Abstract
Purpose
Physical activity may protect against breast cancer. Few prospective studies have evaluated breast cancer mortality in relation to cardiorespiratory fitness, an objective marker of physiologic response to physical activity habits.
Methods
We examined the association between cardiorespiratory fitness and risk of death from breast cancer in the Aerobics Center Longitudinal Study. Women (N=14,811), aged 20 to 83 years with no prior breast cancer history, received a preventive medical examination at the Cooper Clinic in Dallas, TX, between 1970 and 2001. Mortality surveillance was completed through December 31, 2003. Cardiorespiratory fitness was quantified as maximal treadmill exercise test duration and was categorized for analysis as low (lowest 20% of exercise duration), moderate (middle 40%), and high (upper 40%). At baseline, all participants were able to complete the exercise test to at least 85% of their age-predicted maximal heart rate.
Results
A total of 68 breast cancer deaths occurred during follow-up (mean=16 years). Age-adjusted breast cancer mortality rates per 10,000 woman-years were 4.4, 3.2, and 1.8 for low, moderate, and high cardiorespiratory fitness groups, respectively (trend P = 0.008). After further controlling for body mass index, smoking, drinking, chronic conditions, abnormal exercise electrocardiogram responses, family history of breast cancer, oral contraceptive use, and estrogen use, hazard ratios (95% CI) for breast cancer mortality across incremental cardiorespiratory fitness categories were 1.00 (referent), 0.67 (0.35–1.26), 0.45 (0.22–0.95); trend P = 0.04.
Conclusions
These results indicate that cardiorespiratory fitness is associated with a reduced risk of dying from breast cancer in women.
Keywords: Epidemiology, Prevention, Death from breast cancer, Physical activity
Introduction
Breast cancer is the second leading cause of cancer death and the most frequently diagnosed form of cancer among women in the United States. According to the most recent publication from the American Cancer Society, in 2008, an estimated 182,462 new cases of invasive breast cancer will be diagnosed among women, and approximately 40,480 women are expected to die from this disease. The etiology of breast cancer remains to be fully explained, with less than 50% of the cases attributed to known risk factors (1). Several potential risk factors including genetic components, diet, smoking, alcohol intake, and physical activity have been identified (28).
It has been suggested that physical activity, one of the few established modifiable breast cancer risk factors, may protect against both the development of the disease (9, 36, 37, 39) and progression after diagnosis (19, 27). A recent systematic review by Monninkhof and colleagues (30) found evidence of an extreme inverse association between physical activity and breast cancer in postmenopausal women; i.e., equivalent to a 20 to 80% decrease in risk of incident disease. Differences in risk estimates may result from imprecise physical activity assessment collected from self-report instruments, especially in women (3). In addition, most of previous studies have reported either on physical activity and incident (9, 37) or combined fatal/nonfatal (36, 39) breast cancer. Because physical activity protects against incident breast cancer (9, 12, 18, 37), it might be reasonable to expect that physical activity would confer protection against fatal breast cancer. However, this inference can not confidently be drawn from studies of combined nonfatal/fatal events or studies solely reliant on nonfatal outcomes. To the best of our knowledge, no study has been conducted on cardiorespiratory fitness, an objective and reproducible measure that reflects the functional consequences of physical activity habits, short-term effects of disease status (e.g., respiratory tract infections), and genetics (21). We therefore examined the association between the cardiorespiratory fitness, objectively measured by maximal exercise test on a treadmill, and breast cancer mortality in women from the Aerobic Center Longitudinal Study (ACLS).
METHODS
Study sample
The population for the current analysis was composed of primarily white (>99%), married, well-educated women with no prior history of breast cancer. These women, ranging in age from 20–83 years, received a preventive medical examination at the Cooper Clinic in Dallas, TX, between 1970 and 2001 (N=14,811). Study participants came to the clinic for periodic preventive health examinations and to receive counseling regarding diet, exercise, and other lifestyle factors associated with increased risk of chronic disease. Many participants were sent by their employers for the examination; some were referred by their personal physicians; while the rest were self-referred. At baseline, all participants were able to complete an exercise stress test to at least 85% of their age-predicted maximal heart rate (220 minus age in years). The study protocol was approved annually by the Institutional Review Board of the Cooper Institute.
Baseline examination
The baseline clinical examination was performed after receiving written informed consent from each participant and included fasting blood chemistry analyses, personal and family health history, anthropometry, resting blood pressure and electrocardiogram, and a maximal graded exercise test. Examination methods and procedures followed a standard manual of operations, as described previously (6). Briefly, body mass index [BMI=weight(kg)/height(m)2] was computed from measured height and weight. Resting blood pressure was recorded as the first and fifth Korotkof sounds by ausculatatory methods. Serum samples were analyzed for lipids and glucose using standardized automated bioassays by a laboratory that participates in and meets quality control standards of the CDC Lipid Standardization Program. Information on smoking habits (whether a current smoker), alcohol intake (number of drinks per week), personal history of cardiovascular disease (myocardial infarction or stroke), hypertension and diabetes, family history of breast cancer, oral contraceptive use, and exogenous estrogen use was obtained from a standardized questionnaire.
Cardiorespiratory fitness was assessed at the baseline examination as the duration of a symptom-limited maximal treadmill exercise test using a modified Balke protocol (5, 6). The treadmill speed was 88m • min−1 for the first 25 min. During this time the grade was 0% for the first minute, 2% the second minute, and increased 1% each minute until 25 min had elapsed. After 25 min, the grade remained constant while the speed increased 5.4 m • min−1 each minute until test termination. Patients were encouraged to give a maximal effort during the test. The mean (SD) percentage of age-predicted maximal heart rate achieved during exercise was 100.0 (6.3). The duration of the maximal exercise treadmill test on this protocol is highly correlated with directly measured maximal oxygen uptake in women (r = 0.94), an accepted measure of CRF (32). Maximal metabolic equivalents (METs, 1 MET = 3.5 ml O2 uptake • kg−1 • min−1) were estimated from the final treadmill speed and grade (4). We used our previously published age-specific distribution of treadmill duration from the overall ACLS population to define fitness groups as low (lowest 20%), moderate (middle 40%), and high (upper 40%) to maintain consistency in the study methods, and because we have found that a low level of fitness, defined in this way, is an independent predictor of mortality (6) and morbidity (38). The respective cut points for total treadmill time and METs in the low, moderate, and high fitness groups were described in detail in a recent report (38).
Abnormal exercise electrocardiogram (ECG) responses were broadly defined as rhythm and conduction disturbances and ischemic ST-T wave abnormalities as described in detail elsewhere (13). We have found 90% agreement between the ECG interpretation recorded in our database and that of a group of three physicians who read a random sample of 357 patient records (13).
Mortality surveillance
All participants were followed from the date of their baseline examination until their date of death or December 31, 2003. The National Death Index (NDI) was the primary data source for mortality surveillance. The underlying cause of death was determined from the NDI report or by a nosologist’s review of official death certificates obtained from the department of vital records in the decedent’s state of residence. Breast cancer mortality was defined by International Classification of Diseases, Ninth Revision (ICD-9) codes 174 to 175 before 1999 and Tenth Revision (ICD-10) codes C50 during 1999–2003. We computed woman-years of exposure as the sum of follow-up time among decedents and survivors.
Statistical analysis
Baseline characteristics of the population were calculated for the entire cohort and by cardiorespiratory fitness groups. Differences in covariates were assessed using F-tests. Kaplan-Meier plots were used to compare survival curves and Cox proportional hazards models were used to estimate adjusted hazard ratios (HRs), associated 95% confidence intervals (CI), mortality rates ( deaths/10,000 woman-years of follow-up), and linear trends of breast cancer mortality for levels of each fitness category. When calculating HRs, the low fitness group was used as the reference category. Multivariable-adjusted models controlled for the potential confounding effects of baseline age (years), BMI (kg/m2), smoking (current smoker or not), alcohol intake (≥5 drinks/wk or not), chronic conditions (cardiovascular disease, hypertension or diabetes, present or not for each), family history of breast cancer (present or not), abnormal exercise ECG responses (present or not), oral contraceptive use (yes or no), and estrogen use (yes or no). Cumulative hazard plots grouped by exposure suggested no appreciable violations of the proportional hazards assumption.
We also conducted Cox-regression analyses of cardiorespiratory fitness stratified by categories of age, BMI, oral contraceptive use, and estrogen use to assess whether the associations were stronger in particular subgroups. Tests for multiplicative interaction between an increment of 1 minute of maximal exercise duration and other risk factors in relation to risk of breast cancer death were assessed using likelihood ratio tests comparing the models with or without interaction terms. We examined the risk of breast cancer across increments of METs to assess the shape of the fitness-breast cancer curve. Statistical analyses were performed using SAS (version 9.1, SAS Institute, Cary, NC) software. All p values were calculated from two-sided hypothesis tests, and CIs were calculated at the 95% level.
RESULTS
At baseline, the mean (SD) age of the study participants was 43.0 (10.5) years, the mean (SD) treadmill test duration was 13.3 (4.7) minutes, and the mean (SD) maximal METs was 9.5 (2.2). The distribution of participant characteristics for several breast cancer risk factors is given in Table 1 across categories of cardiorespiratory fitness. Women in the high fitness group were more likely to have a lower BMI, to have a more favorable lipid and blood pressure profile, and to have a healthier lifestyle (as reflected by smoking and alcohol intake), and to have a lower rate of chronic conditions (as reflected by cardiovascular disease, hypertension, diabetes, and abnormal exercise ECG), as compared with low cardiorespiratory fitness.
Table 1.
Baseline characteristics according to cardiorespiratory fitness in the Aerobics Center Longitudinal Study, 1970–2001
| Characteristic | All (n=14811) | Cardiorespiratory Fitness
|
P for trend | ||
|---|---|---|---|---|---|
| Low (n=2117) | Moderate (n=5397) | High (n=7297) | |||
| Age, years | 43.0±10.5 | 42.9±10.1 | 42.8±10.4 | 43.1±10.7 | 0.36 |
| Body Mass Index, kg/m2 | 23.0±4.1 | 25.9±6.0 | 23.4±4.0 | 21.9±2.9 | <0.0001 |
| Maximal METs | 9.5±2.2 | 6.7±0.9 | 8.5±1.0 | 11.1±1.8 | <0.0001 |
| Treadmill time duration, minutes | 13.3±4.7 | 7.2±1.9 | 11.2±2.2 | 16.7±3.7 | <0.0001 |
| Lipids, mmol/L | |||||
| Total cholesterol | 5.20±1.07 | 5.40±1.04 | 5.28±1.04 | 5.08±1.08 | <0.0001 |
| HDL-C | 1.59±0.42 | 1.42±0.37 | 1.53±0.37 | 1.66±0.45 | <0.0001 |
| Triglycerides | 1.05±0.85 | 1.28±0.87 | 1.10±0.78 | 0.95±0.87 | <0.0001 |
| Fasting blood glucose, mmol/L | 5.27±4.68 | 5.41±1.10 | 5.23±0.75 | 5.27±6.51 | 0.42 |
| Blood pressure, mmHg | |||||
| Systolic | 113±15 | 117±16 | 113±14 | 111±14 | <0.0001 |
| Diastolic | 75±10 | 78±10 | 76±10 | 75±9 | <0.0001 |
| Current smoker (%) | 1591(10.7) | 367(17.3) | 687(12.7) | 537(7.4) | <0.0001 |
| Alcohol drinker (≥ 5 drinks/week) (%) | 2844(19.2) | 424(20.0) | 1133(21.0) | 1287(17.6) | <0.0001 |
| Hypertension* (%) | 1384(9.3) | 339(16.0) | 501(9.3) | 544(7.5) | <0.0001 |
| Diabetes† (%) | 319(2.2) | 66(3.1) | 106(2.0) | 147(2.0) | 0.004 |
| Cardiovascular disease‡ (%) | 63(0.4) | 17(0.8) | 23(0.4) | 23(0.3) | 0.01 |
| Oral contraceptive use (%) | 3908(26.4) | 347(16.4) | 1356(25.1) | 2205(30.2) | <0.0001 |
| Estrogen use (%) | 2832(19.1) | 371(17.5) | 1041(19.3) | 1420(19.5) | 0.13 |
| Family history of breast cancer (%) | 331(2.2) | 18(0.9) | 85(1.6) | 228(3.1) | <0.0001 |
| Abnormal exercise ECG (%) | 683(4.6) | 133(6.3) | 237(4.4) | 313(4.3) | 0.0004 |
Data shown as Means ± SD unless specified otherwise.
METs= maximal metabolic equivalents achieved during the treadmill test; HDL- C= high density lipoprotein cholesterol; ECG= Electrocardiogram.
Hypertension was defined as systolic blood pressure≥130 mm Hg, diastolic blood pressure ≥85 mm Hg, or history of physician-diagnosed hypertension
Diabetes was defined as glucose≥126 mg/dL or history of physician-diagnosed diabetes
Cardiovascular disease was defined as history of physician-diagnosed myocardial infarction or stroke
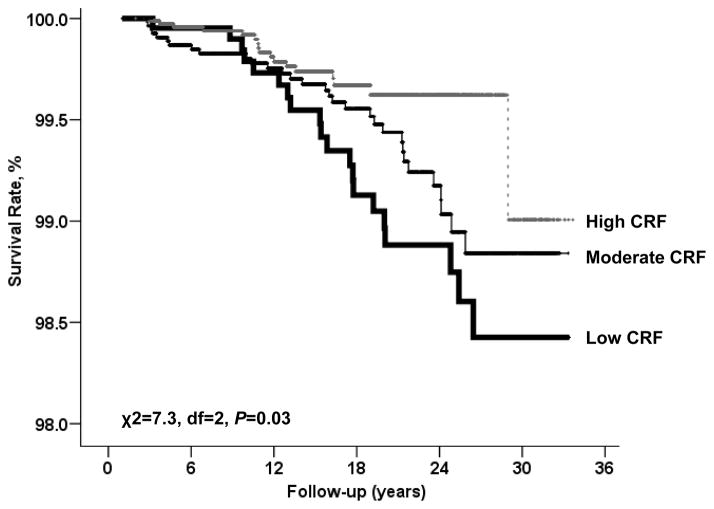
In a mean length of 16.4 years (range 1.01–33.7 years) follow-up and 242,900 woman-years of observation, 68 breast cancer deaths were identified. A steep inverse gradient (ptrend = 0.008) of breast cancer mortality rates was observed across cardiorespiratory fitness groups (Table 2). After adjusting for covariates (age, BMI, current smoking, alcohol intake, chronic conditions, family history of breast cancer, abnormal exercise ECG, and oral contraceptive and estrogen use), women with moderate and high cardiorespiratory fitness had 33% and 55% lower breast cancer risk, respectively, than did women with low cardiorespiratory fitness (ptrend = 0.04). The Kaplan-Meier plot depicts the breast cancer death rates by fitness group (Figure 1).
Table 2.
Rates and hazard ratios for breast cancer mortality by cardiorespiratory fitness (CRF) groups in 14811 women
| Low CRF | Moderate CRF | High CRF | P value for Trend | |
|---|---|---|---|---|
| No. of deaths | 20 | 31 | 17 | |
| No. of woman-years | 42,488 | 96,714 | 103,554 | |
| Rate* | 4.4 | 3.2 | 1.8 | 0.008 |
| Age-adjusted HR (95% CI) | 1.00 | 0.72 (0.41–1.27) | 0.42(0.22–0.80) | 0.008 |
| Multivariate HR (95% CI)† | 1.00 | 0.67 (0.35–1.26) | 0.45(0.22–0.95) | 0.04 |
HR = Hazard ratio; CI = Confidence interval; CRF = cardiorespiratory fitness.
Rate is expressed as per 10,000 woman-years and adjusted for age.
Adjusted for age, body mass index (kg/m2), current smoking (yes or not), alcohol intake (≥5 drinks/wk, or not), chronic conditions (hypertension, diabetes, or cardiovascular disease present or not for each), family history of breast cancer (present or not), abnormal exercise electrocardiogram responses (present or not), oral contraceptive use (yes or no), and estrogen use (yes or no).
Figure 1.
Survival free of breast cancer across cardiorespiratory fitness (CRF) status in a mean 16-year follow-up study of 12411 women.
We next examined whether other risk predictors modified the association between cardiorespiratory fitness and breast cancer death (Table 3). After adjustment for age, we observed significantly lower risk of death per 1-MET increment in exercise capacity among younger women (ptrend = 0.004), women who were normal weight (ptrend = 0.03), and women who never used oral contraceptives (ptrend = 0.03) or estrogen replacement (ptrend = 0.01). The test for interaction was not significant in the subgroups (P for interaction > 0.40 for each). These associations between cardiorespiratory fitness and breast cancer mortality risk according to categories of other risk factors attenuated after controlling each of the other variables shown in the table. The inverse association remained significant only among women who were younger than 55 years old and those who never used estrogen.
Table 3.
Hazard ratios for breast cancer death per 1-MET increment in maximal exercise capacity in different subgroups of women
| No. of deaths | HR† | 95% CI† | p value | HR‡ | 95% CI‡ | p value | |
|---|---|---|---|---|---|---|---|
| Age, years | |||||||
| <55 | 55 | 0.81 | 0.70–0.94 | 0.004 | 0.76 | 0.64–0.91 | 0.002 |
| ≥55 | 13 | 0.72 | 0.50–1.05 | 0.08 | 0.83 | 0.56–1.24 | 0.36 |
| Body mass index, kg/m2 | |||||||
| <25 | 53 | 0.84 | 0.72–0.99 | 0.03 | 0.87 | 0.74–1.02 | 0.08 |
| ≥25 | 15 | 0.87 | 0.61–1.25 | 0.45 | 0.86 | 0.60–1.22 | 0.40 |
| Oral contraceptive use | |||||||
| Never | 58 | 0.84 | 0.72–0.99 | 0.03 | 0.82 | 0.68–0.98 | 0.10 |
| Ever | 10 | 0.85 | 0.60–1.22 | 0.38 | 0.89 | 0.61–1.32 | 0.57 |
| Estrogen use | |||||||
| Never | 60 | 0.83 | 0.71–0.96 | 0.01 | 0.81 | 0.68–0.96 | 0.02 |
| Ever | 8 | 1.02 | 0.67–1.56 | 0.92 | 1.04 | 0.63–1.70 | 0.88 |
HR, hazard ratio; CI, confidence interval.
Adjusted for age.
Adjusted for age and each of the other variables in the table.
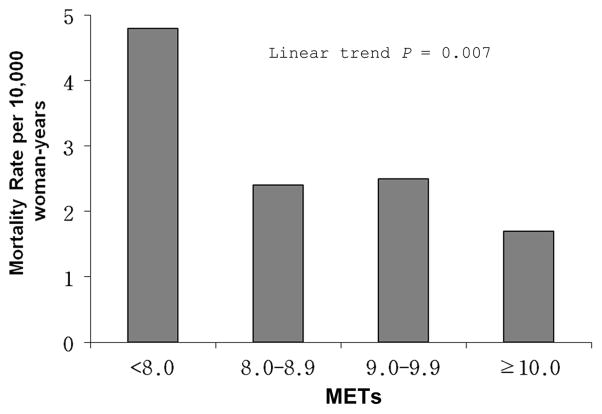
To further examine the dose-response characteristics between cardiorespiratory fitness levels and breast cancer mortality in our population of women, we computed the age-adjusted death rates (per 10,000 woman-yrs) for categories of cardiorespiratory fitness defined by increments of 1 MET across the range of 8 to 10 METs (Figure 2). An exercise capacity of less than 8 METs was associated with a nearly three-fold higher risk of breast cancer mortality compared with women having a capacity of 10 METs and greater (p trend = 0.007). The inverse association remained significant after multivariate adjustment (p trend = 0.02).
Figure 2.
Age- adjusted mortality rates (per 10,000 woman-years) of breast cancer by cardiorespiratory fitness levels quantified in 1-MET increments obtained during a maximal treadmill test in women. Number at risk (and number of cases) in <8.0, 8.0–8.9, 9.0–9.9, and ≥10 was 3345 (34), 3805 (15), 2304 (8), and 5357 (11).
DISCUSSION
In this study, we observed an inverse association between cardiorespiratory fitness and the risk of breast cancer mortality. Women in the moderate and high cardiorespiratory fitness groups demonstrated a 33% and 55% lower risk, respectively, of dying of breast cancer. This association persisted after adjustment of other available potential confounders. Women with an exercise capacity less than 8 METs had a nearly three-fold higher risk of dying of breast cancer compared with those with higher METs level (≥8). These data suggest that an exercise capacity of at least 8 METs may be needed to provide substantially protective benefits.
To the best of our knowledge, this is the first prospective cohort study to assess the association of cardiorespiratory fitness to risk of dying of breast cancer. It is important to note that the vast majority of the literature on breast cancer has focused on incidence, and not mortality of disease. Mortality reflects the combination of incidence with a variety of prognostic factors relating primarily to disease-related characteristics such as stage at diagnosis and grade as well as lifestyle and other factors that might influence outcome. Despite this, death registration is excellent in countries such as the US and mortality is a “hard” outcome that we have used with great success in the past (16). It is also important to note that there are literatures relating factors such as diet, physical activity, and relative body weight to both breast cancer incidence (9, 10, 15, 22) and mortality (8, 31, 40).
Previous cohort studies using self-reported physical activity show lower risks for incident (9, 37) or combined fatal/nonfatal (30, 36, 39) breast cancer ranging from 15% to 40%. However, other studies have reported no association (24, 25, 34) between physical activity and incident breast cancer. The varying range of effects and the inconsistency of results may be due to the imprecise assessment of physical activity derived from the questionnaires, population differences in the study cohorts, differences in breast cancer endpoints used, duration of follow-up after the baseline exposure measurement, or some combination of these.
Beyond a cardiorespiratory fitness level of 8 METs (Figure 2) it did not appear that there were substantial decreases in risk of breast cancer death. This finding of an apparent cardiorespiratory fitness threshold adds insight into the relationship between cardiorespiratory fitness and breast cancer death. A systematic review of physical activity and breast cancer endpoints (including occurrence and mortality) indicates that approximately half of the selected studies observed a dose-response relationship (30). Thune et al (39) found that compared with being sedentary being moderately active during leisure time was associated with a 7% lower breast cancer mortality. Being regularly active during leisure time was associated with a 37% lower breast cancer incidence. These findings indicate an incremental dose-response relationship between self-reported physical activity and breast cancer endpoints, rather than the threshold phenomenon between cardiorespiratory fitness and breast cancer observed in the present study. It is unclear what contributes to this difference, although the differing approaches to measuring physical activity exposures (i.e., self-reported physical activity vs. the more objective cardiorespiratory fitness) likely account for some of the variability. Future studies are needed to confirm our findings.
A functional capacity of 8 METs is considered a low to moderate level of cardiorespiratory fitness for women across the adult age spectrum (6, 38). Most women can attain this level of cardiorespiratory fitness by participating in moderate and/or vigorous intensity physical activities for 30 minutes or more on most days of the week (14). Although cardiorespiratory fitness has a genetic component (21), it is clear that usual physical activity habits are the primary determinant of fitness. Recently, Church et al. (7) reported that women with an activity level as low as 4-kcal/kg per week level (approximately 72 min/wk of moderate intensity walking) had a significant improvement in cardiorespiratory fitness compared with women in the nonexercise control group. Therefore, cardiorespiratory fitness can be enhanced in most individuals through participation in a modest amount of moderate intensity physical activity.
Several biological mechanisms have been proposed to explain how increased physical activity may protect against breast cancer, including the reduction of endogenous reproductive hormone levels, decreases in body weight and adiposity, changes in circulating levels of insulin and insulin-like growth factors (IGFs) and enhanced immune function (12, 17). Unfortunately, we do not have data related to these variables. Therefore, we can not evaluate these biological mechanisms directly. Because of the unique cohort design of this study, however, our investigation has several properties that enable examination of fitness and physical activity in relation to breast cancer mortality. It is rare to have a measure of fitness in a study of breast cancer and rarer still that to obtain that measure prior to a breast cancer diagnosis. One possible hypothesis is that women maintained their level of fitness after their diagnosis, which then resulted in decreased mortality in the high fitness group. However, previous research among cancer survivors demonstrates that physical activity typically decreases after a cancer diagnosis (26). Thus, observational data do not support this supposition. An alternative hypothesis is that “pre-diagnosis” fitness could result in a better breast cancer prognosis (i.e., women are diagnosed with less aggressive disease) which could then favorably impact breast cancer mortality. There is some indirect support for this hypothesis from the literature which has demonstrated a protective effect of physical activity on markers of breast cancer prognosis such as estrogen and progesterone receptor status (2, 11). In these studies, physical activity was protective for estrogen and progesterone receptor-negative breast cancer, which is known to be associated with the poorest prognosis and survival. Examination of the relationship of fitness or physical activity with other breast cancer prognostic indicators could be an interesting avenue for future study.
Strengths of the current study include its prospective design, extensive baseline examinations to detect subclinical disease, careful measurement of risk factors (as opposed to self-report), maximal exercise testing to quantify cardiorespiratory fitness, and a hard endpoint of breast cancer mortality as the study outcome. A limitation is the inability to adjust for dietary factors and menopausal status due to the lack of data in the current study. However, a recent study that has adjusted for daily caloric intake and percentage of calories from fat found that these adjustments did not significantly change conclusions (29). Although there is evidence that dietary fat may be a stronger risk factor for postmenopausal breast cancer than for premenopausal breast cancer (20), Kushi et al. (23) only found a nonsignificant positive association between dietary fat and risk of postmenopausal breast cancer. According to the latest clinical trial results from the Women’s Health Initiative, women following a recommended eating pattern including a reduction in total fat did not experience reduced breast cancer incidence (33). It is possible that residual confounding may exist, although it seems unlikely that it would account for all of the observed association between cardiorespiratory fitness and breast cancer. Another limitation of the present study is that the hormone receptor status (estrogen or progesterone) and the tumor stage (including in situ) and grade were not available. A recent report from the California Teachers Study show an inverse association between self-reported physical activity and risk of both invasive and in situ breast cancer in women and an inverse association on estrogen-negative, but not estrogen-positive breast cancer (9). Additional research is needed to further understand the specificity of associations between cardiorespiratory fitness (or physical activity) and breast cancer subtypes. The current findings are limited to European-American women in the middle and upper socioeconomic strata; the results may not be generalizable to other adult populations. Finally, we had no data on the use of mammography screening and current cancer and treatment. Future studies need to include this information to confirm our findings reported here. Given that the cohort was comprised of well-educated women of a high socio-economic standing, we estimate that the majority of the women were seeking regular mammography screening (35). Thus we would not anticipate a change in our findings upon adjustment for screening.
In summary, we found an inverse association between cardiorespiratory fitness and risk of breast cancer mortality in this cohort of U.S. women and that a certain, relatively low, threshold of cardiorespiratory fitness may be needed. Given the public health burden of breast cancer, future research needs to determine the specific biological aspects of exercise related to breast cancer risk, and if a dose-response relationship exists.
Acknowledgments
Supported by National Institutes of Health grants AG06945 and HL62508. Results of the present study do not constitute endorsement by ACSM.
We thank the Cooper Clinic physicians and technicians for collecting the baseline data, and staff at the Cooper Institute for data entry and data management.
Footnotes
Conflicts of interest
None declared.
References
- 1.Adams SA, Hebert JR, Bolick-Aldrich S, et al. Breast cancer disparities in South Carolina: Early detection, special programs, and descriptive epidemiology. Journal of the South Carolina Medical Association. 2006;102:231–239. [PMC free article] [PubMed] [Google Scholar]
- 2.Adams SA, Matthews CE, Hebert JR, et al. Association of physical activity with hormone receptor status: the Shanghai Breast Cancer Study. Cancer Epidemiol Biomarkers Prev. 2006;15:1170–1178. doi: 10.1158/1055-9965.EPI-05-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ainsworth BE. Issues in the assessment of physical activity in women. Res Q Exerc Sport. 2000;71:S37–S42. [PubMed] [Google Scholar]
- 4.American College of Sports Medicine. ACSM’s Guidelines For Exercise Testing And Prescription. 7. 2005. pp. 291–294. [DOI] [PubMed] [Google Scholar]
- 5.Balke B, Ware RW. An experimental study of physical fitness in Air Force personnel. US Armed Forces Med J. 1959;10:675–688. [PubMed] [Google Scholar]
- 6.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality: a prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 7.Church TS, Earnest CP, Skinner JS, Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: a randomized controlled trial. JAMA. 2007;297:2081–2091. doi: 10.1001/jama.297.19.2081. [DOI] [PubMed] [Google Scholar]
- 8.Daling JR, Malone KE, Doody DR, Johnson LG, Gralow JR, Porter PL. Relation of body mass index to tumor markers and survival among young women with invasive ductal breast carcinoma. Cancer. 2001;92:720–729. doi: 10.1002/1097-0142(20010815)92:4<720::aid-cncr1375>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 9.Dallal CM, Sullivan-Halley J, Ross RK, et al. Long-term recreational physical activity and risk of invasive and in situ breast cancer: the California teachers study. Arch Intern Med. 2007;167:408–415. doi: 10.1001/archinte.167.4.408. [DOI] [PubMed] [Google Scholar]
- 10.Elias SG, Peeters PH, Grobbee DE, van Noord PA. Breast cancer risk after caloric restriction during the 1944–1945 Dutch famine. J Natl Cancer Inst. 2004;96:539–546. doi: 10.1093/jnci/djh087. [DOI] [PubMed] [Google Scholar]
- 11.Enger SM, Ross RK, Paganini-Hill A, Carpenter CL, Bernstein L. Body size, physical activity, and breast cancer hormone receptor status: results from two case-control studies. Cancer Epidemiol Biomarkers Prev. 2000;9:681–687. [PubMed] [Google Scholar]
- 12.Friedenreich CM, Orenstein MR. Physical activity and cancer prevention: etiologic evidence and biological mechanisms. J Nutr. 2002;132:S3456–3464. doi: 10.1093/jn/132.11.3456S. [DOI] [PubMed] [Google Scholar]
- 13.Gibbons LW, Mitchell TL, Wei M, Blair SN, Cooper KH. Maximal exercise test as a predictor of risk for mortality from coronary heart disease in asymptomatic men. Am J Cardiol. 2000;86:53–58. doi: 10.1016/s0002-9149(00)00827-4. [DOI] [PubMed] [Google Scholar]
- 14.Haskell WL, Leon AS, Caspersen CJ, et al. Cardiovascular benefits and assessment of physical activity and physical fitness in adults. Med Sci Sports Exerc. 1992;24:S201–220. [PubMed] [Google Scholar]
- 15.Hebert JR, Hurley TG, Ma Y. The effect of dietary exposures on recurrence and mortality in early stage breast cancer. Breast Cancer Res Treat. 1998;51:17–28. doi: 10.1023/a:1006056915001. [DOI] [PubMed] [Google Scholar]
- 16.Hebert JR, Rosen A. Nutritional, socioeconomic, and reproductive factors in relation to female breast cancer mortality: findings from a cross-national study. Cancer Detect Prev. 1996;20:234–244. [PubMed] [Google Scholar]
- 17.Hoffman-Goetz L, Apter D, mark-Wahnefried W, Goran MI, McTiernan A, Reichman ME. Possible mechanisms mediating an association between physical activity and breast cancer. Cancer. 1998;83:621–628. doi: 10.1002/(sici)1097-0142(19980801)83:3+<621::aid-cncr4>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman-Goetz L, Husted J. Exercise and breast cancer: review and critical analysis of the literature. Can J Appl Physiol. 1994;19:237–252. doi: 10.1139/h94-020. [DOI] [PubMed] [Google Scholar]
- 19.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 20.Howe GR, Hirohata T, Hislop TG, et al. Dietary factors and risk of breast cancer: combined analysis of 12 case-control studies. J Natl Cancer Inst. 1990;82:561–569. doi: 10.1093/jnci/82.7.561. [DOI] [PubMed] [Google Scholar]
- 21.Ingelsson E, Larson MG, Vasan RS, et al. Heritability, linkage, and genetic associations of exercise treadmill test responses. Circulation. 2007;115:2917–2924. doi: 10.1161/CIRCULATIONAHA.106.683821. [DOI] [PubMed] [Google Scholar]
- 22.Kumar NB, Riccardi D, Cantor A, Dalton K, Allen K. A case-control study evaluating the association of purposeful physical activity, body fat distribution, and steroid hormones on premenopausal breast cancer risk. Breast J. 2005;11:266–272. doi: 10.1111/j.1075-122x.2005.21693.x. [DOI] [PubMed] [Google Scholar]
- 23.Kushi LH, Sellers TA, Potter JD, et al. Dietary fat and postmenopausal breast cancer. J Natl Cancer Inst. 1992;84:1092–1099. doi: 10.1093/jnci/84.14.1092. [DOI] [PubMed] [Google Scholar]
- 24.Lee IM, Rexrode KM, Cook NR, Hennekens CH, Burin JE. Physical activity and breast cancer risk: the Women’s Health Study (United States) Cancer Causes Control. 2001;12:137–145. doi: 10.1023/a:1008948125076. [DOI] [PubMed] [Google Scholar]
- 25.Luoto R, Latikka P, Pukkala E, Hakulinen T, Vihko V. The effect of physical activity on breast cancer risk: a cohort study of 30,548 women. Eur J Epidemiol. 2000;16:973–980. doi: 10.1023/a:1010847311422. [DOI] [PubMed] [Google Scholar]
- 26.Lynch BM, Cerin E, Newman B, Owen N. Physical activity, activity change, and their correlates in a population-based sample of colorectal cancer survivors. Ann Behav Med. 2007;34:135–143. doi: 10.1007/BF02872668. [DOI] [PubMed] [Google Scholar]
- 27.Matthews CE, Wilcox S, Hanby CL, et al. Evaluation of a 12-week home-based walking intervention for breast cancer survivors. Support Care Cancer. 2007;15:203–211. doi: 10.1007/s00520-006-0122-x. [DOI] [PubMed] [Google Scholar]
- 28.McPherson K, Steel CM, Dixon JM. ABC of breast diseases. Breast cancer-epidemiology, risk factors, and genetics. BMJ. 2000;321:624–628. doi: 10.1136/bmj.321.7261.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McTiernan A, Kooperberg C, White E, et al. Recreational physical activity and the risk of breast cancer in postmenopausal women: the Women’s Health Initiative Cohort Study. JAMA. 2003;290:1331–1336. doi: 10.1001/jama.290.10.1331. [DOI] [PubMed] [Google Scholar]
- 30.Monninkhof EM, Elias SG, Vlems FA, et al. Physical activity and breast cancer: a systematic review. Epidemiology. 2007;18:137–157. doi: 10.1097/01.ede.0000251167.75581.98. [DOI] [PubMed] [Google Scholar]
- 31.Newman LA, Mason J, Cote D, et al. African-American ethnicity, socioeconomic status, and breast cancer survival: a meta-analysis of 14 studies involving over 10,000 African-American and 40,000 White American patients with carcinoma of the breast. Cancer. 2002;94:2844–2854. doi: 10.1002/cncr.10575. [DOI] [PubMed] [Google Scholar]
- 32.Pollock ML, Foster C, Schmidt D, Hellman C, Linnerud AC, Ward A. Comparative analysis of physiologic responses to three different maximal graded exercise test protocols in healthy women. Am Heart J. 1982;103:363–373. doi: 10.1016/0002-8703(82)90275-7. [DOI] [PubMed] [Google Scholar]
- 33.Prentice RL, Caan B, Chlebowski RT, et al. Low-fat dietary pattern and risk of invasive breast cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:629–642. doi: 10.1001/jama.295.6.629. [DOI] [PubMed] [Google Scholar]
- 34.Rockhill B, Willett WC, Hunter DJ, Manson JE, Hankinson SE, Colditz GA. A prospective study of recreational physical activity and breast cancer risk. Arch Intern Med. 1999;159:2290–2296. doi: 10.1001/archinte.159.19.2290. [DOI] [PubMed] [Google Scholar]
- 35.Ryerson AB, Miller JW, Eheman CR, Leadbetter S, White MC. Recent trends in U.S. mammography use from 2000–2006: A population-based analysis. Prev Med. 2008 doi: 10.1016/j.ypmed.2008.06.010. ahead of print. [DOI] [PubMed] [Google Scholar]
- 36.Sesso HD, Paffenbarger RS, Jr, Lee IM. Physical activity and breast cancer risk in the College Alumni Health Study (United States) Cancer Causes Control. 1998;9:433–439. doi: 10.1023/a:1008827903302. [DOI] [PubMed] [Google Scholar]
- 37.Slattery ML, Edwards S, Murtaugh MA, et al. Physical activity and breast cancer risk among women in the southwestern United States. Ann Epidemiol. 2007;17:342–353. doi: 10.1016/j.annepidem.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sui X, LaMonte MJ, Blair SN. Cardiorespiratory fitness as a predictor of nonfatal cardiovascular events in asymptomatic women and men. Am J Epidemiol. 2007;165:1413–1423. doi: 10.1093/aje/kwm031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thune I, Brenn T, Lund E, Gaard M. Physical activity and the risk of breast cancer. N Engl J Med. 1997;336:1269–1275. doi: 10.1056/NEJM199705013361801. [DOI] [PubMed] [Google Scholar]
- 40.Whiteman MK, Hillis SD, Curtis KM, McDonald JA, Wingo PA, Marchbanks PA. Body mass and mortality after breast cancer diagnosis. Cancer Epidemiol Biomarkers Prev. 2005;14:2009–2014. doi: 10.1158/1055-9965.EPI-05-0106. [DOI] [PubMed] [Google Scholar]