Abstract
Objective
To conduct a therapeutic exploratory clinical trial comparing clinical outcomes of treatment with topical natamycin vs topical voriconazole for fungal keratitis.
Methods
The multicenter, double-masked, clinical trial included 120 patients with fungal keratitis at Aravind Eye Hospital in India who were randomized to receive either topical natamycin or topical voriconazole and either had repeated scraping of the epithelium or not.
Main Outcome Measures
The primary outcome was best spectacle-corrected visual acuity (BSCVA) at 3 months. Other outcomes included scar size, perforations, and a sub-analysis of BSCVA at 3 months in patients with an enrollment visual acuity of 20/40 to 20/400.
Results
Compared with those who received natamycin, voriconazole-treated patients had an approximately 1-line improvement in BSCVA at 3 months after adjusting for scraping in a multivariate regression model but the difference was not statistically significant (P=.29). Scar size at 3 months was slightly greater with voriconazole after adjusting for scraping (P=.48). Corneal perforations in the voriconazole group (10 of 60 patients) were not significantly different than in the natamycin-treated group (9 of 60 patients) (P>.99). Scraping was associated with worse BSCVA at 3 months after adjusting for drug (P=.06). Patients with baseline BSCVA of 20/40 to 20/400 showed a trend toward a 2-line improvement in visual acuity with voriconazole (P=.07).
Conclusions
Overall, there were no significant differences in visual acuity, scar size, and perforations between voriconazole- and natamycin-treated patients. There was a trend toward scraping being associated with worse outcomes.
Application to Clinical Practice
The benefit seen with voriconazole in the subgroup of patients with baseline visual acuity of 20/40 to 20/400 needs to be validated in a confirmatory clinical trial.
Trial Registration
clinicaltrials.gov Identifier: NCT00557362
Infectious keratitis is a leading cause of monocular blindness worldwide.1 In some settings, as many as 50% of all corneal ulcers are due to fungal infection.2 Various centers have reported that an increasing proportion of infectious keratitis is caused by fungus.3–6 Treatment of fungal keratitis is generally more difficult than that of bacterial ulcers, and resulting visual impairment is, on average, more severe.7
Historically, fungal keratitis has been endemic in warmer climates such as India and has been relatively uncommon in temperate regions of the United States. For example, in settings such as South India as many as 50% of infectious ulcers are fungal,2 while they make up approximately 8% of infectious ulcers seen at the Proctor Foundation at the University of California, San Francisco, prior to 2005.8 In 2006, there was an epidemic of contact lens–related keratitis due to Fusarium species in the United States and Asia, and this outbreak heightened concern about how to best care for these patients.3,9,10 Although the peak of the epidemic has subsided, questions regarding the best way to care for patients with fungal keratitis remain.
There has been only a single published randomized trial of antifungal therapy for mycotic keratitis,11 and no new ocular antifungal medications have been approved by the Food and Drug Administration since natamycin was approved in the 1960s. The triazole voriconazole is active against both filamentous fungi and Candida species and has recently become the treatment of choice for systemic diseases such as pulmonary aspergillosis.12 Aspergillus species and other filamentous fungi are common pathogens in fungal keratitis, and use of topical ophthalmic preparations of voriconazole has been described in numerous case reports in the ophthalmic literature.13–25 However, there has been no systematic attempt to determine whether it is more or less effective clinically than the commercially available natamycin. Although there are suggestions in vitro and in vivo that particular fungi respond better to one agent or another, there is little data available for physicians to make an informed, evidence-based decision on choice of antifungal agent. The superior in vitro susceptibility profile and increased penetration of voriconazole compared with natamycin could be an advantage, particularly for corneal ulcers deep in the stroma.24,26–29 In vitro results and case reports may be hypothesis-generating but they are insufficient to answer the question of which drug should be used in patients with fungal keratitis.
We conducted an exploratory therapeutic randomized clinical trial to (1) compare the efficacy of topical natamycin with topical voriconazole, with and without repeat scraping of the epithelium, and (2) to assess for a significant difference in adverse events, in particular, corneal perforations.
METHODS
DESIGN
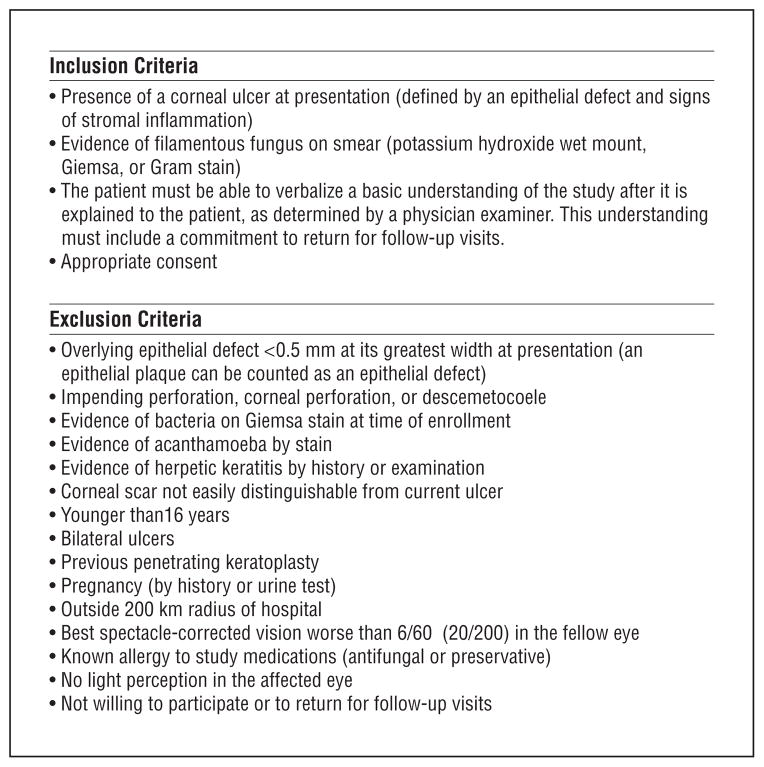
This study was a randomized, double-masked, clinical trial of patients with fungal corneal ulcers. Institutional review board approval was obtained at the University of California, San Francisco, Dartmouth Medical School, and Aravind Eye Hospital. All patients who presented with a corneal ulcer had corneal scraping, which is the standard of care at Aravind Eye Hospital. If fungal elements were present on the corneal scraping, patients were eligible for enrollment. All patients provided written, informed consent for their study participation. Complete inclusion and exclusion criteria are listed in Figure 1. A target enrollment of 120 patients was chosen because this sample size was deemed sufficient to detect a 3-line difference in 3-month visual acuity between the 2 drugs. Specifically, we estimated that 60 patients per arm would provide at least 80% power to detect a 0.3 logMAR effect size (approximately 3 Snellen lines) between the 2 study arms, assuming a residual SD of 0.53 in the 3-month BSCVA (after correcting for enrollment BSCVA, assuming a correlation coefficient of 0.65 between enrollment and 3-month BSCVA), a dropout rate of 15%, and a 2-tailed α of .05.
Figure 1.
Inclusion and exclusion criteria. All inclusion criteria must be met to participate in the study.
INTERVENTION
All patients with a corneal ulcer presenting to Aravind Eye Hospital’s cornea clinics in Madurai and in Pondicherry, India, had corneal scrapings using a Kimura spatula for Gram stain and potassium hydroxide wet mount and had cultures plated on blood, chocolate, and potato dextrose agar. Aravind Eye Hospital is both a primary and tertiary care eye hospital in South India with a well-established cornea subspecialty clinic. If all inclusion criteria and no exclusion criteria were met, the patient was enrolled in the study. Patients were block-randomized in groups of 4 (using the statistical package R; http://www.r-project.org) by T.P. to receive topical natamycin, 5% (Alcon, Fort Worth, Texas), or topical voriconazole, 1% (Pfizer, reconstituted by AuroLab, Madurai, India), with or without repeated scraping of the corneal epithelium at 1 and 2 weeks after enrollment. The antifungal medications were applied topically to the cornea every hour while awake for 1 week, and then every 2 hours while awake until 3 weeks after enrollment. Further continuation was at the discretion of the physician. According to the standard of care at Aravind Eye Hospital, patients were hospitalized from presentation for at least 1 week, with medications given by the ward nurse. Patients randomized to the repeated scraping arm had their epithelium scraped by an ophthalmologist so that the defect was at least as large as the underlying infiltrate at 1 and 2 weeks following enrollment. Following discharge, patients were scheduled for follow-up 3 months after enrollment unless it was deemed medically necessary to see them earlier.
Natamycin is delivered via suspension, while voriconazole is in solution. Double-masking of treatment assignment was achieved by dispensing the medications in identical opaque bottles and by having the ward nurses wipe any white residue from the patient’s eye prior to study assessment. In addition, patients were no longer receiving treatment at 3 months, the time that the primary outcome of final visual acuity was measured. Only the biostatisticians responsible for the randomization coding and the study pharmacist were unmasked.
ASSESSMENTS
Assessments of best spectacle-corrected visual acuity (BSCVA) and clinical characteristics (infiltrate/scar size, epithelial defect size) were performed at enrollment, 3 weeks, and 3 months. Visual acuity measurements were performed according to a protocol adapted from the Age Related Eye Disease Study (AREDS 1999) using a tumbling E chart at 4 m and logMAR visual acuity. Acuities worse than 1.6 logMAR (approximate Snellen equivalent, 20/800) were recorded as counting fingers, 1.7; hand motion, 1.8; light perception, 1.9; and no light perception, 2.0, as in the Herpetic Eye Disease Study.30 A Haag-Streit 900 slitlamp biomicroscope (Haag-Streit AG, Koeniz, Switzerland) was used to assess the size of the infiltrate/scar and epithelial defect at study visits, and ocular adverse events such as corneal perforation. Infiltrate/scar size and epithelial defect size were measured according to a protocol adapted from the Herpetic Eye Disease Study. In brief, the longest dimension was measured, followed by the longest perpendicular to the first measurement. As in the Herpetic Eye Disease Study, no differentiation was made between infiltrate and scar when measuring infiltrate/scar size. Re-epithelialization was defined as an epithelial defect of less than 0.5 mm with administration of fluorescein.
STATISTICAL ANALYSIS
Baseline characteristics were compared between the natamycin and voriconazole groups using the t test for continuous variables and Fisher exact test for categorical variables. The primary efficacy endpoint was BSCVA at 3 months in the study eye, using a linear regression model with 3-month logMAR BSCVA as the outcome variable and treatment arm (voriconazole vs natamycin) and enrollment logMAR BSCVA and scraping (yes or no) as covariates. In addition, we tested for an interaction between drug and scraping. Other prespecified endpoints included BSCVA at 3 weeks, adjusting for enrollment BSCVA, and infiltrate/scar size at 3 weeks and 3 months, adjusting for enrollment infiltrate/scar size. For analysis, infiltrate/scar size was characterized by the geometric mean of the longest dimension and the longest perpendicular. Huber robust regression was conducted to test the robustness of the model. The time to re-epithelialization was compared between the voriconazole and natamycin groups using the Cox proportional hazards model, adjusting for baseline epithelial defect size. Efficacy endpoints were analyzed on an intent-to-treat basis for all randomized patients enrolled in the study. The primary analysis included the actual 3-month data when available and last observation carried forward for missing values. For patients who had a corneal transplant after enrollment, a visual acuity of 1.9 logMAR (light perception) was assigned for all posttransplant visual acuities, and the infiltrate/scar size prior to the transplant was carried forward for postoperative measurements. Sensitivity analyses were also performed in which we separately (1) assigned surgical patients the value 1.7 instead of 1.9, (2) assigned patients with perforation (but no surgery) the value 1.7 or 1.9 (instead of using last observation carried forward), (3) analyzed only patients with complete follow-up, or (4) used multiple imputation (recursive random partitioning-based hot deck method).31 Safety assessments included comparing the incidence of ocular and nonocular adverse events, including corneal perforations, by Fisher exact test. STATA 9.2 (Stata Corporation, College Park, Texas) or R 2.6.1 for MacIntosh (R Foundation for Statistical Computing; http://www.r-project.org) was used to conduct all statistical analyses.
RESULTS
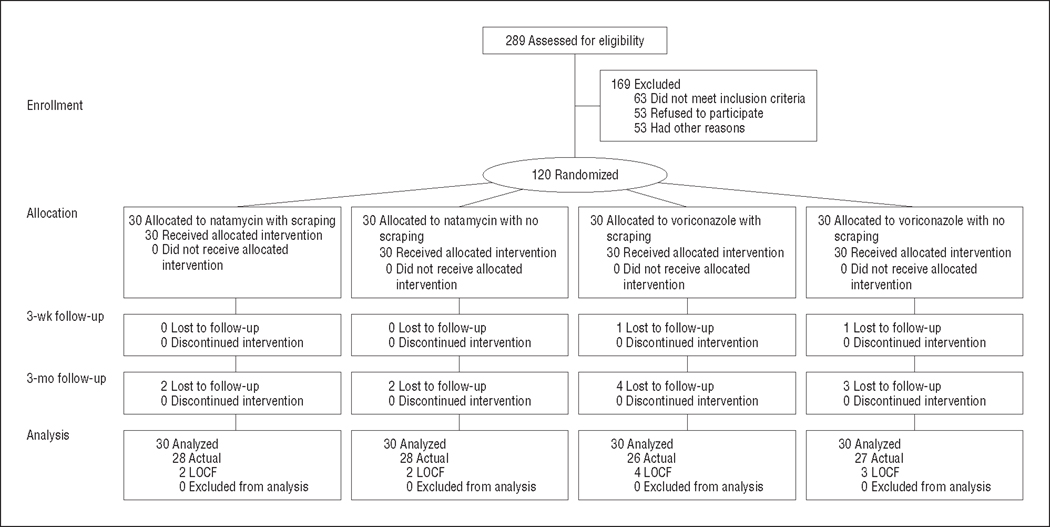
Two hundred eighty-nine patients with a corneal ulcer and fungal elements seen on a smear were assessed for eligibility during the enrollment period from November 27, 2007, to May 12, 2008, and 169 patients were excluded (Figure 1). The most common reasons for exclusion were having an overlying epithelial defect of less than 0.5 mm, impending perforation, visual acuity worse than 20/200 in the fellow eye, living a long distance from the hospital, and unwillingness to participate. One hundred twenty patients with filamentous fungus on corneal scrapings were enrolled; 60 were randomized to natamycin and 60 to voriconazole. For each drug, 30 patients were randomized to repeated epithelial scraping during the course of ulcer treatment and 30 were randomized to no scraping. One hundred seven patients (89%) were followed up at 3 months (Figure 2). Enrollment characteristics for the 120 patients including enrollment BSCVA, infiltrate/scar size, and distribution of organisms were not significantly different between the study arms (Table 1). The enrollment BSCVA and infiltrate/scar size in the patients who did not complete the study were not significantly different between the 2 study arms and did not differ significantly from patients who had complete follow-up.
Figure 2.
CONSORT flowchart. LOCF indicates last observation carried forward.
Table 1.
Baseline Characteristics of Patients (n = 120)
| No. (%)
|
P Value | ||||
|---|---|---|---|---|---|
| Natamycin
|
Voriconazole
|
||||
| Scraping | No Scraping | Scraping | No Scraping | ||
| Age, mean (SD), y | 49.8 (11.9) | 45.9 (13.1) | 47.0 (14.5) | 45.0 (14.5) | .56a |
| Sex | .28b | ||||
| Male | 23 (77) | 19 (63) | 16 (53) | 21 (70) | |
| Female | 7 (23) | 11 (37) | 14 (47) | 9 (30) | |
| Organismsc | .12b | ||||
| Fusarium species | 6 (23.1) | 15 (55.6) | 12 (52.2) | 11 (42.3) | |
| Aspergillus flavus | 2 (7.7) | 3 (11.1) | 2 (8.7) | 4 (14.4) | |
| Aspergillus fumigatus | 4 (15.4) | 1 (3.7) | 0 | 0 | |
| Other Aspergillus species | 1 (3.9) | 0 | 2 (8.7) | 0 | |
| Other filamentous fungi | 13 (50.0) | 8 (29.6) | 7 (30.4) | 11 (42.3) | |
| Enrollment visual acuity, mean (SD), logMAR | 0.87 (0.67) | 0.94 (0.61) | 0.94 (0.66) | 0.96 (0.66) | .96a |
| Enrollment infiltrate/scar diameter, mean (SD), mm | 4.1 (1.8) | 3.9 (1.7) | 3.8 (1.8) | 3.9 (1.8) | .63a |
Analysis of variance.
Fisher exact test.
One culture grew both Aspergillus and Curvularia species (other fungi).
Visual acuity improved in both the natamycin- and voriconazole-treated patients. At baseline, the mean (SD) BSCVA in the natamycin group was 0.91 (0.63) log-MAR (approximate Snellen equivalent, 20/160). The voriconazole group had a mean (SD) baseline BSCVA of 0.95 (0.65) logMAR (approximate Snellen equivalent, 20/200). At 3 weeks, the natamycin group had a mean (SD) BSCVA of 0.73 (0.72) logMAR (approximate Snellen equivalent, 20/100). At 3 months, the BSCVA had improved to a mean (SD) of 0.69 (0.80) logMAR (approximate Snellen equivalent, 20/100). For the voriconazole-treated group, the mean (SD) 3-week BSCVA was 0.73 (0.75) logMAR (approximate Snellen equivalent, 20/100). By 3 months, the voriconazole group exhibited greater improvement, with a mean (SD) BSCVA of 0.63 (0.76) logMAR (Snellen equivalent, 20/80).
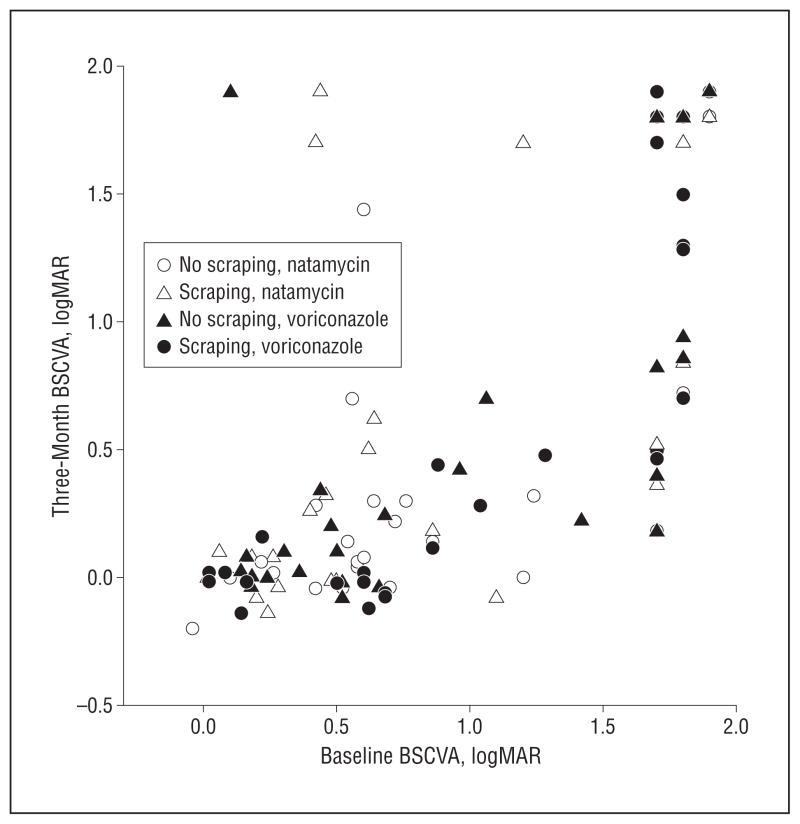
Multiple linear regression model showed that, compared with natamycin treatment, voriconazole treatment was estimated to have 0.043 lower (just under one-half line better) logMAR acuity at 3 weeks (95% CI, −0.18 to 0.09; P =.53), adjusting for enrollment BSCVA and scraping. For the primary analysis (including all 120 patients), multiple linear regression estimated that patients who had voriconazole treatment had 0.098 better logMAR acuity (nearly 1-line benefit) at 3 months (95% CI, −0.28 to 0.083; P=.29). Patients with repeated scraping of the epithelium were estimated to have 0.17 log-MAR worsening (slightly more than a 1.5-line loss) of vision at 3 months (Table 2), although this was not statistically significant. The effect of repeated scraping with voriconazole use was approximately 1 line worse (0.10 logMAR) BSCVA at 3 months, and with natamycin was 2½ lines worse (0.25 logMAR) but the difference was not statistically significant (P=.44). Figure 3 plots the relationship between baseline and 3-month BSCVA for all patients. A subset of our patients (n=78) had hard contact lens overrefraction at 3 months but this was not a prespecified primary outcome. The correlation between contact lens visual acuity and BSCVA at 3 months was 0.99 in this subset of patients.
Table 2.
Multiple Linear Regression Predicting 3-Month logMAR BSCVA (n = 120)
| Covariate | Coefficient (95% CI) | P Value |
|---|---|---|
| Enrollment BSCVA (logMAR) | 0.92 (0.78 to 1.06) | 3.001 |
| Voriconazole (vs natamycin) | −0.098 (−0.28 to 0.083) | .29 |
| Scraping | 0.17 (−0.007 to 0.35) | .06 |
Abbreviations: BSCVA, best spectacle-corrected visual acuity; CI, confidence interval.
Figure 3.
Relationship between baseline and 3-month best spectacle-corrected visual acuity (BSCVA).
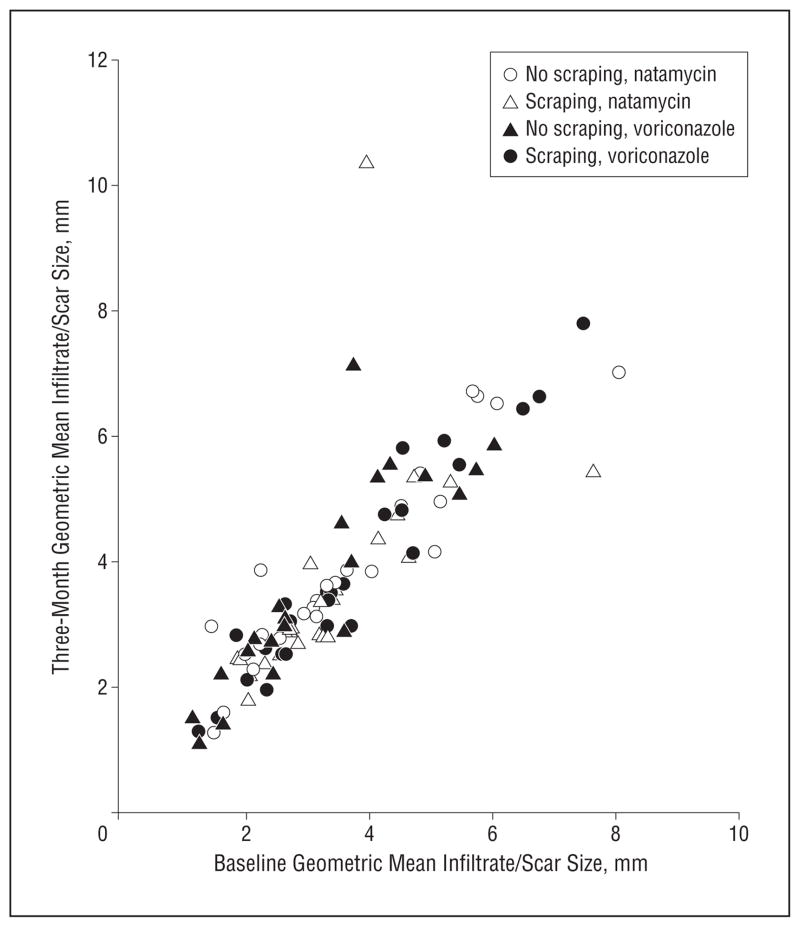
Similar linear regression models were used to predict 3-week and 3-month infiltrate/scar size using enrollment infiltrate/scar size, treatment arm, and scraping as covariates. At 3 weeks, voriconazole treatment was associated with 0.058 mm larger infiltrate/scar size diameter (95% CI, 0.3 mm smaller to 0.42 mm larger; P=.75) compared with the natamycin group. At 3 months, voriconazole treatment was associated with 0.17 mm larger infiltrate/scar size diameter (95% CI, 0.20 mm smaller to 0.53 mm larger; P=.37) compared with the natamycin group. Repeated scraping of the epithelium was associated with a 0.13-mm increase in 3-month infiltrate/scar size, although this result was not statistically significant (95% CI, −0.23 to 0.49; P=.49). The interaction between drug and scraping was not significant (P=.44). Figure 4 shows that the baseline and 3-month geometric mean of the infiltrate/scar size are highly associated.
Figure 4.
Relationship between baseline and 3-month geometric mean infiltrate/scar size.
We also conducted tests to ensure that our linear regression model was valid given the distribution of our data. Results were comparable using Huber robust regression, showing similar coefficients, P values, and standard errors. Each of the 4 sensitivity analyses yielded results consistent with the primary analysis.
Time to re-epithelialization was not significantly different between the voriconazole- and natamycin-treated patients, with voriconazole associated with a hazard ratio of −0.05 (95% CI, −0.13 to 0.14; P=.61).
No systemic adverse events occurred in this study. There were 9 corneal perforations in the natamycin group and 10 in the voriconazole group. Eight patients had corneal transplantation in the voriconazole group and 7 in the natamycin group.
An exploratory post hoc analysis was conducted on the subset of patients who had an enrollment BSCVA of 20/40 to 20/400, which means that they could read at least some letters on the eye chart and that there was room for improvement. In these 55 patients, voriconazole-treated patients were estimated to have a 0.24 logMAR improvement (approximately 2-line benefit) in 3-month BSCVA (95% CI, −0.01 to 0.49; P=.07), while patients with scraping were estimated to have a more than 2-line worsening of final visual acuity (Table 3). Neither estimate was statistically significant.
Table 3.
Multiple Linear Regression Predicting 3-Month logMAR BSCVA in Patients With Enrollment Vision of 20/40 to 20/400 (n = 55)
| Covariate | Coefficient (95% CI) | P Value |
|---|---|---|
| Enrollment BSCVA (logMAR) | 0.25 (−0.28 to 0.77) | .37 |
| Voriconazole (vs natamycin) | −0.24 (−0.49 to 0.01) | .07 |
| Scraping | 0.23 (−0.02 to 0.48) | .08 |
Abbreviations: BSCVA, best spectacle-corrected visual acuity; CI, confidence interval.
COMMENT
In this study of fungal keratitis, the primary analysis showed no significant difference in final visual acuity with voriconazole topical treatment compared with natamycin. A subanalysis of patients who were able to read at least some letters on the eye chart at enrollment (visual acuity, 20/40 to 20/400) demonstrated a trend toward a 2-line benefit with voriconazole treatment. There was no difference in final infiltrate/scar size, time to re-epithelialization, or adverse events between the 2 treatment groups. There was a trend toward scraping being associated with worse clinical outcomes in all of the analyses.
Repeat scraping of the epithelium during the course of treatment has been considered advantageous but this has never been studied in human corneas. Natamycin poorly penetrates an intact epithelium, while voriconazole is thought to have superior permeability through the epithelium.24,27,29 A recent survey of cornea specialists found that, in treating fungal keratitis, more practitioners rescrape with natamycin than with voriconazole.32 We were unable to demonstrate that scraping improved outcomes of natamycin-treated fungal ulcers. In fact, scraping was associated with a 1-line worse BSCVA at 3 months with voriconazole and 2.5-lines worse BSCVA with natamycin, although this interaction was not statistically significant. Given our failure to demonstrate a benefit to corneal scraping in the treatment of fungal keratitis and the fact that scraping nearly reached statistical significance in its association with poor outcomes, the present study does not support this intervention beyond its utility in obtaining a microbiologic sample for culture.
Topical and oral voriconazole are being increasingly used in clinical practice for the treatment of fungal corneal ulcers, though the superiority of these agents compared with natamycin has not been established.* In addition to good penetration of topical voriconazole into the anterior chamber, in vitro antifungal susceptibility testing favors voriconazole over natamycin.22,26,28,29 Topical voriconazole is only available through compounding pharmacies because it is not currently commercially available. If paying out of pocket, the costs of the 2 drugs are comparable (natamycin [Natacyn] ophthalmic suspension, $196.88/15 mL and voriconazole, 1%, compounded ophthalmic solution, $150.00/10 mL, prices courtesy of Leiter’s Pharmacy, San Jose, California). Insurance may cover the cost of natamycin given that it is approved by the Food and Drug Administration drug for treating fungal keratitis, whereas voriconazole is off-label. Overall, we did not find voriconazole to be superior to natamycin in terms of clinical outcomes or adverse events but a subgroup analysis found a trend toward improved outcomes with voriconazole.
We did not investigate the use of oral voriconazole for fungal keratitis. Voriconazole can be given orally and has good systemic penetration into the eye.24 It offers broad-spectrum coverage while avoiding the cost and adverse effects of intravenous antifungal treatment with agents such as amphotericin B. However, because oral voriconazole is expensive and can have systemic adverse effects, its routine use would need to be addressed in a clinical trial with appropriate cost-effectiveness analyses.
This study has many strengths. It was prospective, had a large sample size, and was double-masked. Follow-up and compliance with study treatments were excellent. Limitations include enrollment of patients across the full range of acuity, which may have compromised our ability to truly compare the efficacy of the drugs. In addition, the fact that voriconazole is a solution and natamycin a suspension raises some concern for unmasking, but we took every precaution to maintain masking including using opaque bottles and having ward nurses wipe off any residue prior to study assessments. In addition, our primary outcome of visual acuity at 3 months was measured by refractionists unaware of the treatment assignment and after completion of antifungal therapy, making it even more difficult for them to know the study arm.
Visual acuity may be the most important outcome from a patient standpoint, and we chose it as our primary outcome. Time to re-epithelialization has been used in other studies of corneal ulcers but we did not think it was the optimal primary outcome for this study because one of our interventions was epithelial debridement, and because the epithelium can heal despite an active underlying corneal infiltrate in fungal keratitis, in contrast to most bacterial corneal ulcers. Contact lenses are not practical for many patients in India, so BSCVA was the outcome that best represented final vision. There are some limitations inherent to using BSCVA as an outcome. Little information is gained from including patients with large infiltrates that cover the pupil, for whom there is little reasonable chance of gaining acuity better than 20/400. Also, eyes with ulcers with better than 20/40 visual acuity have little room for improvement. For this reason, we believe that the subgroup of patients with an enrollment visual acuity of 20/40 to 20/400 is a reasonable acuity group to analyze. Other clinical trials, including those on age-related macular degeneration, often have inclusion criteria excluding patients with baseline visual acuities that are very good or very poor.35,36 We found a nearly statistically significant 2-line benefit in 3-month visual acuity with voriconazole in the subgroup of patients with baseline visual acuity of 20/40 to 20/400 but this was an exploratory outcome. It will be important for this finding to be reproduced in a confirmatory therapeutic trial of patients who start with visual acuity in this range. Although voriconazole is increasingly used in clinical practice to treat fungal keratitis on the basis of in vitro and anecdotal results in patients, clinical trials are necessary to provide a rigorous evidence basis to help guide treatment.
Acknowledgments
Funding/Support: This study was supported by That Man May See and the South Asia Research Fund; core grant EY02162 from the National Eye Institute (Department of Ophthalmology at University of California, San Francisco); grant K23EY017897 from the National Eye Institute (Dr Acharya); a Research to Prevent Blindness Career Development Award (Drs Acharya and Lietman); grant U10-EY015114 from the National Eye Institute (Dr Lietman); That Man May See Foundation at University of California, San Francisco (Dr Porco); Alcon Inc; and Pfizer Inc.
Role of the Sponsors: Alcon Inc. donated natamycin and Pfizer Inc. donated voriconazole for the study. The sponsors did not have a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript.
Footnotes
References
- 1.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79(3):214–221. [PMC free article] [PubMed] [Google Scholar]
- 2.Srinivasan M, Gonzales CA, George C, et al. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, South India. Br J Ophthalmol. 1997;81(11):965–971. doi: 10.1136/bjo.81.11.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alfonso EC, Cantu-Dibildox J, Munir WM, et al. Insurgence of Fusarium keratitis associated with contact lens wear. Arch Ophthalmol. 2006;124(7):941–947. doi: 10.1001/archopht.124.7.ecs60039. [DOI] [PubMed] [Google Scholar]
- 4.Rosa RH, Jr, Miller D, Alfonso EC. The changing spectrum of fungal keratitis in south Florida. Ophthalmology. 1994;101(6):1005–1013. doi: 10.1016/s0161-6420(94)31225-5. [DOI] [PubMed] [Google Scholar]
- 5.Xie L, Zhong W, Shi W, Sun S. Spectrum of fungal keratitis in north China. Ophthalmology. 2006;113(11):1943–1948. doi: 10.1016/j.ophtha.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 6.Iyer SA, Tuli SS, Wagoner RC. Fungal keratitis: emerging trends and treatment outcomes. Eye Contact Lens. 2006;32(6):267–271. doi: 10.1097/01.icl.0000249595.27520.2e. [DOI] [PubMed] [Google Scholar]
- 7.Srinivasan M. Fungal keratitis. Curr Opin Ophthalmol. 2004;15(4):321–327. doi: 10.1097/00055735-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Varaprasathan G, Miller K, Lietman T, et al. Trends in the etiology of infectious corneal ulcers at the F. I. Proctor Foundation. Cornea. 2004;23(4):360–364. doi: 10.1097/00003226-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Margolis TP, Whitcher JP. Fusarium: a new culprit in the contact lens case. JAMA. 2006;296(8):985–987. doi: 10.1001/jama.296.8.985. [DOI] [PubMed] [Google Scholar]
- 10.Alfonso EC, Miller D, Cantu-Dibildox J, O’Brien TP, Schein OD. Fungal keratitis associated with non-therapeutic soft contact lenses. Am J Ophthalmol. 2006;142(1):154–155. doi: 10.1016/j.ajo.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 11.Prajna NV, John RK, Nirmalan PK, Lalitha P, Srinivasan M. A randomised clinical trial comparing 2% econazole and 5% natamycin for the treatment of fungal keratitis. Br J Ophthalmol. 2003;87(10):1235–1237. doi: 10.1136/bjo.87.10.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boucher HW, Groll AH, Chiou CC, Walsh TJ. Newer systemic antifungal agents: pharmacokinetics, safety and efficacy. Drugs. 2004;64(18):1997–2020. doi: 10.2165/00003495-200464180-00001. [DOI] [PubMed] [Google Scholar]
- 13.Cretì A, Esposito V, Bocchetti M, et al. Voriconazole curative treatment for Acremonium species keratitis developed in a patient with concomitant Staphylococcus aureus corneal infection: a case report. In Vivo. 2006;20(1):169–171. [PubMed] [Google Scholar]
- 14.Sponsel W, Chen N, Dang D, et al. Topical voriconazole as a novel treatment for fungal keratitis. Antimicrob Agents Chemother. 2006;50(1):262–268. doi: 10.1128/AAC.50.1.262-268.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozbek Z, Kang S, Sivalingam J, Rapuano CJ, Cohen EJ, Hammersmith KM. Voriconazole in the management of Alternaria keratitis. Cornea. 2006;25(2):242–244. doi: 10.1097/01.ico.0000170692.05703.98. [DOI] [PubMed] [Google Scholar]
- 16.Bernal MD, Acharya NR, Lietman TM, Strauss EC, McLeod SD, Hwang DG. Outbreak of Fusarium keratitis in soft contact lens wearers in San Francisco. Arch Ophthalmol. 2006;124(7):1051–1053. doi: 10.1001/archopht.124.7.ecr60006. [DOI] [PubMed] [Google Scholar]
- 17.Klont RR, Eggink CA, Rijs AJ, Wesseling P, Verweij PE. Successful treatment of Fusarium keratitis with cornea transplantation and topical and systemic voriconazole. Clin Infect Dis. 2005;40(12):e110–e112. doi: 10.1086/430062. [DOI] [PubMed] [Google Scholar]
- 18.Kofla G, Ruhnke M. Voriconazole: review of a broad spectrum triazole antifungal agent. Expert Opin Pharmacother. 2005;6(7):1215–1229. doi: 10.1517/14656566.6.7.1215. [DOI] [PubMed] [Google Scholar]
- 19.Hernández Prats C, Llinares Tello F, Burgos San Jose A, Selva Otaolaurruchi J, Ordovas Baines JP. Voriconazole in fungal keratitis caused by Scedosporium apiospermum. Ann Pharmacother. 2004;38(3):414–417. doi: 10.1345/aph.1D128. [DOI] [PubMed] [Google Scholar]
- 20.Lai TF, Malhotra R, Esmail-Zaden R, Galanopoulas A, Chehade M, Selva D. Use of voriconazole in Scedosporium apiospermum keratitis. Cornea. 2003;22(4):391–392. doi: 10.1097/00003226-200305000-00024. [DOI] [PubMed] [Google Scholar]
- 21.Nulens E, Eggink C, Rijs AJ, Wesseling P, Verweij PE. Keratitis caused by Scedosporium apiospermum successfully treated with a cornea transplant and voriconazole. J Clin Microbiol. 2003;41(5):2261–2264. doi: 10.1128/JCM.41.5.2261-2264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dupuis A, Tournier N, Le Moal G, Venisse N. Preparation and stability of voriconazole eye drop solution. Antimicrob Agents Chemother. 2009;53(2):798–799. doi: 10.1128/AAC.01126-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ford JG, Agee S, Greenhaw ST. Successful medical treatment of a case of Paecilomyces lilacinus keratitis. Cornea. 2008;27(9):1077–1079. doi: 10.1097/ICO.0b013e3181783a07. [DOI] [PubMed] [Google Scholar]
- 24.Hariprasad SM, Mieler WF, Lin TK, Sponsel WE, Graybill JR. Voriconazole in the treatment of fungal eye infections: a review of current literature. Br J Ophthalmol. 2008;92(7):871–878. doi: 10.1136/bjo.2007.136515. [DOI] [PubMed] [Google Scholar]
- 25.Mehta H, Mehta HB, Garg P, Kodial H. Voriconazole for the treatment of refractory Aspergillus fumigatus keratitis. Indian J Ophthalmol. 2008;56(3):243–245. doi: 10.4103/0301-4738.40369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lalitha P, Shapiro B, Srinivasan M, et al. Antimicrobial susceptibility of fusarium, aspergillus, and other filamentous fungi isolated from keratitis. Arch Ophthalmol. 2007;125(6):789–793. doi: 10.1001/archopht.125.6.789. [DOI] [PubMed] [Google Scholar]
- 27.O’Day DM, Head WS, Robinson RD, Clanton JA. Corneal penetration of topical amphotericin B and natamycin. Curr Eye Res. 1986;5(11):877–882. doi: 10.3109/02713688609029240. [DOI] [PubMed] [Google Scholar]
- 28.Vemulakonda GA, Hariprasad SM, Mieler WF, Prince RA, Shah GK, Van Gelder RN. Aqueous and vitreous concentrations following topical administration of 1% voriconazole in humans. Arch Ophthalmol. 2008;126(1):18–22. doi: 10.1001/archophthalmol.2007.8. [DOI] [PubMed] [Google Scholar]
- 29.Lau D, Fedinands M, Leung L, et al. Penetration of voriconazole, 1%, eyedrops into human aqueous humor: a prospective open-label study. Arch Ophthalmol. 2008;126(3):343–346. doi: 10.1001/archophthalmol.2007.71. [DOI] [PubMed] [Google Scholar]
- 30.Barron BA, Gee L, Hauck WW, et al. Herpetic Eye Disease Study: a controlled trial of oral acyclovir for herpes simplex stromal keratitis. Ophthalmology. 1994;101(12):1871–1882. doi: 10.1016/s0161-6420(13)31155-5. [DOI] [PubMed] [Google Scholar]
- 31.Iacus SM, Porro G. Missing data imputation, matching and other applications of random recursive partitioning. Comput Stat Data Anal. 2007;52(2):773–789. [Google Scholar]
- 32.Loh AR, Hong K, Lee S, Mannis M, Acharya NR. Practice patterns in the management of fungal corneal ulcers. Cornea. 2009;28(8):856–859. doi: 10.1097/ICO.0b013e318199fa77. [DOI] [PubMed] [Google Scholar]
- 33.Shah KB, Wu TG, Wilhelmus KR, Jones DB. Activity of voriconazole against corneal isolates of Scedosporium apiospermum. Cornea. 2003;22(1):33–36. doi: 10.1097/00003226-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Granados JM, Puerto N, Carrilero MJ. Efficiency of voriconazole in fungal keratitis caused by candida albicans [in Spanish] Arch Soc Esp Oftalmol. 2004;79(11):565–568. doi: 10.4321/s0365-66912004001100011. [DOI] [PubMed] [Google Scholar]
- 35.Rosenfeld PJ, Brown DM, Heier JS, et al. MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355 (14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 36.Brown DM, Kaiser PK, Michels M, et al. ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]