Abstract
Organic anion transporting polypeptides (human: OATPs; rodent: Oatps) were thought to have important functions in bile acid (BA) transport. Oatp1a1, 1a4, and 1b2 are the three major Oatp1 family members in rodent liver. Our previous studies have characterized the BA homeostasis in Oatp1a1-null and Oatp1b2-null mice. The present study investigated the physiological role of Oatp1a4 in BA homeostasis by using Oatp1a4-null mice. Oatp1a4 expression is female-predominant in livers of mice, and thereby it was expected that female Oatp1a4-null mice will have more prominent changes than males. Interestingly, the present study demonstrated that female Oatp1a4-null mice had no significant alterations in BA concentrations in serum or liver, though they had increased mRNA of hepatic BA efflux transporters (Mrp4 and Ostα/β) and ileal BA transporters (Asbt and Ostα/β). In contrast, male Oatp1a4-null mice showed significantly altered BA homeostasis, including increased concentrations of deoxycholic acid (DCA) in serum, liver and intestinal contents. After feeding a DCA-supplemented diet, male but not female Oatp1a4-null mice had higher concentrations of DCA in serum and livers than their WT controls. This suggested that Oatp1a4 is important for intestinal absorption of secondary BAs in male mice. Furthermore, loss of Oatp1a4 function did not decrease BA accumulation in serum or livers of bile-ductligated mice, suggesting that Oatp1a4 is not likely a BA uptake transporter. In summary, the present study for the first time demonstrates that Oatp1a4 does not appear to mediate the hepatic uptake of BAs, but plays an important male-predominant role in secondary BA metabolism in mice.
Keywords: Oatp1a4, bile acid, liver, secondary bile acid
1. Introduction
Bile acids (BAs) are critical in fat absorption and the homeostasis of cholesterol, triglycerides, energy, and glucose. Primary BAs are synthesized from cholesterol in hepatocytes and conjugated with taurine or glycine before excretion into bile and then intestine. Intestinal bacteria metabolize primary BAs to form secondary BAs, which are more hydrophobic and toxic than primary BAs [1]. Approximately 95% of BAs are reabsorbed from the intestine and return back to the liver, which is known as the enterohepatic circulation of BAs. To maintain this process, hepatocytes must transport BAs efficiently from the portal blood into bile. Hepatic uptake of conjugated BAs is thought to be mediated by the sodium-taurocholate cotransporting polypeptide (human: NTCP; rodents: Ntcp) [2]. Organic anion transporting polypeptides (human: OATPs; rodents: Oatps) have been shown to transport unconjugated BAs from blood into liver [3].
Several OATP/Oatps (human: OATP1B1 and 1B3; rodent: Oatp1a1, 1a4, and 1b2) are exclusively or majorly expressed in liver and have been shown to transport BAs in vitro [2, 4]. Furthermore, studies of BAs in humans with OATP polymorphisms and Oatp-null mice further elucidate the important physiological role of these liver OATP/Oatps in transporting BAs. For example, Xiang et al (2009) reported that OATP1B1 polymorphism affects the disposition of several endogenous BAs and markers of BA synthesis[5]. Additionally, the concentration of unconjugated BAs increases about 13-fold in Oatp1a/1b-null mice [6]. The increase of unconjugated BAs in Oatp1a/1b-null mice is likely due to Oatp1b2, which has been shown to transport unconjugated BAs using Oatp1b2-null mice [3]. Oatp1a1 plays an important role in secondary BA metabolism and intestinal bacteria homeostasis as elucidated in Oatp1a1-null mice [7-9]. However, the in vivo role of Oatp1a4 in BA uptake has not been characterized.
A distinct role of Oatp1a4 in BA transport and metabolism has been suggested. Oatp1a4 is predominantly expressed in periportal hepatocytes, whereas Oatp1a1 and 1b2 have a homogeneous lobular distribution [10, 11]. Oatp1a4 is expressed higher in female than male mice, whereas Oatp1a1 is male-predominant and Oatp1b2 shows no gender difference [12]. Mouse Oatp1a4 expression in liver can be induced by xenobiotics via activation of the pregnane X receptor (PXR), whereas Oatp1a1 and 1b2 are not readily inducible [13]. When expressed in Xenopus laevis oocytes, mouse Oatp1a1 can transport taurocholate (TCA) with a Km ~12μM, whereas mouse Oatp1a4 minimally transports TCA [14]. Bile duct ligation (BDL) in mice decreased mRNA of BA uptake transporters Ntcp, Oatp1a1, and Oatp1b2 in liver. In contrast, BDL increased Oatp1a4 mRNA expression in livers of mice [15].
Oatp1a1, 1a4, and 1b2 are majorly expressed in liver of rodents. Our previous studies have characterized the BA homeostasis in Oatp1a1-null and Oatp1b2-null mice. The purpose of the present study was to characterize the physiological role of Oatp1a4 in BA homeostasis by using Oatp1a4-null mice.
2. Materials and Methods
2.1. Chemicals and Reagents
Cholic acid (CA), chenodeoxycholic acid (CDCA), α-muricholic acid (αMCA), βMCA, deoxycholic acid (DCA), lithocholic acid (LCA), and murideoxycholic acid (MDCA), 7-oxo-deoxycholic acid (7-oxoDCA), 12-oxo-chenodeoxycholic acid (12-oxoCDCA) were purchased from Steraloids, Inc. (Newport, Rhode Island). Glycochenodeoxycholic-2,2,4,4-d4 acid (2H4-GCDCA) and chenodeoxycholic-2,2,4,4-d4 acid (2H4-CDCA) were used as internal standards and were purchased from C/D/N Isotopes, Inc. (Pointe-Claire, Quebec, Canada). Tauro-12-epi-deoxycholic acid (T-12epi-DCA) was a generous gift from Dr Alan F Hofmann (University of California, San Diego, CA). The structure and abbreviations of various BAs can be found in our previous manuscript [7]. All other chemicals, unless indicated, were purchased from Sigma-Aldrich (St. Louis, MO).
2.2. Animal Breeding
Eight-week-old adult male and female C57BL/6 mice were purchased from Charles River Laboratories Inc. (Wilmington, MA). Oatp1a4-null mice were bred to homozygosity on the C57BL/6 background as described previously [16]. All mice were housed in an American Animal Associations Laboratory Animal Care (AALAC) accredited facility with a 12:12 hr light:dark cycle and provided chow (Teklad Rodent Diet #8604, Harlan Teklad, Madison, WI) and water ad libitum. The experimental protocol was approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee (IACUC), and humane care of the animals was in accordance with the criteria outlined in the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
2.3. Sample Preparation
Mice (n=5/group) were anesthetized, blood was collected by orbital sinus bleeding, and serum was obtained by centrifuging blood at 6,000 g for 15 min. Gallbladders and contents were collected and stored at −80°C until analysis. Livers were harvested from the same animals, washed with saline, frozen in liquid nitrogen, and stored at −80°C. Small intestine, cecum, and colon were cut open and vortexed in 3 ml of saline to collect contents, respectively. The three small intestine segments, namely duodenum, jejunum, and ileum, as well as cecum and colon, were stored separately at −80°C.
2.4. Deoxycholic Acid Diet Feeding
Pelleted mouse feed (Teklad Rodent Diet #8604, Harlan Teklad, Madison, WI) was ground into a fine powder. The DCA-supplemented diet was prepared by mixing DCA with control ground diet. Individually housed C57BL/6 and Oatp1a4-null mice (n=5/gender/group) were fed a diet supplemented with 0.3% DCA (w/w). The DCA-supplemented diets (40 g) were added to a bowl in each mouse cage daily, and the remaining feed from the previous day was discarded. Cages were replaced daily to minimize contamination of feed with urine and feces. After 7 days, mice were anesthetized, and blood was obtained by orbital sinus bleeding and centrifuged at 6,000 g for 15 min to collect serum. Livers were harvested from the same animals, washed, frozen in liquid nitrogen, and stored at −80°C.
2.5. Bile Duct Ligation
In preliminary studies, Oatp1a4-null BDL mice showed toxic signs after 2-3 days of surgery, whereas WT BDL mice showed no toxic signs. Therefore, to evaluate the early effects of BDL on liver, 24-hr BDL surgeries were performed in both WT and Oatp1a4-null mice. Age-matched male WT and Oatp1a4-null mice (n=5/group) were individually housed in cages. All surgeries were made with aseptic techniques. For induction of anesthesia, mice were placed in a closed-circuit chamber with an inflow of 3% isoflurane and an oxygen flow rate of 1 L/min. After induction of anesthesia, the head of each mouse was positioned into a face mask connected to an anesthesia machine with an inflow of 1% isoflurane and an oxygen flow rate of 1 L/min. Depth of anesthesia was monitored by pinching the footpad of the mice before and throughout surgery. Heart rate and respiratory rate were monitored visually throughout surgery. Body temperatures were maintained at 37°C by means of heating pads. The abdomen of each mouse was shaved and swabbed with betadine and 70% alcohol. Surgical silk thread (7-0) was placed around the common bile duct using micro-dissection forceps. The gallbladder was removed, and the common bile duct was ligated doubly. Sham surgeries were performed similarly without BDL. The abdominal cavity was closed with an interlocking running stitch with 5-0 silk. The skin was then closed with 5-0 nylon suture. After closing the abdomen, the incision was wiped with betadine. All surgeries were performed between 8:30 AM and 1:30 PM. Twenty-four hr after surgery, mice were anesthetized with 4% isoflurane, blood was collected by orbital sinus bleeding, and serum was obtained by centrifuging blood at 6,000 g for 15 min. Livers were washed with saline, frozen in liquid nitrogen, and stored at −80°C.
2.6. BA Analysis
BA extraction and quantification were according to previous methods [7-9].
2.7. Total RNA Isolation
Total RNA was isolated using RNA-Bee reagent (Tel-Test Inc., Friendswood, TX) according to the manufacturer’s protocol. Total RNA concentrations were quantified spectrophotometrically at 260 nm. Integrity of RNA samples was determined by formaldehyde-agarose gel electrophoresis with visualization by ethidium bromide fluorescence under ultraviolet light.
2.8. Multiplex Suspension Array
The mRNA expression was quantified by mutiplex suspension array (Panomics-Affymetrix, Inc., Fremont, CA). Individual gene accession numbers can be accessed at www.panomics.com (panels #21021 and #21151). Assays were performed according to each manufactures’ protocol [7, 9]. RNA data were normalized to Gapdh mRNA. However, Rpl13a was used as the housekeeping gene for BDL samples, as Rpl13a was shown previously to be more stable than Gapdh in livers of BDL mice [9].
2.9. Statistical Analysis
Bars represent Mean ± S.E. (n=5). Differences between mean values were tested for statistical significance (p < 0.05) by a two-tailed Student’s t-test.
3. Results
3.1. Loss of Oatp1a4 function had more effect in female than male mice on mRNA expression of hepatic and ileal transporters
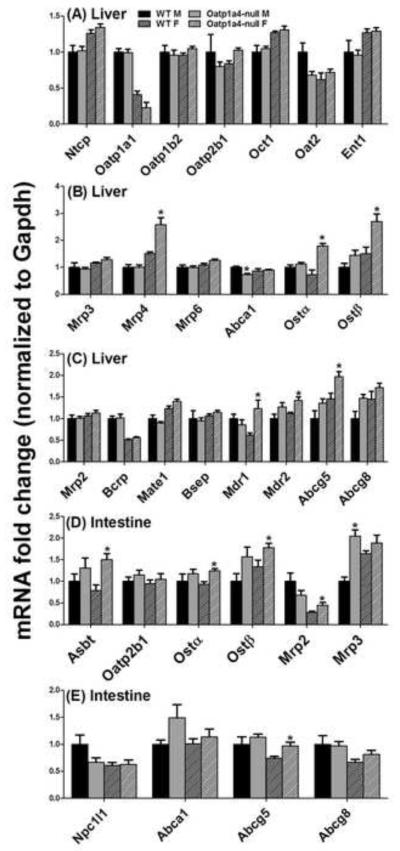
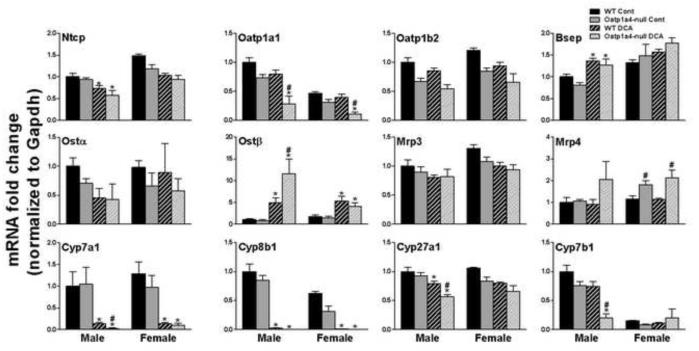
To investigate whether Oatp1a4 deficiency affects the enterohepatic circulation of BAs, the mRNA of major hepatic and ileal transporters were quantified and summarized in Figure 1. Generally, male Oatp1a4-null mice showed no significant alterations in hepatic or ileal transporters, except that they had lower hepatic Abca1 (27%↓) and higher ileal Mrp3 (105%↑) than male WT mice. Compared to female WT mice, female Oatp1a4-null mice had increased mRNA expression of hepatic transporters [Mrp4 (106%↑), Ostα (108%↑), Ostβ (119%↑), Mdr1 (61%↑), Mdr2 (29%↑), and Abcg5 (51%↑)] as well as ileal transporters [Asbt (71%↑), Ostα (30%↑), Ostβ (44%↑), Mrp2 (16%↑), and Abcg5 (23%↑)]. Therefore, loss of Oatp1a4 function showed a female-predominant effect on the mRNA expression of other transporters of the BA enterohepatic circulation.
Figure 1. mRNA expression of BA transporters in livers and ilea of WT and Oatp1a4-null mice.
Total RNA was isolated from livers and ilea of WT and Oatp1a4-null mice. The mRNA expression of hepatic basolateral uptake transporters (A), hepatic basolateral efflux transporters (B), hepatic canaliculus efflux transporters (C), intestinal BA transporters (D) and intestinal cholesterol transporters (E) were analyzed by multiplex suspension array. The mRNAs were normalized to Gapdh. All data are expressed as mean ± S.E. for five mice in each group. Asterisks (*) indicate statistically significant difference between WT and Oatp1a4-null mice within the same gender (p<0.05).
3.2. Serum and liver bile acid concentrations were altered in male but not female Oatp1a4-null mice
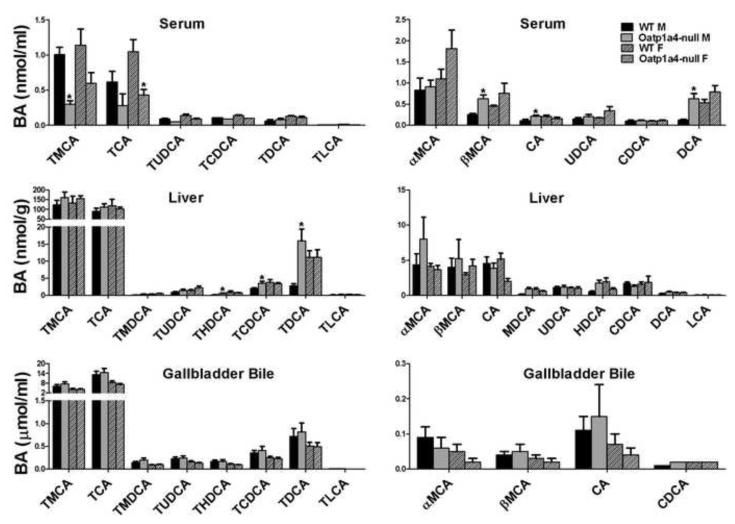
Individual BA concentrations were quantified in serum, livers and gallbladder bile of Oatp1a4-null mice to investigate the effect of Oatp1a4 deficiency on BA homeostasis (Figure 2). Compared to male WT mice, male Oatp1a4-null mice had decreased TMCA (70%↓) and TCA (45%↓) but increased βMCA (150%↑), CA (92%↑), and DCA (430%↑) in serum. In addition, loss of Oatp1a4 function increased THDCA (390%↑), TCDCA (80%↑), and TDCA (480%↑) in livers of male mice. In contrast, Oatp1a4 deficiency had little effect on BA concentrations in serum and livers of female mice, as well as the BA composition in gallbladder bile of both male and female mice. Taken together, loss of Oatp1a4 function showed a male-predominant effect on serum and liver BA concentrations.
Figure 2. Concentrations of conjugated and unconjugated BAs in serum, liver and gallbladder bile of WT and Oatp1a4-null mice.
BA concentrations in serum, liver and gallbladder bile of WT and Oatp1a4-null mice (n=5/group) were analyzed using ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). All data are expressed as mean ± S.E. for five mice in each group. Asterisks (*) indicate statistically significant difference between WT and Oatp1a4-null groups within the same gender (p<0.05).
3.3. Intestinal BA concentrations were altered in male but not female Oatp1a4-null mice
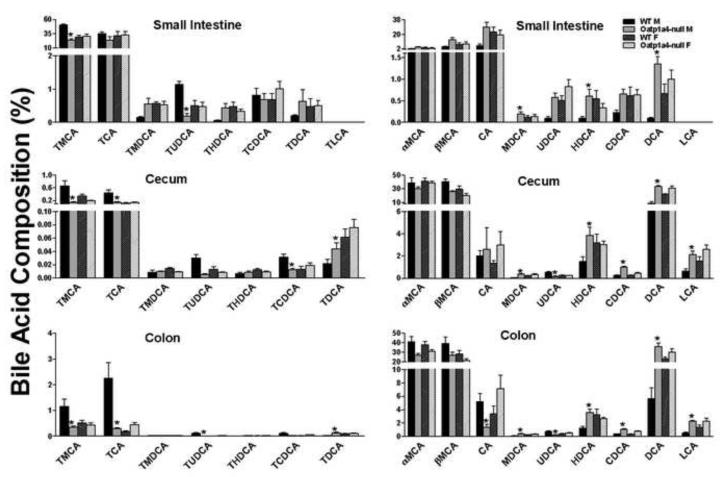
The increase in serum DCA and liver TDCA in male Oatp1a4-null mice suggests an alteration in the intestinal environment. To further characterize the role of Oatp1a4 in BA metabolism, intestinal contents were collected and individual BA concentrations were quantified. Figure 3 summarizes the BA composition of the contents of the small intestine, cecum, and colon of both male and female mice. Marked changes in intestinal BAs of male Oatp1a4-null mice included a decrease in TMCA (52%↓) in small intestinal contents, a decrease of TMCA (68-78%↓) and TCA (67-86%↓) in cecum and colon contents, as well as an increase of MDCA (400-1900% ↑) and HDCA (160-500%↑) in contents of small intestine, cecum and colon. Consistent with serum and liver BAs, TDCA and DCA were increased in intestinal contents of male Oatp1a4-null mice. As shown in Figure 3, TDCA in male Oatp1a4-null mice tended to increase in small intestinal contents, and significantly increased in cecum (110%↑) and colon contents (550%↑). DCA in male Oatp1a4-null mice increased significantly in the contents of all three intestinal segments (1400, 270, and 540%↑, respectively). Taken together, loss of Oatp1a4 function increased the formation of secondary BAs in the intestine of male but not in female mice.
Figure 3. BA composition in the intestinal contents of WT and Oatp1a4-null mice.
The concentrations of conjugated (left panel) and unconjugated BAs (right panel) in the contents of small intestine, cecum and colon were analyzed using ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). All data are expressed as mean ± S.E. for five mice in each group. Asterisks (*) indicate statistically significant difference between WT and Oatp1a4-null groups within the same gender (p<0.05).
3.4. Male Oatp1a4-null mice had higher concentrations of DCA and TDCA in serum and livers than male WT mice after feeding a 0.3% DCA Diet
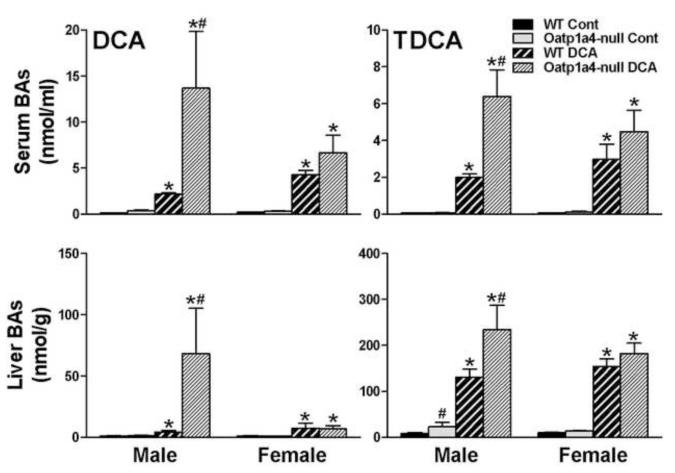
To further investigate the role of Oatp1a4 in secondary BA metabolism, WT and Oatp1a4-null mice were fed a diet supplemented with 0.3 % (w/w %) DCA for 7 days. As shown in Figure 4, feeding DCA increased the concentrations of DCA and TDCA in serum and livers of both WT and Oatp1a4-null mice. After feeding DCA, male Oatp1a4-null mice had higher DCA and TDCA in serum (530%↑ and 220%↑) and livers (1,500%↑ and 79%↑), respectively, than male WT mice. In contrast, although feeding DCA tended to increase the concentrations of TDCA and DCA in serum of female Oatp1a4-null mice, such increase was not significant (Figure 4).
Figure 4. Concentrations of TDCA and DCA in serum and livers of WT and Oatp1a4-null mice fed a 0.3% DCA diet.
BA concentrations in serum and livers of WT and Oatp1a4-null mice (n=5/group) were analyzed by UPLC-MS/MS. All data are expressed as mean ± S.E. for five mice in each group. Asterisks (*) indicate statistically significant difference between control and DCA-treated groups (p<0.05). Pound signs (#) indicate statistically significant difference between the DCA –treated WT and Oatp1a4-null groups (p<0.05).
3.5. Male Oatp1a4-null mice had lower hepatic mRNA expression of the BA uptake transporter (Oatp1a1), higher hepatic mRNA expression of the BA efflux transporter (Ostβ), and lower hepatic mRNA expression of the BA synthetic enzymes (Cyp7a1, 27a1, and 7b1) than male WT mice after feeding a 0.3% DCA Diet
The effects of feeding DCA on BA transporters and synthetic enzymes were investigated in livers of WT and Oatp1a4-null mice (Figure 5). Feeding DCA decreased the major BA uptake transporter Ntcp in male WT (26%↓) and male Oatp1a4-null (39%↓) mice, but not in females. Feeding DCA increased the major BA efflux transporter Bsep in male WT (36%↑) and male Oatp1a4-null (56%↑) mice, but not in females. Feeding DCA decreased Oatp1a1 in Oatp1a4-null (M: 44%↓; F: 65%↓) but not in WT mice. Feeding DCA increased Ostβ in livers of both WT and Oatp1a4-null mice, but the increase of Ostβ was higher in male Oatp1a4-null mice than male WT ones. BA synthetic enzymes Cyp7a1 and 8b1 decreased more than 90% in both WT and Oatp1a4-null mice. Cyp27a1 was slightly decreased (20-30%↓) in male but not in female WT and Oatp1a4-null mice. In contrast, Cyp7b1 was decreased only in male Oatp1a4-null mice (about 56%↓). Taken together, feeding a 0.3% DCA diet had more effect on the mRNA of hepatic BA transporters and synthetic enzymes in male Oatp1a4-null mice than female Oatp1a4-null mice.
Figure 5. mRNA expression of BA transporters and synthetic enzymes in livers of WT and Oatp1a4-null mice fed a 0.3% DCA diet.
Total RNA from livers of WT and Oatp1a4-null mice (n=5/gender/group) was analyzed by mutiplex suspension array. The mRNA expression were normalized to Gapdh and expressed as fold change to male WT control mice. All data are expressed as mean ± S.E. for five mice in each group. Asterisks (*) indicate statistically significant difference between control and DCA-treated groups (p<0.05). Pound signs (#) indicate statistically significant difference between the DCA –treated WT and Oatp1a4-null groups (p<0.05).
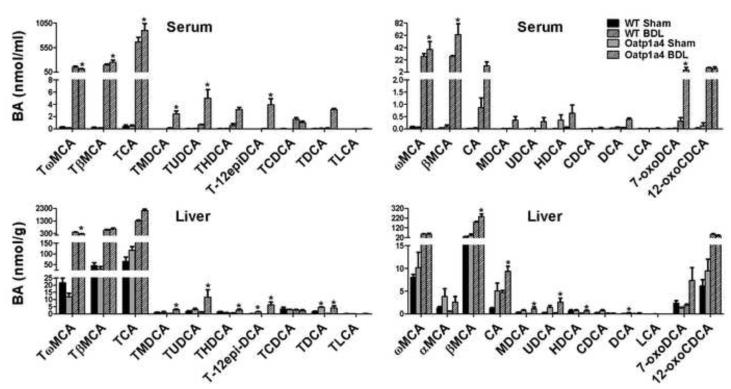
3.6. Male Oatp1a4-null mice had higher BA concentrations in serum and livers than male WT mice after bile duct ligation
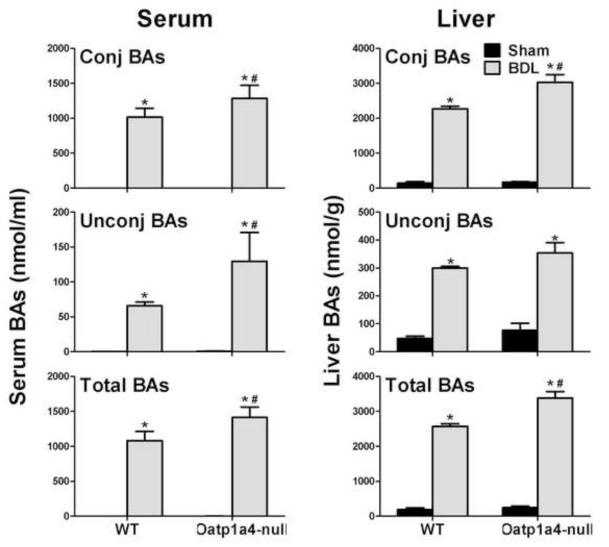
To further investigate the role of Oatp1a4 in BA homeostasis, the common bile ducts of WT and Oatp1a4-null mice were ligated. Male mice were used because the effect of Oatp1a4 deficiency on BA metabolism is more prominent in male than female mice. Figure 6 summarizes the concentrations of individual BAs in serum and livers of WT and Oatp1a4-null mice 24 hr after surgery, respectively. Oatp1a4-null BDL mice had higher TβMCA (30%↑), TCA (35%↑), TMDCA (18-fold↑), TUDCA (670%↑), T-12epiDCA (12400%↑), ωMCA (40%↑), βMCA (1300%↑), and 7-oxoDCA (1600%↑) in serum than WT BDL mice. In addition, Oatp1a4-null BDL mice had higher TMDCA (1200%↑), TUDCA (850%↑), THDCA (420%↑), T-12-epiDCA (78800%↑), TDCA (4000%↑), βMCA (31%↑), CA (99%↑), MDCA (2160% ↑), UDCA (1100%↑), and HDCA (510%↑) in livers than did WT BDL mice. In contrast, Oatp1a4-null BDL mice had lower TωMCA in both serum (29%↓) and livers (36%↓) compared to WT BDL mice. As shown in Figure 7, Oatp1a4-null BDL mice had higher total BA concentrations (30%↑) in serum than WT BDL mice, due to increase in both conjugated (26%↑) and unconjugated (97%↑) BAs. Additionally, Oatp1a4-null BDL mice had higher total BA concentrations (30%↑) in livers than WT BDL mice, mainly due to the increase in conjugated BAs.
Figure 6. Individual BA concentrations in serum and livers of male WT and Oatp1a4-null mice 24 hrs after BDL.
The concentrations of conjugated (left panel) and unconjugated BAs (right panel) in serum and liver were analyzed using ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). All data are expressed as mean ± S.E. for five mice in each group. Asterisks (*) indicate statistically significant difference between BDL WT and BDL Oatp1a4-null mice (p<0.05).
Figure 7. Unconjugated, conjugated, and total BA concentrations in serum and livers of WT and Oatp1a4-null mice 24 hrs after BDL.
All data are expressed as mean ± S.E. for five mice in each group. Asterisks (*) indicate statistically significant difference between sham and BDL groups (p<0.05). Pound signs (#) indicate statistically significant difference between BDL WT and BDL Oatp1a4-null groups (p<0.05).
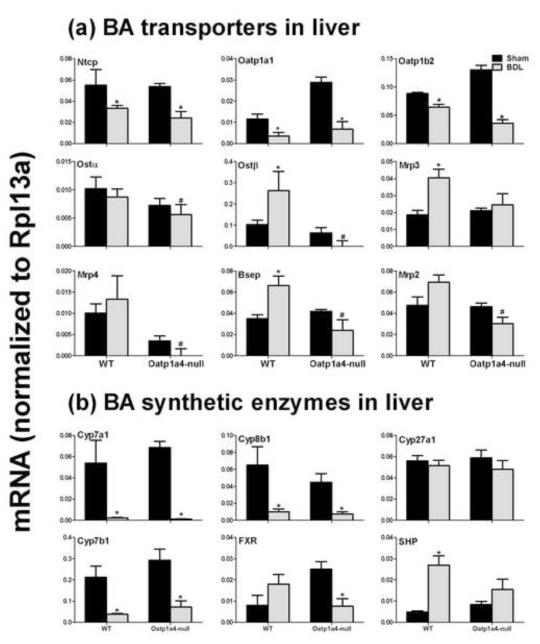
3.7. Male Oatp1a4-null mice had lower mRNA expression of hepatic BA efflux transporters than male WT mice after bile duct ligation
The mRNA expression of BA transporters and synthetic enzymes were quantified in livers of WT and Oatp1a4-null mice after BDL surgery to investigate the effect of Oatp1a4 in liver on the adaptive response during cholestasis (Figure 8). BDL decreased the mRNA of BA uptake transporters (Ntcp, Oatp1a1, and Oatp1b2) in livers of both WT and Oatp1a4-null mice (Figure 8a). In contrast, BDL increased mRNA of BA sinusoidal and canalicular efflux transporters (Mrp3 and Bsep) in livers of WT but not Oatp1a4-null mice. As a result, Oatp1a4-null BDL mice had lower Ostα (20%↓), Ostβ (140%↓), Mrp4 (180%↓), Bsep (64%↓), and Mrp2 (56%↓) than WT BDL mice. In addition, BDL decreased the mRNA of BA synthetic enzymes (Cyp7a1, 8b1, and 7b1) in livers of both WT and Oatp1a4-null mice (Figure 8b). BDL decreased FXR mRNA in Oatp1a4-null but not WT mice, whereas BDL increased SHP, a target of FXR, in WT but not Oatp1a4-null mice. Therefore, Oatp1a4-null BDL mice had similar mRNA expression of BA synthetic enzymes as WT BDL mice.
Figure 8. mRNA expression of BA transporters and BA-synthetic enzymes in livers of male WT and Oatp1a4-null mice 24 hrs after BDL.
Total RNA from livers of sham-operated and BDL mice were analyzed by multiplex suspension array. The mRNAs were normalized to Rpl13a. All data are expressed as mean ± S.E. for five mice in each group. Asterisks (*) indicate statistically significant difference between sham and BDL groups (p<0.05). Pound signs (#) indicate statistically significant difference between BDL WT and BDL Oatp1a4-null groups (p<0.05).
4. Discussion
Oatp1a4 expression is female-predominant in livers of mice, and thus it was expected that the Oatp1a4-null phenotype would have more prominent changes in females than males. Interestingly, loss of Oatp1a4 function shows a female-predominant effect on hepatic and ileal transporters, but a male-predominant effect on BA homeostasis in mice. Oatp1a4 is suggested to be a bidirectional transporter, e.g. Oatp1a4-mediated transport of taurocholate is bidirectional and stimulated by glutathione and its conjugates [17]. The marked alteration of transporters in female Oatp1a4-null mice is almost exclusively an increase in hepatic efflux transporters, suggesting a possible compensation of hepatic efflux function in livers as a result from the lack of Oatp1a4 function (Figure 1). Despite an increase in mRNA of intestinal BA transporters (Asbt and Ostα/β), female Oatp1a4-null mice had almost no significant alterations in BA concentrations in serum or livers (Figure 2). Therefore, the results suggest that Oatp1a4 is more likely to play a role in BA homeostasis in male mice, although the role of Oatp1a4 in BA homeostasis in female mice cannot be completely ignored due to the compensatory changes in the expression of other BA transporters in female Oatp1a4-null mice.
The present study indicates that Oatp1a4 is important to maintain secondary BA metabolism in male but not female mice. DCA is the major secondary BA converted from CA by bacteria in intestine. The increase of both serum DCA and liver TDCA indicates increased activity of intestinal BA metabolism in male Oatp1a4-null mice, which is further evidenced by a marked increase of other secondary BAs in the intestinal contents of male Oatp1a4-null mice (Figure 3). Consistently, DCA and TDCA were also increased in intestinal contents of male but not female Oatp1a4-null mice. In addition to the increased formation of secondary BAs, there is also increased intestinal absorption of secondary BAs in male Oatp1a4-null mice. After feeding a DCA-supplemented diet, Oatp1a4-null male mice had higher DCA and TDCA concentrations in both serum and livers than did WT male mice (Figure 4). Consistent with the higher BA concentration, DCA-treated male Oatp1a4-null mice express more Ostβ to efflux BAs from liver to blood, and less Cyp7a1, Cyp27a1, and Cyp7b1 to decrease BA biosynthesis in liver (Figure 5). In contrast, no marked differences were observed in DCA concentrations in serum and livers, as well as mRNA of BA synthetic enzymes between DCA-treated female WT and DCA-treated female Oatp1a4-null mice. Taken together, loss of Oatp1a4 function increases both formation and intestinal absorption of secondary BAs in male but not female mice.
Loss of Oatp1a4 function does not decrease BA accumulation in serum or livers of male mice during BDL-induced cholestasis. Oatp1a4 is induced markedly during cholestatic liver injury [15]. In the current study, the total BA concentrations in male Oatp1a4-null BDL mice were higher in both serum and livers compared to male WT BDL mice (Figure 7). In contrast to the higher BA concentrations in liver, male Oatp1a4-null BDL mice had lower mRNA expression of hepatic BA efflux transporters (Ostβ, Bsep, Mrp4, and Mrp2) and similar mRNA expression of hepatic BA synthetic enzymes when compared to male WT BDL mice. In addition, mRNA of SHP, a FXR target, increased in male WT BDL mice, but not in male Oatp1a4-null BDL mice. This suggests that loss of Oatp1a4 function might impair the adaptive protective mechanisms in livers of mice during cholestatic injury. The increased secondary BAs, such as TMDCA, TUDCA, and THDCA in serum and livers of male Oatp1a4-null BDL mice further suggest an important role of Oatp1a4 in secondary BA metabolism.
Despite the different lobular distribution and gender-specific expression, Oatp1a4 appears to share similar functions as Oatp1a1 in BA homeostasis. An increase in DCA and/or TDCA concentrations in serum and livers is also observed in male but not female Oatp1a1-null mice [8]. Furthermore, male Oatp1a1 null mice are sensitive to BDL-induced cholestasis [9]. Although DCA feeding and BDL caused less toxicity in Oatp1a4-null than Oatp1a1-null mice (data not shown), these manipulations caused similar BA profile changes in serum and livers in both transporter null models. In addition, the available data suggest that both Oatp1a1 and 1a4 are likely more important for BA metabolism and absorption in the intestine than BA transport in liver. Altered metabolism of secondary BAs in Oatp1a1-null mice is likely the result of alterations in resident intestinal bacteria [7]. Several bacterial species in Clostridium family, such as C. scindens VPI 12708, C. sp. Strain TO-931, and C. hylemonae TN271, have been suggested to possess BA 7a-dehydroxylation activity, which converts primary CA to secondary DCA [18-21]. Several Clostridum species were increased in the small intestinal contents of Oatp1a1-null mice [7]. It is also possible that changes in the intestinal bacteria, in particular, Clostridium species, explain the altered secondary BA metabolism in male Oatp1a4-null mice, because metabolomic profiling suggests alterations of intestinal bacteria in both Oatp1a1- and Oatp1a4-null mice [7, 16].
The gender-different expression of Oatp1a4 in mouse livers is caused by male-matter growth hormone secretion pattern and androgens [22]. Transcription factors such as signal transducer and activator of transcription (STAT)5b, and hepatic nuclear factor (HNF)4a are shown to play essential roles for the sex-dependent effects of growth hormone on the liver gene expression [23]. Gender difference is also observed in the composition of fecal flora in laboratory mice [24, 25]. Consistently, WT female mice have higher concentrations of secondary BAs, such as DCA, in their intestinal contents than WT male mice (Figure 3). The male-specific role of Oatp1a4 in secondary BA metabolism may be due to the gender difference in mouse intestinal bacteria. It is possible that lack of Oatp1a1 or 1a4 alters the disposition of some endogenous substrates other than BAs which are specific to male mice and play an important role in maintaining normal intestinal bacteria homeostasis. Further studies are required to investigate the gender-specific alterations of intestinal bacteria in Oatp1a4-null mice.
In summary, the present study systematically investigates the physiological role of mouse Oatp1a4 in BA metabolism and adds a new perspective on the in vivo functions of OATPs/Oatps. Although considerable emphasis is placed on evaluating hepatic function in many transporter null models, the present work shows that Oatps play an important role in intestinal function, with evidence for altered secondary BAs as well as altered intestinal bacteria. With the BA metabolism data generated from both Oatp1a/1b-null mice and individual Oatp-null mice (lack of Oatp1a1, 1a4 or 1b2), the functions of individual hepatic Oatps in BA homeostasis has been elucidated. Generally, Oatp1b2 mediates the transport of unconjugated BAs into the liver, whereas Oatp1a1 and 1a4 share similar roles in influencing the intestinal secondary BA metabolism.
ACKNOWLEDGMENTS
The authors would like to thank the members in Dr. Curtis Klaassen’s laboratory for their help in tissue collection and manuscript reviewing. This work was supported by NIH grants ES009649 and ES-019487.
Abbreviations
- Abca1
ATP-binding cassette transporter a1
- BA
bile acid
- CA
cholic acid
- Bsep
bile salt-export pump
- CDCA
chenodeoxycholic acid
- Cyp
cytochrome P450
- DCA
deoxycholic acid
- Fxr
farnesoid X receptor
- Gapdh
glyceraldehyde 3-phosphate dehydrogenase
- HDCA
hyodeoxycholic acid
- IS
internal standard
- LCA
lithocholic acid
- MCA
muricholic acid
- MDCA
murideoxycholic acid
- Mrp
multidrug resistance-associated protein
- Ntcp
sodium taurocholate cotransporting polypeptide
- Oatp/OATP
organic anion transporting polypeptide
- Ost
organic solute transporter
- 7-oxoDCA
7-oxo-deoxycholic acid
- Rpl13a
ribosomal protein L13a
- Shp
small heterodimer partner
- TCA
tauro-cholic acid
- T-12-epiDCA
tauro-12-epi deoxycholic acid
- UDCA
ursodeoxycholic acid
- UPLC
ultra performance liquid chromatography
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hofmann AF, Hagey LR. Bile acids: chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell Mol Life Sci. 2008;65(16):2461–83. doi: 10.1007/s00018-008-7568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hagenbuch B, Meier PJ. The superfamily of organic anion transporting polypeptides. Biochim Biophys Acta. 2003;1609(1):1–18. doi: 10.1016/s0005-2736(02)00633-8. [DOI] [PubMed] [Google Scholar]
- 3.Csanaky IL, et al. Organic anion-transporting polypeptide 1b2 (Oatp1b2) is important for the hepatic uptake of unconjugated bile acids: Studies in Oatp1b2-null mice. Hepatology. 2011;53(1):272–81. doi: 10.1002/hep.23984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meier PJ, Stieger B. Bile salt transporters. Annu Rev Physiol. 2002;64:635–61. doi: 10.1146/annurev.physiol.64.082201.100300. [DOI] [PubMed] [Google Scholar]
- 5.Xiang X, et al. Effect of SLCO1B1 polymorphism on the plasma concentrations of bile acids and bile acid synthesis marker in humans. Pharmacogenet Genomics. 2009;19(6):447–57. doi: 10.1097/FPC.0b013e32832bcf7b. [DOI] [PubMed] [Google Scholar]
- 6.van de Steeg E, et al. Organic anion transporting polypeptide 1a/1b-knockout mice provide insights into hepatic handling of bilirubin, bile acids, and drugs. J Clin Invest. 2010;120(8):2942–52. doi: 10.1172/JCI42168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, et al. Dysfunction of organic anion transporting polypeptide 1a1 alters intestinal bacteria and bile acid metabolism in mice. PLoS One. 2012b;7(4):e34522. doi: 10.1371/journal.pone.0034522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, et al. Loss of organic anion transporting polypeptide 1a1 increases deoxycholic acid absorption in mice by increasing intestinal permeability. Toxicol Sci. 2011;124(2):251–60. doi: 10.1093/toxsci/kfr236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, et al. Organic anion transporting polypeptide 1a1 null mice are sensitive to cholestatic liver injury. Toxicol Sci. 2012a;127(2):451–62. doi: 10.1093/toxsci/kfs123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reichel C, et al. Localization and function of the organic anion-transporting polypeptide Oatp2 in rat liver. Gastroenterology. 1999;117(3):688–95. doi: 10.1016/s0016-5085(99)70463-4. [DOI] [PubMed] [Google Scholar]
- 11.Kakyo M, et al. Immunohistochemical distribution and functional characterization of an organic anion transporting polypeptide 2 (oatp2) FEBS Lett. 1999;445(2-3):343–6. doi: 10.1016/s0014-5793(99)00152-0. [DOI] [PubMed] [Google Scholar]
- 12.Cheng X, et al. Tissue distribution and ontogeny of mouse organic anion transporting polypeptides (Oatps) Drug Metab Dispos. 2005a;33(7):1062–73. doi: 10.1124/dmd.105.003640. [DOI] [PubMed] [Google Scholar]
- 13.Cheng X, et al. Regulation of mouse organic anion-transporting polypeptides (Oatps) in liver by prototypical microsomal enzyme inducers that activate distinct transcription factor pathways. Drug Metab Dispos. 2005b;33(9):1276–82. doi: 10.1124/dmd.105.003988. [DOI] [PubMed] [Google Scholar]
- 14.Hagenbuch B, Adler ID, Schmid TE. Molecular cloning and functional characterization of the mouse organic-anion-transporting polypeptide 1 (Oatp1) and mapping of the gene to chromosome X. Biochem J. 2000;345(Pt 1):115–20. [PMC free article] [PubMed] [Google Scholar]
- 15.Slitt AL, et al. Regulation of transporter expression in mouse liver, kidney, and intestine during extrahepatic cholestasis. Biochim Biophys Acta. 2007;1768(3):637–47. doi: 10.1016/j.bbamem.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Gong L, et al. Characterization of organic anion-transporting polypeptide (Oatp) 1a1 and 1a4 null mice reveals altered transport function and urinary metabolomic profiles. Toxicol Sci. 2011;122(2):587–97. doi: 10.1093/toxsci/kfr114. [DOI] [PubMed] [Google Scholar]
- 17.Li L, Meier PJ, Ballatori N. Oatp2 mediates bidirectional organic solute transport: a role for intracellular glutathione. Mol Pharmacol. 2000;58(2):335–40. doi: 10.1124/mol.58.2.335. [DOI] [PubMed] [Google Scholar]
- 18.Mallonee DH, White WB, Hylemon PB. Cloning and sequencing of a bile acid-inducible operon from Eubacterium sp. strain VPI 12708. J Bacteriol. 1990;172(12):7011–9. doi: 10.1128/jb.172.12.7011-7019.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ridlon JM, Kang DJ, Hylemon PB. Isolation and characterization of a bile acid inducible 7alpha-dehydroxylating operon in Clostridium hylemonae TN271. Anaerobe. 2010;16(2):137–46. doi: 10.1016/j.anaerobe.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47(2):241–59. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Wells JE, Hylemon PB. Identification and characterization of a bile acid 7alpha-dehydroxylation operon in Clostridium sp. strain TO-931, a highly active 7alpha-dehydroxylating strain isolated from human feces. Appl Environ Microbiol. 2000;66(3):1107–13. doi: 10.1128/aem.66.3.1107-1113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng X, et al. Endocrine regulation of gender-divergent mouse organic anion-transporting polypeptide (Oatp) expression. Mol Pharmacol. 2006;70(4):1291–7. doi: 10.1124/mol.106.025122. [DOI] [PubMed] [Google Scholar]
- 23.Waxman DJ, Holloway MG. Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol. 2009;76(2):215–28. doi: 10.1124/mol.109.056705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ge Z, et al. Colonization dynamics of altered Schaedler flora is influenced by gender, aging, and Helicobacter hepaticus infection in the intestines of Swiss Webster mice. Appl Environ Microbiol. 2006;72(7):5100–3. doi: 10.1128/AEM.01934-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fushuku S, Fukuda K. Gender difference in the composition of fecal flora in laboratory mice, as detected by denaturing gradient gel electrophoresis (DGGE) Exp Anim. 2008;57(5):489–93. doi: 10.1538/expanim.57.489. [DOI] [PubMed] [Google Scholar]