Abstract
It is often challenging to assess cardiac filling pressure clinically. An improved system for detecting or ruling out elevated cardiac filling pressure may help reduce hospitalizations for heart failure. The blood pressure response to the Valsalva maneuver reflects left heart filling pressure, but its underuse clinically may be due in part to lack of continuous blood pressure recording along with lack of standardization of expiratory effort. In this study, we tested whether Valsalva-induced changes in the pulse amplitude of finger photoplethysmography (PPG), a technology already widely available in medical settings, correlate with invasively measured left ventricular end-diastolic pressure (LVEDP). We tested 33 subjects before clinically scheduled cardiac catheterizations. A finger photoplethysmography waveform was recorded during a Valsalva effort of 20 mmHg expiratory pressure sustained for 10 s, an effort most patients can achieve. Pulse amplitude ratio (PAR) was calculated as the PPG waveform amplitude just before release of expiratory effort divided by the waveform amplitude at baseline. PAR was well correlated with LVEDP (r = 0.68; P < 0.0001). For identifying LVEDP > 15 mmHG, PAR > 0.4 was 85% sensitive [95% confidence interval (95CI): 54–97%] and 80% specific (95CI: 56–93%). In conclusion, finger PPG, a technology already ubiquitous in medical centers, may be useful for assessing clinically meaningful categories of left heart filling pressure, using simple analysis of the waveform after a Valsalva maneuver effort that most patients can achieve.
Keywords: left ventricular end-diastolic pressure
heart failure is the most common hospitalization diagnosis, with over 1,000,000 admissions each year (5). The readmission rate for heart failure is high: 20% by 30 days after discharge and 30% at 60–90 days after discharge. Health care costs due to heart failure total over $35 billion each year (5, 7, 8). It is widely believed that improving the detection of elevated cardiac filling pressure could substantially reduce the number of hospitalizations and repeat hospitalizations for heart failure (7, 18, 35).
Unfortunately, physical examination has important limitations in detecting elevated cardiac filling pressure (2, 31). Various noninvasive diagnostic aids are available for detecting evidence of elevated cardiac filling pressures, including chest X-ray, serum brain natriuretic peptide and pro-brain natriuretic peptide, and echocardiography (4, 25). However, each has important limitations, such as sensitivity, specificity, convenience, or cost (14, 19, 22). A noninvasive diagnostic tool for assessing left heart filling pressure that is accurate, convenient, inexpensive, point-of-care, and immediate could contribute substantially toward reducing admissions and readmissions for heart failure. It would be of further advantage if the tool was already available in most hospitals.
The Valsalva maneuver has been described as an underused bedside biomarker of heart failure (6, 26, 36). The maneuver consists of an expiratory effort against a closed glottis. Normally, the increased intrathoracic pressure during the Valsalva strain reduces venous return and results in a transient drop in average blood pressure and in pulse pressure (20). In the setting of elevated left heart filling pressure, one may detect no change or even an increase in blood pressure during the maneuver. The method of measuring the blood pressure response to the maneuver using an ordinary sphygmomanometer (36) has not gained widespread clinical use, possibly due to several factors that may limit its accuracy, such as lack of standardization of expiratory effort and lack of continuous blood pressure measurement during the maneuver. Use of a continuous arterial waveform obtained invasively during the Valsalva maneuver has been shown to be useful in detecting elevated left heart filling pressure (26, 32, 37). A simple variable determined from the continuous signal is the pulse amplitude ratio, which is the ratio of pulse pressure during Valsalva to the pulse pressure at rest. Noninvasive systems have been developed that continuously measure signals related to continuous blood pressure during a Valsalva maneuver. Research studies have utilized systems employing either a tonometry transducer (17) or a volume-clamp pressure transduction device (Finapres; Finapres Medical Systems, Amsterdam, The Netherlands; Ref. 24), and the systems also allowed the expiratory effort to be monitored. Multiple studies (3, 9, 10, 17, 27, 33) have shown good correlations between characteristics of these noninvasive blood pressure surrogate waveforms and invasively measured indexes of cardiac filling pressure. One recent study (28) suggests that using this technique to guide care may reduce rehospitalization for heart failure.
Unlike the volume-clamp and tonometric devices just described, photoplethysmography is already ubiquitous in medical settings. Finger photoplethysmography is currently used mainly to determine pulse oximetry and heart rate, but the photoplethysmography signal also reflects time-varying finger pulse volume, which reflects peripheral blood flow and peripheral blood pressure (1). Photoplethysmography waveform changes during a Valsalva maneuver closely resemble changes in an invasive arterial blood pressure waveform during the maneuver (34). Furthermore, changes in the photoplethysmography waveform of the ear during Valsalva were shown to contain information indicating left ventricular filling pressure (3). Thus finger photoplethysmography, which is already widely available, may help assess the response to the Valsalva maneuver in evaluating left ventricular filling pressure.
The purpose of this study was to determine if pulse amplitude changes of a finger photoplethysmography waveform during a Valsalva maneuver correlate with left ventricular end-diastolic pressure (LVEDP) measured via cardiac catheterization. We also sought to determine the feasibility of using an expiratory effort that most patients can achieve: 20 mmHg for 10 s.
METHODS
Patients already scheduled to undergo nonemergent cardiac catheterizations were asked to participate in this study. All participants provided written informed consent before involvement. The Johns Hopkins University Institutional Review Board approved the protocol before study initiation. Patients were excluded if they had moderate or severe aortic stenosis, moderate or severe mitral stenosis, uncontrolled hypertension, atrial fibrillation, or hypertrophic obstructive cardiomyopathy. There was no order or pattern of selection for recruiting.
Participants were tested while in the semirecumbent position, within ∼1 h before their catheterization. Participants were asked to sustain an exhalation effort of 20 mmHg for 10 s into a tube connected to a pressure transducer, while a photoplethysmography transducer was attached to a fingertip. The photoplethymography probe was placed on the finger of an open, relaxed hand that was set down at rest. The subject was instructed to use the contralateral hand to hold the tube into which the expiratory effort was produced.
Both transduced signals were input into a digital acquisition system (Biopac Systems, Goleta, CA) and then into a computer. Filtering parameters of the photoplethysmography signal were 10-Hz low pass and 0.01-Hz high pass. The pressure signal was displayed to help guide the participant. At least three Valsalva efforts were recorded. Criteria considered acceptable for inclusion of an effort included maintaining an expiratory effort between 18 and 25 mmHg for ≥10 s. Also, the range must be achieved within ∼3 s of initiating the effort. Waveforms were analyzed using software accompanying the digital acquisition system. For each Valsalva effort, the amplitudes of three typical cycles of the baseline photoplethysmography waveform were averaged. The amplitude of the cycle just before 10 s into the Valsalva maneuver was measured. We did not use multiple cycles when calculating pulse amplitude during the Valsalva maneuver because the amplitude changes continuously during the maneuver. Pulse amplitude ratio (PAR) was calculated as the ratio of the signal amplitude at end Valsalva to the average baseline signal amplitude. The average PAR over all the acceptable efforts was obtained for each subject.
LVEDP was measured via a catheter placed in the left ventricle during the cardiac catheterization procedure. The operators who performed the catheterization measurements were blinded to the photoplethysmography measurements. Standard least-squares linear regression analysis was used to examine the correlation between the PAR and LVEDP measurements. Receiver operating characteristic (ROC) analyses were also performed.
RESULTS
Of 36 participants enrolled, a total of 33 participants (92%) had at least one acceptable expiratory effort. Table 1 shows their baseline characteristics. The average age was 58 (11) years, and they consisted of 22 men and 11 women. Left ventricular ejection fraction was obtained clinically for all but one participant, a median of within 2 days of the catheterization. Ejection fraction was measured either by echocardiogram, left ventriculogram, or nuclear medicine scan.
Table 1.
Subject characteristics
| Number of subjects | 33 |
| Age, yr | 58 (11) |
| Gender, male/female | 22/11 |
| Height, in. | 67 (5) |
| Weight, lb | 201 (71) |
| Body mass index, kg/m2 | 31 (8) |
| Systolic blood pressure, mmHg | 140 (20) |
| Diastolic blood pressure, mmHg | 85 (11) |
| Heart rate, beats/min | 69 (12) |
| Left ventricular ejection fraction, % | 46 (15) |
| Medical problems | |
| Hypertension, % | 26 |
| Diabetes type 2, % | 13 |
| Coronary artery disease, % | 79 |
| Congestive heart failure, % | 7 |
| Medicines | |
| β-Blocker, % | 70 |
| Angiotensin-converting enzyme inhibitor, % | 33 |
| Angiotensin receptor blocker, % | 3 |
| Calcium channel blocker, % | 15 |
| Nitrate, % | 12 |
| Hydrochlorothiazide, % | 4 |
| Furosemide, % | 6 |
| Indication for catheterization | |
| Non-ST elevation myocardial infarction, % | 39 |
| Suspicion of angina, % | 52 |
| Evaluation of cardiomyopathy, % | 9 |
Continuous variables are means (SD).
Participants typically required about three attempts before they could generate an acceptable expiratory effort profile. The range of acceptable expiratory efforts used was 1 (only 2 subjects) to 8. The mean and median number of acceptable expiratory efforts used was 4. Figure 1 shows a photoplethysmography waveform response in a participant whose PAR was 0.4 and LVEDP was normal at 10 mmHg. Figure 2 shows a waveform response in a participant whose PAR was 0.9 and LVEDP was very elevated at 35 mmHg. PAR correlated well with LVEDP (r = 0.68; P < 0.0001; Fig. 3). Figure 4 shows a Bland-Altman plot. An ROC plot for identifying LVEDP > 15 mmHg is shown in Fig. 5. A PAR > 0.4 has an 85% sensitivity and 80% specificity in identifying LVEDP > 15 mmHg, area under the curve 0.83.
Fig. 1.
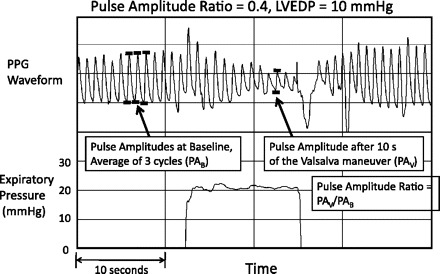
Photoplethysmography (PPG) waveform and expiratory pressure in a subject whose left ventricular end-diastolic pressure (LVEDP) was subsequently measured to be normal at 10 mmHg. Pulse amplitude ratio was 0.4.
Fig. 2.
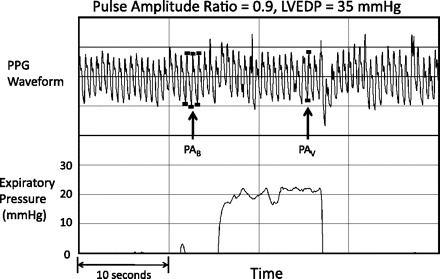
PPG waveform and expiratory pressure in a subject whose LVEDP was subsequently measured to be very elevated at 35 mmHg. Pulse amplitude ratio was 0.9.
Fig. 3.
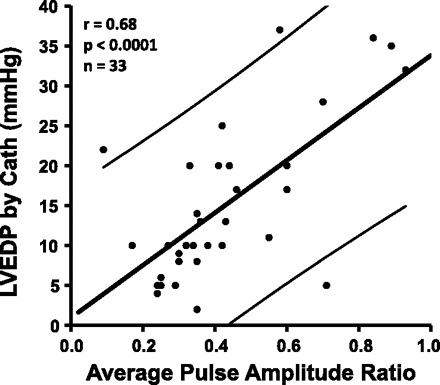
Pulse amplitude ratio (PAR) measured from the PPG waveforms vs. subsequently measured LVEDP from cardiac catheterizations. Thinner, slightly concave lines indicate confidence intervals of the point.
Fig. 4.
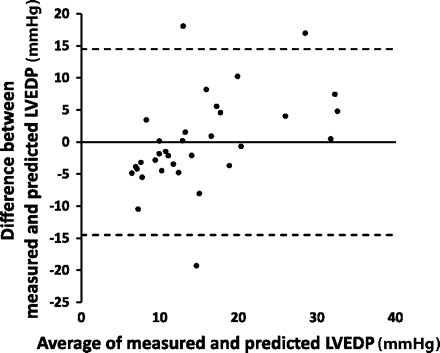
Bland-Altman plot. Predicted LVEDP was calculated from PAR based on the regression equation from measured LVEDP vs. PAR as plotted in Fig. 3.
Fig. 5.
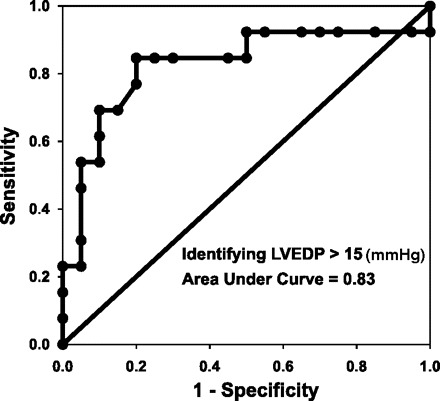
Receiver operating characteristic analysis for identifying LVEDP > 15 (mmHg). Area under the curve = 0.83. PAR > 0.4 has 85% sensitivity (95% confidence interval: 54–97%) and 80% specificity (95% confidence interval: 56–93%).
DISCUSSION
In this study, we investigated the utility of finger photoplethysmography to assess left heart filling pressure noninvasively during the Valsalva maneuver. We found a good correlation between PAR, a simple measure of waveform change during the maneuver, with invasively measured LVEDP. The main new physiological finding is that Valsalva-induced changes in finger perfusion via changes in pressure and flow amplitude as assessed by finger photoplethysmography reflect left ventricular filling pressure. In addition, ROC analysis suggests that PAR by finger photoplethysmography has good sensitivity and specificity for identifying clinical meaningful categories of LVEDP. A PAR cutoff of >0.4 may help identify patients with LVEDP > 15 mmHg, a cutoff value that has mortality implications in heart failure (30).
It has been known for over 50 yr that blood pressure responses to the Valsalva maneuver reflect cardiac volume status (13, 15, 29). The major underlying physiology, as advanced in prior studies, is that the Valsalva maneuver increases intrathoracic pressure and limits systemic venous return, leading to a decrease in left heart filling pressure, thus a decrease in stroke volume, and thus a decrease in peripheral pulse amplitude. However, when there is thoracic fluid congestion and elevated LVEDP, then limiting systemic venous return into the thorax will not significantly limit the fluid available for the left heart to eject. In that situation, peripheral pulse amplitude will be less changed, or not changed at all, by the Valsalva maneuver (16, 20, 33, 38).
The blood pressure response to the Valsalva maneuver consists of four phases. These have been well described using continuous, invasive blood pressure measurement (37, 38). In individuals with normal filling pressure, the four phases consist of: phase 1: transient rise in blood pressure associated with the onset of strain; phase 2: decrease in average blood pressure and pulse amplitude with maintained strain, increased intrathoracic pressure and decreased venous return; phase 3: transient drop in blood pressure upon release of strain; and phase 4: overshoot of blood pressure with restored venous return (37). In individuals with elevated filling pressures, the maneuver may be associated with abnormal responses detectable with a sphygmomanometer: an increase in blood pressure with no subsequent decrease during phase 2 (“square wave response”) and no overshoot upon strain release.
For years, the Valsalva maneuver has been touted as an underused bedside test of heart failure (6, 26, 36). Two disadvantages of performing a bedside Valsalva maneuver with only a sphygmomanometer are that the expiratory effort is not measured and standardized and that a continuous blood pressure signal is not recorded. These drawbacks may limit accuracy because expiratory effort affects blood pressure response (21, 23) and because calculation of pulse amplitude ratio, a continuous variable, requires a continuous signal. These considerations may contribute to a lack of widespread clinical use of the technique. One implication of our study is that the bedside Valsalva test might be improved by employing equipment already available in most hospitals: a finger photoplethysmography transducer and medical tubing attached to a sphygmomanometer to measure a patient's expiratory effort.
Photoplethysmography measures blood perfusion. Therefore, changes in amplitude most directly reflect changes in flow into the digit but as such are also dependent on pressure (1). A variety of noninvasive transducers have been used to measure continuously a physiological response to the Valsalva maneuver that reflects an invasive continuous blood pressure response. The first noninvasive, continuous signal shown to exhibit changes with Valsalva that correlated with LVEDP was photoplethysmography of the ear (3). However, the index used was derived retrospectively from multiple parameters of the photoplethysmography waveform. PAR, a much more simply derived index, was not tested. One study compared PAR of a finger photoplethysmography signal with changes in an impedance cardiographic signal but did not compare them with a direct measurement of cardiac filling pressure (21).
The VeriCor (CVP Diagnostics, Boston, MA) system's sensor is a tonometer, a surface deformation sensor, applied to the finger or wrist (17). The Finapres device (Finapres Medical Systems) is a sophisticated system that employs an inflatable volume clamp with photoplethysmography used as a feedback signal. A reverse transfer function is applied to the detected waveform to provide essentially a calibrated, continuous blood pressure signal (12). Multiple studies (3, 9, 10, 17, 27, 33), each using one of these systems, have shown a very good correlation between noninvasive blood pressure surrogate waveform characteristics and invasively measured indexes of cardiac filling pressure. A recent study (28) using the VeriCor suggests that guiding therapy in heart failure using the Valsalva technique may reduce repeat hospitalizations (28). The present study is the first to investigate the relationship between LVEDP and only the pulse amplitude response of ordinary photoplethysmography of the finger, which can be readily obtained in many medical settings.
We selected parameters of this study that would allow for the simplest and most convenient bedside examination with available hospital equipment, including the use of PAR only, expiratory effort of 20 mmHg, expiratory effort duration of 10 s, and semirecumbent body position. Some studies (27, 28) have employed indexes calculated from multiple descriptors of the recorded physiological waveform. For example, the most recent studies using the Vericor also incorporated the rate of decline of pulse pressure, rate of change of the slope of the systolic pressure, and rate of change of the pressure over time (dP/dt) of the upstroke of the arterial pressure. The ear photoplethysmography study (3) incorporated normalized slope of linear fit of pulse amplitude decay and also various time intervals. However, other studies PAR (9, 17, 23, 33) using the Finapres or the Vericor have shown close correlation between cardiac filling pressure and just PAR, which would be much easier to derive from a bedside photoplethysmography signal than combining multiple variables. Some studies (3, 9, 23, 38) have used a higher expiratory effort, as high as 40 mmHg, and one study (33) used a longer expiratory effort duration of 15 s. The expiratory effort needs to be consistent, because PAR is lower when expiratory effort is higher (10, 21, 32). We chose parameters that patients with heart and/or lung disease would be more likely to achieve (23). We chose to measure the response to Valsalva maneuver in a common examination position, semirecumbent, although PAR may be similar in different body positions (21). Therefore, the PAR measurements taken in a common examination position reflect the LVEDP measurements obtained in the supine position in the catheterization laboratory.
Factors such as skin pigment and thickness, nail polish, overall digit size, and shape affect the photoplethysmography waveform. However, those factors would be the same before and during the Valsalva maneuver. Thus the change in pulse amplitude with the maneuver is caused mainly by the flow and pressure changes from the maneuver.
There are several limitations to our study. One limitation was that this feasibility study was not powered to investigate the independent effects of specific baseline characteristics on PAR. This also means that we could not assess whether left ventricular ejection fraction affects the correlation between PAR and LVEDP, as was suggested by one study (11). Another limitation is that the sensitivity and specificity reported here are based on a threshold of PAR derived from the same set of data. A formal assessment of sensitivity and specificity needs to be done in a separate, prospective study on a larger population. Yet another limitation is that the study was not designed to compare photoplethysmography with other devices that measure PAR, such as the volume-clamp technique (i.e., Finapres). The correlations between LVEDP and PAR by volume-clamp technique and between pulmonary capillary wedge pressure and PAR by tonometry (VeriCor) were higher than in this study (9, 10, 17). However, those studies employed an expiratory effort of 40 mmHg, which may improve the correlation, but which fewer patients can achieve. Future larger studies using photoplethysmography may suggest improvements in the technique that could improve the correlation. It would also be useful in a future study to compare accuracy of the different techniques using similar expiratory pressures. Nevertheless, the main new finding of this study is that Valsalva-induced changes in finger photoplethysmography, a technology already widely used, reflect LVEDP.
Although pulse oximeters are ubiquitous in medical settings, many do not offer a printout of the photoplethysmography waveform, which limits the immediate clinical applicability of this study. However, if larger studies support the clinical utility of Valsalva photoplethysmography, more pulse oximeter models could be designed to include printout or screen-recall capability.
In conclusion, this study suggests that finger photoplethysmography, a technology already widely available in inpatient and outpatient settings and easily amenable to pulse amplitude estimation, may be used during a Valsalva maneuver effort that most patients can achieve to assess left heart filling pressure. The technique may help to detect or rule out elevated left heart filling pressure. Future studies should evaluate clinical utility further, including whether the technique predicts hospitalization or repeat hospitalization for decompensated heart failure.
GRANTS
This study was supported by Johns Hopkins Bayview Cardiology Division discretionary research funds.
DISCLOSURES
H. A. Silber has filed a patent application based in part on work done in this study. His role in the study adheres to a protocol established by the Conflict of Interest Committee of the Johns Hopkins Office of Policy Management.
AUTHOR CONTRIBUTIONS
Author contributions: H.A.S. and E.K.K. conception and design of research; H.A.S., J.C.T., P.V.J., W.L.M., T.R.A., and D.E.B. performed experiments; H.A.S. and N.-Y.W. analyzed data; H.A.S., N.-Y.W., and E.K.K. interpreted results of experiments; H.A.S. prepared figures; H.A.S. drafted manuscript; H.A.S. edited and revised manuscript; H.A.S., J.C.T., P.V.J., W.L.M., N.-Y.W., E.K.K., T.R.A., and D.E.B. approved final version of manuscript.
REFERENCES
- 1.Allen J. Photoplethysmography and its application in clinical physiological measurement. Physiol Meas 28: R1–R39, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Badgett RG, Lucey CR, Mulrow CD. Can the clinical examination diagnose left-sided heart failure in adults? JAMA 277: 1712–1719, 1997 [PubMed] [Google Scholar]
- 3.Bernardi L, Saviolo R, Spodick DH. Noninvasive assessment of central circulatory pressures by analysis of ear densitographic changes during the Valsalva maneuver. Am J Cardiol 64: 787–792, 1989 [DOI] [PubMed] [Google Scholar]
- 4.Dokainish H, Zoghbi WA, Lakkis NM, Al-Bakshy F, Dhir M, Quinones MA, Nagueh SF. Optimal noninvasive assessment of left ventricular filling pressures: a comparison of tissue Doppler echocardiography and B-type natriuretic peptide in patients with pulmonary artery catheters. Circulation 109: 2432–2439, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Fang J, Mensah GA, Croft JB, Keenan NL. Heart failure-related hospitalization in the US, 1979 to 2004. J Am Coll Cardiol 52: 428–434, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Felker GM, Cuculich PS, Gheorghiade M. The Valsalva maneuver: a bedside “biomarker” for heart failure. Am J Med 119: 117–122, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Gheorghiade M, Filippatos G, De Luca L, Burnett J. Congestion in acute heart failure syndromes: an essential target of evaluation and treatment. Am J Med 119: S3–S10, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol 53: 557–573, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Gillard C, Henuzet C, Lallemand J, Moscariello A, Guillaume M, Van Meerhaeghe A. Operating characteristics of the Finapress system to predict elevated left ventricular filling pressure. Clin Cardiol 29: 107–111, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Givertz MM, Slawsky MT, Moraes DL, McIntyre KM, Colucci WS. Noninvasive determination of pulmonary artery wedge pressure in patients with chronic heart failure. Am J Cardiol 87: 1213–1215; A1217, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Hebert JL, Coirault C, Zamani K, Fontaine G, Lecarpentier Y, Chemla D. Pulse pressure response to the strain of the valsalva maneuver in humans with preserved systolic function. J Appl Physiol 85: 817–823, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Imholz BP, Wieling W, van Montfrans GA, Wesseling KH. Fifteen years experience with finger arterial pressure monitoring: assessment of the technology. Cardiovasc Res 38: 605–616, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Judson WE, Hatcher JD, Wilkins RW. Blood pressure responses to the Valsalva maneuver in cardiac patients with and without congestive failure. Circulation 11: 889–899, 1955 [DOI] [PubMed] [Google Scholar]
- 14.Kapoor JR, Perazella MA. Diagnostic and therapeutic approach to acute decompensated heart failure. Am J Med 120: 121–127, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Knowles JH, Gorlin R, Storey CF. Clinical test for pulmonary congestion with use of the Valsalva maneuver. J Am Med Assoc 160: 44–48, 1956 [DOI] [PubMed] [Google Scholar]
- 16.Little WC, Barr WK, Crawford MH. Altered effect of the Valsalva maneuver on left ventricular volume in patients with cardiomyopathy. Circulation 71: 227–233, 1985 [DOI] [PubMed] [Google Scholar]
- 17.McIntyre KM, Vita JA, Lambrew CT, Freeman J, Loscalzo J. A noninvasive method of predicting pulmonary-capillary wedge pressure. N Engl J Med 327: 1715–1720, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Metra M, DeiCas L, Bristow MR. The pathophysiology of acute heart failure–it is a lot about fluid accumulation. Am Heart J 155: 1–5, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Mullens W, Borowski AG, Curtin RJ, Thomas JD, Tang WH. Tissue Doppler imaging in the estimation of intracardiac filling pressure in decompensated patients with advanced systolic heart failure. Circulation 119: 62–70, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray WB, Foster PA. The peripheral pulse wave: information overlooked. J Clin Monit 12: 365–377, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Patterson RP, Zhang J. Impedance cardiographic measurement of the physiological response to the Valsalva manoeuvre. Med Biol Eng Comput 41: 40–43, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Pfisterer M, Buser P, Rickli H, Gutmann M, Erne P, Rickenbacher P, Vuillomenet A, Jeker U, Dubach P, Beer H, Yoon SI, Suter T, Osterhues HH, Schieber MM, Hilti P, Schindler R, Brunner-LaRocca HP. BNP-guided vs symptom-guided heart failure therapy: the Trial of Intensified vs Standard Medical Therapy in Elderly Patients With Congestive Heart Failure (TIME-CHF) randomized trial. JAMA 301: 383–392, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Remmen JJ, Aengevaeren WR, Verheugt FW, Jansen RW. Detection of elevated pulmonary capillary wedge pressure in elderly patients with various cardiac disorders by the Valsalva manoeuvre. Clin Sci (Lond) 111: 153–162, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Remmen JJ, Aengevaeren WR, Verheugt FW, Jansen RW. Normal values of pulmonary capillary wedge pressure and the blood pressure response to the Valsalva manoeuvre in healthy elderly subjects. Clin Physiol Funct Imaging 25: 318–326, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Sanders GP, Mendes LA, Colucci WS, Givertz MM. Noninvasive methods for detecting elevated left-sided cardiac filling pressure. J Card Fail 6: 157–164, 2000 [PubMed] [Google Scholar]
- 26.Schmidt DE, Shah PK. Accurate detection of elevated left ventricular filling pressure by a simplified bedside application of the Valsalva maneuver. Am J Cardiol 71: 462–465, 1993 [DOI] [PubMed] [Google Scholar]
- 27.Sharma GV, Woods PA, Lambrew CT, Berg CM, Pietro DA, Rocco TP, Welt FW, Sacchetti P, McIntyre KM. Evaluation of a noninvasive system for determining left ventricular filling pressure. Arch Intern Med 162: 2084–2088, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Sharma GV, Woods PA, Lindsey N, O'Connell C, Connolly L, Joseph J, McIntyre KM. Noninvasive monitoring of left ventricular end-diastolic pressure reduces rehospitalization rates in patients hospitalized for heart failure: a randomized controlled trial. J Card Fail 17: 718–725, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Sharpey-Schafer EP. Effects of Valsalva's manoeuvre on the normal and failing circulation. Br Med J 1: 693–695, 1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevenson LW. Are hemodynamic goals viable in tailoring heart failure therapy? Hemodynamic goals are relevant. Circulation 113: 1020–1027; discussion 1033, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA 261: 884–888, 1989 [PubMed] [Google Scholar]
- 32.Uehara H, Takenaka I, Aoyama K, Kadoya T, Sata T, Shigematsu A. A new method of predicting pulmonary capillary wedge pressure: the arterial pressure ratio. Anaesthesia 55: 113–117, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Weilenmann D, Rickli H, Follath F, Kiowski W, Brunner-LaRocca HP. Noninvasive evaluation of pulmonary capillary wedge pressure by BP response to the Valsalva maneuver. Chest 122: 140–145, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Weinman J, Ben-Yaakov S, Sapoznikov D. The application of photoplethysmography to the recording of Valsalva maneuver responses. Isr J Med Sci 5: 534–536, 1969 [PubMed] [Google Scholar]
- 35.Wolfel EE. Can we predict and prevent the onset of acute decompensated heart failure? Circulation 116: 1526–1529, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Zema MJ. Diagnosing heart failure by the Valsalva maneuver: isn't it finally time? Chest 116: 851–853, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Zema MJ, Masters AP, Margouleff D. Dyspnea: the heart or the lungs? Differentiation at bedside by use of the simple Valsalva maneuver. Chest 85: 59–64, 1984 [DOI] [PubMed] [Google Scholar]
- 38.Zema MJ, Restivo B, Sos T, Sniderman KW, Kline S. Left ventricular dysfunction–bedside Valsalva manoeuvre. Br Heart J 44: 560–569, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]


