Abstract
Intact Streptococcus pneumoniae, expressing type 14 capsular polysaccharide (PPS14) and type III Streptococcus agalactiae containing a PPS14 core capsule identical to PPS14, exhibit non-covalent associations of PPS14 and bacterial protein, in contrast to soluble covalent conjugates of these respective antigens. Both bacteria and conjugates induce murine PPS14-specific IgG responses dependent on CD4+ T cells. Further, secondary immunization with conjugate and S. agalactiae, although not S. pneumoniae, results in a boosted response. However, in contrast to conjugate, PPS14-specific IgG responses to bacteria lack affinity maturation, utilize the 44.1-idiotype and are dependent on marginal zone B cells. To better understand the mechanism underlying this dichotomy we developed a minimal model of intact bacteria in which PPS14 and pneumococcal surface protein A (PspA) were stably attached to 1 μm (bacteria-sized) latex beads, but not directly linked to each other, in contrast to PPS14-PspA conjugate. PPS14+[PspA] beads, similar to conjugate, induced in mice boosted PPS14-specific IgG secondary responses, dependent on T cells and ICOS-dependent costimulation, and in which priming could be achieved with PspA alone. In contrast to conjugate, but similar to intact bacteria, the primary PPS14-specific IgG response to PPS14+[PspA] beads peaked rapidly, with the secondary response highly enriched for the 44.1-idiotype and lacking affinity maturation. These results demonstrate that non-covalent association in a particle, of polysaccharide and protein, recapitulates essential immunologic characteristics of intact bacteria that are distinct from soluble covalent conjugates of these respective antigens.
Introduction
Encapsulated Gram-positive bacteria, like Streptococcus pneumoniae, express a capsule formed by multiple copies of a single capsular polysaccharide (PS) covalently linked to the underlying cell wall peptidoglycan (1,2). An array of different proteins are also attached to the cell wall through covalent and non-covalent links (3). Thus, PS and proteins are associated, but not directly linked, on the bacterial surface through the cell wall. Although there are some examples of bacterial pathogens expressing surface glycoproteins (4–6), the short glycan modifications usually function to decrease the immunogenicity of the protein or downmodulate host immunity (5). Gram-positive bacteria also contain multiple pathogen associated molecular patterns (PAMPs), such as lipoteichoic acid and lipoproteins in the cell membrane and peptidoglycan in the cell wall (7–9). PAMPS, such as unmethylated CpG-containing DNA (10) and certain toxins (11), are also located intracellularly. These PAMPs can induce innate and subsequent adaptive immunity (12–14) through interactions with Toll-like receptors (TLR) (12), Nod-like receptors (15,16), or surface lectins (17). Microbial pathogens can also express subcapsular components that suppress immunity (18–20). Thus, analyses of PS-specific Ig responses to intact bacteria are complicated by this structural and functional complexity.
Indeed, we recently showed that the composition of the bacterial subcapsular domain dramatically affects in vivo PS-specific Ig responses (21,22). Thus, S. pneumoniae expressing type 14 PS (PPS14) fails to induce PPS14-specific IgG responses after secondary immunization. In contrast, the strain COH1-11 of Streptococcus agalactiae type III (GBS-III) that expresses a PS structurally identical to PPS14 (23), induced highly boosted PPS14-specific IgG responses (22). PPS14-specific IgG responses to both bacteria share similar dependence on CD4+ T cells and marginal zone (MZ) B cells, and a dominant use of the 44.1-idiotype (44.1-Id) with limited avidity maturation (24,25). The mechanism underlying the differences in these two PPS14-specific IgG responses might reflect the presence, in the subcapsular domain, of particular immunostimulatory components in GBS and/or inhibitory components of S. pneumoniae.
The covalent linkage of a T cell-independent (TI) PS to a carrier protein, to create a soluble conjugate vaccine, converts the PS into a T cell dependent (TD) Ag (26). These vaccines have had a major impact on the prevention of infections by encapsulated extracellular bacteria (27,28). Conjugate vaccines of pneumococcal surface protein A (PspA) and PPS14 (PPS14-PspA), similar to intact GBS, induce highly boosted PPS14-specific IgG responses after secondary immunization in a T cell- and ICOS-dependent manner (22,25). However, in contrast to intact bacteria, soluble PPS14-PspA conjugate induces PPS14-specific IgG responses derived from follicular B cells that undergo extensive affinity maturation, and make minimal if any use of the 44.1-Id (24,25,29). Of note, the PPS14-specific IgG response to PPS14-PspA attached to aldehyde/sulfate latex beads is dependent on 44.1-Id+ MZ B cells, but nevertheless still exhibits extensive affinity maturation (24,25). Thus, our data suggested that affinity maturation of the PPS14-specific IgG response requires the presence of a covalent linkage between the PS and the protein, absent in the intact bacteria. This covalent linkage allows for the binding of PS-associated peptide to MHC-II on the surface of the APC after processing (30). This results in CD4+ T cell responses specific for peptide conformations expressed only by the glycopeptide (31), combined saccharide-peptide motifs (32), or the saccharide component alone (33).
In this report, we use bacteria-sized latex beads, as a model for intact bacteria, to determine directly the immunologic consequences of non-covalent associations of PPS14 and PspA expressed on the bacterial surface, by eliminating potential effects of bacterial subcapsular components. We demonstrate that PPS14+[PspA] beads induce highly boosted PPS14-specific IgG secondary responses dependent on T cells and ICOS-dependent costimulation, similar to both intact GBS-III and PPS14-PspA conjugate. However, in contrast to PPS14-PspA, but similar to intact GBS-III, PPS14+[PspA] beads exhibit low affinity maturation in the induced PPS14-specific IgG response. These studies elucidate the immunologic consequences of the non-covalent association of PS and protein on the bacterial surface and establish a minimal model for understanding the mechanism by which intact bacteria elicit PS-specific Ig responses.
Materials and Methods
Mice
Female BALB/c mice were purchased from the National Cancer Institute (Frederick, MD). Female BALB/c athymic nude mice (CByJ.Cg-Foxn1nu/J) and control BALB/c mice (BALB/cByJ), ICOS−/− mice (B6129P2-Icostm1Mak/J) and control C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were used between 8–12 weeks of age. The experiments in this study were conducted in accordance with the principles set forth in the Guide for Care and Use of Laboratory Animals (34), and approved by the Uniformed Services University of the Health Sciences Institutional Animal Use and Care Committee.
Bacterial strains and antigens
Strain R6-14 of S. pneumoniae is an isogenic transformant of strain R6 expressing PPS14 (25). Purified PPS14 was purchased from ATCC (Manassas, VA). Protein contaminants in the PPS14 batches used were undetectable by BCA assay at 60°C (Pierce, Rockford, IL) and were <0.08% of the total PPS14 weight. However, these purified PPS14 preparations contain a contaminating, unidentified TLR2 ligand(s) similar to the 23-valent pneumococcal polysaccharide vaccine (Pneumovax-23) (35). A covalent conjugate of recombinant pneumococcal surface protein A (PspA) and PPS14 (PPS14-PspA) was synthesized as described (25). The PspA in the conjugate contains the first 302 amino acids of the full-length PspA. The PPS14 to PspA ratio of PPS14-PspA is 1:24. The PspA expressed by R6-14 is serologically identical with that used for PPS14-PspA. A stimulatory 30-mer CpG-containing oligodeoxynucleotide (CpG-ODN) was synthetized as previously described (36).
Antibodies
Mouse IgG1κ anti-PPS14 mAb (clone 44.1), and mouse IgG2a anti-PspA mAb (DC10-IA5) were gifts from Dr. Alex Lucas (Children's Hospital Oakland Research Institute, Oakland, CA), and Dr. Rick Schuman (Antibody and Immunoassay Consultants, Rockville, MD), respectively. The preparation and characterization of the anti-Id 2B6.2 IgG1κ mAb specific for an idiotope in or near the paratope of the PPS14-specific 44.1 mAb has been described previously (24). MAbs were oxidized with 10 mM metaperiodate and biotinylated using biotin-LC-hydrazide (Pierce, Rockford, IL) (37). The 44.1 mAb was labeled with Alexa Fluor 488-hydrazide (Invitrogen, Grand Island, NY) using a similar protocol. DC10-IA5 was labeled with Alexa Fluor 633-carboxylic acid succinimidyl ester (Invitrogen). Rat IgG2a anti-mouse CD275 (B7RP-1 or ICOS ligand; clone HK5.3) and a rat IgG2a anti-TNP isotype control mAb (clone 2A3) were purchased from BioXcell (West Lebanon, NH).
Preparation of latex beads coated with PPS14
One hundred micrograms of PPS14 in 0.1 M phosphate buffer, pH 6.5, were covalently linked to 109 surfactant-free aldehyde-sulfate latex beads [0.96 μm in diameter] (Molecular Probes; Invitrogen), by incubation overnight in an orbital shaker. In most of the experiments, covalent attachment of PPS14 to the beads was stabilized by reduction with 200 μg cyanoborohydride (SIGMA, St Louis, MO) per 109 beads. Free binding sites in the latex beads were blocked using 0.1% glycine (SIGMA) in PBS, by incubation for 1 h at room temperature (PPS14+[Gly]-beads). The Gly solution was ultrafiltered through 3 kDa ultrafiltration units (Amicon Ultra, Millipore, Billerica, MA) to remove possible protein contaminants, and sterile filtered through 0.22 μm filters (Millipore). All manipulations during the coating of the beads were carried out under sterile conditions. Beads were washed 5 times with blocking buffer, and resuspended in 0.05% Gly in PBS. Bead density after coupling was determined by densitometry at 630 nm. Beads coated only with Gly ([Gly]-beads), were produced using an identical protocol and were used as a control.
Preparation of latex beads coated simultaneously with PPS14 and PspA
Latex beads initially coated with PPS14 alone, were washed in 0.1 M phosphate buffer pH 6.5, and incubated for 24 h with 8 μg of PspA per 109 latex beads. These conditions result in a similar ratio of PspA to PPS14 on beads as that found in the PPS14-PspA conjugate. However, incubation time and/or PspA concentration were altered in some experiments to change the amount of PspA attached per bead. Beads were then washed 5 times with 50 volumes of PBS, and resuspended in 0.05% Gly in PBS (PPS14+[PspA] beads). Beads coated only with PspA ([PspA] beads) were used as controls. Attachment of PspA to fresh beads was more efficient than to PPS14 coated beads, further supporting that PspA is interacting directly with the bead and not with the PPS14, as PPS14 impairs rather than facilitates the attachment of PspA to the bead.
Preparation of latex beads coated with PPS14-PspA conjugate
Surfactant-free aldehyde-sulfate latex beads coated with PPS14-PspA were prepared as previously reported (25), with slight modifications. Fifty micrograms of PPS14-PspA in 0.1 M phosphate buffer, pH 6.5, were covalently linked to 109 latex beads by incubation overnight in an orbital shaker (PPS14-PspA conjugate beads). Free binding sites in the latex beads were blocked by incubation for 1 h at room temperature with 0.1% Gly in PBS, or maintained in PBS. Blocked beads were washed with 50 volumes of blocking buffer.
Quantitation of PspA, PPS14 and PPS14-PspA attached to beads
The content of PspA, PPS14 and PPS14-PspA conjugate attached to beads was determined by the ELISA methods previously described in detail (25). PspA content was determined by competitive inhibition ELISA using the mAb DC10-IA5, specific for PspA. PPS14 content was determined by a quantitative sandwich ELISA in which mAb 44.1 specific for PPS14 was used as both capture and detection Ab. The content of PPS14-PspA conjugate was determined as the average of the value obtained in two different quantitative assays: 1) a sandwich ELISA essentially identical with that used for the quantitation of PPS14, except for the use of PPS14-PspA conjugate as standard; 2) a capture ELISA in which particles were captured with 5 μg/ml PspA-specific mAb DC10-IA5, and detected with 1 μg/ml biotinylated 44.1 (anti-PPS14 mAb).
Flow cytometric detection of antigen attached to latex beads
Beads (2.5 × 107) were incubated overnight at 4°C with 5 μg of 44.1 mAb labeled with Alexa Fluor 488, with 1 μg of DC10-IA5 labeled with Alexa Fluor 633, or with a mixture of both in PBS plus 2% BSA (PBS-BSA). Bead fluorescence was analyzed on a BD LSRII (BD Biosciences, San Jose, CA). Bead singlets and multiplets were acquired and analyzed.
Mouse immunizations and sera collection
Unless indicated, mice were immunized i.p. at day 0 and boosted on day 14. Latex beads were always washed in PBS immediately before immunization, to remove any traces of Ag released free to the supernatant during storage. In most of the experiments, latex beads were injected mixed only with 25 μg of a stimulatory 30-mer CpG-containing oligodeoxynucleotide (CpG-ODN) as adjuvant. However, in some experiments, 13 μg of alum (Alhydrogel; Brenntag Biosector, Frederikssund, Denmark) were included in the immunization mixture. All mixtures were incubated 1 h at room temperature before immunization. Sera were prepared from blood obtained through the tail vein.
Measurement of serum titers of PPS14- or PspA-specific Ig isotypes by ELISA
Immulon-4 HBX (Dynex Technologies Chantilly, VA) microtiter plates were coated with 0.5 μg/well of PPS14 or PspA in PBS, overnight at 4°C. The plates were washed in PBS containing 0.05% Tween 20 (PBS-T), and non-specific binding was blocked with PBS containing 2% BSA (PBS-BSA). Threefold dilutions of the serum samples in PBS-BSA were added to the wells, and the plates were incubated overnight at 4°C. In most of the experiments, serum samples were diluted in PBS-BSA containing 20 μg/ml of PPS22F and added to the wells. PPS22F is structurally unrelated to PPS14, and is known to be highly contaminated with cell wall C-polysaccharide. Nevertheless, PPS14-specific Ig titers were unaffected by serum preincubation with PPS22F, and there were no detectable serum titers of PC-specific Ig observed in PPS14-coated ELISA plates in the absence of added PPS22F. A high titer, pooled antisera was included in every plate as control The plates were incubated for 1 h at 37°C with polyclonal goat anti-mouse IgM, IgG (γ-chain), IgG1, IgG2a, IgG2b, or IgG3 conjugated to alkaline phosphatase (Southern Biotechnology Associates, Birmingham, AL) in PBS-BSA. Plates were washed five times with PBS-T, and the enzymatic reaction was measured at room temperature using pnitrophenyl phosphate as substrate until absorbance for the standard wells reached predetermined values. Titers were expressed as the dilution of serum giving OD405 nm equal to 1.0. If the absorbances obtained were <1, the titer was extrapolated from the standard curve. Binding of the detected IgG and IgM to PPS14 was specific, and completely inhibited by 10 μg/ml of purified PPS14. For the quantitation of PPS14-specific IgG1 and IgG, serial dilutions of mouse IgG1κ mAb 44.1 (150-0.03 ng/ml) were included in the corresponding plates. The standard curve obtained for 44.1 was used to convert OD405 nm into a final μg/ml concentration, by using a four-parameter logistic regression method (38) with correction for the serum dilution. The specificity of 44.1 mAb has been extensively characterized (24,39).
Inhibition ELISA to quantify serum PPS14-specific Ig expressing the 44.1-Id
The inhibition ELISA for the estimation of the serum content of 44.1-Id+ PPS14-specific IgG1, IgG and IgM, has been previously described (24). Briefly, inhibition mixtures prepared by mixing sera at predetermined dilutions with 10 μg/ml of anti-Id 2B6.2 mAb, and incubated for 24 h at 4°C, were transferred to wells previously coated with 1 μg/ml PPS14 and blocked with PBSBSA. 2B6.2 is specific for the 44.1-Id expressed by the PPS14-specific 44.1 mAb (24). Mixtures of the sera with an unrelated anti-Id mAb (clone 5C11.1), instead of 2B6.2, served as a control for lack of inhibition. In the final step, plates were incubated with alkaline phosphatase-conjugated polyclonal goat anti-mouse IgG1, IgG, or IgM for 1 h at 37°C, until the OD405 nm for the standard wells reached predetermined values. Concentrations (IgG1 and IgG) or titers (IgM) in the absence and presence of inhibitor represent total (44.1-Id+ and 44.1-Id−) and 44.1-Id− alone, respectively, with the difference being the amount of 44.1-Id+ IgG1, IgG or IgM.
Determination of the avidity of serum PPS14-specific IgG
The average avidity of serum PPS14-specific IgG was determined by ELISA using elution with sodium thiocyanate (NaSCN) (40), with the modifications previously described (24). Briefly, PPS14-specific IgG bound to wells coated with 5 μg/ml PPS14, were incubated for 15 min at room temperature with increasing concentrations (0 to 4 M) of NaSCN. Sera were pre-titered and used at the corresponding dilutions producing absorbance readings at the top of the titration curve. Avidities were expressed as avidity index (AI) (i.e., the molar concentration of NaSCN eluting 50% of PPS14-specific IgG). The avidity of the interaction is proportional to the resistance to elution by this chaotrope (40).
Statistics
Data were expressed as geometric mean ± SEM of the individual serum samples. Significance between groups was determined by the Student's t-test. P values <0.05 were considered statistically significant.
Results
Latex beads can be simultaneously coated with PPS14 and a bacterial protein
The surface of S. pneumoniae, is composed of a structured mixture of PS and bacterial proteins, associated within the peptidoglycan cell wall, but not directly linked to each other. In order to reproduce, in a simple manner, this bacterial surface without the potential influence of other bacterial subcapsular components, we covalently coated 0.96-μm in diameter aldehyde/sulfate latex beads with PPS14 alone or combined, within the same particle, with pneumococcal surface protein A (PspA) (PPS14+[PspA] beads) (Fig. 1). This bead size was selected to approximate the size of an intact bacterium. As show in Figure 1A, PPS14+[PspA] beads contained both Ags, at concentrations similar to that in PPS14-PspA conjugate beads, a condition that allows for a direct comparison. Attachment to the bead was homogeneous and stable (Fig. 1A), with no significant bead aggregation observed by microscopy or FACS analysis (73–87% unstained beads were singlets). The content of free PspA was <0.05%, PPS14 <0.13% and conjugate <0.06% of attached Ag. The linkage of PPS14 to the bead involved Schiff base formation, with only a few bonds to the bead per molecule of PPS14, as supported by the increased stability of the attachment of PPS14 by selective reduction with NaBH3CN (Fig. 1B). Without reduction, PPS14 was continually released free during storage at 4°C, and by 5.1 months, only 50% PPS14 remained attached (Fig. 1B). However, once reduced, the linkage to the bead was essentially permanent (>300 years to release 13% PPS14). Reactive aldehyde groups on beads, left unoccupied after coating, were blocked with glycine (Gly) to prevent the undesired attachment of environmental proteins, which could create artifacts. Moreover, the very mild conditions used during coupling ensured that PspA did not conjugate to PPS14, when coupled to the same bead.
Figure 1. Attachment of bacterial antigens to the surface of aldehyde/sulfate latex beads.
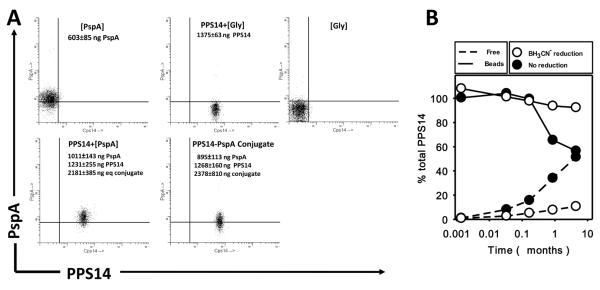
(A) Singlet FACS scatter profile of aldehyde-sulfate latex beads, 0.96 μm in diameter, coated with the different antigens and antigen combinations indicated in the graphs, and stained with Alexa-Fluor 488-conjugated 44.1 (anti-PPS14) mAb and Alexa-Fluor 633 conjugated DC-10IA5 (anti-PspA) mAb. The PspA, PPS14 or PPS14-PspA conjugate content per 109 beads and the amount of PPS14-PspA conjugate equivalents in PPS14+[PspA] beads, as determined by ELISA, is indicated in each of the representative preparations. For beads containing simultaneously PPS14 and PspA, the equivalent weight of PPS14-PspA conjugate is indicated. (B) Kinetics of release of the PPS14 attached to beads with stabilization of the PPS14 attachment (by reduction with cyanoborohydride) or without stabilization. Beads coated with PPS14 were maintained at 4°C in PBS for the time indicated. The amount of PPS14 in the supernatant (Free), and attached to the beads (Beads) once collected by centrifugation, was determined by ELISA and expressed as a percentage of the total amount of PPS14 initially attached to the beads.
PPS14+[PspA] beads induce boosted secondary PPS14-specific Ig responses in a T cell-dependent manner, similar to conjugate vaccines
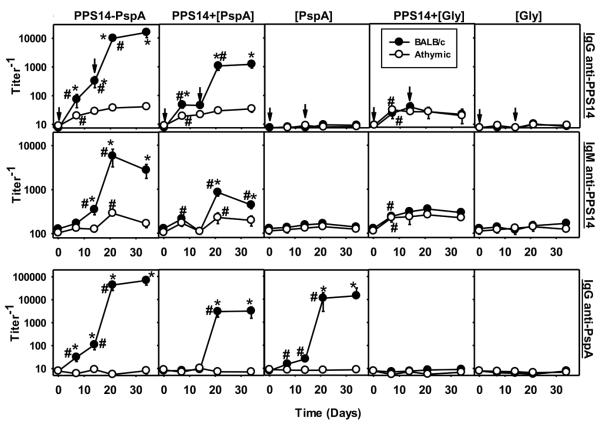
Utilizing PPS14+[PspA] beads, we wished to determine whether non-covalent association of PS and protein, as found in intact bacteria, is sufficient to induce a T cell-dependent (TD) PS-specific Ig response with induction of memory, or whether covalent attachment, a feature of conjugate vaccines is critical. We thus immunized athymic nude or control BALB/c mice with PPS14+[PspA] beads or PPS14-PspA beads in alum + CpG-ODN, with similar boosting on day14. A relatively low dose of alum was initially used in these experiments to facilitate close contact of CpG-ODN with the particles, but this was subsequently determined to be unnecessary (data not shown). We previously showed that the attachment of PPS14-PspA to beads not only preserves, but enhances its ability to induce a boosted secondary IgG anti-PPS14 response (25). As shown in Figure 2, immunization with PPS14+[PspA] beads, similar to PPS14-PspA beads, induced highly boosted secondary IgG and IgM responses specific for both PPS14 and PspA in BALB/c mice, but not in syngeneic athymic nude mice, demonstrating both the T cell-dependent nature of these responses and the induction of memory. In contrast, beads coated with PPS14 alone (PPS14+[Gly]) induced PPS14-specific IgG and IgM responses in BALB/c mice, that were not statistically different to those induced in athymic nude mice (p>0.45; Fig. 2), indicating their T cell-independent (TI) nature. Primary anti-PPS14 Ig responses to PPS14+[Gly] beads were not affected by secondary immunization. In addition, PPS14-specific IgG secondary responses to both bead-associated PPS14-PspA and PPS14+[PspA], exhibited a similar TD IgG isotype profile (Fig. 3A), although serum titers were significantly higher in response to bead-associated PPS14-PspA. In contrast, PPS14-specific IgG responses to PPS14+[Gly] beads were predominantly IgG1 and IgG3 in both, BALB/c and athymic nude mice (Fig. 3A), similar to the IgG responses to free soluble PPS14, a classical TI Ag. Moreover, the levels of PPS14-specific Ig induced by PPS14+[Gly] beads were not significantly different from those induced by the same amount of free PPS14, as determined in a titration assay (data not shown). As expected, beads coated only with PspA ([PspA] beads) or Gly ([Gly] beads) did not induce detectable anti-PPS14 Ig responses (Fig. 2), indicating that latex beads by themselves do not induce PPS14 cross-reactive Igs. Moreover, the use of two different preparations of PPS14 or two different batches of aldehyde latex beads did not affect the results obtained.. Collectively, these results indicate that the presence of associated protein, and not the covalent attachment of PPS14 to the bead or the bead structure by itself, was critical for the TD induction of the boosted secondary PPS14-specific Ig responses to PPS14+[PspA] beads.
Figure 2. PPS14+[PspA] beads induce PPS14-specific Ig responses in a T cell dependent manner.
BALB/c and BALB/c athymic nude mice (n=7), were immunized on days 0 and 14 (arrows) with ≈ 2×108 latex beads, coated with the Ag combinations indicated, in alum + CpG-ODN. Each dose of the corresponding beads contained approximately 230 ng of serologically active PPS14 and/or the equivalent of 425 ng of total PPS14-PspA conjugate. Beads used in this experiment are the same shown in Figure 1A. Serum titers of PPS14-specific and PspA-specific IgG and IgM were determined by ELISA. Values are expressed as geometric mean ± SEM. *p < 0.05 (between the responses elicited in BALB/c versus athymic nude mice), #p < 0.05 (titer−1 relative to the previous bleeding of each mouse group).
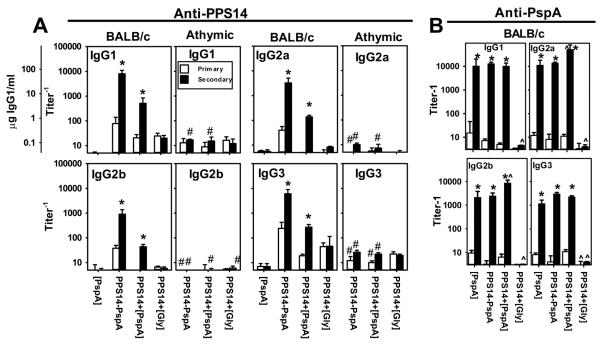
Figure 3. All IgG subclasses are boosted in a TD manner during the PPS14- and PspA-specific secondary responses to PPS14+[PspA] beads.
(A) Titers of PPS14-specific IgG isotypes in sera of BALB/c and BALB/c athymic nude mice (n=7), 14 days after a primary immunization (Primary) and 20 days after a secondary immunization (Secondary) with ≈ 2×108 latex beads, coated with the Ag combinations indicated, in alum + CpG-ODN. For IgG1 determinations a scale in weight units is included as a reference. (B) PspA-specific IgG isotype titers in selected BALB/c mice sera from several independent experiments chosen for having a similar content of PspA-specific IgG. Sera were collected 14 days after a primary immunization (Primary) and 7 days after the secondary immunization (Secondary). Values are expressed as geometric mean ± SEM. *p < 0.05 (titer−1 in primary relative to secondary response), #p < 0.05 (between the responses elicited in BALB/c versus athymic nude mice for each bleeding and group). ^p<0.05 (Only in panel B, relative to mice immunized with [PspA] beads)
PPS14-PspA beads induce a more sustained, TD primary PPS14-specific IgG response relative to PPS14+[PspA] beads
Despite similarities in the secondary PPS14-specific Ig responses to PPS14-PspA and PPS14+[PspA] beads, noted above, key differences were observed for the primary response. Primary PPS14-specific IgM and IgG responses to PPS14+[PspA] beads peaked by day 7 post-immunization (Fig. 2), similar to intact S. pneumoniae (25), whereas the peak response to PPS14-PspA beads peaked at or after day 14 (Fig. 2), similar to free, soluble PPS14-PspA (25). Further, whereas the, PPS14-specific IgG response to PPS14-PspA beads on day 7 was not significantly different (p=0.2) from that induced by PPS14+[PspA] beads, the response to the former was 10-fold higher on day 14 (p=0.031; Fig 2). Indeed, in contrast to PPS14-PspA beads, the peak primary PPS14-specific IgM and IgG responses to PPS14+[PspA] beads were only partially TD, whereas the secondary responses to both bead preparations were fully TD. This is consistent with the predominant IgG1 and IgG3 primary response to PPS14+[PspA] beads, in contrast to the expression of all IgG subclasses during the secondary response (Fig. 3). Both primary and secondary responses to PPS14+[Gly] beads exhibited the TI IgG isotype profile, whereas those to PPS14-PspA beads exhibited the TD IgG isotype profile (Fig. 3). Finally, PPS14-PspA beads induced substantially higher secondary PspA-specific IgG responses relative to PPS14+[PspA] or [PspA] beads (Fig. 2). Collectively, these data suggest that although a direct covalent link between PPS14 and PspA is not critically required to induce boosted PPS14-specific IgG secondary responses, it appears to favor the recruitment of T cell help during the primary response to PPS14 and enhances the Ig responses specific not only for PPS14 but also those specific for the carrier protein in the conjugate.
ICOS-dependent costimulation is critical for the induction of primary and secondary PPS14-specific IgG responses to PPS14+[PspA] beads
The induction of memory typically requires ICOS-dependent costimulation of CD4+ T cells, which promotes T follicular helper cell differentiation and the germinal center reaction (41,42). We thus wished to determine a potential role for ICOS in the TD boosted secondary PPS14-specific IgG response to PPS14+[PspA] beads. Mice genetically deficient in ICOS (ICOS−/−), and C57BL/6 wild type control mice, were immunized i.p. with beads coated with PPS14+[PspA], PPS14-PspA, or PPS14+[Gly], containing identical amounts of PPS14 and PspA. Beads were admixed only with CpG-ODN, in the absence of alum, in this experiment and thereafter, since alum was determined to have no effect on the CpG-ODN-adjuvanted anti-PPS14 IgG responses induced by any type of bead (data not shown). As illustrated in Figure 4A, the primary and secondary PPS14-specific IgG responses to PPS14+[PspA] and PPS14-PspA beads were markedly reduced in ICOS−/− mice, whereas the responses to PPS14+[Gly] were unaffected compared to control C57BL/6 mice. ICOS−/− mice exhibited a more complete reduction in the primary PPS14-specific IgG response to PPS14-PspA relative to PPS14+[PspA] beads, despite comparable inhibition in the PspA-specific IgG responses (Fig. 4A). Similar results were obtained in mice injected with 1 mg of a neutralizing anti-CD275 mAb (anti-ICOS ligand) 24h before primary immunization with PPS14+[PspA] beads (Fig. 4B). Thus, ICOS-dependent T cell costimulation is required for the induction of optimal primary and boosted secondary PPS14-specific IgG responses to beads containing both PspA and PPS14, regardless of the presence of a covalent linkage between these two Ags. The ICOS-dependence of these boosted PPS14-specific IgG responses strongly suggests that they depend upon germinal center formation. ICOS−/− mice immunized with intact S. pneumoniae or free PPS14-PspA exhibit essentially complete abrogation in germinal center formation (43).
Figure 4. ICOS-dependent costimulation is required for optimal induction of primary and secondary PPS14-specific IgG responses to PPS14+[PspA] beads.
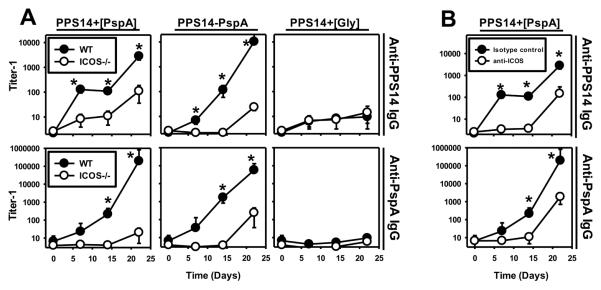
(A) C57BL/6 mice and mice genetically deficient in ICOS (ICOS−/−, n=5) and, (B) Female C57BL/6 mice (n=7) injected 1 day before with 1 mg of neutralizing anti-CD275 (ICOS-ligand) mAb (clone HK5.3) or a rat IgG2a isotype control mAb (clone 2A3), were immunized with 2×108 beads containing equal amounts of serologically active PPS14 (230 ng/dose) attached to beads, alone (PPS14+[Gly]), combined with PspA (PPS14+[PspA]) or in the form of a covalent conjugate with PspA (PPS14-PspA). All beads were administered combined with CpG-ODN, without alum. Serum titers of IgG specific for PPS14 or PspA were determined by ELISA and expressed as geometric mean ± SEM. *p < 0.05, titer−1 (A) ICOS−/− mice relative to C57BL/6 (B) Anti-CD275 treated mice relative to mice injected with isotype control mAb.
Secondary PPS14-specific IgG and IgG1 responses to PPS14+[PspA] beads are highly enriched in the expression of the 44.1-idiotype (44.1-Id)
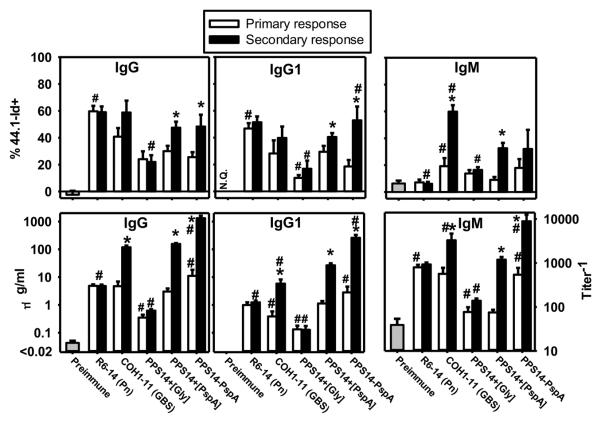
We previously demonstrated that the 44.1-Id, absent in the repertoire of natural PPS14-specific Igs, dominates the PPS14-specific IgG, but not IgM, response to intact S. pneumoniae expressing PPS14, in BALB/c mice (24) (Fig. 5). In distinct contrast, PPS14-specific IgG responses to soluble, free PPS14-PspA exhibits minimal usage of the 44.1-Id, although significant 44.1-Id expression is elicited in response to PPS14-PspA attached to latex particles (24) (Fig. 5). These data indicate a role for particulation in the expression of this Id. In this regard we now observe that the degree of 44.1-Id utilization in the PPS14-specific IgM, IgG1 and IgG responses to PPS14+[PspA] beads is largely similar to that observed using PPS14-PspA beads, with a higher proportion of 44.1-Id usage in the secondary IgG or IgG1 responses, relative to the primary. In contrast, the proportion of 44.1-Id+ anti-PPS14 IgM remained mostly unchanged (p=0.55). (Fig. 5). Notably, 44.1-Id usage in the secondary PPS14-specific IgG or IgG1 responses to PPS14+[Gly] beads, which fail to recruit CD4+ T cells, were not significantly increased relative to the primary, and thus remained a minor contribution to the overall PPS14-specific response (Fig. 5). Similarly, in BALB/c nude mice, the titer and 44.1-Id contribution to the PPS14-specific IgG secondary responses to PPS14+[PspA] beads (p=0.44; 9.8 ± 4.2%) were similar to those of the primary (data not shown). Collectively, these data indicate that the recruitment of 44.1-Id+ B cell clones, likely of MZ B cell origin, by particulate forms of PPS14 requires the recruitment of T cell help during the primary response to PspA in the particle, but without the need for direct covalent attachment between PspA and PPS14.
Figure 5. Secondary PPS14-specific IgG responses to PPS14+[PspA] beads are highly enriched in the expression of the 44.1-Id that dominates the PPS14-specific IgG responses to intact bacteria.
Sera from BALB/c mice (n=14) collected 7–14 days after primary immunization (primary response) or 7 days after secondary immunization (14 days following the primary) (secondary response) with intact S. pneumoniae type 14 (strain R6-14), Group B Streptococcus expressing a PS identical to PPS14 (strain COH1-11), or aldehyde latex beads coated with the Ag combinations indicated, admixed with CpG-ODN without alum, were analyzed by the expression of the 44.1-Id in PPS14-specific IgG, IgG1 and IgM by inhibition ELISA. Sera were collected in three independent experiments. *p < 0.05 (primary relative to secondary response), #p < 0.05 (Each group relative to the responses to PPS14+[PspA] beads).
The PPS14-specific IgG response to PPS14+[PspA] beads undergoes minimal avidity maturation, similar to intact bacteria, but in contrast to PPS14-PspA beads
We previously demonstrated that PPS14-specific IgG responses to free, soluble or bead-associated PPS14-PspA, undergo strong avidity maturation throughout both the primary and secondary responses (24) (Fig. 6). In contrast, the avidity of PPS14-specific IgG secondary responses to intact PPS14-expressing S. pneumoniae or S. agalactiae were unaffected or even reduced relative to the primary response (24) (Fig. 6). We now show that, although early (day 7) in the primary response, PPS14-specific IgG induced in response to PPS14+[PspA] beads shows similar avidity to that induced by PPS14-PspA beads (p=0.43), the avidity in the latter response increases modestly by day 14 (p=0.01). Whereas, avidity maturation during the secondary response to PPS14-PspA beads increased substantially, a decrease after secondary immunization with PPS14-PspA beads (p=0.00004) was observed, indistinguishable to that observed in response to intact S. pneumoniae type 14 (Fig. 6) or the PPS14-expressing COH1-11 strain of GBS-III (24). These data indicate that a direct covalent link between PPS14 and PspA, as found in the conjugate vaccine but not in PPS14+[PspA] beads or intact bacteria, is critical for avidity maturation of the PPS14-specific IgG response, although not necessary for recruitment of CD4+ T cell help or secondary boosting.
Figure 6. Average avidity of PPS14-specific IgG responses following immunization with different antigenic forms of PPS14 attached to latex beads.
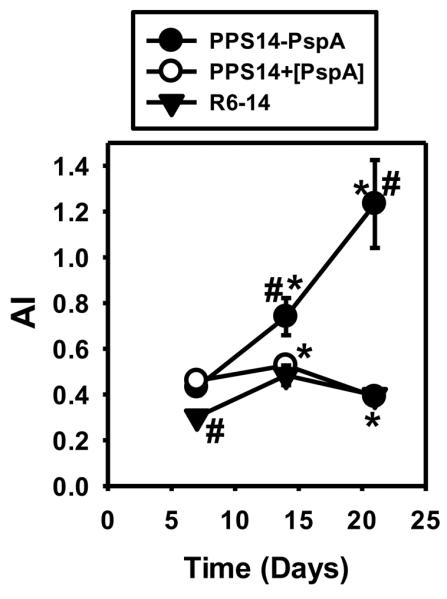
The average avidity of PPS14-specific IgG in serum was estimated by elution with sodium thiocyanate (NaSCN). Mice were immunized at days 0 and 14 with different antigenic forms of PPS14 attached to ≈2×108 latex beads admixed with CpG-ODN or with 2×108 CFU of S. pneumoniae strain R6-14 in saline. Each latex bead dose contained 193±9 ng PspA and/or 230±26 ng PPS14 attached to beads in the form of PPS14-PspA conjugate (PPS14-PspA), as a combination of PPS14 and PspA individually attached to the bead (PPS14+[PspA]), or PPS14 alone (PPS14+[Gly]). Sera tested (n=15) were from three independent experiments. Data show the avidity index (AI), as the molar concentration of NaSCN eluting 50% of the total content of PPS14-specific IgG in the serum sample. *p < 0.05 (AI relative to the previous bleeding of each mouse group), #p < 0.05 (Each group relative to the responses to PPS14+[PspA] beads).
Beads coated only with PspA prime for the PPS14-specific IgG response to PPS14+[PspA] beads
PS-specific IgG responses to conjugate vaccines typically exhibit the carrier effect. Specifically, pre-immunization with a protein, the carrier, primes carrier-specific CD4+ T cells for an enhanced IgG response specific for a hapten that is covalently attached to the same protein (28,44). Thus, we tested whether PPS14-specific IgG responses to PPS14+[PspA] beads exhibits this effect. BALB/c mice preimmunized 14 days earlier with [PspA] beads, were then immunized with PPS14+[PspA] beads and boosted in a similar fashion 17 days later. Naïve mice immunized and boosted 17 days apart with PPS14+[PspA] or [PspA] beads were used as controls. As shown in Fig. 7, both PspA- and PPS14-specific IgG responses to a primary immunization with PPS14+[PspA] beads were highly boosted in mice previously immunized with [PspA] beads, compared with the primary responses in naive mice. In contrast, pre-immunization with PPS14+[Gly] beads, or beads coated with autologous mouse albumin, did not affect the PPS14-specific IgG responses to primary or secondary immunization with PPS14+[PspA] beads (data not shown), indicating that the booster effect is derived from the response to PspA, and not to the beads themselves or to PPS14. The carrier effect extended to the PPS14-specific responses to a secondary immunization with PPS14+[PspA] beads, which were enhanced in mice preimmunized with [PspA] beads relative to controls (Fig. 7). Similarly enhanced PPS14-specific IgG responses were observed in naïve mice after a third immunization with PPS14+[PspA] beads, two months later, but still without showing any significant increase in avidity maturation (data not shown). Thus, the PPS14-specific IgG response to PPS14+[PspA] beads exhibited a carrier effect associated with PspA priming, similar to conjugate vaccines. This further supports the notion that non-covalent association of PspA and PPS14 within a particle is sufficient for recruiting CD4+ T cell help for a PPS14-specific IgG response, although insufficient for inducing avidity maturation.
Figure 7. Effect of priming with [PspA] beads and PPS14+[PspA] beads in the PspA- and PPS14-specific IgG responses to PPS14+[PspA] beads.
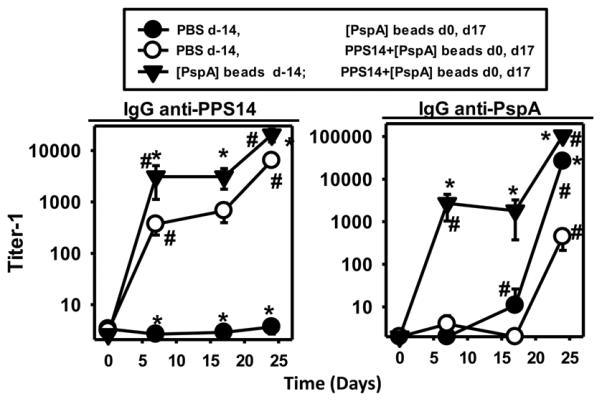
Groups of five naïve BALB/c mice (PBS) and mice previously immunized with ≈2×108 beads coated only with PspA ([PspA] beads; 167 ng per dose) 14 days earlier (d-14), were immunized at days 0 and 17 with 174 ng of PspA in the form of beads coated only with PspA ([PspA] beads) or with beads coated simultaneously with PspA and PPS14 (PPS14+[PspA] beads, admixed with CpG-ODN without alum. *p < 0.05 (Each group relative to the responses of naïve mice to the immunization with PPS14+[PspA] beads at days 0 and 17), #p < 0.05 (titer−1 relative to the previous bleeding in each mouse group).
Discussion
These results demonstrate that non-covalent association of PPS14 and PspA attached to the surface of bacteria-sized aldehyde/sulfate latex beads, recapitulate the essential immunologic characteristics of those Ags in the intact bacterium. Thus, the primary PPS14-specific IgG response to PPS14+[PspA] beads was rapid, peaking on or before day 7, with a highly boosted, TD secondary IgG response, comprising all IgG subclasses and abundantly enriched in the usage of the 44.1-Id, but with minimal avidity maturation. Optimal primary and secondary PPS14-specific IgG responses also required ICOS-dependent costimulation, strongly suggesting a key role for the germinal center reaction. These characteristics of the PPS14-specific Ig response to PPS14+[PspA] beads are essentially identical to those displayed in response to intact GBS expressing PPS14 (22,24).
However, intact S. pneumoniae induces PPS14-specific IgG responses that diverge from this basic pattern in two major ways. Thus, it induces a PPS14-specific IgG response that, although dependent on CD4+ T cells, is ICOS-independent and fails to generate a boosted IgG response upon secondary immunization (43,45). These deviations from this model strongly suggest the presence of an immunosuppressive component(s) in the subcapsular domain of S. pneumoniae. This effect is likely direct and not mediated by CD25+ regulatory T cells (46). However, a deficiency in the expression of SLAM-associated protein (SAP), which impairs the ability of CD4+ T cells to stably interact with cognate B cells early during the immune response (47), results in the complete abrogation of the primary and secondary PPS14-specific IgG responses to S. pneumoniae (43). Thus, this putative inhibitory effect of S. pneumoniae does not prevent early B-T cell cognate interactions, but likely impacts on later events involved in the subsequent generation of a secondary PPS14-specific IgG response. This inhibitory property of intact S. pneumoniae may also be responsible for its ability to markedly suppress TD IgG responses to co-immunized soluble proteins, a property not observed for intact GBS (48). Thus, the distinct nature of the PS-specific IgG responses to intact S. pneumoniae versus GBS underscore the importance of the subcapsular domain in determining the outcome of the humoral response to the associated capsular PS.
The induction of T cell help for generating the PPS14-specific Ig response to PPS14+[PspA] beads is due to the bead-associated PspA. Thus, beads coated only with PspA prime for the boosted PPS14-specific IgG response upon subsequent immunization with PPS14+[PspA] beads. Immunization with PspA, as with other proteins, results in germinal center formation, accounting for the ICOS-dependence of the PPS14-specific IgG response. In contrast, it is highly unlikely that CD4+ T cell help for the PPS14-specific IgG response is induced by bead-associated PPS14. Thus, priming with beads coated only with PPS14, had no effect on the PPS14-specific IgG response to a subsequent immunization with PPS14+[PspA] beads. Of note, PS-specific T cell help for an anti-PS response to a conjugate vaccine has recently been described (33). However, processed peptide covalently linked to the PS seems to be required for the anchoring to MHC-II at the APC surface and for the recruitment of PS-specific T cell help (33). The direct link between PS and protein in the conjugate vaccine that is likely required for this peptide-PS linkage, is absent in PPS14+[PspA] beads and intact bacteria. Moreover, we and others have previously found that DC fail to express PS on their surface following internalization of intact PS-expressing bacteria (49). Thus, optimal PS-specific IgG responses to both intact bacteria and PPS14+[PspA] beads will depend upon cognate B cell-T cell interactions specific only for the associated protein.
The major difference between beads coated simultaneously with PPS14 and PspA, and those coated with PPS14-PspA conjugate, is the absence or presence, respectively, of a covalent linkage between PPS14 and PspA. In this regard, only PPS14-PspA beads induced avidity maturation of the PPS14-specific IgG response. These data strongly suggest that PPS14-specific B cells responding to PPS14+[PspA] beads or intact bacteria, receive either non-cognate, and/or transient cognate help from protein-specific T cells as a result of this non-linkage of PPS14 and PspA. This likely directs PPS14-specific B cells into a rapid, short-lived, extrafollicular plasma cell response (29) that generates little, if any avidity maturation or B cell memory. Thus, the boosted PPS14-specific IgG response following secondary immunization with PPS14+[PspA] beads or intact GBS, may reflect rapid recruitment of an expanded population of protein-specific memory CD4+ T cells generated during the primary response. Engagement of TLR on APC and/or PS-specific B cells by TLR ligands expressed by intact bacteria acts in concert with this CD4+ T cell help for optimal induction of the PS-specific IgG response (reviewed in (50)). In this regard, addition, although not direct attachment, of CpG-ODN to PPS14+[PspA] beads further enhanced, by 3–4 fold, the secondary PPS14-specific IgG response (data not shown). Activation of the inflammasome on the basis of the particulate nature of the latex beads themselves or the intact bacterium (15), could also theoretically enhance the humoral response, although only in the presence of associated protein, since beads coated only with PPS14 induced similar responses as the same amount of free PPS14.
In our bead model, we added CpG-ODN to mimic the TLR activation mediated by bacteria. As with CpG-ODN, bacterial DNA is a potent immunostimulant (10,51), that signals through TLR9 in the endolysosomal compartment (52). Indeed, we previously demonstrated a modest role for TLR9 in S. pneumoniae-mediated cytokine induction (53). Nevertheless, bacteria express multiple TLR-ligands that demonstrate distinctive cellular expression patterns and differing signaling pathways that may result in elicitation of distinctive effector functions (12). However, we do not believe that inclusion of CpG-ODN alone as an adjuvant significantly impacts on the major conclusions of our study. Thus, we have observed that substituting CpGODN for synthetic TLR2 or TLR4 ligands, preserves the essential parameters of the PPS14-specific Ig responses reported in this study, although the IgG isotype profile of the responses affected, as could be expected. Further, we observed similar findings in the absence of added adjuvant, although the serum titers of PPS14-specific Ig were significantly reduced (data not shown).
The ability of PPS14+[PspA] or PPS14-PspA beads, in addition to intact S. pneumoniae or GBS expressing PPS14, to elicit rapid PPS14-specific IgG responses highly enriched for the 44.1-Id likely reflects selective recruitment of MZ B cells into the PS-specific IgG response (25,29). The common feature underlying MZB recruitment is the particulate form of the antigenic stimulus. In contrast, the PPS14-specific IgG response to free soluble PPS14-PspA conjugate, which appears to involve FB cells, develops more slowly and exhibits only minimal 44.1-Id expression. The ability of PPS14-PspA beads to induce avidity maturation of the PPS14-specific IgG response exhibiting 44.1-Id dominance, confirms that MZB cells can be recruited into a GC reaction under the appropriate conditions (54). In rodents, MZ B cells are considered to be naïve, preactivated B cells, with an intrinsic ability to respond rapidly and more strongly than follicular B cells, to activation via BCR and TLR engagement (55). In addition, the BCR repertoire of MZB cells is enriched for cross-reactive specificities for multivalent self or microbial antigens. These features of MZB cells likely account for the rapidity and 44.1-Id dominance of responses to particulate forms of PPS14 and protein.
Covalent conjugation between PS and carrier protein has traditionally been considered to be critical for the induction of TD humoral immune responses to PS (56). However, our results demonstrate that non-covalent associations of protein and PS on inert latex particles can also induce and dramatically boost (over 10-fold) the circulating level of PS-specific IgG in a TD manner, similarly to soluble conjugate vaccines. Similar to humoral responses to soluble conjugates, the Ig responses to PPS14+[PspA] beads also exhibited a protein carrier effect, in which priming could be achieved with the carrier protein alone (28,44). Importantly, circulating levels of PPS14-specific IgG were sustained for more than 3 weeks after secondary stimulation, suggesting that long-lived plasma cells were generated during the response. Collectively, these immunologic characteristics of PPS14+[PspA] beads suggest an alternative approach to the use of conjugate vaccines, particularly for rapidly boosting immunity during an epidemic outbreak or perhaps in response to a known biological warfare agent. This could be accomplished with a single immunization of bead-associated target antigen and carrier protein in individuals previously exposed to the carrier, although repetitive immunizations would likely further increase the level of immunity. The selective targeting of MZB cells by antigens in a particulate form may also select for broadly neutralizing cross-reactive antibodies that might be of value for highly mutating viruses. Although other groups have proposed the use of PS entrapped in biodegradable particles, as a vehicle to maintain a sustained release of Ag (57,58), and the ability of PS encapsulated in cross-linked BSA particles to induce the release of IL-8, TNF-α and IL-1β (57), this is the first report to study the adaptive responses to non-covalent associations of PS and protein on the surface of inert particles.
Our bead-based approach to vaccination has numerous advantages. Non-covalent associations of protein and PS on the surface of latex beads can be produced without the need for the use of organic solvents, complex methods of conjugation or chromatographic separation. Instead, they can be produced using centrifugation or filtration as the only method for separation of uncoupled material. Moreover, pre-assembled beads coated with each serotype-specific PS, could be stored to be later combined with the proper carrier protein. The lack of avidity maturation of the PPS14-specific IgG secondary responses to PPS14+[PspA] beads, a characteristic in common with the IgG responses to intact bacteria, may not a serious drawback for their application as a vaccine, since PS-specific Ig responses to intact S. pneumoniae, of similar avidity, are highly protective (59). Furthermore, in contrast to free soluble PPS14-PspA conjugate, PPS14+[PspA] beads, induce rapid increases and secondary boosting in the circulating levels of both PPS14-specific IgG, as well as IgM. PS-specific IgM responses can also be fully protective against pneumococcal infection (60). In summary, our data on the in vivo immunologic properties of bead-associated PPS14 and PspA suggest a simple model for better understanding PS-specific Ig responses to intact bacteria as well as a novel approach to vaccination.
Abbreviations
- AI
Avidity Index
- PPS14+[Gly] beads
Beads coated with PPS14 and blocked with glycine
- PPS14+[PspA] beads
Beads coated simultaneously with PPS14 and PspA
- PPS14-PspA beads
Beads coated with PPS14-PspA conjugate vaccine
- GBS
Group B Streptococcus [Streptococcus agalactiae]
- PspA
Pneumococcal surface protein A
- PPS14
Capsular polysaccharide of Streptococcus pneumoniae type 14
- Id
Idiotype
- TD
T cell dependent
- TI
T cell independent
Footnotes
This study was supported by NIH grant AI49192 (CMS), the USUHS Dean's Research and Education Endowment Fund (CMS), and NIH grant AI25008 (AHL).
The opinions expressed here are those of the authors and should not be construed as official or reflecting the views of the Uniformed Services University of the Health Sciences or the Department of Defense.
Reference List
- 1.Eberhardt A, Hoyland CN, Vollmer D, Bisle S, Cleverley RM, Johnsborg O, Havarstein LS, Lewis RJ, Vollmer W. Attachment of Capsular Polysaccharide to the Cell Wall in Streptococcus pneumoniae. Microb. Drug Resist. 2012;18:240–255. doi: 10.1089/mdr.2011.0232. [DOI] [PubMed] [Google Scholar]
- 2.Sorensen UB, Henrichsen J, Chen HC, Szu SC. Covalent linkage between the capsular polysaccharide and the cell wall peptidoglycan of Streptococcus pneumoniae revealed by immunochemical methods. Microb. Pathog. 1990;8:325–334. doi: 10.1016/0882-4010(90)90091-4. [DOI] [PubMed] [Google Scholar]
- 3.Jedrzejas MJ. Pneumococcal virulence factors: structure and function. Microbiol. Mol. Biol. Rev. 2001;65:187–207. doi: 10.1128/MMBR.65.2.187-207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanchez CJ, Shivshankar P, Stol K, Trakhtenbroit S, Sullam PM, Sauer K, Hermans PW, Orihuela CJ. The pneumococcal serine-rich repeat protein is an intra-species bacterial adhesin that promotes bacterial aggregation in vivo and in biofilms. PLoS. Pathog. 2010;6:e1001044. doi: 10.1371/journal.ppat.1001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Upreti RK, Kumar M, Shankar V. Bacterial glycoproteins: functions, biosynthesis and applications. Proteomics. 2003;3:363–379. doi: 10.1002/pmic.200390052. [DOI] [PubMed] [Google Scholar]
- 6.Seo HS, Mu R, Kim BJ, Doran KS, Sullam PM. Binding of glycoprotein Srr1 of Streptococcus agalactiae to fibrinogen promotes attachment to brain endothelium and the development of meningitis. PLoS. Pathog. 2012;8:e1002947. doi: 10.1371/journal.ppat.1002947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J. Biol. Chem. 1999;274:17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 8.Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D. Cutting Edge: Recognition of Gram-Positive Bacterial Cell Wall Components by the Innate Immune System Occurs Via Toll-Like Receptor 2. The Journal of Immunology. 1999;163:1–5. [PubMed] [Google Scholar]
- 9.Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–739. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 10.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 11.Malley R, Henneke P, Morse SC, Cieslewicz MJ, Lipsitch M, Thompson CM, Kurt-Jones E, Paton JC, Wessels MR, Golenbock DT. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc. Natl. Acad. Sci. U. S. A. 2003;100:1966–1971. doi: 10.1073/pnas.0435928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 13.Vasilevsky S, Chattopadhyay G, Colino J, Yeh TJ, Chen Q, Sen G, Snapper CM. B and CD4+ T-cell expression of TLR2 is critical for optimal induction of a T-cell-dependent humoral immune response to intact Streptococcus pneumoniae. Eur. J Immunol. 2008;38:3316–3326. doi: 10.1002/eji.200838484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hua Z, Hou B. TLR signaling in B-cell development and activation. Cell Mol. Immunol. 2012;10 doi: 10.1038/cmi.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharp FA, Ruane D, Claass B, Creagh E, Harris J, Malyala P, Singh M, O'Hagan DT, Petrilli V, Tschopp J, O'Neill LA, Lavelle EC. Uptake of particulate vaccine adjuvants by dendritic cells activates the NALP3 inflammasome. Proc. Natl. Acad. Sci. U. S. A. %20. 2009;106:870–875. doi: 10.1073/pnas.0804897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saleh M. The machinery of Nod-like receptors: refining the paths to immunity and cell death. Immunol. Rev. 2011;243:235–246. doi: 10.1111/j.1600-065X.2011.01045.x. [DOI] [PubMed] [Google Scholar]
- 17.Sancho D, Sousa Reis e. Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu. Rev. Immunol. 2012;30:491–529. doi: 10.1146/annurev-immunol-031210-101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashida H, Kim M, Schmidt-Supprian M, Ma A, Ogawa M, Sasakawa C. A bacterial E3 ubiquitin ligase IpaH9.8 targets NEMO/IKKgamma to dampen the host NF-kappaB-mediated inflammatory response. Nat. Cell Biol. 2010;12:66–73. doi: 10.1038/ncb2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gringhuis SI, den DJ, Litjens M, d. van V, Geijtenbeek TB. Carbohydrate-specific signaling through the DC-SIGN signalosome tailors immunity to Mycobacterium tuberculosis, HIV-1 and Helicobacter pylori. Nat. Immunol. 2009;10:1081–1088. doi: 10.1038/ni.1778. [DOI] [PubMed] [Google Scholar]
- 20.Colino J, Snapper CM. Two distinct mechanisms for induction of dendritic cell apoptosis in response to intact Streptococcus pneumoniae. J. Immunol. 2003;171:2354–65. doi: 10.4049/jimmunol.171.5.2354. [DOI] [PubMed] [Google Scholar]
- 21.Arjunaraja S, Massari P, Wetzler LM, Lees A, Colino J, Snapper CM. The nature of an in vivo anti-capsular polysaccharide response is markedly influenced by the composition and/or architecture of the bacterial subcapsular domain. J. Immunol. 2012;188:569–577. doi: 10.4049/jimmunol.1101446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arjunaraja S, Paoletti LC, Snapper CM. Structurally Identical Capsular Polysaccharide Expressed by Intact Group B Streptococcus versus Streptococcus pneumoniae Elicits Distinct Murine Polysaccharide-Specific IgG Responses In Vivo. J. Immunol. 2012;188:5238–5246. doi: 10.4049/jimmunol.1200132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wessels MR, Haft RF, Heggen LM, Rubens CE. Identification of a genetic locus essential for capsule sialylation in type III group B streptococci. Infect. Immun. 1992;60:392–400. doi: 10.1128/iai.60.2.392-400.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colino J, Duke L, Arjunaraja S, Chen Q, Liu L, Lucas AH, Snapper CM. Differential Idiotype Utilization for the In Vivo Type 14 Capsular Polysaccharide-Specific Ig Responses to Intact Streptococcus pneumoniae versus a Pneumococcal Conjugate Vaccine. J. Immunol. 2012;189:575–586. doi: 10.4049/jimmunol.1200599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colino J, Chattopadhyay G, Sen G, Chen Q, Lees A, Canaday DH, Rubtsov A, Torres R, Snapper CM. Parameters underlying distinct T cell-dependent polysaccharide-specific IgG responses to an intact gram-positive bacterium versus a soluble conjugate vaccine. J Immunol. 2009;183:1551–1559. doi: 10.4049/jimmunol.0900238. [DOI] [PubMed] [Google Scholar]
- 26.Guttormsen HK, Wetzler LM, Finberg RW, Kasper DL. Immunologic memory induced by a glycoconjugate vaccine in a murine adoptive lymphocyte transfer model. Infect. Immun. 1998;66:2026–2032. doi: 10.1128/iai.66.5.2026-2032.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seale A, Finn A. What is the best way to use conjugate vaccines? Curr. Opin. Infect. Dis. 2011;24:219–224. doi: 10.1097/QCO.0b013e3283468996. [DOI] [PubMed] [Google Scholar]
- 28.Dagan R, Poolman J, Siegrist CA. Glycoconjugate vaccines and immune interference: A review. Vaccine. 2010;28:5513–5523. doi: 10.1016/j.vaccine.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 29.Chattopadhyay G, Khan AQ, Sen G, Colino J, DuBois W, Rubtsov A, Torres RM, Potter M, Snapper CM. Transgenic expression of Bcl-xL or Bcl-2 by murine B cells enhances the in vivo antipolysaccharide, but not antiprotein, response to intact Streptococcus pneumoniae. J. Immunol. 2007;179:7523–7534. doi: 10.4049/jimmunol.179.11.7523. [DOI] [PubMed] [Google Scholar]
- 30.Lai Z, Schreiber JR. Antigen processing of glycoconjugate vaccines; the polysaccharide portion of the pneumococcal CRM(197) conjugate vaccine co-localizes with MHC II on the antigen processing cell surface. Vaccine. 2009;27:3137–3144. doi: 10.1016/j.vaccine.2009.03.064. [DOI] [PubMed] [Google Scholar]
- 31.Harding CV, Kihlberg J, Elofsson M, Magnusson G, Unanue ER. Glycopeptides bind MHC molecules and elicit specific T cell responses. J. Immunol. 1993;151:2419–2425. [PubMed] [Google Scholar]
- 32.Deck MB, Sjolin P, Unanue ER, Kihlberg J. MHC-restricted, glycopeptide-specific T cells show specificity for both carbohydrate and peptide residues. J. Immunol. 1999;162:4740–4744. [PubMed] [Google Scholar]
- 33.Avci FY, Li X, Tsuji M, Kasper DL. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat. Med. %20. 2011;17:1602–1609. doi: 10.1038/nm.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Research Council . Guide for the care and use of laboratory animals. National Academy Press; Washington, DC: 1996. [Google Scholar]
- 35.Sen G, Khan AQ, Chen Q, Snapper CM. In vivo humoral immune responses to isolated pneumococcal polysaccharides are dependent on the presence of associated TLR ligands. J. Immunol. 2005;175:3084–3091. doi: 10.4049/jimmunol.175.5.3084. [DOI] [PubMed] [Google Scholar]
- 36.Sen G, Flora M, Chattopadhyay G, Klinman DM, Lees A, Mond JJ, Snapper CM. The critical DNA flanking sequences of a CpG oligodeoxynucleotide, but not the 6 base CpG motif, can be replaced with RNA without quantitative or qualitative changes in Toll-like receptor 9-mediated activity. Cell Immunol. 2004;232:64–74. doi: 10.1016/j.cellimm.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 37.O'Shannessy DJ, Quarles RH. Specific conjugation reactions of the oligosaccharide moieties of immunoglobulins. J. Appl. Biochem. 1985;7:347–355. [PubMed] [Google Scholar]
- 38.Plikaytis BD, Turner SH, Gheesling LL, Carlone GM. Comparisons of standard curve-fitting methods to quantitate Neisseria meningitidis group A polysaccharide antibody levels by enzyme-linked immunosorbent assay. J. Clin. Microbiol. 1991;29:1439–1446. doi: 10.1128/jcm.29.7.1439-1446.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colino J, Snapper CM. Dendritic cell-derived exosomes express a Streptococcus pneumoniae capsular polysaccharide type 14 cross-reactive antigen that induces protective immunoglobulin responses against pneumococcal infection in mice. Infect. Immun. 2007;75:220–230. doi: 10.1128/IAI.01217-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pullen GR, Fitzgerald MG, Hosking CS. Antibody avidity determination by ELISA using thiocyanate elution. J. Immunol. Methods. 1986;86:83–87. doi: 10.1016/0022-1759(86)90268-1. [DOI] [PubMed] [Google Scholar]
- 41.Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH, Flavell RA. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 42.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 43.Chen Q, Cannons JL, Paton JC, Akiba H, Schwartzberg PL, Snapper CM. A novel ICOS-independent, but CD28- and SAP-dependent, pathway of T cell-dependent, polysaccharide-specific humoral immunity in response to intact Streptococcus pneumoniae versus pneumococcal conjugate vaccine. J. Immunol. 2008;181:8258–8266. doi: 10.4049/jimmunol.181.12.8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Katz DH, Paul WE, Goidl EA, Benacerraf B. Carrier function in anti-hapten immune responses. I. Enhancement of primary and secondary anti-hapten antibody responses by carrier preimmunization. J. Exp. Med. 1970;132:261–282. doi: 10.1084/jem.132.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu ZQ, Vos Q, Shen Y, Lees A, Wilson SR, Briles DE, Gause WC, Mond JJ, Snapper CM. In vivo polysaccharide-specific IgG isotype responses to intact Streptococcus pneumoniae are T cell dependent and require CD40− and B7-ligand interactions. J Immunol. 1999;163:659–667. [PubMed] [Google Scholar]
- 46.Lee KS, Sen G, Snapper CM. Endogenous CD4+ CD25+ regulatory T cells play no apparent role in the acute humoral response to intact Streptococcus pneumoniae. Infect. Immun. 2005;73:4427–4431. doi: 10.1128/IAI.73.7.4427-4431.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chattopadhyay G, Chen Q, Colino J, Lees A, Snapper CM. Intact bacteria inhibit the induction of humoral immune responses to bacterial-derived and heterologous soluble T cell-dependent antigens. J. Immunol. 2009;182:2011–2019. doi: 10.4049/jimmunol.0802615. [DOI] [PubMed] [Google Scholar]
- 49.Colino J, Shen Y, Snapper CM. Dendritic cells pulsed with intact Streptococcus pneumoniae elicit both protein- and polysaccharide-specific immunoglobulin isotype responses in vivo through distinct mechanisms. J. Exp. Med. 2002;195:1–13. doi: 10.1084/jem.20011432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Snapper CM. Mechanisms underlying in vivo polysaccharide-specific immunoglobulin responses to intact extracellular bacteria. Ann. N. Y. Acad. Sci. 2012;1253:92–101. doi: 10.1111/j.1749-6632.2011.06329.x. 92–101. [DOI] [PubMed] [Google Scholar]
- 51.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 52.Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010;32:305–315. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 53.Lee KS, Scanga CA, Bachelder EM, Chen Q, Snapper CM. TLR2 synergizes with both TLR4 and TLR9 for induction of the MyD88-dependent splenic cytokine and chemokine response to Streptococcus pneumoniae. Cell Immunol. 2007;245:103–110. doi: 10.1016/j.cellimm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song H, Cerny J. Functional heterogeneity of marginal zone B cells revealed by their ability to generate both early antibody-forming cells and germinal centers with hypermutation and memory in response to a T-dependent antigen. J. Exp. Med. 2003;198:1923–1935. doi: 10.1084/jem.20031498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pillai S, Cariappa A. The follicular versus marginal zone B lymphocyte cell fate decision. Nat. Rev. Immunol. 2009;9:767–777. doi: 10.1038/nri2656. [DOI] [PubMed] [Google Scholar]
- 56.Schneerson R, Barrera O, Sutton A, Robbins JB. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J. Exp. Med. 1980;152:361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ubale RV, D'souza MJ, Infield DT, McCarty NA, Zughaier SM. Formulation of meningococcal capsular polysaccharide vaccine-loaded microparticles with robust innate immune recognition. J. Microencapsul. 2013;30:28–41. doi: 10.3109/02652048.2012.692402. [DOI] [PubMed] [Google Scholar]
- 58.Anish C, Goswami DG, Kanchan V, Mathew S, Panda AK. The immunogenic characteristics associated with multivalent display of Vi polysaccharide antigen using biodegradable polymer particles. Biomaterials. 2012;33:6843–6857. doi: 10.1016/j.biomaterials.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 59.Alonso De Velasco E, Dekker BA, Verheul AF, Feldman RG, Verhoef J, Snippe H. Anti-polysaccharide immunoglobulin isotype levels and opsonic activity of antisera: relationships with protection against Streptococcus pneumoniae infection in mice. J Infect Dis. 1995;172:562–565. doi: 10.1093/infdis/172.2.562. [DOI] [PubMed] [Google Scholar]
- 60.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]