Abstract
Snake venom proteins are broadly investigated in the different areas of life science. Direct interaction of these compounds with cells may involve a variety of mechanisms that result in diverse cellular responses leading to the activation or blocking of physiological functions of the cell. In this review, the snake venom components interacting with integrins will be characterized in context of their effect on cellular response. Currently, two major families of snake venom proteins are considered as integrin-binding molecules. The most attention has been devoted to the disintegrin family, which binds certain types of integrins through specific motifs recognized as a tri-peptide structurally localized on an integrin-binding loop. Other snake venom integrin-binding proteins belong to the C-type lectin family. Snake venom molecules bind to the cellular integrins resulting in a modulation of cell signaling and in consequence, the regulation of cell proliferation, migration and apoptosis. Therefore, snake venom research on the integrin-binding molecules may have significance in biomedicine and basic cell biology.
1. Introduction
Snake venoms are natural sources of various biologically active compounds, with the major physiological role of killing and predigesting a prey. Toxic effects of viper venoms on the prey s tissue comprise severe local necrosis and antagonizing of the blood coagulation system. Many viper venom proteins have been characterized as non-toxic, although displaying interesting biological properties. Among them are proteins, which modulate cell interaction with extracellular matrix (ECM) proteins. The most important cellular receptors responsible for cell/ECM interaction are integrins. Viper venoms contain antagonists of integrins, which were structurally classified as disintegrins and C-type lectin proteins (CLP).
Integrins in the functional stage are heterodimeric glycoproteins composed from α and β subunits. Currently, 18 α and 8 β subunits have been identified in mammalian cells, whose association is limited only to 24 heterodimers that strictly determines ligand specificity (Hynes, 2002; Humphries et al., 2006). Expression of integrins in normal cells is characteristic for a specific tissue that determines cell function in a particular organ. For example, cells involved I the construction a tissue scaffold express receptors for structural ECM proteins (e.g. collagen), whereas immune cells express receptors responsible for cell-cell interaction (e.g. VCAM-1), which facilitate their migration upon inflammatory response. Many cellular responses require activation of integrin that is correlated with a signal transduction induced by these cell surface receptors. Activation of integrin reflects induction of their conformational changes in the ligand binding pocket of the extracellular domain, which is initiated by the stimulation of the integrin cytoplasmic tail. This type of signaling is also termed “inside-out” and stimulates receptor for binding to a ligand (Ye et al., 2011). “Inside-out signaling is very important for activation of αIIbβ3 integrin, which following stimulation of platelets by several agonists, gains conformation to bind fibrinogen. “Outside-in” integrin signaling generates signal transductions upon activation by ligand binding. This bi-directional signaling process is dynamic and requires assembly and disassembly of several types of cytosolic proteins to integrin cytoplasmic tail (Harburger and Calderwood, 2009). The physiological outcome of ligand/integrin interaction includes activation of a variety of cytoplasmic pathways, which may contribute to cell spreading, proliferation, migration and differentiation. Attachment of integrin to its endogenous ligands stabilizes cell environment leading to generation of pro-survival signals. However, in certain conditions integrins may be involved in triggering pro-apoptotic signals (Cheresh and Stupack, 2008). That situation may occur when cells attach to degraded ECM proteins, or detach from ECM.
It is well documented that integrins contribute to the initiation and progression of many pathologies such as cancer, cardiovascular disorders and autoimmune diseases. Anti-integrin drugs are in intensive clinical trials and a few of them have already been approved for medical application. First, the αIIbβ3 integrin antagonists were introduced for therapy of ischemic heart diseases (Hook and Bennett, 2012). Currently, three types of platelet αIIbβ3 integrin inhibitors are on the pharmaceutical market. Abciximab is a humanized monoclonal antibody, Eptifibatide is a cyclic heptapeptide, and Tirofiban is a small molecular weight peptidomimetic. Structures of Eptifibatide and Tirofiban were designed based on the molecules of snake venom disintegrins, barbourin and echistatin, respectively (Scarborough et al, 1993; Egbertson et al., 1994).
2. Snake venom disintegrins
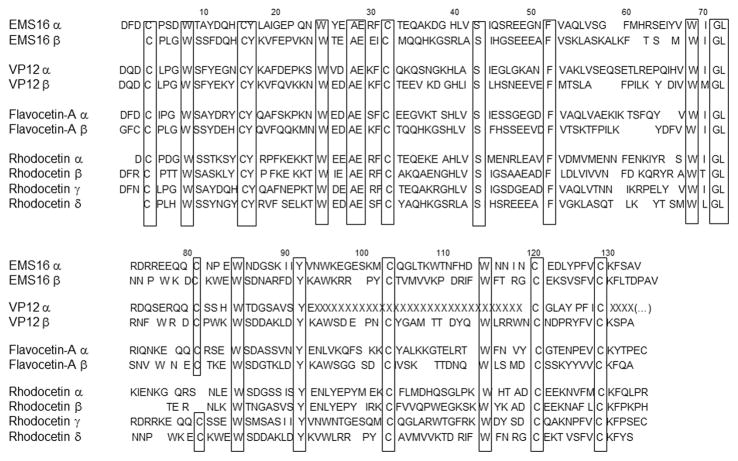
Snake venom disintegrin research began over 25 years ago, when the first report about trigramin was published (Huang et al., 1987). This RGD-containing disintegrin inspired many scientists to search for analogous molecules leading to the discovery of new classes of these proteins (for historical review see: Calvete, 2013). Currently, snake venom disintegrins may be divided into subfamilies according to their structure, as well as a function (Marcinkiewicz, 2005). Structurally, disintegrins are monomers and dimers in homodimeric and heterodimeric forms. Monomeric disintegrins are grouped according to their polypeptide chain length, and the number of cysteines: short disintegrins contain 8 cysteines, medium 12 cysteines and long 14 cysteines. Each subunit of dimeric disintegrins contains 10 cysteines (Fig. 1). Functional classification of snake venom disintegrins was determined by the presence of a tri-peptide motif in the active site. Currently, three functional groups of disintegrins may be distinguished, which express RGD-, MLD- and KTS-sequences (Fig. 1).
Fig. 1.
Amino acid sequences of representative members of disintegrins PII and PIII classes. Functionally active tri-peptidic motifs are in italic and underlined. All sequences were aligned according to the position of active motifs. Cysteines are underlined by a single line.
2.1. Structure of snake venom disintegrins
Disintegrin-related structures in snake venoms in general evolved from precursor molecules containing metalloproteinase domains (Fox and Serrano, 2009; Moura-da-Silva et al., 2007), although separate genes were identified for certain heterodimeric disintegrins (Vija et al., 2009; Okuda et al., 2002). Moreover, analysis of the cDNA library of venom glands of vipers expressing KTS-disintegrins also revealed the absence of metalloproteinase domain (Bazaa et al., 2005). Classical snake venom disintegrins, in a mature form are single domain molecules proteolytically released from PII class of metalloproteinases, whereas disintegrins derived from PIII class are processed together with a cysteine-rich domain. Moreover, PIII disintegrins contain an additional cysteine localized near the sequence, which resembles the active motive of PII disintegrins (Fig. 1).
The structural pattern of disulfide bonds within each class of disintegrins is highly conserved and discloses some evolutionary correlations. Therefore, various disintegrin families appear to have a common ancestor resulting in the homological conformations of their structure (Juarez et al., 2008). The 3-D structure was established for several monomeric disintegrins belonging to the PII class. The molecules of disintegrins were modeled based the NMR coordinates or crystallographic analysis (for review see: McLane et al., 2008). The most important for disintegrin activity is a mobile 14–17Å loop exposed apart from the core of the molecule. This loop, also named as integrin-binding loop, contains an active motif positioned in its central part.
Disintegrins expressing RGD motif bind to RGD-dependent integrins including fibrinogen receptors (αIIbβ3 integrin), vitronectin receptors (αvβ3 integrin) and fibronectin receptor (α5β1 integrin), although selectivity and potency of this binding strongly depends on the amino acid composition surrounding RGD motif. MLD motif has been found exclusively in heterodimeric disintegrins. MLD-disintegrins have been characterized as inhibitors of α4β1, α4β7 and α9β1 integrins, although the presence of another motif in the second subunit such as RGD, determines their activity toward other integrins including α5β1 (Walsh and Marcinkiewicz, 2011). Composition of amino acids surrounding MLD motif also regulates selectively to interact with α4β1, α4β7 or α9β1 integrins. KTS-disintegrins structurally are short monomeric molecules, which potently and selectively bind to the collagen receptor, α1β1 integrin. However, they significantly differ in the organization of integrin-binding loop. KTS loop is two residues shorter than those in RGD or MLD-disintegrins (Fig. 1). Moreover, it does not contain any acidic residues in contrast to other disintegrins, which predominantly express aspartic acid. KTS-disintegrins occur rarely among the viper species. Obtustatin and lebestatin are present in Vipera lebetina obtusa venom (Marcinkiewicz et al., 2003; Olfa et al., 2005), whereas viperistatin is in Vipera palestinae (Kisiel et al., 2004). The fourth member of this family, jerdostatin has lysine substituted to arginine and expresses an RTS motif (Sanz et al., 2005). Substitution of leucine (obtustatin) to arginine (viperistatin) increases inhibitory effect on α1β1 integrin close to two orders of magnitude (Brown et al., 2009). Recent comparison of structure/function relationship of obtustatin and lebestatin revealed, that lebestatin contains a higher flexibility of the C-terminal tail and a greater solvent accessibility of the integrin binding loop than obtustatin (Daidone et al. 2012). These properties reflect higher activity of lebestatin in biological assays.
The disintegrin domain in PIII class proteins is complemented by a cysteine-rich domain. They were isolated as a separated non-enzymatic molecule from only a few venoms. In general, they are a part of larger molecules together with metalloproteinase domain (Ramos and Selistre-de-Araujo, 2006). In comparison to PII class, these disintegrins have a very limited anti-integrin activity. Collagen receptor, α2β1 integrin was reported as a target for alternagin C and the sequence, RSECD, located in the disintegrin-like domain was identified as an active site for this interaction (Souza et al., 2000). More recent studies with leberagin-C showed that it inhibits typical RGD-dependent integrin, αvβ3 (Limam et al., 2010). However, specific integrin-binding motif was not evaluated.
2.2. Interaction of snake venom disintegrins with platelets
Platelets were the first cells investigated in context of snake venom disintegrins. They express αIIbβ3 integrin, which binds fibrinogen through an RGD motif. The majority of discovered snake venom disintegrins contain an RGD or related motif in the active site and they potently block activity of these thrombocytes. The first reported disintegrin, trigramin inhibited ADP-induced platelet aggregation by direct binding to the αIIbβ3 integrin (Huang et al., 1987). Potency of snake venom disintegrins to inhibit platelet aggregation depends on their ability to bind αIIbβ3 integrin. Several studies were performed to map binding epitopes of specific disintegrins on this integrin heterodimer. For example, short monomeric disintegrins echistatin and eristostatin, bind to different but overlapping sites of the fibrinogen receptor (Marcinkiewicz et al., 1996). Interestingly, these two disintegrins do not affect rat platelet aggregation (Basani et al, 2009). This phenomenon is implicated by the structural differences between human and rodent αIIb integrin subunit. The medium monomeric disintegrins, kistrin and elegantin, also bind to different, but closely associated epitopes on αIIbβ3 integrin (Rahman et al., 1995). The presence of a tryptophan residue in the RGD loop appears to be crucial for strength and selectivity of disintegrin to block fibrinogen receptor. Eristostatin, containing RGDW motif binds resting platelets close to a 10 fold stronger than echistatin, which expresses an RGDD motif (McLane et al., 2008). Interestingly, the majority of disintegrins bind activated and non-activated platelets with a similar affinity. This activity of disintegrins may be explained by the generation of changes in the integrin s tertiary and quaternary structure that shifts equilibrium amongst resting, activated and clustered integrins toward a thermodynamically stable oligomeric state (Hantgan et al., 2004).
Natural replacement of RGD sequence with WGD in CC8 makes this heterodimeric disintegrin the strongest blocker of the fibrinogen receptor, although it decreases its selectivity (Calvete et al., 2002). Dimeric disintegrins exhibit another interesting activity: they inhibit the dissociation of platelets aggregates (Okuda and Morita, 2001). Disintegrins also modulate aggregation-dependent tyrosine phosphorylation in platelets cytoplasmic proteins (Table 1). The homodimeric disintegrin, contortrostatin showed stimulatory effect on phosphorylation of Syk (Clark et al., 1994), whereas monomeric disintegrin, saxatilin inhibited collagen-induced activation of this protein in platelets (Jang et al., 2007). In this context, saxatilin also inhibited MAPK Erk1/2 phosphorylation and had no effect on activation of FAK. On the other hand, contortrostatin inhibited thrombin-stimulated phosphorylation of FAK (Clark et al., 1994). Echistatin had opposite effects on the signal transduction in platelets, when used in soluble or solid (immobilized) form. Platelets following adhesion to this disintegrin activated Syk and FAK (Belisario et al, 2000), whereas phosphorylation of these two signaling proteins was blocked by echistatin following adhesion to fibrinogen (Staiano et al, 1997). Similar ability of blocking vs. activation of FAK was observed for other disintegrins used in soluble and solid phase, respectively. Recombinant soluble RGD-disintegrin, DisBa-01 blocked agonist-activated phosphorylation of FAK (Kauskot et al, 2008), while platelet plated on GST–rhodostomin elevated phosphorylation of this protein to an even higher level than after binding to the immobilized fibrinogen (Chang et al., 1999). The phenomenon of opposite effects of disintegrins used in solid or soluble form on platelet signaling may be correlated with inducing different conformational changes within αIIbβ3 integrin. These changes are associated with recruitment of cytoplasmic molecules such as FAK, although reorganization of the cellular membrane also occurs. For example rhodostomin in the solid phase supports platelet spreading and translocation of P-selectin from internal store to the cell membrane (Chang et al., 1999). Accutin activates platelets in soluble form, but only in the presence of complex-dependent monoclonal antibody, AP2 against αIIbβ3 (Huang et al, 2008). In this combinatory treatment monoclonal antibody probably works as a platform to facilitate inducing platelets shape change by disintegrin, and activation of cellular pathways involving FAK, Src, PI3K and Syk. Accutin in the presence of AP2 also induced translocation of P-selectin to the membrane and cytosolic calcium mobilization, which resulted in association of αIIbβ3 integrin with FcγRII receptor (Huang et al, 2008).
Table 1.
Effect of selected snake venom disintegrins on cell signaling in platelets
| Disintegrin | Integrin/binding motif | Regulated cell signaling molecules | References |
|---|---|---|---|
|
| |||
| Echistatin | αIIbβ3/RGD | Activation of FAK and Syk in solid phase; blocking of FAK and Syk activated by fibrinogen | Belisario et al, 2000 |
| Contortrostatin | αIIbβ3/RGD | Activation of Syk; inhibition agonist-induced FAK activation | Clark et al., 1994 |
| Saxatilin | αIIbβ3/RGD | Blocking collagen-induced activation of Syk and MAPK Erk1/2; blocking secretion of PDGF-AB | Jang et al., 2007 |
| DisBa-01 | αIIbβ3/RGD | Blocking agonist-induced activation of FAK | Kauskot et al, 2008 |
| Rhodostomin | αIIbβ3/RGD | Activation of FAK and translocation of P-selectin to the cells membrane in the solid phase | Chang et al., 1999 |
| Accutin | αIIbβ3/RGD | Activation of FAK, Src, PI3K and Syk, translocation of P-selectin and cytosolic calcium mobilization in the presence of AP2 mab | Huang et al, 2008 |
Majority of RGD-heterodimeric disintegrins revealed a low affinity to bind αIIbβ3 integrin including EMF10. However, this disintegrin was very effective to interact with α5β1 integrin modulating megakaryocyte activity. These platelet precursors adhered to fibronectin and EMF10, showing similar spreading and cytoskeleton reorganization (Marcinkiewicz et al., 1999a). Significantly lower interaction with platelets and fibrinogen receptor expressed in CHO cells showed non-RGD expressing heterodimeric disintegrins, such as EC3, VLO5 and EO5 (Bazan-Socha et al., 2004). KTS-disintegrins are the only family of these snake venom proteins, which showed the complete lack of interaction with αIIbβ3 integrin (Kisiel et al., 2004). On the other hand, several disintegrins belonging to the PIII class block collagen-induced platelet aggregation by interacting with α2β1 integrin (Moura-da-Silva et al., 2007).
2.2. Interaction of snake venom disintegrins with leukocytes
Snake venom disintegrins have also been investigated in cells of the immune system. Leukocytes express receptors, which are targeted by disintegrins. Heterodimeric, MLD-disintegrins were identified as the most active blockers of α4β1, α4β7 and α9β1 integrins, which by interaction with endothelial cell-expressed adhesion molecules, facilitate transmigration of leukocytes through the vessel wall (Walsh and Marcinkiewicz, 2011). EC3 was the first disintegrin characterized in this class and it showed inhibitory effect on α4β1 integrin-dependent binding of Jurkat cells and murine splenic lymphocytes to VCAM-1 (Marcinkiewicz et al., 1999b). This disintegrin also blocked transmigration of human neutrophils across an endothelial cell monolayer activated by fMLP (Marcinkiewicz et al., 2000a). VLO5 is structurally similar to the EC3, although it displays increased potency and selectivity toward binding α4β1 and α9β1 integrins (Bazan-Socha et al., 2004). Both MLD-disintegrins showed opposite effects on the modulation of α9β1 integrin-dependent activity and cell signaling in neutrophils. EC3 inhibited activation of MAPK Erk-2 resulting in decreased expression of IL-8 and inducing apoptosis (Coelho et al., 2004). On the contrary, VLO5 inhibited neutrophil apoptosis by up-regulation of the anti-apoptotic proteins Bcl-xL and by increasing the degradation of pro-apoptotic protein Bad (Saldanha-Gama et al., 2010). This discrepancy may be explained by the structural differences between disintegrins, which reflect their selectivity. EC3 showed a significantly higher ability to bind RGD-dependent integrins, especially α5β1 (Bazan-Socha et al., 2004).
Interaction of RGD-monomeric disintegrins with neutrophils appears to be associated with binding to the αMβ2 integrin (Mac-1). Rhodostomin blocked adhesion of these cells to fibrinogen and decreased production of superoxide (Tseng et al., 2004). Jarastatin inhibited Mac-1-dependent neutrophil migration induced in vivo mouse model, as well as human neutrophil chemotaxis stimulated in vitro by IL-8 and fMLP (Coelho et al, 1999). Interestingly, a different cell signaling effect was observed for jarastatin and kistrin than for flavoridin (Coelho et al., 2001). Jarastatin and kistrin supported actin polymerization and FAK phosphorylation, whereas flavoridin was neutral in these processes. Flavoridin inhibited activation of MAPK Erk-2 in contrast to the other two disintegrins, which promoted MAPK Erk2 nuclear translocation. Flavoridin, kistrin and echistatin were also investigated in the human T-cells system (Neto et al., 2007). These disintegrins activated T-lymphocytes by increasing their proliferation, actin cytoskeleton reorganization, and tyrosine phosphorylation. They activated FAK and NF-κB nuclear translocation and c-Fos expression, in PI3K and ERK1/2 activity-dependent manner. Interaction of RGD-disintegrins with T-cells appears to be associated with αvβ3 and α5β1 integrins, although the involvement of other cellular receptors (“cross-talk”) cannot be excluded.
Collagen receptors expressed on the leukocytes have also been investigated as mediators of inflammatory response, and considered as a target for the therapy of autoimmune diseases. Elevated expression of α1β1 and α2β1 integrins was observed on the eosinophils of patients with bronchial asthma. KTS-disintegrins, obtustatin and viperistatin inhibited adhesion of isolated eosinophils to immobilized collagen IV (Bazan-Socha et al., 2006). Transmigration of asthmatic eosinophils was also inhibited by viperistatin, although total peripheral blood mononuclear cells (PBMC) migration was not affected by this disintegrin (Bazan-Socha et al., 2012). The effect of PIII class of disintegrins was examined on another collagen receptor, α2β1 integrin expressed on human neutrophils. The ECD-disintegrin, alternagin-C stimulated activity of these cells by inducing their chemotactic activity in correlation with cytoskeleton rearrangement, and phosphorylation of FAK, PI3K and MAPK Erk-2 (Mariano-Oliveira et al., 2003). Jararhagin-C increased rolling of leukocytes in vivo in a mouse model without interfering with microvasculature haemodynamic. The locally increased level of inflammatory cytokines such as TNF-α, IL-1 and IL-6 confirmed the stimulatory effect of this disintegrin/cysteine-rich containing protein on early inflammatory response (Clissa et al., 2006).
Summary of effect of snake venom disintegrins on leukocytes is presented in Table 2.
Table 2.
Modulation of cellular responses in leukocytes by snake venom disintegrins.
| Disintegrin | Targeted cells | Integrin/binding motif | Type of cellular response | References |
|---|---|---|---|---|
|
| ||||
| Echistatin | T-lymphocytes | αvβ3/RGD α5β1/RGD |
Activation of FAK and PI3K, nuclear translocation of NFκB and c-Fos expression in PI3K and MAPK Erk1/2-dependent manner. | Neto et al., 2007 |
| Kistrin | T-lymphocytes | αvβ3/RGD | Activation of FAK and PI3K, nuclear translocation of NFκB and c-Fos expression in PI3K and MAPK Erk1/2-dependent manner. | Neto et al., 2007 |
| Neutrophils | αMβ2/RGD | Promotion of MAPK Erk2 nuclear translocation, activation of FAK, increasing actin polymerization. | Coelho et al., 2001 | |
| Flavoridin | T-lymphocytes | α5β1/RGD | Activation of FAK and PI3K, nuclear translocation of NFκB and c-Fos expression in PI3K and MAPK Erk1/2-dependent manner. | Neto et al., 2007 |
| Neutrophils | αMβ2/RGD | Blocking activation and nuclear translocation of MAPK Erk2, reduction F-actin content, activation of FAK. | Coelho et al., 2001 | |
| Jarastatin | Neutrophils | αMβ2/RGD | Chemotaxis promotion, induction of nuclear translocation of MAPK Erk2, activation of FAK and PI3K, increasing actin polymerization, increasing expression of IL8. | Coelho et al., 2001 |
| Rhodostomin | Neutrophils | αMβ2/RGD | Blocking adhesion to fibrinogen, decreasing production of superoxide | Tseng et al., 2004 |
| EC3 | T-lymphocytes | α4β1/MLD | Blocking adhesion to VCAM-1 | Marcinkiewicz et al., 1999b |
| Neutrophils | α9β1/MLD | Blocking transmigration through endothelial cells, promotion chemotaxis, activation of FAK and PI3K, blocking activation of MAPK Erk2 and expression of IL8, increasing actin polymerization, apoptosis promotion. | Marcinkiewicz et al., 2000a Coelho et al., 2004 | |
| VLO5 | T-lymphocytes | α4β1/MLD | Blocking adhesion to VCAM-1 | Bazan-Socha et al., 2004 |
| Neutrophils | α9β1/MLD | Activation FAK, PI3K, MAPK Erk2, AKT, induction of nuclear translocation of NFκB, blocking spontaneous apoptosis by up-regulation of Bcl-xL and degradation of Bad | Saldanha-Gama et al., 2010 | |
| Obtustatin/Viperistatin | Eosinophils | α1β1/KTS | Blocking adhesion to collagen IV | Bazan-Socha et al., 2006 |
| Viperistatin | Eosinophils | α1β1/KTS | Blocking migration through endothelial cells | Bazan-Socha et al., 2012 |
| Alternagin-C | Neutrophils | α2β1/ECD | Chemotaxis promotion, increasing actin cytoskeleton polymerization, activation of FAK, PI3K, MAPK Erk2, | Mariano-Oliveira et al., 2003 |
2.3. Interaction of snake venom disintegrins with endothelial cells
Snake venom disintegrin research has been extensively performed with endothelial cells. These major vessel wall cells are exposed to the blood stream and are on the first line of attack of snake venom components during envenomation. Therefore, researchers screened a variety of snake venom species to identify agents that interact with these cells. This strategy allowed separating compounds that regulated angiogenesis, a process of new vessel formation from pre-existing vasculature. Modulation of angiogenesis is very important in the therapy of many diseases. Cancer pathological angiogenesis is targeted to block vascularization of malignant tissue, whereas in cardiovascular disorders it is stimulated to promote vascularization of ischemic tissues. Inhibition or stimulation of endothelial cells to proliferate or migrate is the central point to develop new angiogenesis therapies. Endothelial cells express variety of integrins, which are affected by snake venom disintegrins, including RGD-dependent αvβ3, αvβ5 and α5β1, as well as α4β1 and α9β1, and collagen receptors, α1β1 and α2β1 (Table 3).
Table 3.
Effect of selected snake venom disintegrins on regulation of endothelial cell responses
| Disintegrin | Endothelial cell type | Integrin/binding motif | Regulated cellular activities | References |
|---|---|---|---|---|
|
| ||||
| Echistatin | human umbilical vein endothelial cells (HUVEC) | αvβ3/RGD α5β1/RGD |
Supporting adhesion but not spreading in immobilized form, blocking adhesion to vitronectin and fibronectin, blocking vitronectin-induced FAK and paxillin phosphorylation. |
Juliano et al., 1996 Minamiguchi et al., 2001 |
| Accutin | HUVEC | αvβ3/RGD | Blocking adhesion to fibrinogen, fibronectin and vitronectin, blocking tube formation in Matrigel, induction of apoptosis | Yeh et al., 1998 |
| Rhodostomin | HUVEC | αvβ3/RGD | Inducing cell detachment, blocking activation of FAK and actin cytoskeleton organization, degradation of β-catenin and poly(ADP-ribose) polymerase, induction caspase 3-dependent apoptosis. | Wu et al., 2003 |
| Salmosin | bovine capillary endothelial (BCE) cells | αvβ3/RGD | Blocking bFGF-induced proliferation, disassembling focal adhesion elements, blocking FAK activation, decrease expression of paxillin and p130CAS, induction caspase 3-dependent apoptosis. | Hong et al., 2003 |
| Contortrostatin | HUVEC | αvβ3/RGD | Supporting adhesion and spreading in immobilized form without apoptosis, detaching from vitronectin and inducing apoptosis, blocking migration through Matrigel barrier, blocking tube formation in Matrigel, inhibiting FAK and paxillin phosphorylation, disrupting actin cytoskeleton organization and VE-cadherin distribution in cell-cell contact. | Zhou et al., 1999 Golubkov et al., 2003 Swenson et al., 2007 |
| VLO5 | dermal human microvascular endothelial cells (dHMVEC) | α9β1/MLD | Blocking FBS-induced proliferation and heptotaxis. | Staniszewska et al., 2007 |
| glioma human microvascular endothelial cells (gHMVEC) | α9β1/MLD | Blocking adhesion to NGF, blocking NGF-induced proliferation and migration, blocking tube formation in Matrigel | Walsh et al., 2012 | |
| Obtustatin | dHMVEC | α1β1/KTS | Blocking FBS- and VEGF-induced proliferation, induction caspase 8/3-dependent apoptosis | Brown et al., 2008a |
| Lebestatin | dHMVEC (cell line) | α1β1/KTS | Partial blocking adhesion and heptotaxis to collagen IV. | Olfa et al., 2005 |
| Alternagin-C | HUVEC | α2β1/ECD | Modulation of proliferation and expression of VEGFR1 and VEGFR2 (low doses stimulate, high inhibit). | Ramos et al., 2007 |
The majority of disintegrin research has been directed to search for an inhibitory effect on the pathological vessel growth in solid cancers. Several RGD-disintegrins were investigated as endothelial cell blockers in angiogenesis assays in vitro and in vivo. Two endothelial cells-expressed RGD-dependent integrin, αvβ3 and α5β1 were in the focus of disintegrin research. Initial screening performed on the human umbilical cord endothelial cells (HUVEC) showed that monomeric disintegrins kistrin, flavoridin, echistatin and mambin bind αvβ3 integrin, whereas only echistatin and flavoridin interacted with α5β1 integrin (Juliano et al., 1996). More complex examinations with HUVEC were performed using accutin and rhodostomin (Yeh et al., 1998; Huang et al., 2001). These RGD-disintegrins inhibited angiogenesis in vitro in the Matrigel tube formation assay, and in vivo in the chorioallantoic membrane (CAM) chicken assay. Rhodostomin induced apoptosis in the caspase-3-dependent manner and generated the degradation of β-catenin and poly(ADP-ribose) polymerase. Moreover, FAK phosphorylation and actin cytoskeleton were affected upon rhodostomin treatment (Wu et al., 2003). Monomeric RGD-disintegrin, salmosin inhibited proliferation of bovine microvascular endothelial cells by the blocking of αvβ3 integrin-dependent activation of FAK and decreasing expression of paxillin and p130CAS. This disintegrin also blocked angiogenesis in vitro and in vivo, including those induced by the cancer cells (Hong et al., 2003). Recent studies revealed that recombinant disintegrin, DisBa-01 suppressed releasing of VEGF by endothelial cells and decreased expression of receptors of this growth factor (Montenegro et al, 2012).
Contortrostatin, a homodimeric RGD-disintegrin was intensively investigated in angiogenesis. It targets αvβ3 on endothelial cells, although in contrast to the monomeric disintegrins it does not induce direct apoptosis. HUVEC apoptosis was detected only in cells detached from vitronectin by contortrostatin, but the viability of cells attached to fibronectin or Matrigel was not altered by this disintegrin (Swenson et al., 2007). Mechanistic studies revealed that contortrostatin blocks HUVEC migration, proliferation and tube formation in Matrigel, as well as angiogenesis in vivo in chicken CAM model and various mouse cancer models. Contortrostatin decreased the level of phosphorylation of focal adhesion elements such as FAK and paxillin. It resulted in disruption of actin cytoskeleton and altered VE-cadherin distribution in cell-cell contact (Golubkov et al., 2003).
MLD-disintegrin, VLO5 also showed cross-reactivity with endothelial cells. This heterodimeric disintegrin binds to α9β1 integrin, which is expressed on activated endothelium during intense angiogenesis. It blocked proliferation and migration of dermal human microvascular endothelial cells (dHMVEC) (Staniszewska et al., 2007). Interestingly, VLO5 blocked also FBS-induced proliferation of brain human microvascular endothelial cells (bHMVEC), which do not express α9β1 integrin, suggesting its binding to other receptor(s). Possibly, the effect of VLO5 may occur through other integrin, α4β1, although we cannot exclude non-integrin receptor, which is involved in this process. VLO5 effectively blocked angiogenesis in vivo in the quail CAM embryonic system and Matrigel plug mouse assay. Recent studies revealed that MLD-disintegrins, VLO5 and bitisgabonin-2 block pathological angiogenesis through their interaction with glioma human microvascular endothelial cells (gHMVEC). These disintegrins effectively inhibited the binding of pro-angiogenic nerve growth factor (NGF) to α9β1 integrin in cell adhesion, migration, proliferation, and Matrigel tube formation assays of gHMVEC. Moreover, they also blocked NGF-induced and glioma-induced angiogenesis in vivo in the quail CAM embryonic system (Walsh et al., 2012).
Anti-angiogenic effect was also observed for KTS-disintegrins. Obtustatin potently inhibited angiogenesis in vivo in various systems including CAM quail and chicken and mouse Matrigel plug assays, as well as angiogenesis-dependent experimental melanoma and Levis lung carcinoma tumor growth (Marcinkiewicz et al., 2003; Brown et al., 2008a). Mechanistic studies revealed a direct binding of obtustatin to the endothelial cells and induction of apoptosis in a caspase 8/3-dependent manner (extrinsic pathway). Lebestatin, which is closely similar in structure to obtustatin, also inhibited angiogenesis by binding to dermal human microvascular endothelial cell line through α1β1 integrin (Olfa et al., 2005).
Alternagin-C is a PIII class of disintegrins, which modulated angiogenic activity of endothelial cells by binding to α2β1 integrin. Interestingly, a low concentration of this disintegrin stimulated angiogenesis process by elevating the expression of VEGFR2 on endothelial cells. However, in higher concentrations, alternagin-C inhibited angiogenesis and decreased level of VEGFR2 and VEGFR1 on endothelial cells (Ramos et al., 2007). This activity is analogous to recently published results showing the blocking of αvβ3 and αvβ5 integrins on the endothelial cells by short, synthetic RGD-peptides (Reynold et al., 2009). The proposed explanation of this phenomenon includes possibility of recycling of receptors by low doses of integrin inhibitors. However, for molecules containing higher molecular weight such as disintegrins, releasing of metabolic (proteolytic) fragments should also be considered. These fragments may have an opposite activity stimulating pro-angiogenic cellular responses. Higher concentration of disintegrin could not be proteolitycally processed and blocks integrin-dependent angiogenesis. These observations decrease enthusiasm for safety and efficiency of anti-integrin therapy, especially for the cancer related complications.
2.4. Interaction of snake venom disintegrins with cancer cells
The effect of RGD-disintegrins on various cancer cells has been broadly investigated in vitro and in vivo. Cancer metastasis was studied in context of tumoral cell adhesion to the extracellular matrix, as well as cell migration and proliferation. The first published report showed that albolabrin, a medium size monomeric RGD-disintegrin, inhibited adhesion of mouse B16F10 melanoma cells to fibronectin and laminin, as well as blocked hematogenous metastasis of these cells in mouse model (Soszka et al., 1991). The adhesive properties of the various cancer cells to the RGD-containing extracellular matrix proteins were blocked by several other monomeric disintegrins such as triflavin (Sheu et al., 1996), ussuristatin 1 (Oshikawa and Terada, 1999), rhodostomin (Yang et al., 2005), colombistatin (Sanches at al., 2009), and viridistatin 2 (Lucena et al., 2012). Monomeric RGD-disintegrins also blocked cancer cell proliferation and migration, and several of them were tested in vivo in B16F10 melanoma hematogenous metastasis and tumor growth (Kang et al., 2000; Zhou et al., 2004; Oliva et al., 2007; Lucena et al., 2011). Eristostatin is a short monomeric RGD-disintegrin, which was also recognized as an inhibitor of melanoma metastasis (Morris et al., 1995). However, this disintegrin has no effect on αvβ3 integrin, which was targeted in this process by other RGD-disintegrins. The mechanism of action of eristostatin in lung melanoma colonization is not fully understood. One study suggests that α4β1 integrin, expressed on these cancer cells, is inhibited by eristostatin (Danen et al., 1998). Another possibility may be related to involvement of fibronectin-binding integrins in metastasis process (Tian et al, 2007). Recently published results revealed that eristostatin after binding to the surface of melanoma cells exposes them to the lysis by natural killer cells (Hailey et al., 2013).
A panel of studies with different tumor types was performed for contortrostatin. This homodimeric RGD-disintegrin inhibited growth and metastasis of MDA-MB-435 cell-induced orthotropic xenograft in nude mouse model (Zhou et al., 2000), ovarian cancer dissemination in the nude mouse model (Markland et al., 2001), as well as glioma growth implanted intracranially to mouse brain (Pyrko et al., 2005). Combination of contortrostatin with the chemotherapeutic drug docetaxel completely inhibited growth of PC-3 prostate cancer cells in vitro and in vivo in an additive fashion (Lin et al., 2010). Contortrostatin mainly targeted αvβ3 and αvβ5 integrins expressed on the various cancer cells, although another RGD-dependent integrin α5β1 was also affected, especially in glioma cell lines (Schmitmeier et al., 2003). The mechanistic studies revealed that this disintegrin strongly blocks migration and invasiveness of cancer cells, although does not induce apoptosis following binding to the cells. Interestingly, contortrostatin mimics the activity of fibronectin in intracellular signaling pathways of glioma cells (Schmitmeier et al., 2005). It activates FAK phosphorylation, which recruits Src for further phosphorylation of paxillin and p130Cas. However, when attached to fibronectin and cells were treated with soluble contortrostatin, FAK was down-regulated leading to disruption of the cytoskeleton and cellular detachment.
Investigation of non-RGD containing disintegrins, in context of their interaction with cancer cells, is in the early stage. VLO5 was found as an inhibitor of glioma growth by antagonizing α9β1 integrin. This MLD-heterodimeric disintegrin blocked α9β1 integrin-dependent glioma development (Brown et al., 2008b). VLO5 blocked proliferation and migration of glioma cell line, LN229, which endogenously expresses α9β1 integrin. This disintegrin induced apoptosis in cancer cells, which is a major mechanistic difference when compared with the anti-glioma activity of the RGD-homodimeric disintegrin, contortrostatin. Interestingly, the anti-tumoral and pro-apoptotic effect of this disintegrin was significantly diminished by NGF. This growth factor was described earlier as a ligand for α9β1 integrin, which displays pro-survival and pro-proliferative activities (Staniszewska et al., 2008). Another brain tumor type, medulloblastoma was also inhibited by VLO5 (Fiorilli et al., 2008). VLO5 was a potent inhibitor of α9β1 integrin-dependent adhesion of these cells to specific extracellular matrix proteins, whereas echistatin had very little effect in this assay. VLO5 appeared to be an excellent tool as a blocker of α9β1 integrin to investigate the importance of this integrin in tumor growth (Gupta et al., 2013). Small cell lung cancer increased a functionally relevant epithelial-mesenchymal transition (EMT) phenotype when α9β1 integrin was expressed. α9β1-associated EMT increased cancer cells pro-adhesive, pro-migratory and pro-invasive activities, which were blocked by VLO5.
The interaction of KTS-disintegrins with tumoral cells was investigated in the melanoma system. Viperistatin blocked α1β1 integrin-dependent adhesion of human melanoma cell line, HS.939T to collagen type IV, and the transmigration of this cell line through dermal human microvascular endothelial cells (Staniszewska et al., 2009). Partial inhibitory effect of this disintegrin was observed for B16F10 cells colonization in the lung during hematogenous metastasis in a mouse model. Simultaneous blocking of another collagen receptor, α2β1 integrin, by CLP, VP12 enhanced the anti-adhesive property of viperistatin on melanoma cells in vitro, whereas in metastasis in vivo was not altered. Interestingly, obtustatin injected alone was effective to completely block B16F10 hematogenous metastasis in a mouse model (author s personal communication). This discrepancy between activity of obtustatin and viperistatin in vivo is confusing in light of close to two orders of magnitude higher inhibitory effect of viperistatin in vitro on α1β1 integrin binding to collagen IV (Kisiel et al., 2004). The explanation may be correlated with a small difference in amino acid sequences of both disintegrins presents in the C-terminal tail (Fig. 1). In that part, obtustatin contains PLYP sequence, whereas viperistatin PVYQ, respectively. PXXP motif is characteristic for SH3 domain binding proteins, and this domain is expressed by a variety of cytoplasmic cell signaling molecules regulating proliferation, survival and apoptosis processes. Therefore, direct effect of obtustatin on these cytoplasmic molecules may be crucial for blocking cellular responses, whereas the effect of viperistatin is only resembled with protection against integrin/ligand interaction. The validation of this hypothesis requires confirmation that disintegrins are internalized. Our unpublished data suggests that soluble snake venom disintegrins following binding to the integrins are internalized in the endosome-like fashion. This type of internalization is not observed when disintegrins are present in the solid phase (immobilized). Endocytic migration may be helpful to explain the opposite cell signaling induced by the same disintegrin used in soluble or immobilized forms (Tables 1–4). Direct interaction of disintegrins with cytoplasmic cell molecules may interfere with integrin-generated signaling, leading to arresting cell proliferation, survival and induction of apoptosis. In the immobilized form disintegrins mimic ECM proteins and through binding to integrins produce similar to this natural ligands cell signaling.
Table 4.
Effect of selected snake venom disintegrins on the activities of various cancer cell lines
| Disintegrin | Cancer cell type | Integrin/binding motif | Cellular response following treatment | References |
|---|---|---|---|---|
|
| ||||
| Echistatin | human osteosarcoma LM7 line | αvβ3/RGD | Blocking adhesion to vitronectin, blocking chemotaxis, | Duan et al., 2004 |
| human colon adenocarcinoma DLD-1 line | αvβ3/RGD | Blocking of FAK activation, no effect on MAPK Erk1/2 activation, no effect on prolidase, HIF-1α and β1 integrin subunit expression | Karna et al, 2012 | |
| Human lung carcinoma H1299, A549 lines | αvβ3/RGD | Blocking pituitary tumor transforming gene-induced activation of FAK | Shah et al, 2012 | |
| Eristostatin | mouse melanoma B16F10 line | ????/RGD | Blocking lung colonization (hematogenous metastasis) in mouse, no effect on proliferation. | Morris et al, 1995 |
| human melanoma MV3 cell line | α4β1/RGD | Supporting adhesion in immobilized form, blocking adhesion to VCAM-1 in soluble form, blocking lung colonization (hematogenous metastasis) in nude mouse | Danen et al, 1998 | |
| various human melanoma cell lines | ????/RGD | Blocking fibronectin-dependent migration, no effect on proliferation, direct binding to cell surface, exposing for lysing by natural killer cells |
Tian et al, 2007 Hailey et al., 2013 |
|
| Rhodostomin | human breast carcinoma MDA-MB-231, MCF-7 lines, human prostate carcinoma PC-3 line | αvβ3/RGD | Blocking adhesion to bone matrixes, blocking chemotaxis and heptotaxis, no effect on proliferation and apoptosis | Yang et al., 2005 |
| Colombistatin | human urinary carcinoma T24 line, human melanoma SK-Mel-28 line | αvβ3/RGD α5β1/RGD |
Blocking adhesion to fibronectin, blocking migration | Sanches at al., 2009 |
| Viridistatin 2 | various human and mouse carinomas | αvβ3/RGD α5β1/RGD |
Blocking adhesion to various ECM proteins, blocking migration, blocking hematogenous metastasis of B16F10 cells in mice | Lucena et al., 2012 |
| Jarastatin | mouse melanoma B16F10 line | α5β1/RGD | Blocking hematogenous metastasis in mice, low inhibiting proliferation, inducing actin cytoskeleton rearrangement and FAK phosphorylation, blocking NFκB nuclear translocation, no effect on MAPK Erk2. | Oliva et al., 2007 |
| Contortrostatin | human glioma U87 line | αvβ3/RGD α5β1/RGD |
Blocking intracranial tumor development in nude mice | Pyrko et al., 2005 |
| human melanoma M21 line | α5β1/RGD | Blocking tumor growth in nude mice in combination with chemotherapeutic drug araC | Schwartz et al, 2008 | |
| prostate carcinoma PC-3 line | αvβ5/RGD α5β1/RGD |
Blocking tumor growth in mice in combination with chemotherapeutic drug docetaxel, blocking migration and proliferation | Lin et al., 2010 | |
| human glioma A172, U87 lines | αvβ3/RGD α5β1/RGD |
Activating FAK, Src, p130Cas and paxillin in immobilized form and blocking in soluble form, no effect on proliferation | Schmitmeier et al., 2005 | |
| VLO5 | human glioma LN229 line | α9β1/MLD | Blocking cell proliferation and migration, inducing caspase 9/3-dependent apoptosis, inhibiting tumor development in quail embryonic CAM assay. | Brown et al., 2008b |
| human medulloblastoma D283 line | α9β1/MLD | Blocking of adhesion to cell-produced matrix and to Tenascin-C | Fiorilli et al., 2008 | |
| various human lung cancer lines | α9β1/MLD | Blocking EMT-dependent proliferation, migration and invasion | Gupta et al., 2013 | |
| Viperistatin | human melanoma MV3, HS.939T lines | α1β1/KTS | Inhibiting collagen I and IV-dependent adhesion, blocking transmigration through dHMVC layer, inhibiting hematogenous metastasis of B16F10 cells in mice | Staniszewska et al., 2009 |
3. C-type lectin proteins interacting with integrins
C-type lectin proteins (abbreviated: CLPs, CTLs or Snaclecs) belong to another family of snake venom proteins, which have also been characterized as integrin-binding molecules. They are broadly spread among different species of vipers, having diverse effects on the circulatory system of the victim. Major attention has been committed to platelets as a potential target for CLPs, although some proteins in the blood coagulation system such as factors IX/X or von Willebrand factor were also affected by these snake venom compounds. The binding of CLPs to certain receptors expressed on the platelet surface may result in an agonistic or antagonistic effect on thrombosis (Clemetson, 2010). Among them is only one integrin, α2β1, which was characterized as a receptor for CLPs. Endothelial cells and certain types of cancer cells have also been investigated in interaction with CLPs.
3.1. Structure of snake venom CLPs
Structurally, snake venom CLPs are similar (15–40% homology) to the carbohydrate recognition domains of C-type lectins. However, snake venom CLPs do not encompass the functional binding of carbohydrates, because their structure is absent of the classic calcium/sugar binding loop. CLPs are composed from two subunits α and β, which in the majority of proteins create a heterodimeric structure connected by cysteine-dependent disulfide bounds (Fig. 2). However, some of these heterodimers have been found in higher oligomeric forms linked by an extra S-S bridge between cysteines located on the C-terminal and N-terminal parts of α and β subunits, respectively. This oligomerization occurs in the cyclic fashion by the connection of each heterodimer through a “head-to-tail” arrangement for tetrameric (αβ)4 or dimeric dimeric (αβ)2 organization (Morita, 2005). There are some initial studies suggesting that cyclic trimers of heterodimeric associates (αβ)3 may also be present among CLPs. Mass spectroscopy analysis indicated that this type of CLP molecule may occur in Bitis nasicornis venom (Calvete et al., 2007b). Our recent studies revealed that sochicetin-A, a newly isolated CLP may also be oligomerized in the (αβ)3 fashion (Jakubowski et al., 2013). However, crystallography studies are required to confirm the presence of these types of CLP oligomers. The X-ray crystallography studies were performed for several single homodimers including EMS16, a CLP interacting with α2β1 integrin (Hori et al., 2003). The model of EMS16 showed that this heterodimer is involved in domain swapping of the central loop as observed in the structures of other CLPs. Uniquely, EMS16 has a positively charged electrostatic potential patch on a concave surface. The crystallographic analysis of the complex with A-domain of α2 subunit of α2β1 integrin revealed that EMS16 spatially blocks collagen-binding and possibly stabilizes the closed conformation of the α2A-domain (Horii et al., 2004). In contrast to snake venom disintegrins, there are no reports showing a specific amino acid motif that is responsible for binding CLP to a receptor. This suggests that specific conformation of both α and β subunits, which must be associated in hetrodimeric structure, are required for receptor recognition. Reduction and separation of CLP subunits resulted in complete loss of activity to bind α2β1 integrin (author s personal communication). Therefore, interaction of CLP with receptor is rather dependent on spatially formulated domain, which may involve amino acids localized in different areas of subunits polypeptide chains, in contrast to simple tri-peptide motif binding, which is localized on disintegrins integrin-binding loop. Comparison of amino acids present in subunits of CLPs showed conserved alignment of cysteines and other residues such as tryptophan (Fig. 2). Therefore, it is difficult to evaluate specific areas involved in the integrin binding, because of a high diversity of these proteins to interact with various receptors. Moreover, recent work with flavocetin-A revealed that selectivity of CLP could be less strict than previously assumed (Arlinghaus and Eble, 2013). This CLP was initially characterized as an inhibitor of binding of von Willebrand to platelet GPIb receptor, and it also blocks α2β1 integrin binding to collagen I. Further studies including mutation in recombinant molecules or competition with synthetic peptides are required for mapping biologically active domains within CLP molecules.
Fig. 2.
Comparison of amino acid sequences of the C-type lectin proteins interacting with α2β1 integrin. The conservative amino acid were aligned and framed. X, unidentified yet amino acids in VP12 α subunit.
3.2. Functional characteristic of CLPs as integrin antagonists
The functional interaction of CLPs with platelets occurs through non-integrin related receptors, GPIb and GPIV, as well as by collagen receptor, α2β1 integrin. CLPs, which selectively interact with α2β1 including EMS16, have been characterized as inhibitors of platelet aggregation induced by collagen but not by other agonists such as ADP, thromboxane analog (U46619), or TRAP (Marcinkiewicz et al., 2000b). Interestingly, platelet aggregation induced by another CLP convulxin, which inhibits another collagen receptor, GPVI, was not affected by EMS16. Significant work has been performed with rhodocetin, which was initially recognized as a heterodimeric CLP blocking collagen-induced platelet aggregation (Wang et al., 1999). Recently published results revealed that it forms a heterotetramer composed from four subunits α, β, γ and δ. (Eble et al., 2009). This tetramer binds α2β1 integrin and after this binding, αβ subunits are released from the complex. Dissociated αβ subunits have no binding ability to integrins (Bracht et al., 2011). Other α2β1 integrin-binding CLP, VP12 was purified from Vipera palestinae venom (Staniszewska et al., 2009). Although the activity of VP12 was not investigated in collagen-induced platelet aggregation, another CLP purified from the same venom, VP-i showed inhibitory activity in this assay (Arlinghaus et al., 2013). However, structural correlations between VP12 and VP-i have not yet been established. Potent inhibitory effect on collagen-induced platelet aggregation was observed for sochicetin-A, a CLP isolated from Echis sochureki venom, which is most likely oligomerized in a trimeric heterodimer structure (Jakubowski et al., 2013). In the same venom, two α2β1 integrin-interacting CLPs were identified. These molecules, sochicetin-B and sochicetin-C are classical heterodimers, but significantly differ in their affinity to bind α2A-domain of integrin. Sochicetin-A and sochicetin-B appeared to be very potent antagonists of α2β1 integrin and their activities were comparable in ELISA and cell adhesion assays. On the other hand, sochicetin-C was a very weak inhibitor of α2β1 integrin.
Collagen receptors, including α2β1 integrin are highly expressed on endothelial cells. Initial experiments with EMS16 revealed that this CLP potently inhibited radial migration of HUVEC in a 2-D collagen matrix (Marcinkiewicz et al., 2000b). Recent VP12 (Vixapatin) studies showed its α2β1 integrin-dependent cross-reactivity with dermal human endothelial cells (Momic et al., 2012). It antagonized these cells by blocking their proliferation and Matrigel tube formation. Moreover, VP12 blocked angiogenesis in vivo in the embryonic quail system when this process was induced by exogenously added bFGF or pathologically, by implantation of C6 glioma cells. Lebectin is another CLP, which was characterized as a blocker of pro-angiogenic activities of endothelial cells (Pilorget et al., 2007). It inhibited proliferation, migration and adhesion to fibronectin of the brain human microvascular endothelial cell line. Blocking of angiogenesis in vitro in the Matrigel tube formation assay, as well as in vivo in the chicken CAM and mouse Matrigel plug assays was also observed for this CLP. Interestingly, the anti-angiogenic effect appears to be associated with blocking of α5β1 and αv integrins, although direct studies of this CLP with purified integrins were not published. Lebectin seems to be a multifunctional ligand, because inhibition of GPIb-dependent platelet agglutination was also reported (Sarray et al., 2003).
Integrin-dependent interaction of CLPs was also reported for several cancer cells. VP12 blocked collagen-dependent adhesion and migration of human melanoma cell lines in vitro (Staniszewska et al., 2009). This effect was potentiated by the presence of α1β1 integrin inhibitor viperistatin, suggesting the importance of both integrins in the development of this tumor. However, any additive or synergistic effect was not observed on melanoma hematogenous metastasis in a mouse model, although both inhibitors of collagen receptors when used separately effectively blocked lung colonization of B16F10 cells to about 50%. Rhodocetin showed an inhibitory effect on experimental liver tumor growth and metastasis (Rosenow et al., 2008). This data indicated that collagen receptor, α2β1 integrin is important in the extravasation into the liver stroma and micrometastasis of hepatocellular carcinoma, HepG2 and the colorectal carcinoma, HT29LMM cells, and may be an attractive target for the development of new therapy. Our unpublished studies revealed that glioma cells express α2β1 integrin in vitro and in vivo. Adhesion of two glioma cell lines, LN18 and LBC3 to collagen I was inhibited by sochicetin-A, although this effect was not completed due to the presence of other collagen receptors, such as α1β1 integrin (Jakubowski et al., 2013). Glioma cells adhered to sochicetin-A, but their spreading was very poor if compared with spreading on collagen I. This indicates that CLPs induces different cell signaling pathways than collagen, although both of these ligands interact with the same integrin.
4. Conclusions and perspectives
Snake venom inhibitors of integrins appear to be very useful tools in pharmacology in the development of new pharmaceuticals, and in cell biology to understand the mechanisms, which regulate cell physiology. A summary of their specific interaction with integrins expressed on various cell types is illustrated in Fig. 3. Disintegrins and C-type lectin proteins have receptors on practically all types of blood cells, as well as on endothelial cells and cancer cells. Although, we currently classified only two families of snake venom proteins as integrin-binding molecules, further studies of integrins and snake venom components should extend the list of families of proteins interacting with these cell surface receptors. For example, nerve growth factor (NGF) and vascular endothelial growth factor (VEGF) are direct ligands for α9β1 integrin (Staniszewska et al., 2008; Vlahakis et al., 2007). Structurally related molecules for mammalian NGF and VEGF were localized in a variety of snake venoms (Yamazaki and Morita, 2006; Trummal et al., 2011). These snake venom growth factors interact with mammalian receptor tyrosine kinases (RTK), and they should also bind integrins. A similar situation occurs for secretory phospholipases A2 (PLA2). Mammalian PLA2 was identified as a ligand for αvβ3 and α4β1 integrins and receptor-binding sites have already been localized (Saegusa et al., 2008). Recent studies revealed that PLA2 from Cerastes cerastes and Macrovipera lebetina transmediterranea snake venoms also inhibit αvβ3 and α5β1 integrins, although binding sites for these molecules remain unknown (Bazaa et al., 2009; Kessentini-Zouari et al., 2010).
Fig. 3.
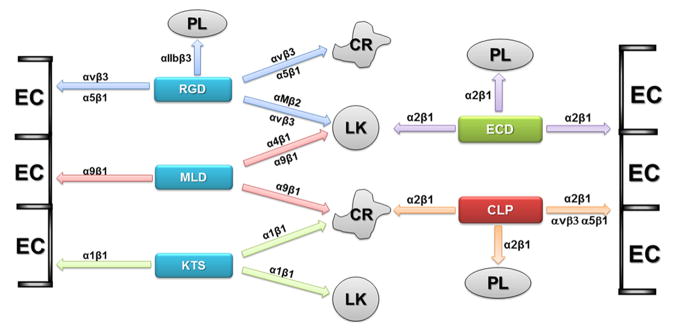
Summary of interaction of snake venom proteins with integrins expressed on the specific cells. Functionally classified snake venom disintegrins of PII class are presented in the blue boxes. PIII class of disintegrin is in the green box. C-type lectin protein family is in the red box. Cell types are abbreviated: PL – platelets; LK – leukocytes; EC – endothelial cells; CR – cancer cells.
In this review, we focused on cell types presented in Fig. 3. However, research on snake venom molecules may be extended to other types of mammalian cells. Effect of RGD-disintegrin, echistatin was investigated in fibroblast-like cells, showing inhibitory effect on TGFβ- and IGF-1 stimulated activation of MAPK and Src family members (Sekimoto et al., 2005; Surazynski et al., 2005; Pechkovsky et al., 2008). Moreover, echistatin as the most investigated disintegrin was tested in other cell types such as osteoblasts (Long et al, 2011), chondrocytes (Belisario et al., 2005), neuronal progenitors (Harper et al., 2010) and smooth muscle cells (Davenpeck et al., 2001). We have already initiated studies on neuronal cells, which express α1β1 integrin and are sensitive for obtustatin treatment (Wang et al., 2007). A completely unexplored area is stem cell research. Stem cells express a variety of integrins including those, which were identified as targets for snake venom proteins. Snake venom integrin ligands may be used not only as a pattern for developing new therapeutics to block cancer stem cell invasion and differentiation, but may be investigated as potential components of biomaterials to support attachment and differentiation of stem cells in regenerative medicine. Therefore, research on the snake venom molecules, which interact with integrins, appears to have perspective in the application in medicine and basic cell biology.
Acknowledgments
This work was supported in part by NIH NCI grant R01CA100145 and NIH NCI grant R01CA133262. Special thanks Rachel Chiaverelli for reading and help in preparation of the manuscript. Author apologizes to the many other important investigators, who published articles in the field and were not citied due to space limitations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arlinghaus FT, Momic T, Ammar NA, Shai E, Spectre G, Varon D, et al. Identification of α2β1integrin inhibitor VP-i with anti-platelet properties in the venom of Vipera palaestinae. Toxicon. 2013;64:96–105. doi: 10.1016/j.toxicon.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Arlinghaus FT, Eble JA. The collagen-binding integrin α2β1 is a novel interaction partner of the Trimeresurus flavoviridis venom protein flavocetin-A. Journal of Biological Chemistry. 2013;288:947–55. doi: 10.1074/jbc.M112.399618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basani RB, Zhu H, Thornton MA, Soto CS, Degrado WF, Kowalska MA, et al. Species differences in small molecule binding to alpha IIb beta 3 are the result of sequence differences in 2 loops of the alpha IIb beta propeller. Blood. 2009;113:902–10. doi: 10.1182/blood-2008-09-177337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazaa A, Marrakchi N, El Ayeb M, Sanz L, Calvete JJ. Snake venomics: comparative analysis of the venom proteomes of the Tunisian snakes Cerastes cerastes, Cerastes vipera and Macrovipera lebetina. Proteomics. 2005;5:4223–35. doi: 10.1002/pmic.200402024. [DOI] [PubMed] [Google Scholar]
- Bazaa A, Luis J, Srairi-Abid N, Kallech-Ziri O, Kessentini-Zouari R, Defilles C, et al. MVL-PLA2, a phospholipase A2 from Macrovipera lebetina transmediterranea venom, inhibits tumor cells adhesion and migration. Matrix Biology. 2009;28:188–93. doi: 10.1016/j.matbio.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Bazan-Socha S, Kisiel DG, Young B, Theakston RDG, Calvete JJ, Sheppard D, et al. Structural requirements of MLD-containing disintegrins for functional interaction with α4β1 and α9β1 integrins. Biochemistry. 2004;43:1639–1647. doi: 10.1021/bi035853t. [DOI] [PubMed] [Google Scholar]
- Bazan-Socha S, Bukiej A, Pulka G, Marcinkiewicz C, Musial J. Increased expression of collagen receptors: alpha1beta1 and alpha2beta1 integrins on blood eosinophils in bronchial asthma. Clinical and Experimental Allergy. 2006;36:1184–91. doi: 10.1111/j.1365-2222.2006.02540.x. [DOI] [PubMed] [Google Scholar]
- Bazan-Socha S, Zuk J, Plutecka H, Marcinkiewicz C, Zareba L, Musial J. Collagen receptors α(1)β(1) and α(2)β(1) integrins are involved in transmigration of peripheral blood eosinophils, but not mononuclear cells through human microvascular endothelial cells monolayer. Journal of Physiology and Pharmacology. 2012;63:373–9. [PubMed] [Google Scholar]
- Belisario MA, Tafuri S, Di Domenico C, Della Morte R, Squillacioti C, Lucisano A, et al. Immobilised echistatin promotes platelet adhesion and protein tyrosine phosphorylation. Biochimica et Biophysica Acta. 2000;1497:227–36. doi: 10.1016/s0167-4889(00)00061-6. [DOI] [PubMed] [Google Scholar]
- Belisario MA, Tafuri S, Pontarelli G, Staiano N, Gionti E. Modulation of chondrocyte adhesion to collagen by echistatin. European Journal of Cell Biology. 2005;8410:833–42. doi: 10.1016/j.ejcb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Bracht T, Figueiredo de Rezende F, Stetefeld J, Sorokin LM, Eble JA. Monoclonal antibodies reveal the alteration of the rhodocetin structure upon α2β1 integrin binding. Biochemical Journal. 2011;440:1–11. doi: 10.1042/BJ20110584. [DOI] [PubMed] [Google Scholar]
- Brown MC, Staniszewska I, Del Valle L, Tuszynski GP, Marcinkiewicz C. Angiostatic activity of obtustatin as alpha1beta1 integrin inhibitor in experimental melanoma growth. International Journal of Cancer. 2008;123:2195–203.a. doi: 10.1002/ijc.23777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, Staniszewska I, Lazarovici P, Tuszynski GP, Del Valle L, Marcinkiewicz C. Regulatory effect of nerve growth factor in alpha9beta1 integrin-dependent progression of glioblastoma. Neuro-Oncology. 2008;10:968–80.b. doi: 10.1215/15228517-2008-047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, Eble JA, Calvete JJ, Marcinkiewicz C. Structural requirements of KTS-disintegrins for inhibition of α1β1 integrin. Biochemical Journal. 2009;417:95–101. doi: 10.1042/BJ20081403. [DOI] [PubMed] [Google Scholar]
- Calvete JJ, Fox JW, Agelan A, Niewiarowski S, Marcinkiewicz C. The presence of the WGD motif in CC8 heterodimeric disintegrin increases its inhibitory effect on alphaII(b)beta3, alpha(v)beta3, and alpha5beta1 integrins. Biochemistry. 2002;41:2014–21. doi: 10.1021/bi015627o. [DOI] [PubMed] [Google Scholar]
- Calvete JJ, Moreno-Murciano MP, Theakston RD, Kisiel DG, Marcinkiewicz C. Snake venom disintegrins: novel dimeric disintegrins and structural diversification by disulphide bond engineering. Biochemical Journal. 2003;372:725–34. doi: 10.1042/BJ20021739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvete JJ. The continuing saga of snake venom disintegrins. Toxicon. 2013;62:40–9. doi: 10.1016/j.toxicon.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Chang HH, Lin CH, Lo SJ. Recombinant rhodostomin substrates induce transformation and active calcium oscillation in human platelets. Experimental Cell Research. 1999;250:387–400. doi: 10.1006/excr.1999.4547. [DOI] [PubMed] [Google Scholar]
- Cheresh DA, Stupack DG. Regulation of angiogenesis: apoptotic cues from the ECM. Oncogene. 2008;27:6285–98. doi: 10.1038/onc.2008.304. [DOI] [PubMed] [Google Scholar]
- Clark EA, Trikha M, Markland FS, Brugge JS. Structurally distinct disintegrins contortrostatin and multisquamatin differentially regulate platelet tyrosine phosphorylation. Journal of Biological Chemistry. 1994;269:21940–3. [PubMed] [Google Scholar]
- Clemetson KJ. Snaclecs (snake C-type lectins) that inhibit or activate platelets by binding to receptors. Toxicon. 2010;56:1236–46. doi: 10.1016/j.toxicon.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Clissa PB, Lopes-Ferreira M, Della-Casa MS, Farsky SH, Moura-da-Silva AM. Importance of jararhagin disintegrin-like and cysteine-rich domains in the early events of local inflammatory response. Toxicon. 2006;47:591–6. doi: 10.1016/j.toxicon.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Coelho AL, de Freitas MS, Oliveira-Carvalho AL, Moura-Neto V, Zingali RB, Barja-Fidalgo C. Effects of jarastatin, a novel snake venom disintegrin, on neutrophil migration and actin cytoskeleton dynamics. Experimental Cell Research. 1999;251:379–87. doi: 10.1006/excr.1999.4583. [DOI] [PubMed] [Google Scholar]
- Coelho AL, de Freitas MS, Mariano-Oliveira A, Oliveira-Carvalho AL, Zingali RB, Barja-Fidalgo C. Interaction of disintegrins with human neutrophils induces cytoskeleton reorganization, focal adhesion kinase activation, and extracellular-regulated kinase-2 nuclear translocation, interfering with the chemotactic function. FASEB Journal. 2001;15:1643–5. doi: 10.1096/fj.00-0812fje. [DOI] [PubMed] [Google Scholar]
- Coelho AL, De Freitas MS, Mariano-Oliveira A, Rapozo DC, Pinto LF, Niewiarowski S, et al. RGD and MLD-disintegrins, jarastatin and EC3, activate integrin-mediated signaling modulating the human neutrophils chemotaxis, apoptosis and IL-8 gene expression. Experimental Cell Research. 2004;292:371–384. doi: 10.1016/j.yexcr.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Daidone I, Aschi M, Patamia M, Bozzi A, Petruzzelli R. Structural and dynamical properties of KTS-disntegrins: A comparison between obtustatin and lebestatin. Biopolymers. 2012;99:47–54. doi: 10.1002/bip.22138. [DOI] [PubMed] [Google Scholar]
- Danen EH, Marcinkiewicz C, Cornelissen IM, van Kraats AA, Pachter JA, Ruiter DJ, et al. The disintegrin eristostatin interferes with integrin alpha 4 beta 1 function and with experimental metastasis of human melanoma cells. Experimental Cell Research. 1998;238:188–96. doi: 10.1006/excr.1997.3821. [DOI] [PubMed] [Google Scholar]
- Davenpeck KL, Marcinkiewicz C, Wang D, Niculescu R, Shi Y, Martin JL, et al. Regional differences in integrin expression: role of alpha(5)beta(1) in regulating smooth muscle cell functions. Criculation Research. 2001;88:352–8. doi: 10.1161/01.res.88.3.352. [DOI] [PubMed] [Google Scholar]
- Duan X, Jia SF, Zhou Z, Langley RR, Bolontrade MF, Kleinerman ES. Association of alphavbeta3 integrin expression with the metastatic potential and migratory and chemotactic ability of human osteosarcoma cells. Clinical and Experimental Metastasis. 2004;21:747–53. doi: 10.1007/s10585-005-0599-6. [DOI] [PubMed] [Google Scholar]
- Eble JA, Niland S, Bracht T, Mormann M, Peter-Katalinic J, Pohlentz G, Stetefeld J. The alpha2beta1 integrin-specific antagonist rhodocetin is a cruciform, heterotetrameric molecule. FASEB Journal. 2009;23:2917–27. doi: 10.1096/fj.08-126763. [DOI] [PubMed] [Google Scholar]
- Egbertson MS, Chang CT, Duggan ME, Gould RJ, Halczenko W, Hartman GD, et al. Non-peptide fibrinogen receptor antagonists. 2 Optimization of a tyrosine template as a mimic for Arg-Gly-Asp. Journal of Medicinal Chemistry. 1994;37:2537–51. doi: 10.1021/jm00042a007. [DOI] [PubMed] [Google Scholar]
- Fiorilli P, Partridge D, Staniszewska I, Wang JY, Grabacka M, So K, et al. Integrins mediate adhesion of medulloblastoma cells to tenascin and activate pathways associated with survival and proliferation. Laboratory Investigation. 2008;88:1143–56. doi: 10.1038/labinvest.2008.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JW, Serrano SMT. Timeline of key events in snake venom metalloproteinase research. Journal of Proteomics. 2009;72:200–9. doi: 10.1016/j.jprot.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Golubkov V, Hawes D, Markland FS. Anti-angiogenic activity of contortrostatin, a disintegrin from Agkistrodon contortrix contortrix snake venom. Angiogenesis. 2003;6:213–24. doi: 10.1023/B:AGEN.0000021396.47009.b0. [DOI] [PubMed] [Google Scholar]
- Gupta SK, Oommen S, Aubry MC, Williams BP, Vlahakis NE. Integrin α9β1promotes malignant tumor growth and metastasis by potentiating epithelial-mesenchymal transition. Oncogene. 2013;32:141–50. doi: 10.1038/onc.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailey S, Adams E, Penn R, Wong A, McLane MA. Effect of the disintegrin eristostatin on melanoma-natural killer cell interactions. Toxicon. 2013;61:83–93. doi: 10.1016/j.toxicon.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantgan RR, Stahle MC, Connor JH, Lyles DS, Horita DA, Rocco M, Nagaswami C, Weisel JW, McLane MA. The disintegrin echistatin stabilizes integrin alphaIIbbeta3’s open conformation and promotes its oligomerization. Journal of Molecular Biology. 2004;342:1625–36. doi: 10.1016/j.jmb.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Harburger DS, Calderwood DA. Integrin signaling at a glance. Journal of Cell Science. 2009;122:159–63. doi: 10.1242/jcs.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper MM, Ye EA, Blong CC, Jacobson ML, Sakaguchi DS. Integrins contribute to initial morphological development and process outgrowth in rat adult hippocampal progenitor cells. Journal of Molecular Neuroscience. 2010;40:269–83. doi: 10.1007/s12031-009-9211-x. [DOI] [PubMed] [Google Scholar]
- Hong SY, Lee H, You WK, Chung KH, Kim DS, Song K. The snake venom disintegrin salmosin induces apoptosis by disassembly of focal adhesions in bovine capillary endothelial cells. Biochemical and Biophysical Research Communications. 2003;302:502–508. doi: 10.1016/s0006-291x(03)00213-4. [DOI] [PubMed] [Google Scholar]
- Hook KM, Bennett JS. Glycoprotein IIb/IIIa antagonists. Handbook of Experimental Pharmacology. 2012;210:199–223. doi: 10.1007/978-3-642-29423-5_8. [DOI] [PubMed] [Google Scholar]
- Horii K, Okuda D, Morita T, Mizuno H. Structural characterization of EMS16, an antagonist of collagen receptor (GPIa/IIa) from the venom of Echis multisquamatus. Biochemistry. 2003;42:12497–502. doi: 10.1021/bi034890h. [DOI] [PubMed] [Google Scholar]
- Horii K, Okuda D, Morita T, Mizuno H. Crystal structure of EMS16 in complex with the integrin alpha2-I domain. Journal of Molecular Biology. 2004;341:519–27. doi: 10.1016/j.jmb.2004.06.036. [DOI] [PubMed] [Google Scholar]
- Huang TF, Holt JC, Lukasiewicz H, Niewiarowski Trigramin. A low molecular weight peptide inhibiting fibrinogen interaction with platelet receptors expressed on glycoprotein IIb-IIIa complex. Journal of Biological Chemistry. 1987;262:16157–63. [PubMed] [Google Scholar]
- Huang TF, Yeh CH, Wu WB. Viper venom components affecting angiogenesis. Haemostasis. 2001;31:192–206. doi: 10.1159/000048063. [DOI] [PubMed] [Google Scholar]
- Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. Journal of Cell Science. 2006;119:3901–3. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TF, Chang CH, Ho PL, Chung CH. FcgammaRII mediates platelet aggregation caused by disintegrins and GPIIb/IIIa monoclonal antibody, AP2. Experimental Hematology. 2008;36:1704–13. doi: 10.1016/j.exphem.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: Bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Jakubowski P, Calvete JJ, Eble JA, Lazarovici P, Marcinkiewicz C. Identification of inhibitors of α2β1 integrin, members of C-type lectin proteins, in Echis sochureki venom. Toxicology and Applied Pharmacology. 2013;269:34–42. doi: 10.1016/j.taap.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang Y-J, Jeon O-K, Kim D-S. Saxatilin, a snake venom disintegrin, regulates platelet activation associated with human vascular endothelial cell migration and invasion. Journal of Vascular Research. 2007;44:129–37. doi: 10.1159/000098519. [DOI] [PubMed] [Google Scholar]
- Juarez P, Comas I, González-Candelas F, Calvete JJ. Evolution of snake venom disintegrins by positive Darwinian selection. Molecular Biology and Evolution. 2008;25:2391–407. doi: 10.1093/molbev/msn179. [DOI] [PubMed] [Google Scholar]
- Juliano D, Wang Y, Marcinkiewicz C, Rosenthal LA, Stewart GJ, Niewiarowski S. Disintegrin interaction with alpha V beta 3 integrin on human umbilical vein endothelial cells: expression of ligand-induced binding site on beta 3 subunit. Experimental Cell Research. 1996;225:132–42. doi: 10.1006/excr.1996.0164. [DOI] [PubMed] [Google Scholar]
- Kang IC, Kim DS, Jang Y, Chung KH. Suppressive mechanism of salmosin, a novel disintegrin in B16 melanoma cell metastasis. Biochemical and Biophysical Research Communications. 2000;275:169–73. doi: 10.1006/bbrc.2000.3130. [DOI] [PubMed] [Google Scholar]
- Karna E, Szoka L, Palka J. Thrombin-dependent modulation of β1-integrin-mediated signaling up-regulates prolidase and HIF-1α through p-FAK in colorectal cancer cells. Molecular and Cellular Biochemistry. 2012;361:235–41. doi: 10.1007/s11010-011-1108-7. [DOI] [PubMed] [Google Scholar]
- Kauskot A, Cominetti MR, Ramos OH, Bechyne I, Renard JM, Hoylaerts MF, et al. Hemostatic effects of recombinant DisBa-01, a disintegrin from Bothrops alternatus. Frontiers in Biosciences. 2008;13:6604–16. doi: 10.2741/3176. [DOI] [PubMed] [Google Scholar]
- Kessentini-Zouari R, Jebali J, Taboubi S, Srairi-Abid N, Morjen M, Kallech-Ziri O, et al. CC-PLA2-1 and CC-PLA2-2, two Cerastes cerastes venom-derived phospholipases A2, inhibit angiogenesis both in vitro and in vivo. Laboratory Investigation. 2010;90:510–9. doi: 10.1038/labinvest.2009.137. [DOI] [PubMed] [Google Scholar]
- Kisiel DG, Calvete JJ, Katzhendler J, Fertala A, Lazarovici P, Marcinkiewicz C. Structural determinants of the selectivity of KTS-disintegrins for the α1β1 integrin. FEBS Letters. 2004;577:478–82. doi: 10.1016/j.febslet.2004.10.050. [DOI] [PubMed] [Google Scholar]
- Limam I, Bazaa A, Srairi-Abid N, Taboubi S, Jebali J, Zouari-Kessentini R, et al. Leberagin-C, A disintegrin-like/cysteine-rich protein from Macrovipera lebetina transmediterranea venom, inhibits alphavbeta3 integrin-mediated cell adhesion. Matrix Biology. 2010;29:1217–26. doi: 10.1016/j.matbio.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Lin E, Wang Q, Swenson S, Jadvar H, Groshen S, Ye W, et al. The disintegrin contortrostatin in combination with docetaxel is a potent inhibitor of prostate cancer in vitro and in vivo. Prostate. 2010;70:1359–70. doi: 10.1002/pros.21173. [DOI] [PubMed] [Google Scholar]
- Long RK, Nishida S, Kubota T, Wang Y, Sakata T, Elalieh HZ, et al. Seletal unloading-induced insulin-like growth factor 1 (IGF-1) nonresponsiveness is not shared by platelet-derived growth factor: the selective role of integrins in IGF-1 signaling. Journal of Bone and Mineral Research. 2011;26:2948–58. doi: 10.1002/jbmr.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucena S, Sanchez EE, Perez JC. Anti-metastatic activity of the recombinant disintegrin, r-mojastin 1, from the Mohave rattlesnake. Toxicon. 2011;57:794–802. doi: 10.1016/j.toxicon.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucena SE, Jia Y, Soto JG, Parral J, Cantu E, Brannon J, et al. Anti-invasive and anti-adhesive activities of a recombinant disintegrin, r-viridistatin 2, derived from the Prairie rattlesnake (Crotalus viridis viridis) Toxicon. 2012;60:31–9. doi: 10.1016/j.toxicon.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkiewicz C, Rosenthal LA, Mosser DM, Kunicki TJ, Niewiarowski S. Immunological characterization of eristostatin and echistatin binding sites on alpha IIb beta 3 and alpha V beta 3 integrins. Biochemical Journal. 1996;317:817–25. doi: 10.1042/bj3170817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkiewicz C, Calvete JJ, Vijay-Kumar S, Marcinkiewicz MM, Raida M, Schick P, et al. Structural and functional characterization of EMF10, a heterodimeric disintegrin from Eristocophis macmahoni venom that selectively inhibits alpha 5 beta 1 integrin. Biochemistry. 1999;38:13302–9a. doi: 10.1021/bi9906930. [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz C, Calvete JJ, Marcinkiewicz M, Raida M, Vijay-Kumar S, Huang Z, et al. EC3, a novel heterodimeric disintegrin from Echis carinatus venom, inhibits α4 nad α5 integrins in an RGD-independent manner. Journal of Biological Chemistry. 1999;274:12468–73b. doi: 10.1074/jbc.274.18.12468. [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz C, Taooka Y, Yokosaki Y, Calvete JJ, Marcinkiewicz MM, Lobb RR, et al. Inhibitory effects of MLDG-containing heterodimeric disintegrins reveal distinct structural requirements for interaction of the integrin alpha9beta1 with VCAM-1, tenascin-C, and osteopontin. Journal of Biological Chemistry. 2000;275:31930–37a. doi: 10.1074/jbc.M003209200. [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz C, Lobb RR, Marcinkiewicz MM, Daniel JL, Smith JB, Dangelmaier C, et al. Isolation and characterization of EMS16, a C-type lectin protein from Echis multisquamatus venom, a potent and selective inhibitor of the α2β1 integrin. Biochemistry. 2000;39:9859–67b. doi: 10.1021/bi000428a. [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz C, Wainreb PH, Calvete JJ, Kisiel DG, Mousa SA, Tuszynski GP, et al. Obtustatin, a potent inhibitor of α1β1 integrin in vitro and angiogenesis in vivo. Cancer Research. 2003;63:2020–3. [PubMed] [Google Scholar]
- Marcinkiewicz C. Functional characteristic of snake venom disintegrins: Potential therapeutic implication. Current Pharmaceutical Design. 2005;11:815–27. doi: 10.2174/1381612053381765. [DOI] [PubMed] [Google Scholar]
- Mariano-Oliveir A, Coelho AL, Terruggi CH, Selistre-de-Araujo HS, Barja-Fidalgo C, de Freitas MS. Alternagin-C, a nonRGD-disintegrin, induces neutrophil migration via integrin signaling. European Journal of Biochemistry. 2003;270:4799–808. doi: 10.1046/j.1432-1033.2003.03867.x. [DOI] [PubMed] [Google Scholar]
- Markland FS, Shieh K, Zhou Q, Golubkov V, Sherwin RP, Richters V, et al. A novel snake venom disintegrin that inhibits human ovarian cancer dissemination and angiogenesis in an orthotopic nude mouse model. Haemostasis. 2001;31:183–91. doi: 10.1159/000048062. [DOI] [PubMed] [Google Scholar]
- McLane MA, Joerger T, Mahmoud A. Disintegrins in health and disease. Frontiers in Bioscience. 2008;13:6617–37. doi: 10.2741/3177. [DOI] [PubMed] [Google Scholar]
- Minamiguchi K, Kumagai H, Masuda T, Kawada M, Ishizuka M, Takeuchi T. Thiolutin, an inhibitor of HUVEC adhesion to vitronectin, reduces paxillin in HUVECs and suppresses tumor cell-induced angiogenesis. International Journal of Cancer. 2001;93:307–16. doi: 10.1002/ijc.1321. [DOI] [PubMed] [Google Scholar]
- Momic T, Cohen G, Reich R, Arlinghaus FT, Eble JA, Marcinkiewicz C, et al. Vixapatin (VP12), a c-type lectin-protein from Vipera xantina palestinae venom: characterization as a novel anti-angiogenic compound. Toxins (Basel) 2012;4:862–77. doi: 10.3390/toxins4100862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenegro CF, Salla-Pontes CL, Ribeiro JU, Machado AZ, Ramos RF, Figueiredo CC, et al. Blocking αvβ3 integrin by a recombinant RGD disintegrin impairs VEGF signaling in endothelial cells. Biochimie. 2012;94:1812–20. doi: 10.1016/j.biochi.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Morita T. Structures and functions of snake venom CLPs (C-type lectin-like proteins) with anticoagulant-, procoagulant-, and platelet modulating activities. Toxicon. 2005;45:1099–114. doi: 10.1016/j.toxicon.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Morris VL, Schmidt EE, Koop S, MacDonald IC, Grattan M, Khokha R, et al. Effects of the disintegrin eristostatin on individual steps of hematogenous metastasis. Experimental Cell Research. 1995;219:571–8. doi: 10.1006/excr.1995.1266. [DOI] [PubMed] [Google Scholar]
- Moura-da-Silva AM, Butera D, Tanjoni I. Importance of snake venom metalloproteinases in cell biology: Effects on platelets, inflammatory and endothelial cells. Current Pharmaceutical Design. 2007;13:2893–905. doi: 10.2174/138161207782023711. [DOI] [PubMed] [Google Scholar]
- Neto EH, Coelho AL, Sampaio AL, Henriques MD, Marcinkiewicz C, de Freitas MS, et al. Activation of human T lymphocytes via integrin signaling induced by RGD-disintegrins. Biochimica et Biophysica Acta. 2007;1773:176–84. doi: 10.1016/j.bbamcr.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Okuda D, Morita T. Purification and characterization of a new RGD/KGD-containing dimeric disintegrin, piscivostatin, from the venom of Agkistrodon piscivorus piscivorus: the unique effect of piscivostatin on platelet aggregation. Journal of Biochemistry. 2001;130:407–15. doi: 10.1093/oxfordjournals.jbchem.a003000. [DOI] [PubMed] [Google Scholar]
- Okuda D, Koike H, Morita T. A new gene structure of the disintegrin family: a subunit of dimeric disintegrin has a short coding region. Biochemistry. 2002;41:14248–54. doi: 10.1021/bi025876s. [DOI] [PubMed] [Google Scholar]
- Oliva IB, Coelho RM, Barcellos GG, Saldanha-Gama R, Wermelinger LS, Marcinkiewicz C, et al. Effect of RGD-disintegrins on melanoma cell growth and metastasis: involvement of the actin cytoskeleton, FAK and c-Fos. Toxicon. 2007;50:1053–63. doi: 10.1016/j.toxicon.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Oshikawa K, Terada S. Ussuristatin 2, a novel KGD-bearing disintegrin from Agkistrodon ussuriensis venom. Journal of Biochemistry. 1999;125:31–5. doi: 10.1093/oxfordjournals.jbchem.a022264. [DOI] [PubMed] [Google Scholar]
- Olfa KZ, Luis J, Daoud S, Bazaa A, Srairi Abid N, Andreotti N, et al. Lebestatin, a disintegrin from Macrovipera venom, inhibits integrin-mediated cell adhesion, migration and angiogenesis. Laboratory Investigation. 2005;85:1507–16. doi: 10.1038/labinvest.3700350. [DOI] [PubMed] [Google Scholar]
- Pechkovsky DV, Scaffidi AK, Hackett TL, Ballard J, Shaheen F, Thompson PJ, et al. Transforming growth factor beta1 induces alphavbeta3 integrin expression in human lung fibroblasts via a beta3 integrin-, c-Src-, and p38 MAPK-dependent pathway. Journal of Biological Chemistry. 2008;283:12898–908. doi: 10.1074/jbc.M708226200. [DOI] [PubMed] [Google Scholar]
- Pilorget A, Conesa M, Sarray S, Michaud-Levesque J, Daoud S, Kim KS, et al. Lebectin, a Macrovipera lebetina venom-derived C-type lectin, inhibits angiogenesis both in vitro and in vivo. Journal of Cell Physiology. 2007;211:307–15. doi: 10.1002/jcp.20935. [DOI] [PubMed] [Google Scholar]
- Pyrko P, Wang W, Markland FS, Swenson SD, Schmitmeier S, Schönthal AH, et al. The role of contortrostatin, a snake venom disintegrin, in the inhibition of tumor progression and prolongation of survival in a rodent glioma model. Journal of Neurosurgery. 2005;103:526–37. doi: 10.3171/jns.2005.103.3.0526. [DOI] [PubMed] [Google Scholar]
- Rahman S, Lu X, Kakkar VV, Authi KS. The integrin alpha IIb beta 3 contains distinct and interacting binding sites for snake-venom RGD (Arg Gly Asp) proteins. Evidence that the receptor-binding characteristics of snake-venom RGD proteins are related to the amino acid environment flanking the sequence. RGD Biochemical Journal. 1995;312:223–32. doi: 10.1042/bj3120223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos OH, Selistre-de-Araujo HS. Snake venom metalloproteases-structure and function of catalytic and disintegrin domains. Comparative Biochemistry and Physiology. 2006;142:328–46. doi: 10.1016/j.cbpc.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Ramos OH, Terruggi CH, Ribeiro JU, Cominetti MR, Figueiredo CC, Bérard M, et al. Modulation of in vitro and in vivo angiogenesis by alternagin-C, a disintegrin-like protein from Bothrops alternatus snake venom and by a peptide derived from its sequence. Archives of Biochemistry and Biophysics. 2007;461:1–6. doi: 10.1016/j.abb.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Reardon DA, Cheresh DA. Cilengitide: A prototypic integrin inhibitor for the treatment of glioblastoma and other malignancies. Genes Cancer. 2011;2:1159–65. doi: 10.1177/1947601912450586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AR, Hart IR, Watson AR, Welti JC, Silva RG, Robinson SD, et al. Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nature Medicine. 2009;15:392–400. doi: 10.1038/nm.1941. [DOI] [PubMed] [Google Scholar]
- Rosenow F, Ossig R, Thormeyer D, Gasmann P, Schlüter K, Brunner G, et al. Integrins as antimetastatic targets of RGD-independent snake venom components in liver metastasis [corrected] Neoplasia. 2008;10:168–76. doi: 10.1593/neo.07898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saegusa J, Akakura N, Wu CY, Hoogland C, Ma Z, Lam KS, et al. Pro-inflammatory secretory phospholipase A2 type IIA binds to integrins alphavbeta3 and alpha4beta1 and induces proliferation of monocytic cells in an integrin-dependent manner. Journal of Biological Chemistry. 2008;283:26107–15. doi: 10.1074/jbc.M804835200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha-Gama R, Moraes JA, Mariano-Oliveira A, Coelho AL, Walsh EM, Marcinkiewicz C, et al. alpha(9)beta(1) integrin engagement inhibits neutrophil spontaneous apoptosis: Involvement of Bcl-2 family members. Biochimica et Biophysica Acta. 2010;1803:848–57. doi: 10.1016/j.bbamcr.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Sarray S, Srairi N, Hatmi M, Luis J, Louzir H, Regaya I, et al. Lebecetin, a potent antiplatelet C-type lectin from Macrovipera lebetina venom. Biochimica et Biophysica Acta. 2003;1651:30–40. doi: 10.1016/s1570-9639(03)00232-2. [DOI] [PubMed] [Google Scholar]
- Sanchez EE, Rodriguez-Acosta A, Palomar R, Lucena SE, Bashir S, Soto JG, et al. Colombistatin: a disintegrin isolated from the venom of the South American snake (Bothrops colombiensis) that effectively inhibits platelet aggregation and SK-Mel-28 cell adhesion. Archives of Toxicology. 2009;83:271–9. doi: 10.1007/s00204-008-0358-y. [DOI] [PubMed] [Google Scholar]
- Sanz L, Chen RQ, Perez A, Hilario R, Juarez P, Marcinkiewicz C, et al. cDNA cloning and functional expression of jerdostatin, a novel RTS-disintegrin from Trimeresurus jerdonii and a specific antagonist of the α1β1 integrin. Journal of Biological Chemistry. 2005;208:40714–22. doi: 10.1074/jbc.M509738200. [DOI] [PubMed] [Google Scholar]
- Schmitmeier S, Markland FS, Ritter MR, Sawcer DE, Chen TC. Functional effect of contortrostatin, a snake venom disintegrin, on human glioma cell invasion in vitro. Cell Communication and Adhesion. 2003;10:1–16. doi: 10.1080/15419060302062. [DOI] [PubMed] [Google Scholar]
- Schmitmeier S, Markland FS, Schönthal AH, Chen TC. Potent mimicry of fibronectin-induced intracellular signaling in glioma cells by the homodimeric snake venom disintegrin contortrostatin. Neurosurgery. 2005;57:141–53. doi: 10.1227/01.neu.0000163426.25227.56. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, McRoberts K, Coyner M, Andarawewa KL, Frierson HF, Jr, Sanders JM, et al. Integrin agonists as adjuvants in chemotherapy for melanoma. Clinical Cancer Research. 2008;14:6193–7. doi: 10.1158/1078-0432.CCR-08-1285. [DOI] [PubMed] [Google Scholar]
- Sekimoto H, Eipper-Mains J, Pond-Tor S, Boney CM. (alpha)v(beta)3 integrins and Pyk2 mediate insulin-like growth factor I activation of Src and mitogen-activated protein kinase in 3T3-L1 cells. Molecular Endocrinology. 2005;19:1859–67. doi: 10.1210/me.2004-0481. [DOI] [PubMed] [Google Scholar]
- Shah PP, Fong MY, Kakar SS. PTTG induces EMT through integrin αVβ3-focal adhesion kinase signaling in lung cancer cells. Oncogene. 2012;31:3124–35. doi: 10.1038/onc.2011.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu JR, Lin CH, Peng HC, Huang TF. Triflavin, an Arg-Gly-Asp-containing peptide, inhibits the adhesion of tumor cells to matrix proteins via binding to multiple integrin receptors expressed on human hepatoma cells. Proceedings of the Society for Experimental Biology and Medicine. 1996;213:71–9. doi: 10.3181/00379727-213-44038. [DOI] [PubMed] [Google Scholar]
- Soszka T, Knudsen KA, Beviglia L, Rossi C, Poggi A, Niewiarowski S. Inhibition of murine melanoma cell-matrix adhesion and experimental metastasis by albolabrin, an RGD-containing peptide isolated from the venom of Trimeresurus albolabris. Experimental Cell Research. 1991;196:6–12. doi: 10.1016/0014-4827(91)90449-5. [DOI] [PubMed] [Google Scholar]
- Souza DH, Iemma MR, Ferreira LL, Faria JP, Oliva ML, Zingali RB, et al. The disintegrin-like domain of the snake venom metalloprotease alternagin inhibits alpha2beta1 integrin-mediated cell adhesion. Archives of Biochemistry and Biophysics. 2000;348:341–50. doi: 10.1006/abbi.2000.2120. [DOI] [PubMed] [Google Scholar]
- Staiano N, Della Morte R, Di Domenico C, Tafuri S, Squillacioti C, Belisario MA. Echistatin inhibits pSyk and pp125FAK phosporylation in fibrinogen-adherent platelets. Biochimie. 1997;79:769–73. doi: 10.1016/s0300-9084(97)86935-0. [DOI] [PubMed] [Google Scholar]
- Staniszewska I, Zaveri S, Del Valle L, Oliva I, Rothman VL, Croul SE, et al. Interaction of α9β1 integrin with thrombospondin-1 promotes angiogenesis. Circulation Research. 2007;100:1308–16. doi: 10.1161/01.RES.0000266662.98355.66. [DOI] [PubMed] [Google Scholar]
- Staniszewska I, Sariyer IK, Lecht S, Brown MC, Tuszynski GP, Safak M, et al. The integrin α9β1 is a receptor for nerve growth factor and other neurotrophins. Journal of Cell Science. 2008;121:504–13. doi: 10.1242/jcs.000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staniszewska I, Walsh EM, Rothman VL, Gaathon A, Tuszynski GP, Calvete JJ, et al. Effect of VP12 and viperistatin on inhibition of collagen-receptor-dependent melanoma metastasis. Cancer Biology and Therapy. 2009;8:1507–16. doi: 10.4161/cbt.8.15.8999. [DOI] [PubMed] [Google Scholar]
- Surazynski A, Sienkiewicz P, Wolczynski S, Palka J. Differential effects of echistatin and thrombin on collagen production and prolidase activity in human dermal fibroblasts and their possible implication in beta1-integrin-mediated signaling. Pharmacological Research. 2005;51:217–21. doi: 10.1016/j.phrs.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Swenson S, Ramu S, Markland FS., Jr Anti-angiogenesis and RGD-containing snake venom disintegrins. Current Pharmaceutical Design. 2007;13:2860–71. doi: 10.2174/138161207782023793. [DOI] [PubMed] [Google Scholar]
- Tian J, Paquette-Straub C, Sage EH, Funk SE, Patel V, Galileo D, et al. Inhibition of melanoma cell motility by the snake venom disintegrin eristostatin. Toxicon. 2007;49:899–908. doi: 10.1016/j.toxicon.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trummal K, Tonismagi K, Paalme V, Jarvekulg L, Siigur J, Siigur E. Molecular diversity of snake venom nerve growth factors. Toxicon. 2011;58:363–8. doi: 10.1016/j.toxicon.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Varner JA, Cheresh DA. Integrins and cancer. Current Opinion in Cell Biology. 1996;8:724–30. doi: 10.1016/s0955-0674(96)80115-3. [DOI] [PubMed] [Google Scholar]
- Vija H, Samel M, Siigur E, Aaspõllu A, Tonismagi K, Trummal K, et al. VGD and MLD-motifs containing heterodimeric disintegrin viplebedin-2 from Vipera lebetina snake venom. Purification and cDNA cloning Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biololgy. 2009;153:572–80. doi: 10.1016/j.cbpb.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Vlahakis NE, Young BA, Atakilit A, Hawkridge AE, Issaka RB, Boudreau N, et al. Integrin alpha9beta1 directly binds to vascular endothelial growth factor (VEGF)-A and contributes to VEGF-A-induced angiogenesis. Journal of Biological Chemistry. 2007;282:15187–96. doi: 10.1074/jbc.M609323200. [DOI] [PubMed] [Google Scholar]
- Walsh EM, Marcinkiewicz C. Non-RGD-containing snake venom disintegrins, functional and structural relations. Toxicon. 2011;58:355–62. doi: 10.1016/j.toxicon.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Walsh EM, Kim R, Del Valle L, Weaver M, Sheffield J, Lazarovici P, et al. Importance of interaction between nerve growth factor and α9β1 integrin in glial tumor angiogenesis. Neuro-Oncology. 2012;14:890–901. doi: 10.1093/neuonc/nos119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Kini RM, Chung MC. Rhodocetin, a novel platelet aggregation inhibitor from the venom of Calloselasma rhodostoma (Malayan pit viper): synergistic and noncovalent interaction between its subunits. Biochemistry. 1999;38:7584–93. doi: 10.1021/bi982132z. [DOI] [PubMed] [Google Scholar]
- Wang YJ, Gualco E, Peruzzi F, Sawaya B, Passiatore G, Marcinkiewicz C, et al. Interaction between serine phosphorylated IRS-1 and β1-integrin affects the stability of neuronal processes. Journal of Neuroscience Research. 2007;85:2360–73. doi: 10.1002/jnr.21400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WB, Peng HC, Huang TF. Disintegrin causes proteolysis of beta-catenin and apoptosis of endothelial cells. Involvement of cell-cell and cell-ECM interactions in regulating cell viability. Experimental Cell Research. 2003;286:115–27. doi: 10.1016/s0014-4827(03)00105-8. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Morita T. Molecular and functional diversity of vascular endothelial growth factors. Molecular Diversity. 2006;10:515–27. doi: 10.1007/s11030-006-9027-3. [DOI] [PubMed] [Google Scholar]
- Yang RS, Tang CH, Chuang WJ, Huang TH, Peng HC, Huang TFWM, et al. Inhibition of tumor formation by snake venom disintegrin. Toxicon. 2005;45:661–9. doi: 10.1016/j.toxicon.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Ye F, Kim C, Ginsberg MH. Molecular mechanism of inside-out integrin regulation. Journal of Thrombosis and Haemostasis. 2011;9:20–5. doi: 10.1111/j.1538-7836.2011.04355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh CH, Peng HC, Huang TF. Accutin, a new disintegrin, inhibits angiogenesis in vitro and in vivo by acting as integrin alphavbeta3 antagonist and inducing apoptosis. Blood. 1998;92:3268–76. [PubMed] [Google Scholar]
- Zhou Q, Sherwin RP, Parrish C, Richters V, Groshen SG, Tsao-Wei D, et al. Contortrostatin, a dimeric disintegrin from Agkistrodon contortrix contortrix, inhibits breast cancer progression. Breast Cancer Research and Treatment. 2000;61:249–60. doi: 10.1023/a:1006457903545. [DOI] [PubMed] [Google Scholar]
- Zhou XD, Jin Y, Chen RQ, Lu QM, Wu JB, Wang WY, Xiong YL. Purification, cloning and biological characterization of a novel disintegrin from Trimeresurus jerdonii venom. Toxicon. 2004;43:69–75. doi: 10.1016/j.toxicon.2003.10.023. [DOI] [PubMed] [Google Scholar]