Abstract
Context
Thienopyridines are among the most widely prescribed medications, but their use can be complicated by the unanticipated need for surgery. Despite increased risk of thrombosis, guidelines recommend discontinuing thienopyridines 5–7 days prior to surgery to minimize bleeding.
Objective
To evaluate the use of cangrelor, an intravenous, reversible P2Y12 platelet inhibitor for bridging thienopyridine-treated patients to coronary artery bypass grafting (CABG).
Design, Setting, and Patients
Prospective, randomized double-blind, placebo-controlled, multicenter trial, in patients (n=210) with an acute coronary syndrome (ACS) or treated with a coronary stent on a thienopyridine awaiting CABG to receive either cangrelor or placebo after an initial open-label, dose-finding phase (n=11) conducted between January 2009 and April 2011.
Interventions
Thienopyridines were stopped and patients administered cangrelor or placebo for at least 48 hours, which was discontinued 1–6 hours prior to CABG.
Main outcome measures
The primary efficacy endpoint was platelet reactivity (measured in P2Y12 Reaction Units [PRU]), assessed daily with the VerifyNow™ P2Y12 assay. The main safety endpoint was excessive CABG-related bleeding.
Results
The dose of cangrelor determined in the open-label stage was 0.75 µg/kg/min. In the randomized phase, a greater proportion of patients treated with cangrelor had low levels of platelet reactivity throughout the entire treatment period compared with placebo (primary endpoint, PRU<240: 98.8% (83/84) vs. 19.0% (16/84); relative risk [RR]: 5.2, 95% confidence interval [CI]:3.3–8.1, p<0.001). Excessive CABG-related bleeding occurred in 11.8% (12/102) vs. 10.4% (10/96) in the cangrelor and placebo groups, respectively (RR=1.1, 95% CI: 0.5–2.5, p=0.763). There were no significant differences in major bleeding prior to CABG, although minor bleeding was numerically higher with cangrelor.
Conclusions
Among patients who must wait for cardiac surgery after thienopyridine discontinuation, the use of cangrelor compared with placebo resulted in a higher rate of maintenance of platelet inhibition.
Dual antiplatelet therapy with aspirin and an oral P2Y12 receptor inhibitor is the standard of care to prevent the short and long-term risk of recurrent atherothrombotic events in high-risk settings, such as patients with an acute coronary syndrome (ACS) and those undergoing percutaneous coronary intervention (PCI) (1–4). However, the ischemic benefit associated with more intense platelet blockade in these high-risk settings occurs at the expense of an increased risk of bleeding complications. Importantly, given that the risk of bleeding is significantly increased in patients undergoing surgical procedures, in particular coronary artery bypass grafting (CABG) surgery, discontinuation of antiplatelet therapy for a time frame that allows recovery of platelet function is warranted (1–4). However, premature discontinuation of antiplatelet therapy in these settings has been associated with an increase in ischemic complications (5–8). These concerns are magnified in patients treated with drug-eluting stents (DES) in whom thrombotic occlusions occurring as a consequence of antiplatelet treatment discontinuation, in particular P2Y12 receptor blockers, are associated with substantial morbidity and mortality (9–12). These findings underscore the need to define strategies of platelet inhibition that allow to safely “bridge” patients to their surgical procedure with minimum risk of ischemic events or bleeding complications.
Cangrelor, a nonthienopyridine adenosine triphosphate analogue, is an intravenous (IV) antagonist of the P2Y12 receptor characterized by rapid, potent, predictable, and reversible platelet inhibition with quick offset of effect (13). Therefore, this compound possesses desirable pharmacodynamic (PD) properties to be considered for bridging patients to surgery in whom discontinuation of antiplatelet therapy, particularly a P2Y12 receptor inhibitor, can lead to catastrophic consequences (e.g. stent thrombosis) while preserving normal hemostasis at the time of surgery (14). In the current trial we hypothesized that cangrelor may be a safe and effective drug to bridge patients from irreversible platelet P2Y12 inhibitors to open heart surgery.
METHODS
Study Oversight
The trial was designed and led by an Executive Committee that was chaired by EJT and included academic investigators in conjunction with the Sponsor, The Medicines Company. Pertinent national regulatory authorities and ethics committee at participating centers approved the protocol. All patients provided written informed consent. The data were analyzed independently by the investigators and the Sponsor. The analysis was also independently validated by a faculty statistician at the Penn State University using entire raw datasets. Results reported were consistent with the independent analysis. The trial protocol is available with the full text of this article at jama.ama-assn.org. All the authors assume responsibility for the accuracy and completeness of the data and the analyses.
Trial Design
The BRIDGE trial consisted of two independent stages. Eligibility criteria were the same for both stages. Stage I was conducted between January 2009 and August 2009 and represented an open-label phase of the study aimed to identify the dose of cangrelor that achieved a desired antiplatelet effect after thienopyridine discontinuation. Specifically, cangrelor IV infusion was to be administered to cohorts of 5 patients at a time in a step-wise fashion at pre-determined doses (0.5 µg/kg/min, 0.75 µg/kg/min, 1.0 µg/kg/min and 1.5 µg/kg/min) until percent platelet inhibition as measured by VerifyNow™ P2Y12 was > 60% in 80% of daily samples or a dose of 2.0 µg/kg/min was reached.
Stage II was a prospective, randomized, double-blind, placebo-controlled phase of the study enrolling patients independent of Stage I conducted between October 2009 and April 2011.. The aim was to assess whether a cangrelor IV infusion (at the dose determined in Stage I) would maintain levels of platelet reactivity <240 P2Y12 Reaction Units (PRU) throughout the pre-operative period as measured by the VerifyNow™ P2Y12 assay. This level approximated the levels of platelet reactivity expected to be maintained if a thienopyridine had not been discontinued (15–16). Eligible patients were randomly assigned in a 1:1 ratio by an interactive voice-response system (IVRS) to receive cangrelor infusion plus standard of care or placebo infusion plus standard of care, using the cangrelor dose determined in Stage I. The fixed, blocked, permuted randomization schedule was generated with a block size of 4 and was stratified according to the anticipated delay until CABG (≤ 3 days or > 3 days). Study drug was provided in blinded kits and allocated by IVRS. Study drug infusion was initiated after thienopyridine therapy was discontinued and was continued throughout the pre-operative period up to 1–6 hours prior to surgical incision. Study drug was not administered during or after CABG. It was recommended that patients wait 5 days after discontinuation of ticlopidine and clopidogrel, and 7 days after prasugrel, before undergoing surgery in accordance with practice guidelines (1–4). However, the timing of surgery was left to the discretion of the investigator with a minimum of 48 hours and an allowed maximum of 7 days of study drug infusion. Aspirin therapy was maintained at a dosing regimen as per routine local practice.
Safety analyses were carried out on data from patients who had received at least one dose of study drug. A dose confirmation analysis and safety data review was conducted by an independent and unblinded Data Safety Monitoring Board (DSMB) based on the pre-specified plan. According to pre-specified DSMB Charter, the DSMB reviewed the dosing data for the first 24 patients randomized into Stage II on April 20th 2010. In order to allow for a better assessment of the optimal dose further unblinded review was requested by DSMB. This review confirmed the dose,however, given the unblinded review, the DSMB and Executive Committee recommended exclusion of these patients from primary efficacy analysis.
Study Population
Patients at least 18 years of age planned to undergo non-emergent CABG were eligible to be enrolled. All patients had to have received a thienopyridine (at least 500 mg ticlopidine, 75 mg of clopidogrel or 10 mg of prasugrel) within at least 72 hours prior to randomization either for the treatment of an ACS or for long-term preventive therapy following coronary stent implantation, DES or bare metal stents. CABG surgery, either on-pump or off-pump, had to occur no sooner than 48 hours but no longer than 7 days from randomization, with patients hospitalized until planned CABG. Exclusion criteria are described in the Appendix.
Blood Sampling and Platelet Function Testing
Platelet function was assessed with the VerifyNow™ P2Y12 (Accumetrics, San Diego CA) point of care test according to manufacturer instructions before, during, and after study drug infusion (15–16). Blood sampling for platelet function testing during study drug infusion was performed daily; it was recommended that blood sampling be drawn daily at the same time. Study drug infusion was discontinued 1–6 hours prior to CABG and the last on-infusion sample for platelet function testing had to be within 12 hours prior to infusion stop. Blood sampling after study drug stop had to be just prior to surgical incision. This test has been previously described in detail (15–16). In brief, this test measures adenosine diphosphate (ADP)-induced platelet agglutination as an increase in light transmittance and utilizes a proprietary algorithm to report values in PRU. A higher PRU result reflects greater P2Y12-mediated reactivity. A second activator, iso-thrombin-receptor activating peptide (iso-TRAP), is incorporated into a second channel of the assay device and provides an estimated inhibition (percent VerifyNow inhibition) without a pre-thienopyridine sample by reporting the ratio of the results of the ADP and iso-TRAP channels. Specialized software developed for the trial encrypted the platelet function results to maintain double blinding.
Study Endpoints
The primary efficacy endpoint of Stage I was maintenance of platelet inhibition in at least 80% of patient samples above 60% as determined by VerifyNow™ P2Y12 point of care test measured during study drug infusion prior to surgery. The primary efficacy endpoint of Stage II was the proportion (%) of patients with platelet reactivity <240 PRU for all samples assessed during study drug infusion prior to surgery. Additional efficacy endpoints included: the percentage of total patient samples that maintained >60% platelet inhibition during study drug infusion; the percentage of total patient samples that maintained platelet reactivity <240 PRU during study drug infusion; the percentage of patients who maintained platelet reactivity <240 PRU in their last on-treatment sample prior to surgery; and the percentage of patients in whom all platelet reactivity evaluations during study drug infusion prior to surgery were less than or equal to their baseline platelet reactivity prior to receipt of study drug.
The main safety endpoint (secondary endpoint) of the trial was excessive CABG-related bleeding, as defined by the occurrence of one or more of the following 3 components during the CABG procedure through hospital discharge: surgical re-exploration, 24 hour chest tube output of >1.5 liters, or packed red blood cell transfusion >4 units. In addition to the protocol defined endpoint of excessive CABG-related bleeding, the Bleeding Academic Research Consortium (BARC)-defined CABG-related bleeding was assessed, i.e. the occurrence of one or more of the following during the CABG procedure through hospital discharge: fatal bleeding; perioperative intracranial bleeding within 48 hours; reoperation following closure of sternotomy for the purpose of controlling bleeding; transfusion of ≥ 5 units of whole blood or packed red blood cells within a 48 hour period; chest tube output ≥ 2 L within a 24 hour period (17). Pre-operative bleeding defined according to the Thrombolysis in Myocardial Infarction (TIMI), Global Use of Strategies To Open coronary arteries (GUSTO), and Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) definitions (18–20), as well all blood product transfusions up to 7 days post-CABG or discharge, whichever was sooner, were also collected. Additional safety observations included the incidence of combined ischemic endpoint of death, myocardial infarction (MI), stroke or need for urgent revascularization from the time of randomization until discontinuation of study drug and within 30 days following CABG. Incidence of other adverse events and serious adverse events were recorded up to 7 days post CABG or discharge, whichever occurred sooner (See Appendix for Protocol Definitions for Efficacy and Safety Assessments). All safety and ischemic events were site reported and non-adjudicated.
Statistical analysis
We estimated that, assuming 30% of placebo-treated patients and at least 60% of cangrelor-treated patients would reach the primary endpoint, a sample size of 106 patients (53 each arm) would provide 90% power to detect a statistically significant difference between the treatment arms at a two-sided alpha of 0.05 using a Chi-squared test. The sample size calculation was performed with PASS 2008 software. We selected a sample size of up to 100 patients per treatment arm to further evaluate the safety of cangrelor before and after CABG.
Two analysis populations were defined and used in the analysis and presentation of the data. The safety population included patients who received any study drug, and patients were classified according to the actual treatment received. The intention to treat (ITT) population was defined as all patients randomized into the trial in Stage II who received study drug excluding the first 24 patients whose data were unblinded for the DSMB review in dose confirmation. Treatment classification for ITT analysis was based on the randomized treatment. VerifyNow P2Y12 results were considered valid if at least one sample was confirmed drawn while on infusion and if the sample was analyzed within the manufacturer recommended time window. Per protocol, missing data were not imputed; therefore, the efficacy analysis was performed only on ITT patients with valid PRU data. A sensitivity analyses including a per protocol analysis (excluding major deviations) and an analysis with safety was also performed.
Baseline difference of quantitative factors, such as duration of infusion, was compared using Wilcoxon test. The primary efficacy endpoint, the percentage of patients who maintained PRU <240 during study drug infusion prior to surgery, was analyzed using logistic regression adjusted for the expected days to surgery (either ≤3 days or >3 days) with the ITT population. The Chisquare test was performed for other efficacy endpoints measured in proportions. Analysis of variance was used to compare quantitative endpoints, such as PRU value, between the treatment groups. No missing data imputation or multiple comparison alpha adjustment was applied. SAS version 9.2 for Windows was used for all statistical analysis.
RESULTS
Patient Population and Follow-up
A total of 11 patients from 4 sites in the United States participated in the open-label dose-finding phase of the trial (Stage I). Of these, 10 patients had evaluable data for the purpose of dose considerations; 5 patients completed the first cohort at a dose of 0.5 µg/kg/min, and 5 the second at a dose of 0.75 µg/kg/min (See Appendix for Supplementary Table on PRU results of Stage I). The 0.75 µg/kg/min dose of cangrelor met the efficacy endpoint of maintenance of platelet inhibition in at least 80% of patient samples above 60% (94.4% (17/18), 95% confidence interval [CI]: 83.9% – 100%), and was considered for the randomized, double-blind, placebo-controlled phase of the trial. This dose was confirmed by designated unblinded review of data of the first 24 patients randomized to Stage II.
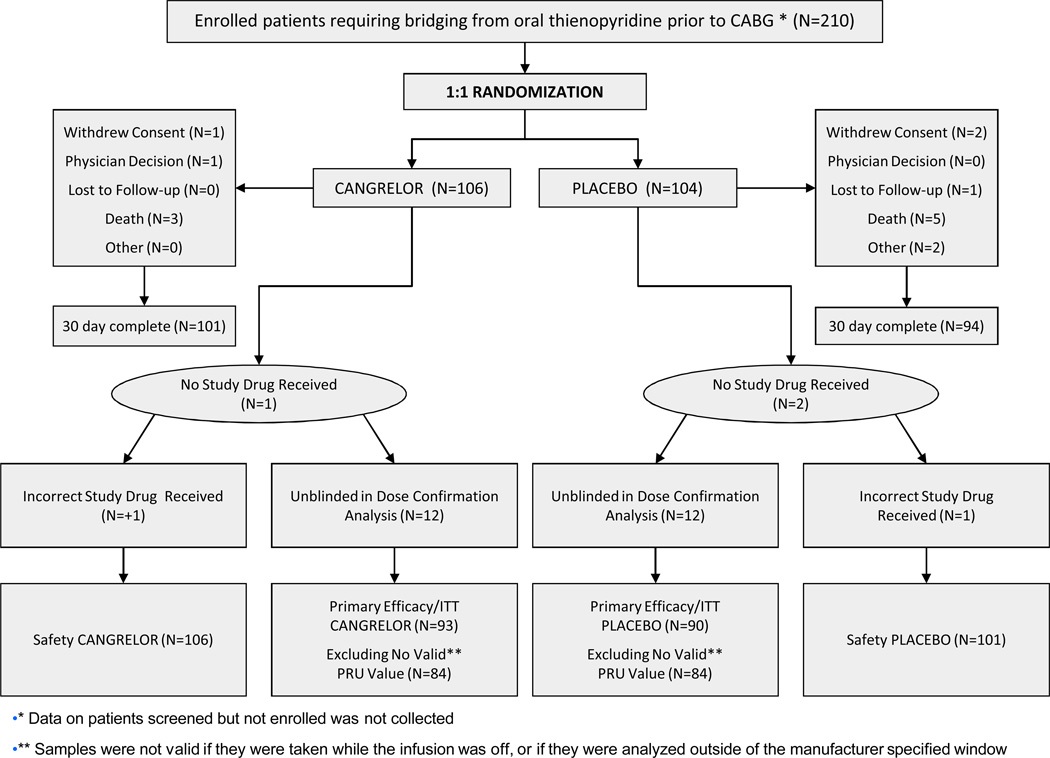
A total of 210 patients from 34 global sites (See Appendix for Participating Centers) requiring bridging from oral thienopyridine therapy to CABG were randomly assigned in the blinded strata (Stage II) to either cangrelor (n=106) or placebo (n=104). One patient in the cangrelor arm and 2 in the placebo did not receive study medication; 1 patient in the placebo arm was erroneously given an active medication kit. Therefore, a total of 106 and 101 patients treated with cangrelor and placebo, respectively, represented the safety population of this study. Due to the unblinding of the first 24 patients (12 per group) by the DSMB, these were excluded from the efficacy analyses. Therefore, the efficacy/ITT population was composed of 93 and 90 patients treated with cangrelor and placebo, respectively. Only 1 patient (0.9%), randomly assigned to placebo, was lost to 30-day follow-up. Patient disposition is illustrated in Figure 1.
Figure 1. BRIDGE CONSORT diagram.
CABG indicates coronary artery bypass grafting. ITT indicates intention to treat
The treatment groups were well balanced with regard to baseline clinical and demographic characteristics (Table 1). In the cangrelor arm, 15.1% of patients had presented with an ST-elevation MI and 32.1% with a non-STEMI, while in the placebo arm, 11.9% of patients had presented with an ST-elevation MI and 44.5% with a non-STEMI; the remaining patients were enrolled in a non-acute setting. CABG was performed in 102 patients (96%) and 96 patients (95%) in cangrelor and placebo groups, respectively. The median time and inter-quartile range from thienopyridine discontinuation to study drug infusion was 29.1 (11–38) and 29.5 (14–39) hours in cangrelor and placebo, respectively (Wilcoxon p=0.796). The median duration and inter-quartile range of infusion of cangrelor versus placebo was 2.8 (2.5 – 3.8) and 3.4 (2.6 – 4.7) days (Wilcoxon p=0.046). The median time and inter-quartile range from discontinuation of study drug infusion to surgical incision was 3.2 (2– 5) and 3.2 (2– 5) hours in cangrelor and placebo, respectively (Wilcoxon p=0.818).
Table 1.
Baseline characteristics and study treatments
| Cangrelor (N= 106) |
Placebo (N= 101) |
|
|---|---|---|
| Age, yrs | 65.0 (42, 84) | 62.0 (39, 89) |
| Sex, No. (%) | ||
| Male | 80 (75.5) | 74 (73.3) |
| Female | 26 (24.5) | 27 (26.7) |
| Race, No. (%) | ||
| White | 93 (87.7) | 94 (93.1) |
| Asian | 3 (2.8) | 0 (0.0) |
| Black | 6 (5.7) | 5 (5.0) |
| Hispanic | 4 (3.8) | 2 (2.0) |
| Other | 0 (0.0) | 0 (0.0) |
| Weight, kg | 88.0 (58, 154) | 85.0 (51, 139) |
| Height, cm | 172.9 (152, 190) | 172.7 (150, 193) |
| Body Mass Index | 29.3 (20, 50) | 28.2 (19, 47) |
| Medical history, No. (%) | ||
| Diabetes mellitus | 49 (46.2) | 47 (46.5) |
| Current smoker | 31 (29.2) | 38 (37.6) |
| Hypertension | 87 (82.1) | 83 (82.2) |
| Hyperlipidemia | 76 (71.7) | 77 (76.2) |
| Stroke/TIA | 9 (8.5) | 4 (4.0) |
| Family history of CAD | 47 (44.3) | 49 (48.5) |
| Prior MI | 46 (43.4) | 36 (35.6) |
| Prior PCI | 53 (50.0) | 46 (45.5) |
| Prior CABG | 3 (2.8) | 1 (1.0) |
| Congestive HF | 16 (15.1) | 6 (5.9) |
| PAD | 14 (13.2) | 12 (11.9) |
| Last Thienopyridine therapy | 0 (0.0) | |
| Clopidogrel, No. (%) | 105 (99.1) | 93 (92.1) |
| 75mg, No. (%) | 88 / 105 (83.8) | 65 / 93 (69.9) |
| 150mg, No. (%) | 1 / 105 (1.0) | 2 / 93 (2.2) |
| 300mg, No. (%) | 4 / 105 (3.8) | 12 / 93 (12.9) |
| 600mg, No. (%) | 12 / 105 (11.4) | 13 / 93 (14.0) |
| 900mg, No. (%) | 0 / 105 (0.0) | 1 / 93 (1.1) |
| Prasugrel, | 1 (0.9) | 8 (7.9) |
| 10mg, No. (%) | 1 / 1 (100) | 6 / 8 (75.0) |
| 60mg, No. (%) | 0 / 1 (0.0) | 1 / 8 (12.5) |
| Unknown, No. (%) | 0 / 1 (0.0) | 1 / 8 (12.5) |
| Ticlopidine | 0 (0.0) | 0 (0.0) |
| Other Antithrombotic Medications (Pre Surgery) | ||
| Aspirin, No. (%) | 105 (99.1) | 97 (96.0) |
| Low molecular weight heparin, No. (%) |
38 (35.8) | 43 (42.6) |
| Unfractionated heparin, No. (%) | 51 (48.1) | 49 (48.5) |
| Bivalirudin, No. (%) | 0 (0) | 1 (1.0) |
| Fondaparinux, No. (%) | 3 (2.8) | 4 (4.0) |
Variables are presented as median (25th, 75th) unless otherwise indicated.
CABG denotes coronary artery bypass graft surgery; CAD, coronary artery disease; HF, heart failure; MI, myocardial infarction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; TIA, transient ischemic attack.
Efficacy Endpoints
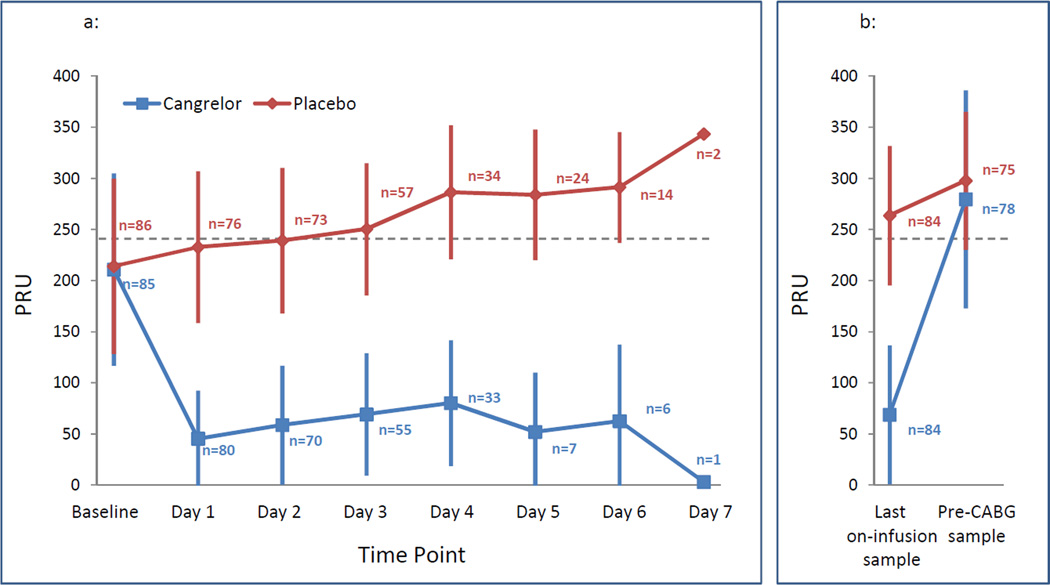
Valid PRU results were not available for 15 patients (9 in cangrelor and 6 in placebo groups) in the planned ITT analysis population during the infusion period. At baseline, prior to infusion of study medication, the levels of platelet reactivity (p=0.817) and percent of patients with platelet reactivity <240 PRU (p=0.185) were not different between groups (Table 2). The primary efficacy endpoint of percent of patients with platelet reactivity <240 PRU throughout the entire infusion of study drug was significantly higher in the cangrelor compared with the placebo arm (98.8% (83/84) [95% CI: 96.5–100%] vs. 19.0% (16/84) [95% CI: 10.7–27.4%], p<0.0001; crude relative risk [RR]: 5.2, 95% CI: 3.3 – 8.1; RR: 5.2, 95% CI 3.3 – 8.0 adjusted for expected days to surgery). These outcomes were yielded independent of prior thienopyridine dose and time of discontinuation (RR: 4.3, 95% CI: 2.8–6.6) and were consistent with the sensitivity analysis [95.9% (93/97) vs. 20.0% (19/95); RR=4.8, 95% CI: 3.2–7.2]. Adjusting for both expected days to surgery and duration of infusion did not alter the effect of cangrelor, RR: 5.1, 95% CI: 3.3 – 8.0. The percent of overall samples displaying platelet reactivity <240 PRU, patients with all samples ≤ baseline PRU value, total patient samples that maintained > 60% platelet inhibition during study drug infusion were all greater with cangrelor compared with placebo (all p<0.001; Table 2). Accordingly, the last sample during study drug infusion showed lower PRU values and a greater percent of patients with platelet reactivity <240 PRU with cangrelor (all p<0.001; Table 2). Following discontinuation of study medication infusion prior to surgical incision, PRU levels (p=0.212) and the percent of patients with platelet reactivity <240 PRU (p=0.313) were similar between groups (Table 2). The distribution of platelet reactivity during the overall study time course is illustrated in Figure 2.
Table 2.
Pharmacodynamic data in the Intention To Treat Population
| Cangrelor (N= 93) |
Placebo (N= 90) |
Relative Risk (95% CI) |
p-value | |
|---|---|---|---|---|
| Prior to Study Drug Infusion | ||||
| Patients with platelet reactivity <240 PRU, % (95% CI, N) |
62.4% (52.1–72.7%, 53/85) |
52.3% (41.8–62.9%, 45/86) |
1.2 (0.9 – 1.5) | 0.185 |
| PRU values, Mean ± SD | 210.9 ± 94.0 | 214.1 ± 85.9 | NA | 0.817 |
| During Study Drug Infusion | ||||
| Patients with platelet reactivity <240 PRU throughout entire |
98.8% (96.5–100%, 83/84) | 19.0% (10.7–27.4%, 16/84) |
Crude: 5.2 (3.3 – 8.1) |
<0.001 |
| infusion period (Primary Endpoint), % (95% CI, N) |
Adjusted: 5.2 (3.3 – 8.0) |
<0.001 | ||
| Samples with platelet reactivity <240 PRU, % (95% CI, N) |
99.6% (98.9–100%, 258/259) |
33.3% (27.9–38.7%, 98/294) |
3.0 (2.5 – 3.5) | <0.001 |
| Total samples that maintained > 60% platelet inhibition, % (95% CI, N) |
83.8% (79.3–88.3%, 217/259) |
3.7% (1.6–5.9%, 11/294) | 22.4 (12.5 – 40.1) | <0.001 |
| Patients with all samples ≤ baseline PRU value, % (95% CI, N) |
92.1% (86.0–98.2%, 70/76) |
12.3% (5.2–19.5%, 10/81) |
7.5 (4.2 – 13.4) | <0.001 |
| Patients with last sample during infusion with platelet reactivity <240 PRU, % (95% CI, N) |
98.8% (96.5–100%, 83/84) | 31.0% (21.1–40.8%, 26/84) |
3.2 (2.3 – 4.4) | <0.001 |
| PRU values last sample during infusion, Mean ± SD |
68.9 ± 67.8 | 263.7 ± 68.3 | NA | <0.001 |
|
Following Discontinuation of Study Drug Infusion |
||||
| Patients with platelet reactivity <240 PRU, % (95% CI, N) |
26.9% (17.1–38.2%, 21/78) |
20.0% (11.0–29.1%, 15/75) |
1.3 (0.8 – 2.4) | 0.313 |
| PRU values, Mean ± SD | 279.7 ± 106.5 | 297.8 ± 67.3 | NA | 0.212 |
Chi-square test was performed for proportions. Logistic regression was performed adjusted for the expected days to surgery (either ≤3 days or >3 days). Analysis of variance was used for PRU value. NA – Not Applicable.
Figure 2. Distribution of platelet reactivity during the overall study time course.
Platelet reactivity as assessed by PRU using the VerifyNow P2Y12 assay during the study time course (at baseline and up to 7 days of study drug infusion) (Figure 2A) and at last on-infusion sample and prior to CABG (Figure 2B). Data are presented as mean and standard deviations. Blue box: cangrelor. Red circle: placebo. PRU indicates P2Y12 Reactivity Units. N indicates number of patients with valid samples in the intention to treat population. CABG indicates coronary artery bypass grafting.
Safety Endpoints
Study-defined excessive CABG-related bleeding occurred in a total of 22 patients and was not significantly different between patients randomly assigned to cangrelor (11.8% [12/102]) or placebo (10.4% [10/96]) (RR=1.1, 95% CI: 0.5–2.5, p=0.763). There were no differences in BARC-defined CABG-related bleeding occurring during the surgical procedure until patient discharge (p=0.886, RR=0.9, 95% CI: 0.4 – 2.2) (Table 3). Pre-CABG major bleeding events were rare and not different between treatment arms; minor bleeding events were numerically more frequent with cangrelor (Table 3). Minor bleeding during study drug infusion was mostly attributed to ecchymosis at the site of venipuncture (See Appendix for Supplementary Table on ACUITY minor bleeding). Ischemic endpoints were low, 2.8% (3/106) and 4.0% (4/101) in cangrelor and placebo, respectively, prior to surgery (See Appendix for Supplementary Tables on ischemic endpoints). Adverse events occurred similarly in both groups. Incidence of dyspnea was low; 1.9% with cangrelor vs. 1.0% with placebo (3 events total). Incidence of post-baseline clinically significant laboratory tests in hematology and serum chemistry was low and similar in both groups (See Appendix for Supplementary Tables on adverse events and serious adverse events).
Table 3.
CABG-related and pre-operative bleeding events in the Safety Population
| Cangrelor (N= 106) |
Placebo (N= 101) |
Relative Risk (95% CI) |
p-value | |
|---|---|---|---|---|
|
Excessive CABG-related bleeding (primary safety endpoint) (during the CABG procedure through hospital discharge) |
||||
| Protocol-defined, No. (%) | 12/ 102(11.8) |
10/ 96(10.4) |
1.1(0.5,2.5) | 0.763 |
| surgical re-exploration | 2/ 102( 2.0) | 2/ 96( 2.1) | 0.9(0.1,6.5) | 0.951 |
| 24 hour chest tube output of >1.5 liters |
8/ 102( 7.8) | 5/ 96( 5.2) | 1.5(0.5,4.4) | 0.457 |
| incidence of PRBC transfusions > 4 units |
6/ 102( 5.9) | 8/ 96( 8.3) | 0.7(0.3,2.0) | 0.503 |
| BARC-defined, No. (%) | 10/ 102( 9.8) | 10/ 96(10.4) |
0.9 (0.4,2.2) | 0.886 |
| fatal bleeding | 0/ 102( 0.0) | 0/ 96( 0.0) | NA | NA |
| Perioperative intracranial bleeding within 48 hours |
0/ 102( 0.0) | 0/ 96( 0.0) | NA | NA |
| reoperation following closure of sternotomy for the purpose of controlling bleeding |
2/ 102( 2.0) | 2/ 96( 2.1) | 0.9(0.1,6.5) | 0.951 |
| transfusion of ≥ 5 units of whole blood or PRBC within a 48 hour period |
7/ 102( 6.9) | 8/ 96( 8.3) | 0.8(0.3,2.2) | 0.696 |
| chest tube output ≥ 2 L within a 24 hour period |
3/ 102( 2.9) | 4/ 96( 4.2) | 0.7(0.2,3.1) | 0.642 |
|
Pre-operative related bleeding (from randomization until surgical incision) |
||||
| ACUITY, No. (%) | ||||
| Major | 3/ 106( 2.8) | 1/ 101( 1.0) | 2.9(0.3,27.0) | 0.358 |
| Minor | 19/ 106(17.9) | 10/ 101( 9.9) | 1.8(0.9,3.7) | 0.101 |
| GUSTO, No. (%) | ||||
| Severe/Life threatening | 0/ 106( 0.0) | 0/ 101( 0.0) | NA | NA |
| Moderate | 2/ 106( 1.9) | 1/ 101( 1.0) | 1.9(0.2,20.7) | 0.596 |
| Mild | 19/ 106(17.9) |
10/ 101( 9.9) |
1.8(0.9,3.7) | 0.101 |
| TIMI, No. (%) | ||||
| Major | 1/ 106( 0.9) | 0/ 101( 0.0) | NA | NA |
| Minor | 1/ 106( 0.9) | 0/ 101( 0.0) | NA | NA |
CABG: coronary artery bypass graft surgery; BARC: Bleeding Academic Research Consortium; PRBC: packed red blood cell
ACUITY: Acute Catheterization and Urgent Intervention Triage Strategy; GUSTO: Global Use of Strategies To Open coronary arteries; TIMI: Thrombolysis in Myocardial Infarction
NA – Not Applicable.
COMMENT
We compared the antiplatelet efficacy of cangrelor with placebo in patients undergoing CABG who were being treated with a thienopyridine for secondary prevention of recurrent events following an ACS or coronary stent implantation. Cangrelor at an infusion dose of 0.75 µg/kg/min consistently achieved and maintained platelet inhibition at levels known to be associated with a low risk of thrombotic events compared with placebo. Bridging with a prolonged infusion of cangrelor did not increase major bleeding prior to surgery, as defined according to several established classifications, although minor bleeding was numerically higher. The rapid recovery of platelet function after discontinuing cangrelor infusion is shown by the similar levels of platelet inhibition compared with placebo prior to CABG and is consistent with the very short half-life of cangrelor (3–6 minutes). In turn, there was no excess in CABG-related bleeding with cangrelor. In addition, there was no increased incidence of non-bleeding adverse events (including dyspnea) or laboratory abnormalities despite extended dosing. These observations support the hypothesis that intravenous cangrelor is a feasible management strategy, providing prolonged platelet P2Y12 inhibition in patients who must wait for cardiac surgery after thienopyridine discontinuation.
The early benefits associated with oral P2Y12 receptor inhibition, in particular clopidogrel, have made its upstream use the standard of care in ACS patients (1–4, 21–22). These cardiovascular benefits have also been observed in patients requiring CABG not only with clopidogrel, but also with the more potent novel P2Y12 receptor blockers prasugrel and ticagrelor (23–25). Thienopyridines irreversibly block the P2Y12 receptor for the life-span of the platelet; therefore, the trade-off of this treatment strategy is the increased risk of bleeding complications in patients requiring surgery if they have been exposed to thienopyridine therapy within the prior 5–7 days. Similar safety concerns exists for ticagrelor, an oral non-thienopyridine P2Y12 inhibiting agent, which despite its reversible effects has an offset of action which is relatively slow requiring a wash-out period of 5–7 days prior to surgery (25–26). It is important to note that consistent with reports assessing on-treatment platelet reactivity with clopidogrel (27), even after discontinuation of thienopyridine therapy there was a broad variability in platelet reactivity. In fact, in the placebo group there were a considerable number of patients with a last PRU <240, indicating that not only a large number of patients are not adequately protected when stopping thienopyridine therapy for up to a week, but there are also a large number of patients exposed to an increased risk of perioperative bleeding due to ongoing platelet inhibition at the time of CABG (28). A strategy to maintain adequate platelet inhibition until the time of surgery, while avoiding the complications of both coronary thrombosis and surgical bleeding, is currently lacking.
The controversy has been propelled because premature discontinuation of thienopyridine treatment is associated with an increased risk of stent thrombosis that often leads to myocardial infarction and death (9–12). Cessation of the thienopyridine for nearly a week before surgery, with patients not hospitalized or monitored, but carrying an excess risk of major ischemic events, has been a troubling and not infrequent problem for clinicians, since it is estimated that approximately 5% of patients will require some type of surgery within the first 12 months after stent implantation or an acute coronary syndrome (14). Furthermore, in retrospective reviews it has been estimated that 1–2% and, prospectively, as many as 12.5% of patients develop recurrent complications in this waiting period (23, 29) – complications that might either further delay definitive surgical therapy, prompt emergent interventions in unfavorable situations, or force alternative treatment strategies that might result in less than ideal outcomes, while potentially increasing the complexity, length and cost of hospitalization.
Various approaches using currently available intravenous antithrombotic drugs, such as heparin and GP IIb/IIIa inhibitors, have been proposed for bridging strategies (14). However, these are associated with important drawbacks. Anticoagulants do not reduce the incidence of stent thrombosis (30) and in the case of heparin, can enhance platelet reactivity (31). Glycoprotein IIb/IIIa inhibitors, in particular the small molecules tirofiban and eptifibatide, present some of the advantage of cangrelor including potency, rapid onset of action and consistent platelet inhibitory effects (32). However, small molecule GP IIb/IIIa inhibitors have a slower offset of action requiring 4–6 hours to return to baseline platelet function, which is achieved within 1 hour with cangrelor (13, 32). In addition, these agents are used at dosing regimens recommended for ACS treatment and dose-finding studies targeting lower levels of platelet inhibition that would minimize bleeding complications, known to be increased with prolonged GP IIb/IIIa inhibiting therapy (33), particularly while bridging patients to surgery are lacking. The overall favorable safety profile with prolonged infusion (up to 7 days) of cangrelor at a dose shown to be below thresholds associated with thrombotic risk but not excessive to minimize bleeding as identified in this trial is reassuring. Ultimately, cangrelor represents a more natural bridging strategy as it selectively targets the P2Y12 receptor. Although the drug is not yet commercially available, it has been extensively studied in large scale trials of patients with coronary artery disease (34–35).
Study Limitations
This trial only enrolled patients undergoing open heart surgery, and did not assess the strategy for common non-cardiac surgical procedures, such as orthopedic or gastrointestinal operations. However, different non-cardiac surgeries have variable bleeding risk and their inclusion could have limited the ability to adequately assess the safety of cangrelor. Indeed, the selection of a homogenous surgical cohort with high bleeding risk such as CABG limited such confounding. The present study was not powered to assess if more platelet blockade with cangrelor prior to CABG would reduce the risk of ischemic events compared with placebo. Similarly, the trial was not powered to assess differences in bleeding event rates, although the similarity of the results is reassuring. Further, it may be argued that in Stage I platelet inhibition was used to assess the antiplatelet efficacy of cangrelor, while Stage II considered on-treatment platelet reactivity. However, this was due to an evolving of our understanding of platelet function testing in which studies have shown that levels of on-treatment platelet reactivity have better prognostic implications than percentage inhibition (36 Whether similar outcomes are achieved with cangrelor in patients with prior exposure to the oral non-thienopyridine ticagrelor cannot be extrapolated from our analysis.
CONCLUSION
In this trial, cangrelor consistently achieved and maintained target levels of platelet inhibition known to be associated with a low risk of thrombotic events compared with placebo, without any significant excess in bleeding complications. Our data support the hypothesis that intravenous cangrelor is a feasible management strategy in patients waiting for cardiac surgery who require prolonged platelet P2Y12 inhibition after thienopyridine discontinuation
Supplementary Material
Acknowledgement
Funded by The Medicines Company
The first author (DJA) and senior author (EJT) were responsible for the overall coordination of the trial, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All the authors participated in the design of the trial or planning of the analyses. The first author (DJA) wrote the first draft of the manuscript, and all the authors participated in subsequent revisions and approved the final version of the manuscript.
Footnotes
Maintenance of platelet inhibition with cangrelor after discontinuation of thienopyridines in patients undergoing surgery (BRIDGE)
DISCLOSURES
Dominick J. Angiolillo reports receiving: honoraria for lectures from Bristol Myers Squibb; Sanofi-Aventis; Eli Lilly Co; Daiichi Sankyo, Inc; consulting fees from Bristol Myers Squibb; Sanofi-Aventis; Eli Lilly Co; Daiichi Sankyo, Inc.; The Medicines Company; Portola; Novartis; Medicure; Accumetrics; Arena Pharmaceuticals; Astra Zeneca; Abbott Vascular; research grants from Bristol Myers Squibb; Sanofi-Aventis; GlaxoSmithKline; Otsuka; Eli Lilly Co; Daiichi Sankyo, Inc., The Medicines Company; Portola; Accumetrics; Schering-Plough; Astra-Zeneca; Eisai.
Michael S. Firstenberg reports receiving speaking fees from Sanofi-Aventis.
Matthew J. Price reports receiving: honoraria for research support from Bristol Myers Squibb/sanofi aventis, Quest Diagnostics,and Accumetrics; consulting fees from Bristol Myers Squibb/sanofi aventis, Accumetrics, AstraZeneca, Daiichi Sankyo/Eli Lilly & Co, Medicure; honoraria for lectures from Daiichi Sankyo/Eli Lilly & Co, AstraZeneca, Medtronic, Boston Scientific, and St. Jude
Pradyumna E. Tummala reports receiving: consulting fees from Medtronic; Cordis/Johnson and Johnson; research grants from Abbott Vascular; Bayer Healthcare; Bristol Myers Squibb ; CardioMems; Cordis/Johnson and Johnson; Corindus; Eli Lilly Co; Foxhollow; Genentech; GlaxoSmithKline; The Medicines Company; Novartis; Regado Biosciences; Sanofi-Aventis; Schering-Plough; Stereotaxis.
Martin Hutyra reports no disclosures.
Ian J. Welsby reports receiving grant support from IIT CSL Behring and consultant fees from CSL Behring.
Michele D. Voeltz reports no disclosures.
Harish Chandna reports speaking fees from Bristol Myers Squibb, Sanofi-Aventis and Eli Lilly.
Chandrashekhar Ramaiah reports no disclosures.
Miroslav Brtko reports no disclosures.
Louis Cannon reports no disclosures.
Cornelius Dyke reports consulting fees from The Medicines Company (Non-paying for last 2 years) Equity ownership: The Medicines Company (<$5000, speaker fees from Sanofi-Aventis, Bristol Myers Squibb
Tiepu Liu is an employee of The Medicines Company
Steven V. Manoukian reports no disclosures.
Dr. G. Montalescot reports receiving grant support from Abbott Vascular, Boston Scientific, Cordis, EliLilly, Fédération Française de Cardiologie, Fondation de France, Guerbet Medical, INSERM, ITC Edison, Medtronic, Pfizer, Sanofi-Aventis, Société Française de Cardiologie, Stago; consulting or board fees and lecture fees from AstraZeneca, Bayer, Boehringer Ingelheim, Cardiovascular Research Foundation, Cleveland Clinic Research Foundation, Daiichi-Sankyo, Duke Institute, Eli Lilly, Europa, Lead-up, GSK, Institut de Cardiologie de Montreal, Menarini, Nanospheres, Novartis, Pfizer, Portola, Sanofi-Aventis, The Medicines Company, TIMI study group.
Jayne Prats is an employee of The Medicines Company.
Eric J. Topol reports receiving grant support from The Medicines Company, Sanofi-Aventis and NIH/NCRR UL1 RR025774 (PI, CTSA Award).
REFERENCES
- 1.Anderson JL, Adams CD, Antman EM, et al. WRITING GROUP MEMBERS; ACCF/AHA TASK FORCE MEMBERS. 2011 ACCF/AHA Focused Update Incorporated Into the ACC/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011 May 10;123(18):e426–e579. doi: 10.1161/CIR.0b013e318212bb8b. [DOI] [PubMed] [Google Scholar]
- 2.Kushner FG, Hand M, Smith SC, Jr., et al. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction (updating the 2004 Guideline and 2007 Focused Update) and ACC/AHA/SCAI Guidelines on Percutaneous Coronary Intervention (updating the 2005 Guideline and 2007 Focused Update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009 Dec 1;120(22):2271–2306. doi: 10.1161/CIRCULATIONAHA.109.192663. [DOI] [PubMed] [Google Scholar]
- 3.Wijns W, Kolh P, Danchin N, et al. European Association for Percutaneous Cardiovascular Interventions/ESC Committee for Practice Guidelines. Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2010 Oct;31(20):2501–2555. doi: 10.1093/eurheartj/ehq277. [DOI] [PubMed] [Google Scholar]
- 4.Hamm CW, Bassand JP, Agewall S, et al. ESC Committee for Practice Guidelines. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2011 Aug 26; doi: 10.1093/eurheartj/ehr236. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Ho PM, Peterson ED, Wang L, et al. Incidence of death and acute myocardial infarction associated with stopping clopidogrel after acute coronary syndrome. JAMA. 2008 Feb 6;299(5):532–539. doi: 10.1001/jama.299.5.532. [DOI] [PubMed] [Google Scholar]
- 6.Boggon R, van Staa TP, Timmis A, et al. Clopidogrel discontinuation after acute coronary syndromes: frequency, predictors and associations with death and myocardial infarction--a hospital registry-primary care linked cohort (MINAP-GPRD) Eur Heart J. 2011 Aug 29; doi: 10.1093/eurheartj/ehr340. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collet JP, Montalescot G, Blanchet B, et al. Impact of prior use or recent withdrawal of oral antiplatelet agents on acute coronary syndromes. Circulation. 2004 Oct 19;110(16):2361–2367. doi: 10.1161/01.CIR.0000145171.89690.B4. [DOI] [PubMed] [Google Scholar]
- 8.Ho PM, Tsai TT, Wang TY, et al. Adverse events after stopping clopidogrel in post-acute coronary syndrome patients: Insights from a large integrated healthcare delivery system. Circ Cardiovasc Qual Outcomes. 2010 May;3(3):303–308. doi: 10.1161/CIRCOUTCOMES.109.890707. [DOI] [PubMed] [Google Scholar]
- 9.Pfisterer M, Brunner-La Rocca HP, Buser PT, et al. BASKET-LATE Investigators. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J Am Coll Cardiol. 2006 Dec 19;48(12):2584–2591. doi: 10.1016/j.jacc.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005 May 4;293(17):2126–2130. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- 11.Spertus JA, Kettelkamp R, Vance C, et al. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement: results from the PREMIER registry. Circulation. 2006 Jun 20;113(24):2803–2809. doi: 10.1161/CIRCULATIONAHA.106.618066. [DOI] [PubMed] [Google Scholar]
- 12.Rossini R, Capodanno D, Lettieri C, et al. Prevalence, predictors, and long-term prognosis of premature discontinuation of oral antiplatelet therapy after drug eluting stent implantation. Am J Cardiol. 2011 Jan 15;107(2):186–194. doi: 10.1016/j.amjcard.2010.08.067. [DOI] [PubMed] [Google Scholar]
- 13.Ferreiro JL, Ueno M, Angiolillo DJ. Cangrelor: a review on its mechanism of action and clinical development. Expert Rev Cardiovasc Ther. 2009 Oct;7(10):1195–1201. doi: 10.1586/erc.09.101. [DOI] [PubMed] [Google Scholar]
- 14.Brilakis ES, Banerjee S, Berger PB. Perioperative management of patients with coronary stents. J Am Coll Cardiol. 2007 Jun 5;49(22):2145–2150. doi: 10.1016/j.jacc.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 15.Price MJ. Bedside evaluation of thienopyridine antiplatelet therapy. Circulation. 2009 May 19;119(19):2625–2632. doi: 10.1161/CIRCULATIONAHA.107.696732. [DOI] [PubMed] [Google Scholar]
- 16.Malinin A, Pokov A, Spergling M, Defranco A, Schwartz K, Schwartz D, Mahmud E, Atar D, Serebruany V. Monitoring platelet inhibition after clopidogrel with the VerifyNow-P2Y12(R) rapid analyzer: the VERIfy Thrombosis risk ASsessment (VERITAS) study. Thromb Res. 2007;119(3):277–284. doi: 10.1016/j.thromres.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 17.Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG, White H. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011 Jun 14;123(23):2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449. [DOI] [PubMed] [Google Scholar]
- 18.Rao AK, Pratt C, Berke A, Jaffe A, Ockene I, Schreiber TL, Bell WR, Knatterud G, Robertson TL, Terrin ML. Thrombolysis in Myocardial Infarction (TIMI) Trial-phase I: hemorrhagic manifestations and changes in plasma fibrinogen and the fibrinolytic system in patients treated with recombinant tissue plasminogen activator and streptokinase. J Am Coll Cardiol. 1988 Jan;11(1):1–11. doi: 10.1016/0735-1097(88)90158-1. [DOI] [PubMed] [Google Scholar]
- 19.The GUSTO investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med. 1993 Sep 2;329(10):673–682. doi: 10.1056/NEJM199309023291001. [DOI] [PubMed] [Google Scholar]
- 20.Stone GW, McLaurin BT, Cox DA, Bertrand ME, Lincoff AM, Moses JW, White HD, Pocock SJ, Ware JH, Feit F, Colombo A, Aylward PE, Cequier AR, Darius H, Desmet W, Ebrahimi R, Hamon M, Rasmussen LH, Rupprecht HJ, Hoekstra J, Mehran R, Ohman EM. ACUITY Investigators. Bivalirudin for patients with acute coronary syndromes. N Engl J Med. 2006 Nov 23;355(21):2203–2216. doi: 10.1056/NEJMoa062437. [DOI] [PubMed] [Google Scholar]
- 21.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001 Aug 16;345(7):494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 22.Sabatine MS, Cannon CP, Gibson CM, López-Sendón JL, Montalescot G, Theroux P, Lewis BS, Murphy SA, McCabe CH, Braunwald E. Clopidogrel as Adjunctive Reperfusion Therapy (CLARITY)-Thrombolysis in Myocardial Infarction (TIMI) 28 Investigators. Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA. 2005 Sep 14;294(10):1224–1232. doi: 10.1001/jama.294.10.1224. [DOI] [PubMed] [Google Scholar]
- 23.Fox KA, Mehta SR, Peters R, Zhao F, Lakkis N, Gersh BJ, Yusuf S. Clopidogrel in Unstable angina to prevent Recurrent ischemic Events Trial. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation. 2004 Sep 7;110(10):1202–1208. doi: 10.1161/01.CIR.0000140675.85342.1B. [DOI] [PubMed] [Google Scholar]
- 24.Smith PK, Despotis GJ, Goodnough LT, Levy JH, Poston RS, Short MA, Weerakkody GJ, LeNarz LA. Mortality benefit with prasugrel in TRITON-TIMI 38 coronary artery bypass grafting (CABG) cohort: Risk adjusted retrospective data analysis. Circulation. 2010;122:A10881. doi: 10.1016/j.jacc.2012.03.030. (Abstract Supplement) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Held C, Asenblad N, Bassand JP, Becker RC, Cannon CP, Claeys MJ, Harrington RA, Horrow J, Husted S, James SK, Mahaffey KW, Nicolau JC, Scirica BM, Storey RF, Vintila M, Ycas J, Wallentin L. Ticagrelor versus clopidogrel in patients with acute coronary syndromes undergoing coronary artery bypass surgery: results from the PLATO (Platelet Inhibition and Patient Outcomes) trial. J Am Coll Cardiol. 2011 Feb 8;57(6):672–684. doi: 10.1016/j.jacc.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 26.Gurbel PA, Bliden KP, Butler K, Tantry US, Gesheff T, Wei C, Teng R, Antonino MJ, Patil SB, Karunakaran A, Kereiakes DJ, Parris C, Purdy D, Wilson V, Ledley GS, Storey RF. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation. 2009 Dec 22;120(25):2577–2585. doi: 10.1161/CIRCULATIONAHA.109.912550. [DOI] [PubMed] [Google Scholar]
- 27.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, et al. Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J Am Coll Cardiol. 2007 Apr 10;49(14):1505–1516. doi: 10.1016/j.jacc.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 28.Price MJ, Coleman JL, Steinhubl SR, Wong GB, Cannon CP, Teirstein PS. Onset and offset of platelet inhibition after high-dose clopidogrel loading and standard daily therapy measured by a point-of-care assay in healthy volunteers. Am J Cardiol. 2006 Sep 1;98(5):681–684. doi: 10.1016/j.amjcard.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 29.Akowuah E, Shrivastava V, Jamnadas B, et al. Comparison of two strategies for the management of antiplatelet therapy during urgent surgery. Ann Thorac Surg. 2005 Jul;80(1):149–152. doi: 10.1016/j.athoracsur.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Leon MB, Baim DS, Popma JJ, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med. 1998 Dec 3;339(23):1665–1671. doi: 10.1056/NEJM199812033392303. [DOI] [PubMed] [Google Scholar]
- 31.Mascelli MA, Kleiman NS, Marciniak SJ, Jr., Damaraju L, Weisman HF, Jordan RE. Therapeutic heparin concentrations augment platelet reactivity: implications for the pharmacologic assessment of the glycoprotein IIb/IIIa antagonist abciximab. Am Heart J. 2000 Apr;139(4):696–703. doi: 10.1016/s0002-8703(00)90050-4. [DOI] [PubMed] [Google Scholar]
- 32.Bhatt DL, Topol EJ. Current role of platelet glycoprotein IIb/IIIa inhibitors in acute coronary syndromes. JAMA. 2000 Sep 27;284(12):1549–1558. doi: 10.1001/jama.284.12.1549. [DOI] [PubMed] [Google Scholar]
- 33.Tricoci P, Newby LK, Hasselblad V, et al. Upstream use of small-molecule glycoprotein iib/iiia inhibitors in patients with non-ST-segment elevation acute coronary syndromes: a systematic overview of randomized clinical trials. Circ Cardiovasc Qual Outcomes. 2011 Jul 1;4(4):448–458. doi: 10.1161/CIRCOUTCOMES.110.960294. [DOI] [PubMed] [Google Scholar]
- 34.Bhatt DL, Lincoff AM, Gibson CM, et al. CHAMPION PLATFORM Investigators. Intravenous platelet blockade with cangrelor during PCI. N Engl J Med. 2009 Dec 10;361(24):2330–2341. doi: 10.1056/NEJMoa0908629. [DOI] [PubMed] [Google Scholar]
- 35.Harrington RA, Stone GW, McNulty S, et al. Platelet inhibition with cangrelor in patients undergoing PCI. N Engl J Med. 2009 Dec 10;361(24):2318–2329. doi: 10.1056/NEJMoa0908628. [DOI] [PubMed] [Google Scholar]
- 36.Bonello L, Tantry US, Marcucci R, et al. Working Group on High On-Treatment Platelet Reactivity. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol. 2010 Sep 14;56(12):919–933. doi: 10.1016/j.jacc.2010.04.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.