Abstract
Background
The prevalence of ischemia and its prediction of events are unclear in outpatient diabetic patients in the modern era of intensive medical management. We sought to identify the prevalence of ischemia, subsequent cardiac events, and impact of gender, stress type, and symptom status on these findings in a cohort of outpatient, stable diabetic patients referred for SPECT myocardial perfusion imaging (MPI).
Methods and Results
The study cohort included 575 consecutive diabetic outpatients who underwent quantitative, gated-SPECT MPI. Clinical information, stress MPI variables, and cardiac events were prospectively collected and analyzed. The study population was at intermediate risk of coronary artery disease (CAD) or had known CAD (40.3%); 29% were asymptomatic at the time of stress testing. Scintigraphic ischemia and significant (≥10%) left ventricular (LV) ischemia were present in 126 (21.9%) and 29 (5.0%), respectively, and <1% had early revascularization. The risk of ischemia was increased >2-fold by male gender (p<0.001) but was not impacted by pharmacologic stress (p=0.15) or presence of symptoms (p=0.89). Over median 4.4 years follow-up, the rate of cardiac death/nonfatal myocardial infarction (MI) was moderate at 2.6%/year (cardiac death 0.8%/year) in the total cohort but was 5.7%/year in those with ischemia (p<0.001). Pharmacologic stress predicted a higher cardiac event rate (p<0.001) but symptoms did not (p=0.55).
Conclusions
This stable outpatient diabetic SPECT referral cohort had low rates of significant ischemia and early revascularization; an initially-low initial cardiac event rate increased after 2 years. Independent predictors of cardiac death/nonfatal MI were known CAD, pharmacologic stress, and MPI ischemia. Nearly one-third of those with events had a normal MPI, indicating a need for improved risk stratification.
Keywords: myocardial perfusion imaging, coronary artery disease, prognosis, diabetes mellitus
Diabetes mellitus (DM) is a highly-prevalent comorbidity that affects more than 194 million worldwide and is closely associated with coronary artery disease (CAD).1 CAD is the leading cause of mortality in diabetic patients (accounting for 65–70% of deaths) and is also associated with high morbidity.2, 3 The diagnosis of CAD is complicated by the often atypical presentation of diabetic patients due to concomitant autonomic neuropathy and other disorders. Moreover, it is important to identify CAD early in these patients to optimize medical therapy and lifestyle modifications. It is especially important to identify and aggressively treat those at the highest risk of events. For these reasons, there is a high rate of referral for myocardial perfusion imaging (MPI) when symptoms develop in this population. Similarly, many asymptomatic diabetic patients deemed at high risk for silent ischemia are referred for stress imaging. The prognostic impact of ischemia together with other clinical and stress variables has previously been reported.4–7 However, the prevalence of ischemia and its ability to predict those who experience future cardiac events is less clear in a consecutive group of stable outpatient diabetic patients with or without symptoms referred for MPI in the current era.
Accordingly, we sought to identify the prevalence and predictors of significant scintigraphic ischemia and subsequent cardiac events and assess the impact of gender, type of stress, and symptom status on these findings in a cohort of stable outpatients with diabetes referred for SPECT MPI.
Methods
This study comprised a retrospective analysis of prospectively collected data from the University of Virginia Nuclear Databank with subsequent prospective collection of outcomes data.
Study Cohort
All outpatients undergoing 99mTc-sestamibi single-photon emission computed tomography (SPECT) stress MPI from 2/1/2006 – 1/31/2007 for the detection of myocardial ischemia were identified. Those referred for indications other than detection of ischemia, such as viability assessment or post-acute coronary syndromes, were excluded. As shown in Figure 1, only those patients with DM, complete data, and follow-up of at least 1 year or a known event within the first year were considered for study inclusion. The cohort was stratified by presence of symptoms. Subjects were considered symptomatic if they were experiencing chest pain or shortness of breath thought to be of possible cardiac origin. A total of 575 consecutive patients comprised the final study cohort. Five subjects with early revascularization were censored prior to outcomes analysis, leaving 570 subjects as the follow-up cohort.
Figure 1.
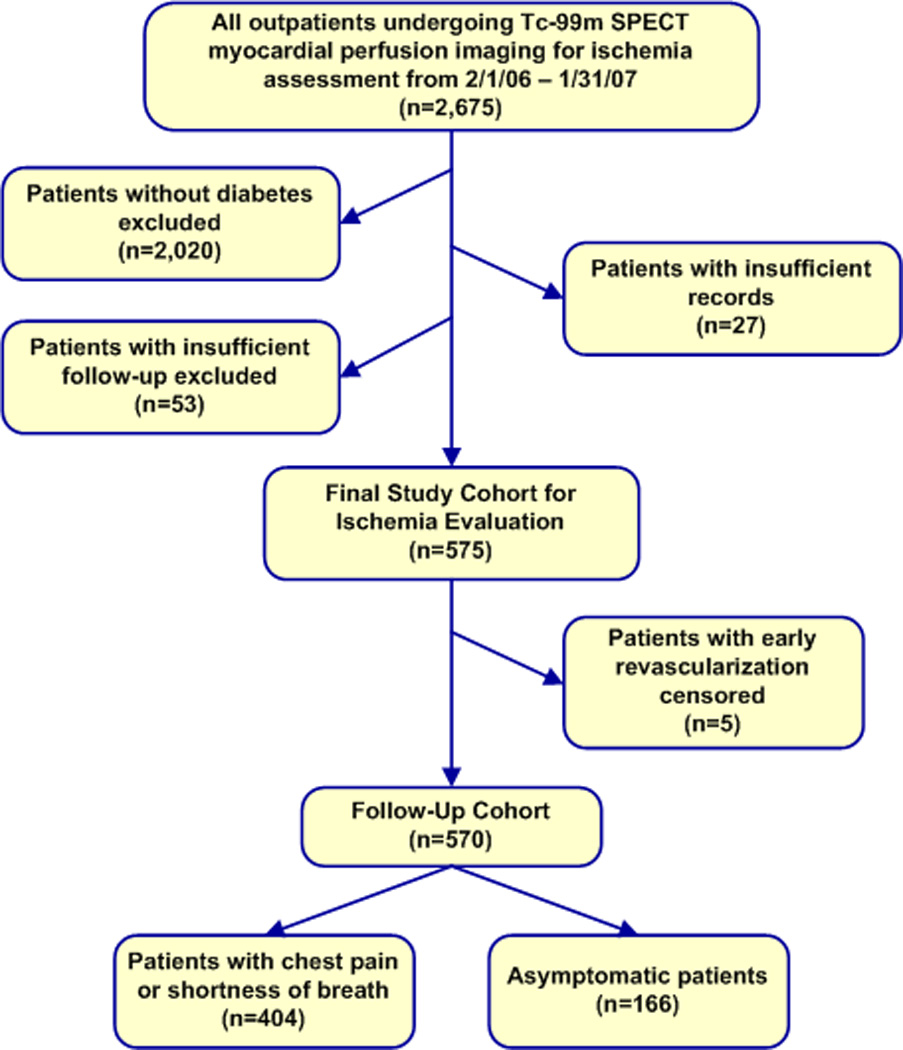
Study cohort derivation flowchart.
Clinical Information Collection and Management
Key data including baseline demographics, comorbidities, medication usage, physical examination findings, and baseline electrocardiographic findings were collected at the time of testing. This information was entered into the University of Virginia Nuclear Databank along with exercise and stress electrocardiographic parameters and imaging results. Collected imaging parameters included myocardial volumes, regional and global function, and perfusion variables. Protocol approval and waiver of informed consent were obtained from the University of Virginia Institutional Review Board.
Stress Myocardial Perfusion Imaging
Subjects underwent either exercise (n=200, 34.8%) or pharmacologic stress (n=375, 65.2%) with either adenosine (n=364, 97.1% of those undergoing pharmacologic testing, 63.3% of the entire cohort) or dobutamine (n=11, 2.9% of those undergoing pharmacologic testing, 1.9% of the entire cohort). Exercise stress was treadmill-based and was performed according to a Bruce or modified Bruce protocol in 99% of cases. Testing was symptom-limited unless prematurely terminated for reasons recommended in the most recent guidelines on exercise testing.8 Positive electrocardiographic evidence of ischemia was defined as horizontal or down-sloping ST-depression of ≥1mm measured 80ms after the J-point for 3 consecutive beats.
All subjects underwent stress gated-SPECT MPI according to a one- (for body mass index (BMI) <36) or two-day protocol (for BMI≥36) as described previously.9 One-day imaging was performed with 10mCi and 30mCi of 99mTc Sestamibi at rest and stress, respectively. Patients with a BMI of 36–45 received 30 mCi rest and stress doses over two days, and those with a BMI >45 received 45 mCi at rest and with stress.
Supine images were acquired with a dual-head GE Infinia camera with low-energy, high-resolution collimators. Each camera head acquired 180° of data by 60 projections at 30–40 seconds per projection using a standard 99mTc energy window. The data from the 2 heads were combined to give 360° of coverage.
Radionuclide Image Processing
All radionuclide images and associated data were processed according to standard protocols using proprietary V-Quant software (Charlottesville, VA).10 Myocardial perfusion was calculated as the relative percent tracer uptake in each of the 17 segments of a standard model.11 Uptake deviating ≥2 standard deviations from institution-derived gender-specific normal databases was flagged as abnormal in a reversible or fixed pattern. The transient ischemic dilation (TID) ratio was defined as the ratio of left ventricular (LV) volumes on ungated images at stress and rest.12 A TID ratio ≥1.24 was considered abnormal.13
Gated images were used to generate systolic and diastolic volumes, end-systolic and end-diastolic volumes indexed to body surface-area (ESVI, EDVI), segmental and global LV thickening fractions, and the LV ejection fraction (EF).10 Thickening fractions >2 standard deviations below the normal database mean were considered abnormal. The LVEF was measured on the post-stress gated images.
Experienced nuclear cardiology specialists used this quantitative data as well as visual image analysis to interpret each MPI study as described previously and briefly summarized here.9, 10 All borderline or abnormal studies were reclassified by the consensus of two additional readers blinded to additional patient information. Readers assigned a score to each segment (0–4 for normal, mild, moderate, severe, and absent uptake respectively). The semi-quantitative summed stress, rest, and difference scores were calculated from these segmental values, with the five apical segments receiving 40% weighting (each apical segmental score x 0.4) to partially correct for the over-representation of the apex in the standard 17-segment model.
Finally, the “percent myocardial ischemia” was obtained by dividing the difference between summed stress and summed rest scores by 56, the maximum possible difference. This score, modified from Hachamovitch et al, provides a logical semi-quantitative measure which combines both extent and severity of LV inducible ischemia.14, 15 LV ischemia of 1–9% was considered mild-moderate, and ≥10% LV ischemia was considered significant.
Coronary Angiography
Coronary angiography was performed and interpreted by experienced operators using computer-assisted quantitative angiography (AGFA Heartlabs; Greenville, SC). Stenoses of ≥70% or equivocal stenoses of 50–69% with a fractional flow reserve of<0.8 were considered significant. Operators had access to all clinical data and SPECT results during study interpretation.
Follow-Up
Data on all-cause and cardiac mortality, nonfatal MI, and both early and late revascularization were collected initially through mailed questionnaires. All subjects not responding received at least 3 follow-up telephone contact attempts, with additional calls made to their primary cardiologist and primary care physician. Intensive chart review was subsequently performed and events were confirmed when possible. A documented visit to a cardiologist or an annual visit with a primary care physician was considered sufficient to rule-out an intervening event if none was described. Death certificates were obtained when available for deaths outside the healthcare system.
Events were classified by a reviewer blinded to the stress SPECT results to minimize bias. Cardiac death was defined as any death with a cardiac cause or without a clear non-cardiac cause. Indeterminate deaths were classified as cardiac. A nonfatal MI was recorded for any troponin elevation ≥2 times the upper limit of normal, with or without typical ischemic electrocardiographic changes, in the setting of a history consistent with an acute coronary syndrome. Coronary revascularization procedures included percutaneous coronary interventions and coronary artery bypass grafting. Late revascularization was designated as any coronary revascularization performed later than 12 weeks after the initial MPI study unless documentation linked it to the original MPI results.
Statistical Analyses
The clinical characteristics, medication usage, findings on exercise stress and MPI, and clinical outcomes were given as percentages for categorical variables and as medians with 25th and 75th percentiles for continuous variables. Continuous and categorical variables were compared using Wilcoxon rank-sum and chi-square analysis or Fisher’s Exact Testing where appropriate.
Event rates were calculated through person-years analysis. The total events in a subgroup over the entire study period were divided by the sum of the years of follow-up for all patients in that subgroup. This value was adjusted for one person-year of follow-up to give an annualized rate. Subjects with an event within the first year were included in the analysis. Subjects with non-cardiac death were censored at the time of death. Unadjusted survival analysis was performed using Kaplan-Meier methodology.16 Survival endpoints included freedom from cardiac death, cardiac death/nonfatal MI, and cardiac death/nonfatal MI/late revascularization. Survival free of cardiac death/nonfatal MI was also stratified by the %LV ischemia on SPECT imaging, presence or absence of ischemia, and type of stress. Cox proportional hazards modeling was performed to identify the significant predictors of cardiac death or nonfatal MI. Candidate variables with p<0.10 on univariable analysis were entered into a multivariable Cox proportional hazards model using forward stepwise selection with age and gender forced into the model. Prior MI was not included in the multivariable model, as a similar variable, known CAD, had a higher Chi-square on univariable analysis. The incidences of cardiac death and nonfatal MI by degree of ischemia were compared by Chi-square analysis or Fisher’s Exact Testing where appropriate. Incremental Chi-square analysis was performed by assessing the change in global Chi-square with the stepwise addition of symptoms and ischemia to a Cox proportional hazards model of the following clinical variables found to be significant univariable predictors of cardiac death/nonfatal MI: age, gender, dyslipidemia, known CAD, history of MI, prior revascularization, and type of stress.
All analyses were completed using SAS version 9.2.2. (Cary, NC) and Med Calc version 12.3 (Maria kerke, Belgium).
Results
Study Population
The study population consisted of 575 patients with DM referred for SPECT imaging for the evaluation of ischemia. Of these, 409 were symptomatic, the majority with chest pain (74.0%). Dyspnea was the sole presenting symptom in the remaining 26.0%, and 42.5% had it as one of their presenting complaints. Angina was classified as typical in 58.4% of those with chest pain. In the symptomatic subgroup, preoperative risk assessment was an additional reason for referral in 34 patients (8.3%), whereas it was a much more frequent reason in asymptomatic subjects (58.2%).The clinical characteristics of this cohort are provided in Table 1 stratified by presence of symptoms of chest pain or shortness of breath. Symptomatic subjects were more often female but had a higher prevalence of CAD risk factors including hyperlipidemia, obesity, and tobacco use. They were more likely to be on aspirin, statins, and oral hypoglycemic agents. Only 3 symptomatic patients (0.6%) were considered low pretest probability for CAD by the Diamond and Forrester criteria. The remaining symptomatic patients were at intermediate or high probability.8 The majority of patients had type 2 DM (97.3%), with a mean HgA1c of 7.5. The aggregate rate of microvascular complications was 32.7%, with 6.3% having retinopathy, 13.6% neuropathy, and 22.0% nephropathy.
Table 1.
Clinical characteristics of the study cohort stratified by presence or absence of chest pain or shortness of breath
| Clinical Characteristic | Total Cohort (n (%)) |
Symptoms (n (%)) |
Asymptomatic (n (%)) |
P-Value |
|---|---|---|---|---|
| Total patients | 575 | 409 | 166 | |
| Age (median (25th, 75th percentiles)) | 61 (53, 68) | 61 (53, 68) | 59 (52, 68) | 0.87 |
| Male | 295 (51.3) | 193 (47.2) | 102 (61.5) | 0.004* |
| Hypertension | 490 (85.2) | 346 (84.6) | 144 (86.8) | 0.51 |
| Hyperlipidemia | 431 (75.0) | 321 (78.5) | 110 (66.3) | 0.002* |
| Obesity (BMI ≥30)† | 383 (66.6) | 297 (72.6) | 86 (51.8) | <0.001* |
| Tobacco use | 163 (28.3) | 128 (31.3) | 35 (21.1) | 0.014* |
| Known coronary artery disease | 232 (40.3) | 160 (39.1) | 72 (43.4) | 0.35 |
| Prior myocardial Infarction | 134 (23.3) | 96 (23.5) | 38 (22.9) | 0.88 |
| Prior revascularization | 184 (32.0) | 130 (31.8) | 54 (33.1) | 0.76 |
| Medication usage | ||||
| ACE-inhibitor or ARB† | 372 (64.7) | 284 (69.4) | 88 (53.0) | <0.001* |
| Aspirin | 346 (60.7) | 259 (63.3) | 87 (54.0) | 0.041* |
| Beta-blocker | 322 (56.0) | 224 (54.8) | 98 (59.0) | 0.35 |
| Day of Test | 81 (14.1) | 53 (13.0) | 28 (16.9) | 0.22 |
| Clopidogrel | 44 (7.7) | 36 (8.8) | 8 (4.8) | 0.10 |
| Statin | 339 (59.0) | 257 (62.8) | 82 (50.9) | 0.009* |
| Insulin | 270 (47.0) | 187 (45.7) | 83 (50.0) | 0.37 |
| Oral hypoglycemic agent | 329 (57.2) | 256 (62.6) | 74 (44.6) | <0.001* |
ACE=angiotensin converting enzyme; ARB=Angiotensin receptor blocker; BMI=body-mass index.
Stress and Perfusion Imaging Findings
Stress parameters and imaging findings by symptom status are provided in Table 2, separated into exercise versus pharmacologic stress. Asymptomatic patients undergoing exercise stress were more likely to achieve a high exercise workload of ≥10 METS (58.3% versus 23.1%;p =0.007). Despite being able to attain a higher workload, asymptomatic patients were more likely to have a summed stress score >3 (29.7% versus 12.9%, p=0.011). There were no significant differences in summed stress or rest scores by symptom status in subjects undergoing pharmacologic stress.
Table 2.
Findings on exercise and pharmacologic stress and myocardial perfusion imaging dichotomized by presence or absence of chest pain or shortness of breath
| Characteristic | Exercise Stress (n=200) | Pharmacologic Stress (n=375) | ||||
|---|---|---|---|---|---|---|
| Symptomatic (n (%)) |
Asymptomatic (n(%)) |
P-value | Symptomatic (n(%)) |
Asymptomatic (n(%)) |
P-value | |
| Total patients | 163 | 37 | 246 | 129 | ||
| METS achieved (median (25th 75th percentiles)* | 8.0 (6.6, 10.0) | 8.6 (7.0, 10.1) | 0.61 | Not applicable. | ||
| ≥10 METS achieved* | 37 (23.1) | 7 (58.3) | 0.007 | |||
| Peak exercise heart rate | 144 (133, 157) | 140 (132, 151) | 0.31 | |||
| Maximum systolic BP* | 199 (180, 222) | 204 (178, 223) | 0.64 | |||
| Maximum diastolic BP* | 85 (75, 97) | 89 (73, 100) | 0.49 | |||
| Chest pain during stress | 23 (14.1) | 1 (2.7) | 0.054 | |||
| ≥1mm stress ST-depression | 19 (11.7) | 7 (18.9) | 0.28 | 8 (3.2) | 5 (3.9) | 0.77 |
| LVEF* | 63 (59, 67) | 62 (58, 67) | 0.28 | 63 (54, 68) | 63 (54, 68) | 0.73 |
| LVEF<45%* | 8 (4.9) | 5 (13.5) | 0.068 | 25 (10.2) | 7 (5.4) | 0.12 |
| ESV index ≥25 mL/m2 *,† | 19 (12.0) | 8 (22.9) | 0.11 | 44 (18.0) | 29 (22.7) | 0.28 |
| Summed stress score | 0.023 | 0.51 | ||||
| 0–3 | 142 (87.1) | 26 (70.3) | 202 (82.1) | 109 (84.5) | ||
| 4–8 | 9 (5.5) | 6 (16.2) | 16 (6.5) | 10 (7.8) | ||
| >8 | 12 (7.4) | 5 (13.5) | 28 (11.4) | 10 (7.8) | ||
| Summed rest score | 0.084 | 0.51 | ||||
| 0–3 | 154 (94.5) | 33 (89.2) | 219 (89.0) | 119 (92.3) | ||
| 4–8 | 7 (4.3) | 1 (2.7) | 13 (5.3) | 6 (4.7) | ||
| >8 | 2 (1.2) | 3 (8.1) | 14 (5.7) | 4 (3.1) | ||
BP=blood pressure; ESV=end-systolic volume; LVEF=left-ventricular ejection fraction; METS=metabolic equivalents.
End-systolic volume indexis missing in 3asymptomatic and 5 symptomatic patients in the exercise cohort and 1 asymptomatic and 1 symptomatic patient in the pharmacologic cohort.
In the total cohort, ischemia was present in 126 individuals (21.9%). The majority of these (97/126;77.0 % of the ischemic subgroup, 16.9% of the overall cohort) had mild-moderate ischemia (<10% of the LV). Significant LV ischemia of ≥10% was present in 29 patients (23.0% of those with ischemia, 5.0% of the entire cohort). Of these 29, 14 had ≥15% LV ischemia, of whom 10 had known angiographic CAD and 9 had a history of prior revascularization. Only 6 subjects (4.8% of those with ischemia and 1.5% of the entire cohort) had high levels of LV ischemia defined as ≥20%. Fixed perfusion defects were found in 62 patients (10.8%). No perfusion defects were observed in the remaining 412 patients (71.7% of the entire cohort).
An abnormal resting electrocardiogram leading to non-diagnostic stress electrocardiography was present in 80 (13.9%), of whom 16 (2.8%) had a LBBB, 8 (1.4%) had a paced rhythm, and 56 (9.7%) had resting ST-depression ≥1mm. Seventeen (3.0%) had electrocardiograms with left ventricular hypertrophy but minimal baseline ST-changes that were still considered diagnostic.
Non-Perfusion Imaging Variables
In patients without perfusion defects, 90 patients (21.8%) had one or more high-risk non-perfusion markers for CAD on their imaging study. These markers included a post-stress LVEF <50% in 19 subjects (4.6%) and an ESVI ≥25 mL/m2 in 29 subjects (7.0%). Of the 412, 337 patients had both stress and rest images for the measurement of TID (the remainder had stress-only imaging). Of these, 14 (4.2%) had TID by quantitative criteria. The most prevalent non-perfusion finding was the presence of segmental wall-motion abnormalities involving ≥4 segments, which occurred in 71 patients (17.2%). Of note, 12 of these 71 (16.9%) had a paced rhythm or LBBB that may explain the abnormal findings.
Relationship of Symptoms, Type of Stress, Gender, and Exercise Workload to Ischemia
The prevalence and degree of scintigraphic ischemia are stratified by symptom status, type of stress, gender, and exercise workload achieved in Figure 2. There were no significant differences in any or significant ischemia (≥10%) based on either presence of absence of symptoms (Figure 2a) or type of stress (Figure 2b). However, male gender was associated with a >2-fold increase in the prevalence of both any and significant LV ischemia (Figure 2c). We have previously shown that high cardiac workload is associated with a low risk of significant LV ischemia.9 In the subgroup of 200 patients who underwent exercise stress, there were 1.6-fold and 3.8-fold increases in any and ≥10% LV ischemia in those unable to reach 10-METS of exercise workload (Figure 2d). These differences were not statistically significant, likely due to decreased power from the low absolute number of individuals who achieved ≥10 METS on exercising testing.
Figure 2.
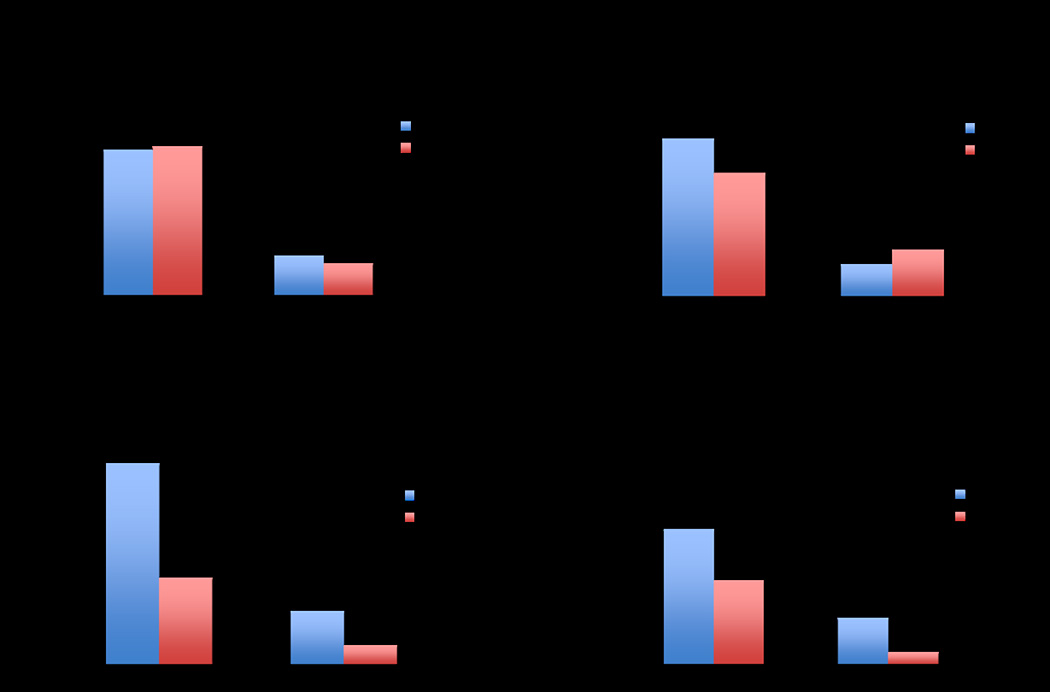
Prevalence of any and significant (≥10%) LV ischemia stratified by: (a) presence of symptoms; (b) type of stress; (c) gender; and (d) exercise workload attained (in the 200 patients undergoing exercise stress). Asympt=asymptomatic; LV=left ventricular; METS=metabolic equivalents; Pharm=pharmacologic.
Follow-Up and Outcomes
Early catheterization within 12 weeks as a result of the stress findings occurred in 12/575 patients (2.1%), of whom 8 had obstructive coronary artery disease (66.7%). Of these 8, five had early revascularization (percutaneous coronary interventions in 4, 1 with bypass surgery). Thus, the rate of early revascularization in the entire cohort was 0.9% (5/575). These five patients were censored from the subsequent outcomes analysis. However, it should be noted that 2 of the 5 (40%) undergoing early revascularization had subsequent nonfatal MIs.
The remaining 570 patients comprised the follow-up cohort; the median follow-up time was 4.4 years (25th and 75th percentiles 2.5 and 5.1 years, respectively). Median follow-up time was slightly longer for the asymptomatic cohort (4.9 versus 4.3 years). There were 60 total deaths, of which 18 were classified as cardiac (30.0%). The remaining 42 deaths were due to cancer (approximately 60%) or other non-cardiac causes. The incidences of cardiac death, nonfatal MI, and late revascularization over the median 4.4-year follow-up period are provided in Table 3 stratified by the presence or absence of chest pain or shortness of breath. There were 92 total cardiac events. No difference in incidence of events was observed between symptomatic and asymptomatic patients. The annualized rate of cardiac death/nonfatal MI was 10-fold higher in those receiving pharmacologic (6.1%/year) versus exercise stress (0.6%/year).
Table 3.
Total incidence of cardiac death or nonfatal MI over 4.4years median follow-up and corresponding annualized event rates
| Outcome | Total (n (%/yr)) |
Symptomatic (n (%/yr)) |
Asymptomatic (n (%/yr)) |
P-value |
|---|---|---|---|---|
| Total patients | 570 | 404 | 166 | |
| Cardiac death | 18 (0.8) | 11 (0.7) | 7 (1.1) | 0.32 |
| Nonfatal myocardial infarction | 39 (1.8) | 30 (2.0) | 9 (1.4) | 0.15 |
| Late revascularization | 35 (1.6) | 24 (1.6) | 11 (1.7) | 0.76 |
| Total Cardiac Events | 92 (4.2) | 65 (4.3) | 27 (4.1) | 0.96 |
Survival Analysis
The annualized rate of cardiac death or nonfatal MI was 2.6%/year. Total cardiac events (cardiac death, nonfatal MI, and late revascularization) occurred at a rate of 4.2%/year. The majority of these events were nonfatal MIs, with an annual cardiac death rate of only 0.8%. Kaplan-Meier analyses of the cumulative incidences of cardiac death, cardiac death/nonfatal MI, and cardiac death/nonfatal MI/late revascularization are depicted in Figure 3.There is a visually-apparent low rate of each of these events through the first two years of follow-up, at which time the rate appears to increase. The majority of events (77.2%) occurred 2 or more years after the index MPI study.
Figure 3.
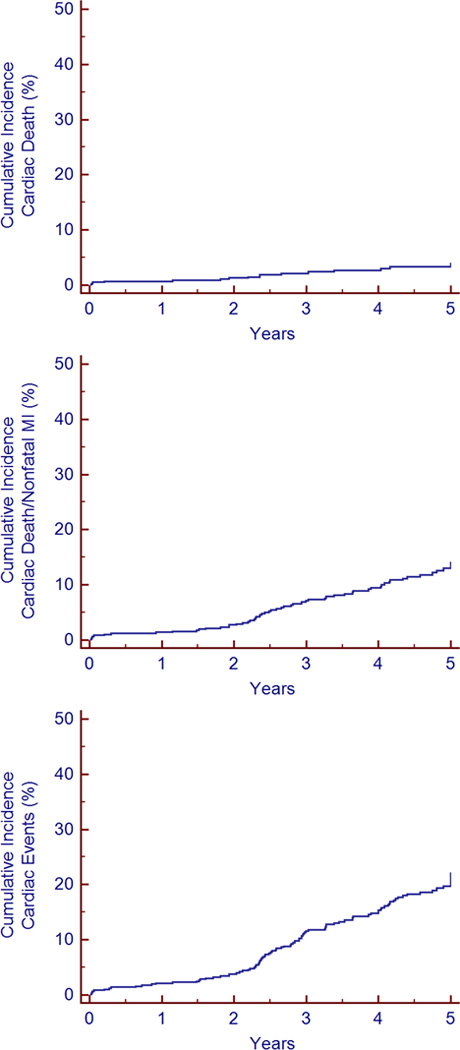
Kaplan-Meier analyses of the cumulative incidence of cardiac death, cardiac death/nonfatal MI, and cardiac events (cardiac death, nonfatal MI, and late revascularization) over 5 years of follow-up. MI=myocardial infarction.
Predictors of Hard Cardiac Events
The results of the univariable and multivariable Cox proportional hazards analyses predicting cardiac death or nonfatal MI are given in Table 4. The predictors that remained significant after multivariable adjustment were known CAD, pharmacologic stress, and a 5% increase in LV ischemia. When age and gender were not forced into the model, a 5% decrease in LVEF became significant (p=0.036) with a hazard ratio of 1.12 (1.01–1.25). Kaplan-Meier survival curves stratified by presence of symptoms and type of stress are given in Figure 4. Symptoms were not a significant predictor of cardiac death/nonfatal MI on Cox analysis (p=0.55), and the Kaplan-Meier curves are closely aligned (Figure 4a).In contrast, the Kaplan-Meier curves stratified by type of stress show an early divergence (p<0.001, Figure 4b). Only 8 of the 57 cardiac deaths and nonfatal MIs (14.0%) occurred in patients undergoing exercise stress. Exercise variables were not included in the Cox model, as only 197/570 (34.6%) underwent exercise stress, and there were only 8 events in the exercise subgroup versus 49 in the pharmacologic stress patients. Although there is limited power due to the low event rate in the exercise subgroup, SPECT ischemia was not a significant predictor of cardiac death/nonfatal MI in univariable Cox proportional hazards analysis. The annual cardiac death or MI rate was 1.4% for those with exercise-induced ischemia versus 0.9% for those without ischemia (p=0.63). Moreover, only 1 nonfatal MI and no cardiac deaths occurred in patients achieving ≥10 METS of exercise workload. If late revascularization is included in a combined endpoint with cardiac death and nonfatal MI, then ischemia becomes a significant predictor of this endpoint (hazard ratio 5.2 (1.1–26.0, p=0.043)).
Table 4.
Univariable and multivariable Cox proportional hazards analysis predicting cardiac death or nonfatal myocardial infarction
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Predictors | X2 | Hazard Ratio (95% CI)* |
p-value | X2 | Hazard Ratio (95% CI) |
p-value |
| Age (increase by 10) | 9.6 | 1.31 (1.10–1.56) | 0.002† | 1.2 | 1.14 (0.91–1.43) | 0.27 |
| Male gender | 5.5 | 1.93 (1.12–3.36) | 0.008† | 1.9 | 1.51 (0.85–2.69) | 0.16 |
| Hypertension | 0.0 | 1.05 (0.59–1.86) | 0.86 | |||
| Hyperlipidemia | 6.1 | 2.05 (1.16–3.63) | 0.013 | |||
| Tobacco use | 0.1 | 0.72 (0.69–1.71) | 0.72 | |||
| Ischemic symptoms | 0.3 | 1.14 (0.72–1.81) | 0.58 | |||
| Body mass index ≥30 | 1.8 | 0.70 (0.41–1.18) | 0.18 | |||
| Known CAD* | 33.4 | 3.75 (2.40–5.88) | <0.001† | 10.6 | 2.76 (1.50–5.08) | <0.001† |
| Prior MI* | 13.5 | 2.20 (1.44–3.34) | <0.001† | |||
| Prior revascularization | 19.9 | 2.56 (1.69–3.87) | <0.001† | |||
| Pharmacologic stress | 9.7 | 3.65 (1.62–8.24) | 0.002† | 11.7 | 3.76 (1.76–8.03) | 0.001† |
| ST-Depression ≥1mm | 0.2 | 1.17 (0.54–2.52) | 0.70 | |||
| LVEF (5% Decrements)*ǂ | 18.1 | 1.19 (1.10–1.29) | <0.001† | |||
| LV Ischemia (5% Increments)*ǂ | 15.1 | 1.46 (1.21–1.77) | <0.001† | 6.5 | 1.32 (1.07–1.62) | 0.003† |
CI=confidence interval; CAD=coronary artery disease; EF=ejection fraction; LV=left ventricular; MI=myocardial infarction.
Prior MI was considered to provide redundant information to known CAD and was not included in the multivariable model.
LVEF and LV ischemia are coded in 5% increments so that the hazard ratio represents the increased risk of events with a 5% decrease in LVEF and 5% increase in LV ischemia, respectively.
Figure 4.
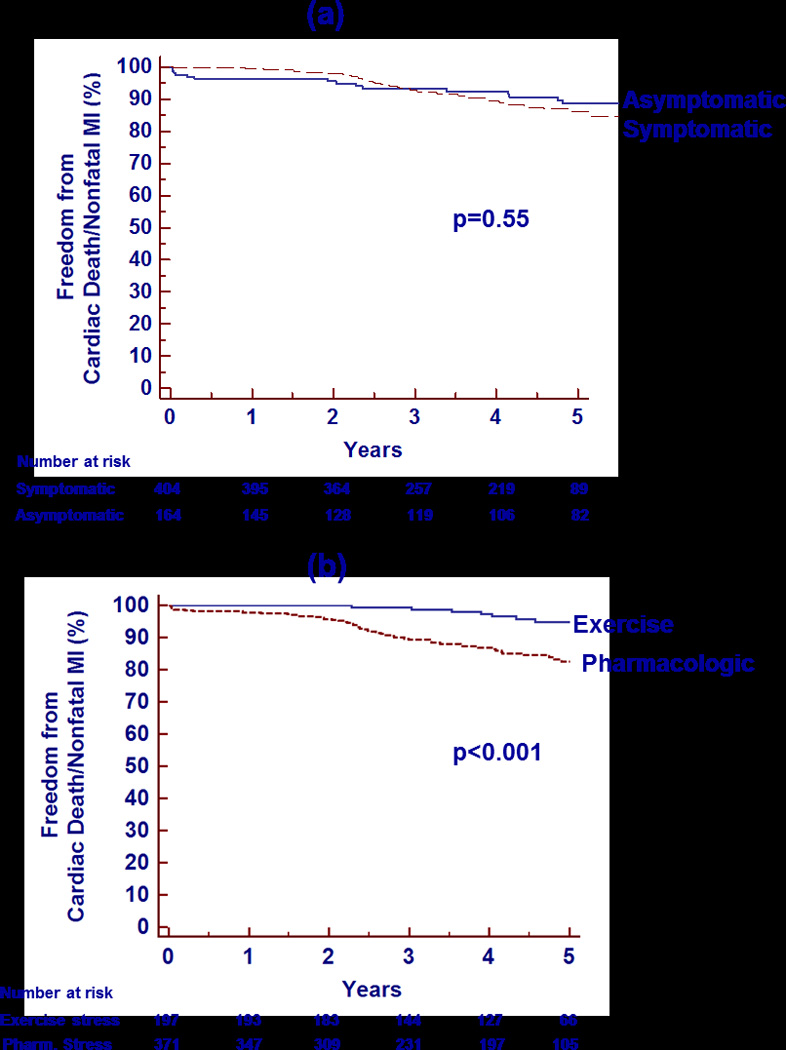
Kaplan-Meier analysis of survival free from cardiac death/nonfatal MI over 5 years of follow-up stratified by the presence of symptoms (a) and the type of stress (b). MI=myocardial infarction.
Relationship Between Events and Ischemia
Incremental chi-square analysis showed that the presence or absence of symptoms did not provide incremental prognostic value (p=0.24), while the presence of ischemia on the index SPECT MPI study did have incremental value (p=0.007). Kaplan-Meier survival analysis of cardiac death/nonfatal MI stratified by degree of ischemia shows increased events for patients with both mild (1–9% of the LV) and significant (≥10% of the LV) ischemia on the initial MPI study (p<0.001, Figure 5). Compared with no ischemia on the index MPI, the annualized rate of cardiac death/nonfatal MI was significantly higher for 1–9% LV ischemia (6.0% versus 1.8%, p<0.001) and trended higher for ≥10% LV ischemia (4.5% versus 1.8%, p=0.05), but did not differ significantly between the two ischemia subgroups (p=0.57). The annualized rate for patients with any ischemia was 5.7%/year (p<0.001 versus no ischemia).
Figure 5.
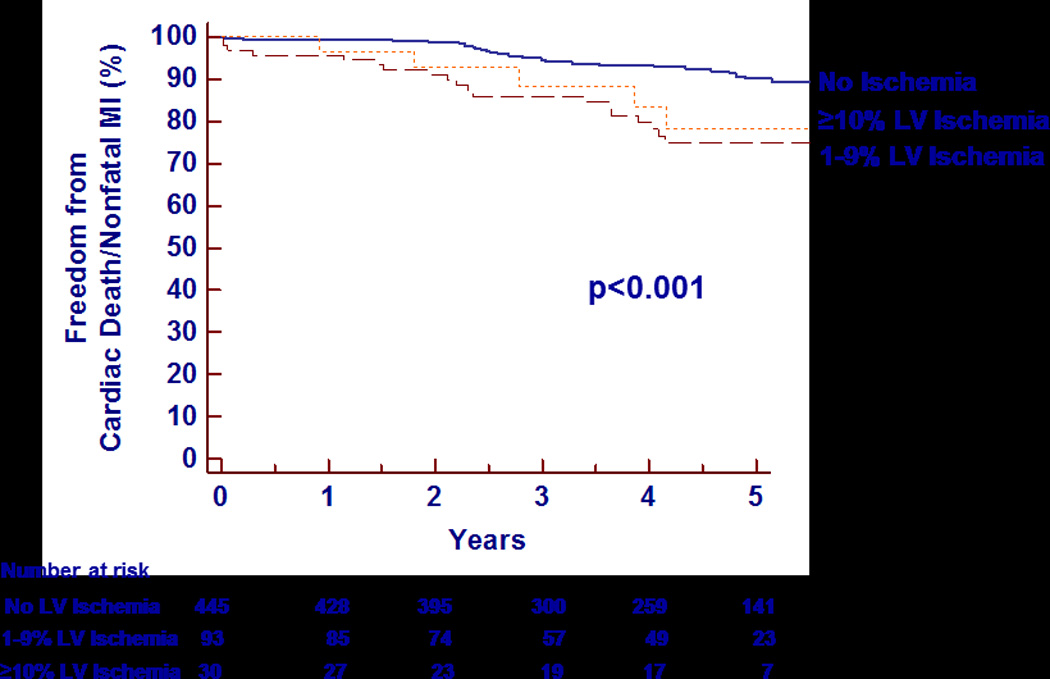
Kaplan-Meier analysis of survival free from cardiac death/nonfatal MI over 5 years of follow-up stratified by the degree of left ventricular ischemia. LV=left ventricular; MI=myocardial infarction.
Despite the higher event rates in those with ischemia, 56.1% of the cardiac death/nonfatal MIs (32/57) occurred in the 446 patients without any ischemia. Some had nonischemic scan abnormalities as discussed below. Only 2 of the 18 (11.1%) patients suffering a subsequent cardiac death had significant (≥10%) LV ischemia present on their index MPI. Some deaths in these patients may be attributed to acute plaque ruptures in the absence of high-grade stenoses.
High Risk Findings Other Than Reversible Perfusion Defects
Of the 32 patients without ischemia but who experienced a cardiac death or nonfatal MI, 3 had fixed perfusion defects. In the remaining 29 patients with normal perfusion and a subsequent hard cardiac event, a moderate number (12, 41.3%) had a high-risk non-perfusion finding on their index MPI study. One (3.4%) had ischemic ST-depression on his stress electrocardiogram. A LV ejection fraction <50% was measured in 3 of these 29 subjects (10.3%). Segmental wall-motion abnormalities were more common but still infrequent, with 7 (24.1%) having ≥4 segments with abnormal wall motion. An ESVI ≥ 25 mL/m2 was present in 4 patients (13.8%). Finally, 2 (6.9%) subjects had a TID ratio ≥1.24. Thus, of all 57 patients who experienced a cardiac death/nonfatal MI in the follow-up cohort, 17 (29.8%) had completely normal stress MPI scans without perfusion defects or other high-risk non-perfusion findings.
Discussion
The prognosis of patients with diabetes mellitus and acute coronary syndromes or known ischemia has been documented in multiple prior studies, most notably the BARI-2D study.4, 17–19 The prevalence of ischemia and outcomes has also been studied extensively in symptomatic and asymptomatic diabetic patients.20–26 In this study of consecutive ambulatory diabetic patients referred for stress SPECT MPI, we report a low overall cardiac death/nonfatal MI rate of 2.6% annually. Only 5% of this diabetic cohort had ≥10% LV ischemia. As expected, the event rate of patients with ischemia was significantly greater than seen in patients with normal scans. The event rate was lower than observed in diabetic patients with normal or abnormal scans as reported in a pooled analysis of studies published before 2004, many of which were performed in the 1990s.7 One presumed explanation for this lower event rate is that more diabetic patients without known CAD are now placed on medications such as statins, ACE-inhibitors, and beta-blockers than in prior years. Another potential explanation for the difference in our event rates versus those reported in the pooled analysis 7 is that our cohort was solely comprised of outpatients, and the event rates analyzed were for ischemia versus nonischemia rather than normal versus abnormal scans. Furthermore, our study showed no difference in prevalence of ischemia or event incidence in symptomatic versus asymptomatic patients. Patients who underwent pharmacologic stress had a higher event rate than those undergoing exercise stress.
Population Makeup
This study population was evenly split between the sexes with an intermediate age range and high levels of cardiovascular risk factors, as seen in both DIAD and BARI-2D.17, 21, 27 Approximately 30% were asymptomatic at the time of testing. A moderate number had known CAD and prior revascularization. Since 23% had a prior MI, 40% had known CAD, and nearly one-third had prior revascularization, the study cohort can be considered at intermediate pretest risk. Only 3 patients (0.6%) were deemed low-risk by the Diamond and Forrester criteria, indicating that this cohort either had known CAD or was at intermediate-to-high risk for CAD. Of those who were symptomatic, 42.5% had dyspnea, a high-risk marker in diabetic patients.28 The majority of subjects were taking appropriate medications, such as a statin in 61% and an ACE-inhibitor or angiotensin-receptor blocker in 68%.
Prevalence of Ischemia
The prevalence of ischemia in this cohort of stable outpatient symptomatic diabetic patients referred for SPECT MPI was somewhat lower than expected, with only 5% having significant LV ischemia of ≥10%. Seventy-eight percent of the entire group had no evidence of ischemia by MPI. This finding suggests a reduction in inducible ischemia in stable diabetic patients compared to prior published studies. The total prevalence of ischemia of 21.9% in our cohort of symptomatic and asymptomatic diabetic patients is similar to the 22% prevalence of ischemia in the DIAD study of asymptomatic patients with diabetes21, the 21% prevalence in asymptomatic diabetics studied by De Lorenzo et al29, and the 17% prevalence of ischemia in asymptomatic diabetic patients in the J-ACCESS 2 study.30 The rate of ischemia is also lower than the 47% rate seen in an analysis of diabetic patients with stable symptoms recruited in the discontinued MERIDIAN trial (performed in 2002–2004)31 and the 59.5% prevalence of an abnormal SPECT study in symptomatic diabetic patients from the Mayo Clinic cohort.23
As suggested previously, it is possible that the contemporary increased aggressive use of medications that suppress ischemia or lower LDL cholesterol or blood pressure may be attenuating the degree of ischemia, both macro- and microvascular. Similarly, many diabetic patients currently take aspirin even though they have not experienced a prior cardiac event. Accordingly, a contemporary assessment of silent ischemia may even show a lower prevalence of ischemia in asymptomatic patients with diabetes than perceived in the past. In accordance with the low ischemia rate, the rates of catheterization as a result of the index MPI study (2.1%) and early revascularization (0.9%) were extremely low. Another explanation for the low early revascularization rate is that 10 of the 14 patients with ≥15% LV ischemia had known CAD and 9 had a prior history of revascularization. The imaging studies in this subgroup were ordered to assess any significant changes in perfusion, presumably to guide therapy.
Symptom State and Mode of Stress
The prevalence of any ischemia and significant ischemia (≥10% of the LV) was similar in symptomatic and asymptomatic patients. We found no difference in the incidence of cardiac death, nonfatal MI, or late revascularization between symptomatic and asymptomatic patients, and symptom state was not a significant variable predicting cardiac death or nonfatal MI by univariable or multivariable Cox Proportional Hazards analysis. Event-free survival (see Figure 4) was comparable in symptomatic and asymptomatic diabetic patients. These findings are different from those of Zellweger et al who reported a higher event rate for symptomatic diabetic patients with abnormal scans.28 In that study, patients with dyspnea and abnormal MPI studies had a higher event rate than asymptomatic patients or patients presenting with angina. It should be emphasized that the asymptomatic patients in our study were judged to be so at the time of testing. They may have had symptoms in the past. The asymptomatic diabetic subgroup in our study was at significantly higher clinical baseline risk before testing than asymptomatic patients in the DIAD study.21 This is evidenced by a 43% prevalence of known CAD, a 23% prevalence of prior MI, and a 33% prevalence of prior revascularization. More than 50% were referred for preoperative risk assessment, further suggesting a high baseline risk as compared to the DIAD cohort of asymptomatic patients. Thus, it is not surprising that the event rates and prevalence of high risk scans were similar between asymptomatic and symptomatic patients in our study.
We found a higher rate of cardiac death/nonfatal MI in patients who had pharmacologic stress versus exercise stress, as previously reported in related populations undergoing stress imaging.7, 32 The higher risk is not surprising, as pharmacologic stress is often performed in patients with comorbidities such as older age, physical deconditioning, and other causes of weakness or gait instability that may be unapparent but increase the risk of cardiac events. In addition, patients initially referred for exercise testing with imaging are not injected with tracer if they fail to achieve ≥85% of their maximum age-predicted heart rate. Such patients are switched to pharmacologic stress imaging if no ischemic symptoms or ST-depression are noted. This is one explanation for the larger number of patients undergoing pharmacologic versus exercise imaging (375 versus 200). In the setting of the low event rate in the exercise subgroup and questionable predictive ability of SPECT ischemia, the utility of SPECT imaging in stable diabetic patients able to exercise to target heart rates may be limited. Further research in a larger number of diabetic patients will be necessary to identify which subset of patients able to undergo exercise stress will benefit from the addition of MPI imaging to exercise electrocardiography (e.g. older age, known prior MI, poor exercise workload, eGFR<60, and long duration of diabetes). In a recent study, 20% of patients with known or suspected CAD and normal exercise ECG testing had reversible perfusion defects on SPECT MPI.33
Outcomes and Impact of Ischemia
The low prevalence of ischemia in this population corresponded with a low incidence of cardiac death, nonfatal MI, and late revascularization over a long-term follow-up (median 4.4 years). The rate of cardiac death was very low at 0.8% per year. A composite of all cardiac events had a similarly-low incidence of 4.2% per year. The 2.6% yearly incidence of cardiac death or nonfatal MI in the total cohort was intermediate between that of the DIAD study (0.6%/year) and both BARI-2D (4.7%/year, including stroke) and a prospective cohort of symptomatic diabetic patients undergoing stress MPI assembled by Giri et al (5.7%/year).5, 17, 22 Given that patients were asymptomatic in DIAD and had ischemia by design in BARI-2D, this is not unexpected. The symptomatic cohort reported by Giri et al predated the more widespread practice of statin therapy to lower LDL cholesterol to below 100 mg/dL for primary prevention of CAD in diabetic patients. The annual rate of cardiac death/nonfatal MI in this study in those with ischemia was 5.7%, which is 1.5–2-fold lower than reported in a pooled analysis of studies in the literature by Shaw and Iskandrian, which included an analysis of diabetic individuals undergoing SPECT MPI.7 The potential explanations for the difference in event rates were previously discussed. The more than 2-fold increase in events in those with ischemia in our cohort (Figure 4) is comparable to that observed in prior studies.5, 28, 34
The multivariable predictors of cardiac death/nonfatal MI in this cohort (Table 4) were not unexpected. Multiple prior studies have shown ischemia to be a significant predictor of cardiac events, and it remained so in this cohort of outpatient diabetics.29, 35 Patients with known CAD are much more likely to have progressive disease and thus subsequent events. Not unexpectedly, referral for pharmacologic stress was an independent predictor of cardiac death or nonfatal MI. Duration of diabetes and type of therapy (insulin versus oral agents) have been reported to provide independent and incremental prognostic information to perfusion and functional variables.36 These variables were not investigated in the present study.
Impact of Exercise Capacity
High exercise workload (≥10 METS) was associated with a low risk of significant ischemia and cardiac events in this diabetic cohort. No patients achieving this high workload had ≥10% LV ischemia, and there was only 1 hard cardiac event. These findings are consistent with multiple prior studies showing the powerful positive impact of exercise capacity on prognosis.9, 37–39
Other High Risk Findings
It is possible that some patients without evidence of regional ischemia on their nuclear MPI study are falsely negative, with unappreciated balanced ischemia or microvascular disease. We therefore closely examined variables other than perfusion defects that might identify underlying ischemia.40, 41 The prevalence of these other high risk findings was also relatively low. Only 21.8% had evidence of a reduced LV ejection fraction (<50%), an elevated ESVI (≥25 mL/m2), TID, or ≥4 segments with abnormal thickening fractions. Moreover, only 6.7% had ischemic ST-depression on their stress electrocardiogram.
Study Limitations
The data in this study emanate from a single center and may apply only to this specific referral cohort. It may be that some of the higher risk patients were directly referred for cardiac catheterization rather than first undergoing noninvasive imaging. A larger median follow-up may have yielded a higher cardiac event rate with more patients crossing over to revascularization strategies. The use of chart review to identify follow-up in some patients prevented precise adjudication of events. However, documentation was available in the majority of subjects.
Conclusions
The prevalence of significant (≥10%) LV ischemia was only 5%in this cohort of stable, predominately symptomatic outpatients with diabetes. The increased use of aggressive risk-factor modification by primary care physicians and cardiovascular specialists and use of evidence-based medicines may be partly responsible for the lower event rates in patients with and without ischemia in the present study. Reaching a high cardiac workload of ≥10 METS predicts a very low risk of significant ischemia on SPECT imaging in this population of patients with diabetes, similar to what was observed in a mixed population of patients referred for exercise testing.9
The event free survival was similar in patients with mild to moderate ischemia and those who exhibited ≥10% LV ischemia. Thus, any inducible ischemia on stress SPECT should be of concern. The rate of cardiac death/nonfatal MI in patients with no ischemia was 1.8% yearly, which is higher than seen in nondiabetic patients without ischemia. Interestingly, the prevalence of ischemia and the cardiac event rates were similar in asymptomatic and symptomatic diabetic patients, although the asymptomatic patients in this study were at intermediate pretest risk.
Finally, compared to those who underwent exercise stress, patients who underwent pharmacologic stress had a worse event-free survival. An increased risk of hard events in those with ischemia undergoing exercise stress could not be demonstrated. Further studies in a large population of diabetic patients referred for stress imaging might indicate which patients would benefit from SPECT imaging (e.g. markedly abnormal Duke Treadmill Score). Since 30% of the patients without SPECT abnormalities had events during follow-up, other noninvasive technologies such as quantitative PET and cardiac magnetic resonance MPI should be explored for identifying a larger number of diabetic patients at risk for an adverse outcome.
Acknowledgments
Sources of Funding
This project was partially completed while Dr. Bourque was funded by an NIH NRSA Training Grant: T32 EB003841-04.
Abbreviations
- BMI
body mass index
- CAD
coronary artery disease
- EDVI
end-diastolic volume index
- ESVI
end-systolic volume index
- LBBB
left bundle-branch block
- LV (EF)
left ventricular (ejection fraction)
- MET
metabolic equivalent
- MI
myocardial infarction
- MPI
myocardial perfusion imaging
- SPECT
single photon-emission computed tomography
- TID
transient ischemic dilatation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
References
- 1.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: Prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Roger VL, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: A report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 3.National Diabetes Information Clearinghouse (a service of the National Institute of Diabetes and Digestive and Kidney Diseases NIH. Diabetes across the United States. http://www.diabetes.niddk.nih.gov/populations/index.htm.
- 4.Malmberg K, Yusuf S, Gerstein HC, Brown J, Zhao F, Hunt D, Piegas L, Calvin J, Keltai M, Budaj A. Impact of diabetes on long-term prognosis in patients with unstable angina and non-q-wave myocardial infarction: Results of the OASIS (Organization to Assess Strategies for Ischemic Syndromes) registry. Circulation. 2000;102:1014–1019. doi: 10.1161/01.cir.102.9.1014. [DOI] [PubMed] [Google Scholar]
- 5.Giri S, Shaw LJ, Murthy DR, Travin MI, Miller DD, Hachamovitch R, Borges-Neto S, Berman DS, Waters DD, Heller GV. Impact of diabetes on the risk stratification using stress single-photon emission computed tomography myocardial perfusion imaging in patients with symptoms suggestive of coronary artery disease. Circulation. 2002;105:32–40. doi: 10.1161/hc5001.100528. [DOI] [PubMed] [Google Scholar]
- 6.Navare SM, Mather JF, Shaw LJ, Fowler MS, Heller GV. Comparison of risk stratification with pharmacologic and exercise stress myocardial perfusion imaging: A meta-analysis. J Nucl Cardiol. 2004;11:551–561. doi: 10.1016/j.nuclcard.2004.06.128. [DOI] [PubMed] [Google Scholar]
- 7.Shaw LJ, Iskandrian AE. Prognostic value of gated myocardial perfusion SPECT. J Nucl Cardiol. 2004;11:171–185. doi: 10.1016/j.nuclcard.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, Mark DB, McCallister BD, Mooss AN, O'Reilly MG, Winters WL, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Hiratzka LF, Jacobs AK, Russell RO, Smith SC. ACC/AHA 2002 guideline update for exercise testing: Summary article. A report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee to update the 1997 exercise testing guidelines) J Am Coll Cardiol. 2002;40:1531–1540. doi: 10.1016/s0735-1097(02)02164-2. [DOI] [PubMed] [Google Scholar]
- 9.Bourque JM, Holland BH, Watson DD, Beller GA. Achieving an exercise workload of ≥10 metabolic equivalents predicts a very low risk of inducible ischemia: Does myocardial perfusion imaging have a role? J Am Coll Cardiol. 2009;54:538–545. doi: 10.1016/j.jacc.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watson DD, Smith WH. The role of quantitation in clinical nuclear cardiology: The University of Virginia approach. J Nucl Cardiol. (2nd) 2007;14:466–482. doi: 10.1016/j.nuclcard.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 11.ASNC. Updated imaging guidelines for nuclear cardiology procedures, part 1. J Nucl Cardiol. 2001;8:G5–G58. doi: 10.1067/mnc.2001.112538. [DOI] [PubMed] [Google Scholar]
- 12.Raff GL, Abidov A, Achenbach S, Berman DS, Boxt LM, Budoff MJ, Cheng V, DeFrance T, Hellinger JC, Karlsberg RP. SCCT guidelines for the interpretation and reporting of coronary computed to mographic angiography. J Cardiovasc Comput Tomogr. 2009;3:122–136. doi: 10.1016/j.jcct.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Marcassa C, Galli M, Baroffio C, Campini R, Giannuzzi P. Transient left ventricular dilation at quantitative stress-rest sestamibi tomography: Clinical, electrocardiographic, and angiographic correlates. J Nucl Cardiol. 1999;6:397–405. doi: 10.1016/s1071-3581(99)90005-3. [DOI] [PubMed] [Google Scholar]
- 14.Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed to mography. Circulation. 2003;107:2900–2907. doi: 10.1161/01.CIR.0000072790.23090.41. [DOI] [PubMed] [Google Scholar]
- 15.Hachamovitch R, Rozanski A, Hayes SW, Thomson LE, Germano G. Friedman JD, Cohen I, Berman DS. Predicting therapeutic benefit from myocardial revascularization procedures: Are measurements of both resting left ventricular ejection fraction and stress-induced myocardial ischemia necessary? J Nucl Cardiol. 2006;13:768–778. doi: 10.1016/j.nuclcard.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 17.Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, Orchard TJ, Chaitman BR, Genuth SM, Goldberg SH, Hlatky MA, Jones TL, Molitch ME, Nesto RW, Sako EY, Sobel BE. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–2515. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilcox I, Freedman SB, Allman KC, Collins FL, Leitch JW, Kelly DT, Harris PJ. Prognostic significance of a predischarge exercise test in risk stratification after unstable angina pectoris. J Am Coll Cardiol. 1991;18:677–683. doi: 10.1016/0735-1097(91)90789-c. [DOI] [PubMed] [Google Scholar]
- 19.Woodfield SL, Lundergan CF, Reiner JS, Greenhouse SW, Thompson MA, Rohrbeck SC, Deychak Y, Simoons ML, Califf RM, Topol EJ, Ross AM. Angiographic findings and outcome in diabetic patients treated with thrombolytic therapy for acute myocardial infarction: The GUSTO-I experience. J Am Coll Cardiol. 1996;28:1661–1669. doi: 10.1016/s0735-1097(96)00397-x. [DOI] [PubMed] [Google Scholar]
- 20.Wackers FJ, Chyun DA, Young LH, Heller GV, Iskandrian AE, Davey JA, Barrett EJ, Taillefer R, Wittlin SD, Filipchuk N, Ratner RE, Inzucchi SE. Resolution of asymptomatic myocardial ischemia in patients with type 2 diabetes in the Detection of Ischemia in Asymptomatic Diabetics (DIAD) study. Diabetes Care. 2007;30:2892–2898. doi: 10.2337/dc07-1250. [DOI] [PubMed] [Google Scholar]
- 21.Wackers FJ, Young LH, Inzucchi SE, Chyun DA, Davey JA, Barrett EJ, Taillefer R, Wittlin SD, Heller GV, Filipchuk N, Engel S, Ratner RE, Iskandrian AE. Detection of silent myocardial ischemia in asymptomatic diabetic subjects: The DIAD study. Diabetes Care. 2004;27:1954–1961. doi: 10.2337/diacare.27.8.1954. [DOI] [PubMed] [Google Scholar]
- 22.Young LH, Wackers FJ, Chyun DA, Davey JA, Barrett EJ, Taillefer R, Heller GV, Iskandrian AE, Wittlin SD, Filipchuk N, Ratner RE, Inzucchi SE. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: The DIAD study: A randomized controlled trial. JAMA. 2009;301:1547–1555. doi: 10.1001/jama.2009.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller TD, Rajagopalan N, Hodge DO, Frye RL, Gibbons RJ. Yield of stress single-photon emission computed to mography in asymptomatic patients with diabetes. Am Heart J. 2004;147:890–896. doi: 10.1016/j.ahj.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Rajagopalan N, Miller TD, Hodge DO, Frye RL, Gibbons RJ. Identifying high-risk asymptomatic diabetic patients who are candidates for screening stress single-photon emission computed tomography imaging. J Am Coll Cardiol. 2005;45:43–49. doi: 10.1016/j.jacc.2004.06.078. [DOI] [PubMed] [Google Scholar]
- 25.Scholte AJ, Schuijf JD, Kharagjitsingh AV, Dibbets-Schneider P, Stokkel MP, van der Wall EE, Bax JJ. Prevalence and predictors of an abnormal stress myocardial perfusion study in asymptomatic patients with type 2 diabetes mellitus. Eur J Nucl Med Mol Imaging. 2009;36:567–575. doi: 10.1007/s00259-008-0967-y. [DOI] [PubMed] [Google Scholar]
- 26.Acampa W, Petretta M, Evangelista L, Daniele S, Xhoxhi E, De Rimini ML, Cittanti C, Marranzano F, Spadafora M, Baldari S, Mansi L, Cuocolo A. Myocardial perfusion imaging and risk classification for coronary heart disease in diabetic patients. The idis study: A prospective, multicentre trial. Eur J Nucl Med Mol Imaging. 2012;39:387–395. doi: 10.1007/s00259-011-1983-x. [DOI] [PubMed] [Google Scholar]
- 27.Wackers FJ. Diabetes and coronary artery disease: The role of stress myocardial perfusion imaging. Cleve Clin J Med. 2005;72:21–25. 29–33. doi: 10.3949/ccjm.72.1.21. [DOI] [PubMed] [Google Scholar]
- 28.Zellweger MJ, Hachamovitch R, Kang X, Hayes SW, Friedman JD, Germano G, Pfisterer ME, Berman DS. Prognostic relevance of symptoms versus objective evidence of coronary artery disease in diabetic patients. Eur Heart J. 2004;25:543–550. doi: 10.1016/j.ehj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 29.De Lorenzo A, Lima RS, Siqueira-Filho AG, Pantoja MR. Prevalence and prognostic value of perfusion defects detected by stress technetium-99m sestamibi myocardial perfusion single-photon emission computed tomography in asymptomatic patients with diabetes mellitus and no known coronary artery disease. Am J Cardiol. 2002;90:827–832. doi: 10.1016/s0002-9149(02)02702-9. [DOI] [PubMed] [Google Scholar]
- 30.Nakajima K, Yamasaki Y, Kusuoka H, Izumi T, Kashiwagi A, Kawamori R, Shimamoto K, Yamada N, Nishimura T. Cardiovascular events in japanese asymptomatic patients with type 2 diabetes: A 1-year interim report of a J-access 2 investigation using myocardial perfusion imaging. Eur J Nucl Med Mol Imaging. 2009;36:2049–2057. doi: 10.1007/s00259-009-1207-9. [DOI] [PubMed] [Google Scholar]
- 31.Wiersma JJ, Verberne HJ, Trip MD, ten Holt WL, van Eck-Smit BL, Piek JJ, Tijssen JG. Prevalence of myocardial is chaemia as assessed with myocardial perfusion scintigraphy in patients with diabetes mellitus type 2 and mild anginal symptoms. Eur J Nucl Med Mol Imaging. 2006;33:1468–1476. doi: 10.1007/s00259-006-0165-8. [DOI] [PubMed] [Google Scholar]
- 32.Rozanski A, Gransar H, Hayes SW, Friedman JD, Hachamovitch R, Berman DS. Comparison of long-term mortality risk following normal exercise vs adenosine myocardial perfusion SPECT. J Nucl Cardiol. 2010;17:999–1008. doi: 10.1007/s12350-010-9300-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schinkel AF, Boiten HJ, van der Sijde JN, Ruitinga PR, Sijbrands EJ, Valkema R, van Domburg RT. Prediction of 9-year cardiovascular outcomes by myocardial perfusion imaging in patients with normal exercise electrocardiographic testing. Eur Heart J Cardiovasc Imaging. 2012;13:900–4. doi: 10.1093/ehjci/jes104. [DOI] [PubMed] [Google Scholar]
- 34.Wiersma JJ, Verberne HJ, ten Holt WL, Radder IM, Dijksman LM, van Eck-Smit BL, Trip MD, Tijssen JG, Piek JJ. Prognostic value of myocardial perfusion scintigraphy in type 2 diabetic patients with mild, stable angina pectoris. J Nucl Cardiol. 2009;16:524–532. doi: 10.1007/s12350-009-9111-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanzetto G, Halimi S, Hammoud T, Fagret D, Benhamou PY, Cordonnier D, Denis B, Machecourt J. Prediction of cardiovascular events in clinically selected high-risk niddm patients. Prognostic value of exercise stress test and thallium-201 single-photon emission computed tomography. Diabetes Care. 1999;22:19–26. doi: 10.2337/diacare.22.1.19. [DOI] [PubMed] [Google Scholar]
- 36.Barmpouletos D, Stavens G, Ahlberg AW, Katten DM, O'Sullivan DM, Heller GV. Duration and type of therapy for diabetes: Impact on cardiac risk stratification with stress electrocardiographic-gated SPECT myocardial perfusion imaging. J Nucl Cardiol. 2010;17:1041–1049. doi: 10.1007/s12350-010-9293-4. [DOI] [PubMed] [Google Scholar]
- 37.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 38.Dutcher JR, Kahn J, Grines C, Franklin B. Comparison of left ventricular ejection fraction and exercise capacity as predictors of two- and five-year mortality following acute myocardial infarction. Am J Cardiol. 2007;99:436–441. doi: 10.1016/j.amjcard.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 39.Bourque JM, Charlton GT, Holland BH, Belyea CM, Watson DD, Beller GA. Prognosis in patients achieving ≥10 mets on exercise stress testing: Was SPECT imaging useful? J Nucl Cardiol. 2011;18:230–7. doi: 10.1007/s12350-010-9323-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharir T, Kang X, Germano G, Bax JJ, Shaw LJ, Gransar H, Cohen I, Hayes SW, Friedman JD, Berman DS. Prognostic value of poststress left ventricular volume and ejection fraction by gated myocardial perfusion SPECT in women and men: Gender-related differences in normal limits and outcomes. J Nucl Cardiol. 2006;13:495–506. doi: 10.1016/j.nuclcard.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 41.Emmett L, Van Gaal WJ, Magee M, Bass S, Ali O, Freedman SB, Van der Wall H, Kritharides L. Prospective evaluation of the impact of diabetes and left ventricular hypertrophy on the relationship between ischemia and transient ischemic dilation of the left ventricle on single-day adenosine Tc-99m myocardial perfusion imaging. J Nucl Cardiol. 2008;15:638–643. doi: 10.1016/j.nuclcard.2008.06.005. [DOI] [PubMed] [Google Scholar]


