Abstract
Nonobese diabetic (NOD) mice develop spontaneous autoimmune Type 1 diabetes (T1D) that results from the destruction of insulin secreting β cells by diabetogenic T cells. The activation of autoreactive T cells occurs in the pancreatic lymph nodes (PLN) from where effector T cells migrate to the pancreas. This study was designed to explore whether T cell populations in the NOD PLN expand in a predictable and reproducible way during disease progression. Complementary determining region (CDR) 3 length spectratype analysis of 19 TCR Vβ families was used to identify the relative frequency of T populations in PLN of 4 and 10 week old NOD mice and mice at T1D onset. Significant and highly reproducible changes in specific T cell populations were detected in 14 of Vβ families tested at all stages of disease. However, of these, the CDR3 spectratype of only four Vβ families was significantly more perturbed at T1D onset than in 10 week old mice. Intriguingly, when diabetes was induced in 10 week old mice with cyclophosphamide (CYP) the same four Vβ families, Vβ5.1, Vβ9, Vβ10, and Vβ15, were again significantly more perturbed than in the untreated non-diabetic age matched mice. Taken together the data show that while T cell responses in PLN of NOD mice are heterogeneous, they are ordered and consistent throughout disease development. The finding that within this heterogeneous response four Vβ families are significantly more perturbed in diabetic mice, whether spontaneous or induced, strongly suggests their selection as part of the disease process.
Keywords: Autoimmune disease, TCR, CDR3, Diabetes, Pancreatic lymph nodes, Perturbation
1. Introduction
Type 1 diabetes (T1D) in both humans and NOD mice is the result of a slowly progressing destruction of the insulin-producing β cells within pancreatic islets by auto-reactive T cells (Anderson and Bluestone, 2005; Tisch and McDevitt, 1996; Large et al., 1995; Makino et al., 1980). Pancreatic lymph nodes (PLN) contain APC that present β-cell antigens which migrate from islets into the draining PLN (Höglund et al., 1999; Sarukhan et al., 1999). There, auto-reactive T cells are primed as early as 2 weeks of age (Turley et al., 2003; Gagnerault et al., 2002; Höglund et al., 1999; Fabien et al., 1995). However, changes in the T cell repertoire in the PLN during the disease process have not been closely examined. In this study, we compared the PLN TCR repertoire between early and late stages of disease to determine whether T cell populations expand randomly in the periphery, or if there is an ordered sequence of events in which the same T cell populations expand in PLN of all, or the majority of NOD mice during disease development.
T cell specificity is determined by the variable (Vα and Vβ) and constant (Cα and Cβ) regions of the TCR. Both the Vβ and the Vα chains are encoded by variable (V) and junctional (J) gene segments, and the Vβ chain has additional diversity (D) gene segments. The diverse TCRβ repertoire is generated by random association of V-D-J segments during somatic gene rearrangement. Junctional diversity created by nucleotide deletions and insertions at the V-J and D-J junction results in CDR3s of different lengths and with different sequences (Davis and Bjorkman, 1988; Tonegawa, 1983). Since the CDR3 region interacts most closely with peptide-MHC molecules the heterogeneity of the CDR3 correlates with T cell diversity. Therefore, CDR3 length spectratyping can identify changes in T cell diversity by determining the relative frequency of T cell populations based on their CDR3 length distribution (Nikolich-Zugich et al., 2004; Ria et al., 2001; Pannetier et al., 1995; Cibotti et al., 1994; Pannetier et al., 1993, Cochet et al., 1992). In the unprimed animal the CDR3 length distribution is Gaussian-like, usually composed of T cell populations with 8-10 different CDR3 lengths (Kronenberg et al, 1986). In contrast, antigen-primed animals exhibit clonal expansion that can be identified by an increase in relative frequency of T cells with one CDR3 length and perturbation away from the Gaussian distribution.
Several analyses of the TCR repertoire of pancreatic T cells have shown a restricted TCR repertoire in islet infiltrates from young NOD mice, with biased usage of particular TCR Vβ regions and/or conserved amino acid sequences of the TCR CDR3 (Baker et al., 2002; Yang et al., 1996; Galley and Danska, 1995; Sarukhan et al., 1994a; Drexler et al., 1993; Maeda et al., 1991). However, in islet infiltrates of older mice such biased TCR Vβ usage remains inconclusive with some reports showing Vβ restriction and others showing no restriction (Simone et al., 1997; Galley and Danska, 1995; Sarukhan et al., 1994a; Berschick et al., 1993; Drexler et al., 1993; Koide et al., 1993; Waters et al., 1992; Candéias et al., 1991; Maeda et al., 1991; Nakano et al., 1991). The data to date suggest that the TCR repertoire in the islet of older NOD mice and in the lymph node of mice at any age is heterogeneous (Baker et al., 2002; Yang et al., 1996; Galley and Danska, 1995; Drexler et al., 1993; Nakano et al., 1991). Consistent with data from other investigators we have shown that significant perturbations of the TCR Vβ repertoire occur in PLN before 4 weeks of age (Petrovic et al., 2008). In an attempt to identify disease related responses in the PLN, in this study we ask whether within a heterogeneous T cell population there are consistent differences in the TCR repertoire in PLN of pre-diabetic mice and mice at TID onset.
Treatment with Cyclophosphamide (CYP) is a well-established method used to accelerate diabetes onset in NOD mice (Bai et al., 2006; Harada and Makino, 1984). Different mechanisms are involved in this effect, including depletion of CD4+CD25+Foxp3+ regulatory cells (Tregs) (Brode et al., 2006). CYP was used in this study to determine whether differences in the TCR repertoire between 10 week old mice and mice with T1D are associated with age or disease. We show that within a heterogeneous response, T cells in a limited number of Vβ families expand in PLN in both spontaneously diabetic mice and in CYP-induced diabetes compared to their respective non-diabetic controls. These data strongly suggest that diabetes-associated T cell responses are selected in the PLN, controlled by Tregs, and then expand when Treg function is overcome at the onset of disease. Our data suggest that a better understanding of the T cell response in PLN might identify T cell populations that cause T1D.
2. Materials and Methods
2.1. Mice
Female NOD/ShiLtJ (NOD) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were maintained in a specific pathogen-free animal facility at the Torrey Pines Institute for Molecular Studies (San Diego, CA). Mice were used between 4 and 20 weeks of age. All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) and were performed in accordance with institutional guidelines for animal care.
2.2. Diabetes onset
Mice were monitored for diabetes twice a week by measuring urine glucose using Chemstrip uGK Urine Test Strips (Roche Diagnostics, Indianapolis, IN). After a positive urine test, hyperglycemia was verified by measuring blood glucose levels using Accu-Check Compact Plus (Roche Diagnostics). Mice with glucose levels >250 mg/dl were tested the next day and were considered diabetic if the second reading was also >250 mg/dl. Diabetes onset in diabetic NOD mice included in this study ranged from 12 weeks to 17 weeks of age with a mean at 15 weeks of age. In this group, PLN were isolated the same day that the mice were considered diabetic.
2.3. Cell preparation
Single cell suspensions of PLN and thymus were prepared in RPMI 1640 (GIBCO, Invitrogen, San Diego, CA) using 70 μm nylon mesh cell strainer (BD Biosciences). After one wash cell viability was determined by trypan blue exclusion
2.4. Cyclophosphamide (CYP) treatment
CYP (Sigma, St Louis, Mo) was prepared in 0.9% normal saline at 1 mg/ml immediately before administration. Female NOD mice (8 weeks of age) were injected intraperitoneally twice at a 1-week interval at a dose of 200 mg/Kg. Mice were monitored for diabetes biweekly as described above. In our colony all CYP-treated mice become diabetic one week after the second CYP dose.
2.5. Extraction of RNA and cDNA synthesis
Total RNA was extracted from single cell suspensions of either PLN or thymus of individual mice using the RNeasy Mini Kit (Qiagen, Valencia, CA). The cDNA was synthesized from total RNA (1 to 2 μg) using 0.5 μg Oligo(dT)12-18, 200 units SuperScript II RT (Invitrogen, San Diego, CA), 10 mM of each dNTP, in a total volume of 20 μl. The cDNA was stored at −80°C before used as the template for PCR amplification.
2.7. Measurement of TCR Vβ usage and CDR3 length
CDR3 length spectratype analysis was performed using modifications of the protocol described by Pannetier el al. (1993). Primer sequences for mouse Vβ and Cβ segments were synthesized at Operon Technologies (Alameda, CA). The first-round PCR amplification reactions for each of the 19 Vβ gene families tested were carried out with 1 μl of cDNA in 25 μl reaction mixtures containing 1 μM of each forward Vβ-specific primer (Pannetier et al., 1993), 0.5 μM of the reverse Cβ145 primer (CACTGATGTTCTGTGTGACA), 1X PCR buffer II (Applied Biosystems, Foster City, CA), 1.5 mM MgCl2 (Applied Biosystems), 0.25 mM dNTP mixture (Applied Biosystems), and 0.75 U of AmpliTaq DNA Polymerase (Applied Biosystems) at 94°C for 5 min, one cycle; then 94°C for 45 s, 60°C for 45 s, 72°C for 45 s, 39 cycles, and a final elongation step at 72°C for 10 min. Two microliters of the PCR products were used as templates for second-round PCR amplification in 25 μl of reaction using 1 μM of fluorescent-conjugated (6-FAM) Cβ5 primer (Pannetier et al., 1993), 1X PCR buffer II, 3.25 mM MgCl2, 0.2 mM dNTP mixture and 0.5 U of Taq DNA polymerase (New England BioLabs, Ipswich, MA) at 94°C for 30 sec, one cycle; then 94°C for 45 s, 55°C for 45 s, 72°C for 1 min, 15 cycles, and a final elongation step at 72°C for 5 min. All PCR reactions were performed on a 96-well GeneAmp PCR System 9700 (Applied Biosystems). The mixture containing 2 μl fluorescent PCR products was denatured in deionized formamide with a GeneScan 400 HD [ROX] size standard (Applied Biosystems) at 94°C for 2 min, and cooled on ice for 5 min. Fragment analysis of the denatured products was performed using ABI PRISM 3100 Genetic Analyzer. The data were analyzed by GeneMapper Software V4.0 (Applied Biosystems) to obtain the CDR3 spectratype profile for each Vβ family in individual mice.
2.8. Identification of T cell populations that have expanded in PLN using CDR3 length and Vβ usage
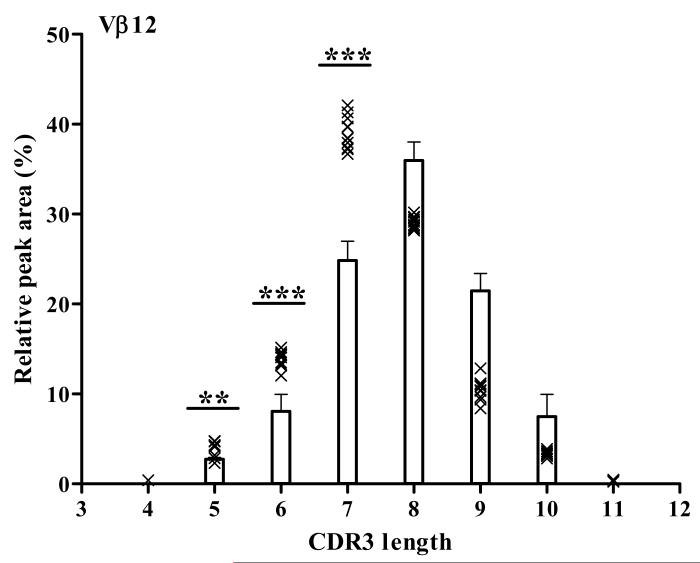
The CDR3 spectratype profile is seen as a series of peaks where each peak corresponds to a given CDR3 length. Each peak may contain multiple CDR3 sequences representing different T cell clones. The area underneath a single peak is proportional to the number of T cells that share that particular CDR3 length within a given Vβ-Cβ pairing. Thus, when expressed as a percentage of the total area under all peaks in the spectratype, the area of a single peak can be used to determine the contribution of T cells with that particular CDR3 length to the profile. Since the TCR repertoire in the thymus is relatively unaffected by peripheral antigen we expect that maximum diversity, indicated by a Gaussian distribution in the CDR3 spectratype, will be seen in thymus. Therefore, we compared the percentage of total area of each peak in PLN with that in thymus from 8 week old female NOD mice to determine the expansion of T cells within a Vβ family in PLN. PLN T cell populations within a CDR3 spectratype are considered significantly expanded if the area of any peak within that spectratype exceeds 3 SDs above the mean of the corresponding peak for the thymus (Ahmed et al., 2009; Scott Killian et al., 2002). An example of data analyzed using this method is shown for the TCR Vβ12 CDR3 spectratype in PLN and thymus in Fig. 1. The bars represent T cell populations in the thymus with CDR3 lengths of 4-11 amino acids (x axis). The relative peak area (%) is the contribution of each T cell population with a given CDR3 length to the total T cell population expressing Vβ12 (y axis). The bars show the mean and 3SD of the relative peak area for thymus from 5 mice. Each symbol (x) represents the relative peak area of each T cell population identified by CDR3 length in PLN of individual mice (n=11). Of note, three peaks corresponding to T cell populations with CDR3 lengths of 5, 6, and 7 amino acids were significantly greater in PLN compared to thymus. In summary, this method identifies T cell populations by their TCR Vβ usage and CDR3 length. The contribution of each T cell population to the PLN is compared to the contribution of the same total T cell population in the thymus. A significant increase in the contribution of any T cell population in the PLN compared to thymus indicates an expansion in that T cell population.
Figure 1. Identification of expanded T cell populations in the TCR Vβ 12 family in PLN.
In this example, the relative areas of individual CDR3 peaks were calculated as a percentage of the total area under all peaks for Vβ12. The mean and SD of each relative peak area for Vβ12 was calculated for thymus (n=5) and the mean ± 3 SD was plotted for each peak (bars). The relative peak area for T cell populations with different CDR3 lengths in individual 4 week old NOD mice (n=11) are plotted as x in the graph. The peaks in PLN that are significantly different from the equivalent peak in thymus are indicated with asterisk above the respective bar: ***, p<0.0001; **, p<0.001 or *, p<0.01 by ANOVA. The x-axis shows the CDR3 length in amino acid and the y-axis shows the relative peak area (%).
2.9. Statistical Analysis
Spectratype data from PLN of NOD mice at 4 and 10 weeks of age and at T1D onset were compared to thymus using one-way analysis of variance (ANOVA), followed by either Dunnett’s or Bonferroni’s multiple comparison tests. Comparisons of total perturbation and global perturbation indexes between groups were performed using the unpaired t test. The strategy for calculating the total and gobal perturbation indexes are shown in Supplementary Fig. 1. Statistical significance is represented as *=p<0.05, **= p<0.01, and ***=p< 0.001. Significance was determined using the data presented in each figure.
3. Results
3.1. The majority of 4 week old NOD mice display the same altered TCR Vβ repertoire in pancreatic lymph nodes
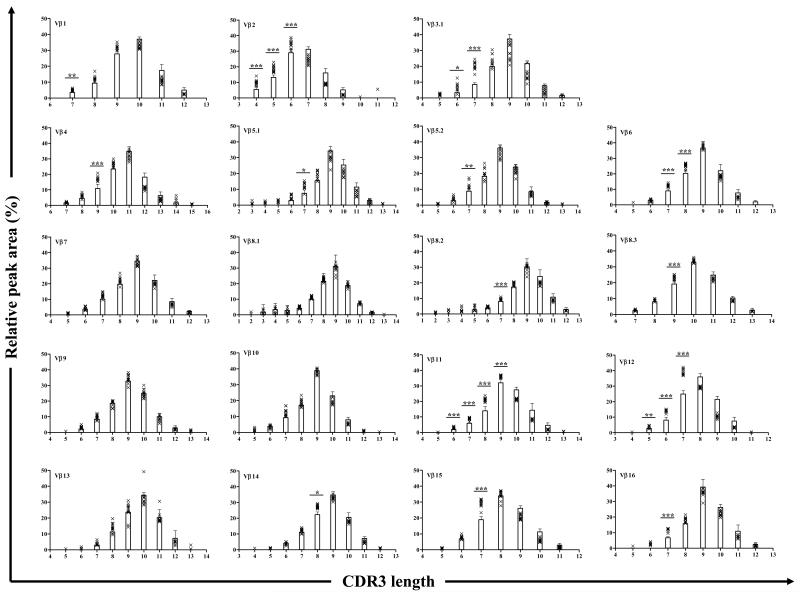
To identify T cell populations that are naturally expanded in young pre-diabetic NOD mice the TCR repertoire of PLN T cells of 4 week old mice was compared to that of thymus using TCR Vβ and CDR3 length analysis as described in the Materials and Methods. As expected the thymus CDR3 spectratypes are Gaussian-like indicating a diverse repertoire (Fig. 2). These data are consistent with a previous report on TCR repertoire diversity in the thymus of NOD mice (Sarukhan et al., 1994b). Although the spectratype of each Vβ family in PLN displayed a Gaussian-like distribution, we found that 23 CDR3 peaks within 14 Vβ families are significantly larger in PLN compared to thymus. Moreover, these T cell populations were present in at least 80% of mice tested suggesting dominant responses. Thus, Vβ1, Vβ4, Vβ5.1, Vβ5.2, Vβ8.2, Vβ8.3, Vβ14, Vβ15 and Vβ16 are significantly different at a single peak, and Vβ2, Vβ3.1, Vβ6, Vβ11 and Vβ12 are significantly different at two or more peaks (Fig. 2). In the Vβ2 and Vβ12 families the dominant central peak is different in PLN and thymus. Thus, for Vβ2 the dominant central peak has a CDR3 length of 7 amino acids in the thymus but 6 amino acids in the PLNs. Likewise, for Vβ12 the dominant peak has a CDR3 length of 8 amino acids in the thymus but 7 amino acids in PLN (Supplementary Fig. 2). No significant differences are found between thymus and PLN in Vβ7, Vβ8.1, Vβ9, Vβ10 and Vβ13. The spectratypes of thymus and PLN for all Vβ families, including those that are not significantly different between PLN and thymus, are shown in Supplementary Fig. 3.
Figure 2. The majority of 4 week old NOD mice display the same altered TCR Vβ repertoire in pancreatic lymph nodes.
The Vβ CDR3 spectratype of 19 Vβ families was compared between thymus (n=5) and PLN (n=11) of 4 week old NOD mice. The bars represent the mean ± 3 SD of relative peak area for each CDR3 length in each Vβ family for thymus. The relative peak area for each CDR3 length in each Vβ family for PLN in individual mice is indicated by an x on the same plot. Peaks in PLN that are significantly different from the equivalent peak in thymus in at least 80% of mice tested are indicated with asterisk above the respective bar: ***, p<0.0001; **, p<0.001 or *, p<0.01 by ANOVA. The x-axis shows the CDR3 length in amino acid and the y-axis shows the relative peak area (%).
3.2. A limited number of T cell populations that expand by 4 weeks of age contract and expand as disease progresses
To investigate whether the T cell populations that expand in PLN by 4 weeks of age continue to expand as disease progresses, CDR3 spectratype analysis was performed on PLN of female NOD mice at 10 weeks of age and at diabetes onset and compared to thymus. The data in Table 1 show that all of the T cell populations (CDR3 peaks) that expand at 4 weeks of age, except for Vβ1 and Vβ5.1, also expand at 10 weeks and T1D onset compared to thymus. When comparing T cell populations (identified by Vβ usage and CDR3 length) in PLN at 4 and 10 weeks of age and at T1D onset we found that within the Vβ1 family a T cell population corresponding to a CDR3 length of 7 amino acids is significantly reduced at 10 weeks of age compared to 4 week old mice. This population decreases from 5.1 % of the spectratype at 4 weeks of age to 4.4% at 10 weeks of age in 13 out of 16 mice (81%). Within the Vβ15 family, a T cell population with a CDR3 length of 7 amino acids is reduced from 29.3% of the spectratype at 4 weeks of age to 27.7% at 10 weeks of age, but recovers at T1D onset (29.3%) when compared to 10 weeks of age. The reduction at 10 weeks of age and expansion at T1D onset of this T cell population was observed in 100% of mice. In addition, T cell populations within the Vβ2 and Vβ4 families are significantly reduced at T1D onset compared to 4 weeks of age and the reduction was found in 90% and 100% of mice, respectively. T cell populations within the Vβ3.1 and Vβ5.1 families also increased at T1D onset compared to 10 weeks of age although this increase did not quite reach significance.
Table 1.
T cell populations that expand at 4 and 10 weeks of age and at T1D onset1
| Relative peak area (%) | Relative peak area (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||
| CDR3 length2 |
p 3 | Thymus | 4 weeks | 10 weeks | T1D onset |
CDR3 length |
p | Thymus | 4 weeks | 10 weeks | T1D onset |
||
|
|
|
||||||||||||
| Vβ1 | 7 | 0.0117 | 3.5±0.34 | 5.1±0.6 | 4.4±0.85 | 4.6±0.3 | Vβ8.2 | 7 | <0.0001 | 8.0±0.4 | 9.7±0.6 | 10.1±0.4 | 9.8±0.5 |
| Vβ2 | 4 | <0.0001 | 5.5±0.5 | 9.9±1.8 | 9.2±0.9 | 8.8±0.86 | Vβ8.3 | 9 | <0.0001 | 19.2±0.7 | 23.3±1.3 | 24.1±1.9 | 23.9±1.1 |
| 5 | <0.0001 | 13.2±2.1 | 18.7±2.1 | 20.4±2.7 | 19.4±0.7 | ||||||||
| 6 | <0.0001 | 28.9±0.8 | 34.5±2.7 | 35.0±2.8 | 35.6±1.7 | Vβ11 | 6 | 0.0007 | 1.9±0.2 | 2.9±0.6 | 2.5±0.4 | 2.6±0.3 | |
| 7 | <0.0001 | 5.9±0.6 | 8.9±0.8 | 8.9±1.1 | 8.7±0.8 | ||||||||
| Vβ3.1 | 6 | 0.0002 | 3.3±0.5 | 7.1±2.7 | 8.8±1.5 | 9.8±3.3 | 8 | <0.0001 | 13.9±0.9 | 21.1±1.3 | 20.2±1.3 | 20.1±1.3 | |
| 7 | <0.0001 | 8.4±0.4 | 20.2±2.7 | 20.0±2.7 | 21.2±5.1 | 9 | <0.0001 | 32.0±0.9 | 36.2±1.1 | 36.1±1.3 | 36.6±0.9 | ||
| Vβ4 | 9 | <0.0001 | 10.8±1.0 | 17.2±1.5 | 16.0±1.6 | 15.3±1.86 | Vβ12 | 5 | 0.0028 | 2.7±0.1 | 3.9±0.8 | 4.0±0.5 | 3.7±0.6 |
| 6 | <0.0001 | 8.1±0.6 | 13.9±0.9 | 13.6±1.0 | 13.9±1.2 | ||||||||
| Vβ5.1 | 7 | 0.0085 | 7.5±0.7 | 12.1±2.5 | 10.8±2.8 | 12.8±3.5 | 7 | <0.0001 | 24.8±0.7 | 39.0±1.8 | 38.7±2.0 | 39.0±2.0 | |
| Vβ5.2 | 7 | 0.0009 | 8.8±0.5 | 12.7±2.3 | 12.7±1.6 | 11.5±2.1 | Vβ14 | 8 | 0.0253 | 22.5±0.4 | 27.4±1.0 | 27.1±3.2 | 27.7±2.6 |
| Vβ6 | 7 | <0.0001 | 9.0±0.4 | 12.5±0.8 | 12.3±0.9 | 12.8±1.2 | Vβ15 | 7 | <0.0001 | 18.9±0.7 | 29.3±2.5 | 27.7±1.55 | 29.3±2.17 |
| 8 | <0.0001 | 20.3±0.7 | 25.2±1.4 | 24.9±1.4 | 24.5±1.4 | ||||||||
| Vβ16 | 7 | <0.0001 | 6.7±0.3 | 11.6±1.0 | 11.5±1.3 | 11.3±1.1 | |||||||
The TCRVβ repertoire was analyzed by CDR3 length spectratype in PLN T cells from individual NOD mice at 4 weeks of age (n=11), 10 weeks of age (n=18), and at T1D onset (n=14) and the relative peak area for each peak in each Vβ family was calculated.
CDR3 length in amino acid
Significance by ANOVA compared to thymus (n=5) for all groups
Mean of relative peak area ± SD for each peak length for each Vβ
Peaks that are significantly reduced at 10 weeks of age compared to 4 weeks of age
Peaks that are significantly reduced at T1D onset compared to 4 weeks of age
Peak that increase between 10 weeks of age and T1D onset
The relative peak areas in shaded boxes are significantly different from the relative peak area of the same peak in thymus using Dunnett’s Multiple Comparison test
3.3. The TCR repertoire is more diverse at 10 weeks of age than at 4 weeks of age
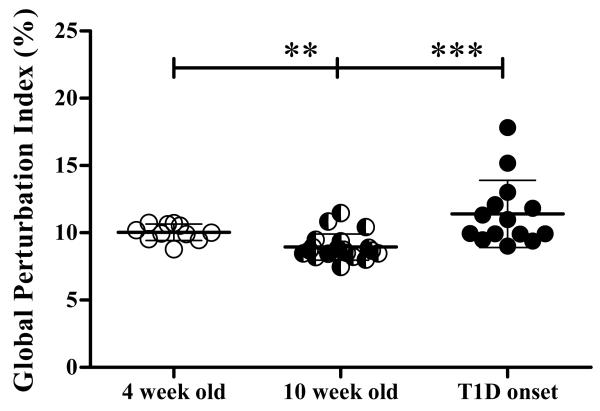
To quantify the contribution that all peaks make to the TCR Vβ repertoire, the difference between the area of all CDR3 spectratype peaks for each Vβ family in PLN and that of the thymus was calculated for all Vβ family. The sum of the differences for individual Vβ families is called the total perturbation index, and the global perturbation index is the mean of the total perturbation index for all 19 Vβ families tested for each mouse (Gorochov et al., 1998). The strategy to calculate both total and global perturbation is described in Supplementary Fig. 1. It is important to note that the global perturbation index takes into account differences in all peaks in the CDR3 spectratype and is calculated for all 19 Vβ families tested. As such, the global perturbation index includes the contribution of changes in all peaks in all Vβ families tested to the total response. This is different from the strategy used to analyze data in Fig. 2 that shows individual peaks that are significantly different in PLN and thymus. In the latter, the strategy analyses the contribution of single peaks to the total response. A significant decrease in global perturbation index was detected at 10 weeks of age (8.9±0.9) compared to 4 week old mice (10.0±0.6) (p= 0.0021). Furthermore, the global perturbation index at 10 weeks of age was significantly less than at T1D onset (11.4±2.5; p< 0.0001) (Fig. 3). These results indicate higher TCR repertoire diversity at 10 weeks of age suggesting that significant changes in the TCR repertoire occur between 4 and 10 weeks of age and then again between 10 weeks of age and T1D onset.
Figure 3. The TCR repertoire is more diverse at 10 weeks of age than at 4 weeks of age.
The global perturbation index was calculated for each mouse at 4 weeks of age (n=11), 10 weeks of age (n=20) and at diabetes onset (n=14). Each dot represents an individual mouse. The mean and SD is shown for each group. Statistical differences between groups are calculated using unpaired t test and are shown as *** p<0.0001 or ** p<0.001.
3.4. The reduction in global perturbation index between 4 and 10 weeks of age is not reflected by a reduction in total perturbation index of individual Vβ families.
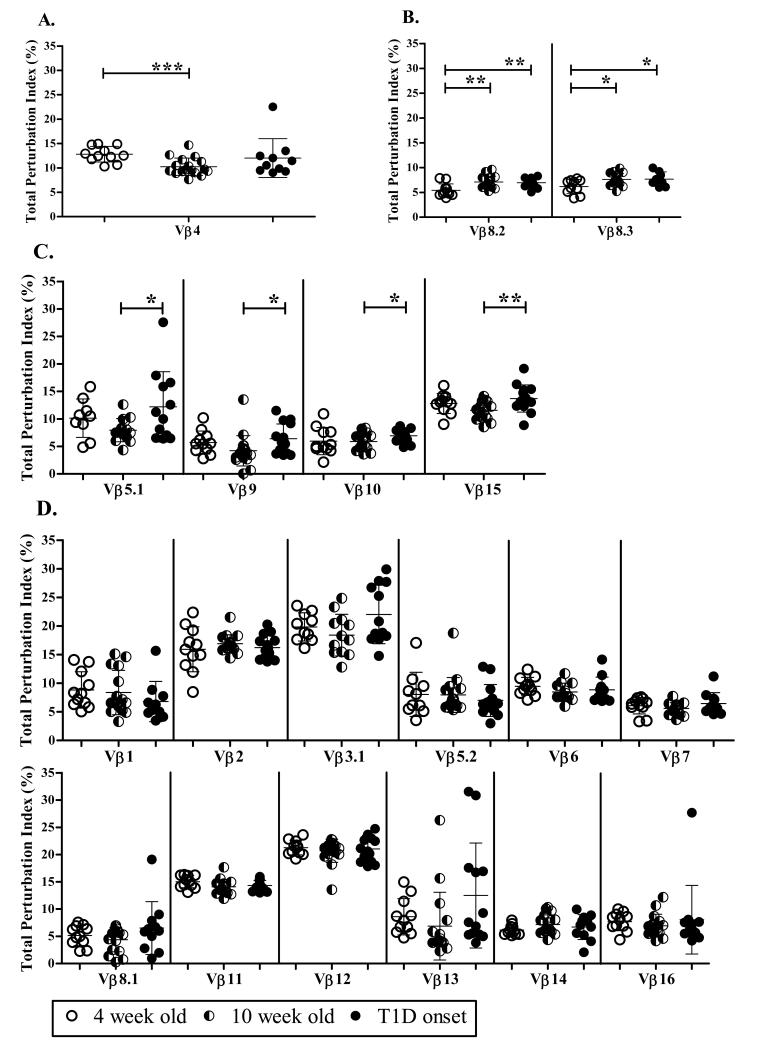
To determine which Vβ families contribute to the decrease in the global perturbation at 10 weeks of age, we calculated the total perturbation index for each of the 19 Vβ families tested in PLN at 4 weeks and 10 weeks of age and at T1D onset. Our data show that the total perturbation index is only significantly reduced at 10 weeks of age (10.2±1.8) (p= 0.0006) compared to 4 weeks (12.8±1.6) in the Vβ4 family (Fig. 4A). In contrast, Vβ8.2 and Vβ8.3 show a significant increase in total perturbation index at 10 weeks of age: 5.4±1.3 (p= 0.0017) for Vβ8.2 and 6.2±1.4 (p=0.0122) for Vβ8.3 compared to 7.1±1.2 and 7.6±1.2 at 4 weeks of age for Vβ8.2 and Vβ8.3, respectively (Fig. 4B). A significant increase in total perturbation index was also detected for Vβ8.2 (6.9±0.9) (p=0.0020) and Vβ8.3 (7.7±1.4) (p= 0.0433) in PLN at T1D onset compared to 4 weeks of age (Fig. 4B). Moreover, a similar increase in total perturbation index was detected in Vβ5.1, Vβ9, Vβ10, and Vβ15 at T1D onset compared to 10 weeks of age (Fig. 4C). The total perturbation index of the remaining 12 Vβ families did not vary between time points (Fig. 4D). The total perturbation index for most Vβ families at 10 weeks of age was below 10% indicating a highly diverse TCR repertoire. However, the total perturbation index for Vβ2, Vβ3.1, Vβ11 and Vβ12, four of the families that did not significantly change over time, was consistently greater than 15% suggesting a continuous selection of these families within the TCR repertoire at all time points. Overall, these data might suggest that either Vβ4 is a major contributor to the reduced global perturbation seen at 10 weeks of age compared to 4 weeks, or that many small changes contribute to the global perturbation.
Figure 4. The reduction in global perturbation index between 4 and 10 weeks of age is not reflected by a reduction in total perturbation index of individual Vβ families.
The total perturbation index (%) for each Vβ family in each mouse was calculated for 4 (n=11) and 10 week old mice (n=20), and mice at diabetes onset (n=14). The mean and SD is shown for each Vβ family, and each dot represents an individual mouse. Vβ4 is significantly less perturbed at 10 weeks than at 4 weeks of age (A), while the total perturbation index of Vβ8.2 and Vβ8.3 increased during disease progression (B). Other Vβ families are either more perturbed at T1D onset (C) or do not change during disease progression (D). Significant changes between time points was calculated using unpaired t test and are shown as *** p<0.0001; ** p<0.001 or * p<0.01.
3.5. Cyclophosphamide (CYP) accelerates changes that naturally occur between 10 weeks of age and T1D onset
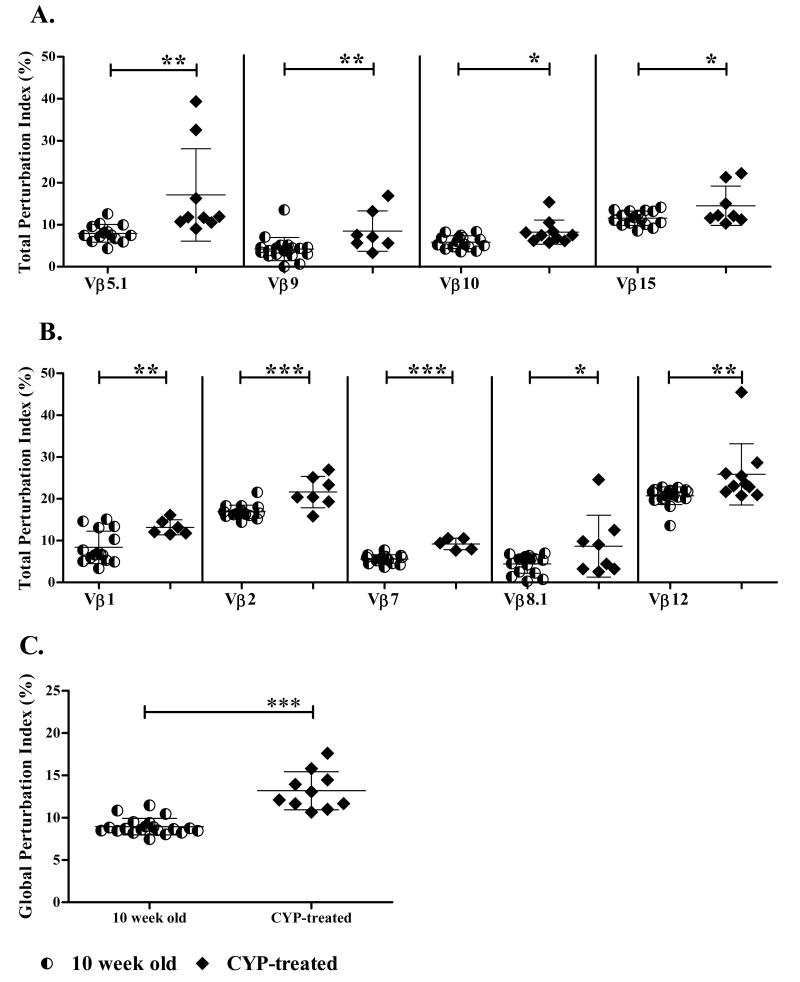
To determine whether the increase in total perturbation index seen at T1D onset in Vβ5.1, Vβ9, Vβ10, and Vβ15 is associated with disease or age of the mouse, we used CYP treatment to accelerate diabetes onset in NOD mice. Specifically, 8 week old NOD mice were treated with CYP and monitored twice a week for diabetes. Two weeks later, when all treated mice were diabetic, the TCR Vβ repertoire in PLN was analyzed. CYP treatment resulted in a significant increase in the perturbation index of Vβ5.1, Vβ9, Vβ10, and Vβ15 compared to untreated age matched mice (Fig. 5A). We predicted that the Vβ4 family might be susceptible to CYP treatment since this was the only family that showed a decrease in total perturbation index between 4 and 10 weeks of age. Surprisingly, the total perturbation index for Vβ4 was not different after CYP treatment (data not shown) suggesting that TCR diversity in Vβ4 at 10 weeks of age might not be due to regulation. Interestingly, five Vβ families, Vβ1, Vβ2, Vβ7, Vβ8.1, and Vβ12 that showed no change in total perturbation at any time point in untreated mice were significantly more perturbed after CYP treatment (Fig. 5B) suggesting that clones within these Vβ families may normally be controlled by Tregs.
Figure 5. Cyclophosphamide (CYP) accelerates changes that naturally occur between 10 weeks of age and TID onset.
Eight week old female NOD mice were treated with (n=10) or without (n=20) CYP and monitored for diabetes onset. One week after the last CYP injection when all treated mice were diabetic, PLN were isolated and the CDR3 spectratype for all 19 TCR Vβ families determined for individual mice. (A) Total perturbation index for Vβ families that expand naturally and after CYP treatment between 10 weeks of age and TID onset. (B) Total perturbation index for Vβ families that expand only after CYP treatment. The total perturbation index for each Vβ family was calculated for each mouse. The mean and SD is shown for each Vβ family, and each dot represents an individual mouse. (C) Global perturbation index for treated and untreated mice. The global perturbation index was calculated for each mouse and the mean ± SD is shown. Statistical analysis was performed using unpaired t test and statistical differences between the corresponding groups is shown as *** p<0.0001; ** p<0.001 or * p<0.01.
The effect of CYP treatment on the global perturbation index of PLN from 10 week old CYP-treated mice was determined by comparing to untreated age matched mice. A significant increase in global perturbation index was detected in PLN T cells of CYP treated NOD mice (13.2±2.2) compared to untreated mice (8.9±0.9, p<0.0001) (Fig. 5C). Furthermore, the global perturbation index in CYP-treated 10 week old mice was also significantly greater than in untreated mice at 4 weeks of age (10.0±0.6; p= 0.0002).
4. Discussion
Studies by others have shown limited TCR usage in the islets of NOD mice between 2 and 4 weeks of age with a more heterogeneous T cell contribution in the islets of older NOD mice. Thus, preferential usage of Vβ1 and Vβ12 for CD4+ T cells with a CDR3 length restricted to 9 amino acids for 50% of Vβ1 sequences and to 8 amino acids for 67% of Vβ12 sequences is seen in islets of 14 to 18 day old female NOD mice (Baker et al., 2002). Consistent with these data Drexler et al. (1993) also showed prevalence of Vβ1 transcripts in islets of 4 week old NOD mice with 39% and 35% of sequences having a CDR3 length of 9 and 10 amino acids respectively. In addition, limited Vβ diversity was detected in the CDR3 of Vβ3 and Vβ7 clones isolated from islets of 28 to 30-day-old NOD mice (Galley and Danska, 1995), and Yang et al. (1996) described a predominance of Vβ8.2 T cells in islet isolated from 14-day-old female NOD mice. In contrast, T cell cloning studies have reported heterogeneous usage of TCR Vβ gene products by islet-infiltrating T cells from 7 to 11 week old NOD mice (Nakano et al., 1991). These data suggest that the TCR repertoire of islet infiltrating cells becomes more diverse with age and/or with disease progression. In contrast to findings in the islets, heterogeneous expression of Vβ families have been described for lymph node cells of NOD mice (Petrovic et al., 2008; Yang et al., 1996; Galley and Danska, 1995; Drexler et al., 1993). Moreover, islet-specific T cell clones isolated from spleen and LN from 3 to 5 month old NOD mice were highly heterogeneous (Candéias et al., 1991). Overall, these data implicate a limited number of Vβ families in the T cell response within the islets of young mice that is more heterogeneous in islets of older mice and in LN cells. Our data show that within an ordered but heterogeneous T cell response in PLN a limited number of Vβ families expand in both spontaneously diabetic mice and in CYP-induced diabetes suggesting that diabetes-associated T cell responses are selected in the PLN.
Limited TCR Vβ usage by islet infiltrating T cells in young NOD mice suggests preferential priming of T cells that express particular TCR Vβ in the PLN, the site for islet antigen-specific T cell priming. Here we show that distinct T cell populations within a diverse group of Vβ families significantly expand in PLN at 4 and 10 weeks of age and at T1D onset indicating heterogeneity at each stage in the disease process. In addition, we show that there is an ordered and predetermined selection of a large number of T cell population identified by their Vβ CDR3 usage in the PLN as early as 4 weeks of age and that these T cell populations are present in greater than 80% of mice tested. The finding that three Vβ families, Vβ3.1, Vβ5.1, and Vβ15, contain T cell populations that decrease at 10 weeks of age compared to 4 weeks of age and then increase again at T1D onset support the notion that a disease-related response in PLN restricted to cell populations in Vβ3.1, Vβ5.1, and Vβ15 families is evident as early as 4 weeks of age, and that these responses decline and then expand again before T1D onset. The significant increase in total perturbation index in Vβ5.1 and Vβ15 at T1D onset compared to 10 weeks of age likely reflects the contribution of single T cell populations identified by their CDR3 length for these Vβ families. In contrast, the significant increase in total perturbation in Vβ9, Vβ10, Vβ8.2 and Vβ8.3 suggests that perturbation of a Vβ family can also be the result of a large number of smaller responses.
The finding that the total perturbation within the Vβ5.1, Vβ9, Vβ10 and Vβ15 families is significantly greater in CYP-induced diabetes in 10 week old NOD mice compared to age matched untreated mice indicates that these Vβ families are associated with both spontaneous and acute T1D. These data further support the idea that pathogenic T cells within these Vβ families are naturally and efficiently selected in the periphery to contribute to an aggressive autoimmune response. CYP treatment depletes CD4+CD25+Foxp3+ Tregs and this contributes to the mechanism of accelerated T1D in CYP treated mice (Brode et al., 2006). This suggests that diabetogenic T cell responses within these four Vβ families are suppressed at 10 weeks of age by Tregs, and that in order to develop T1D spontaneously this regulation is overcome resulting in disease. Additional data suggest that T cell responses within the Vβ1, Vβ2, Vβ7, Vβ8.1, and Vβ12 families are also susceptible to immune regulation since the total perturbation index within these families is also significantly enhanced after CYP treatment. However, since significant differences were not detected between 10 weeks of age and spontaneous T1D onset in these families it is possible that T cell responses within the Vβ1, Vβ2, Vβ7, Vβ8.1 and Vβ12 families are naturally under tighter control by Tregs than the T cells within the Vβ5.1, Vβ9, Vβ10 and Vβ15 families.
We predicted that the Vβ4 family would be the most susceptible to regulation because perturbation is significantly reduced at 10 weeks of age compared to 4 weeks of age in this family. However, Vβ4 is not affected by CYP treatment suggesting that T cells expressing Vβ4 are resistant to Tregs. Interestingly, the BDC2.5 CD4+ T cell clone, which was established from the spleen of diabetic NOD mice (Bergman and Haskins, 1994), and that is diabetogenic upon adoptive transfer into young NOD or NOD.scid recipients expresses Vβ4 (Peterson and Haskins, 1996; Haskins and McDuffie, 1990). In addition, T cell clones specific for 530-543 of GAD65 that arise spontaneously in islets of NOD mice preferentially utilize Vβ4 (Quinn et al., 2001). These data might suggest that the decrease in perturbation in Vβ4 at 10 weeks of age compared to 4 weeks of age might be the result of export from the periphery to the target (pancreas) tissue.
Our data show that the CDR3 spectratype of Vβ2 and Vβ12 in PLN is generally shorter than that it is in thymus with the central peak in PLN having a shorter CDR3 length than the central peak in thymus. This is consistent with data from Yassai et al. (2002) who have demonstrated that in several H2u and H2b mouse strains the CDR3 spectratype of peripheral CD4+ T cells is shifted towards a shorter TCR β-chain CDR3 length compared to thymocytes. This reduction in CDR3 length has been described as a consequence of positive selection for nTregs in NOD mice (Ferreira et al., 2009) suggesting that either expanded Vβ2 and Vβ12 populations are rich in nTregs or that the same strategy is used by non-nTregs when they are exported from the thymus. Extensive analyses of TCR Vβ usage in spleen and thymus of 6 to 8 week old NOD mice have shown that transcripts for Vβ2, Vβ12, and Vβ14 are significantly more abundant in the spleen than thymus in the CD4+ T cell compartment (Sarukhan et al., 1994b). This is consistent with our data that show a significant expansion within the Vβ2 and Vβ12 families, in addition to other Vβ families, in PLN of both 4 and 10 week old NOD mice compared to thymus.
A recent study using TCR CDR3 spectratype analysis revealed a significant decrease in the global perturbation in the PLN and inguinal LN between 10 day old and 22 day old NOD mice indicating that changes in the TCR repertoire take place in the periphery as early as 10 days of age (Petrovic et al., 2008). In the Petrovic study inguinal LN was used as the putative non-stimulated population. No differences were found between the PLN and inguinal LN suggesting that no specific events occurred in the PLN in NOD mice at this age. However, a significant reduction in perturbation was seen between 10 and 22 days of age. When we compared our data with these published data we found that at 4 weeks of age there is a recovery of the global perturbation index compared to the published data in PLN from 22 day old NOD mice, followed by a significant decrease in global perturbation again between 4 and 10 weeks of age. These data suggest that 22 days and 10 weeks of age might be important time points in the control of the diabetogenic TCR repertoire. Alternatively, it is possible that the value for global perturbation in the previously published study (Petrovic et al., 2008) and the study described here cannot be compared because different reference groups were used for the analysis of the perturbation, thymus in ours and inguinal LN in the published study. Nevertheless, the decrease in global perturbation seen in older compared to younger mice is consistent in the two studies. It is possible that the decrease in global perturbation seen at 10 weeks of age in our study might be a direct consequence of Tregs that attempt to control disease development by suppressing clonal expansion. The increase in TCR diversity seen at 10 weeks of age might also play a role in promoting Tregs since a high TCR diversity in Treg populations is critical for Treg expansion and in vivo suppressive function (Föhse et al., 2011, Wing et al., 2011). Consistent with this notion is our finding that treatment of 10 week old NOD mice with CYP, a well-established protocol to deplete Tregs and accelerate diabetes onset (Bai et al., 2006; Harada and Makino, 1984) results in significantly greater perturbation of the TCR Vβ repertoire in PLN that is naturally expanded in mice with diabetes.
Since PLN are an important location for recruitment, priming, and activation of anti-β cell autoreactive T cells as early as 3 weeks of age (Gagnerault et al., 2002), we infer that changes in the PLN T cell repertoires as early as 4 weeks of age might be due to early islet antigen presentation during β cell remodeling (Trudeau et al., 2000). Alternatively, T cell populations that expand by 4 weeks of age might be due to homeostatic expansion that is more efficient for some T cells than others. The expansion of a limited number of T cell populations in PLN at 10 weeks of age and after T1D onset suggest that dominant islet-specific epitopes might play a role in driving their expansion and survival (Correia-Neves et al., 2001). Since T cells within the Vβ5.1, Vβ9, Vβ10, and Vβ15 families expand during spontaneous and accelerated T1D we suggest that T cell responses within these families are the strongest candidates for pathogenicity. On the other hand, Vβ2, Vβ3.1, Vβ11 and Vβ12 are the most perturbed throughout disease progression also suggesting these families as candidates for pathogenic T cell responses. The expansion of dominant T cell populations in the T cell repertoire might be influenced by many factors including precursor frequency of individual TCRs, TCR affinity for the peptide-MHC complex, and availability of specific and cross-reactive epitopes (Li et al., 2008; Venturi et al., 2008). The presence of dominant T cell populations in the TCR Vβ repertoire in the PLN is consistent with a role for any or all of these factors in the disease process.
The TCR αβ repertoire is estimated to range in size from 107 in mice and 108 in humans (Casrouge et al., 2000; Arstila et al., 1999) and a major contributor to repertoire diversity is the CDR3 region (Kedzierska et al., 2008; Turner et al., 2006). Typically, histograms of CDR3 lengths from a diverse T cell population follow a Gaussian distribution and deviations from a Gaussian distribution are seen after antigen stimulation. This deviation can be given a numerical value by calculating the percentage of total perturbation for each Vβ family compared to a non stimulated T cell population, in the case of this study, the thymus. By adding together all the differences in all Vβ families tested, an index of global perturbation can be calculated, reflecting the overall change in the repertoire in an individual mouse. Using this approach, we have identified dominant and conserved changes in the TCR Vβ repertoire of the NOD mouse strain that take place naturally in the PLN as disease progresses. We also show that 10 weeks of age might be an important checkpoint for control of the TCR repertoire by Tregs. It is likely that a combination of CDR3 spectratyping, cytokine analysis, and surface phenotype will be necessary to determine which of these T cell populations play a role in driving or triggering T1D in the NOD mouse. Such identification and fine characterization of dominant T cell populations in the NOD mice might contribute to the development of strategies to attenuate pathogenic T cell activity.
Supplementary Material
Supplementary Figure 1. Strategy used to calculate the total and global perturbation. The CDR3 spectratype is shown for Vβ12 in thymus (A) and PLN (B). The relative peak area for each CDR3 peak (C) and the difference (D) between the two are shown for thymus and PLN. The sum of the differences where the relative peak area for the PLN is greater than thymus (total positive perturbation) is the total perturbation index for Vβ12. The global perturbation index is the mean of all total positive perturbation indexes for all Vβ analyzed.
Supplementary Figure 2. CDR3 length distribution of Vβ2 and Vβ12 families in PLN of 4 week old NOD mice compared to thymus. The mean and SD of the relative peak area for each CDR3 length is shown for PLN from 4 week old NOD mice (shaded bars, n=11) and thymus from NOD mice (open bars, n=5). CDR3 length in amino acid is shown on the x-axis, and the relative peak area is shown on the y-axis.
Supplementary Figure 3. CDR3 spectratype of thymus and PLN from NOD mice. The CDR3 spectratype for each Vβ family is shown for one representative example of thymus and two of PLN from NOD mice at 4 weeks of age. The shaded peaks are those that have significantly different relative peak areas between PLN and thymus. The peak corresponding to a CDR3 length of 10 amino acids is shown on each spectratype plot.
Acknowledgements
This paper is dedicated by I. Marrero to the memory of Dr. Eli E. Sercarz for his inspirational role as a mentor. This study was supported by a postdoctoral fellowship from the Juvenile Diabetes Research Foundation (3-2005-336) and a Junior Faculty Award from the American Diabetes Association (1-11-JF-29) to I.M., and a grant from National Institutes of Health (AI065937) to E.E.S.
Abbreviations
- NOD
nonobese diabetic
- T1D
Type 1 Diabetes
- PLN
pancreatic lymph nodes
- CDR3
complementary determining region 3
- CYP
cyclophosphamide
- Tregs
regulatory T cells
- ANOVA
analysis of variance
Footnotes
Disclosures
The authors have no financial conflict of interest.
References
- Ahmed M, Lanzer KG, Yager EJ, Adams PS, Johnson LL, Blackman MA. Clonal expansions and loss of receptor diversity in the naive CD8 T cell repertoire of aged mice. J. Immunol. 2009;182:784–792. doi: 10.4049/jimmunol.182.2.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu. Rev. Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- Arstila TP, Casrouge A, Baron V, Even J, Kanellopoulos J, Kourilsky P. A direct estimate of the human alphabeta T cell receptor diversity. Science. 1999;286:958–961. doi: 10.1126/science.286.5441.958. [DOI] [PubMed] [Google Scholar]
- Bai Y, Robinson E, Chai R, Ross JM, Reddy S. Immunohistochemical study of monocyte chemoattractant protein-1 in the pancreas of NOD mice following cyclophosphamide administration and during spontaneous diabetes. J. Mol. Histol. 2006;37:101–113. doi: 10.1007/s10735-006-9045-6. [DOI] [PubMed] [Google Scholar]
- Baker FJ, Lee M, Chien YH, Davis MM. Restricted islet-cell reactive T cell repertoire of early pancreatic islet infiltrates in NOD mice. Proc. Natl. Acad. Sci. USA. 2002;99:9374–9379. doi: 10.1073/pnas.142284899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman B, Haskins K. Islet-specific T-cell clones from the NOD mouse respond to beta-granule antigen. Diabetes. 1994;43:197–203. doi: 10.2337/diab.43.2.197. [DOI] [PubMed] [Google Scholar]
- Berschick P, Fehsel K, Weltzien HU, Kolb H. Molecular analysis of the T-cell receptor Vβ5 and Vβ8 repertoire in pancreatic lesions of autoimmune diabetic NOD mice. J. Autoimm. 1993;6:405–422. doi: 10.1006/jaut.1993.1034. [DOI] [PubMed] [Google Scholar]
- Brode S, Raine T, Zaccone P, Cooke A. Cyclophosphamide-induced type-1 diabetes in the NOD mouse is associated with a reduction of CD4+CD25+Foxp3+ regulatory T cells. J. Immunol. 2006;177:6603–6612. doi: 10.4049/jimmunol.177.10.6603. [DOI] [PubMed] [Google Scholar]
- Candéias S, Katz J, Benoist C, Mathis D, Haskins K. Islet-specific T-cell clones from nonobese diabetic mice express heterogeneous T-cell receptors. Proc. Natl. Acad. Sci. USA. 1991;88:6167–6170. doi: 10.1073/pnas.88.14.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casrouge A, Beaudoing E, Dalle S, Pannetier C, Kanellopoulos J, Kourilsky PJ. Size estimate of the alpha beta TCR repertoire of naive mouse splenocytes. Immunol. 2000;164:5782–5787. doi: 10.4049/jimmunol.164.11.5782. [DOI] [PubMed] [Google Scholar]
- Cibotti R, Cabaniols JP, Pannetier C, Delarbre C, Vergnon I, Kanellopoulos JM, Kourilsky P. Public and private Vβ T cell receptor repertoires against hen egg white lysozyme (HEL) in nontransgenic versus HEL transgenic mice. J. Exp. Med. 1994;180:861–872. doi: 10.1084/jem.180.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochet M, Pannetier C, Regnault A, Darche S, Leclerc C, Kourilsky P. Molecular detection and in vivo analysis of the specific T cell response to a protein antigen. Eur. J. Immunol. 1992;22:2639–2647. doi: 10.1002/eji.1830221025. [DOI] [PubMed] [Google Scholar]
- Correia-Neves M, Waltzinger C, Mathis D, Benoist C. The shaping of the T cell repertoire. Immunity. 2001;14:21–32. doi: 10.1016/s1074-7613(01)00086-3. [DOI] [PubMed] [Google Scholar]
- Davis MM, Bjorkman PJ. T-cell antigen receptor genes and T-cell recognition. Nature. 1988;334:395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Drexler K, Burtles S, Hurtenbach U. Limited heterogeneity of T-cell receptor Vβ gene expression in the early stage of insulitis in NOD mice. Immunol. Letters. 1993;37:187–196. doi: 10.1016/0165-2478(93)90030-6. [DOI] [PubMed] [Google Scholar]
- Fabien N, Bergerot I, Maguer-Satta V, Orgiazzi J, Thivolet C. Pancreatic lymph nodes are early targets of T cells during adoptive transfer of Diabetes in NOD mice. J. Autoimm. 1995;8:323–334. doi: 10.1006/jaut.1994.0025. [DOI] [PubMed] [Google Scholar]
- Ferreira C, Singh Y, Furmanski AL, Wong FS, Garden OA, Dyson J. Non-obese diabetic mice select a low-diversity repertoire of natural regulatory T cells. Proc. Natl. Acad. Sci. USA. 2009;106:8320–8325. doi: 10.1073/pnas.0808493106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Föhse L, Suffner J, Suhre K, Wahl B, Lindner C, Lee CW, Schmitz S, Haas JD, Lamprecht S, Koenecke C, Bleich A, Hämmerling GJ, Malissen B, Suerbaum S, Förster R, Prinz I. High TCR diversity ensures optimal function and homeostasis of Foxp3+ regulatory T cells. Eur J Immunol. 2011;41:3101–3113. doi: 10.1002/eji.201141986. [DOI] [PubMed] [Google Scholar]
- Gagnerault MC, Luan JJ, Lotton C, Lepault F. Pancreatic lymph nodes are required for priming of beta cell reactive T cells in NOD mice. J. Exp. Med. 2002;196:369–377. doi: 10.1084/jem.20011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galley KA, Danska JS. Peri-islet infiltrates of young non-obese diabetic mice display restricted TCR β-chain diversity. J. Immunol. 1995;154:2969–2982. [PubMed] [Google Scholar]
- Gorochov G, Neumann AU, Kereveur A, Parizot C, Li T, Katlama C, Karmochkine M, Raguin G, Autran B, Debré P. Perturbation of CD4+ and CD8+ T-cell repertoires during progression to AIDS and regulation of the CD4+ repertoire during antiviral therapy. Nat. Med. 1998;4:215–221. doi: 10.1038/nm0298-215. [DOI] [PubMed] [Google Scholar]
- Harada M, Makino S. Promotion of spontaneous diabetes in non-obese diabetes-prone mice by cyclophosphamide. Diabetologia. 1984;27:604–606. doi: 10.1007/BF00276978. [DOI] [PubMed] [Google Scholar]
- Haskins K, McDuffie M. Acceleration of diabetes in young NOD mice with a CD4+ islet-specific T cell clone. Science. 1990;249:1433–1436. doi: 10.1126/science.2205920. [DOI] [PubMed] [Google Scholar]
- Höglund P, Mintern J, Waltzinger C, Heath W, Benoist C, Mathis D. Initiation of autoimmune diabetes by developmentally regulated presentation of islet cell antigens in the pancreatic lymph nodes. J. Exp. Med. 1999;189:331–339. doi: 10.1084/jem.189.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedzierska K, La Gruta NL, Stambas J, Turner SJ, Doherty PC. Tracking phenotypically and functionally distinct T cell subsets via T cell repertoire diversity. Mol. Immunol. 2008;45:607–618. doi: 10.1016/j.molimm.2006.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide Y, Kaidoh T, Yanagawa T, Yoshida TO. A comparative study on T cell receptor Vβ gene usages: spleen cells from the non-obese diabetic (NOD) mouse and its non-diabetic sister strain, the ILI mouse, and infiltrating T cells into pancreata of NOD mice. Microbiol. Immunol. 1993;37:653–659. doi: 10.1111/j.1348-0421.1993.tb01688.x. [DOI] [PubMed] [Google Scholar]
- Kronenberg M, Siu G, Hood LE, Shastri N. The molecular genetics of the T-cell antigen receptor and T-cell antigen recognition. Annu Rev Immunol. 1986;4:529–591. doi: 10.1146/annurev.iy.04.040186.002525. [DOI] [PubMed] [Google Scholar]
- Larger E, Bécourt C, Bach JF, Boitard C. Pancreatic islet beta cells drive T cell-immune responses in the nonobese diabetic mouse model. J. Exp. Med. 1995;181:1635–1642. doi: 10.1084/jem.181.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wang B, Frelinger JA, Tisch R. T-cell promiscuity in autoimmune diabetes. Diabetes. 2008;57:2099–2106. doi: 10.2337/db08-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Sumida T, Kurasawa K, Tomioka H, Itoh I, Yoshida S, Koike T. T-lymphocyte-receptor repertoire of infiltrating T lymphocytes into NOD mouse pancreas. Diabetes. 1991;40:1580–1585. doi: 10.2337/diab.40.12.1580. [DOI] [PubMed] [Google Scholar]
- Makino S, Kunimoto K, Muraoka Y, Mizushima Y, Katagiri K, Tochino Y. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu. 1980;29:1–13. doi: 10.1538/expanim1978.29.1_1. [DOI] [PubMed] [Google Scholar]
- Nakano N, Kikutani H, Nishimoto H, Kishimoto T. T cell receptor V gene usage of islet β cell-reactive T cells is not restricted in non-obese diabetic mice. J. Exp. Med. 1991;173:1091–1097. doi: 10.1084/jem.173.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolich-Zugich J, Slifka MK, Messaoudi I. The many important facets of T-cell repertoire diversity. Nat. Rev. Immunol. 2004;4:123–132. doi: 10.1038/nri1292. [DOI] [PubMed] [Google Scholar]
- Pannetier C, Even J, Kourilsky P. T-cell repertoire diversity and clonal expansions in normal and clinical samples. Immunol. Today. 1995;16:176–181. doi: 10.1016/0167-5699(95)80117-0. [DOI] [PubMed] [Google Scholar]
- Pannetier C, Cochet M, Darche S, Casrouge A, Zöller M, Kourilsky P. The sizes of the CDR3 hypervariable regions of the murine T-cell receptor beta chains vary as a function of the recombined germ-line segments. Proc. Natl. Acad. Sci. USA. 1993;90:4319–4323. doi: 10.1073/pnas.90.9.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JD, Haskins K. Transfer of diabetes in the NOD-scid mouse by CD4 T-cell clones. Differential requirement for CD8 T-cells. Diabetes. 1996;45:328–36. doi: 10.2337/diab.45.3.328. [DOI] [PubMed] [Google Scholar]
- Petrovic J, Mariotti-Ferrandiz E, Rosmaraki E, Hall H, Cazenave PA, Six A, Höglund P. TCR repertoire dynamics in the pancreatic lymph nodes of non-obese diabetic (NOD) mice at the time of disease initiation. Mol. Immunol. 2008;45:3059–3064. doi: 10.1016/j.molimm.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Quinn A, McInerney B, Reich EP, Kim O, Jensen KP, Sercarz EE. Regulatory and effector CD4 T cells in nonobese diabetic mice recognize overlapping determinants on glutamic acid decarboxylase and use distinct V beta genes. 2001;166:2982–2991. doi: 10.4049/jimmunol.166.5.2982. [DOI] [PubMed] [Google Scholar]
- Ria F, van den Elzen P, Madakamutil LT, Miller JE, Maverakis E, Sercarz EE. Molecular characterization of the T cell repertoire using immunoscope analysis and its possible implementation in clinical practice. Curr. Mol. Med. 2001;1:297–304. doi: 10.2174/1566524013363690. [DOI] [PubMed] [Google Scholar]
- Sarukhan A, Lechner O, von Boehmer H. Autoimmune insulitis and diabetes in the absence of antigen-specific contact between T cells and islet beta-cells. Eur. J. Immunol. 1999;29:3410–3416. doi: 10.1002/(SICI)1521-4141(199910)29:10<3410::AID-IMMU3410>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Sarukhan A, Bedossa P, Garchon HJ, Bach JF, Carnaud C. Molecular analysis of TCR junctional variability in individual infiltrated islets of non-obese diabetic mice: evidence for the constitution of largely autonomous T cell foci within the same pancreas. Int. Immunol. 1994a;l7:139–146. doi: 10.1093/intimm/7.1.139. [DOI] [PubMed] [Google Scholar]
- Sarukhan A, Gombert JM, Olivi M, Bach JF, Carnaud C, Garchon HJ. Anchored polymerase chain reaction based analysis of the V beta repertoire in the non-obese diabetic (NOD) mouse. Eur. J. Immunol. 1994b;24:1750–1756. doi: 10.1002/eji.1830240805. [DOI] [PubMed] [Google Scholar]
- Scott Killian M, Matud J, Detels R, Giorgi JV, Jamieson BD. MaGiK method of T-cell receptor repertoire analysis. Clin. Diagn. Lab. Immunol. 2002;9:858–863. doi: 10.1128/CDLI.9.4.858-863.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone E, Daniel D, Schloot N, Gottlieb P, Babu S, Kawasaki E, Wegmann D, Eisenbarth GS. T cell receptor restriction of diabetogenic autoimmune NOD T cells. Proc. Natl. Acad. Sci. USA. 1997;94:2518–2521. doi: 10.1073/pnas.94.6.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisch R, McDevitt H. Insulin-dependent diabetes mellitus. Cell. 1996;85:291–297. doi: 10.1016/s0092-8674(00)81106-x. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983;302:575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Trudeau JD, Dutz JP, Arany E, Hill DJ, Fieldus WE, Finegood DT. Neonatal beta-cell apoptosis: a trigger for autoimmune diabetes? Diabetes. 2000;49:1–7. doi: 10.2337/diabetes.49.1.1. [DOI] [PubMed] [Google Scholar]
- Turley S, Poirot L, Hattori M, Benoist C, Mathis D. Physiological beta cell death triggers priming of self-reactive T cells by dendritic cells in a type-1 diabetes model. J. Exp. Med. 2003;198:1527–1537. doi: 10.1084/jem.20030966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner SJ, Doherty PC, McCluskey J, Rossjohn J. Structural determinants of T-cell receptor bias in immunity. Nat. Rev. Immunol. 2006;6:883–894. doi: 10.1038/nri1977. [DOI] [PubMed] [Google Scholar]
- Venturi V, Price DA, Douek DC, Davenport MP. The molecular basis for public T-cell responses? Nat. Rev. Immunol. 2008;8:231–238. doi: 10.1038/nri2260. [DOI] [PubMed] [Google Scholar]
- Waters SH, O’Neil JJ, Melican DT, Appel MC. Multiple TCR V beta usage by infiltrates of young NOD mouse islets of Langerhans. A polymerase chain reaction analysis. Diabetes. 1992;41:308–12. doi: 10.2337/diab.41.3.308. [DOI] [PubMed] [Google Scholar]
- Wing JB, Sakaguchi S. TCR diversity and Treg cells, sometimes more is more. Eur. J. Immunol. 2011;41:3097–3100. doi: 10.1002/eji.201142115. [DOI] [PubMed] [Google Scholar]
- Yang Y, Charlton B, Shimada A, Dal Canto R, Fathman CG. Monoclonal T cells identified in early NOD islets infiltrates. Immunity. 1996;4:189–194. doi: 10.1016/s1074-7613(00)80683-4. [DOI] [PubMed] [Google Scholar]
- Yassai M, Ammon K, Goverman J, Marrack P, Naumov Y, Gorski J. A molecular marker for thymocyte-positive selection: selection of CD4 single-positive thymocytes with shorter TCRB CDR3 during T cell development. J. Immunol. 2002;168:3801–3807. doi: 10.4049/jimmunol.168.8.3801. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Strategy used to calculate the total and global perturbation. The CDR3 spectratype is shown for Vβ12 in thymus (A) and PLN (B). The relative peak area for each CDR3 peak (C) and the difference (D) between the two are shown for thymus and PLN. The sum of the differences where the relative peak area for the PLN is greater than thymus (total positive perturbation) is the total perturbation index for Vβ12. The global perturbation index is the mean of all total positive perturbation indexes for all Vβ analyzed.
Supplementary Figure 2. CDR3 length distribution of Vβ2 and Vβ12 families in PLN of 4 week old NOD mice compared to thymus. The mean and SD of the relative peak area for each CDR3 length is shown for PLN from 4 week old NOD mice (shaded bars, n=11) and thymus from NOD mice (open bars, n=5). CDR3 length in amino acid is shown on the x-axis, and the relative peak area is shown on the y-axis.
Supplementary Figure 3. CDR3 spectratype of thymus and PLN from NOD mice. The CDR3 spectratype for each Vβ family is shown for one representative example of thymus and two of PLN from NOD mice at 4 weeks of age. The shaded peaks are those that have significantly different relative peak areas between PLN and thymus. The peak corresponding to a CDR3 length of 10 amino acids is shown on each spectratype plot.