with endometrial cancer. Despite high numbers of endometrial cancer survivors, insufficient data exist on the association between obesity, assessed before or after diagnosis, and mortality after endometrial cancer diagnosis. If the association between obesity and endometrial cancer survival is as strong in survivor populations as suggested in prospective mortality studies (among women healthy at baseline), it would suggest that obesity is a stronger mortality risk factor among this population than in the general population. Thus, interventions geared toward BMI change could be a viable lifestyle means to improve endometrial cancer survivorship.
METHODS
This systematic review focuses on studies examining obesity among endometrial cancer survivors. We performed a medline search using exploded Mesh keywords ‘exp endometrial neoplasms/’ and (‘exp body mass index/’ or ‘exp obesity/’) and (‘exp survival analysis/’ or ‘exp mortality/’ or (survivor* or survival*).mp.). We also inspected bibliographies of relevant papers to identify publications on obesity and endometrial cancer survival. Studies with exposures other than obesity were excluded, as were studies in populations other than endometrial cancer survivors. Publications reporting different outcomes or analyses in the same population were only included in a single instance. We reviewed bibliographies of relevant papers and reviews to identify additional studies to be included. Publication year was not considered in exclusion criteria. Studies to be included in this review were subject to the following criteria: (1) human subjects (2) English language (3) original data collection (4) exposure assessment of obesity (5) analysis of endometrial cancer survivors (as opposed to primary prevention studies) (6) outcome of overall survival.
RESULTS
Our medline search strategy produced 73 abstracts published between 1995 and 2011 to be reviewed (Figure 1).15 Of the 73 studies identified in the original search, 4 were duplicates, 13 explored risk of endometrial cancer, 16 examined survival by exposures other than those specified, 13 studied endometrial cancer outcomes other than overall survival such as response to treatment or quality of life, 3 did not include information on endometrial cancer, 7 were not primary data collection (reviews or trend analyses) and 3 were in languages other than English. This left the authors with 14 papers to review in full. Three further papers were excluded upon full inspection due to the following reasons: reported exposures did not include obesity, the analysis was on mortality among women without a history of disease rather than endometrial cancer survivors, and the study was limited to normal-weight women, precluding potential to assess the association between obesity and survival. Examination of bibliographies of related literature generated one additional paper to be included in this review. Table 1 summarizes the 12 included publications. Of the included studies on endometrial cancer survivors, all relied on BMI assessed before or at diagnosis; only one study16 relied on self-reported BMI, while the others were based on measured BMI. Post-diagnosis BMI was not assessed in studies that met review criteria and thus is not discussed in this review.
Figure 1.
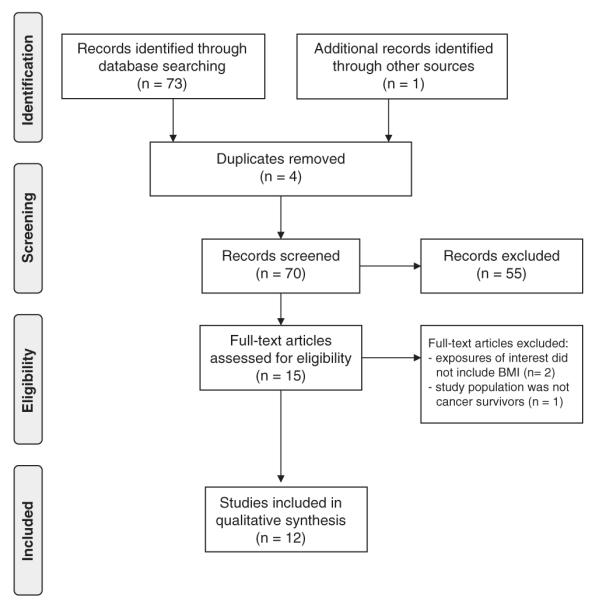
PRISMA flow diagram of search and manuscript review.
Table 1.
Characteristics of included studies on obesity and endometrial cancer survivorship (N=12)
| Reference | Characteristics | BMI measure |
Total, N |
Recurrence/ total deaths, N |
OS (BMI categories in kgm−2) listed
where appropriate) |
Covariates included | PFS or DSS | |
|---|---|---|---|---|---|---|---|---|
| Anderson et al.21 |
Period: 1978–1993 Context: chart review of patients at the University of Iowa Hospitals and Clinics Tumor Registry Median FU=3.97 years |
Abstracted from medical charts for BMI at diagnosis |
492 | 80/100 | Cox PH—no association, HR not reported; Kaplan–Meier: improved survival with higher continuous BMI, P=0.0645 |
Cox PH model adjusted for grade, myometrial invasion, stage |
PFS: time to recurrence longer with increasing continuous BMI, P=0.0136; nonsignificant association between recurrence and BMI overall DSS: not reported |
|
| Chia et al.16 | Period: 1991–1994 Context: chart review of patients identified through Wisconsin statewide mandatory cancer registry Mean FU=9.3 years |
Telephone interview queried on height and weight two years prior |
745 | ?/166 | <25 25–29.9 ≥30 |
1.0 1.2 (0.8–2.0) 1.6 (1.0–2.5) |
Cox PH model adjusted for age at diagnosis, stage, menopausal status, BMI, diabetes, smoking history, OC use, parity, PMH use |
PFS: not reported DSS: HR=2.0 (95% CI: 0.8–5.1) comparing BMI ≥30 with BMI <25 |
| Everett et al.22 | Period: 1990–2000 Context: chart review of patients treated at the University of Washington Median FU=27 months |
Abstracted from medical charts for BMI at diagnosis |
396 | 42/51 | No differences between BMI<30, 30–40 and >40 for OS (P=0.18) |
Univariate chi-squared analysis performed |
PFS: trend toward more recurrence for BMI >40 but not significant (P=0.065) DSS: no difference between BMI groups |
|
| Gates et al.20 | Period: 1992–2001 Context: tumor registry abstraction at the Morehouse School of Medicine, GA FU=5-year survival |
Abstracted from medical charts for BMI at diagnosis |
165 | ?/11 | <25 >25 |
1.00 5.4 (0.49–59.00) |
Cox PH model adjusted for age, race, parity, progesterone receptor status, HER-2 status |
Not reported |
| Jeong et al.23 | Period: 2000–2006 Context: tumor registries of eight tertiary medical centers in Korea FU=not reported |
Abstracted from medical charts for BMI at diagnosis |
937 | Not reported |
<23 23–25 ≥25 |
1.00 0.93 (0.37–2.29) 0.87 (0.41–1.83) |
Cox PH models adjusted for age, menopause, tumor stage, grade, adjuvant treatment and LVSI |
Not reported |
| Kodama et al.24 | Period: 1992–2002 Context: surgical treatment at the Okayama University Graduate School of Medicine, Japan FU=5-year survival |
BMI abstracted from chart review at the time of surgery |
242 | 34/30 | Log-rank test P=0.07 with 5 years OS=84.3% among BMI<25 and OS=93.0% among BMI ≥25; nonsignificant in Cox PH models |
Cox PH model adjusted for stage, grade, histological cell type and lower abdominal pain |
Log-rank test P=0.34 with 5-year PFS=84.4% among BMI<25 and PFS=88.8% among BMI≥25 DSS: not reported |
|
| Mauland et al.18 |
Period: 1981–2009 Context: treatment at the Haukeland University Hospital, Norway Median FU=4.9 years |
Measured BMI at diagnosis abstracted from baseline records |
905 | ?/338 | Kaplan–Meier curve showed nonsignificant (P=0.18) survival differences comparing BMI<25 to BMI≥25; continuous BMI, HR=1.02 (95% CI: 1.00–1.04, P=0.035) |
Cox PH model for continuous BMI adjusted for age at diagnosis, FIGO stage, histological subtype and grade |
PFS: not reported DSS: HR=1.01 (95% CI: 0.98–1.04) on continuous scale |
|
| Modesitt et al.17 |
Period: 1981–2009 Context: data on advanced* or recurrent** or endometrial cancer from patients treated on five Gynecologic Oncology Group trials Median FU=13.6 months |
BMI gathered from individual trial data |
949 | 533/772 | <25* 25–29.9 30–39.9 ≥40 <25** 25–29.9 30–39.9 ≥40 |
1.00 0.96 (0.69–1.33) 1.33 (0.96–1.84) 1.86 (1.16–2.99) 1.00 1.02 (0.80–1.30) 0.88 (0.70–1.12) 1.0 (0.71–1.40) |
Cox PH model adjusted for age, race, performance status, histology and tumor grade and treatment protocol |
|
| Münstedt et al.26 |
Period: 1986–2005 Context: patients at the Department of Obstetrics and Gynecology of the Justus-Liebig University Giessen, Germany Median FU: 6.2 years |
Measured BMI using preoperative weight at diagnosis abstracted from medical records |
1180 | Not reported |
Kaplan–Meier curve showed better survival with higher BMI (P=0.004); nonsignificant improved survival with higher continuous BMI, HR=0.98 (95% CI: 0.95–1.00), P=0.087 |
Cox PH model for continuous BMI adjusted for age, grade and stage |
Not reported | |
| Studzinski and Zajewski27 |
Period: 1984–1995 Context: patients treated for endometrial cancer at the Regional Hospital in Slupsk, Poland FU=5-year survival |
Abstracted from medical charts for BMI at diagnosis |
121 | Not reported |
87.4% of obese patients lived to 5 years compared with 73.4% of nonobese patients; highest percentage 5-year survival among those with a BMI 25–29 |
Multivariate models not used |
Not reported | |
| Temkin et al.25 | Period: 1982–2003 Context: patients treated for endometrial cancer at the State University of New York (SUNY), Downstate and Kings County Hospital Mean FU=9.05 years |
Abstracted from medical charts for BMI at diagnosis |
442 | ?/167 | Continuous BMI HR=0.99 (0.97–1.05) | Cox PH adjusted for age, race, grade, stage, and chemotherapy |
P=0.041 comparing those who died of other causes, died of disease, were alive with disease or were alive with no evidence of disease comparing BMI categories; HR not reported for PFS or DSS |
|
| Von Gruenigen et al.19 |
Period: 1987–1995 Context: RCT of surgery with or without adjuvant radiation therapy Median FU=5.42 years |
Measured at baseline | 380 | 44/61 | <30 30–39 ≥40 |
1.00 1.47 (0.81–2.67) 2.76 (1.20–6.33) |
Cox PH model, adjusted for age, race, performance status, stage, histology, tumor grade, myometrial invasion, LVSI, intervention arm |
PFS: HR=0.41 (0.09–1.83) comparing BMI ≥40 with BMI<30 DSS: morbidly obese women had a higher percentage of deaths due to other causes compared with women with BMI <40, HR not reported |
Abbreviations: BMI, body mass index; CI, confidence interval; DSS, disease-specific survival; FU, follow up; HR, hazard ratio; LVSI, lymphovascular space involvement; OC, oral contraceptive; OS, overall survival; PFS, progression free survival; PH, proportional hazards; PMH, post-menopausal hormone; RCT, randomized controlled trial.
Advanced disease.
Recurrent disease.
Overall survival
Four of the included studies16–19 showed marginally or statistically significant associations between obesity and all cause mortality (Table 1). Of the studies reporting a statistically significant association between BMI and all cause mortality, the magnitude of association ranged from a HR=1.86 (95% CI: 1.16–2.99)17 to a HR=2.76 (95% CI: 1.20–6.33)19 comparing those with a BMI ≥40 kg m−2 to those with a BMI <30 kg m−2. An additional study comparing women with a BMI≥30 kg m−2 to those with a BMI <25 kg m−2 reported a nominally nonsignificant association of HR=1.6 (95% CI: 1.0–2.5).16 Although another smaller study of 165 women reported HR=5.4 comparing those with a BMI≥25 kg m−2 to those with a BMI <25 kg m−2, the 95% CI was quite wide (0.49–59.00) with only 11 deaths in the study.20 On a continuous scale a study of 905 women with 338 deaths reported a HR=1.02 (95% CI: 1.00–1.04) for an one unit change in BMI.18 The remaining seven studies reviewed reported no association between BMI and overall survival, with study sample sizes ranging from 121 women to 1180; the number of total deaths was not reported for all studies.21–27
Progression-free survival and disease-specific survival were not uniformly reported. Five of the 12 studies reported on endometrial cancer recurrence in relation to BMI, none of which showed a significant association.17,19,21,22,24 Only two studies reported a HR for obesity and disease-specific survival, neither of which were significant;16,18 a third study reported no difference in disease-specific survival by obesity status but did not present a HR estimate.22
Prognostic characteristics
Eight of the reviewed papers found a statistically significant association between higher BMI and lower tumor stage17,18,20–23,25,26 and six found an association between higher BMI and lower tumor grade,17,18,21,22,25,26 suggesting that some tumor characteristics were less aggressive among obese women.
There was a lack of uniformity of methods (Chi-squared, log-rank and Cox proportional hazards), BMI categorizations and adjustment covariates among the included papers. Four of the papers did not report HR estimates,21,22,24,27 three reported only continuous HRs18,25,26 and the remaining five studies used different BMI cutoffs for estimating risk.16,17,19,20,23 As estimates were thus not comparable, we did not perform a meta-analysis.
DISCUSSION
We identified 12 published manuscripts on obesity and endometrial cancer survival, 4 of which suggested that obesity assessed by BMI at diagnosis is associated with worse survival among women diagnosed with endometrial cancer, with magnitudes of risk ranging from 1.86(ref. 17)–2.76(ref. 19) for women with a BMI ≥40 kg m−2 compared with non-obese weight women. On the basis of the five papers reporting on BMI and cancer recurrence and two presenting HRs on disease-specific mortality there was insufficient evidence to suggest an association. Many of the included publications indicated that obesity is associated with better tumor differentiation and lower stage, provoking further questions about the nature of the relationship between obesity and survival in this population and causes of death.
To our knowledge, this is the first systematic review paper summarizing the evidence on obesity and mortality among women with endometrial cancer. Our review does not include unpublished literature and thus is limited to published manuscripts. Although papers were assessed for potential sources of bias, our review is limited by the information provided in published manuscripts. A lack of uniform methods precluded a meta-analysis and thus a funnel plot was not produced to assess publication bias. Future studies should uniformly report unadjusted and adjusted associations, and consider following published guidelines for quality of reporting such as the STROBE statement.28 Of the reviewed papers, few included sufficiently detailed methods or reported unadjusted and adjusted results for overall and disease-specific survival. Studies using non-standard BMI cutoffs should include justification for classification of BMI. In addition, the association between BMI and mortality may not be linear, and most studies did not separately categorize underweight women and did not report on the association between low BMI and overall mortality. Grouping underweight women with normal-weight women may lessen the ability to observe an association between BMI and mortality. Also, some of the researchers failed to discuss limitations and interpret the study cautiously given the context in which the research was performed. Of the papers that reported an association between higher BMI and mortality more detailed methods and robust methodology were outlined, thus substantiating the suggestion of an association.
Mechanisms relating obesity to endometrial cancer survival
Various mechanisms have been suggested to explain the relationship between obesity and mortality among endometrial cancer survivors. Some studies point toward surgical complications among obese women,29 whereas other studies show longer operating times and higher blood loss but no differences in length of hospital stay or intraoperative or postoperative complications.22 As endometrial tumors in obese women have been linked to favorable histology or shown no association, the relationship between higher BMI and worse survival also cannot be attributed to obesity-related tumor prognostic characteristics.
Studies on BMI and mortality in the general population suggest increased risk of death among the obese due to medical complications such as hypertension, type 2 diabetes, cardiovascular disease and cancer.30 Physiological changes such as insulin resistance, lipid abnormalities, hormonal changes and chronic inflammation have been suggested as mediators in this relationship.31
Although some of these mechanisms may also influence survival among women with endometrial cancer, the relationship between obesity and mortality in the survivor population may be characterized by disease-specific changes. There are well-established hypotheses that obese women are at increased risk for endometrial cancer due to mitogenic effects of unopposed estrogens.32 Obese women have more adipose tissue, which increases synthesis and bioavailability of endogenous sex steroids.31 Also more frequent anovulation among obese premenopausal women lead to progesterone deficiency and unopposed estrogen exposure.33 Excess estrogens unopposed by progesterone can cause endometrial cell proliferation, inhibition of apoptosis, and an increased number of DNA replication errors and somatic mutations that may lead to cancer.33 Obesity is also linked to decreased production of sex hormone binding globulin in the liver, which in turn increases bioavailable estrogens that diffuse into endometrial tissue.31 Although lifetime exposure to estrogens increases risk of endometrial cancer,32 how this exposure also affects endometrial cancer recurrence and mortality is not clear.
There is suggestive evidence not only for an association between BMI and mortality as described above, but also for additional consequences such as decreased quality of life. Blanchard et al.34 found that endometrial cancer survivors who met more lifestyle recommendations on physical activity, diet and smoking reported higher health-related quality of life using the RAND 36 health status inventory (P<0.001). Other studies also report statistically significant, clinically important differences in quality of life between those who met public health guidelines for physical activity and body weight, and those who did not using the FACT-A and SF 36 scales.14,35 The authors suggest that physical activity may affect quality of life by improving physical fitness and treatment-related side effects, and reducing risk of other chronic diseases while improving body image, social interaction and providing a positive distraction from stressful events.
Future research directions
Future research is needed to better clarify the association between the obesity and endometrial cancer survival, and to investigate other lifestyle factors that may affect survival. A recent review on weight, physical activity, diet and survival in breast and gynecologic cancers showed a lack of publications on lifestyle factors other than overweight and obesity on endometrial cancer survival.13 Observational studies should gather data on both pre- and post-diagnosis diet, physical activity and obesity to parse out whether timing of lifestyle factors changes risk. Also, sedentary time is emerging in the literature as an independent risk factor for various cancers,36 including endometrial cancer,37 but has not yet been analyzed with respect to mortality. Various measures of obesity such as waist circumference, body fat measured via dual energy X-ray absorptiometry could be used to better pinpoint the magnitude of the association between obesity and survival. A randomized clinical trial among endometrial cancer survivors shows the feasibility of an intervention to increase physical activity levels and lose weight in this population.38 In a randomized controlled trial one could attempt to better understand the independent effects of obesity, physical activity and diet on serum biomarkers, and thus better explain some of the increased mortality risk among this survivor population.
CONCLUSION
In summary, this review presents emerging evidence that normal-weight women with endometrial cancer may have better survival than obese survivors, and that the magnitude of association is comparable to the association between obesity and all cause mortality in the general population. More research is needed to confirm this association and endometrial cancer-specific mortality risk in this population. Previous review papers on endometrial cancer and obesity focused on incidence rather than survival or did not perform a comprehensive review of relevant literature. As over 70% of women with type I endometrial cancer are obese, health consequences associated with obesity are a major concern among this population.35,39 Also, future research should investigate the significance of post-diagnosis BMI, as this knowledge could affect cancer care after diagnosis. To our knowledge, no published studies have examined diet and physical activity with survival among women with endometrial cancer, yet these studies would significantly add to the survivorship literature.
ACKNOWLEDGEMENTS
This work was supported in part by the training grant T32 CA105666.
Footnotes
CONFLICT OF INTEREST The authors declare no conflict of interest.
REFERENCES
- 1.National Cancer Institute . What You Need to Know About Cancer of the Uterus. National Cancer Institute; Bethesda: 2012. Endometrial Cancer. [Google Scholar]
- 2.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 3.Terry P, Baron JA, Weiderpass E, Yuen J, Lichtenstein P, Nyren O. Lifestyle and endometrial cancer risk: a cohort study from the Swedish twin registry. Int J Cancer. 1999;82:38–42. doi: 10.1002/(sici)1097-0215(19990702)82:1<38::aid-ijc8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 4.Spurdle AB, Thompson DJ, Ahmed S, Ferguson K, Healey CS, O’Mara T, et al. Genome-wide association study identifies a common variant associated with risk of endometrial cancer. Nat Genet. 2011;43:451–454. doi: 10.1038/ng.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366:491–505. doi: 10.1016/S0140-6736(05)67063-8. [DOI] [PubMed] [Google Scholar]
- 6.Obermair A, Manolitsas TP, Leung Y, Hammond IG, McCartney AJ. Total laparoscopic hysterectomy for endometrial cancer: patterns of recurrence and survival. Gynecol Oncol. 2004;92:789–793. doi: 10.1016/j.ygyno.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 8.Fader AN, Arriba LN, Frasure HE, von Gruenigen VE. Endometrial cancer and obesity: epidemiology, biomarkers, prevention and survivorship. Gynecol Oncol. 2009;114:121–127. doi: 10.1016/j.ygyno.2009.03.039. [DOI] [PubMed] [Google Scholar]
- 9.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 10.Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335:1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 12.Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 13.McTiernan A, Irwin M, Vongruenigen V. Weight, physical activity, diet, and prognosis in breast and gynecologic cancers. J Clin Oncol. 2010;28:4074–4080. doi: 10.1200/JCO.2010.27.9752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basen-Engquist K, Scruggs S, Jhingran A, Bodurka DC, Lu K, Ramondetta L, et al. Physical activity and obesity in endometrial cancer survivors: associations with pain, fatigue, and physical functioning. Am J Obstet Gynecol. 2009;200:288.e1–288.e8. doi: 10.1016/j.ajog.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Medicine: A Peer-Reviewed, Independent, Open-Access Journal. 2009;3:e123–e130. [PMC free article] [PubMed] [Google Scholar]
- 16.Chia VM, Newcomb PA, Trentham-Dietz A, Hampton JM. Obesity, diabetes, and other factors in relation to survival after endometrial cancer diagnosis. Int J Gynecol Cancer. 2007;17:441–446. doi: 10.1111/j.1525-1438.2007.00790.x. [DOI] [PubMed] [Google Scholar]
- 17.Modesitt SC, Tian C, Kryscio R, Thigpen JT, Randall ME, Gallion HH, et al. Impact of body mass index on treatment outcomes in endometrial cancer patients receiving doxorubicin and cisplatin: a Gynecologic Oncology Group study. Gynecol Oncol. 2007;105:59–65. doi: 10.1016/j.ygyno.2006.10.045. [DOI] [PubMed] [Google Scholar]
- 18.Mauland KK, Trovik J, Wik E, Raeder MB, Njolstad TS, Stefansson IM, et al. High BMI is significantly associated with positive progesterone receptor status and clinico-pathological markers for non-aggressive disease in endometrial cancer. Br J Cancer. 2011;104:921–926. doi: 10.1038/bjc.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Gruenigen VE, Tian C, Frasure H, Waggoner S, Keys H, Barakat RR. Treatment effects, disease recurrence, and survival in obese women with early endometrial carcinoma. Cancer. 2006;107:2786–2791. doi: 10.1002/cncr.22351. [DOI] [PubMed] [Google Scholar]
- 20.Gates EJ, Hirschfield L, Matthews RP, Yap OWS. Body mass index as a prognostic factor in endometrioid adenocarcinoma of the endometrium. J Natl Med Assoc. 2006;98:1814. [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson B, Connor JP, Andrews JI, Davis CS, Buller RE, Sorosky JI, et al. Obesity and prognosis in endometrial cancer. Am J Obstet Gynecol. 1996;174:1171–1178. doi: 10.1016/s0002-9378(96)70659-2. discussion 1178–1179. [DOI] [PubMed] [Google Scholar]
- 22.Everett E, Tamimi H, Greer B, Swisher E, Paley P, Mandel L, et al. The effect of body mass index on clinical/pathologic features, surgical morbidity, and outcome in patients with endometrial cancer. Gynecol Oncol. 2003;90:150–157. doi: 10.1016/s0090-8258(03)00232-4. [DOI] [PubMed] [Google Scholar]
- 23.Jeong NH, Lee JM, Lee JK, Kim JW, Cho CH, Kim SM, et al. Role of body mass index as a risk and prognostic factor of endometrioid uterine cancer in Korean women. Gynecol Oncol. 2010;118:24–28. doi: 10.1016/j.ygyno.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Kodama J, Seki N, Ojima Y, Nakamura K, Hongo A, Hiramatsu Y. Correlation of presenting symptoms and patient characteristics with endometrial cancer prognosis in Japanese women. Int J Gynaecol Obstet. 2005;91:151–156. doi: 10.1016/j.ijgo.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Temkin SM, Pezzullo JC, Hellmann M, Lee YC, Abulafia O. Is body mass index an independent risk factor of survival among patients with endometrial cancer? Am J Clin Oncol. 2007;30:8–14. doi: 10.1097/01.coc.0000236047.42283.b8. [DOI] [PubMed] [Google Scholar]
- 26.Munstedt K, Wagner M, Kullmer U, Hackethal A, Franke FE. Influence of body mass index on prognosis in gynecological malignancies. Cancer Causes Control. 2008;19:909–916. doi: 10.1007/s10552-008-9152-7. [DOI] [PubMed] [Google Scholar]
- 27.Studzinski Z, Zajewski W. Factors affecting the survival of 121 patients treated for endometrial carcinoma at a Polish hospital. Arch Gynecol Obstet. 2003;267:145–147. doi: 10.1007/s00404-001-0288-x. [DOI] [PubMed] [Google Scholar]
- 28.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 29.Foley K, Lee RB. Surgical complications of obese patients with endometrial carcinoma. Gynecol Oncol. 1990;39:171–174. doi: 10.1016/0090-8258(90)90427-m. [DOI] [PubMed] [Google Scholar]
- 30.Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, the obesity society. Am J Clin Nutr. 2005;82:923–934. doi: 10.1093/ajcn/82.5.923. [DOI] [PubMed] [Google Scholar]
- 31.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 32.Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev. 2002;11:1531–1543. [PubMed] [Google Scholar]
- 33.Akhmedkhanov A, Zeleniuch-Jacquotte A, Toniolo P. Role of exogenous and endogenous hormones in endometrial cancer. Ann NY Acad Sci. 2001;943:296–315. doi: 10.1111/j.1749-6632.2001.tb03811.x. [DOI] [PubMed] [Google Scholar]
- 34.Blanchard CM, Courneya KS, Stein K. Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society’s SCS-II. J Clin Oncol. 2008;26:2198–2204. doi: 10.1200/JCO.2007.14.6217. [DOI] [PubMed] [Google Scholar]
- 35.Courneya KS, Karvinen KH, Campbell KL, Pearcey RG, Dundas G, Capstick V, et al. Associations among exercise, body weight, and quality of life in a population-based sample of endometrial cancer survivors. Gynecol Oncol. 2005;97:422–430. doi: 10.1016/j.ygyno.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Lynch BM. Sedentary behavior and cancer: a systematic review of the literature and proposed biological mechanisms. Cancer Epidemiol Biomarkers Prev. 2010;19:2691–2709. doi: 10.1158/1055-9965.EPI-10-0815. [DOI] [PubMed] [Google Scholar]
- 37.Moore SC, Gierach GL, Schatzkin A, Matthews CE. Physical activity, sedentary behaviours, and the prevention of endometrial cancer. Br J Cancer. 2010;103:933–938. doi: 10.1038/sj.bjc.6605902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.von Gruenigen VE, Courneya KS, Gibbons HE, Kavanagh MB, Waggoner SE, Lerner E. Feasibility and effectiveness of a lifestyle intervention program in obese endometrial cancer patients: a randomized trial. Gynecol Oncol. 2008;109:19–26. doi: 10.1016/j.ygyno.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 39.Sorosky JI. Endometrial cancer. Obstet Gynecol. 2008;111(2 Pt 1):436–447. doi: 10.1097/AOG.0b013e318162f690. [DOI] [PubMed] [Google Scholar]


