Abstract
BK virus is associated with hemorrhagic cystitis after hematopoietic stem cell transplantation (HSCT), although evidence supporting a causal relationship remains limited. Although BK viruria is common after HSCT, BK viremia may better predict clinically significant cystitis, similar to its predictive value for nephropathy after kidney transplantation. We hypothesized that BK viremia would precede hemorrhagic cystitis in a cohort of 88 consecutive children prospectively enrolled to originally study thrombotic microangiopathy in the first 100 days after allogeneic HSCT. Cox regression models with time-varying covariates assessed the association between different BK viremia cutoffs and the development of hemorrhagic cystitis, defined as at least macroscopic hematuria. Subjects with a peak plasma BK viral load 1 to 9999 copies/mL had an adjusted hazard ratio of 4.2 (95% confidence interval (CI), 1.3 to 13.7) for the development of hemorrhagic cystitis. Those with peak BK viremia >100,000 copies/mL had an adjusted hazard ratio of 116.8 (95% CI, 12 to 1136) for cystitis. Other independent risk factors for hemorrhagic cystitis included age >7 years and HHV-6 viremia. Neither graft-versus-host disease nor achieving engraftment increased the risk for cystitis. If therapeutic strategies are found to be effective, these observations may support screening for BK viremia after HSCT, as currently recommended for other DNA viruses.
Keywords: BK virus, Hemorrhagic cystitis, Transplantation, Pediatrics
Introduction
Hemorrhagic cystitis is a significant complication after hematopoietic stem cell transplantation (HSCT) and is associated with prolonged hospitalization, urinary tract obstruction, and possibly death [1-4]. Early-onset cystitis, occurring within 72 hours of conditioning, is typically related to chemotherapy [5,6]. With the use of mesna and aggressive hydration, late-onset cystitis (>72 hours after conditioning) has become the more common posttransplantation bladder complication [3,7,8]. Potential risk factors for late-onset cystitis include the type of conditioning chemotherapy, timing of engraftment, development of graft-versus-host disease (GVHD), presence of BK virus infection in the blood or urine, and other viral infections [3,7,9].
BK virus identified in the urine (viruria) is common after HSCT and occurs even in the absence of hemorrhagic cystitis [10]. Up to 80% of HSCT recipients are noted to have BK viruria [2,4], but only 10% to 25% of all patients develop clinically significant cystitis [3,8,11,12]. The mechanism by which BK virus infection leads to hemorrhagic cystitis in a subset of transplantation patients is unknown, but may be related to immune-mediated bladder injury, GVHD, or later effects of conditioning chemotherapy, such as cyclophosphamide [3,8,9].
BK viremia (≥10,000 copies/mL) is more specific than viruria for predicting nephropathy after kidney transplantation [13]. Kidney transplantation guidelines recommend BK testing of plasma, not urine, as the most effective screening approach [14]. Studies in adults have assessed if BK viremia also predicts hemorrhagic cystitis after HSCT, with conflicting results [15-17]. We previously reported that peak BK viremia, but not viruria, in the first year after HSCT was associated with higher grade cystitis in a retrospective study of 21 children [18]. To increase the causal evidence for an association between infection and disease, we hypothesized that BK viremia would precede cystitis in an independent, prospective cohort of children undergoing HSCT.
Materials and Methods
Study Population
We analyzed a previously completed prospective cohort of 100 consecutive children receiving HSCT at Cincinnati Children's Hospital Medical Center from September 2010 to December 2011. This cohort was originally developed to study thrombotic microangiopathy (TMA) in the first 100 days after transplantation. We included subjects who consented to have their information used for any scientific purpose (98 of 100 subjects) and the research was approved by the institutional review boards at Cincinnati Children's Hospital Medical Center and The Children's Hospital of Philadelphia. We further restricted our analyses to the 88 allogeneic recipients to decrease the heterogeneity of the study population.
Outcome Definition
Cases of hemorrhagic cystitis were first identified by reviewing discharge summaries. Additionally, as part of the original protocol to study TMA, subjects had routine urinalyses performed weekly while admitted. To supplement case ascertainment, we reviewed nursing assessments and daily progress notes for subjects with ≥50 red blood cells (RBC) per high-power field on these protocol urinalyses. Each subject's maximum grade of hemorrhagic cystitis was defined according to the criteria of Bedi et al. [8], with grade 1 representing microscopic hematuria; grade 2, macroscopic hematuria; grade 3, the presence of clots; and grade 4, urinary tract obstruction. We defined hemorrhagic cystitis as grade ≥2 to identify the most clinically significant cases, consistent with prior reports [8,15]. Subjects could be diagnosed with hemorrhagic cystitis only once, defined as the first date recorded in the medical record.
BK Virus PCR Testing
New onset BK viremia was the primary, time-varying risk factor. Assays for BK viremia were performed as clinically indicated (primarily for unexplained microscopic hematuria on urinalysis, gross hematuria and/or an elevation in serum creatinine) or using stored plasma at predefined intervals over the course of follow-up. Plasma specimens were stored (−80° C) at baseline before conditioning, weekly while admitted, when TMA was diagnosed, and at day 100. To approximate BK viremia from day 0 until day 100, stored samples were tested so that each subject had at least 3 plasma BK virus PCR results, with at least 1 measured between days 0 to 14, 15 to 85, and 100 ± 14 days after HSCT.
Because viral replication is often evaluated on a logarithmic scale, we assessed BK viremia at the following cut-off values before the development of hemorrhagic cystitis: (1) 0; (2) 1 to 9999 copies/mL; (3) 10,000 to 99,999 copies/mL; and (4) ≥ 100,000 PCR plasma copies/mL. Subjects were placed into 1 of these 4 threshold categories based on their peak plasma PCR result before the development of hemorrhagic cystitis or their peak value during the first 100 days (in those without cystitis). For subjects with ≥1 identical peak value, the first date was used in the analysis. In this cohort, clinical testing for BK viruria was performed in HSCT recipients with hematuria or symptoms of cystitis; a systematic assessment for BK viruria was not performed.
BK virus testing for clinical indications and on stored specimens were both performed in the Cincinnati Children's Hospital Medical Center, Molecular Pathology Laboratory. Viral DNA was extracted from plasma samples on the Roche MagNA Pure 96 System using the Total Nucleic Acid Kit. The input sample volume was 200 μL and the final volume was 100 μL of DNA eluate. A real-time PCR reaction (Applied Biosystems 7500 FAST System) used primers targeting the VP3 gene for BK virus with a sequence-specific TaqMan hydrolysis probe labeled with FAM reporter molecules on the 5′ end and TAMRA quencher molecules on the 3′ end [19]. The reaction used 23 μL of enzyme/oligonucleotide master mix with 5 μL of DNA template for patient samples, as well as positive and negative controls. A quantified standard material obtained from Advanced Biotechnologies, Inc. was serially diluted, and the known concentrations of the dilutions were programmed into the instrument software to allow for quantification of the patient samples, which were then compared to the standard curve. The final viral concentrations were determined using a calculation accounting for the amount of sample used for DNA extraction, DNA eluate obtained from the extraction, and DNA eluate used in the reaction. All of the PCR assays were run with a separate housekeeping control to exclude the presence of any inhibitors. For analysis purposes, values less than the limit of detection (ie, <500 copies/mL) were converted by dividing by the square root of 2 [20].
Covariates
We prospectively captured demographic information and transplantation data starting 3 weeks before conditioning until day 114 (day 100 + 2 weeks). Age was analyzed both as a continuous variable and as a dichotomous variable at a cutoff above and below the median age of the cohort (7 years). To test current theories on risk factors for hemorrhagic cystitis, the development of GVHD and the timing of engraftment were analyzed as time-varying covariates assessed before the development of hemorrhagic cystitis. Engraftment was analyzed separately as the first day when (1) the absolute neutrophil count was >500/μL for 3 consecutive days, (2) the platelet count was >20,000/μL for 7 days without transfusion, or (3) the platelet count was >50,000/μL for 7 days without transfusion. Each subject's absolute lymphocyte count was examined on the day of peak viremia, the day of peak viruria, and the day hemorrhagic cystitis was diagnosed. Transplantation complications were defined using clinical criteria consistent with published guidelines for GVHD [21], sinusoidal obstruction syndrome [22], and TMA [23,24]. As part of routine clinical care, plasma PCR testing for Epstein-Barr virus (EBV), cytomegalovirus (CMV), and adenovirus, was performed at least weekly during the first 100 days. Testing for HHV-6 viremia was ordered at the discretion of the treating physician, but was typically performed for unexplained fever. Infection with these viruses was considered to be present the first day a plasma PCR was >0 copies/mL.
Statistical Analysis
Descriptive statistics were reported as medians, interquartile ranges (IQR), and frequencies. Differences in categorical and continuous variables were assessed with the Fisher exact and Wilcoxon rank sum tests, respectively. We used a time-to-event analysis with Cox regression models to calculate hazard ratios (HR) and their associated 95% confidence intervals (CI) for the outcome of hemorrhagic cystitis, censoring at time of death or the end of the follow-up period at day 114, whichever came first. Time-dependent variables, before the development of hemorrhagic cystitis, were initially evaluated in univariate models and included BK viremia at the different threshold levels, GVHD, TMA, engraftment, EBV, CMV, adenovirus, and HHV-6 viremia. Any variable (time-dependent or not) with a univariate P value < .2 was considered for inclusion in the multivariate analyses. Study data were collected using REDCap (Research Electronic Data Capture) hosted at Cincinnati Children's Hospital Medical Center [25]. Statistical analyses were performed using STATA statistical software (version 12, College Station, Texas).
Results
Hemorrhagic Cystitis Identification
A total of 1438 urinalyses were performed on the 88 allogeneic recipients during the first 100 days. Seventeen of 88 subjects (19%) developed grade ≥2 hemorrhagic cystitis based on review of these urinalyses and the clinical record. The median time to diagnosis of cystitis was day +25 (IQR, 18 to 42 days). Grade 2 cystitis was diagnosed in 7 of 17 (41%), grade 3 in 9 of 17 (53%), and grade 4 in 1 of 17 (6%). An additional 12 of 88 subjects (14%) had at least 1 urinalysis with >50 RBC per high-power field and/or symptoms of dysuria without gross hematuria, and were therefore classified as <grade 2 cystitis and not included in our case definition.
Based on clinical indication, testing for BK viruria was performed in 41 of 88 (47%) allogeneic recipients: 14 who developed grade ≥2 cystitis and 27 who did not. Among these 27 subjects without grade ≥2 cystitis, 18 of 27 (67%) had at least 1 positive urine PCR (>0 copies/mL) during the first 100 days. Among the 14 subjects with grade ≥2 cystitis and urine PCR testing, 13 of 14 (93%) had a positive urine PCR (>0 copies/mL) anytime during the first 100 days, with 9 of 13 (69%) positive before the diagnosis of cystitis.
Cohort Characteristics
The pretransplantation characteristics of the cohort are shown in Table 1. By univariate analyses, subjects with hemorrhagic cystitis were significantly older and more likely to have received antithymocyte globulin conditioning. Underlying diagnosis, type of preparatory regimen, and specific conditioning agents (ie, antithymocyte globulin) were strongly correlated, making it difficult to assess the independent association of these variables with hemorrhagic cystitis.
Table 1. Characteristics of the Study Cohort.
| Hemorrhagic Cystitis (n = 17) | Controls (n = 71) | P Value* | |
|---|---|---|---|
| Age, yr, median (IQR) | 10.2 (7.8 to 14.0) | 5.7 (1.8 to 15.2) | .04 |
| Male gender | 11 (64.7%) | 45 (63.4%) | 1.00 |
| Diagnosis group | .07 | ||
| Malignancy | 4 (23.5%) | 20 (28.2%) | |
| Immunodeficiency | 5 (29.4%) | 31 (43.7%) | |
| Bone marrow failure | 5 (29.4%) | 19 (26.8%) | |
| Genetic/metabolic | 2 (11.8%) | 1 (1.4%) | |
| Benign hematologic | 1 (5.9%) | 0 (0%) | |
| Donor cell source | .77 | ||
| Unrelated | 13 (76.5%) | 50 (70.4%) | |
| Related | 4 (23.5%) | 21 (29.6%) | |
| Donor cell product | .50 | ||
| Marrow | 12 (70.6%) | 56 (78.9%) | |
| Peripheral blood | 2 (11.8%) | 9 (12.7%) | |
| Cord blood | 3 (17.7%) | 6 (8.4%) | |
| Conditioning therapy | .11 | ||
| Myeloablative | 12 (70.6%) | 34 (47.9%) | |
| Reduced Intensity | 5 (29.4%) | 37 (52.1%) | |
| Conditioning agents received (yes versus. no) | |||
| Testicular radiation | 1 (5.9%) | 3 (4.2%) | 1.00 |
| Total body irradiation | 2 (11.85) | 10 (14.1%) | 1.00 |
| Busulfan | 9 (52.9%) | 23 (32.4%) | .16 |
| Cyclophosphamide | 12 (70.6%) | 34 (47.9%) | .11 |
| Antithymocyte globulin | 11 (64.7%) | 23 (32.4%) | .02 |
| Fludarabine | 7 (41.2%) | 44 (62.0%) | .17 |
| Melphalan | 5 (29.4%) | 38 (53.5%) | .10 |
| Alemtuzumab | 5 (29.4%) | 34 (47.9%) | .19 |
Data presented as n (%) unless otherwise indicated. Bold typeface indicates statistical significance.
Categorical variables with Fisher exact test, continuous variables with Mann-Whitney U.
The posttransplantation characteristics of the cohort are shown in Table 2. Subjects with hemorrhagic cystitis were more likely to develop adenoviremia at any time during the first 100 days and receive more platelet transfusions. Although both groups had low absolute lymphocyte counts at the time of peak viremia and peak viruria, there was no difference in these values between the groups. In the subjects with cystitis, 9 of 17 (53%) received cidofovir. Cidofovir was prescribed for persistent BK viremia after the date of hemorrhagic cystitis in 6 of 9 subjects and before cystitis for adenoviremia in 3 of 9. There was no significant difference between the groups in GVHD prophylaxis (Table 2). Only 2 children (both in the noncystitis group) received prophylaxis with tacrolimus or sirolimus. There was no significant difference between the groups in the maximum grade of acute GVHD, sinusoidal obstruction syndrome (2 of 71, 3% in control group, 0 of 17 in cystitis group), or the need for dialysis. Fluoroquinolones and intravenous immunoglobulin are reported as having potential activity against BK virus [26-30]. There was no difference in fluoroquinolone use between the groups and all patients received intravenous immunoglobulin to maintain the IgG level in the normal range for their age. No subject had their immunosuppression reduced based on the diagnosis of hemorrhagic cystitis. The median follow-up time for the entire cohort was 122 days (IQR, 85.5 to 128 days).
Table 2. Posttransplantation Characteristics of the Study Cohort in the First 100 Days.
| Hemorrhagic Cystitis (n = 17) | Controls (n = 71) | P Value* | |
|---|---|---|---|
| GVHD prophylaxis | |||
| Cyclosporine | 17 (100%) | 65 (91.65) | .59 |
| Antithymocyte globulin | 2 (11.8%) | 5 (7.0%) | .62 |
| Methylprednisolone | 9 (52.9%) | 44 (62.0%) | .58 |
| Methotrexate | 5 (29.4%) | 18 (25.3%) | .76 |
| Mycophenolate mofetil | 1 (5.9%) | 9 (12.7%) | .68 |
| Engraftment, median (IQR) | |||
| Neutrophil day+ | 12 (10 to 21) | 12 (10 to 20) | .40 |
| Platelet 20,000/μL day+ | 26 (19 to 38) | 25 (18 to 36) | .62 |
| Platelet 50,000/μL day+ | 31 (26.5 to 53.5) | 28 (19 to 43) | .18 |
| ALC at peak viremia (K/mcL) | 100 (60 to 150) | 120 (40 to 380) | .29 |
| ALC at peak viruria (K/mcL) | 395 (100 to 580) | 150 (40 to 340) | .20 |
| ALC at cystitis (K/mcL) | 200 (100 to 580) | - | - |
| Antimicrobials | |||
| Ciprofloxacin | 13 (76.5%) | 45 (63.4%) | .40 |
| Levofloxacin | 0 (0%) | 3 (4.2%) | 1.00 |
| Cidofovir | 9 (52.9%) | 12 (16.9%) | <.01 |
| GVHD | 7 (41.2%) | 23 (32.4%) | .57 |
| Thrombotic microangiopathy | 10 (58.8%) | 27 (38.0%) | .17 |
| Dialysis | 1 (5.9%) | 6 (8.4%) | 1.00 |
| Platelet transfusions, median (IQR) | 14 (12 to 17) | 11 (4 to 17) | .02 |
| Red cell transfusions, median (IQR) | 4 (2 to 7) | 4 (2 to 6) | .34 |
| BK viremia, median (IQR) | Before cystitis | In the first 100 days | |
| Number of plasma tests | 2 (2 to 4) | 3 (3 to 12) | <.01 |
| Peak copies/mL | 884 (0 to 1653) | 354 (0 to 2424) | .54 |
| Peak viremia threshold groups | |||
| 0 copies/mL | 6 (35%) | 35 (49%) | .42 |
| 1 to 9999 copies/mL | 9 (53%) | 27 (38%) | .28 |
| 10,000 to 99,999 copies/mL | 0 (0%) | 5 (7%) | .58 |
| ≥ 100,000 copies/mL | 2 (12%) | 4 (6%) | .33 |
| Other DNA viremia | Anytime in the first 100 days | ||
| Adenovirus | 9 (52.9%) | 18 (25.3%) | .04 |
| CMV | 4 (23.5%) | 18 (25.3%) | 1.00 |
| EBV | 7 (41.2%) | 17 (23.9%) | .22 |
| HHV-6 | 5 (29.4%) | 10 (14.1%) | .16 |
GVHD indicates graft-versus-host disease; ALC, absolute lymphocyte count; CMV, cytomegalovirus; EBV, Epstein-Barr virus. Data are presented as n (%) unless otherwise indicated. Bold typeface indicates statistical significance.
Categorical variables with Fisher exact test, continuous variables with Mann-Whitney U.
Plasma BK PCR Results
The 88 allogeneic recipients underwent a total of 883 plasma BK virus PCR tests (summarized in Table 2). The median time from the peak plasma PCR to the diagnosis of cystitis was 11.5 days (IQR, 9 to 14 days) in the 2 subjects with a peak >100,000 copies/mL and 4 days (IQR, 3 to 13 days) in the 9 subjects with a peak plasma PCR 1 to 9999 copies/mL. The significance of this difference is unknown given the 2 subjects with a peak plasma PCR ≥ 100,000 copies/mL before hemorrhagic cystitis had plasma testing initially performed for acute elevations in serum creatinine. Both of these children had a 2-log increase in their plasma PCR in the 2 to 3 months before the onset of cystitis. No other subject with cystitis had a log increase in their BK plasma PCR before symptoms.
Univariate Analysis of Time-Varying Exposures
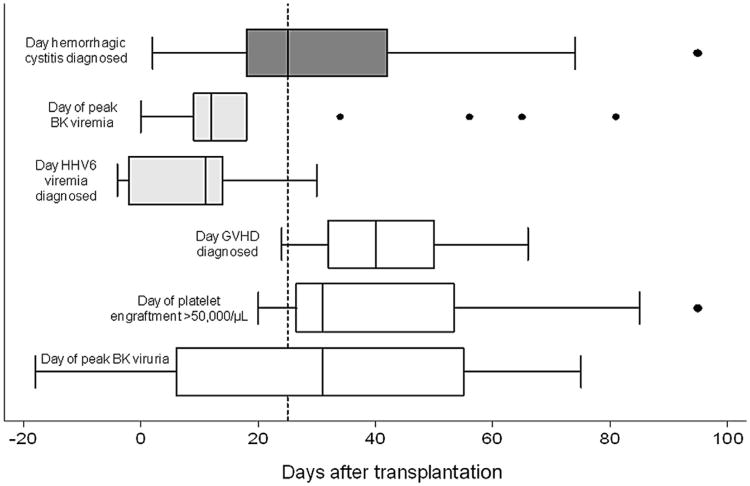
Subjects without BK viremia were not at increased risk for hemorrhagic cystitis, whereas those with a peak plasma PCR of 1 to 9999 copies/mL and ≥100,000 copies/mL did have a significantly higher risk of later cystitis (Table 3). We could not assess the cutoff level of 10,000 to 99,999 copies/mL because no cystitis subject had a peak PCR meeting this threshold before cystitis. There was no significant association between GVHD and the development of subsequent hemorrhagic cystitis. Point estimates suggested that achieving platelet engraftment was associated with a lower risk of cystitis at both a platelet count of >20,000/μL and a platelet count >50,000/μL, although not statistically significant. Subjects with HHV-6 viremia were significantly more likely to develop hemorrhagic cystitis with a HR of 7.6 (95% CI, 2.6 to 22.2). Figure 1 illustrates the timing of BK viremia, HHV-6 viremia, GVHD, platelet engraftment, and BK viruria in the 17 subjects with hemorrhagic cystitis.
Table 3. Univariate Analyses Assessing the Association of Time-Varying Exposures with Subsequent Hemorrhagic Cystitis.
| Variable | Hazard Ratio (95% Confidence Interval) | P Value |
|---|---|---|
| Primary Exposures | ||
| Peak BK plasma PCR* | ||
| 0 copies/mL | 0.9 (.3 to 2.5) | .83 |
| 1 to 9999 copies/ml | 5.3 (2.0 to 14.6) | <.01 |
| 10,000 to 99,999 copies/mL | No cystitis subject met this criterion | |
| ≥100,000 copies/mL | 34.3 (4.6 to 256.1) | <.01 |
| Secondary Exposures | ||
| Graft-versus-host disease | 1.0 (.2 to 4.9) | .99 |
| Thrombotic microangiopathy | 2.2 (.7 to 6.9) | .18 |
| Neutrophil engraftment | .6 (.1 to 2.7) | .49 |
| Platelet engraftment > 20,000/μL | .4 (.1 to 1.3) | .12 |
| Platelet engraftment > 50,000/μL | .3 (.1 to 1.4) | .14 |
| Adenoviremia > 0 copies/mL | 1.3 (.4 to 4.7) | .70 |
| CMV viremia > 0 copies/mL | 1.6 (.4 to 5.8) | .48 |
| EBV viremia > 0 copies/mL | 2.1 (.6 to 7.8) | .26 |
| HHV-6 viremia > 0 copies/mL | 7.6 (2.6 to 22.2) | <.01 |
CMV indicates cytomegalovirus; EBV, Epstein-Barr virus. Boldface indicates statistically significant.
Peak BK plasma PCR before cystitis in the hemorrhagic cystitis group and anytime during the first 100 days in the noncystitis group. For subjects with more than 1 identical peak value, the first date was used in the analysis.
Figure 1.
Timing of covariates in relation to the diagnosis of hemorrhagic cystitis. For each variable, box plots summarize data from the 17 subjects diagnosed with hemorrhagic cystitis. The dotted vertical line shows the median time to the diagnosis of cystitis after stem cell infusion (day +25). The median time to peak BK viremia and HHV-6 viremia occurred before cystitis was diagnosed. The median time to GVHD, platelet engraftment >50,000/μL, and peak BK viruria occurred after cystitis was diagnosed.
Multivariate Analysis
After adjusting for age, HHV-6 viremia, achieving platelet engraftment, EBV viremia, antithymocyte globulin, cyclophosphamide, and busulfan, BK viremia at cutoff levels of 1 to 9999 copies/mL and ≥ 100,000 copies/mL were independently associated with later hemorrhagic cystitis (Table 4). Age >7 years and HHV-6 viremia were also independently associated with hemorrhagic cystitis. Neither conditioning with busulfan or cyclophosphamide, the type of conditioning (myeloablative versus reduced intensity) received, or the development of TMA were significantly associated with later hemorrhagic cystitis after adjustment (data not shown).
Table 4. Adjusted Multivariate Models Exploring the Association between BK Viremia and Subsequent Hemorrhagic Cystitis*.
| Variable | Peak BK Viremia 1 to 9999 copies/mL | Peak BK Viremia ≥ 100,000 copies/mL | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Model 1 | Model 2 | Model 3 | Model 4 | Model 1 | Model 2 | Model 3 | Model 4 | |
| BK viremia | 5.2 (1.9 to 14.5) p<.01 | 4.8 (1.7 to 13.6) P <.01 | 6.4 (2.2 to 19.0) P<.01 | 4.2 (1.3 to 13.7) P = .02 | 41.4(5.1 to 336) P<.01 | 44.2 (5.6 to 346) P<.01 | 67.9 (7.1 to 651) P <.01 | 116.8 (12.0 to 1136) P <.01 |
| Age > 7 yr | 3.1 (1.0 to 9.4) P = .05 | 2.9 (.9 to 8.9) P = .06 | 3.8 (1.2 to 12.3) P = .02 | 7.7 (1.9 to 31.8) P <.01 | 3.5 (1.1 to 10.9) P = .03 | 3.9 (1.2 to 12.6) P = .02 | 3.5 (1.1 to 11.3) P = .04 | 9.2 (2.3 to 37.4) P <.01 |
| Antithymocyte globulin conditioning | - | 2.9(1.1 to 7.9) P = .04 | 33(1.2 to 9.2) P = .02 | 2.3 (.8 to 6.9) P = .13 | - | 3.3(1.2 to 9.0) P = .02 | 3.7 (1.3 to 10.5) P = .01 | 2.7 (.9 to 8.3) P = .09 |
| Platelet engraftment (> 50,000/μL) | - | - | .2(.05 to .9) P = .03 | .3 (.06 to 1.3) P = .ll | - | - | 0.2 (.04 to 1.2) P =.08 | 0.3 (.06 to 1.8) P = .21 |
| HHV-6 viremia | _ | _ | _ | 7.2 (1.8 to 29.8) P <.01 | _ | _ | _ | 16.9 (4.2 to 68.1) P <.01 |
Data presented as hazard ratios (95% confidence intervals). Bold typeface indicates statistical significance.
Adjusted for all variables listed. Busulfan, cyclophosphamide, type of conditioning (myeloablative versus reduced intensity), and posttransplantation thrombotic microangiopathy were not significant covariates when added to the models.
Discussion
We observed that BK viremia independently predicted the development of hemorrhagic cystitis in children receiving allogeneic HSCT. Analyzing BK viremia as a time-varying exposure, children with increasing peak plasma PCRs had a higher risk for cystitis in the first 100 days after transplantation. Neither GVHD nor achieving engraftment, when also considered as time-varying covariates, were associated with an increased risk of hemorrhagic cystitis. In the multivariate model, older patient age and HHV-6 viremia were also associated with cystitis.
Several adult reports have examined the association between BK viremia and hemorrhagic cystitis after HSCT, with conflicting results. Leung et al. [17] prospectively identified 6 of 50 (12%) patients developing grade ≥2 cystitis after engraftment. The level of viremia did not predict hemorrhagic cystitis, although formal testing for temporal associations was not performed. Erard et al. [16] reported BK viremia was associated with postengraftment hemorrhagic cystitis (n = 3) in 132 consecutive adults in a univariate, time-dependent analysis. Later, the same group used a case-control design to retrospectively examine 30 allogeneic recipients with grade ≥2 cystitis identified over a 24-year period [15]. Cases had BK virus plasma testing within 3 weeks before or during hemorrhagic cystitis and were matched to 81 controls on age. BK viremia >10,000 copies/mL had a sensitivity of 63% and a specificity of 95% for diagnosing hemorrhagic cystitis. Finally, Wong et al. [31] randomly selected 20 patients for BK virus plasma testing from a prospective cohort of 140 adults. BK viremia remained low (100 copies/mL) in the first 2 months after HSCT, precluding assessment of the relationship between viremia and clinical outcomes.
The conflicting associations between BK viremia and clinical disease reported in the literature may, in part, result because each institution uses its own PCR assay and BK virus exists as at least 4 distinct genotypes. Without standardized procedures, these technical issues regarding BK virus testing make comparisons across studies difficult. Along these lines, a recent case report of a kidney transplantation recipient demonstrated variable PCR results depending on which laboratory analyzed the specimens [32].
To the best of our knowledge, this is the largest study to analyze temporal associations between BK viremia and subsequent hemorrhagic cystitis in children after HSCT. Cesaro et al. [4] prospectively observed that 4 of 15 (27%) children receiving allogeneic transplantation developed grade ≥2 cystitis, and viremia >1000 copies/mL (which preceded cystitis by a median of 17 days) had a sensitivity of 40% and specificity of 93% for the detection of hemorrhagic cystitis. Others have evaluated the association between BK viremia and hemorrhagic cystitis, but not their timing. Gorcyznska et al. [2] prospectively examined 102 children and found 10 of 102 (10%) had at least 1 positive qualitative PCR for BK viremia, with 8 of 10 developing cystitis. Similar to our findings, hemorrhagic cystitis was less common in those <5 years of age, and there was no association between GVHD or total body irradiation and cystitis. Gaziev et al. [1] prospectively followed children undergoing HSCT for hemoglobinopathies from baseline until day 100. Of 64 patients, 34 tested had BK viremia on at least 1 specimen and 18 of 64 (28%) on at least 2 samples. Independent risk factors for hemorrhagic cystitis were antithymocyte globulin, peak BK viruria >100,000, and acute GVHD, but not BK viremia. Finally, our preliminary retrospective assessment in 21 children with BK viremia found that a peak plasma PCR >10,000 copies/mL in the first transplantation year was associated with higher grade cystitis, but we did not assess the timing of infection in relation to symptoms [18].
Children may have a different risk for BK virus-related disease compared with adults [33]. Older age was a significant risk factor for hemorrhagic cystitis in our patient population, as shown by others [2]. In fact, the median age of our subjects with hemorrhagic cystitis was 10 years, consistent with data from the general population, in which the BK seroprevalence rate is reported to reach 90% by about 10 years of age [34]. Although only speculation, this finding supports the hypothesis that BK virus infection after HSCT is secondary to latent viral reactivation rather than primary infection [10]. In a study of 140 adult HSCT recipients, higher pretransplantation BK virus IgG titers, a possible marker of latent infection, were associated with subsequent peaking of urine BK virus, but the authors were unable to assess its relationship with clinical disease because of their small sample size [31]. In contrast, a recent study in 50 children undergoing allogeneic HSCT found that 5 of 6 subjects with hemorrhagic cystitis were seronegative before transplantation [10]. Seroconversion after transplantation was associated with decreasing BK viremia and clinical improvement in the patients with cystitis.
The exact mechanism of bladder injury from BK virus after HSCT remains unknown. Leung et al. [9] proposed that conditioning chemotherapy first damages the bladder. The combination of decreased viral-specific immunity from chemotherapy and GVHD prophylaxis allows BK virus to reactivate, which becomes apparent as increasing BK viruria. Finally, donor immune cells attack the bladder epithelium, leading to hemorrhagic cystitis. This “immune reconstitution” phenomenon is reported to occur after engraftment in the presence of GVHD [1,3,7,31]. However, hemorrhagic cystitis is not always associated with GVHD [2,15,17,35], and others have refuted this conceptual model given that hemorrhagic cystitis occurs during periods of lymphopenia [15]. Additionally, high-dose corticosteroids, which would presumably attenuate an immune reconstitution response, have also been associated with an increased risk of hemorrhagic cystitis and BK viremia [16].
We did not find that GVHD or achieving engraftment increased the risk for hemorrhagic cystitis in our time-varying analyses. Myeloablative conditioning and/or the use of cyclophosphamide or busulfan were also not associated with hemorrhagic cystitis, in contrast to prior reports [15,35]. Antithymocyte globulin was associated with cystitis in our univariate analyses, possibly supporting the role of significant immunosuppression and decreased virus-specific T cells in the pathogenesis of disease. As patients often received combinations of these conditioning therapies, it was not possible to determine the risk associated with individual agents in the multivariate analyses. Nevertheless, we speculate that latent BK virus reactivation occurs in the absence of virus-specific T cells that normally prevent viral replication, leading to subclinical bladder cell lysis and detectable viremia before the onset of overt cystitis [10]. Although we did not observe a difference in absolute lymphocyte counts between the groups, the role of BK-specific T cells in the pathogenesis of BK virus-associated genitourinary disease is supported by preliminary studies after HSCT and kidney transplantation and deserves further study [10,36-38].
The potential role of virus-specific immunity is also strengthened by our finding that HHV-6 viremia predicted the development of hemorrhagic cystitis. EBV, CMV, adenovirus, and HHV-6, like BK virus, are also DNA viruses frequently contributing to disease in transplantation recipients. Adenovirus and CMV are less commonly associated with hemorrhagic cystitis, alone or in combination with BK virus [3,9,39]. Wang et al. reported that HHV-6 viremia (HHV-6B variant) may be associated with hemorrhagic cystitis in 72 allogeneic recipients (HR, 2.6; CI, 1.0 to 7.0; P = .06), but did not assess for BK virus infection in their patients [40]. One case report also describes an association between HHV-6 viremia and hemorrhagic cystitis [41]. We observed that 6 of 17 of our subjects with hemorrhagic cystitis did not have BK viremia of any degree before symptoms, suggesting either that BK virus is not the sole cause of bladder injury or that we missed the window of detection in these subjects. The role of HHV-6 infection after HSCT is still being defined, but typically involves central nervous system disease [42]. The strong association between HHV-6 viremia, BK viremia, and hemorrhagic cystitis we observed may point to a novel viral cause of bladder injury or simply reflect the degree of immunosuppression after HSCT. Interestingly, HHV-6 has been reported to decrease human leukocyte antigen class I expression in an effort to evade cytotoxic T cell attack, potentially increasing the susceptibility to other infections [42-44].
The strengths of our analysis include the prospective enrollment of consecutive HSCT recipients combined with the prospective collection of samples and clinical data. This allowed for the rigorous assessment of multiple potential covariates using time-dependent survival methods. Past studies using only dichotomous characterization of dynamic variables (ie, GVHD and engraftment) may have lead to biased inferences. Finally, the inclusion of children permitted adjustment for age in a patient population that may have a different seroprevalence of infection compared with adults [10,34].
Although we leveraged data from a completed prospective cohort, the cases of hemorrhagic cystitis were identified by reviewing the medical record. We believe the likelihood of significant misclassification bias was low as the review of nursing note assessments and physician progress notes was supplemented by urinalysis results collected prospectively on all participants at weekly intervals while admitted. It is possible that ascertainment bias resulted from more frequent plasma PCR testing in sicker patients, especially because plasma BK testing was typically ordered in those with microscopic hematuria. However, when analyzing only PCR results occurring before hemorrhagic cystitis, we actually found that patients with cystitis had less frequent BK plasma testing. Finally, we did not have sufficient urine PCR results to test the association between viruria and hemorrhagic cystitis. Prior studies examining BK viruria and hemorrhagic cystitis after HSCT have reported that a ≥3-log increase in BK viruria over baseline was associated with the development of cystitis [17,29,31]. Future research is needed to systematically measure viruria, viremia, and outcomes in all subjects and in a larger study population.
After kidney transplantation, BK viremia >10,000 copies/mL is highly predictive of biopsy-proven nephropathy [13]. Similar to HSCT, BK viruria is likely nonspecific after kidney transplantation [45]. Therefore, current consensus kidney transplantation guidelines recommend BK plasma PCR screening monthly for the first 3 to 6 months, then every 3 months until the first year after kidney transplantation [14]. Similar guidelines are currently not available in the HSCT population as the optimal frequency of BK plasma testing is not known [42]. It is possible that monthly screening after HSCT is not sufficient and that similar to CMV and EBV, at least weekly screening is required in the immediate posttransplantation period in children.
Retrospective studies in HSCT recipients suggest that BK viremia >10,000 copies/mL may also be associated with hemorrhagic cystitis [15,18]. Although we were unable to demonstrate that this 10,000 copy/mL cutoff was associated with disease, we did observe that even higher BK plasma viral loads independently predicted subsequent cystitis. It also remains unknown why hemorrhagic cystitis is more common after HSCT compared with after kidney transplantation. Hemorrhagic cystitis may result from a more profound decrease in virus-specific T cells, supported by our observations that concomitant viremia (HHV-6), delayed engraftment, and T cell depleting therapy were potentially associated with cystitis.
In the first 100 days after HSCT, severe infections affect up to 15% of patients [46] and infection remains a common cause of death in allogeneic recipients [47]. Decreasing the burden of viral disease would hopefully improve outcomes after HSCT. The strong association between BK viremia and clinically significant hemorrhagic cystitis justifies the prospective assessment of prophylactic and treatment strategies [3]. Causal evidence would be strengthened if treating or preventing BK viremia resulted in a lower risk of hemorrhagic cystitis [9]. Preliminary reports have suggested that fluoroquinolone prophylaxis (because of its antitopoisomerase activity) reduces BK viruria, but not hemorrhagic cystitis, after HSCT and BK viremia after kidney transplantation [27-29]. In children receiving kidney transplantations, early reduction of immunosuppression after the detection of BK viremia has also prevented BK virus nephropathy [48]. If these or other strategies are shown to be effective in HSCT recipients, it may support frequent plasma monitoring for BK virus, as currently recommended for other DNA viruses such as CMV and adenovirus [42]. Finally, further research is needed to determine if HHV-6 viremia causes hemorrhagic cystitis or if our observed effect simply reflects an overall degree of immunosuppression.
Acknowledgments
Financial disclosure: B.L.L. and this work are supported by a Career Development Award in Comparative Effectiveness Research (KM1CA156715-01). The REDCap database is supported by a Cincinnati Children's Hospital Center for Clinical and Translational Science and Training grant (UL1-RR026314-01 NCRR/NIH). M.D. is supported by K23DK093556 and the Nephcure Foundation-American Society of Nephrology Research Grant. S.F. is supported by K24DK078737 and U01DK066174. None of these funding sources had any input in the study design, analysis, manuscript preparation, or decision to submit for publication.
Footnotes
Financial disclosure: See Acknowledgements on page 7.
References
- 1.Gaziev J, Paba P, Miano R, et al. Late-onset hemorrhagic cystitis in children after hematopoietic stem cell transplantation for thalassemia and sickle cell anemia: a prospective evaluation of polyoma (BK) virus infection and treatment with cidofovir. Biol Blood Marrow Transplant. 2010;16:662–671. doi: 10.1016/j.bbmt.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Gorczynska E, Turkiewicz D, Rybka K, et al. Incidence, clinical outcome, and management of virus-induced hemorrhagic cystitis in children and adolescents after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2005;11:797–804. doi: 10.1016/j.bbmt.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Dropulic LK, Jones RJ. Polyomavirus BK infection in blood and marrow transplant recipients. Bone Marrow Transplant. 2008;41:11–18. doi: 10.1038/sj.bmt.1705886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cesaro S, Facchin C, Tridello G, et al. A prospective study of BK-virus-associated haemorrhagic cystitis in paediatric patients undergoing allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41:363–370. doi: 10.1038/sj.bmt.1705909. [DOI] [PubMed] [Google Scholar]
- 5.Miano M, Faraci M, Dini G, Bordigoni P. Early complications following haematopoietic SCT in children. Bone Marrow Transplant. 2008;41(Suppl 2):S39–S42. doi: 10.1038/bmt.2008.53. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto R, Kusumi E, Kami M, et al. Late hemorrhagic cystitis after reduced-intensity hematopoietic stem cell transplantation (RIST) Bone Marrow Transplant. 2003;32:1089–1095. doi: 10.1038/sj.bmt.1704261. [DOI] [PubMed] [Google Scholar]
- 7.Decker DB, Karam JA, Wilcox DT. Pediatric hemorrhagic cystitis. J Pediatr Urol. 2009;5:254–264. doi: 10.1016/j.jpurol.2009.02.199. [DOI] [PubMed] [Google Scholar]
- 8.Bedi A, Miller CB, Hanson JL, et al. Association of BK virus with failure of prophylaxis against hemorrhagic cystitis following bone marrow transplantation. J Clin Oncol. 1995;13:1103–1109. doi: 10.1200/JCO.1995.13.5.1103. [DOI] [PubMed] [Google Scholar]
- 9.Leung AY, Yuen KY, Kwong YL. Polyoma BK virus and haemorrhagic cystitis in haematopoietic stem cell transplantation: a changing paradigm. Bone Marrow Transplant. 2005;36:929–937. doi: 10.1038/sj.bmt.1705139. [DOI] [PubMed] [Google Scholar]
- 10.Koskenvuo M, Dumoulin A, Lautenschlager I, et al. BK polyomavirusassociated hemorrhagic cystitis among pediatric allogeneic bone marrow transplant recipients: Treatment response and evidence for nosocomial transmission. J Clin Virol. 2013;56:77–81. doi: 10.1016/j.jcv.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Arthur RR, Shah KV, Baust SJ, et al. Association of BK viruria with hemorrhagic cystitis in recipients of bone marrow transplants. N Engl J Med. 1986;315:230–234. doi: 10.1056/NEJM198607243150405. [DOI] [PubMed] [Google Scholar]
- 12.Hale GA, Rochester RJ, Heslop HE, et al. Hemorrhagic cystitis after allogeneic bone marrow transplantation in children: clinical characteristics and outcome. Biol Blood Marrow Transplant. 2003;9:698–705. doi: 10.1016/s1083-8791(03)00269-6. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch HH, Knowles W, Dickenmann M, et al. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med. 2002;347:488–496. doi: 10.1056/NEJMoa020439. [DOI] [PubMed] [Google Scholar]
- 14.KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1–S155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 15.Erard V, Kim HW, Corey L, et al. BK DNA viral load in plasma: evidence for an association with hemorrhagic cystitis in allogeneic hematopoietic cell transplant recipients. Blood. 2005;106:1130–1132. doi: 10.1182/blood-2004-12-4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erard V, Storer B, Corey L, et al. BK virus infection in hematopoietic stem cell transplant recipients: frequency, risk factors, and association with postengraftment hemorrhagic cystitis. Clin Infect Dis. 2004;39:1861–1865. doi: 10.1086/426140. [DOI] [PubMed] [Google Scholar]
- 17.Leung AY, Suen CK, Lie AK, et al. Quantification of polyoma BK viruria in hemorrhagic cystitis complicating bone marrow transplantation. Blood. 2001;98:1971–1978. doi: 10.1182/blood.v98.6.1971. [DOI] [PubMed] [Google Scholar]
- 18.Haines HL, Laskin BL, Goebel J, et al. Blood, and not urine, BK viral load predicts renal outcome in children with hemorrhagic cystitis following hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:1512–1519. doi: 10.1016/j.bbmt.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Watzinger F, Suda M, Preuner S, et al. Real-time quantitative PCR assays for detection and monitoring of pathogenic human viruses in immunosuppressed pediatric patients. J Clin Microbiol. 2004;42:5189–5198. doi: 10.1128/JCM.42.11.5189-5198.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hornung RW, Reed L. Estimation of average concentration in the presence of nondetectable values. ApplOccup Environ Hyg. 1990;5:46–51. [Google Scholar]
- 21.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coppell JA, Richardson PG, Soiffer R, et al. Hepatic veno-occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biol Blood Marrow Transplant. 2010;16:157–168. doi: 10.1016/j.bbmt.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho VT, Cutler C, Carter S, et al. Blood and marrow transplant clinical trials network toxicity committee consensus summary: thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:571–575. doi: 10.1016/j.bbmt.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Ruutu T, Barosi G, Benjamin RJ, et al. Diagnostic criteria for hematopoietic stem cell transplant-associated microangiopathy: results of a consensus process by an International Working Group. Haematologica. 2007;92:95–100. doi: 10.3324/haematol.10699. [DOI] [PubMed] [Google Scholar]
- 25.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Randhawa PS, Schonder K, Shapiro R, et al. Polyomavirus BK neutralizing activity in human immunoglobulin preparations. Transplantation. 2010;89:1462–1465. doi: 10.1097/tp.0b013e3181daaaf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gabardi S, Waikar SS, Martin S, et al. Evaluation of fluoroquinolones for the prevention of BK viremia after renal transplantation. Clin J Am Soc Nephrol. 2010;5:1298–1304. doi: 10.2215/CJN.08261109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Randhawa PS. Anti-BK virus activity of ciprofloxacin and related antibiotics. Clin Infect Dis. 2005;41:1366–1367. doi: 10.1086/497080. author reply 1367. [DOI] [PubMed] [Google Scholar]
- 29.Leung AY, Chan MT, Yuen KY, et al. Ciprofloxacin decreased polyoma BK virus load in patients who underwent allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2005;40:528–537. doi: 10.1086/427291. [DOI] [PubMed] [Google Scholar]
- 30.Sener A, House AA, Jevnikar AM, et al. Intravenous immunoglobulin as a treatment for BK virus associated nephropathy: one-year follow-up of renal allograft recipients. Transplantation. 2006;81:117–120. doi: 10.1097/01.tp.0000181096.14257.c2. [DOI] [PubMed] [Google Scholar]
- 31.Wong AS, Chan KH, Cheng VC, et al. Relationship of pre transplantation polyoma BK virus serologic findings and BK viral reactivation after hematopoietic stem cell transplantation. Clin Infect Dis. 2007;44:830–837. doi: 10.1086/511863. [DOI] [PubMed] [Google Scholar]
- 32.Trofe-Clark J, Sparkes T, Gentile C, et al. BK Virus Genotype Variance and Discordant BK Viremia PCR Assay Results. Am J Transplant. 2013;13:1112–1113. doi: 10.1111/ajt.12169. [DOI] [PubMed] [Google Scholar]
- 33.Egli A, Infanti L, Dumoulin A, et al. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis. 2009;199:837–846. doi: 10.1086/597126. [DOI] [PubMed] [Google Scholar]
- 34.Knowles WA, Pipkin P, Andrews N, et al. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol. 2003;71:115–123. doi: 10.1002/jmv.10450. [DOI] [PubMed] [Google Scholar]
- 35.Giraud G, Priftakis P, Bogdanovic G, et al. BK-viruria and haemorrhagic cystitis are more frequent in allogeneic haematopoietic stem cell transplant patients receiving full conditioning and unrelated-HLA-mismatched grafts. Bone Marrow Transplant. 2008;41:737–742. doi: 10.1038/sj.bmt.1705962. [DOI] [PubMed] [Google Scholar]
- 36.Schneidawind D, Schmitt A, Wiesneth M, et al. Polyomavirus BK-specific CD8+ T cell responses in patients after allogeneic stem cell transplant. Leuk Lymphoma. 2010;51:1055–1062. doi: 10.3109/10428191003746323. [DOI] [PubMed] [Google Scholar]
- 37.Schachtner T, Muller K, Stein M, et al. BK virus-specific immunity kinetics: a predictor of recovery from polyomavirus BK-associated nephropathy. AmJ Transplant. 2011;11:2443–2452. doi: 10.1111/j.1600-6143.2011.03693.x. [DOI] [PubMed] [Google Scholar]
- 38.Comoli P, Binggeli S, Ginevri F, Hirsch HH. Polyomavirus-associated nephropathy: update on BK virus-specific immunity. Transpl Infect Dis. 2006;8:86–94. doi: 10.1111/j.1399-3062.2006.00167.x. [DOI] [PubMed] [Google Scholar]
- 39.O'Donnell PH, Swanson K, Josephson MA, et al. BK virus infection is associated with hematuria and renal impairment in recipients of allogeneic hematopoetic stem cell transplants. Biol Blood Marrow Transplant. 2009;15:1038–1048. e1. doi: 10.1016/j.bbmt.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang LR, Dong LJ, Zhang MJ, Lu DP. The impact of human herpesvirus 6B reactivation on early complications following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006;12:1031–1037. doi: 10.1016/j.bbmt.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 41.Kim YJ, Kim DW, Lee DG, et al. Human herpesvirus-6 as a possible cause of encephalitis and hemorrhagic cystitis after allogeneic hema-topoietic stem cell transplantation. Leukemia. 2002;16:958–959. doi: 10.1038/sj.leu.2402403. [DOI] [PubMed] [Google Scholar]
- 42.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glosson NL, Hudson AW. Human herpesvirus-6A and -6B encode viral immunoevasins that downregulate class I MHC molecules. Virology. 2007;365:125–135. doi: 10.1016/j.virol.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 44.Gerdemann U, Keukens L, Keirnan JM, et al. Immunotherapeutic strategies to prevent and treat human herpesvirus 6 reactivation after allogeneic stem cell transplantation. Blood. 2013;121:207–218. doi: 10.1182/blood-2012-05-430413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laskin BL, Goebel J. Cost-efficient screening for BK virus in pediatric kidney transplantation: a single-center experience and review of the literature. Pediatr Transplant. 2010;14:589–595. doi: 10.1111/j.1399-3046.2010.01318.x. [DOI] [PubMed] [Google Scholar]
- 46.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplant recipients: a global perspective. Preface Bone Marrow Transplant. 2009;44:453–455. doi: 10.1038/bmt.2009.254. [DOI] [PubMed] [Google Scholar]
- 48.Ginevri F, Azzi A, Hirsch HH, et al. Prospective monitoring of polyomavirus BK replication and impact of pre-emptive intervention in pediatric kidney recipients. AmJ Transplant. 2007;7:2727–2735. doi: 10.1111/j.1600-6143.2007.01984.x. [DOI] [PubMed] [Google Scholar]