Abstract
Embryonic stem cell therapy has been proposed as a therapeutic strategy to restore β-cell mass and function in T1DM. Recently, a group from Novocell (now ViaCyte) reported successful development of glucose-responsive islet-like structures after implantation of pancreatic endoderm (PE) derived from human embryonic stem cells (hESC) into immune-deficient mice. Our objective was to determine whether implantation of hESC-derived pancreatic endoderm from Novocell into athymic nude rats results in development of viable glucose-responsive pancreatic endocrine tissue. Athymic nude rats were implanted with PE derived from hESC either via implantation into the epididymal fat pads or by subcutaneous implantation into TheraCyte encapsulation devices for 20 wk. Blood glucose, weight, and human insulin/C-peptide secretion were monitored by weekly blood draws. Graft β-cell function was assessed by a glucose tolerance test, and graft morphology was assessed by immunohistochemistry and immunofluorescence. At 20 wk postimplantation, epididymal fat-implanted PE progressed to develop islet-like structures in 50% of implants, with a mean β-cell fractional area of 0.8 ± 0.3%. Human C-peptide and insulin were detectable, but at very low levels (C-peptide = 50 ± 26 pmol/l and insulin = 15 ± 7 pmol/l); however, there was no increase in human C-peptide/insulin levels after glucose challenge. There was no development of viable pancreatic tissue or meaningful secretory function when human PE was implanted in the TheraCyte encapsulation devices. These data confirm that islet-like structures develop from hESC differentiated to PE by the protocol developed by NovoCell. However, the extent of endocrine cell formation and secretory function is not yet sufficient to be clinically relevant.
Keywords: type 1 diabetes, insulin secretion, pancreatic ducts
type 1 diabetes mellitus (T1DM) develops as a consequence of autoimmune-mediated loss of β-cell mass and function (1). Survival for individuals with type 1 diabetes requires daily exogenously administered insulin by injection. Although it is now possible for individuals with T1DM to maintain blood glucose concentrations close to goal much of the time, this is accomplished only with a substantial intrusion on quality of life and at the risk of recurrent hypoglycemia (9). For these reasons, the concept of a cell-based therapy has been explored as a strategy to restore β-cell mass and function in T1DM (2, 20). To be useful, such a cell-based therapy would require an abundant supply of cells that could be implanted safely, avoid immune rejection, and secrete insulin in a glucose-dependent manner that closely recapitulates the dose response of the endocrine pancreas in vivo.
Human embryonic stem cells have the property of self-renewal, thus potentially providing an unlimited supply. However, the development of human embryonic stem cells into functional pancreatic β-cells is not a trivial challenge. Several efforts have been reported with varying degrees of success (7, 10, 11, 13, 24). To date, the most promising data come from the NovoCell (now ViaCyte) group that reported development of islet-like structures after implantation of pancreatic endoderm (PE) derived from human embryonic stem cells into immune-deficient mice (13). However, a number of important hurdles remain before this advancement can be exploited as a potential therapy for humans. First, as with all scientific advances, it is necessary to reproduce the advance. Second, given the propensity of human embryonic stem cell-derived implants to develop teratomas, a means must be developed to implant the cells that are protected against tumor formation. Third, given the fact that any implant of human embryonic stem cell-derived tissue would be an allograft, some means to provide protection of the implanted cells must be established. Finally, given the narrow therapeutic window for insulin secretion, newly formed cells must secrete insulin in a tightly regulated glucose-sensitive manner, particularly to prevent fatal hypoglycemia.
To date, there have been limited physiological studies of insulin secretion in animals implanted with human embryonic stem cell-derived endoderm, in part because the implants have exclusively been in mice with a limited blood volume (7, 13, 24). The issue of immunoprotection of allograft transplants has recently been examined by use of the TheraCyte encapsulation devices developed originally in the hope of permitting islet xenotransplantation (15, 23, 25). When these devices were loaded with human fetal islet cell-like clusters (fetal age 18–24 wk) and implanted into immunodeficient mice, it was reported that functional β-cells developed within the devices (15). These authors suggested that the same devices might accommodate human embryonic stem cell-derived PE, thereby offering both immune protection of the allogenic endoderm as well as the means to limit the risk for teratoma or other tumor spread from the site of implantation (15).
In the present study, our first objective was to reproduce and extend the studies of Novocell by implanting human embryonic stem cell-derived PE from Novocell into the epididymal fat pad of athymic nude rats. The rat permits more comprehensive physiological studies than mice due to the greater blood volume. This facilitated our second objective, which was to establish the metabolic characteristics of the athymic nude rat (insulin sensitivity and glucose-stimulated insulin secretion), since this model has not previously been characterized for metabolic studies. Having established this, we then sought to determine whether β-cells derived from the implanted endoderm in the nude rat are responsive to glucose stimulation and how this compares with physiological insulin secretion in the same model. Our final objective was to test the hypothesis that human embryonic stem cell-derived PE can develop when encapsulated in the TheraCyte immunoisolation encapsulation device and, if so, provide an approach to overcome the barriers of immunoprotection of the graft and protection against teratoma or other tumor formation from the human embryonic-derived endoderm.
RESEARCH DESIGN AND METHODS
Study design.
A total of 15 congenitally athymic “nude” male rats (Crl: NIH-Foxn1) and six wild-type male (Sprague-Dawley) rats at 5 mo of age were used in the current study. Wild-type rats were bred and housed individually at the University of California Los Angeles (UCLA) animal housing facility and subjected to standard 12:12-h light-dark cycle. Metabolic data for wild-type rats has previously been published elsewhere (18). Athymic nude rats were purchased from Charles River Laboratories and upon arrival to UCLA kept in laminar flow units inside the certified “pathogen-free” UCLA vivarium facility and subjected to a standard 12:12-h light-dark cycle. The UCLA Institutional Animal Care and Embryonic Stem Cell Research Oversight Committees approved all experimental procedures. First, to validate the nude rat model for the study of glucose metabolism, we examined fasting metabolic characteristics, glucose-stimulated insulin secretion, and insulin sensitivity in athymic nude rats compared with age-matched wild-type controls. Second, we implanted athymic nude rats with PE derived from human embryonic stem cells (Novocell) either by implantation into the epididymal fat pads (n = 5) or by subcutaneous implantation into TheraCyte encapsulation devices (n = 5). Following PE implantation, animals were subjected to sequential blood draws every 2 wk to monitor blood glucose and anticipated appearance of human insulin and C-peptide secretion.
In vivo measurements of insulin secretion and insulin sensitivity.
To validate the athymic nude rat model for the study of glucose metabolism, we performed hyperglycemic clamps to examine glucose-stimulated insulin secretion and hyperinsulinemic euglycemic clamps to determine insulin sensitivity. Animals were first anesthetized with isoflourane (2.5%) by inhalation, and indwelling catheters were then inserted into the right internal jugular vein and left carotid artery for subsequent in vivo metabolic studies, as described previously (19). After surgery, all rats were allowed 5 days to recover, and they all maintained preoperative body weight and had normal food intake and mean hematocrit (>40%). To assess glucose and arginine-stimulated insulin secretion, wild-type and athymic nude rats underwent a hyperglycemic clamp followed by an arginine bolus injection, as described previously (17). In brief, following a 30-min equilibration period (−30 to 0 min), plasma samples were taken for measurement of baseline fasting glucose and insulin. Thereafter, animals received an intravenous glucose bolus (375 mg/kg) followed by a variable 50% (wt/vol) glucose infusion to clamp arterial glucose concentrations at ∼250 mg/dl (0–70 min). At t = 60 min, rats received a bolus injection of l-arginine solution (1 mmol/kg; Sigma, St. Louis, MO). Arterial blood samples (50 μl) were taken at baseline (−30 and 0 min), 1 min, and 5 min and every 15 min thereafter during the clamp for immediate determination of plasma glucose and subsequent analysis for insulin. Two days later, the same group of animals underwent a hyperinsulinemic euglycemic clamp to measure insulin sensitivity. Following an equilibration period, a constant infusion of regular human insulin (Novolin; Novo Nordisk, Princeton, NJ) at 4 mU·kg−1·min−1 was initiated and continued until the end of the clamp (0–120 min). Plasma glucose levels were determined every 10 min, and glucose (50% wt/vol) was infused (5–120 min) to clamp plasma glucose levels at ∼100 mg/dl. Rates of exogenous glucose infusion were recorded to assess whole body insulin sensitivity.
PE implantation.
All implantation procedures were conducted by representatives from ViaCyte. PE implantation methods were based on a protocol published by Kroon et al (13). In short, for epididymal fat implantation, rats were anesthetized with isoflourane (2.5%) by inhalation until effect, and the right epididymal fat pad was carefully externalized. Subsequently, three Gelfoam discs each containing 15 μl of cell aggregate slurry (0.5 × 107 cells each) were wrapped in the epididymal fat pad tissue and secured by veterinary adhesive. For TheraCyte device implantation, each sterile TheraCyte encapsulation device was first loaded with 45 μl of cell aggregate slurry (1.5 × 107 cells each) and subsequently implanted in left dorsal subcutaneous space using blunt forcep dissection. All animals recovered fully from the procedure and maintained consistent body weight and food intake following either fad pad or TheraCyte implantation.
Glucose tolerance test.
To assess graft β-cell function, we performed a glucose tolerance test 18 wk after original graft implantation. All rats were fasted overnight for 12 h prior to glucose administration. Subsequently, glucose solution (50% dextrose) was administered by intraperitoneal injection at 2 mg/kg body wt. Blood samples (100 μl) were taken by venopuncture at baseline and 30 min postinjection for immediate determination of blood glucose and subsequent analysis for human insulin and human C-peptide plasma concentrations.
Immunohistochemistry and immunofluorescence.
At 20 wk past implantation, rats were euthanized by injection of pentobarbital sodium (120 mg/kg), and both epididymal and TheraCyte grafts were removed from euthanized rats and fixed in 4% paraformaldehyde overnight at 4°C. Paraffin-embedded pancreatic sections were obtained, deparaffinized in xylene, rehydrated in ethanol gradient, and stained in Harris Hematoxylin Solution (HHS16; Sigma) and Eosin Y Solution (HT110132; Sigma). For immunofluorescence, antigen retrieval was performed via microwave heating in citrate buffer (H-3300; Vector Laboratories, Burlingame, CA). Slides were blocked in TBS (3% BSA, 0.2% Triton X-100, 2% donkey serum) for 1 h. The following primary antibodies were used for 12-h incubation: insulin (guinea pig, 1:100; Zymed, Carlsbad, CA), somatostatin (rabbit, 1:50; DAKO, Carpinteria, CA), glucagon (rabbit, 1:500; ImmunoStar, Hudson, WI), pancreatic polypeptide (rabbit, 1:50; DAKO), cytokeratin (mouse, 1:50; Sigma), amylase (goat, 1:50; Santa Cruz Biotechnology, Santa Cruz, CA), and pancreatic duodenal homeobox-1(PDX-1; rabbit, 1:1,000; Millipore, St. Louis, MO). Secondary antibodies labeled with Cy3 and FITC were obtained from Jackson Laboratories (West Grove, PA) and used at dilutions of 1:100 for 1-h incubation. Images were obtained and imaged using Leica DM600 microscope (Leica Microsystems, Wetzlar, Germany) and acquired using OpenLab software (Improvision). Tiled images were made into montages using Image J software (National Institutes of Health).
Analytical procedures.
Plasma glucose concentrations were measured by the glucose oxidase method (YSI, Yellow Springs, OH). Rat plasma insulin was measured using competitive colomiteric enzyme-linked immunosorbent assay (Alpco Diagnostics, Salem, NH). Human insulin and human C-peptide were measured by competitive colomiteric enzyme-linked immunosorbent assays (Mercodia, Uppsala, Sweden). The limit of detection for human insulin was 6 pmol/l and for human C-peptide 15 pmol/l.
Statistical analysis.
Statistical analysis was performed using ANOVA with Fisher's post hoc analysis (Statistica, version 6; Statsoft, Tulsa, OK). Data in graphs and tables are presented as means ± SE. Findings were assumed to be statistically significant at P < 0.05.
RESULTS
Metabolic characteristics, insulin sensitivity, and insulin secretion of the athymic nude rat.
At 5 mo of age, fasting glucose concentrations were comparable in athymic nude rats and age-matched wild-type controls (98 ± 2 vs. 106 ± 4 mg/dl; Supplemental Fig. S1; Supplemental Material for this article can be found online at the AJP-Endocrinology and Metabolism web site). However, despite comparable glucose levels, systemic fasting plasma insulin concentrations were lower in nude rats (69 ± 16 vs. 299 ± 57 pmol/l, P < 0.05; Supplemental Fig. S1), implying increased insulin sensitivity. Consistent with this, nude rats exhibited reduced body weight (364 ± 25 vs. 571 ± 27 g, P < 0.05; Supplemental Fig. S1).
To evaluate glucose-mediated insulin secretion, we next performed hyperglycemic clamp studies in which by design plasma glucose levels were maintained at ∼250 mg/dl (Supplemental Fig. S2A). In response to hyperglycemia, both nude and wild-type rats demonstrated a robust increase above basal in glucose- and arginine-stimulated insulin secretion (Supplemental Fig. S2B). However, absolute insulin secretion (in response to glucose and arginine) was (50–80%) lower in nude rats compared with wild type (Supplemental Fig. S2B). To elucidate insulin sensitivity, we next performed hyperinsulinemic euglycemic clamp studies. By design, plasma glucose and insulin concentrations were comparable during the hyperinsulinemic euglycemic clamps in nude and wild-type rats (Supplemental Fig. S2C). Whole body insulin sensitivity, assessed by the mean glucose infusion rates during the hyperinsulinemic clamp, was 50% higher in nude vs. wild-type rats (Supplemental Fig. S2D). Consequently, the disposition index (calculated as a product of insulin secretion and insulin sensitivity) was comparable in athymic nude rats and age-matched wild-type controls (Supplemental Fig. S2E). Therefore, these studies established that nude rats demonstrate robust glucose-stimulated insulin secretion and higher insulin sensitivity compared with age-matched wild-type rats.
Human insulin, C-peptide, and blood glucose concentrations with time in implanted nude rats.
Having established the basic glucose metabolic parameters of the nude rat, we next implanted nude rats with PE derived from human embryonic stem cells either by implantation into the epididymal fat pads (n = 5) or by subcutaneous implantation into TheraCyte encapsulation devices (n = 5). We subsequently monitored changes in body weight and fasting glycemia. Neither body weight nor fasting glucose levels changed significantly vs. baseline during the 18-wk period postimplantation (Table 1). In contrast to previous studies in mice (13) in which robust fasting human C-peptide levels were observed by 10 wk postimplantation (values ranged from 333 to 835 pmol/l), human C-peptide and insulin were undetectable in any animal by biweekly testing until 18 wk after implantation. At 18 wk postimplantation, fasting human C-peptide and insulin were detectable, but at very low levels (C-peptide range: 7–152 pmol/l; insulin range: 1–38 pmol/l) in rats in which endoderm was implanted into the fat pad (Fig. 1, A–C). However, there was no increase in human C-peptide and insulin levels after glucose challenge in those animals (Fig. 1, A–C). At 18 wk postimplantation, fasting C-peptide and insulin were not detected in any of the five rats in which endoderm was implanted into the TheraCyte encapsulation device (Fig. 1, D–F) and detected in only 1 rat following the glucose challenge, but only at the level slightly above the limit of assay detection (e.g., C-peptide = 25 pmol/l with a detection limit of 15 pmol/l). Although our initial objective was to perform additional comprehensive metabolic studies to examine glucose- and arginine-stimulated insulin secretion as well as insulin sensitivity in these rats, the absence of meaningful C-peptide/insulin secretion from the endodermal implants 18 wk postimplantation rendered these additional studies futile.
Table 1.
Changes in body weight and fasting glucose for 18 wk following either fat pad or TheraCyte device graft implantation in “nude” rats
| Weeks Postimplantation |
||||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 12 | 18 | ||
| Fasting glucose, mmol/l | ||||||||
| Fat pad | 4.2 ± 0.3 | 4.2 ± 0.2 | 4.1 ± 0.1 | 4.1 ± 0.1 | 4.3 ± 0.2 | 3.9 ± 0.2 | 3.7 ± 0.2 | |
| Device | 4.9 ± 0.1 | 3.9 ± 0.1 | 4.2 ± 0.1 | 4.1 ± 0.1 | 4.4 ± 0.2 | 4.2 ± 0.2 | 3.6 ± 0.2 | |
| Body weight, g | ||||||||
| Fat pad | 417 ± 13 | 387 ± 12 | 395 ± 12 | 398 ± 12 | 401 ± 11 | 404 ± 11 | 402 ± 11 | |
| Device | 403 ± 9 | 379 ± 12 | 388 ± 12 | 392 ± 11 | 389 ± 12 | 412 ± 7 | 402 ± 14 | |
Data are expressed as means ± SE.
Fig. 1.
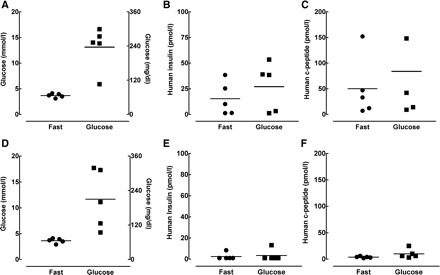
Glucose tolerance test in “nude” rats at 18 wk following either epididymal fat pad or TheraCyte device pancreatic endoderm implantation. A–C: individual data points for glucose, human insulin, and human C-peptide at fasting (fast) and 30 min after intraperitoneal (ip) glucose injection in nude rats implanted with pancreatic endoderm into the epididymal fat pad. D–F: individual data points for glucose, human insulin, and human C-peptide at fasting and 30 min after ip glucose injection in nude rats with subcutaneously implanted pancreatic endoderm in TheraCyte devices.
Pancreatic endocrine and exocrine cell development in PE-implanted nude rats.
After the completion of metabolic studies, we removed the epididymal fat pad transplants or the TheraCyte devices from the nude rats to analyze the fate of transplanted cells. Fibrous masses were removed from the fat pads of rats that were transplanted with Gelfoam discs and cell slurry and were fixed, dehydrated, and processed for immunohistochemical analysis. Hematoxylin and eosin staining of sections from fibrotic fat pad implants revealed extensive duct-like epithelial structures within a stromal matrix (Fig. 2). Implants from animals that had detectable human insulin were larger than implants than those in which human insulin was not detectable (Fig. 2, A and B). In the former implants the ductal structures were smaller, the stromal matrix was denser, and small epithelial cell clusters were scattered throughout the implant. To determine whether the implants contained any pancreatic cells, sections of the implants were evaluated by immunofluorescence for all of the mature pancreatic endocrine and exocrine cell types. In total, 14 of 15 (3 grafts/animal were implanted) epididymal grafts were retrieved successfully at the completion of the study. The fraction of the implant positive for insulin in these three grafts varied from 0 to 3.6%, with a mean of 0.8 ± 0.3% (Fig. 3). In total, seven of 14 grafts were completely devoid of any detectable islet cells. Further evaluation of the implants that contained pancreatic endocrine cells revealed the presence of all four major endocrine hormone-expressing cells. Most endocrine cells were together in clusters (Fig. 4A), whereas some appeared to “stream” or migrate from pancreatic ductal-like structures, reminiscent of islet formation during embryogenesis (Fig. 4B). Within the endocrine clusters, most cells were positive for a single hormone, but double-hormone-positive cells were also present (Fig. 4C, white arrow), reminiscent of islets during embryogenesis. The implants rarely contained exocrine cells that were amylase positive, and any amylase cells detected were scattered single cells (Fig. 5A). Implants that contained pancreatic endocrine cells also contained PDX-1-positive duct cells scattered throughout the pancreas, suggestive of ongoing β-cell neogenesis (Fig. 5, B and C). Fractional β-cell area and β-cell mass of the endogenous pancreas were not significantly different among control nude rats or nude rats implanted with PE into either epididymal fat pads or the TheraCyte device (Supplemental Fig. S4).
Fig. 2.
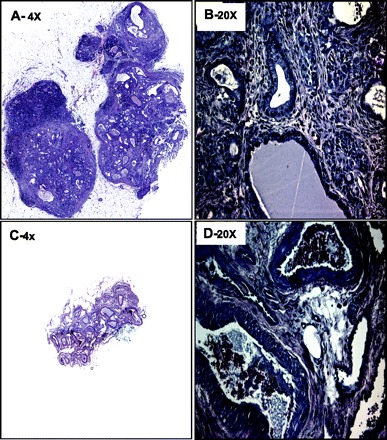
Examples of epididymal pancreatic endodermal grafts stained for hematoxylin and eosin extracted at 20 wk posttransplantation from nude rats with detectable vs. undetectable human insulin/C-peptide levels. A and B: examples of epididymal pancreatic endodermal grafts stained for hematoxylin and eosin at ×4 (A) and ×20 (B) from a nude rat that demonstrated detectable levels of human insulin and C-peptide following glucose challenge. C and D: examples of epididymal pancreatic endodermal grafts stained for hematoxylin and eosin at ×4 (C) and ×20 (D) from a nude rat that demonstrated undetectable levels of human insulin and C-peptide following glucose challenge.
Fig. 3.
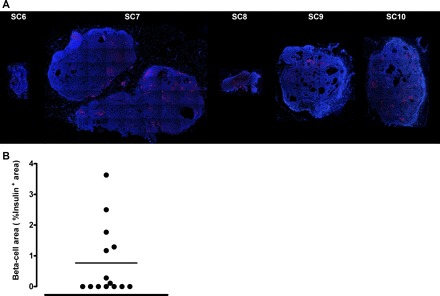
A: examples of epididymal pancreatic endodermal grafts extracted at 20 wk posttransplantation from nude rats. Representative examples of 6 epididymal pancreatic endodermal grafts stained by immunofluorescence for insulin (red) and nuclei (blue) at ×4 magnification taken from 5 different nude rats. B: quantification of insulin positive area from 14 different epididymal pancreatic endodermal grafts extracted from 5 nude rats after euthanization. Note that, in total, 3 grafts/animal (total of 15) were implanted at the beginning of the study, with 14 successfully retrieved following the completion of the study.
Fig. 4.
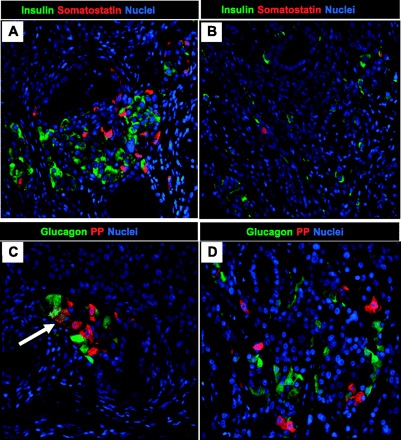
Examples of pancreatic endocrine cell expression in epididymal pancreatic endodermal grafts extracted at 20 wk posttransplantation from a nude rat with detectable human insulin/C-peptide levels. Representative images of epididymal pancreatic endodermal grafts stained for insulin/somatostatin (A and B) and glucagon/pancreatic polypeptide (PP; C and D) presented at ×20 magnification. Arrow denotes presence of glucagon and PP double-positive cells.
Fig. 5.
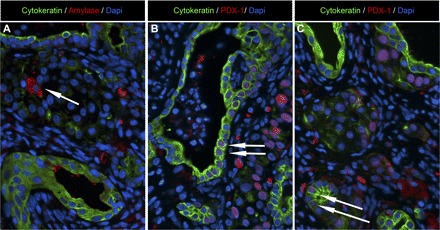
Examples of pancreatic exocrine cell expression in epididymal endodermal grafts extracted at 20 wk posttransplantation from a nude rat with detectable human insulin/C-peptide levels. A: representative image of epididymal pancreatic endodermal grafts stained for ductal cell marker (cytokeratin; green), exocrine cell marker (amylase; red), and nuclear marker DAPI (blue) presented at ×20 magnification. Arrow denotes presence of a single amylase positive cell. B and C: representative images of epididymal pancreatic endodermal grafts stained for ductal cell marker (cytokeratin; green), endocrine cell marker [pancreatic duodenal homeobox-1 (PDX-1); red], and nuclear marker DAPI (blue) presented at ×20 magnification. Arrows denote the presence of PDX-1/cytokeratin-positive cells throught the graft, including exocrine ductal tissue.
TheraCyte devices removed from the subcutaneous space were encased in fibrotic tissue (Supplemental Fig. S3A). Small samples of each device were split open to expose the inner cell compartment (Supplemental Fig. S3B). There was no evidence of islet-like clusters or fibrotic growths in the inner cell compartment by hematoxylin and eosin staining (Supplemental Fig. S3C). However, since in one rat we did observe detectable human C-peptide levels (albeit marginally above the detection limit of the assay) following glucose challenge, it is possible that the small amount of implanted tissue in this rat was lost during tissue processing.
DISCUSSION
In the present study, our first objective was to reproduce the findings of a prior report with the human embryonic stem cell-derived endoderm implanted into the nude mice reported to develop glucose-sensitive insulin secretion (13). Unfortunately, the present studies only partially reproduce those findings, showing only limited endocrine cell development over a much longer time period than required in the prior report and only in a subset of rats. Moreover, we were unable to identify any endocrine cells histologically or any meaningful human C-peptide/human insulin secretion in rats in which the human embryonic stem cell-derived endoderm was implanted after encapsulation in the TheraCyte devices.
Although the findings in the present study are disappointing, they do serve as a reminder of the barriers and challenges that remain before human embryonic stem cell-derived therapy can be seriously contemplated for therapy in type 1 diabetes. The failure of pancreatic endoderm to develop into significant functional endocrine tissue when implanted into the same site (fat pad) in the nude rat as implanted previously in mice can be due to several possible causes. It is unlikely to be due to insufficient cells implanted or technical issues of implantation since representatives from Novocell provided and participated in the implantation of the endoderm in the present experiments and the cell numbers were adequately scaled up to be at least equivalent to that in the prior mouse studies. It is conceivable that following implantation of the pancreatic endoderm the nude rat model is a less accommodating host than the athymic nude mouse model. It is reported here for the first time that the athymic nude rat is relatively insulin sensitive compared with the Sprague-Dawley rat, and it is possible that this metabolic environment is not ideal to foster islet formation. Additionally, in nude rats (as in nude mice) occasional immune cells with T cell characteristics begin to emerge as the animal ages (14).
However, similar low efficiency of differentiation to insulin-expressing cells from human embryonic stem cells using an analogous protocol have been reported previously (8). It is important to provide a clarification about the methods used and results obtained in this study. After our current studies were only partially successful in generating β-cells, our former collaborators at NovoCell informed us that the specific protocol they used to develop the endoderm supplied for the current studies differed from that used in the cited paper of Kroon et al. (13). They also stated that the endoderm arising from this altered protocol did not develop in their own mice, as reported previously. However, because we were not provided with details of the changes in the Kroon et al. (13) protocol used to generate the endoderm supplied by NovoCell for the studies reported here, nor were the data obtained by NovoCell with this endoderm, we are unable to provide them.
The histology of the implants that contained elements of pancreas conveyed several interesting parallels with pancreas during embryonic development and the early postnatal period. When islet-like structures were present, they were often related to exocrine ducts, with the appearance of budding of islets from ducts previously described in pancreatic development (4). Similarly, we observed frequent expression of PDX-1-positive cells within pancreatic exocrine ducts. Endocrine cells when present were occasionally double-hormone positive as present in human islets before 10 wk of gestation (3, 21). The majority of the implants even in those that contained abundant endocrine cells were positive for cytokeratin and assumed small pancreatic duct-like structures with very high rates of replication, whereas pancreatic acinar cells were rarely present (4).
In summary, epididymal fat-implanted pancreatic endoderm derived from human embryonic stem cells progressed to develop predominantly exocrine duct-like tissue (∼99% of implant) and occasional islet-like structures (∼0.8% of implants) in seven of 14 retrieved mesenteric fat pad implants. Human insulin and C-peptide were detectable in some rats but only at low, clinically insufficient levels, and insulin secretion in implanted rats was not glucose responsive. There was no development of viable tissue when human pancreatic endoderm was implanted in the TheraCyte encapsulation devices. These studies affirm that islet-like structures do develop from human embryonic cells differentiated to pancreatic endoderm ex vivo by the protocol developed and published by NovoCell. However, the extent of islet formation and its function is not yet sufficiently reproducible to be clinically useful. Moreover, effective strategies are not yet available to protect human embryonic stem cell-derived endoderm implants from immune rejection or the risk of dissemination of any tumor arising from the implant.
GRANTS
These studies were supported by a gift from Peter and Valerie Kampaniez and the Larry Hillblom Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to NovoCell for providing the endoderm and assisting with its implantation. We are also grateful to Ryan Galasso for excellent technical support and acknowledge the support and excellent suggestions of our colleagues at the Larry Hillblom Islet Research Center, Drs. Alexandra Butler and Tatyana Gurlo.
REFERENCES
- 1. Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet 358: 221–229, 2001. [DOI] [PubMed] [Google Scholar]
- 2. Baetge EE. Production of beta-cells from human embryonic stem cells. Diabetes Obes Metab 10, Suppl 4: 186–194, 2008. [DOI] [PubMed] [Google Scholar]
- 3. Bocian-Sobkowska J, Zabel M, Wozniak W, Surdyk-Zasada J. Polyhormonal aspect of the endocrine cells of the human fetal pancreas. Histochem Cell Biol 112: 147–153, 1999. [DOI] [PubMed] [Google Scholar]
- 4. Bouwens L, Lu WG, De Krijger R. Proliferation and differentiation in the human fetal endocrine pancreas. Diabetologia 40: 398–404, 1997. [DOI] [PubMed] [Google Scholar]
- 5. Brauker J, Martinson LA, Young SK, Johnson RC. Local inflammatory response around diffusion chambers containing xenografts. Nonspecific destruction of tissues and decreased local vascularization. Transplantation 61: 1671–1677, 1996. [DOI] [PubMed] [Google Scholar]
- 6. Brauker JH, Carr-Brendel VE, Martinson LA, Crudele J, Johnston WD, Johnson RC. Neovascularization of synthetic membranes directed by membrane microarchitecture. J Biomed Mater Res 29: 1517–1524, 1995. [DOI] [PubMed] [Google Scholar]
- 7. Brolen GK, Heins N, Edsbagge J, Semb H. Signals from the embryonic mouse pancreas induce differentiation of human embryonic stem cells into insulin-producing beta-cell-like cells. Diabetes 54: 2867–2874, 2005. [DOI] [PubMed] [Google Scholar]
- 8. Courtney ML, Jones PM, Burns CJ. Importance of quantitative analysis in the generation of insulin-expressing cells from human embryonic stem cells. Pancreas 39: 105–107, 2010. [DOI] [PubMed] [Google Scholar]
- 9. Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care 26: 1902–1912, 2003. [DOI] [PubMed] [Google Scholar]
- 10. D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol 23: 1534–1541, 2005. [DOI] [PubMed] [Google Scholar]
- 11. D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 24: 1392–1401, 2006. [DOI] [PubMed] [Google Scholar]
- 12. Elliott RB, Escobar L, Calafiore R, Basta G, Garkavenko O, Vasconcellos A, Bambra C. Transplantation of micro- and macroencapsulated piglet islets into mice and monkeys. Transplant Proc 37: 466–469, 2005. [DOI] [PubMed] [Google Scholar]
- 13. Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D'Amour KA, Carpenter MK, Baetge EE. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 26: 443–452, 2008. [DOI] [PubMed] [Google Scholar]
- 14. Kung JT, Thomas CA., 3rd Athymic nude CD4+8- T cells produce IL-2 but fail to proliferate in response to mitogenic stimuli. J Immunol 141: 3691–3696, 1988. [PubMed] [Google Scholar]
- 15. Lee SH, Hao E, Savinov AY, Geron I, Strongin AY, Itkin-Ansari P. Human beta-cell precursors mature into functional insulin-producing cells in an immunoisolation device: implications for diabetes cell therapies. Transplantation 87: 983–991, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Loudovaris T, Jacobs S, Young S, Maryanov D, Brauker J, Johnson RC. Correction of diabetic nod mice with insulinomas implanted within Baxter immunoisolation devices. J Mol Med 77: 219–222, 1999. [DOI] [PubMed] [Google Scholar]
- 17. Matveyenko AV, Butler PC. Beta-cell deficit due to increased apoptosis in the human islet amyloid polypeptide transgenic (HIP) rat recapitulates the metabolic defects present in type 2 diabetes. Diabetes 55: 2106–2114, 2006. [DOI] [PubMed] [Google Scholar]
- 18. Matveyenko AV, Gurlo T, Daval M, Butler AE, Butler PC. Successful versus failed adaptation to high-fat diet-induced insulin resistance: the role of IAPP-induced beta-cell endoplasmic reticulum stress. Diabetes 58: 906–916, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matveyenko AV, Veldhuis JD, Butler PC. Adaptations in pulsatile insulin secretion, hepatic insulin clearance, and β-cell mass to age-related insulin resistance in rats. Am J Physiol Endocrinol Metab 295: E832–E841, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meier JJ, Bhushan A, Butler PC. The potential for stem cell therapy in diabetes. Pediatr Res 59: 65R–73R, 2006. [DOI] [PubMed] [Google Scholar]
- 21. Meier JJ, Köhler CU, Alkhatib B, Sergi C, Junker T, Klein HH, Schmidt WE, Fritsch H. Beta-cell development and turnover during prenatal life in humans. Eur J Endocrinol 162: 559–568, 2010. [DOI] [PubMed] [Google Scholar]
- 22. Rafael E, Wernerson A, Arner P, Wu GS, Tibell A. In vivo evaluation of glucose permeability of an immunoisolation device intended for islet transplantation: a novel application of the microdialysis technique. Cell Transplant 8: 317–326, 1999. [DOI] [PubMed] [Google Scholar]
- 23. Rafael E, Wu GS, Hultenby K, Tibell A, Wernerson A. Improved survival of macroencapsulated islets of Langerhans by preimplantation of the immunoisolating device: a morphometric study. Cell Transplant 12: 407–412, 2003. [DOI] [PubMed] [Google Scholar]
- 24. Segev H, Fishman B, Ziskind A, Shulman M, Itskovitz-Eldor J. Differentiation of human embryonic stem cells into insulin-producing clusters. Stem Cells 22: 265–274, 2004. [DOI] [PubMed] [Google Scholar]
- 25. Suzuki K, Bonner-Weir S, Hollister-Lock J, Colton CK, Weir GC. Number and volume of islets transplanted in immunobarrier devices. Cell Transplant 7: 47–52, 1998. [DOI] [PubMed] [Google Scholar]
- 26. Yang Z, Chen M, Fialkow LB, Ellett JD, Wu R, Nadler JL. Survival of pancreatic islet xenografts in NOD mice with the theracyte device. Transplant Proc 34: 3349–3350, 2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


