Abstract
Background
Up to 20% of people initiating antiretroviral therapy (ART) in sub- Saharan Africa die during the first year of treatment. Understanding the clinical conditions associated with mortality could potentially lead to effective interventions to prevent these deaths.
Methods
We examined data from participants aged ≥18 years in the Home-Based AIDS Care project in Tororo, Uganda, to describe mortality over time and to determine clinical conditions associated with death. Survival analysis was used to examine variables associated with mortality at baseline and during follow-up.
Results
A total of 112 (9.4%) deaths occurred in 1,132 subjects (73% women) during a median of 3.0 years of ART. Mortality was 15.9 per 100 person-years (PYR) during the first 3 months and declined to 0.3 per 100 PYR beyond 24 months after ART initiation. Tuberculosis (TB) was the most common condition associated with death (21% of deaths), followed by Candida disease (15%). In 43% of deaths no specific clinical diagnosis was identified. Deaths within 3 months after ART initiation were associated with WHO clinical stage III or IV at baseline, diagnosis of TB at baseline, a diagnosis of a non-TB opportunistic infection (OI) in follow-up and a body mass index (BMI) ≤ 17 kg/m2 during follow-up. Mortality after 3 months of ART was associated with CD4 cell counts <200 cells/µL, a diagnosis of TB or other OI, adherence to therapy <95%, and low hemoglobin levels during follow-up.
Conclusion
Potentially remediable conditions and preventable infections were associated with mortality while receiving ART in Uganda.
Keywords: antiretroviral therapy, mortality, Africa, anemia, tuberculosis
Introduction
Mortality among HIV-infected individuals during the first year of antiretroviral therapy (ART) in Africa varies from 5 to 19 deaths per 100 person-years [1],[2–7]. These rates are much higher than reported from industrialized countries [8, 9], and may be due to the late stage at which most patients start ART in low- or middle-income countries, the negative health effects of under-nutrition and poverty, and/ or the more common occurrence of infectious diseases [10, 11]. Previous studies have highlighted the importance of low baseline CD4 cell counts, body mass index (BMI) and anemia in association with increased risk for mortality on ART in Zambia [12], Senegal[13], South Africa[14] and Mozambique, Malawi and Tanzania[7]. However, there is a lack of data on specific clinical conditions which are associated with mortality on ART in Africa which greatly limits the ability of programs to design specific interventions to prevent these early deaths.
The risk of mortality is greatest in the first few months after ART initiation when the full effect of therapy has not yet been obtained and subsequently declines rapidly. Conventionally, this early period of mortality has been stated as within 3 [12, 15], 4[14] or 6 months[2] of ART initiation. The time from ART initiation to when risk for mortality declines, however, has not been substantiated on the basis of objective findings. In addition, it is not known if the determinants of mortality during ART differ between “early” and “late” deaths.
We analyzed data from the Home-Based AIDS Care (HBAC) project, a study of three different monitoring strategies for HIV-infected individuals receiving ART in rural Uganda. We identified specific diseases associated with death within the first three years on ART as well as factors at baseline associated with early and later mortality.
Methods
Study Design
The HBAC project is a clinical trial examining three different monitoring strategies for patients receiving ART in rural Uganda. Clients of The AIDS Support Organization (TASO), a local HIV/AIDS care and support organization in Tororo and Busia districts, were invited to be screened for ART eligibility. The study includes participants from a prior diarrhea prevention and cotrimoxazole study described elsewhere [16], as well as newly recruited clients. The study included 3 arms: one with clinical monitoring only, one with clinical monitoring and quarterly CD4+ T-lymphocyte counts, and one with clinical monitoring, quarterly CD4+ T-lymphocyte counts, and HIV viral loads. Laboratory data were collected quarterly on all participants, but reported to treating physicians per protocol arm. The study was approved by the Science and Ethics Committee of the Uganda Virus Research Institute and the Institutional Review Boards of the Centers for Disease Control and Prevention and the University of California, San Francisco. The results of the randomized clinical trial have been reported elsewhere [17].
Participants
All subjects were screened for ART eligibility between May 2003 and December 2006, through clinical and laboratory assessments, including complete blood count with differential, aspartate aminotransferase (AST), alanine aminotransferase (ALT), serum creatinine, HIV viral load and CD4+ T-lymphocyte (CD4) counts. Patients with a CD4 count ≤250 cells/µL or those who had symptomatic HIV infection (WHO stage III or IV, excluding isolated pulmonary tuberculosis [TB]) were offered ART, with nevirapine, stavudine and lamivudine as the standard regimen. Potential candidates were excluded on the basis of serum chemistries only if measured AST or ALT was greater than 5 times the upper limit of normal or if estimated serum creatinine clearance was <25 mL/min. Participants were screened for active TB, malaria and other common infections by history and clinical examination. Those with symptoms or signs of TB had sputum examined for acid-fast bacilli (AFB) and chest radiographs taken. Participants diagnosed with TB were provided with home-based TB treatment. Malaria was diagnosed through blood smears and treated with chloroquine and sulfamethoxazole/pyrimethamine, according to Ugandan Ministry of Health (MoH) recommendations.
Diagnosis, classification and management of TB cases followed the guidelines of the National TB and Leprosy Program of the Ugandan MoH: patients who were ART eligible but were diagnosed with TB, completed the first two months of TB treatment (when rifampicin is used) prior to initiating ART. If they had CD4 counts <50 cells/µL they were offered ART using efavirenz, rather than nevirapine, and concomitantly began TB therapy. Participants who were diagnosed with TB while on ART were changed to efavirenz-based therapy, and were maintained on ART unless they were severely symptomatic from their TB, in which case they were offered an ART treatment interruption of one to four weeks. All participants received ART education and counseling on adherence and reduction of sexual and vertical HIV transmission risks. All participants identified medicine companions whose role was to support drug adherence for at least the first six months of therapy.
Data Collection
Participants received drug delivery and monitoring by trained lay field officers through weekly home visits and referrals as needed to see clinicians and counselors at the HBAC clinic, and they had the option of seeking acute care at the study clinic at any time. We offered sputum screening for TB and recommended visiting clinic physicians for clients complaining of a cough lasting &gt;3 weeks or other potential symptoms of TB. Study physicians at Tororo District Hospital collected clinical information during screening, at acute clinic visits and hospitalizations using standardized instruments.
Pulmonary TB was defined as two sputum smears positive for AFB or negative sputum smears, but with a chest radiograph compatible with TB and a lack of response to a 2 week trial of antibiotic therapy. Extra-pulmonary TB was diagnosed by clinical presentation and infrequently by lymph node biopsy. Cryptococcal meningitis was diagnosed by serum cryptococcal antigenemia testing. Blood smears for malaria were also collected at home for clients who complained of fever within the previous two days. Other diagnoses were made on the basis of history and physical exam with chest X-rays, sputum smear results, complete blood counts (CBC), malaria smears and urinalysis available to assist physicians in diagnosis. Diagnoses of Kaposi’s sarcoma, lymphoma and cervical cancer were based on biopsy results. Physicians responsible for patients in the study arms that included routine viral loads and/or CD4 counts received these results on a quarterly basis.
All physicians received monthly weights on all patients. Clients with CD4 counts ≤100 cells/µL had stored baseline serum samples tested for cryptococcal antigen as part of a later sub-study[18]. Monitoring or diagnostic procedures for the occurrence of intercurrent infections did not differ between study arms. All laboratory testing results were transmitted electronically from the CDC laboratory in Entebbe to clinicians in Tororo and into the study database. Field workers completed weekly client monitoring forms that included information on client symptoms, pill counts, and other information which might impact participant health until February 28, 2007. If field workers determined that a participant was seriously ill at a home visit, they had the ability to call to request transportation for the participant to the study clinic. All diagnoses of WHO stage III or IV illnesses were presented at a weekly medical case conference and reviewed by the medical team. Deaths were verified by home or hospital visits. Clinical conditions associated with death were determined by verbal autopsy, review of clinic and hospital records, and consensus of the medical team during weekly case conferences. Formal autopsies were not conducted. We defined conditions as being associated with death if they were diagnosed within one month prior to or at the time of death, or were thought to be related to death after review of the medical records. More than one diagnosis was possible for each death. We double-entered clinical and questionnaire data using Epi Info 2004 (Centers for Disease Control and Prevention, Atlanta, GA, USA).
Data Analysis
We performed descriptive statistics of the population at baseline, comparing those who died with those who did not die using the Chi-squared or Fischer’s exact test for categorical variables and the Kruskal-Wallis test for continuous variables. We calculated mortality rates, with observation time stratified into before ART (but after screening), first three months after ART, three to six months, and at six-month intervals thereafter. Weibull piece-wise survival models were fitted to Kaplan-Meier survival curves in order to determine time points where hazards for mortality changed significantly over the first 2 years of ART [19]. This procedure is described in more detail elsewhere[20]. Analyses were implemented in Stata 9.0 (Stata Corporation, College Station, TX). These change points were then used to define data-derived mortality periods for further analysis.
Opportunistic infections (OIs) and other clinical conditions present at death, thought to be associated with death or diagnosed within one month prior to death were tabulated for each period and were compared using Chi-squared or Fischer’s exact test. Adherence to therapy was calculated using a combination of pill count and pharmacy refill data, known as the medication possession ratio [21]. Cox proportional hazards modeling using both baseline and time-updated variables was used to examine factors associated with mortality for both early and late mortality periods. Variables collected in follow-up were included in statistical models using a last-observation-carried-forward approach. All variables significantly associated (p<0.05) with mortality in univariate Cox models were assessed in multivariate models. The final models were chosen by a forward stepwise selection method. These analyses were conducted in SAS version 9.0 (SAS Institute, Cary, NC).
Results
A total of 1,154 individuals were found to be ART eligible and 1,132 participants (73% women) initiated ART. The median CD4 cell count at initiation of ART was 128 cells/µL (interquartile range [IQR] = 65–194). Thirty nine percent of participants had WHO stage III disease and 8% of subjects had WHO stage IV disease. Subjects were followed for a median of 3.0 years and 112 died. Among enrollees who initiated ART, those who died in follow-up were more likely to have had WHO stage III or IV disease (77.7% vs. 43.6%; p <0.001), have lower median CD4 cell counts at baseline (74 cells/µL vs. 134 cells/µL; p<0.001), lower hemoglobin (Hb) values (10.1 vs. 11.4 g/dL; p < 0.001) and lower median BMI (18.2 vs. 19.9 kg/m2 ; p <0.001) (Table 1). Subjects who died were also more likely to have elevated AST, TB and non-TB opportunistic infections and higher log10 viral loads at baseline than subjects who survived (p <0.05 for all). There were no significant differences between participants who died and those who survived with respect to assigned monitoring arm, gender, age, median total lymphocyte counts, creatinine, proportion with ALT elevations, or malaria parasitemia at baseline.
Table 1.
Baseline characteristics and mortality among 1132 persons initiating ART, Home-based AIDS Care Program, Tororo, Uganda.
| Characteristic | Survival status |
|||
|---|---|---|---|---|
| Dead | Alive | P value | ||
| Participants N (% of total) | 112 (9.9) | 1020 (90.1) | ||
| Treatment arm N (%) | ||||
| Viral load, CD4 and clinical monitoring | 31 (27.7) | 345 (33.8) | 0.241 | |
| CD4 and clinical monitoring | 36 (32.1) | 341 (33.4) | ||
| Clinical monitoring only | 45 (40.2) | 334 (32.7) | ||
| Male - N (%) | 38 (33.9) | 268 (26.3) | 0.093 | |
| AST > 37 IU/mL - N (%) | 56 (50.9) | 405 (40.4) | 0.041 | |
| ALT > 40 IU/mL- N (%) | 23 (20.9) | 147 (14.7) | 0.093 | |
| Creatinine ≥1.5 mg/dL- N (%) | 8 (7.3) | 44 (4.4) | 0.156 | |
| WHO stage IV disease - N (%) | 19 (17.0) | 69 (6.8) | <0.001 | |
| WHO stage III or IV disease - N (%) | 87 (77.7) | 445 (43.6) | <0.001 | |
| **TB - N (%) | 22 (19.6) | 68 (6.7) | <0.001 | |
| Non-TB OI | 8 (7.1) | 26 (2.6) | 0.014 | |
| Malaria parasetemia - N (%) | 0 (0.0) | 2 (0.2) | >0.999 | |
| Age - median (IQR) | 38.5 (32.5- 45.0) | 38.0 (32.0–44.0) | 0.692 | |
| CD4 count cells/µL – median (IQR) | 74 (22–143) | 134 (73 –196) | <0.001 | |
| log10 VL – median (IQR) | 5.52 (5.11–5.88) | 5.27 (4.76–5.67) | <0.001 | |
| Total lymphocyte count – median (IQR) | 1420 (1030–2110) | 1570 (1170–2030) | 0.166 | |
| Hemoglobin – median (IQR) | 10.1 (9.1–11.5) | 11.4 (10.2–12.6) | <0.001 | |
| Body mass index kg/m2 – median (IQR) | 18.2 (16.1–19.7) | 19.9 (18.4–21.7) | <0.001 | |
| Distance from home to study clinic | ||||
| ≤ 10 km | 55 (49.1) | 513 (50.3) | 0.979 | |
| 11–30 km | 35 (31.3) | 311 (30.5) | ||
| > 30 km | 22 (19.6) | 196 (19.2) | ||
“Baseline” is the closest value at or before ART initiation
TB diagnosed at screening or within one month of starting ART or already receiving TB treatment
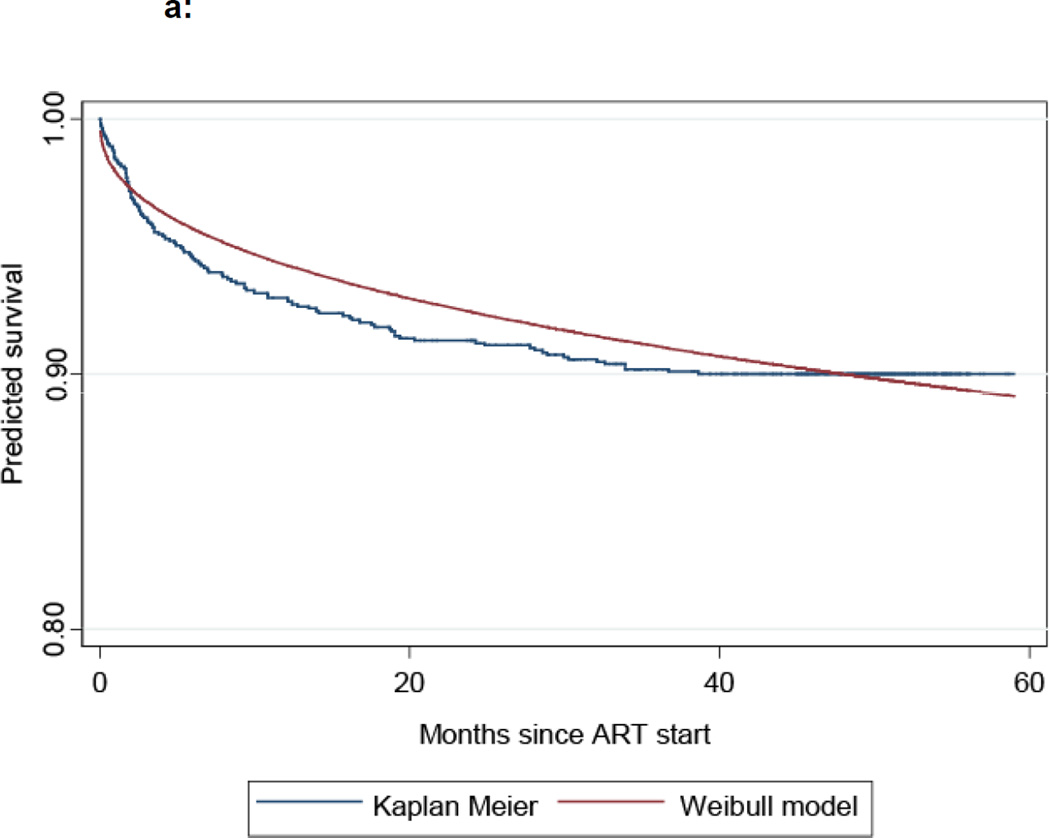
A total of 16 ART-eligible subjects died prior to ART initiation in a median follow-up time of 28 days, resulting in a mortality rate of 15.1 per 100 person-years (PYR) of follow-up (Table 2). Among participants who initiated ART, mortality was highest during the first 3 months of treatment (15.9 per 100 PYR) and declined steadily over time to reach 0.3 per 100 PYR beyond 24 months from ART initiation. We examined parametric survival models with zero, one, or more changepoints in order to determine which models best fit the observed mortality data. Figure 1 compares the observed Kaplan-Meier survival curve with a two change-point, piece-wise Weibull survival model that best approximated the observed mortality data. The estimated locations of the two changepoints were at 3 and 10 months after ART initiation. On this basis, we conducted subsequent analyses defining early mortality as deaths which occurred within 3 months of ART initiation. We defined late mortality by aggregating into a single category all deaths which occurred after 3 months of therapy as few deaths occurred after 10 months of ART.
Table 2.
Mortality over time for 1154 ART-eligible subjects, Home-based AIDS Care Project, Tororo, Uganda.
| Pre-ART | 0 – 3 months post-ART |
3 – 6 months |
6 – 12 months |
12 – 18 months |
18 – 24 months |
>24 months |
|
|---|---|---|---|---|---|---|---|
| Number of subjects at start of period |
1,154 | 1,132 | 1,087 | 1,068 | 1,046 | 1,030 | 1,006 |
| Number of deaths (%) |
16 (13) | 44 (34) | 17 (13) | 18 (14) | 13 (10) | 6 (5) | 14 (11) |
| Follow-up time (person-years) |
105.7 | 276.8 | 269.4 | 527.2 | 519.1 | 508.2 | 105.7 |
| Mortality per 100 person-years (95% confidence interval) |
15.1 (8.7 – 24.6) |
15.9 (11.6 – 21.3) |
6.3 (3.7 – 10.1) |
3.4 (2.0 – 5.4) |
2.5 (1.3 – 4.3) |
1.2 (0.4 – 2.6) |
0.3 (0.2–0.6) |
Figure 1.
a: Kaplan-Meier vs Weibull survival model – unmodified
b: Kaplan –Meier vs Weibull survival model with 2 changepoints
Note: Arrows indicate change points at 3 months (upper) and 10 months (lower) after ART initiation.
Among the participants who initiated ART, TB was the most common clinical condition associated with death (21% of deaths) throughout the study, followed by oral or esophageal candidiasis (15%), cryptococcal disease (12%), Pneumocystis jiroveci pneumonia (PCP) (8%) and Kaposi’s sarcoma (KS) (6%) (Figure 2). In 43% of cases no specific clinical condition was identified while 17.2% of all cases had 2 diseases and 2.4% of all cases had more than two diseases associated with death. HIV wasting disease was the only disease category which was distributed differently between the two time periods (9% of deaths ≤ 3 months of ART vs. 0% of deaths after 3 months; p = 0.022). A smaller proportion of deaths associated with candida (23% vs. 10%; p = 0.105) and cryptococcal disease (18% vs. 7%; p = 0.129) occurred after 3 months of ART compared with before, however these differences were not statistically significant.
Figure 2.
Clinical conditions associated with death before and after 3 months of ART, Home-based AIDS Care Project, Tororo, Uganda.
Notes for Figure 2:
N of deaths = 44 for ≤ 3 months and 68 for > 3 months
Percentages reflect the proportion of deaths where the clinical condition was identified prior to death.
More than one clinical condition was possible, so totals add to >100%
TB = tuberculosis; Candida = oropharygeal or esophageal Candida infection
Crypto = cryptococcal meningitis; PCP = Pneumoscystis jiroveci pneumonia
KS = Kaposi’s sarcoma
The final multivariate Cox proportional hazard model examining factors independently associated with mortality during the first 3 months of ART revealed associations with baseline TB diagnosis (adjusted hazard ratio [AHR] = 2.25; 95% confidence interval [CI] 1.03–4.92), a diagnosis of an opportunistic infection other than TB during follow-up (AHR = 20.43; 95% CI 10.40–40.16), baseline WHO stage III or IV disease (AHR = 4.01; 95% CI 1.51–10.40), and BMI during follow-up ≤17 kg/m2 (AHR = 2.54; 95% CI 1.32–4.90) (Table 3). In adjusted models, baseline CD4 cell count was not associated with early mortality.
Table 3.
Cox proportional hazards modeling of baseline and time-dependent variables associated with mortality within 3 months of ART initiation, Home-based AIDS Care Project, Tororo, Uganda
| Variable* | Unadjusted Hazard Ratio (95% confidence interval) |
P value | Adjusted Hazard Ratio (95% confidence interval) |
P value | |
|---|---|---|---|---|---|
| Treatment arm | |||||
| Viral load, CD4, and clinical monitoring | 0.59(0.27–1.28) | 0.182 | |||
| CD4 and clinical monitoring | 1.01 (0.52–1.98) | 0.979 | |||
| Clinical monitoring | 1.00 | ||||
| Baseline CD4 count (cells/ml) | |||||
| < 50 | 2.78 (1.22–6.35) | 0.015 | |||
| 50–200 cells/ml | 0.81 (0.35–1.88) | 0.630 | |||
| >200 cells/ml | 1.00 | ||||
| Baseline VL (log copies/ml) | 2.03 (1.14–3.61) | 0.016 | |||
| *VL in follow-up (log copies/ml) | NA | ||||
| Baseline hemoglobin (per unit decrease) | 1.33 (1.15–1.53) | <0.001 | |||
| *Hemoglobin in follow-up | NA | ||||
| Adherence to therapy (< 95%) | 2.80 (1.32–5.94) | 0.007 | |||
| **Baseline TB diagnosis | 3.60 (1.78–7.29) | < 0.001 | 2.25 (1.03–4.92) | 0.042 | |
| TB diagnosis in follow-up | 2.65 (1.46–4.83) | 0.001 | |||
| Baseline non-TB OI diagnoses | 6.70 (2.99–15.04) | <0.001 | |||
| Non-TB OI diagnoses in follow-up | 22.19(11.9041.39) | <0.001 | 20.43 (10.40–40.16) | <0.001 | |
| Baseline BMI (≤17 kg/m2 vs. >17 kg/m2) | 3.37 1.756.51 | <0.001 | |||
| BMI in follow-up (≤17 vs. >17 kg/m2) | 5.61(3.0010.46) | <0.001 | 2.54 (1.32–4.90) | 0.005 | |
| Baseline AST (≤37 IU/dL vs. >37 IU/dL) | 1.87 (1.03 – 3.40) | 0.039 | |||
| Baseline ALT (≤40 IU/dL vs. >40 IU/dL) | 2.35 (1.23–4.50) | 0.010 | |||
| Baseline Cr (<1.5 IU/dL vs. ≥1.5 IU/dL) | 2.10 (0.75–5.86) | 0.158 | |||
| Baseline WHO stage (III–IV versus I–II) | 9.07 (3.57–23.01) | <0.001 | 4.01 (1.51–10.40) | 0.005 | |
Viral load and hemoglobin scores during follow-up are not included in this analysis because they were first measured after 3 months from ART initiation
TB diagnosed at screening or within one month of initiating ART or already receiving TB treatment at screening
Mortality after 3 months of ART was associated with time-updated CD4 cell counts <50 cells/µL (AHR= 4.02; 95% CI 1.57–10.32) and 50–200 cells/µL (AHR= 2.77; 95% CI 1.58 – 4.86) compared with CD4 counts &gt;200 cells/ µ L, follow-up Hb values (AHR= 1.38 per unit decrease; 95% CI 1.23–1.54), adherence to therapy <95% (AHR = 2.85; 95% CI 1.43–5.68), a diagnosis of TB during follow-up (AHR = 2.34; 95% CI 1.34–4.08) and other opportunistic infection in follow-up (AHR= 2.99; 95% CI 1.76–5.07) (Table 4). Neither baseline CD4 cell counts nor WHO clinical stage at baseline was associated with mortality after 3 months in adjusted models. Notably, assigned treatment monitoring arm was not associated with early or late mortality on ART.
Table 4.
Cox proportional hazards modeling of baseline and time-dependent variables associated with mortality after 3 months of ART, Home-based AIDS Care Program, Tororo, Uganda
| Variables | Unadjusted Hazard Ratio (95% confidence interval) |
P value | Adjusted Hazard Ratio (95% confidence interval) |
P value | |
|---|---|---|---|---|---|
| Treatment arm | |||||
| Viral load, CD4, and clinical monitoring | 0.74 (0.42–1.31) | 0.303 | |||
| CD4 and clinical monitoring | 0.68 (0.38–1.22) | 0.196 | |||
| Clinical monitoring | 1.00 | ||||
| Baseline CD4 count (cells/ml) | |||||
| < 50 | 4.25 (1.71–10.57) | 0.002 | |||
| 50–200 cells/ml | 2.64 (1.12–6.21) | 0.027 | |||
| >200 cells/ml | 1.00 | ||||
| CD4 in follow-up (cells/ml) | |||||
| < 50 | 29.0 (12.04–70.01) | <0.001 | 4.02 (1.57–10. 32) | 0.004 | |
| 50–200 cells/ml | 5.09 (3.00–8.63) | <0.001 | 2.77 (1.58 – 4.86) | <0.001 | |
| >200 cells/ml | 1.00 | 1.00 | |||
| Baseline VL (log copies/ml) | 2.09 (1.31–3.34) | 0.002 | |||
| VL in follow-up (log copies/ml) | 1.96 (1.55–2.48) | <0.001 | |||
| Baseline hemoglobin (per unit decrease) | 1.31 1.16–1.47) | <0.001 | |||
| Hemoglobin in follow-up (per unit decrease) | 1.71 (1.57–1.87) | <0.001 | 1.38 (1.23–1.54) | <0.001 | |
| Adherence to therapy (<95%) | 8.88 ( 5.09 – 15.48) | <0.001 | 2.85 (1.43–5.68) | 0.003 | |
| *Baseline TB diagnosis | 3.05 (1.89–4.91) | <0.001 | |||
| TB diagnosis in follow-up | 2.92 (1.56–5.44) | <0.001 | 2.34 (1.34–4.08) | 0.003 | |
| Baseline non-TB OI diagnoses | 0.57 (0.079–4.11) | 0.577 | |||
| Non-TB OI diagnoses in follow-up | 6.46 (3.98–10.48) | <0.001 | 2.99 (1.76–5.07) | <0.001 | |
| Baseline BMI (≤17 kg/m2 vs. >17 kg/m2) | 3.44 (2.02–5.87) | <0.001 | |||
| BMI in follow-up (≤17 vs. >17 kg/m2) | 9.90 (5.92–16.53) | <0.001 | 3.21 (1.74–5.91) | <0.001 | |
| Baseline AST (≤37 IU/dL vs. >37 IU/dL) | 1.29 (0.79–2.08) | 0.310 | |||
| Baseline ALT (≤40 IU/dL vs. >40 IU/dL) | 1.03 (0.53–2.02) | 0.925 | |||
| Baseline Cr (<1.5 IU/dL vs. ≥1.5 IU/dL) | 1.42 (0.52–3.91) | 0.497 | |||
| Baseline WHO stage (3–4 versus 1–2) | 3.02 (1.79–5.08) | <0.001 | |||
TB diagnosed at screening or within one month of initiating ART or already receiving TB treatment at screening
Discussion
Among HIV-infected individuals initiating ART in rural Uganda, the risk of mortality was greatest in the month prior to ART initiation up until 3 months on treatment. An intermediate risk for mortality was observed between 3 and 10 months and mortality declined further after 10 months on ART. Potentially preventable conditions such as TB and cryptococcal disease were associated with about half of the deaths on ART, but many deaths were associated with no obvious clinical diagnosis. Other potentially remediable conditions such as low BMI and anemia also contributed to death on ART.
Many of the variables we examined were associated with mortality in univariate analyses, but were not independently associated with death in multivariate models. Of note, baseline CD4 cell counts were not associated with early mortality, presumably because WHO clinical staging was a better indicator of advanced HIV disease. However, both baseline CD4 cell counts and WHO clinical stage appear to be less predictive of late mortality on ART than time-updated CD4 cell counts, which were retained in multivariate models. In addition, baseline markers of renal and liver dysfunction were not associated with mortality after adjustment for other predictive factors.
There may be implications of our findings with respect to optimizing HIV care and treatment programs. Firstly, identifying HIV-infected individuals at an early stage of their disease will allow access to quality HIV care services for all HIV-infected persons. Such individuals should then be able to initiate ART soon after meeting CD4 cell count eligibility criteria and before symptomatic HIV disease develops and the associated risk of mortality increases. Initiating ART shortly after individuals meet immunological eligibility criteria may also reduce some of the early mortality seen in this and other studies. Adoption of the 2010 WHO guidelines for the initiation of ART which recommend ART for all HIV-infected individuals with CD4 cell counts ≤350 cells/ µL[22] may assist in getting more individuals initiating treatment before they become severely immuno-compromised. However, this also must be tempered with the limited ability of the Uganda to provide ART to all individuals who have CD4 cell counts ≤250 cells/ µL, the threshold currently recommended for non-pregnant adults[23].
Pre-ART care such as the provision of cotrimoxazole prophylaxis may also prevent mortality on ART by preventing malaria and several HIV-associated opportunistic infections and slowing CD4 cell count decline [16]. It might also contribute to reducing malaria-induced anemia, so that when individuals do initiate ART, they will not do so in the presence of potentially life-threatening anemia.
However, as access HIV testing in sub-Saharan African remains sub-optimal, it is likely that many individuals will continue to be diagnosed with HIV only at very late stage of their disease. As many deaths were associated with TB or cryptococcal disease, which have the potential to be prevented through the use of prophylactic isoniazid or fluconazole, respectively. Isoniazid preventive therapy (IPT) has been extensively studied as an intervention to prevent the development of symptomatic TB disease in HIV infected individuals not receiving ART [24]. A large published study from Brazil, found lower TB incidence rates in subjects receiving ART who were cluster randomized to clinics which provided IPT to TB- and HIV co-infected individuals in comparison to those who did not (0.8 per 100 person-years vs. 1.9 per 100 person-years)[25]. Similarly, fluconazole prophylaxis has been shown to reduce the incidence of cryptococcal disease among HIV infected individuals not receiving ART in the United States[26] and Thailand[27]. However, primary fluconazole prophylaxis has been little studied in the context of ART programs in African populations. Both of these interventions warrant further study in this context to see if their use can actually result in decreased incidence of these diseases and reduced mortality.
Screening and aggressively treating anemia through micronutrient supplementation and treating malaria may also reduce the contribution of anemia towards mortality in individuals receiving ART. The predominance of women in our study may partially explain the importance of anemia in our study, although, in fact women were not at higher risk for mortality in comparison to men. Lastly, food supplementation for subjects with BMI below critical thresholds should be considered to determine if the hazardous effects of low BMI can be mitigated. It is interesting to note that adherence to therapy was not independently associated with early mortality, but was with later mortality. Presumably this suggests that much of the mortality in the first 3 months of ART is due to factors which are less remediable by ART, and more related to physical and medical conditions which exist prior to ART initiation.
Our findings are similar to a study from another trial in Uganda which found that tuberculosis was the leading cause of death among patients receiving ART for a median of 2 years [5]. While cryptococcal disease was the third leading cause of death in this study, unspecified febrile illnesses were the second leading cause of death. In our study we also observed that both early and late deaths were associated with oropharyngeal or esophageal Candida infections. However, we think that it is unlikely that Candida itself is a leading cause of death, but is more likely a marker for advanced HIV disease. Note that we did not explicitly collect data on inflammatory immune reconstitution syndrome (IRIS). However, anecdotally, IRIS appeared to occur very rarely, an observation supported by other cohort studies[28–30] and is unlikely to account for much of the unexplained mortality in our study.
This study has a number of limitations. We had limited diagnostic tools available which may have reduced our ability to identify specific causes of illness; nearly half of deaths occurred without a diagnosed clinical cause. While it would be helpful to have a larger array of diagnostic tools available along with autopsies to establish precise causes of death, such infrastructure would be difficult to establish in most rural African settings. Furthermore, the relatively small number of deaths in the early and late time-periods also limited out ability to detect differences between them. Despite these limitations, this analysis has provided some of the most complete information on factors associated with mortality among HIV infected individuals receiving ART in an African setting.
In summary, while mortality is greatly reduced in HIV-infected individuals receiving ART, deaths occur at a higher rate than is generally found in high-income countries. Initiating ART immediately after an individual becomes clinically eligible, supporting adherence to therapy and retaining patients within ART programs remain the most important determinants of survival on ART. As need for ART is likely to continue to greatly outstrip availability in sub-Saharan Africa, in the near future, most HIV-infected individuals are likely to initiate ART at lower than optimal CD4 cell counts. In such a context, many deaths could potentially be prevented through expanded use of prophylactic medications, micronutrient support or targeted food supplementation. The evaluation of these additional interventions within ART programs in sub-Saharan Africa should be an area of intensive future research. As well, more effective and better diagnostic tools are needed in these settings to assist in further clarifying the precise causes of death among this population.
Acknowledgements
The authors would like to thank the field officers, counselors and clinical staff who care for patients in the Home Based AIDS Care project, the informatics and laboratory team at CDC-Uganda who compiled the data for analysis, and the participants in the Home-Based AIDS Care project. We would also like to acknowledge the support of the Ugandan Ministry of Health and The AIDS Support Organization. HBAC is funded by CDC through the President’s Emergency Plan for AIDS Relief (PEPFAR). The work of two authors, CTY and BSM, was partially supported by the International Epidemiologic Databases to Evaluate AIDS (IeDEA) grant AI 69911 (NIAID) and PEPFAR. DM is supported by the Canadian Institutes for Health Research through a New Investigator Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coetzee D, Hildebrand K, Boulle A, Maartens G, Louis F, Labatala V, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. Aids. 2004;18:887–895. doi: 10.1097/00002030-200404090-00006. [DOI] [PubMed] [Google Scholar]
- 2.Ferradini L, Jeannin A, Pinoges L, Izopet J, Odhiambo D, Mankhambo L, et al. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet. 2006;367:1335–1342. doi: 10.1016/S0140-6736(06)68580-2. [DOI] [PubMed] [Google Scholar]
- 3.Laurent C, Diakhate N, Gueye NF, Toure MA, Sow PS, Faye MA, et al. The Senegalese government's highly active antiretroviral therapy initiative: an 18-month follow-up study. Aids. 2002;16:1363–1370. doi: 10.1097/00002030-200207050-00008. [DOI] [PubMed] [Google Scholar]
- 4.Wools-Kaloustian K, Kimaiyo S, Diero L, Siika A, Sidle J, Yiannoutsos CT, et al. Viability and effectiveness of large-scale HIV treatment initiatives in sub-Saharan Africa: experience from western Kenya. Aids. 2006;20:41–48. doi: 10.1097/01.aids.0000196177.65551.ea. [DOI] [PubMed] [Google Scholar]
- 5.Munderi P, Watera C, Nakiyingi J, Kasirye A, Walker S, French N, et al. 16th International AIDS Conference. Toronto: 2006. Survival and Causes of Death, 2 years after introduction of Antiretroviral Therapy in Africa: a historical cohort comparison in Entebbe, Uganda. [Google Scholar]
- 6.Wester CW, Kim S, Bussmann H, Avalos A, Ndwapi N, Peter TF, et al. Initial response to highly active antiretroviral therapy in HIV-1C–infected adults in a public sector treatment program in Botswana. J Acquir Immune Defic Syndr. 2005;40:336–343. doi: 10.1097/01.qai.0000159668.80207.5b. [DOI] [PubMed] [Google Scholar]
- 7.Marazzi MC, Liotta G, Germano P, Guidotti G, Altan AD, Ceffa S, et al. Excessive early mortality in the first year of treatment in HIV type 1-infected patients initiating antiretroviral therapy in resource-limited settings. AIDS Res Hum Retroviruses. 2008;24:555–560. doi: 10.1089/aid.2007.0217. [DOI] [PubMed] [Google Scholar]
- 8.Hogg RS, Yip B, Chan KJ, Wood E, Craib KJ, O'Shaughnessy MV, Montaner JS. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. Jama. 2001;286:2568–2577. doi: 10.1001/jama.286.20.2568. [DOI] [PubMed] [Google Scholar]
- 9.Chene G, Sterne JA, May M, Costagliola D, Ledergerber B, Phillips AN, et al. Prognostic importance of initial response in HIV-1 infected patients starting potent antiretroviral therapy: analysis of prospective studies. Lancet. 2003;362:679–686. doi: 10.1016/s0140-6736(03)14229-8. [DOI] [PubMed] [Google Scholar]
- 10.Attia A, Huet C, Anglaret X, Toure S, Ouassa T, Gourvellec G, et al. HIV-1-related morbidity in adults, Abidjan, Cote d'Ivoire: a nidus for bacterial diseases. J Acquir Immune Defic Syndr. 2001;28:478–486. doi: 10.1097/00042560-200112150-00012. [DOI] [PubMed] [Google Scholar]
- 11.Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A, Miotti P, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 12.Stringer JS, Zulu I, Levy J, Stringer EM, Mwango A, Chi BH, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. Jama. 2006;296:782–793. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 13.Etard JF, Ndiaye I, Thierry-Mieg M, Gueye NF, Gueye PM, Laniece I, et al. Mortality and causes of death in adults receiving highly active antiretroviral therapy in Senegal: a 7-year cohort study. Aids. 2006;20:1181–1189. doi: 10.1097/01.aids.0000226959.87471.01. [DOI] [PubMed] [Google Scholar]
- 14.Lawn SD, Myer L, Harling G, Orrell C, Bekker LG, Wood R. Determinants of mortality and nondeath losses from an antiretroviral treatment service in South Africa: implications for program evaluation. Clin Infect Dis. 2006;43:770–776. doi: 10.1086/507095. [DOI] [PubMed] [Google Scholar]
- 15.Lawn SD, Myer L, Orrell C, Bekker LG, Wood R. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. Aids. 2005;19:2141–2148. doi: 10.1097/01.aids.0000194802.89540.e1. [DOI] [PubMed] [Google Scholar]
- 16.Mermin J, Lule J, Ekwaru JP, Malamba S, Downing R, Ransom R, et al. Effect of co-trimoxazole prophylaxis on morbidity, mortality, CD4-cell count, and viral load in HIV infection in rural Uganda. Lancet. 2004;364:1428–1434. doi: 10.1016/S0140-6736(04)17225-5. [DOI] [PubMed] [Google Scholar]
- 17.Coutinho A, Mermin J, Ekwaru J, Were W, Degerman R, Bunnell R, et al. 15th Conference on Retroviruses and Opportunistic Infections. Boston, USA: 2008. Utility of routine viral load, CD4 cell count, and clinical monitoring among HIV-infected adults in Uganda: a randomized trial. [Google Scholar]
- 18.Liechty CA, Solberg P, Were W, Ekwaru JP, Ransom RL, Weidle PJ, et al. Asymptomatic serum cryptococcal antigenemia and early mortality during antiretroviral therapy in rural Uganda. Trop Med Int Health. 2007;12:929–935. doi: 10.1111/j.1365-3156.2007.01874.x. [DOI] [PubMed] [Google Scholar]
- 19.Noura AA, Read KLQ. Proportional hazards changepoint models in survival analysis. Applied Statistics. 1990;39:241–253. [Google Scholar]
- 20.Yiannoutsos CT. Modeling AIDS survival after initiation of antiretroviral treatment by Weibull models with changepoints. J Int AIDS Soc. 2009;12:9. doi: 10.1186/1758-2652-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weidle PJ, Wamai N, Solberg P, Liechty C, Sendagala S, Were W, et al. Adherence to antiretroviral therapy in a home-based AIDS care programme in rural Uganda. Lancet. 2006;368:1587–1594. doi: 10.1016/S0140-6736(06)69118-6. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents in resource-limited settings: Recommendations towards a public health approach. Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 23.Katabira E, Kamya M, Kalyesubula I, Namale A, editors. Uganda Ministry of Health. National antiretroviral treatment and care guidelines for adults, adolescents and children. 2nd Edition. Kampala: Ministry of Health; 2008. [Google Scholar]
- 24.Bucher HC, Griffith LE, Guyatt GH, Sudre P, Naef M, Sendi P, Battegay M. Isoniazid prophylaxis for tuberculosis in HIV infection: a meta-analysis of randomized controlled trials. Aids. 1999;13:501–507. doi: 10.1097/00002030-199903110-00009. [DOI] [PubMed] [Google Scholar]
- 25.Golub JE, Saraceni V, Cavalcante SC, Pacheco AG, Moulton LH, King BS, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. Aids. 2007;21:1441–1448. doi: 10.1097/QAD.0b013e328216f441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Havlir DV, Dube MP, McCutchan JA, Forthal DN, Kemper CA, Dunne MW, et al. Prophylaxis with weekly versus daily fluconazole for fungal infections in patients with AIDS. Clin Infect Dis. 1998;27:1369–1375. doi: 10.1086/515018. [DOI] [PubMed] [Google Scholar]
- 27.Chetchotisakd P, Sungkanuparph S, Thinkhamrop B, Mootsikapun P, Boonyaprawit P. A multicentre, randomized, double-blind, placebo-controlled trial of primary cryptococcal meningitis prophylaxis in HIV-infected patients with severe immune deficiency. HIV Med. 2004;5:140–143. doi: 10.1111/j.1468-1293.2004.00201.x. [DOI] [PubMed] [Google Scholar]
- 28.Muller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis. 10:251–261. doi: 10.1016/S1473-3099(10)70026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murdoch DM, Venter WD, Feldman C, Van Rie A. Incidence and risk factors for the immune reconstitution inflammatory syndrome in HIV patients in South Africa: a prospective study. Aids. 2008;22:601–610. doi: 10.1097/QAD.0b013e3282f4a607. [DOI] [PubMed] [Google Scholar]
- 30.Castelnuovo B, Manabe YC, Kiragga A, Kamya M, Easterbrook P, Kambugu A. Cause-specific mortality and the contribution of immune reconstitution inflammatory syndrome in the first 3 years after antiretroviral therapy initiation in an urban African cohort. Clin Infect Dis. 2009;49:965–972. doi: 10.1086/605500. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents in resource-limited settings: Recommendations for a publc health approach. Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 32.World Health Organization. Antiretroviral therapy of HIV infection in infants and children: Towards universal access. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]