Abstract
The adrenal glands are the primary source of mineralocorticoids, glucocorticoids and the so-called adrenal androgens. Under physiological conditions, cortisol and adrenal androgen synthesis are controlled primarily by adrenocorticotrophic hormone (ACTH). Although it is well established that ACTH can stimulate steroidogenesis in the human adrenal gland, the effect of ACTH on overall production of different classes of steroid hormones has not been defined. In this study, we examined the effect of ACTH on the production of twenty-three steroid hormones in adult adrenal (AA) primary cultures and 20 steroids in the adrenal cell line, H295R. Liquid chromatography/tandem mass spectrometry analysis (LC-MS/MS) revealed that in primary cultures that cortisol and corticosterone were the two most abundant steroid hormones produced with or without ACTH treatment (48 h). Cortisol production responded the most to ACTH treatment, with a 64-fold increase. Interestingly, the production of two androgens, androstenedione and 11β-hydroxyandrostenedione, that were also produced in large amounts under basal conditions significantly increased after ACTH incubation. In H295R cells 11-deoxycortisol and androstenedione were the major products under basal conditions. Treatment with forskolin increased the percentage of 11β-hydroxylated products including cortisol and 11β-hydroxyandrostenedione. This study illustrates that normal adrenal cells respond to ACTH through the secretion of a variety of steroid hormones, thus supporting the role of adrenal cells as a source of both corticosteroids and androgens.
Keywords: Adrenal cortex, ACTH, Steroidogenesis, LC-MS/MS
Introduction
The human adult adrenal cortex can be divided into three functionally distinct zones, namely the zona glomerulosa (ZG), zona fasciculata (ZF) and zona reticularis (ZR). Each zone exhibits independent regulation and function as illustrated by the selective production of glucocorticoids in the ZF, mineralocorticoids in the ZG and adrenal androgens in the ZR. Adrenal androgens include mainly DHEA, DHEA-S and androstenedione, which exhibit only weak sex hormone activity but can serve as precursors for estrogens and active androgens such as testosterone (Branchaud et al. 1983; Haning et al. 1985; Chen et al. 1996; Labrie et al. 1998; Burger 2002). In addition to the end products of steroid hormone synthesis, adrenal steroid intermediates can also be detected in blood and used as biomarkers for adrenal disease diagnosis.
Adrenocorticotrophic hormone (ACTH) by binding to its specific G-protein coupled receptor MC2R has been shown to induce adrenal cortex proliferation and steroidogenesis (Ney et al. 1969; Sharma 1973; Mahaffee et al. 1974; Hornsby & Gill 1977; Di Blasio et al. 1990). ACTH can stimulate aldosterone, cortisol and DHEA production in the adrenal gland (Hornsby et al. 1973; Hung & LeMaire 1988; Arvat et al. 2000; Sirianni et al. 2005). However, it is not clear if these steroids represent the most abundant steroids secreted in response to ACTH. O’Hare et al., using high-pressure liquid chromatography (HPLC), demonstrated the production of cortisol, corticosterone, 11-deoxycortisol, 11-deoxycorticosterone, androstenedione, 11β-hydroxyandrostenedione (11OHA) and 16α-hydroxyprogesterone in primary cultures of human adrenocortical cells (O'Hare et al. 1976; Morgan & O'Hare 1979). However, the amounts of individual steroids were not quantified and effects of ACTH were examined only for a few steroids.
Given the critical role of ACTH in adrenal development, steroidogenesis and disease, it is appropriate to further determine the detailed effects of ACTH on steroid biosynthesis. In this study, we used liquid chromatography/tandem mass spectrometry analysis (LC-MS/MS) to measure the production of twenty steroid hormones in primary cultures of adrenocortical cells under basal conditions and in response to ACTH treatment. The study has characterized the metabolomic pattern of adrenocortical cells and defined a family of ACTH-responsive steroids thus expanding our knowledge of ACTH action in the human adrenal.
Materials and methods
Tissue collection
Human adult adrenal glands were obtained from cadaveric kidney donors that were transplanted at the Medical College of Georgia (Augusta, GA). Informed consent was obtained from the family of the donor by LifeLink of Georgia. The use of these tissues was approved by the Institutional Review Board of Medical College of Georgia.
Cell culture and treatment
Adult adrenocortical cells were isolated with collagenase-dispase digestion as described previously (Bassett et al. 2004). Briefly, adult adrenals were minced and dissociated into a single cell suspension by repeated exposure of the tissue fragments to DMEM/F12 medium (Invitrogen, Carlsbad, CA) containing 1 mg/mL collagenase dispase and 0.25 mg/mL DNase-1 (F. Hoffmann-La Roche Ltd., Switzerland). Digestion and mechanical dispersion were carried out four times for 1 h each, at 37°C. Cells were collected between each digestion and combined, then plated at a density of 300,000 cells/well in 24-well Falcon cell culture plates.
Steroid production was measured AA cells grown for 5 days in complete growth medium [DMEM/F12 medium with 10% Cosmic Calf serum (Hyclone, Logan, UT) and antibiotics]. Prior to ACTH stimulation, the cells were cultured in experimental medium (0.1% Cosmic Calf serum and antibiotics) overnight and then treated with vehicle or 10 nM ACTH (Organon, Bedford, OH) for 48 h. The cells were lysed for protein assay and media were collected for steroid assays as described below.
H295R cells were cultured using the same complete growth medium as AA cells, while HAC13 and HAC15 cells were grown in DME/F12 medium supplemented with 10% cosmic calf serum, 1% insulin/transferrin/selenium Premix (ITS, BD Biosciences) and antibiotics. All three lines were plated at a density of 400,000 cells per well in 12 well dish and treated with vehicle or forskolin (10 µM, Sigma-Aldrich, St. Louis, MO) following the same protocol.
Protein extraction and protein assay
Cells were lysed in 100 µl Mammalian Protein Extraction Reagent (Pierce Chemical Co., Rockford, IL), and the protein content determined by the bicinchoninic acid (BCA) protein assay using the micro BCA protocol (Pierce Chemical Co.).
Liquid chromatography/tandem mass spectrometry (LC-MS/MS)
Deuterium labeled 4-androstenedione, corticosterone, estradiol, pregnenolone, and testosterone were purchased from CDN Isotopes (Pointe-Claire, Canada). All other steroids were purchased from Steraloids (Newport, RI). HPLC solvents and water were of HPLC analytical grade and filtered (0.2 µm) before use. All other reagents were purchased from Sigma (St. Louis, MO) and were of analytical grade or better.
Calibration curves were prepared by serial dilution of analytical standards in water. Quality control (QC) samples were prepared for each steroid analyzed. QC samples were prepared in experimental medium (0.1% Cosmic Calf serum, 1% antibiotics) spiked with analyte at concentrations near the lower, mid, and upper region of the range of quantification. Calibration curves and QC samples were analyzed in parallel with experimental samples. Steroid concentrations were calculated by Masslynx software (Waters, Beverly, MA), with the assumed concentrations for the standards calculated from the calibration curve regression parameters in comparison to theoretical values. The accuracy of QC samples was determined after first subtracting the endogenous concentration determined in experimental medium from the total concentration found in the QC sample.
Steroids of interest were assigned to one of three categories for quantification by LC-MS/MS based on the need for pre-column derivatization to achieve an appropriate lower limit quantification (LLQ) and/or chromatographic resolution: 1) no pre-column derivatization needed, 2) keto-steroids amenable to condensation with hydroxylamine, and 3) estradiol and estrone. Each method used one or more deuterium-labeled internal standards. Calibration curves and QC samples used for each method were prepared with the corresponding pre-column derivatization method. (See supplemental information Tables 3.2.S1, S2, and S3 for details regarding internal standards, retention times, quantified mass transitions, assay performance parameters and statistics, and detection limits for steroid measurement.)
Method 1
LC-MS/MS without pre-column derivatization (5-androstenediol, androstenedione, corticosterone, cortisol, DHEA, 11-deoxycorticosterone, 11-deoxycortisol, 17α-hydroxyprogesterone, 20α-hydroxyprogesterone, and testosterone). Samples (100 µL) were extracted with 4 mL methyl-tert-butyl-ether (MTBE). The organic phase was evaporated to dryness and reconstituted in 200 µL of water/acetonitrile (75:25, v:v). 20 µL of the reconstituted samples was applied to an Xterra C18 column (2.1×250 mm, 5 µm, Waters, Beverly, MA), and eluted with a mobile phase gradient of 30–85% acetonitrile in water, with 0.1% formic acid. The column temperature was maintained at 40°C. The column eluent was subjected to positive-mode electrospray ionization (ES+), and the analytes were detected with a tandem quadrupole mass spectrometer (Waters, Beverly, MA).
Method 2
LC-MS/MS analysis of oxime derivatives of keto-steroids (aldosterone, dihydrotestosterone, 11OHA, 7α-DHEA, 16α-DHEA, 17α-hydroxypregnenolone, pregnenolone, and progesterone). Samples (100 µL) were extracted with 4 mL MTBE, and the organic phase was evaporated to dryness. The residue was dissolved in 1 M aqueous hydroxylamine hydrochloride, and incubated for 1 h at 60˚C. The reaction mixtures were extracted with 2.5 mL MTBE, dried, and reconstituted in 200 µL of water/acetonitrile (75:25, v:v), and analyzed as above using a mobile phase gradient of 25–60% acetonitrile in water, with 0.1% formic acid.
Method 3
LC-MS/MS analysis of dansylated estradiol and estrone. Samples (100 µL) were extracted and dried as above, dissolved in 0.2 mL of 1 mg/mL dansyl chloride in 1 M aqueous sodium bicarbonate, and incubated for 1 h at 60˚C. The reaction mixtures (20 µL) were analyzed by LC-MS/MS as above using an Xbridge Phenyl column (2.1×150mm, 5 µm, Waters, Beverly, MA) with a mobile phase gradient of 35–90% acetonitrile in water, with 0.1% formic acid.
Steroid Immunoassay
Steroid concentrations in the medium were determined using enzyme immunoassay (EIA) for cortisol (Alpco, Salem, NH), and corticosterone (DRG International Inc., Mountainside, NJ) following the manufacturer recommendation, except that standard curves were prepared in the experimental cell culture medium. Immunoassay for 11OHA was developed in our laboratory. Briefly, plates were coated with rabbit IgG 1 µg/100 µL (LAMPIRE Biological Laboratories, Inc. Pipersville, PA) overnight in a 1 M NaCl, 10 mM phosphate pH 7.4 buffer. Plates were then washed four times in PBS buffer and incubated with 100 µL goat anti-rabbit IgG antisera (LAMPIRE Biological Laboratories) 1/500 in PBS for one hour. Before each assay, coated plates were washed four times in PBS and 50 µL of standards and sample were added to each well. 50 µL of 11OHA antibody was added to each well at a 1:10,000 dilution (in PBS buffer) and mixed on shaker for 5 min. 50 µL of biotin-labeled 11OHA (1:12,000,000 dilution in PBS) was added to each well and mixed for another 5 min. The plate was then placed at 4˚C and allowed to incubate for 18 h. Plates were washed four times in PBS and 150 µL of avidin-HRP (Vector laboratories, Burlingame, CA) was added at a 1:2500 dilution to each well. Plates were shaken for 30 min, followed by four-time washes in PBS and developed for 15 min with 100 µL per well of tetramethylbenzidine substrate (Thermo scientific, Waltham, MA). The reaction was stopped with 50 µL per well of 1 N sulfuric acid. Absorbance was measured at 450 nm. Results were normalized to protein per tissue culture well and shown as fold changes compared to basal.
Statistical Analysis
Individual experiments were repeated three times, using cells isolated from adrenal glands obtained from different donors. Results are given as means ± S.E.M. Statistics were calculated using the two-tail paired Student’s t test (GraphPad Prism 3.0,GraphPad Software, Inc. San Diego, CA). P<0.05 was considered statistically significant.
Results
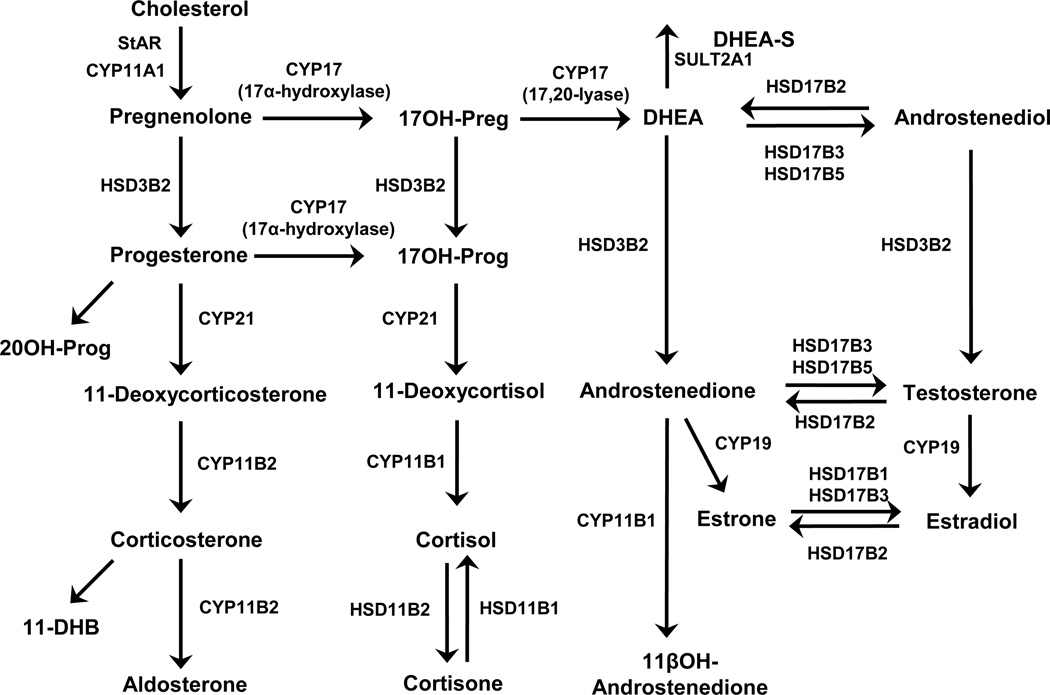
The major steroidogenic pathways for adrenal and gonadal tissues are shown in Figure 1. Twenty-three steroid hormones were quantified in the medium from AA cells with and without ACTH stimulation, using LC-MS/MS and RIA (Table 1).Under basal conditions, the three most abundant steroids produced by AA cells were cortisol (31%), cortisone (15%) and corticosterone (13%). Interestingly, two androgens, androstenedione and 11OHA were also produced by adrenal cells in relatively high amounts (4% and 9 %).
Fig. 1. Steroid biosynthesis pathways.
Summary of the steroidogenic pathways leading to synthesis of glucocorticoids, mineralocorticoids, androgens and estrogens.
Table 1. Steroids produced under basal and ACTH treatments.
AA cells were incubated with/without ACTH (10 nM) for 48 h. Steroids were grouped according to carbon number and property. Concentrations of steroids were measured using LC-MS/MS method and normalized to protein amount. Data represent results from three independent experiments and shown as means ± S.E.M. P values were calculated using the paired t-test with 95% confidence intervals.
| Steroid | Pre-ACTH pmole /mg protein |
Post ACTH pmole /mg protein |
Fold Change |
p-value |
|---|---|---|---|---|
| 21 Carbon Steroids | ||||
| Pregnenolone | 9.6±4.1 | 136.6±74.6 | 13.4±3.6 | 0.07 |
| 17α-Hydroxypregnenolone | 16.9±10.0 | 392.0±211.1 | 32.2±14.0 | 0.15 |
| Progesterone | 12.1±4.0 | 73.0±31.3 | 5.3±1.4 | 0.09 |
| 17α-Hydroxyprogesterone | 88.7±27.3 | 1167.4±512.0 | 15.6±6.2 | 0.14 |
| 20α-Hydroxyprogesterone | ND | ND | - | - |
| Deoxycorticosterone | 60.5±30.9 | 613.1±272.3 | 12.8±2.7* | 0.05 |
| Corticosterone | 243.8±85.8 | 9183.9±3392.5 | 37.0±1.3* | 0.001 |
| Aldosteronea | 21.8±1.4 | 201.3±18.2 | 9.3±1.1* | 0.02 |
| 11-Deoxycortisol | 147.2±41.0 | 3406.6±968.2 | 23.1±0.7* | 0.001 |
| Cortisol | 514.6±94.4 | 31464.4±5609.2 | 63.4±13.3* | 0.04 |
| Cortisone | 259.3±81.5 | 988.0±340.2 | 3.7±0.5* | 0.04 |
| 11-Dehydrocorticosterone | 111.5±81.5 | 535.7±239.7 | 4.9±0.7 | 0.03 |
| 19 Carbon Steroids | ||||
| DHEA | 8.5±2.7 | 188.3±142.3 | 17.6±9.2 | 0.21 |
| DHEA-S | ND | 582.8±83.4 | - | - |
| 7α-DHEA | ND | ND | - | - |
| 16α-DHEA | ND | ND | - | - |
| Androstenediol | ND | ND | - | - |
| Androstenedione | 84.1±44.6 | 2059.6±999.1 | 25.7±1.2* | 0.002 |
| 11OHA | 174.7±40.4 | 2844.0±545.5 | 16.6±1.1* | 0.005 |
| Testosterone | ND | 11.43 + 6.08 | - | - |
| Dihydrotestosterone | ND | ND | - | - |
| 18 Carbon Steroids | ||||
| Estrone | ND | 23.6+12.3 | - | - |
| Estradiol | ND | ND | - | - |
P<0.05; ND, not detectable.
Note
Aldosterone is shown as RIA data due to the low concentration present in experiment medium. As a conjugated steroid, DHEA-S was also measured by immunoassay. The detection limit for DHEA-S is 140.
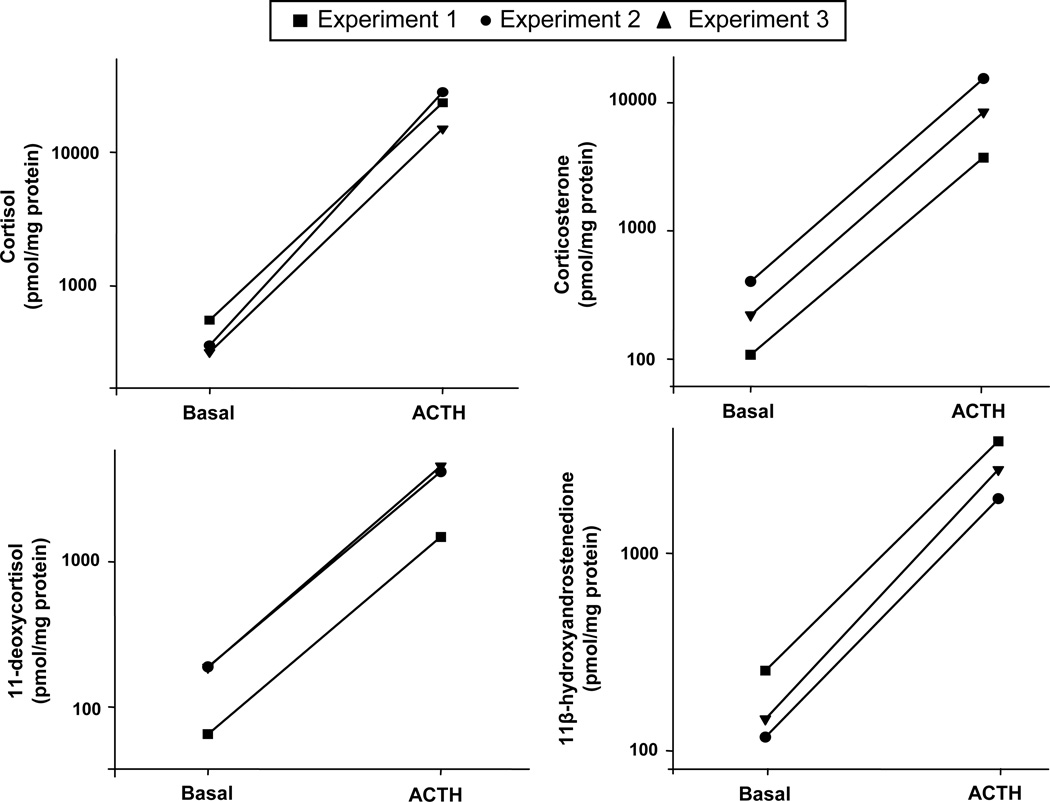
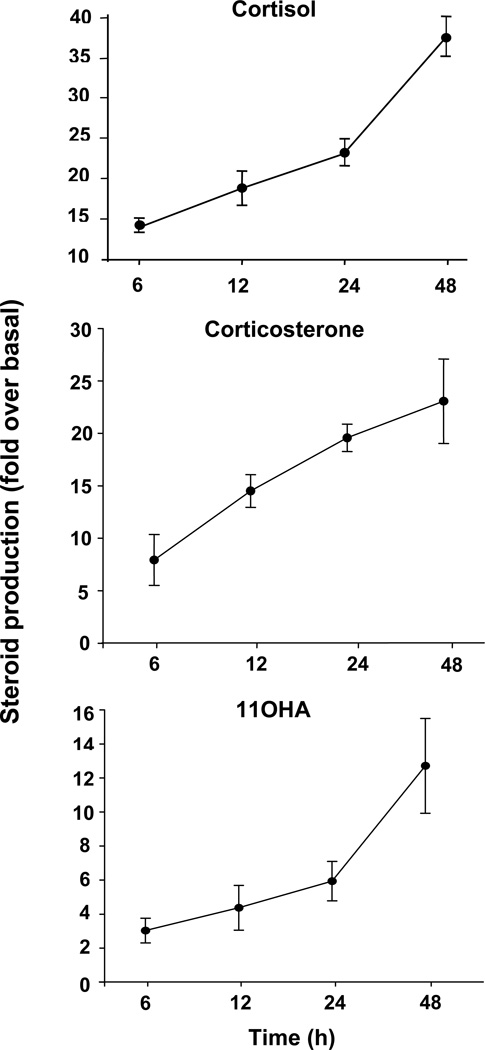
Following treatment with ACTH for 48 h, the production of aldosterone, cortisol, DHEA and all detectable intermediate products of each steroidogenic pathway increased by approximately 4–60 fold. (Table 1). The most highly stimulated steroids were cortisol (63-fold), corticosterone (37-fold) and 11-deoxycortisol (23-fold). Production of the adrenal androgens, androstenedione (26-fold), DHEA (18-fold) and 11OHA (17-fold), was also stimulated by ACTH treatment. The results of individual experiments using AA cells isolated from three different donors, are shown for cortisol, corticosterone, 11-deoxycortisol, and 11OHA (Fig 2). EIA was used to confirm the effects of ACTH on the steroid production in AA cells (Fig 3). The relative increases of cortisol, corticosterone and 11OHA were approximately 2-fold lower with EIA compared to LC-MS/MS, but both analytical methods indicated significant increases in the production of all the three steroids after ACTH treatment.
Fig. 2. Major steroids produced by AA cells with/without ACTH treatment.
Primary human adrenal culture cells were isolated as described in Materials and Methods, and plated at a density of 300,000 cells/well in 24-well dishes. Cells were treated with/without ACTH (10 nM) for 48 h before harvest. The concentration of steroids in the medium was measured by LC-MS/MS and normalized to the amount of protein. The amount of four major steroids produced by AA cells, cortisol, corticosterone, 11-deoxycortisol and 11OHA are shown in the graphs with each line representing an individual, independent experiment.
Fig. 3. Time-dependent stimulation of three major steroids produced in AA cells by ACTH treatment.
Primary human adrenal culture cells were isolated as described in Materials and Methods, and plated at a density of 300,000 cells/well in 24-well dishes. Cells were treated with/without ACTH (10 nM) for indicated times. The concentration of steroids (cortisol, corticosterone and 11OHA) in the medium was measured by immunoassay and expressed as fold changes over basal level. Results represent means ± S.E.M of data from at least three experiments using cells isolated from different adrenal glands.
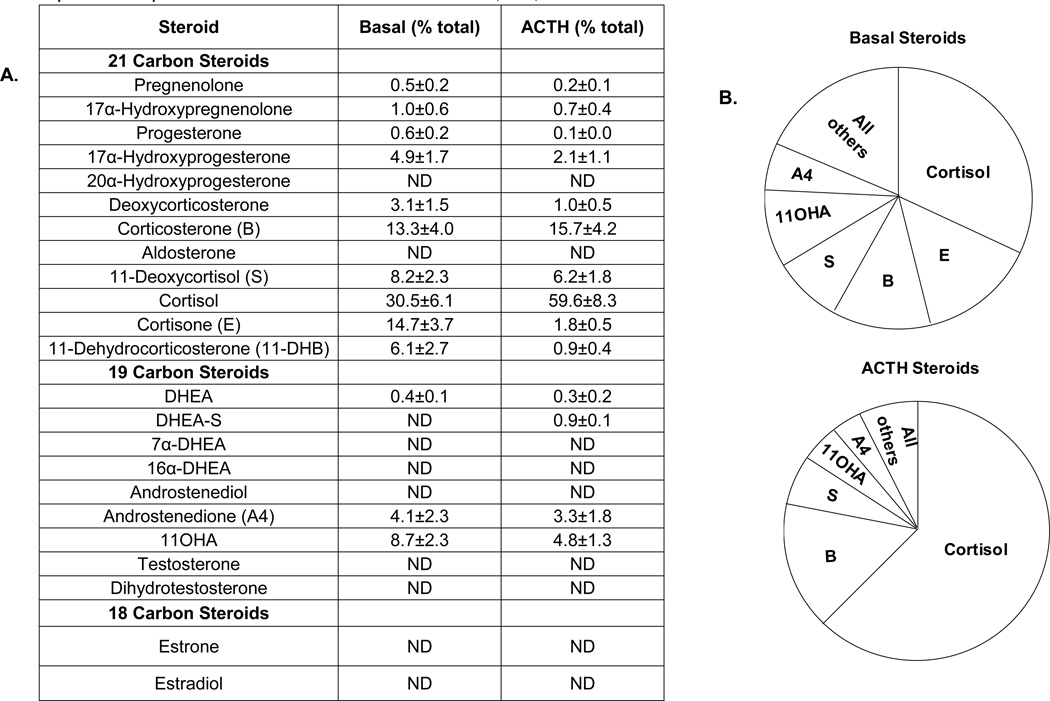
Comparison of the ratio of individual steroids produced by AA cell primary culture shows that cortisol represents 30% of total steroids made under basal conditions and 61% after ACTH treatment (Figure 4). Increased cortisol production following treatment confirmed that ACTH is a potent stimulator of glucocorticoid synthesis that greatly increases the capacity of the adrenal to produce cortisol under the control of the HPA axis. The global decrease in the production of other steroidogenic intermediates after ACTH treatment were consistent with the known effects of ACTH on steroidogenesis. The pie chart summarizing the ratio of individual steroids to total steroids before and after ACTH treatment is shown in Table 4.
Figure 4. Percentage of major steroids produced by AA cells with/ without ACTH treatment.
Primary human adrenal cells were isolated as described in Materials and Methods, and plated at a density of 300,000 cells/well in 24-well dishes. Cells were treated with/without ACTH (10nM) in 0.1% experimental medium for 48 h before harvest. The concentration of steroids in the medium was measured by LC-MS/MS and normalized to the amount of protein. Percentage of each steroid was calculated by dividing the amount of individual steroid with total steroid. A. Table summarizing the percentage of individual steroid compared to total steroid produced by AA cells. B. Pie chart summarizing the relative percentage of the major steroids produced in AA cells. Data represent results from three independent experiments and shown as means ± S.E.M; ND, not detectable.
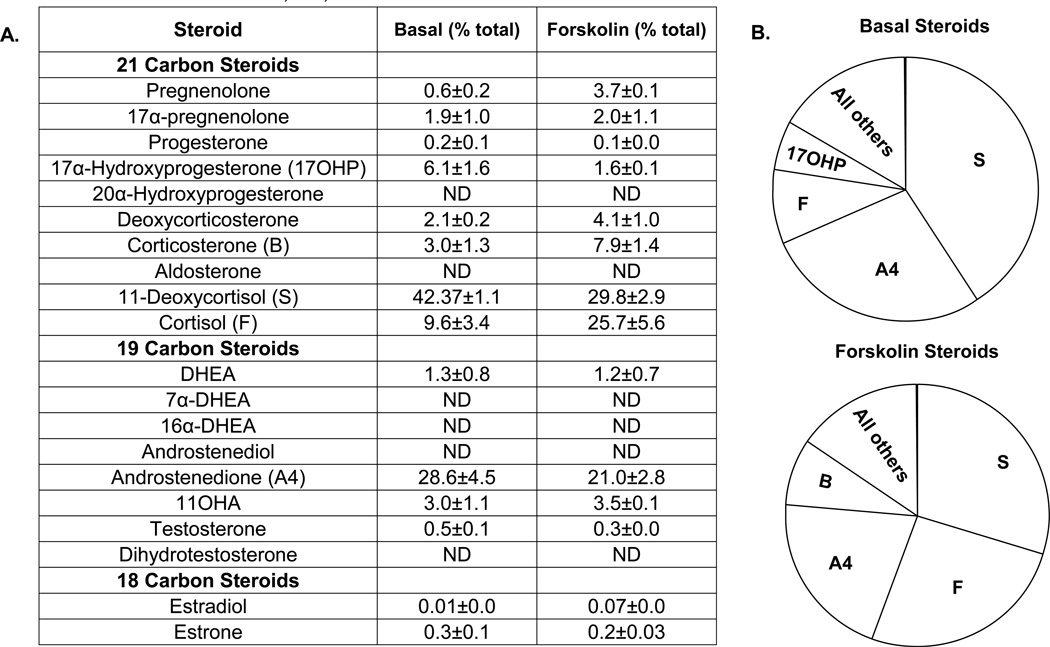
Similar experiments were performed in three different H295R cell models, followed by the same analysis of metabolomic profile (Figure 5). The only experimental change was the use of forskolin instead of ACTH as an agonist for steroidogenesis, because the HAC15, HAC13 and H295R only have minor response to ACTH. Different from primary cultures, H295R cells mainly produced 11-deoxycortisol under both basal and stimulated conditions (42 % versus 30 %), while androstenedione was also produced in large amount (29 % at basal versus 21 % after forskolin treatment). In addition, cortisol was a significant product of the H295R cells, representing 10 % of the steroids measured under basal conditions. The reason for a greater output of 11-deoxycortisol than cortisol may relate to the low basal expression of 11β-hydroxylase (CYP11B1) in H295R cells (data not shown). Treatment with forskolin increased the relative output of cortisol production to 26%, which corresponded to increases in CYP11B1 expression (data not shown). The forskolin stimulating effects on CYP11B1 was also supported by increases in other 11-hydroxylated steroids (11-fold for cortisol, 13-fold for corticosterone and 5-fold for 11-hydroxyandrostenedione). Although these steroids represent only a small portion of the total steroid made by the H295R (Figure 5), the two steroids with the greatest forskolin stimulated fold increase were estrone (20-fold) and estradiol (19-fold), confirming that forskolin activates aromatase expression and activity (Watanabe & Nakajin 2004). Cortisone and 11-dehydrocorticosterone concentrations were not quantified in this experiment.
Figure 5. Percentage of major steroids produced by H295R cells with/ without forskolin treatment.
Three independent strains of the H295R adrenal cell model were cultured as described in Materials and Methods, and plated at a density of 400,000 cells/well in 12-well dishes. Cells were treated with/without forskolin (10 µM) in 0.1% experimental medium for 48 h before harvest. The concentration of steroids in the medium was measured by LC-MS/MS. Percentage of each steroid was calculated by dividing the amount of individual steroid with total steroid. A. Table summarizing the percentage of individual steroid compared to total steroid produced by H295R cells. B. Pie chart summarizing the relative percentage of four major steroids produced in H295R cells. Data represent results from three independent experiments and shown as means ± S.E.M; ND, not detectable.
Discussion
Adrenal steroidogenesis has been extensively studied over the past 50 years (Grant 1962; Mason & Fraser 1975; Lebrethon et al. 1994a; Lebrethon et al. 1994b; Fottner et al. 1998), however the breadth of analytes considered in previous studies was limited by the available analytical technology. Previously reported in vitro studies have used isolated human adrenal cells as short term dispersed cultures as well as long-term monolayer cultures. Short term experiments using suspension cultures of human adrenocortical cells found a dose-dependent stimulatory effect of ACTH on cortisol, corticosterone, androstenedione and DHEA/DHEAS synthesis (Keymolen et al. 1976). Long-term monolayer cultures have been useful to study steroid production, however the steroid synthesis profile in those cells were not well characterized. Fottner, et al. tested the stimulating effects of ACTH only on cortisol and DHEA-S (Fottner et al. 1998), while Guillon tested four steroids (aldosterone, corticosterone, cortisol and 17-hydroxyprogesterone), but only after short term ACTH treatment (2 h) (Guillon et al. 1995). The studies by Lebrethon, et al. both used 2 h treatment times for ACTH and only changes in cortisol, aldosterone or DHEA were measured in individual studies (Lebrethon et al. 1994a; Lebrethon et al. 1994b). In the current study, we used primary cultures of human adrenocortical cells as a model to describe the broad effects of ACTH on steroidogenesis. Our LC-MS/MS quantification of 23 steroids in response to ACTH stimulation is a significant extension of previous reports with regard to the model, the effects of ACTH, and the analytical methodology, and provides a comprehensive overview of chronic ACTH effects on the steroid profile.
Our LC/MS-MS data coincides with previous reports that cortisol is the major steroid stimulated by ACTH (O'Hare & Neville 1974; Israeli et al. 1975; Kolanowski & Crabbe 1976). All the intermediates of the cortisol-producing pathway, including 17α-hydroxypregnenolone, 17α-hydorxyprogesterone and 11-deoxycortisol, were detectable under basal conditions and the presence of high levels of 11-deoxycortisol (147 pmol/mg protein) suggested that CYP11B1 is a late rate-limiting step in controlling cortisol production as has been suggested for the enzyme aldosterone synthase in aldosterone biosynthesis. After ACTH treatment for 48 h, cortisol production increased more than 60-fold, far exceeding that of its precursors, confirming the activating effect of ACTH on CYP11B1. Analysis of steroid production pre and post stimulation in the H295R cells also support a role for CYP11B1 expression as a gate-keeper for cortisol biosynthesis. Both cortisol and 11-hydroxyandrostenedione were significantly up-regulated by 48 h forskolin treatment and represented two of the major steroids produced by H295R cells after stimulation.
Human adrenocortical cells in vitro produce approximately 1/5 as much cortisone as cortisol in response to ACTH (Kolanowski & Crabbe 1976). Higher levels of cortisone and 11-dehydrocorticosterone were reported in adrenal slices (Mazzocchi et al.1998). Our data confirm that primary human adrenal cells can produce significant amounts of cortisone (16%) and 11-dehydrocorticosterone (6%) under basal condition, indicating the presence of functioning 11β-hydroxysteroid dehydrogenase in normal adrenocortical cells. LC-MS/MS data from adrenal vein samples confirmed the ability of adrenal to make cortisone (unpublished data). However, the relatively higher production of cortisone in vitro suggested the possibility of alternations in the 11-dehydrogenase activity that has been reported to occur during the isolation process (O'Hare 1973).
While both DHEA and DHEA-S responded well to ACTH stimulation, the relative production of these steroids was lower than might be expected from their reported abundance in systemic circulation. Several factors may influence the production of Δ5 C19 steroids by primary adrenal cell cultures, including alterations that occurred in vivo prior to cell isolation and the relative proportion of zona fasciculata and zona reticularis cells in the culture. According to the clinical data supplied for two of the adrenal donors, the adrenal glands came from young patients (age range 21–22 year old) who had sustained severe automobile accident injuries and died of intracranial hemorrhage/stroke. Adrenal glands were collected 2–3 days after brain death. Studies from several independent groups have reported decreased DHEA and DHEA-S levels in severely ill patients, while androstenedione and cortisol concentrations remained stable (Parker et al. 1985; Luppa et al. 1991; Beishuizen et al. 2002). Our data from primary cultures coincide with previous findings by showing decreased levels of DHEA in both basal and ACTH treated groups. Regarding cell types, zona reticularis cells mainly release DHEA (Endoh et al. 1996), which during our incubation period may be transformed by in the mixed fasciculata / reticularis cultures to Δ4 steroids (androstenedione and 11OHA), decreasing the apparent production of DHEA. However, the potential contribution of this activity to our results was not established.
Interestingly, androstenedione and 11OHA were produced in large amounts even in unstimulated AA cells (84 and 174 pmol/mg protein, respectively). Compared to the data of Fearon and colleagues (Fearon et al. 1998), our cells have a lower cortisol/androstenedione ratio (6 versus 20). The difference may relate to their use of freshly isolated cells or their treatment time of only 2 h. Alternatively, AA cells in monolayer culture may produce more androstenedione under chronic ACTH treatment.
Androstenedione is more readily converted to testosterone than DHEA or DHEA-S (Zipser et al. 1981). Testosterone functions as a more active androgen than androstenedione in circulation. Several studies have shown that the adrenal gland contributes to testosterone production either by direct secretion or peripheral conversion of adrenal-derived precursor (Keymolen et al. 1976; Macdonald & Matt 1984; Fenske 1986; Canonaco et al. 1989; Nakamura et al. 2009). In addition, the contribution of adrenal gland to circulating testosterone in women is particularly important. However, in the present study, we were not able to detect testosterone in the medium. However, this may relate to our relative low limit for detection of 50 ng/ml. The lack of detection in the primary cultures contrasts with our findings in the H295R adrenocortical cell line, which show detectable levels of testosterone even under unstimulated conditions (Nakamura et al. 2009). Our data support the concept that the H295R cancer cells produce more testosterone and estrone than the primary adrenal cell cultures. This is likely due to the high expression of 17β-hydroxysteroid dehydrogenase type 5 (HSD17B5) and significant expression of aromatase (CYP19) in the H295R cells compared to normal adrenal cells (Staels et al. 1993; Nakamura et al. 2009). It should also be noted that our primary cultures of adrenal cells only produced low levels of DHEA, suggesting that the mixed cortical cells may exhibit more of a fasciculata phenotype, while H295R produces a variety of adrenal steroids, including mineralocorticoids, glucocorticoids, estrogens and androgens. Further experiments will be needed to differentiate the role of ZF and ZR in testosterone production in human adrenal glands.
In summary, using the LC-MS/MS for steroid analysis we characterized the relative production of a wide range of steroids in primary cultures of normal adrenal cells and the H295R adrenal cell lines. This represents the first comprehensive study using LC- MS/MS to examine the production of steroids by adrenal cells in culture. The current report should serve as a reference for researchers studying adrenal steroidogenesis using either normal cells in culture or the H295R adrenal cell line.
Supplementary Material
Acknowledgments
We would like to thank Medical College of Georgia Adrenal Center for helping to collect adrenal samples and Dr. Mary Bassett who provided professional editorial assistance in the writing of the manuscript. This work was supported by National Institute of Health grants DK069950 (WER), DK43140 (WER), MCG Cardiovascular Discovery Institute Synergy Award (WER and MAE), and a Diabetes and Obesity Discovery Institute Pilot Grant (MAE). The authors declare there is no conflict of interest that would prejudice the impartiality of this scientific work.
Reference
- Arvat E, Di Vito L, Lanfranco F, Maccario M, Baffoni C, Rossetto R, Aimaretti G, Camanni F, Ghigo E. Stimulatory effect of adrenocorticotropin on cortisol, aldosterone, and dehydroepiandrosterone secretion in normal humans: dose-response study. J Clin Endocrinol Metab. 2000;85:3141–3146. doi: 10.1210/jcem.85.9.6784. [DOI] [PubMed] [Google Scholar]
- Bassett MH, Suzuki T, Sasano H, De Vries CJ, Jimenez PT, Carr BR, Rainey WE. The orphan nuclear receptor NGFIB regulates transcription of 3beta-hydroxysteroid dehydrogenase. implications for the control of adrenal functional zonation. J Biol Chem. 2004;279:37622–37630. doi: 10.1074/jbc.M405431200. [DOI] [PubMed] [Google Scholar]
- Beishuizen A, Thijs LG, Vermes I. Decreased levels of dehydroepiandrosterone sulphate in severe critical illness: a sign of exhausted adrenal reserve? Crit Care. 2002;6:434–438. doi: 10.1186/cc1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchaud CL, Goodyer CG, Lipowski LS. Progesterone and estrogen production by placental monolayer cultures: effect of dehydroepiandrosterone and luteinizing hormone-releasing hormone. J Clin Endocrinol Metab. 1983;56:761–766. doi: 10.1210/jcem-56-4-761. [DOI] [PubMed] [Google Scholar]
- Burger HG. Androgen production in women. Fertil Steril. 2002;77(Suppl 4):S3–S5. doi: 10.1016/s0015-0282(02)02985-0. [DOI] [PubMed] [Google Scholar]
- Canonaco M, Ando S, Valenti A, Tavolaro R, Panno ML, Maggiolini M, Dessi-Fulgheri F. The in-vitro transformation of [3H]dehydroepiandrosterone into its principal metabolites in the adrenal cortex of adult castrated male rats and following steroid treatment. J Endocrinol. 1989;121:419–424. doi: 10.1677/joe.0.1210419. [DOI] [PubMed] [Google Scholar]
- Chen C, Belanger A, Labrie F. Adrenal steroid precursors exert potent androgenic action in the hamster sebaceous glands of flank organs and ears. Endocrinology. 1996;137:1752–1757. doi: 10.1210/endo.137.5.8612511. [DOI] [PubMed] [Google Scholar]
- Di Blasio AM, Fujii DK, Yamamoto M, Martin MC, Jaffe RB. Maintenance of cell proliferation and steroidogenesis in cultured human fetal adrenal cells chronically exposed to adrenocorticotropic hormone: rationalization of in vitro and in vivo findings. Biol Reprod. 1990;42:683–691. doi: 10.1095/biolreprod42.4.683. [DOI] [PubMed] [Google Scholar]
- Endoh A, Kristiansen SB, Casson PR, Buster JE, Hornsby PJ. The zona reticularis is the site of biosynthesis of dehydroepiandrosterone and dehydroepiandrosterone sulfate in the adult human adrenal cortex resulting from its low expression of 3 beta-hydroxysteroid dehydrogenase. J Clin Endocrinol Metab. 1996;81:3558–3565. doi: 10.1210/jcem.81.10.8855801. [DOI] [PubMed] [Google Scholar]
- Fearon U, Clarke D, McKenna TJ, Cunningham SK. Intra-adrenal factors are not involved in the differential control of cortisol and adrenal androgens in human adrenals. Eur J Endocrinol. 1998;138:567–573. doi: 10.1530/eje.0.1380567. [DOI] [PubMed] [Google Scholar]
- Fenske M. Basal and HCG-stimulated testosterone production by interstitial cells of the Mongolian gerbil (Meriones unguiculatus)--II. Influence of glucocorticosteroids and steroidal precursors. Comp Biochem Physiol A Comp Physiol. 1986;85:273–279. doi: 10.1016/0300-9629(86)90250-1. [DOI] [PubMed] [Google Scholar]
- Fottner C, Engelhardt D, Weber MM. Regulation of steroidogenesis by insulinlike growth factors (IGFs) in adult human adrenocortical cells: IGF-I and, more potently, IGF-II preferentially enhance androgen biosynthesis through interaction with the IGF-I receptor and IGF-binding proteins. J Endocrinol. 1998;158:409–417. doi: 10.1677/joe.0.1580409. [DOI] [PubMed] [Google Scholar]
- Grant JK. Studies on the biogenesis of the adrenal steroids. Br Med Bull. 1962;18:99–105. doi: 10.1093/oxfordjournals.bmb.a069973. [DOI] [PubMed] [Google Scholar]
- Guillon G, Trueba M, Joubert D, Grazzini E, Chouinard L, Cote M, Payet MD, Manzoni O, Barberis C, Robert M, et al. Vasopressin stimulates steroid secretion in human adrenal glands: comparison with angiotensin-II effect. Endocrinology. 1995;136:1285–1295. doi: 10.1210/endo.136.3.7867583. [DOI] [PubMed] [Google Scholar]
- Haning RV, Jr., Austin CW, Carlson IH, Kuzma DL, Zweibel WJ. Role of dehydroepiandrosterone sulfate as a prehormone for ovarian steroidogenesis. Obstet Gynecol. 1985;65:199–205. [PubMed] [Google Scholar]
- Hornsby PJ, O'Hare MJ, Neville AM. Effect of ACTH on biosynthesis of aldosterone and corticosterone by monolayer cultures of rat adrenal zona glomerulosa cells. Biochem Biophys Res Commun. 1973;54:1554–1559. doi: 10.1016/0006-291x(73)91163-7. [DOI] [PubMed] [Google Scholar]
- Hornsby PJ, Gill GN. Hormonal control of adrenocortical cell proliferation. Desensitization to ACTH and interaction between ACTH and fibroblast growth factor in bovine adrenocortical cell cultures. J Clin Invest. 1977;60:342–352. doi: 10.1172/JCI108782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung TT, LeMaire WJ. The effects of corticotropin, opioid peptides and crude pituitary extract on the production of dehydroepiandrosterone and corticosterone by mature rat adrenal cells in tissue culture. J Steroid Biochem. 1988;29:721–726. doi: 10.1016/0022-4731(88)90174-4. [DOI] [PubMed] [Google Scholar]
- Israeli E, Levy J, Rosental E, Auslaender L, Peleg I, Barzilai D. A human adrenocortical adenoma in tissue culture. Morphology and hormone secretion. Isr J Med Sci. 1975;11:1106–1113. [PubMed] [Google Scholar]
- Keymolen V, Dor P, Borkowski A. Output of oestrogens, testosterone and their precursors by isolated human adrenal cells as compared with that of glucocorticosteroids. J Endocrinol. 1976;71:219–229. doi: 10.1677/joe.0.0710219. [DOI] [PubMed] [Google Scholar]
- Kolanowski J, Crabbe J. Characteristics of the response of human adrenocortical cells to ACTH. Mol Cell Endocrinol. 1976;5:255–267. doi: 10.1016/0303-7207(76)90088-5. [DOI] [PubMed] [Google Scholar]
- Labrie F, Belanger A, Luu-The V, Labrie C, Simard J, Cusan L, Gomez JL, Candas B. DHEA and the intracrine formation of androgens and estrogens in peripheral target tissues: its role during aging. Steroids. 1998;63:322–328. doi: 10.1016/s0039-128x(98)00007-5. [DOI] [PubMed] [Google Scholar]
- Lebrethon MC, Jaillard C, Naville D, Begeot M, Saez JM. Regulation of corticotropin and steroidogenic enzyme mRNAs in human fetal adrenal cells by corticotropin, angiotensin-II and transforming growth factor beta 1. Mol Cell Endocrinol. 1994a;106:137–143. doi: 10.1016/0303-7207(94)90195-3. [DOI] [PubMed] [Google Scholar]
- Lebrethon MC, Jaillard C, Naville D, Begeot M, Saez JM. Effects of transforming growth factor-beta 1 on human adrenocortical fasciculata-reticularis cell differentiated functions. J Clin Endocrinol Metab. 1994b;79:1033–1039. doi: 10.1210/jcem.79.4.7962271. [DOI] [PubMed] [Google Scholar]
- Luppa P, Munker R, Nagel D, Weber M, Engelhardt D. Serum androgens in intensive-care patients: correlations with clinical findings. Clin Endocrinol (Oxf) 1991;34:305–310. doi: 10.1111/j.1365-2265.1991.tb03771.x. [DOI] [PubMed] [Google Scholar]
- Macdonald GJ, Matt DW. Adrenal and placental steroid secretion during pregnancy in the rat. Endocrinology. 1984;114:2068–2073. doi: 10.1210/endo-114-6-2068. [DOI] [PubMed] [Google Scholar]
- Mahaffee D, Reitz RC, Ney RL. The mechanism of action of adrenocroticotropic hormone. The role of mitochondrial cholesterol accumulation in the regulation of steroidogenesis. J Biol Chem. 1974;249:227–233. [PubMed] [Google Scholar]
- Mason PA, Fraser R. Estimation of aldosterone, 11-deoxycorticosterone, 18-hydroxy-11-deoxy-corticosterone, corticosterone, cortisol and 11-deoxycortisol in human plasma by gas-liquid chromatography with electron capture detection. J Endocrinol. 1975;64:277–288. doi: 10.1677/joe.0.0640277. [DOI] [PubMed] [Google Scholar]
- Morgan MW, O'Hare MJ. Cytotoxic drugs and the human adrenal cortex: a cell culture study. Cancer. 1979;43:969–979. doi: 10.1002/1097-0142(197903)43:3<969::aid-cncr2820430328>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Hornsby PJ, Casson P, Morimoto R, Satoh F, Xing Y, Kennedy MR, Sasano H, Rainey WE. Type 5 17beta-hydroxysteroid dehydrogenase (AKR1C3) contributes to testosterone production in the adrenal reticularis. J Clin Endocrinol Metab. 2009;94:2192–2198. doi: 10.1210/jc.2008-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ney RL, Hochella NJ, Grahame-Smith DG, Dexter RN, Butcher RW. Abnormal regulation of adenosine 3',5'-monophosphate and corticosterone formation in an adrenocortical carcinoma. J Clin Invest. 1969;48:1733–1739. doi: 10.1172/JCI106139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare MJ. Corticosterone metabolism by rat adrenocortical cell suspensions and monolayer cultures: the effect of damage on 11-dehydrogenase activity. J Endocrinol. 1973;59:141–150. doi: 10.1677/joe.0.0590141. [DOI] [PubMed] [Google Scholar]
- O'Hare MJ, Neville AM. The trophic responses of cultured adrenocortical cells to ACTH. Proc R Soc Med. 1974;67:747. doi: 10.1177/003591577406700826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare MJ, Nice EC, Magee-Brown R, Bullman H. High-pressure liquid chromatography of steroids secreted by human adrenal and testis cells in monolayer culture. J Chromatogr. 1976;125:357–367. doi: 10.1016/s0021-9673(00)93831-7. [DOI] [PubMed] [Google Scholar]
- Parker LN, Levin ER, Lifrak ET. Evidence for adrenocortical adaptation to severe illness. J Clin Endocrinol Metab. 1985;60:947–952. doi: 10.1210/jcem-60-5-947. [DOI] [PubMed] [Google Scholar]
- Sharma RK. Regulation of steroidogenesis by adrenocorticotropic hormone in isolated adrenal cells of rats. J Biol Chem. 1973;248:5473–5476. [PubMed] [Google Scholar]
- Sirianni R, Mayhew BA, Carr BR, Parker CR, Jr., Rainey WE. Corticotropin-releasing hormone (CRH) and urocortin act through type 1 CRH receptors to stimulate dehydroepiandrosterone sulfate production in human fetal adrenal cells. J Clin Endocrinol Metab. 2005;90:5393–400. doi: 10.1210/jc.2005-0680. [DOI] [PubMed] [Google Scholar]
- Staels B, Hum DW, Miller WL. Regulation of steroidogenesis in NCI-H295 cells: a cellular model of the human fetal adrenal. Mol Endocrinol. 1993;7:423–433. doi: 10.1210/mend.7.3.8387159. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Nakajin S. Forskolin up-regulates aromatase (CYP19) activity and gene transcripts in the human adrenocortical carcinoma cell line H295R. J Endocrinol. 2004;180:125–133. doi: 10.1677/joe.0.1800125. [DOI] [PubMed] [Google Scholar]
- Zipser RD, Davenport MW, Martin KL, Tuck ML, Warner NE, Swinney RR, Davis CL, Horton R. Hyperreninemic hypoaldosteronism in the critically ill: a new entity. J Clin Endocrinol. 1981;53:867–873. doi: 10.1210/jcem-53-4-867. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.