Abstract
Lysophosphatidic acid (LPA) is a ligand for three endothelial differentiation gene family G protein-coupled receptors, LPA1–3. We performed computational modeling-guided mutagenesis of conserved residues in transmembrane domains 3, 4, 5, and 7 of LPA1–3 predicted to interact with the glycerophosphate motif of LPA C18:1. The mutants were expressed in RH7777 cells, and the efficacy (Emax) and potency (EC50) of LPA-elicited Ca2+ transients were measured. Mutation to alanine of R3.28 universally decreased both the efficacy and potency in LPA1–3 and eliminated strong ionic interactions in the modeled LPA complexes. The ala-nine mutation at Q3.29 decreased modeled interactions and activation in LPA1 and LPA2 more than in LPA3. The mutation W4.64A had no effect on activation and modeled LPA interaction of LPA1 and LPA2 but reduced the activation and modeled interactions of LPA3. The R5.38A mutant of LPA2 and R5.38N mutant of LPA3 showed diminished activation by LPA; however, in LPA1 the D5.38A mutation did not, and mutation to arginine enhanced receptor activation. In LPA2, K7.36A decreased the potency of LPA; in LPA1 this same mutation increased the Emax. In LPA3, R7.36A had almost no effect on receptor activation; however, the mutation K7.35A increased the EC50 in response to LPA 10-fold. In LPA1–3, the mutation Q3.29E caused a modest increase in EC50 in response to LPA but caused the LPA receptors to become more responsive to sphingosine 1-phosphate (S1P). Surprisingly micromolar concentrations of S1P activated the wild type LPA2 and LPA3 receptors, indicating that S1P may function as a weak agonist of endothelial differentiation gene family LPA receptors.
Lysophosphatidic acid (LPA)2 and sphingosine 1-phosphate (S1P) are structurally related lysophospholipid growth factors that mediate a variety of cellular effects, including regulation of cellular proliferation, survival, migration, and morphology (1–3). LPA has been shown to play an important role in a variety of diseases including ovarian cancer, prostate cancer, breast cancer, and cardiovascular disease (4–14). Many of the biological effects of LPA are mediated through cell surface receptors of the endothelial differentiation gene (EDG) family of G protein-coupled receptors (GPCRs).
The EDG family of GPCRs includes eight closely related genes that show the conserved GPCR topology of an extracellular amino terminus followed by seven α-helical transmembrane domains (TMs) (15). Three of these genes (LPA1–3) are cellular receptors for LPA and share 55% overall homology in humans. The other five (S1P1–5) are cellular receptors for S1P and share 50% homology in humans. The two subclusters are 35% homologous with each other. The transmembrane domains of human LPA1–3 where ligand binding takes place show 81% homology with each other. LPA has also been shown to elicit cellular responses through binding to three non-EDG family GPCRs, p2y9/LPA4, GPR92/LPA5, and GPR87/LPA6, which are more closely related to the purinoreceptor cluster of GPCRs (16–19).
Modeling and mutagenesis studies of S1P receptors in the EDG family have demonstrated that conserved residues can play either conserved or non-conserved roles in different family members. A validated computational model of S1P1 was developed that successfully identified residues in TM3 and TM7 of S1P1 that participated in ligand binding. A critical role for residues R3.28, E3.29, and R7.34 of S1P1 in ligand binding and receptor activation was experimentally confirmed using a site-directed mutagenesis strategy (20). Later studies determined that in S1P4 the residues R3.28, E3.29, W4.64, and K5.38 were critical for ligand binding and receptor activation (21), whereas K5.38 was not essential in S1P1(22). Based upon the high sequence homology within the EDG family, the experimentally validated S1P1 model was used as a template to map the ligand-binding pocket of the LPA-specific EDG receptors. Computational modeling predicted that the residues R3.28, Q3.29, R5.38, and K7.35 of LPA3 form critical interactions with the polar head group of LPA, and this was confirmed experimentally (23). These previous studies suggest a conserved and essential role for R3.28 in all receptors examined so far but a variable role for K5.38 in two family members.
Position 3.29, which is conserved as a glutamine in LPA-specific EDG receptors and a glutamate in S1P-specific EDG receptors, was computationally identified and experimentally validated as a key residue that determines receptor selectivity for LPA or S1P in the S1P1 and LPA1 receptor pair (24). The E3.29Q mutant of S1P1 responded to LPA rather than S1P; the reciprocal Q3.29E mutation in LPA1 showed diminished activation by LPA but was activated by S1P, indicating the involvement of additional residues in ligand recognition. Alanine mutation at this position diminished activation by either ligand in both receptors (24). Similarly the E3.29Q mutation in S1P4 conferred responsiveness to LPA but decreased responsiveness to S1P (25).
Most cell types express multiple EDG receptor subtypes (26). The role of the different LPA receptor subtypes in physiological and pathophysiological processes is often difficult to determine becauseofthelackofLPAreceptorsubtype-specificreagents.Sub-type-specific agonists and antagonists could elucidate the role of the different LPA receptor subtypes in physiological and disease states as well as function as lead compounds in drug development. To aid in the development of subtype-specific reagents, it is important to identify differences between the EDG family LPA receptor subtypes in the ligand-binding pocket.
The fundamental assumption underlying homology modeling and comparative sequence analysis is that identical residues fulfill the same role in homologous proteins. Given the very high degree of sequence identity, especially in the transmembrane domains of the EDG receptors, one would hypothesize that the function of those residues validated to play a role in ligand recognition applies universally within the family. The variable importance of K5.38 in the S1P1 and S1P4 receptors, however, suggests that this assumption is not always accurate (21, 22). In the present study we carried out a comparative analysis of the conserved key residues experimentally validated to be involved in ligand recognition in one or another LPA-or S1P-specific EDG family receptor to find that many of these head group-interacting residues play different roles. We extended our analysis to include all three of the LPA-specific EDG receptors and generated mutations at sites that are computationally predicted to impact ligand recognition: R3.28, Q3.29, W4.64, D/R5.38, K7.35, and K/R7.36. We determined the effect of each mutation upon the potency (EC50) and efficacy (Emax) of LPA relative to the activation elicited in the wild type receptors. We also evaluated the impact of some of these mutations on receptor activation by the related lipid mediator S1P. Experimental results were correlated to predictions based upon computational modeling of the wild type and mutant receptors docked with ligand. These studies reveal that major differences exist between the different LPA receptor subtypes in the functional utilization of several conserved residues in the predicted ligand-binding pocket. Only one residue when mutated to alanine identically impacted the three receptor subtypes; the mutation R3.28A universally reduced both the efficacy and potency in LPA1–3 and eliminated strong ionic interactions in the modeled LPA complexes. The different roles that conserved residues can play among the highly homologous members of the EDG family provide insight into nature’s diverse answers for high affinity molecular recognition and challenge the concept that automatically assigns identical function to homologous residues.
EXPERIMENTAL PROCEDURES
Reagents—All analogs of LPA and S1P were purchased from Avanti Polar Lipids (Alabaster, AL). Lipids were prepared before use as a 1 mm stock in PBS containing 1 mm charcoal-stripped bovine serum albumin (BSA). Alexa Fluor 488-conjugated goat anti-mouse IgG was purchased from Molecular Probes (Eugene, OR). Anti-FLAG M2 monoclonal antibody was purchased from Sigma.
Residue Nomenclature—Amino acids in the TMs were assigned index positions by the method of Ballesteros and Weinstein (27) based upon homology found in the seven helical TMs of GPCRs. Index positions are in the format X.YY where X refers to the number of the TM in which that residue is found and YY refers to the position within that TM relative to the most highly conserved residue in that TM throughout the GPCR superfamily, which is arbitrarily designated position 50 (27).
Computational Homology Modeling—Previously developed computational models of LPA1, LPA2, and LPA3(23, 28) were used for mutation studies. LPA C18:1 was docked into each receptor with a −2 charge because previous quantum mechanical studies suggest that is appropriate for phospholipid binding sites with multiple cationic residues (22). Docking studies were done using Autodock 3.0 (29). Default docking parameters were used except for number of runs (15), energy evaluations (9.0 × 1010), generations (30,000), and local search iterations (3000). The complex with the greatest number of cationic interactions with the LPA phosphate group was chosen and subjected to molecular dynamics simulations using a 1-fs time step at 500 ps. The lowest energy structure from the simulation was geometry-optimized and used as the wild type receptor for mutation studies. Mutation studies were done as described previously (22). Mutant models were generated by amino acid side chain replacement. Each mutant was modeled with LPA bound. The models were refined using the MOE (Molecular Operating Environment) software (version 2004.03, Chemical Computing Group, Montreal, Canada). The models were subjected to molecular dynamics and geometry optimization. The MMFF94 force field (30) was used for all force field simulations. Default parameters for molecular dynamics simulations were used with the exception of the total simulation length, which was 1 ns. LPA was removed from each mutant receptor and docked back into the receptor using Autodock 3.0. The best LPA complex with each mutant was selected as the one with the most cationic interactions with the phosphate group.
Site-directed Mutagenesis—Amino-terminal FLAG epitope-tagged LPA1, LPA2, and LPA3 receptor constructs were sub-cloned into pcDNA3.1 vector (Invitrogen). Receptor constructs were mutated at residues computationally predicted to participate in ligand recognition using the QuikChange II XL site-directed mutagenesis kit (Stratagene, La Jolla, CA). In some cases, a PCR-based site-directed mutagenesis strategy was used to generate the desired mutation as described previously (23). TOP10 competent cells (Invitrogen) were transformed with the mutant constructs, and clones were verified by complete sequencing of the inserts.
Cell Culture and Transfection—LPA does not elicit Ca2+ transients in the parental McArtl rat hepatoma 7777 (RH7777) cells (31) (ATCC, Manassas, VA). RH7777 cells and rat hepatoma HTC4 cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% (v/v) fetal bovine serum, 100 units/ml penicillin, 10 μg/ml streptomycin, and 2 mm glutamine. RH7777 cells stably expressing LPA1 and LPA3 receptors have been characterized elsewhere (32). RH7777 cells stably expressing LPA2 were a generous gift from Dr. Fumikazu Okajima (Gunma University, Gunma, Japan) and were characterized previously (33). Stable transfectants were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% (v/v) fetal bovine serum, 100 units/ml penicillin, 10 μg/ml streptomycin, 2 mm glutamine, and 250 μg/ml G418. Transient transfections of RH7777 cells and HTC4 cells were performed using Effectene transfection reagent (Qiagen, Valencia, CA).
Flow Cytometric Analysis—Expression of all receptor constructs on the cell surface was confirmed by flow cytometric analysis using indirect immunofluorescence staining with anti-FLAG M2 antibody. RH7777 cells were transfected with FLAG epitope-tagged LPA receptor constructs, replated after 16 h, and cultured for an additional 24 h. The culture medium was replaced with Krebs buffer (120 mm NaCl, 5 mm KCl, 0.62 mm MgSO4, 1.8 mm CaCl2, 6 mm glucose, 10 mm HEPES, pH 7.4) for 4 h before collection; cells were detached using HyQTase Cell Detachment Solution (Hyclone Laboratories) and collected on ice. Cells were washed with PBS that contained 3% BSA and incubated for 30 min in PBS that contained 5% BSA and 5% normal donkey serum. The cells were washed with PBS that contained 3% BSA, incubated with anti-FLAG M2 monoclonal antibody (1:200) in PBS containing 5% BSA for 1 h followed by two washes in PBS with 3% BSA, and incubated with Alexa Fluor 488-conjugated goat anti-mouse IgG (1:1000) in PBS that contained 5% BSA for 30 min. Cells were washed two times with PBS that contained 3% BSA and resuspended in PBS that contained 1% BSA. Cells were analyzed using an LSR II flow cytometer (BD Biosciences), and data were analyzed using FlowJo software.
Receptor Activation Assays—FLAG-tagged LPA1, LPA2, and LPA3 receptor constructs were transiently expressed in LPA-nonresponsive RH7777 cells using Effectene transfection reagent (Qiagen). Cells were replated in poly-l-lysine-coated 96-well microplates 16 h after transfection at a density of 30,000 cells/well and cultured for 24 h. The culture medium was replaced with Krebs buffer for 4–6 h before assays. The transfected cells were loaded with Fura-2/AM in Krebs buffer containing 0.001% pluronic acid for 30 min and rinsed with Krebs buffer, and the Ca2+ response to LPA C18:1 or S1P was measured using a FlexStation II fluorescence plate reader (Molecular Devices, Sunnyvale, CA). The ratio of peak emissions at 510 nm after 2 min of ligand addition was determined for excitation wavelengths of 340/380 nm. All samples were run in triplicate, and assays were performed at least three times for each receptor construct. The responses to LPA by the wild type and mutant receptors were measured and reported in terms of maximal activation (Emax) and efficacy (EC50) ± S.D.
Radioligand Binding Assay—HEK293T cells were plated in 24-well plates at 4 × 105/well and the following day transiently transfected with 0.4 μg of receptor constructs using Lipofectamine 2000 (Invitrogen). Two days later cells were washed with ice-cold binding buffer (50 mm Tris, pH 7.4, 150 mm NaCl). Cells were then incubated in binding buffer containing 4 mg/ml fatty acid-free BSA and [32P]S1P ranging from 10 nm to 1 μm in the presence or absence of 10 μm unlabeled S1P as a competitor on ice for 45 min. After washing twice with cold binding buffer containing 0.4 mg/ml BSA, cells were lysed in 0.5% SDS, and binding was quantified by scintillation counting. Triplicate samples were measured for each condition.
Receptor Internalization Assays—RH7777 cells were transiently transfected with FLAG-tagged LPA2 using Effectene transfection reagent. Cells were serum-starved for 4 and then incubated with a 10 μm concentration of either LPA, S1P, ATP, or vehicle for 30 min at 37 °C before collection on ice and subsequent anti-FLAG flow cytometric analysis. The assay was repeated three times with similar results.
RESULTS
Theoretical Models of LPA1, LPA2, and LPA3: Mutation Site Selection—In LPA1–3, we evaluated the effect of alanine mutation of residues in TM3, TM4, TM5, and TM7 that were computationally predicted to impact binding to LPA C18:1. The summary of the computationally predicted ionic ligand-receptor interactions in the wild type receptors as well as in the mutants is summarized in Table 1. The number of interactions that were predicted to occur over distances of less than 4.5 Å between the polar head group of LPA and charged residues in TM3, TM5, and TM7 varied between the receptors and were two, two, and three for LPA1, LPA2, and LPA3, respectively. Mutation of R3.28 to alanine universally diminished these interactions in each receptor model. Mutation of other residues had variable impact on the number of ionic interactions with the alanine mutants of the five residues R3.28, Q3.29, W4.64, D/R5.38, K7.35, and K7.36 we examined (Table 1).
TABLE 1.
The computationally predicted number of close charge-charge interactions between LPA receptor constructs and LPA
The number of interactions that occur over distances of less than 4.5 Å between the polar head group of LPA and nitrogen atoms of charged residues in TM3, TM5, and TM7 was calculated based upon computational models. NA, not applicable; the residue to be mutated does not occur or that mutation was not made in that receptor.
| Construct | LPA1 | LPA2 | LPA3 |
|---|---|---|---|
| Wild type | 2 | 2 | 3 |
| R3.28A | 0 | 0 | 0 |
| Q3.29E | 1 | 2 | 0 |
| Q3.29A | 0 | 0 | 3 |
| W4.64A | 1 | 2 | 0 |
| D5.38A | 2 | NA | NA |
| D5.38R | 2 | NA | NA |
| R5.38A | NA | 1 | 0 |
| R5.38N | NA | NA | 0 |
| K7.36A | 1 | 2 | NA |
| R7.36A | NA | NA | 3 |
| K7.35A | NA | NA | 0 |
Mutation and Cell Surface Expression of LPA1, LPA2, and LPA3 Receptor Constructs—To validate and refine our computational models of the ligand head group-binding pocket of the EDG family LPA receptors, we generated alanine point mutants of amino-terminal FLAG epitope-tagged LPA1, LPA2, and LPA3 receptor constructs at residues in TM3, TM4, TM5, and TM7 that were computationally identified to surround the glycerophosphate portion of LPA C18:1 in the wild type LPA1–3 complexes. Additionally in LPA1, LPA2, and LPA3 Q3.29 was also mutated to glutamate, the residue occurring at this position throughout the S1P receptors in the EDG receptor family; previously this mutation was shown to change the ligand specificity of LPA1 from LPA to S1P (24).
Expression and surface targeting of the receptor constructs was confirmed by indirect immunofluorescence flow cytometric analysis using antibodies directed against the amino-terminal FLAG epitope present in the constructs (Table 2). All receptor constructs showed targeting to the cell surface when transiently transfected into RH7777 cells except for the R5.38A mutant of LPA3, which was not expressed at a detectable level. However, the asparagine mutant R5.38N of LPA3 did show cell surface expression and was used instead of R5.38A in our studies (23).
TABLE 2.
Cell surface expression of wild type and mutant LPA1–3 receptor constructs determined by flow cytometry
Surface expression of receptors was measured by flow cytometry using anti-FLAG epitope antibody staining in RH7777 cells transiently transfected with wild type or mutant LPA1–3 receptor constructs. Cells transfected with pcDNA3.1 vector showed 5.0% positive staining. NA, not applicable; the residue to be mutated does not occur or that mutation was not made in that receptor.
| Construct | Anti-FLAG-stained cells, percentage of total cells | ||
|---|---|---|---|
|
| |||
| LPA1 | LPA2 | LPA3 | |
| % | |||
| Wild type | 42.1 | 55.2 | 18.1 |
| R3.28A | 51.6 | 66.1 | 39.7 |
| Q3.29E | 48.6 | 27.4 | 15.0 |
| Q3.29A | 11.9 | 20.5 | 19.9 |
| W4.64A | 43.8 | 47.6 | 17.6 |
| D5.38A | 54.7 | NA | NA |
| D5.38R | 53.0 | NA | NA |
| R5.38A | NA | 10.7 | 6.2 |
| R5.38N | NA | NA | 13.2 |
| K7.36A | 54.7 | 41.7 | NA |
| R7.36A | NA | NA | 38.3 |
| K7.35A | NA | NA | 32.3 |
Some variability in percentage of cells expressing the receptor mutants on their surface was noted among the FLAG-tagged receptor constructs. Although the LPA1, LPA2, and LPA3 receptor constructs that we used were all subcloned into the pcDNA3.1 vector, surface expression tended to be higher for LPA1 and LPA2 constructs than for LPA3 constructs (Table 2). This variation was possibly due to receptor subtype-specific differences in processing, cell surface targeting, and/or stability; however, these variances in receptor surface expression levels did not correlate with absolute measurements of maximal receptor activation. The absolute values of our ratiometric measurements of LPA-induced calcium mobilization were consistently higher by ∼2-fold for cells transfected with wild type LPA3 than for cells transfected with wild type LPA1 or LPA2 despite the lower surface expression of the LPA3 (Table 2). This lack of correlation between surface expression and maximal receptor activation may reflect an excess of receptor expression relative to the endogenous G proteins that couple to the activated receptors to mediate calcium mobilization as well as different coupling efficiencies among the EDG family receptor subtypes for these G proteins. Indeed in our transient transfection system, heterologous expression of S1P1 is insufficient to cause calcium mobilization in response to S1P unless G16 is cotransfected (data not shown).
Variation in surface expression was also noted among several mutant constructs compared with the wild type receptors (Table 2). For LPA1 constructs, cells transfected with Q3.29A showed low (11.9% of cells) surface expression compared with cells transfected with wild type (42.1%) likely due to diminished cell surface targeting or stability of this construct. In the case of LPA2, surface expression levels of two mutants, Q3.29A (20.5%) and R5.38A (10.7%), were less than half of the level measured for the wild type receptor (55.2%). In LPA3, surface expression of R5.38A was not detected above background (6.2% compared with 5.0% for empty vector-transfected cells); the R5.38N mutant did show cell surface expression although somewhat less than the wild type receptor (13.2 versus 18.1% for wild type).
Because of the relatively high expression levels of the receptors in our transient transfection system, the variation in cell surface expression of the different constructs should have only minor effects on receptor activation as measured in our calcium mobilization assays. In particular the measure of potency (EC50) was not expected to vary much with receptor levels because this is a measurement that reflects the affinity of the ligand for the receptor and is the most informative measured parameter in our assays as far as indicating how mutation of a residue affects ligand recognition.
To assess the impact of LPA receptor surface expression levels on potency and efficacy of the measured LPA response, we transfected RH7777 cells with different ratios of FLAG-tagged LPA1 to vector (pcDNA3.1). Transfection of RH7777 cells with LPA1 diluted with different amounts of vector showed that the variations we observed in our wild type and mutant constructs should have no effect on potency and only a minor effect on efficacy (Table 3). The measured EC50 was not significantly affected by up to 20-fold dilution of the LPA1 plasmid; the measured Emax showed some decrease with dilution of receptor by vector but still retained 51% of Emax when diluted 20-fold with empty vector plasmid (Table 3).
TABLE 3.
The effect of receptor expression levels on potency (EC50) and maximal response (Emax) to LPA
EC50 values (mean ± S.D.) and Emax values were determined in RH7777 cells transiently transfected with different ratios of FLAG-LPA1 to vector (pcDNA3.1). Emax values are expressed relative to the maximal response for Ca2+ mobilization measured in cells transfected with 100% FLAG-LPA1 plasmid.
| LPA1:vector (percentage of LPA1) | EC50 ± S.D. | Relative Emax | Percentage of anti-FLAG-stained cells |
|---|---|---|---|
| nm | % | % | |
| 1:0 (100%) | 145 ± 29 | 100 | 34.5 |
| 1:1 (50%) | 107 ± 37 | 95 | 24.3 |
| 1:4 (20%) | 180 ± 77 | 72 | 20.9 |
| 1:19 (5%) | 105 ± 46 | 51 | 12.0 |
| 0:1 (0%) | No activation | No activation | 5.0 |
Effect of Point Mutations on Receptor Activation—We evaluated the impact of each mutation on the potency (EC50) and efficacy (Emax) elicited by LPA as measured by Ca2+ mobilization in transiently transfected RH7777 cells. The effects of these mutations on the pharmacological properties of the receptors are summarized in Table 4.
TABLE 4.
Properties of LPA receptors and mutants designed to alter LPA head group interaction
EC50 values (mean ± S.D., n = 3) and Emax values were determined in RH7777 cells transiently expressing LPA receptor constructs. 100% represents the maximal response for Ca2+ mobilization of the wild type receptor activated by LPA C18:1. NA, not applicable; the residue to be mutated does not occur or that mutation was not made in that receptor. NT, not tested because cell surface expression was not detected.
| Construct | EC50 ± S.D. (nm), Emax (percentage of wild type) | Nature of amino acid replacement | ||
|---|---|---|---|---|
|
| ||||
| LPA1 | LPA2 | LPA3 | ||
| Wild type | 186 ± 59, 100% | 9 ± 1, 100% | 57 ± 11, 100% | None |
| R3.28A | >1000, 71%a | >1000, 76%b | >1000, 42%a | Positive to nonpolar |
| Q3.29E | 353 ± 74, 76% | 31 ± 7, 100% | 218 ± 52, 98% | Polar to negative |
| Q3.29A | Not activated | 37 ± 14, 51% | 74 ± 23, 76% | Polar to nonpolar |
| W4.64A | 219 ± 51, 97% | 11 ± 2, 98% | 460 ± 66, 71% | Aromatic to nonpolar |
| D5.38A | 223 ± 29, 98% | NA | NA | Negative to nonpolar |
| D5.38R | 149 ± 27, 132% | NA | NA | Negative to positive |
| R5.38A | NA | 98 ± 18, 90% | NT | Positive to nonpolar |
| R5.38N | NA | NA | 476 ± 104, 84% | Positive to polar |
| K7.36A | 292 ± 70, 156% | 439 ± 58, 117%b | NA | Positive to nonpolar |
| R7.36A | NA | NA | 56 ± 12, 121% | Positive to nonpolar |
| K7.35A | NA | NA | 598 ± 105, 86% | Positive to nonpolar |
Maximal activation that was observed with 10 μm LPA.
Maximal activation that was observed with 3 μm LPA.
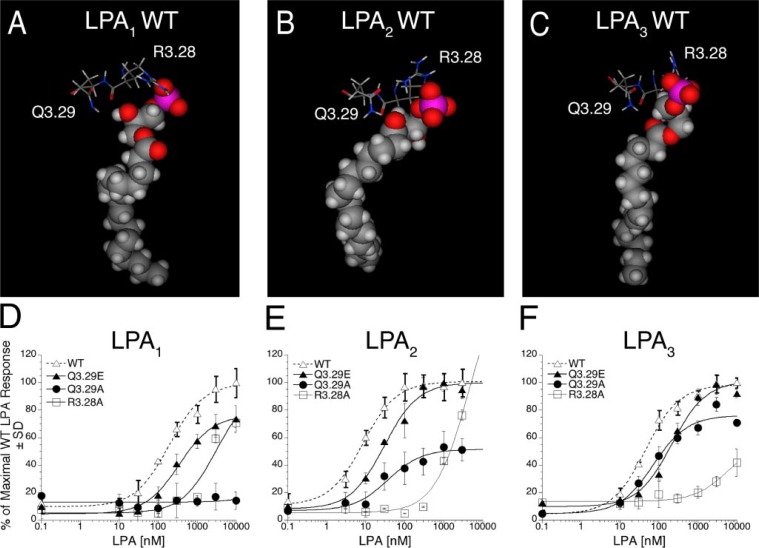
Characteristics of LPA1, LPA2, and LPA3 Receptor Mutations at Strictly Conserved Sites—Three strictly conserved residues surround the glycerophosphate head group of LPA in the modeled LPA receptor complexes: R3.28, Q3.29 (Fig. 1, A–C), and W4.64 (Fig. 2, A–C). Alanine mutants at only one of these sites showed a universal effect across the three receptors. Modeled complexes of LPA with the R3.28A mutant of all three receptors showed a complete lack of close ionic interactions (Table 1). Likewise the LPA-induced Ca2+ responses in the R3.28A mutants showed right-shifted dose-response curves with EC50 values >1000 nm and Emax values less than 80% at the highest concentrations tested at each receptor (Fig. 1, D–F, and Table 4).
FIGURE 1.
Models of the receptor-ligand complex and the effect of mutations in TM3 of LPA1, LPA2, and LPA3 on LPA-induced receptor activation. Models are shown of LPA C18:1 docked in wild type (WT) LPA1 (A), LPA2 (B), and LPA3 (C). Amino acids from TM3 mutated in this study are shown as stick figure models; space-filling models are used to represent LPA C18:1. Intracellular Ca2+ transients were measured in response to LPA in RH7777 cells transiently transfected with mutated or wild type LPA1 (D), LPA2 (E), or LPA3 (F). 100% represents the maximal Ca2+ response to LPA in the wild type receptor. Samples were run in triplicate, and the mean ± S.D. (n = 3) was plotted. The data are representative of at least three independent experiments.
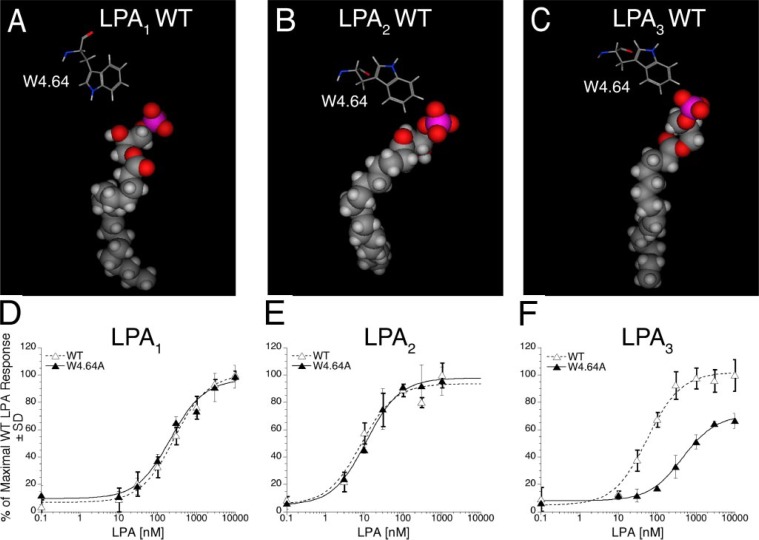
FIGURE 2.
Models of the receptor-ligand complex and the effect of mutation in TM4 of LPA1, LPA2, and LPA3 on LPA-induced receptor activation. Models are shown of LPA C18:1 docked in wild type (WT) LPA1 (A), LPA2 (B), and LPA3 (C). Amino acids from TM4 mutated in this study are shown as stick figure models; space-filling models are used to represent LPA C18:1. Intracellular Ca2+ transients were measured in response to LPA in RH7777 cells transiently transfected with mutated W4.64A or wild type LPA1 (D), LPA2 (E), or LPA3 (F). 100% represents the maximal Ca2+ response to LPA in the wild type receptor. Samples were run in triplicate, and the mean ± S.D. (n = 3) was plotted. The data are representative of at least three independent experiments.
Receptor-dependent effects were observed for mutations at positions Q3.29 and W4.64. Modeled complexes of LPA with the Q3.29A mutant showed a lack of ionic interactions in the LPA1 and LPA2 receptors but retention of ionic interactions in the LPA3 receptor (Table 1). The Q3.29A mutation produced a more pronounced decrease in activation in LPA1 and LPA2 than in LPA3 (Fig. 1, D–F, and Table 4). In LPA3 alanine replacement shifted the EC50 from 57 ± 11 to 76 ± 23 nm while reducing Emax by 34%. In LPA2, replacement of Q3.29 with alanine increased the EC50 4-fold and decreased the Emax by 49%. The Q3.29A mutant of LPA1 showed no activation. The Q3.29E mutation, which changes a glutamine residue conserved in LPA-specific EDG receptors to a glutamate that is conserved in S1P-specific EDG receptors, decreased the potency of LPA in all three receptor subtypes with the largest potency shift occurring in the LPA3 receptor (Fig. 1, D–F, and Table 4). This is reflected in the LPA complexes, which show ionic interactions comparable to wild type in the LPA1 and LPA2 receptors, but absent in the LPA3 receptor (Table 1). The Q3.29E mutation decreased the LPA-induced maximal activation of LPA1 by 24% but had negligible effect on the Emax of LPA2 and LPA3. The impact of the mutation W4.64A in LPA1, LPA2, and LPA3 on Ca2+ mobilization in transiently transfected RH7777 cells is shown in Fig. 2, D–F. This mutation had no impact on the activation of LPA1 and LPA2; however, in LPA3 this mutation decreased the potency (460 ± 66 versus 57 ± 11 nm for wild type) and maximal activation (71% of wild type Emax) of receptor activation by LPA (Table 4). This result corresponds to the observed impact of mutating W4.64 to ala-nine in the receptor models (Fig. 2, A–C). Ionic interactions similar to wild type were observed in LPA1 and LPA2 but were completely absent in LPA3 (Table 1).
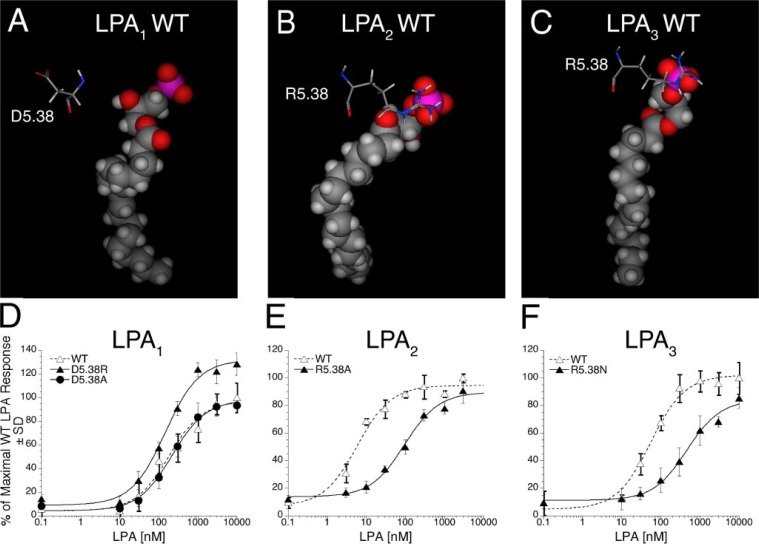
Characteristics of LPA1, LPA2, and LPA3 Receptor Mutations at Partially Conserved Sites—The charged residue at the top of TM5 varies in the three receptors (Fig. 3, A–C). LPA1 contains aspartic acid at position 5.38, whereas LPA2 and LPA3 both have a cationic amino acid, arginine. The R5.38A mutant of LPA2 showed a decrease in the number of close cationic interactions as did the R5.38N mutant of LPA3 (Table 1). Both are expected to show increases in EC50 values, whereas the alanine mutation of the anionic D5.38 in LPA1 is unlikely to be detrimental. We evaluated the impact of mutations in TM5 on receptor activation in transiently transfected RH7777 cells. The mutations R5.38A in LPA2 and R5.38N in LPA3 strongly decreased receptor activation, increasing EC50 by 11-and 8-fold, respectively; however, the alanine mutation of the corresponding position in LPA1, D5.38A, had no impact on receptor activation by LPA (Fig. 3, D–F, and Table 4). The mutation D5.38R in LPA1, which replaces an aspartate residue in LPA1 with an arginine residue that occurs at this position in LPA2 and LPA3, showed enhanced activation by LPA relative to wild type LPA1, shifting the Emax to 132% of wild type LPA1 and decreasing EC50 from 186 to 149 nm (Table 4).
FIGURE 3.
Models of the receptor-ligand complex and the effect of mutations in TM5 of LPA1, LPA2, and LPA3 on LPA-induced receptor activation. Models are shown of LPA C18:1 docked in wild type (WT) LPA1 (A), LPA2 (B), and LPA3 (C). Amino acids from TM5 mutated in this study are shown as stick figure models; space-filling models are used to represent LPA C18:1. Intracellular Ca2+ transients were measured in response to LPA in RH7777 cells transiently transfected with mutated or wild type LPA1 (D), LPA2 (E), or LPA3 (F). 100% represents the maximal Ca2+ response to LPA in the wild type receptor. Samples were run in triplicate, and the mean ± S.D. (n = 3) was plotted. The data are representative of at least three independent experiments.
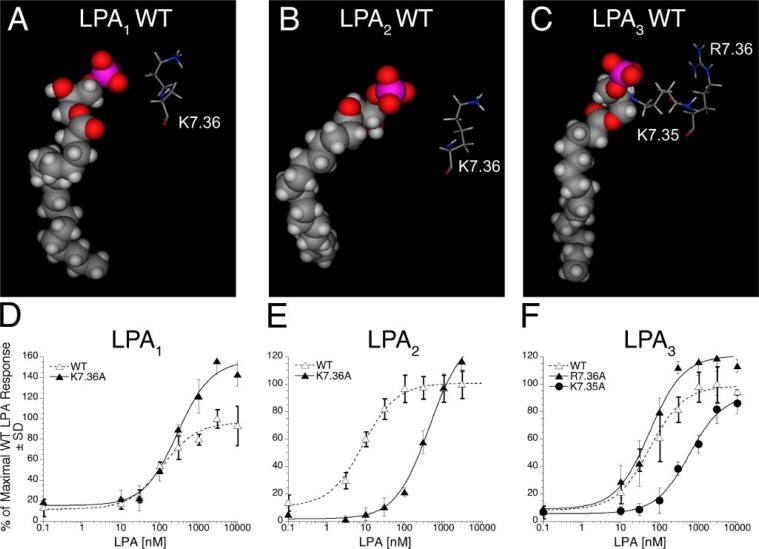
In TM7, LPA1 and LPA2 both contain lysine at position 7.36, which is predicted to be oriented away from the phosphate-binding pocket (Fig. 4, A and B). The modeled alanine mutations at this position show similar interactions between the LPA phosphate group and the cationic residues in the receptor (Table 1), suggesting that EC50 values should be similar to wild type values. The K7.36A mutant of LPA1 showed enhanced maximal activation by LPA (Fig. 4D), whereas the K7.36A mutant of LPA2 showed diminished activation by LPA, causing a 49-fold increase in EC50 (Fig. 4E and Table 4). This apparent inconsistency between model-derived hypothesis and experimental result may result from either incorrect position of K7.36 in the LPA2 receptor model or an indirect role for K7.36. The K7.36 residue in the LPA2 model forms an ion pair with D1.32. Mutation of K7.36 in LPA2 may therefore have an impact on overall receptor structure that our 1-ns molecular dynamics simulations are not long enough to capture. LPA3 contains two cationic amino acids in TM7, R7.36 and K7.35 (Fig. 4C). Computational modeling of the K7.35A LPA3 mutant showed a loss of all cationic interactions with the LPA phosphate group, whereas the R7.36A mutant was predicted to retain the three cationic interactions with the LPA phosphate group observed in the wild type receptor (Table 1). An EC50 increase is expected only for the K7.35A LPA3 mutant. The alanine mutation of R7.36A did not diminish receptor activation by LPA; however, alanine mutation of the adjacent residue, K7.35A, diminished activation of LPA3 by LPA, shifting the EC50 from 57 ± 11 to 598 ± 105 nm (Fig. 4F and Table 4).
FIGURE 4.
Models of the receptor-ligand complex and the effect of mutations in TM7 of LPA1, LPA2, and LPA3 on LPA-induced receptor activation. Models are shown of LPA C18:1 docked in wild type (WT) LPA1 (A), LPA2 (B), and LPA3 (C). Amino acids from TM7 mutated in this study are shown as stick figure models; space-filling models are used to represent LPA C18:1. Intracellular Ca2+ transients were measured in response to LPA in RH7777 cells transiently transfected with mutated or wild type LPA1 (D), LPA2 (E), or LPA3 (F). 100% represents the maximal Ca2+ response to LPA in the wild type receptor. Samples were run in triplicate, and the mean ± S.D. (n = 3) was plotted. The data are representative of at least three independent experiments.
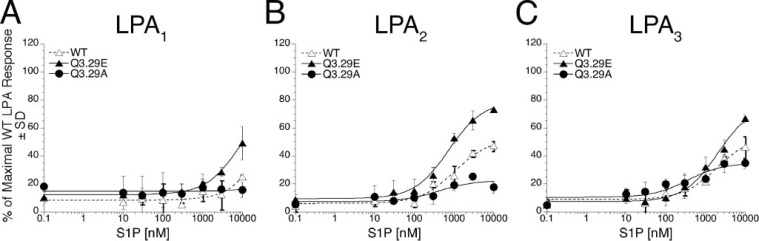
Impact of TM3 Mutations on Ligand Selectivity Between LPA and S1P—Residue 3.29 is a conserved glutamine in LPA-specific EDG receptors and a glutamate in S1P-specific EDG receptors. We previously reported that the Q3.29E mutant of LPA1 showed diminished activation by LPA but gained responsiveness to S1P; the reciprocal E3.29Q mutant of S1P1 responded to LPA rather than S1P (24). We evaluated the effects of Q3.29A and Q3.29E mutants on activation by S1P in LPA1–3 as measured by Ca2+ mobilization in transiently transfected RH7777 cells; the results of these experiments are summarized in Table 5. S1P did not activate the Q3.29A mutant of LPA1. Wild type LPA1 showed only weak activation at the highest S1P concentration tested (10 μm), but the Q3.29E mutant was activated by 10 μm S1P to 39% of the maximal LPA-induced Ca2+ response (Fig. 5A). Unexpectedly expression of wild type LPA2 and LPA3 receptors allowed the RH7777 cells to be activable by S1P. Wild type LPA2 was activated by as little as 300 nm S1P, and a 10 μm concentration yielded 43% of the maximal LPA-induced Ca2+ response. This response was enhanced in the Q3.29E LPA2 mutant, which was activated by 10 μm S1P to 64% of the maximal LPA-induced Ca2+ response. The Q3.29A mutant of LPA2 showed almost no activation by S1P (Fig. 5B). Wild type LPA3 was activated by 10 μm S1P to 40% of the LPA-induced maximal LPA-induced Ca2+ response. The mutation Q3.29E enhanced the response to 10 μm S1P to 62% of the LPA-induced maximal LPA-induced Ca2+ response, and the mutation Q3.29A diminished receptor activation in response to S1P to 30% of the LPA-induced maximal LPA-induced Ca2+ response (Fig. 5C).
TABLE 5.
Activation of LPA receptors by S1P
Emax values in response to 10 μm S1P were determined in RH7777 cells transiently expressing LPA receptor constructs. 100% represents the maximal response for Ca2+ mobilization of the wild type receptor activated by LPA C18:1.
| Construct | Emax | ||
|---|---|---|---|
|
| |||
| LPA1 | LPA2 | LPA3 | |
| % | |||
| Wild type | 15 | 43 | 40 |
| Q3.9E | 39 | 64 | 62 |
| Q3.29A | Not activated | 12 | 30 |
FIGURE 5.
The effect of the mutations Q3.29A and Q3.29E of LPA1, LPA2, and LPA3 on S1P-induced receptor activation. Intracellular Ca2+ transients were measured in response to S1P in RH7777 cells transiently transfected with mutated or wild type (WT) LPA1 (A), LPA2 (B), or LPA3 (C). 100% represents the maximal Ca2+ response to LPA in the wild type receptor. Samples were run in triplicate, and the mean ± S.D. (n =3) was plotted. The data are representative of at least three independent experiments.
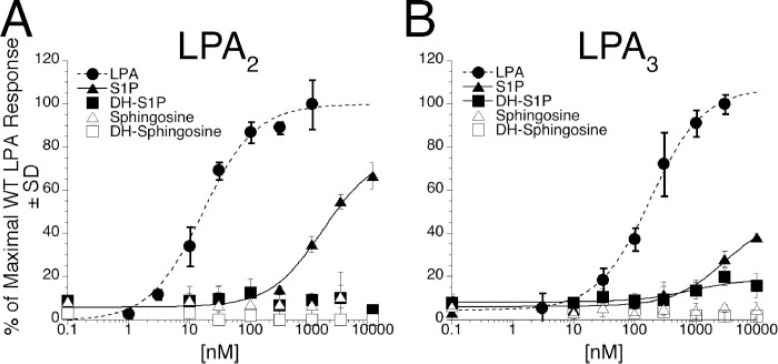
To further investigate the weak agonism we observed of S1P for LPA2 and LPA3, we examined the effect of expression of these receptors on calcium mobilization in response to dihydrosphingosine 1-phosphate (DH-S1P), sphingosine, and dihydrosphingosine as well as in response to S1P (Fig. 6, A and B). Whereas S1P has been reported to release Ca2+ and activate intracellular targets mediating an antiapoptotic response, DH-S1P lacks these effects (34–36). Both LPA2 and LPA3 were completely unresponsive to the non-phosphorylated sphingoid bases sphingosine and dihydrosphingosine, highlighting the importance of the phosphate moiety for receptor recognition. DH-S1P was less potent than S1P in activating LPA3 (Fig. 6A) and was ineffective in activating LPA2 (Fig. 6B), indicating that although both receptors prefer S1P to dihydrosphingosine 1-phosphate, LPA3 may have a greater tolerance for a saturated hydrophobic tail than does LPA2.
FIGURE 6.
The effect of sphingoid bases on LPA2 and LPA3 receptor activation. Intracellular Ca2+ transients were measured in response to S1P, DH-S1P, sphingosine, or dihydrosphingosine (DH-Sphingosine) in RH7777 cells transiently transfected with mutated or wild type (WT) LPA2 (A) or LPA3 (B). 100% represents the maximal Ca2+ response to LPA in the wild type receptor. Samples were run in triplicate, and the mean ± S.D. (n =3) was plotted.
We also examined the Ca2+ response to S1P in clonally derived RH7777 cells, which had been stably transfected with wild type LPA1–3 (supplemental Fig. 1, A–C). LPA3 stable transfectants responded to as little as 1 μm S1P with a measurable Ca2+ response, whereas the LPA2 stable transfectants required 3 μm S1P to elicit a response; LPA1 stable transfectants barely responded to S1P except at the highest (10 μm) concentration tested. Cells stably expressing LPA1, LPA2, and LPA3 were activated by 10 μm S1P to 25, 28, and 44% of the maximal LPA-induced Ca2+ response, respectively. The relative responsiveness to S1P conferred by expression of LPA1, LPA2, or LPA3 differed somewhat from the results we observed in transiently transfected cells, perhaps reflecting the lower expression levels or clonally derived nature of the stable transfectants. Non-transfected RH7777 cells treated with S1P or LPA were completely unresponsive in our assays (supplemental Fig. 1D). To confirm that S1P responsiveness in LPA2-transfected cells is not limited only to the RH7777 cell line, heterologous expression of LPA2 was done in another rat hepatoma cell line, HTC4. Transfection of LPA2 into this endogenously S1P-nonresponsive cell line (37, 38) also introduced S1P-induced calcium mobilization that reached 37% of the maximal LPA-induced response with 10 μm S1P (supplemental Fig. 2).
During the course of the current study, we attempted radioligand binding assays with the LPA receptors using radiolabeled S1P. Although we were able to detect specific binding of radiolabeled S1P to HEK293T cells transfected with S1P1, we could not detect specific binding of radiolabeled S1P to cells transfected with wild type LPA1, LPA2, or LPA3 (data not shown). Radioligand binding studies with LPA and S1P are technically difficult due to the lipophilic nature of the ligand, which tends to form micelles and partition into the phospholipid bilayer, causing high levels of nonspecific binding and background (39). The relatively high amounts of S1P required to elicit a Ca2+ response in our assays suggests that S1P might be a low affinity agonist of LPA2 and LPA3. Our inability to detect specific binding is likely due to high nonspecific binding as well as the low affinity of S1P for LPA2 and LPA3.
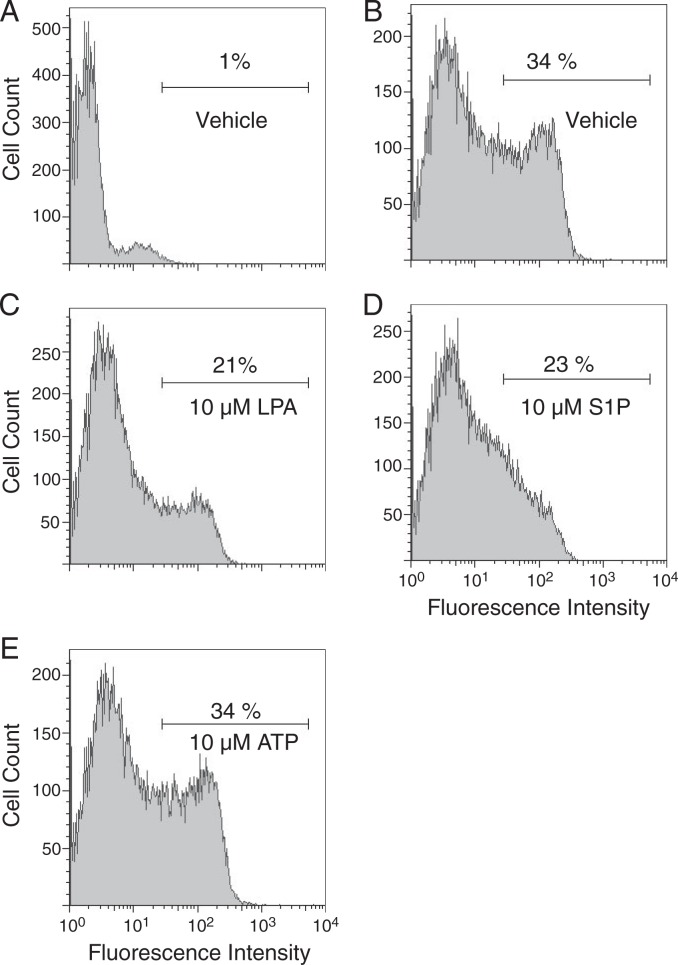
Ligand-induced activation of GPCRs may result in receptor internalization and down-regulation of receptor surface expression. To further investigate whether S1P was directly interacting with LPA2, we examined ligand-induced receptor internalization by using anti-FLAG flow cytometric analysis to compare cell surface expression of FLAG-tagged LPA2 in transiently transfected RH7777 cells exposed to vehicle, LPA, S1P, or ATP for 30 min (Fig. 7). Treatment with ATP, which produces robust calcium transients in RH7777 cells through non-LPA receptors, had no effect on surface expression of LPA2. Treatment with LPA resulted in a marked decrease in LPA2 surface expression from 34 to 21%. A decrease of similar magnitude of receptor internalization to 23% was measured after treatment with S1P, indicating ligand-induced receptor internalization in support of the agonist properties of S1P on LPA2.
FIGURE 7.
Surface expression of heterologously expressed LPA2 after treatment with LPA, S1P, or ATP. RH7777 cells transiently transfected with vector (A) or wild type FLAG-tagged LPA2 (B–E) were treated with vehicle (A and B), 10 μm LPA (C), 10 μm S1P (D), or 10 μm ATP (E) for 30 min before flow cytometric analysis of FLAG surface expression.
DISCUSSION
In the present study, using a combination of computational homology modeling and site-directed mutagenesis experiments, we undertook a comprehensive analysis of residues in LPA1, LPA2, and LPA3 that are computationally predicted to interact with the glycerophosphate moiety of LPA; the complex hydrophobic tail interactions were ignored. The transmembrane domains of LPA1–3 show a high (81%) homology with each other, whereas major sequence diversity is present in the amino and carboxyl termini. Although most of the residues that we mutated are conserved in the three LPA receptor subtypes, contrary to the hypothesis that assigns similar roles to these residues in ligand recognition, in most cases we found fundamental differences in their impact on potency and efficacy in the different receptor subtypes. These differences can be rationalized with our computational modeling studies of the wild type and mutant receptors docked to LPA. The modeling results agree completely with the experimental findings and indicate previously unrecognized differences between the LPA receptor subtypes. The present results show that mutations of even strictly conserved residues that interact with the polar head group of the ligand may have very different effects on receptor-ligand interactions within the spatial geometry of the ligand-binding pocket (Table 1).
We found only one residue whose mutation to alanine exerts an identical effect in the three LPA GPCRs of the EDG family. In all three EDG family LPA receptors, R3.28 ion pairs with the phosphate of LPA, and mutation to alanine of this residue abolished activation by submicromolar concentrations of LPA. This residue is also conserved in the S1P-preferring members of the EDG family and has been found to abolish ligand activation when mutated to alanine in S1P1 and S1P4(21, 24). Thus, R3.28 is a residue that is required for ligand recognition of both LPA and S1P in the EDG family of receptors by making a salt bridge with the phosphate group.
An additional alanine mutation, Q3.29A, was identified that displayed a qualitatively similar but quantitatively distinct role across the three EDG family LPA receptors. Glutamine 3.29 is predicted to interact with the hydroxyl group of LPA. Mutation to alanine of this residue abolished activation of LPA1 by LPA and dramatically decreased activation of LPA2 and, to a lesser extent, LPA3, pointing to the similar role but different impact of this conserved residue in LPA1–3. The modeling studies indicate that residues remaining in the ligand-binding pocket of LPA3 after mutation of Q3.29 to alanine are better able to compensate for the loss of the interaction between Q3.29 and LPA than those in LPA2 and LPA1. We have previously observed a similar compensating effect for loss of an ion pair in our studies of the S1P1 receptor: the mutation K5.38A resulted in wild type behavior due to optimization of other ion pairing interactions in the ligand-binding pocket (22).
The present study identified a new interaction between W4.64 of LPA3 and LPA. This interaction seems to be unique to LPA3 among the LPA-specific EDG family receptors; the W4.64A mutation substantially reduced the ability of LPA3 to be activated by LPA but had almost no effect on the activation of LPA1 or LPA2. W4.64 is conserved in all of the EDG receptors and has been shown to form cation-π interactions with the ammonium group of S1P in S1P1 and S1P4(21). W4.64 is also conserved in the cannabinoid receptors, CB1 and CB2. In CB2, mutation of W4.64 to phenylalanine or tyrosine retained binding to an aromatic, uncharged agonist, whereas mutation to alanine or leucine resulted in loss of agonist binding (41). Taken together, these results indicate that W4.64 is found near the ligand-binding pocket of both EDG and cannabinoid receptors and may interact with either charged or aromatic ligands.
K5.38 is conserved in the EDG family S1P receptors and has been shown to ion pair with the phosphate of S1P in the S1P1 and S1P4 complexes, although it is essential for S1P recognition only in S1P4(21, 22). Our results indicate that in LPA2 and LPA3 an arginine at position 5.38 ion pairs with the phosphate of LPA, whereas an aspartate residue at this position in LPA1 does not contribute to ligand binding. Replacement of this aspartate with arginine (D5.38R) in LPA1 confers increased receptor activation by LPA, underscoring the importance of this polar interaction not only in the S1P4 receptor but also in LPA2 and LPA3 receptors.
We previously demonstrated that in S1P1 a positively charged lysine in TM7, K7.34, forms critical interactions with the phosphate of S1P (20). In LPA1–3, position 7.36 is occupied by a positively charged residue: in LPA1 and LPA2 this residue is a lysine; in LPA3 it is an arginine. The mutation K7.36A enhanced activation of LPA1 but diminished activation of LPA2 by LPA (Fig. 4, D and E), and the mutation R7.36A in LPA3 had no effect on receptor activation by LPA. However, alanine mutation of the adjacent residue in LPA3, K7.35, markedly diminished activation by LPA (Fig. 4F). The models show that the cationic residue at position 7.35 (Fig. 4C) is oriented toward the phosphate group of LPA, but the cationic residue at position 7.36 (Fig. 4, A and B) is not. This apparent discrepancy seems to point to another potential role for K7.36 in LPA2 where a detrimental impact of the mutation was observed. The model indicates that K7.36 in LPA2 forms an ion pair with D1.31, a residue unique to LPA2 (not shown). Mutation of K7.36 in LPA2 likely results in a structural change in the receptor that our molecular dynamics simulations were too brief to observe.
Our previous studies established that position 3.29 determines the ligand specificity of LPA1 and S1P1 receptors. Q3.29, which is conserved in LPA1–3, is predicted to hydrogen bond with the hydroxyl group of LPA, and E3.29, which is conserved in S1P1–5, ion pairs with the ammonium of S1P. The mutation Q3.29E was shown to allow LPA1 to be activated by S1P and diminished activation by LPA. The reciprocal mutation in S1P1 or S1P4, E3.29Q, changed receptor specificity from S1P to LPA. Alanine mutants abolished receptor activation by either ligand as measured by GTPγS binding assays (24, 25). In this study, using more sensitive Ca2+ mobilization assays for monitoring receptor activation, we examined activation by S1P in wild type as well as Q3.29E and Q3.29A mutants in all three EDG family LPA receptor subtypes. The Q3.29E mutations increased receptor activation in response to 10 μm S1P in LPA1, LPA2, and LPA3 to 39, 64, and 62%, respectively, of the maximum LPA-induced Ca2+ response measured in the wild type receptors; the same mutations decreased receptor activation by LPA in LPA1–3, supporting the conserved key role of residue 3.29 in determining ligand specificity among the EDG receptors.
Unexpectedly we found that heterologously expressed wild type LPA2 or LPA3 conveyed responsiveness to submicromolar or low micromolar concentrations of S1P, respectively. Wild type LPA1 conferred only weak activation at the highest S1P concentration tested (10 μm). In RH7777 cells that were transiently transfected with wild type LPA2 or LPA3, S1P concentrations of 10 μm induced Ca2+ mobilization responses that were ∼40% of the maximal LPA-induced responses. Ca2+ responses to S1P were also measured in clonally derived RH7777 cell lines that had been stably transfected with wild type LPA1, LPA2, or LPA3. These cell lines are highly sensitive to LPA as measured by Ca2+ mobilization, although the expression levels of the transfected receptors are much lower than the expression levels in transiently transfected cells. The LPA1–3 stable transfectants were activated by S1P similarly to the LPA1–3 transiently transfected cells, suggesting that S1P might function as a weak agonist of EDG family LPA receptors even when the receptors are expressed at physiological levels. We also confirmed that heterologous expression of LPA2 can allow S1P-induced calcium mobilization in HTC4 cells, another naturally S1P-nonresponsive rat hepatoma cell line.
The LPA2-mediated responses to S1P were corroborated by S1P-induced LPA2 receptor internalization (Fig. 7). Further-more DH-S1P, a ligand with a saturated hydrophobic tail, could activate LPA3 but not LPA2, further supporting the selectivity of the individual receptor subtype in this cross-responsiveness between the three related natural ligands (Fig. 6). The importance of the phosphate head group in this response was underscored by the fact that sphingosine and dihydrosphingosine both failed to activate either receptor.
In the report that originally identified LPA2 as an LPA receptor, S1P at a concentration of 1 μm failed to generate significant increases of a serum response element-driven reporter gene (42). The original reports that identified LPA3 as an LPA receptor indicated that S1P was not a ligand for LPA3: 1 μm S1P did not activate LPA3 expressed in HEK293T cells as measured by GTPγS binding assays (43), 1 μm S1P did not elicit Ca2+ transients in SF9 cells expressing LPA3(44), and S1P did not compete with [3H]LPA for binding to LPA3-expressing SF9 cells (44). A plausible explanation for the discrepancy between our results, which indicate that S1P is a weak agonist of LPA2 and LPA3, and results from those original studies is that our Ca2+ assays are more sensitive than the radioligand binding assays, GTPγS binding assays, and reporter gene assays used in those studies. Other factors may also influence the cellular responses measured under different conditions, such as the formulation of the ligand with BSA carrier (45) and the host cell lines used.
S1P1 has been shown to function as a low affinity receptor for LPA (46). S1P1-transfected HEK293 cells were activated by relatively high concentrations of LPA (20–50 μm) as measured by receptor phosphorylation, cellular morphogenetic differentiation, and P-cadherin expression. Lower concentrations of LPA (2.5 μm) induced S1P1-mediated mitogen-activated protein kinase activation (46). We do not know the biological significance of the weak agonism of the LPA receptors by S1P that we observed. Certainly it raises caution in interpreting experimental data and ascribing cellular responses to a particular receptor when concentrations in excess of 300 nm S1P are used. In our assays, the concentrations of S1P required to activate LPA2 and LPA3 partially overlap with physiological ranges that have been reported for S1P in human plasma (703 ± 41 nm) or mouse plasma (1310 ± 190 nm) (47).
It has been suggested that human platelets possess a receptor that responds to both LPA and S1P (48–50). Specific binding of [3H]S1P to platelets was inhibited by LPA (48), and the platelet aggregation response to LPA was desensitized by S1P (48–50). Similarly to the relatively high S1P concentrations required to activate LPA2 and LPA3 in our Ca2+ mobilization assays, the platelet aggregation response to LPA was desensitized only by preincubation with relatively high concentrations of S1P (5–40 μm) (48–50); preincubation with 1 μm S1P had no effect (48). S1P is a relatively weak platelet-aggregating agent compared with LPA (49–51), and platelets contain LPA1–3 transcripts (50). The weak agonism of S1P toward LPA2 and LPA3 observed in the present study supports the possibility that the cross-desensitization reported for platelets might be mediated by an EDG family LPA receptor.
The structure of LPA can be described as having a polar head group, a glycerol backbone, and a hydrophobic tail. Recently the residues deeper in the transmembrane helices of S1P1 that interact with the hydrophobic tail of S1P have been computationally identified and experimentally validated, thus mapping the hydrophobic ligand-binding pocket of S1P1(40). That study will serve in the future as a template in modeling the hydrophobic interactions in the other EDG receptors. The present study focused on amino acid residues that interact with the polar head group of LPA. These are the most thoroughly characterized receptor-ligand interactions among the EDG family of LPA receptors and have been shown to be critical for receptor activation (23, 24, 28); modifications to the polar head group are the least well tolerated by the LPA receptors in pharmacophore development. Nevertheless the present studies demonstrate that the replacement of the hydroxyl group of LPA with the ammonium group in S1P does not completely abolish LPA receptor activation. This indicates that common recognition elements for both ligands occur at positions other than 3.29, pointing to the common lineage of the EDG receptors from an ancestral gene. The present studies also highlight the divergences that have occurred within the EDG receptors through gene duplication and molecular evolution, resulting in a family of receptors with unique ligand specificities and cellular functions. Given the central role of these receptors in a variety of physiological and disease states, the EDG receptors are even more compelling to study when we consider the extraordinary complexity of these receptors that recognize the simplest phospholipid in nature.
Acknowledgments
We thank Dr. Kary Latham for excellent technical assistance with the flow cytometric analysis.
Footnotes
This work was supported by United States Public Health Service Grants HL61469 (to G. T.), CA921160 (to G. T.), HL084007 (to A. L. P.), and HL07641-19 (to W. J. V.) and by Southeast American Heart Association Postdoctoral Fellowship 0625325 (to Y. F.) and predoctoral fellowship (to J. I. F.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The abbreviations used are: LPA, lysophosphatidic acid; EDG, endothelial differentiation gene; GPCR, G protein-coupled receptor; S1P, sphingosine 1-phosphate; TM, transmembrane domain; PBS, phosphate-buffered saline; BSA, bovine serum albumin; RH7777, rat hepatoma 7777; DH-S1P, dihydrosphingosine 1-phosphate; GTPγS, guanosine 5′-3-O-(thio)triphosphate.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
REFERENCES
- 1.Ishii I, Fukushima N, Ye X, Chun J. Annu Rev Biochem. 2004;73:321–354. doi: 10.1146/annurev.biochem.73.011303.073731. [DOI] [PubMed] [Google Scholar]
- 2.Moolenaar WH, van Meeteren LA, Giepmans BN. BioEssays. 2004;26:870–881. doi: 10.1002/bies.20081. [DOI] [PubMed] [Google Scholar]
- 3.Radeff-Huang J, Seasholtz TM, Matteo RG, Brown JH. J Cell Biochem. 2004;92:949–966. doi: 10.1002/jcb.20094. [DOI] [PubMed] [Google Scholar]
- 4.Xu Y, Fang XJ, Casey G, Mills GB. Biochem J. 1995;309:933–940. doi: 10.1042/bj3090933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Y, Gaudette DC, Boynton JD, Frankel A, Fang XJ, Sharma A, Hurteau J, Casey G, Goodbody A, Mellors A. Clin Cancer Res. 1995;1:1223–1232. [PubMed] [Google Scholar]
- 6.Xu Y, Shen Z, Wiper DW, Wu M, Morton RE, Elson P, Kennedy AW, Belinson J, Markman M, Casey G. J Am Med Assoc. 1998;280:719–723. doi: 10.1001/jama.280.8.719. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz BM, Hong G, Morrison BH, Wu W, Baudhuin LM, Xiao YJ, Mok SC, Xu Y. Gynecol Oncol. 2001;81:291–300. doi: 10.1006/gyno.2001.6124. [DOI] [PubMed] [Google Scholar]
- 8.Sliva D, Mason R, Xiao H, English D. Biochem Biophys Res Commun. 2000;268:471–479. doi: 10.1006/bbrc.2000.2111. [DOI] [PubMed] [Google Scholar]
- 9.Goetzl EJ, Dolezalova H, Kong Y, Zeng L. Cancer Res. 1999;59:4732–4737. [PubMed] [Google Scholar]
- 10.Qi C, Park JH, Gibbs TC, Shirley DW, Bradshaw CD, Ella KM, Meier KE. J Cell Physiol. 1998;174:261–272. doi: 10.1002/(SICI)1097-4652(199802)174:2<261::AID-JCP13>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 11.Guo R, Kasbohm EA, Arora P, Sample CJ, Baban B, Sud N, Sivashanmugam P, Moniri NH, Daaka Y. Endocrinology. 2006;147:4883–4892. doi: 10.1210/en.2005-1635. [DOI] [PubMed] [Google Scholar]
- 12.Karliner JS. Biochim Biophys Acta. 2002;1582:216–221. doi: 10.1016/s1388-1981(02)00174-9. [DOI] [PubMed] [Google Scholar]
- 13.Durgam GG, Virag T, Walker MD, Tsukahara R, Yasuda S, Liliom K, van Meeteren LA, Moolenaar WH, Wilke N, Siess W, Tigyi G, Miller DD. J Med Chem. 2005;48:4919–4930. doi: 10.1021/jm049609r. [DOI] [PubMed] [Google Scholar]
- 14.Zhang C, Baker DL, Yasuda S, Makarova N, Balazs L, Johnson LR, Marathe GK, McIntyre TM, Xu Y, Prestwich GD, Byun HS, Bittman R, Tigyi G. J Exp Med. 2004;199:763–774. doi: 10.1084/jem.20031619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chun J, Goetzl EJ, Hla T, Igarashi Y, Lynch KR, Moolenaar W, Pyne S, Tigyi G. Pharmacol Rev. 2002;54:265–269. doi: 10.1124/pr.54.2.265. [DOI] [PubMed] [Google Scholar]
- 16.Noguchi K, Ishii S, Shimizu T. J Biol Chem. 2003;278:25600–25606. doi: 10.1074/jbc.M302648200. [DOI] [PubMed] [Google Scholar]
- 17.Kotarsky K, Boketoft A, Bristulf J, Nilsson NE, Norberg A, Hansson S, Owman C, Sillard R, Leeb-Lundberg LM, Olde B. J Pharmacol Exp Ther. 2006;318:619–628. doi: 10.1124/jpet.105.098848. [DOI] [PubMed] [Google Scholar]
- 18.Lee CW, Rivera R, Gardell S, Dubin AE, Chun J. J Biol Chem. 2006;281:23589–23597. doi: 10.1074/jbc.M603670200. [DOI] [PubMed] [Google Scholar]
- 19.Tabata K, Baba K, Shiraishi A, Ito M, Fujita N. Biochem Biophys Res Commun. 2007;363:861–866. doi: 10.1016/j.bbrc.2007.09.063. [DOI] [PubMed] [Google Scholar]
- 20.Parrill AL, Baker DL, Wang DA, Fischer DJ, Bautista DL, Van Brocklyn J, Spiegel S, Tigyi G. Ann N Y Acad Sci. 2000;905:330–339. doi: 10.1111/j.1749-6632.2000.tb06573.x. [DOI] [PubMed] [Google Scholar]
- 21.Inagaki Y, Pham TT, Fujiwara Y, Kohno T, Osborne DA, Igarashi Y, Tigyi G, Parrill AL. Biochem J. 2005;389:187–195. doi: 10.1042/BJ20050046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naor MM, Walker MD, Van Brocklyn JR, Tigyi G, Parrill AL. J Mol Graph Model. 2007;26:519–528. doi: 10.1016/j.jmgm.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujiwara Y, Sardar V, Tokumura A, Baker D, Murakami-Murofushi K, Parrill A, Tigyi G. J Biol Chem. 2005;280:35038–35050. doi: 10.1074/jbc.M504351200. [DOI] [PubMed] [Google Scholar]
- 24.Wang DA, Lorincz Z, Bautista DL, Liliom K, Tigyi G, Parrill AL. J Biol Chem. 2001;276:49213–49220. doi: 10.1074/jbc.M107301200. [DOI] [PubMed] [Google Scholar]
- 25.Holdsworth G, Osborne DA, Pham TT, Fells JI, Hutchinson G, Milligan G, Parrill AL. BMC Biochem. 2004;5:12. doi: 10.1186/1471-2091-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fischer DJ, Liliom K, Guo Z, Nusser N, Virag T, Murakami-Murofushi K, Kobayashi S, Erickson JR, Sun G, Miller DD, Tigyi G. Mol Pharmacol. 1998;54:979–988. doi: 10.1124/mol.54.6.979. [DOI] [PubMed] [Google Scholar]
- 27.Ballesteros JA, Weinstein H. In: Methods in Neurosciences. Conn PM, Sealfon SC, editors. Academic Press; San Diego, CA: 1995. pp. 366–428. [Google Scholar]
- 28.Sardar VM, Bautista DL, Fischer DJ, Yokoyama K, Nusser N, Virag T, Wang DA, Baker DL, Tigyi G, Parrill AL. Biochim Biophys Acta. 2002;1582:309–317. doi: 10.1016/s1388-1981(02)00185-3. [DOI] [PubMed] [Google Scholar]
- 29.Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ. J Comput Chem. 1998;19:1639–1662. [Google Scholar]
- 30.Halgren TA. J Comput Chem. 1996;17:490–519. [Google Scholar]
- 31.Fukushima N, Kimura Y, Chun J. Proc Natl Acad Sci U S A. 1998;95:6151–6156. doi: 10.1073/pnas.95.11.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischer DJ, Nusser N, Virag T, Yokoyama K, Wang D, Baker DL, Bautista D, Parrill AL, Tigyi G. Mol Pharmacol. 2001;60:776–784. [PubMed] [Google Scholar]
- 33.Ohta H, Sato K, Murata N, Damirin A, Malchinkhuu E, Kon J, Kimura T, Tobo M, Yamazaki Y, Watanabe T, Yagi M, Sato M, Suzuki R, Murooka H, Sakai T, Nishitoba T, Im DS, Nochi H, Tamoto K, Tomura H, Okajima F. Mol Pharmacol. 2003;64:994–1005. doi: 10.1124/mol.64.4.994. [DOI] [PubMed] [Google Scholar]
- 34.Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, Spiegel S. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- 35.Meyer Zu, Heringdorf D. J Cell Biochem. 2004;92:937–948. doi: 10.1002/jcb.20107. [DOI] [PubMed] [Google Scholar]
- 36.Meyer zu Heringdorf D, Liliom K, Schaefer M, Danneberg K, Jaggar JH, Tigyi G, Jakobs KH. FEBS Lett. 2003;554:443–449. doi: 10.1016/s0014-5793(03)01219-5. [DOI] [PubMed] [Google Scholar]
- 37.An S, Bleu T, Zheng Y, Goetzl EJ. Mol Pharmacol. 1998;54:881–888. doi: 10.1124/mol.54.5.881. [DOI] [PubMed] [Google Scholar]
- 38.An S, Bleu T, Zheng Y. Mol Pharmacol. 1999;55:787–794. [PubMed] [Google Scholar]
- 39.Hecht JH, Weiner JA, Post SR, Chun J. J Cell Biol. 1996;135:1071–1083. doi: 10.1083/jcb.135.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujiwara Y, Osborne DA, Walker MD, Wang DA, Bautista DA, Liliom K, Van Brocklyn JR, Parrill AL, Tigyi G. J Biol Chem. 2007;282:2374–2385. doi: 10.1074/jbc.M609648200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rhee MH, Nevo I, Bayewitch ML, Zagoory O, Vogel Z. J Neurochem. 2000;75:2485–2491. doi: 10.1046/j.1471-4159.2000.0752485.x. [DOI] [PubMed] [Google Scholar]
- 42.An S, Bleu T, Hallmark OG, Goetzl EJ. J Biol Chem. 1998;273:7906–7910. doi: 10.1074/jbc.273.14.7906. [DOI] [PubMed] [Google Scholar]
- 43.Im DS, Heise CE, Harding MA, George SR, O’Dowd BF, Theodorescu D, Lynch KR. Mol Pharmacol. 2000;57:753–759. [PubMed] [Google Scholar]
- 44.Bandoh K, Aoki J, Hosono H, Kobayashi S, Kobayashi T, Murakami-Murofushi K, Tsujimoto M, Arai H, Inoue K. J Biol Chem. 1999;274:27776–27785. doi: 10.1074/jbc.274.39.27776. [DOI] [PubMed] [Google Scholar]
- 45.Hama K, Bandoh K, Kakehi Y, Aoki J, Arai H. FEBS Lett. 2002;523:187–192. doi: 10.1016/s0014-5793(02)02976-9. [DOI] [PubMed] [Google Scholar]
- 46.Lee MJ, Thangada S, Liu CH, Thompson BD, Hla T. J Biol Chem. 1998;273:22105–22112. doi: 10.1074/jbc.273.34.22105. [DOI] [PubMed] [Google Scholar]
- 47.Jiang X, Han X. J Lipid Res. 2006;47:1865–1873. doi: 10.1194/jlr.D600012-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yatomi Y, Yamamura S, Ruan F, Igarashi Y. J Biol Chem. 1997;272:5291–5297. doi: 10.1074/jbc.272.8.5291. [DOI] [PubMed] [Google Scholar]
- 49.Gueguen G, Gaige B, Grevy JM, Rogalle P, Bellan J, Wilson M, Klaebe A, Pont F, Simon MF, Chap H. Biochemistry. 1999;38:8440–8450. doi: 10.1021/bi9816756. [DOI] [PubMed] [Google Scholar]
- 50.Motohashi K, Shibata S, Ozaki Y, Yatomi Y, Igarashi Y. FEBS Lett. 2000;468:189–193. doi: 10.1016/s0014-5793(00)01222-9. [DOI] [PubMed] [Google Scholar]
- 51.Yatomi Y, Ruan F, Hakomori S, Igarashi Y. Blood. 1995;86:193–202. [PubMed] [Google Scholar]