Abstract
Acid-sensing ion channels (ASICs) are proton-activated sodium channels of the nervous system. Mammals express four ASICs, and orthologs of these genes have been found in all chordates examined to date. Despite a high degree of sequence conservation of all ASICs across species, the response to a given increase in external proton concentration varies markedly: from large and slowly inactivating inward currents to no detectable currents. The underlying bases of this functional variability and whether it stems from differences in proton-binding sites or in structures that translate conformational changes have not been determined yet. We show here that the ASIC1 ortholog of an early vertebrate, lamprey ASIC1, does not respond to protons; however, only two amino acid substitutions for the corresponding ones in rat ASIC1, Q77L and T85L, convert lamprey ASIC1 into a highly sensitive proton-activated channel with apparent H+ affinity of pH50 7.2. Addition of C73H increases the magnitude of the currents by fivefold, and W64R confers desensitization similar to that of the mammalian counterpart. Most amino acid substitutions in these four positions increase the rates of opening and closing the pore, whereas only few, namely, the ones in rat ASIC1, slow the rates. The four residues are located in a contiguous segment made by the β1-β2-linker, β1-strand, and the external segment of the first transmembrane helix. We conclude that the segment thus defined modulates the kinetics of opening and closing the pore and that fast kinetics of desensitization rather than lack of acid sensor accounts for the absence of proton-induced currents in the parent lamprey ASIC1.
Keywords: epithelial sodium channel/degenerin, proton sensor, proton-sensing ion channel, channel kinetics, activation by low pH, acid-sensing ion channel
the acid-sensing ion channels (ASICs) are proton-activated sodium channels expressed in neurons and glial cells (1, 14). Several functional roles have been attributed to the ASICs: ASIC1 has been implicated in modulation of synaptic transmission (22) and it may mediate fear responses (25), whereas ASIC3 may modulate nociception in sensory neurons (17). In pathological conditions such as ischemia, activation of ASIC1 enhances cell injury due to influx of calcium into neurons (24). Four independent ASIC genes in the mammalian genome give rise to four subunits, ASIC1 to ASIC4, and differential splicing of the four genes generates additional subunits (5, 16), each one with distinct properties: affinity to protons, kinetics of activation and desensitization, sensitivity to Zn2+ (4), polyamines (3), neuropeptides (2, 23), inhibition by toxins (10, 19), and responses to other modulators. The most salient difference is the variability in the proton response itself. A drop in external pH from 7.4 to 6.5 opens 50% of the mammalian ASIC1 channels, whereas a drop in pH to 4.0 fails to elicit currents in ASIC4 and in the spliced form ASIC2b expressed in the same cell and measured under identical conditions. Despite ubiquitous expression in the brain and the important functions mediated by ASICs, these proteins remain a poorly understood family of ion channels. Particularly, little is known of how they work at the molecular level and what is the physiological meaning of the wide variation in properties.
Structurally, the ASICs comprise three identical or homologous subunits; each subunit has two transmembrane segments, a large extracellular domain (ECD), and short intracellular NH2 and COOH termini. A significant advance in the field came with the resolution of the atomic structure of chicken ASIC1 (11, 13) that gave rise to several hypotheses for mechanisms of proton activation. A cluster of negatively charged residues (D237 and E238 interacting with D345 and D349) located in two adjacent subdomains in the ECD was initially proposed to form the “proton sensor”, i.e., the site where H+ binding triggers conformational changes that open the pore. According to this hypothesis, channels without one or more negative charges in the sensor would exhibit low or no affinity for protons. However, neutralization of conserved charged residues in the extracellular domain either singly or several residues together, including the ones in the putative proton sensor, do not eliminate proton sensitivity from rat ASIC1 (15, 18), suggesting that other elements in the protein play an important role in defining the response to protons.
In this work we approached the question of what gives proton sensitivity to ASIC1 not by eliminating proton response from a functional ASIC but by finding residues and structures that endow the typical proton response to ASICs. For that purpose we examined ASIC1 from the jawless fish lamprey; this protein exhibits high degree of sequence identity with rat ASIC1 but does not respond to protons (7). Thus the lamprey channel is a suitable paradigm to investigate the structural elements underlying the differences in proton sensitivity of the ASICs.
MATERIALS AND METHODS
Site-Directed Mutagenesis and DNA Constructs
Mutagenesis was conducted with the QuickChange method (Agilent). PCR and overlapping primers of rat and lamprey sequences were used to make chimeras of transmembrane segement 1 (TM1). All constructs were confirmed by DNA sequencing.
Surface Biotinylation and Western Blot Analysis
Surface biotinylation and Western blotting were conducted in oocytes as described previously using FLAG monoclonal antibody coupled to horseradish peroxidase (7).
Electrophysiology
Two-electrode voltage clamp of oocytes.
Xenopus laevis oocytes (Nasco) were injected with 5 ng cRNA in a volume of 50 nl and used after 2–4 days of incubation at 16°C. For two-electrode voltage-clamp (TEVC) experiments, oocytes were placed in a recording chamber (400 μl) perfused by gravity at a rate of 4 ml/min. Oocytes were impaled with two glass microelectrodes filled with 3 M KCl having resistance lower than 1 MΩ. Membrane potential was held at −60 mV, and whole cell currents were recorded with a Clamp OC-725B (Warner Instruments, Hamden, CT) and digitized at a sampling rate of 2 kHz (PowerLab/200, ADInstruments). The composition of the standard bath solution was (in mM) 120 NaCl, 2 KCl, 1.5 CaCl2, and 10 HEPES-MES, adjusted to pH 8.0. Activating solutions were administered by a perfusion system positioned directly in front of the oocyte. The composition of activating solutions was (in mM) 120 NaCl, 2 KCl, 1.5 CaCl2, and 15 HEPES-MES or 15 MES, adjusted to pH 7.4 to 4.0. Where indicated, 120 mM NaCl was replaced by equal concentrations of LiCl, KCl, or CsCl.
Patch clamp.
Currents from outside-out patches were recorded with an EPC-9 amplifier and the Pulse acquisition program (HEKA Electronic). Patch pipettes were pulled from PG150T glass (Warner Instruments) to tip diameters of 2–5 μm after heat polishing. Bath solution for patch-clamp recording was (in mM) 120 NaCl, 2 KCl, 1.5 CaCl2, and 10 HEPES, adjusted to pH 8.0. The pipette solution was of identical composition as the bath but it did not contain CaCl2. Activating solutions were buffered with MES to pH 7.4, 7.2, 7.0. 6.5, 6.0, 5.0, 4.0, or 3.5. Application of activation solutions was performed using the SF 77A Perfusion Fast-Step device (Warner Instruments) under control of the program Pulse.
Calculation of apparent pH50.
Oocytes were exposed sequentially to solutions of decreasing pH from 7.4 to 5.0. Between test applications, oocytes were perfused with solution of pH 8.0 for at least 30 s. If possible, each oocyte was tested twice with the whole range of pH solutions. The average of the peak currents from each pH was normalized to the value of pH that produced maximal current. The mean and standard deviation were obtained and the pH50 was calculated according to Eq. 1
| (1) |
where I is the normalized value for the test pH, EC50 is the concentration of protons that induces half-maximal currents, and n is the Hill coefficient. KaleidaGraph (Synergy Software) was used to draw graphs and for curve fitting.
Calculation of relative permeabilities to monovalent cations.
Currents from voltage-clamped oocytes were measured at various voltages from −100 to 100 mV in 10-mV steps while the bathing solution contained single monovalent cations: 120 Na+, Li+, K+, or Cs+ buffered at pH 6.5. Data from oocytes exposed to the four different solutions were used for calculations of relative permeabilities according to Eq. 2
| (2) |
where ErevA indicates the reversal potential for ion A, RT/zF has the usual meaning, PA is the permeability of ion A, and [A]o is the concentration of the ion in the external bath, 120 mM. ErevB, PB, and [B]o refer to sodium in the bath (12).
Activation and desensitization rates.
The activation and desensitization rates were calculated by fitting the rise and decay phases of proton-activated currents from outside-out patches to a single exponential; time constants τ = 1/λ.
RESULTS
Replacement of Two Residues in the Extracellular Domain Converts Lamprey ASIC1 Into a Proton-Sensitive Ion Channel
We started the analysis of lamprey ASIC1 by focusing on residues that are different in the ECD of rat and lamprey proteins. The ECD of lamprey differs from that of rat in 88 out of 370 amino acids, with most of the variability concentrated in the first one-third of the ECD (7). We first reconstituted the putative proton sensor because lamprey ASIC1 does not have two out of the four negatively charged residues that form the sensor. After the substitutions N345D and M349D were made, lamprey ASIC1 with an intact proton sensor remained unresponsive to external protons. Next, we conducted extensive single-residue substitutions of the ECD, replacing each different residue of lamprey for the corresponding one in rat ASIC1. This strategy produced no functional channels. Lamprey ASIC1 was converted into a functional proton-activated channel only after the substitutions were sequentially added, starting from the NH2 terminus of the ECD. Two substitutions, Q77L and T85L, were sufficient and necessary for protons to evoke currents in lamprey ASIC1, but addition of C73H increased the magnitude of the currents by approximately fivefold, from a mean of 5 ± 1.5 to 25 ± 10 μA/cell, n ≥ 70 (Fig. 1). Further permutations of these residues showed that the three residues together are essential to render lamprey ASIC1 into a proton-activated channel. We refer to the functional lamprey channel with these three substitutions (3M) as lamprey ASIC1.
Fig. 1.
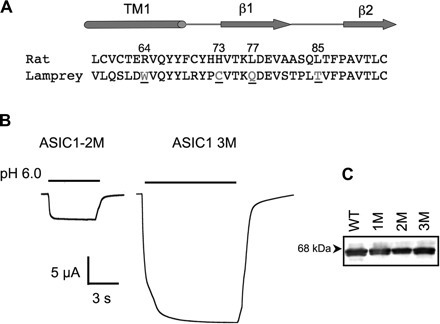
Conversion of a lamprey acid-sensing ion channel (ASIC) into a proton-activated channel. A: alignment of the amino acid sequences of rat and lamprey corresponding to transmembrane segment 1 (TM1), the β1-strand, a linker between β1 and β2 according to the crystal structure of chicken ASIC1. Underlined are residues that when substituted for the corresponding ones in rat produced functional channels with properties similar to rat ASIC1. B: representative current evoked by pH 6.0 from functional lamprey with two mutations, Q77L, T85L (2M); and three mutations, C73H, Q77L, T85L (3M). C: Western blot showing surface expression of wild-type (WT) and mutant channels with one (1M = Q77L), two (2M = Q77L, T85L), or the three substitutions (3M = C73H, Q77L, T85L) probed with FLAG monoclonal.
Properties of Functional Lamprey ASIC1
The functional lamprey ASIC1 expressed in Xenopus oocytes produced large inward currents and mean amplitude of 25 ± 10 μA/oocyte. All oocytes injected with lamprey cRNA had depolarized membrane potential (Vm ∼0 mM) and expressed an inward current of 1 to 5 μA/oocytes at −60 mv. This baseline current was not suppressed by pH 9.0 but was completely and reversibly blocked by 0.1 mM amiloride, a general open channel blocker of epithelial sodium channel/degenerin channels (Fig. 2A), indicating that the baseline current arises from lamprey ASIC1.
Fig. 2.
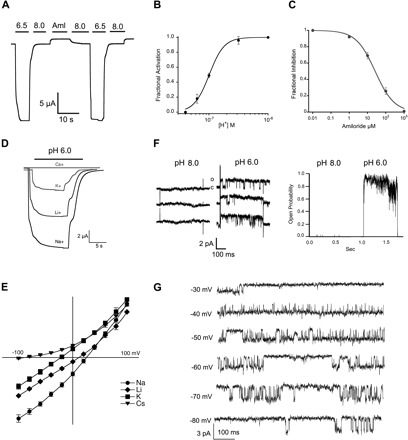
Functional properties of lamprey ASIC1. A: representative current of functional lamprey ASIC1 (3M) evoked by a change in pH from 9.0 to 6.5. A small baseline current was blocked reversibly by 100 μM amiloride (Aml). B: fractional activation by external protons. Whole cell currents of lamprey ASIC1 expressed in Xenopus oocytes were measured by two-electrode voltage clamp (TEVC) in the presence of increasing concentrations of external protons. The lines represent fit of the data to Eq. 1. Each data point is the mean ± SD of 12 cells. C: fractional inhibition by amiloride. Increasing concentrations of amiloride were applied to lamprey ASIC1 currents activated by pH 6.5. The calculated Ki was 26 ± 2 μM, n = 6. D: representative current traces of lamprey ASIC1 activated by pH 6.0 in the presence of 120 mM Na+, Li+, K+, or Cs+ as the only external monovalent cation. Membrane potential, −60 mV. Time (s) and current amplitude (μA) scales are shown with scale bars below traces. E: current-voltage relations of lamprey ASIC1 activated by pH 6.5 in the presence of 120 mM Na+, Li+, K+, or Cs+ as the only external monovalent cation. Data points are normalized currents of at least 4 independent cells. The change in reversal potential was used to calculate the relative permeability to monovalent cations using Eq. 2. F, left: unitary channel currents from an outside-out patch sequentially perfused with pH 8.0 or 6.0. Right: instantaneous open probability calculated from the same patch containing a single channel subjected to 30 consecutive sweeps exposed to pH 8.0 and 46 sweeps to pH 6.0. Membrane potential, −60 mV. G: representative examples of unitary currents evoked by pH 6.8 and recorded at the membrane voltages indicted on the left.
The apparent half-maximal activation by protons or pH50A was 7.1 (Fig. 2B). The affinity for amiloride was similar to rat ASIC1, Ki of 26 ± 2 μM (Fig. 2C). The selectivity of monovalent cations followed the sequence Na+<Li+<K+<>Cs+ calculated from changes in reversal potential (PNa/PLi = 1.5, PNa/PK = 6.0, PNa/PCs = 30), which is also similar to rat ASIC1 (Fig. 2, D and E). The unitary currents in membrane patches in the outside-out configuration showed single-channel conductance of 33 pS examined in symmetric 120 mM Na+ and in the voltage range of −80 to −20 mV (Fig. 2G). The absolute open probability was pH dependent, reaching a value of 0.9 at pH 6.0 (Fig. 2F). The biophysical properties of the unitary currents of lamprey ASIC1 channel were indistinguishable to those of rat ASIC1; hence the parent lamprey protein is indeed an ASIC1 channel. However, lamprey ASIC1 exhibits a much higher apparent affinity for protons than rat ASIC1 indicated by channel openings at pH 8.0. In addition, lamprey ASIC1 remains open in the continuous presence of protons in contrast to rat ASIC1, which exhibits desensitization.
Residues 73 and 77 in the β1-Strand Induce Desensitization in Lamprey ASIC1
From the 370 residues forming the ECD, only two substitutions from the mammalian channel are required to convert lamprey ASIC1 into a proton-gated channel and a third one potentiates the magnitude of the currents. These three residues are all located in a short segment of the protein that connects the ECD to the TM1: C73 and Q77 are in the β1-strand that follows TM1 and T85 in the linker that connects β1 and β2. Previous studies have shown that substitutions of residue H73 in rat ASIC1 and ASIC2a decreased the apparent affinity to protons (4, 18). Here we identified an additional position in the β1-strand Q77 that is critical for channel function. The sequences of many ASIC channels cloned to date show that the amino acid in position 77 is not absolutely conserved: L, V, and rarely M are found. To shed light on the properties of the side chain that make lamprey ASIC1 functional, we examined the effect of several amino acids in position 77. Figure 3A shows the average inward current evoked by pH 6.5 from lamprey ASIC1, with the following amino acids in position 77: L, I, F, V, M, and H, which produced currents, and Q, N, E, C, and A, which did not. Channels that did not respond to pH 6.5 were further tested with pH 5.0 and 4.0, but no currents were detected at the latter cases. Among the functional mutants, ASIC1-Q77H showed small currents that desensitize (Fig. 3B). Comparison of the time course of the currents shown in Figs. 1B and 3B makes evident that histidine in position 77 changes the kinetics: the rise of the current becomes fast (time constant of 0.3 s compared with 1 s) and the peak current decays without inducing complete desensitization. These results indicate that amino acids with large and hydrophobic side chains produce functional channels, whereas the ones with polar or small side chains do not. Histidine combines properties of both groups, allowing expression of small currents with incomplete desensitization.
Fig. 3.
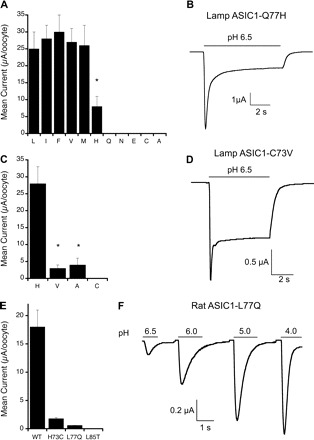
Functional effects of amino acids 77 and 73 in the β1-strand of lamprey ASIC1. A: lamprey ASIC1 substitutions in position 77. Average peak whole cell current evoked by external protons is shown. Cells were exposed to pH 6.5, 5.0, and 4.0. The largest value is plotted in the graph. Each bar represents the mean ± SD of at least 8 cells. *Significant difference (P = 0.01, t-test). B: representative example of the time course of lamprey (Lamp) ASIC1-Q77H current evoked by pH 6.5. C: lamprey ASIC1 substitutions in position 74. Values are means ± SD; n = 8 to 10 cells from 2 independent oocyte batches. *Significant difference compared with H73, P = 0.001. D: representative time course of current from ASIC1-C73V. E: peak currents of rat ASIC1 mutants evoked by pH 6.5. Values are means ± SD; n = 15 from 3 independent frog batches. F: representative example of rat ASIC1-L77Q activated by increasing concentrations of external protons.
We also examined the effect of substituting C73 by amino acids present in other ASICs, such as alanine, valine, and threonine. All of these substitutions produced proton-activated channels, but the currents were of small magnitude and exhibited rapid and incomplete desensitization (Fig. 3, C and D). Replacement of T85 by other residues besides leucine yielded channels with no detectable proton-induced currents; thus further analysis was not possible.
In the converse experiments, i.e., introduction of lamprey residues into rat ASIC1a, H73C, L77Q, and L85T produced channels with markedly reduced proton-evoked current or no current at all. The mutant L77Q had a mean peak current of 1.8 ± 0.5 μA/oocyte, the time course of the macroscopic currents was faster than wild type, and the apparent half-maximal activation by protons was shifted to the acidic range, pH50 5.5 (Fig. 3, E and F).
Taken together, the results suggest that the absence of current in the parent lamprey ASIC1 stems from the additive effect of residues in the β1-strand and β1-β2-linker speeding the rates of activation and desensitization. As a consequence, channel openings become too short to be detected by the recording system.
TM1 Modulates Desensitization of Lamprey ASIC1
Protons induce desensitization of rat ASIC1 channels from the open and the closed states, whereas the currents of lamprey ASIC1 (3M) remain stable for long periods of time despite the continuous presence of protons (Fig. 1A and Fig. 4A); thus lamprey ASIC1 undergoes little desensitization from the open state. We also examined whether protons induce steady-state desensitization (desensitization from the closed state) by the protocol shown in Fig. 4A. Channels were activated by a drop in pH from 8.0 to 7.0 or from 8.0 to 6.0 in the initial part of the protocol, whereas the drop in pH was sequential from 8.0 to 7.0 then to 6.0 in the second part of the protocol. The level of current reached with pH 6.0 was the same independent of the sequence, indicating none or negligible steady-state desensitization.
Fig. 4.
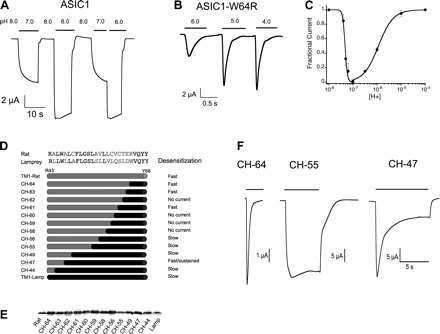
Composition of TM1 changes the kinetics of desensitization of lamprey ASIC1. A: lamprey ASIC1 currents remain stable under prolonged exposure to protons and are not sensitive to the preconditioning pH. B: the substitution W64R in TM1 makes channels desensitize rapidly in the presence of external protons. C: concentration dependence of activation and steady-state desensitization by protons of ASIC1-W64R. Each data point is the mean ± SD of ≥8 cells. D, top: the amino acid sequence comparison of rat and lamprey TM1. In gray are shown different residues. In the schematic representation of rat-lamprey chimeras of TM1, gray represents rat sequences and black represents lamprey sequences. The number on the left is the residue of the crossover. E: Western blot of surface expression of chimeras using FLAG-monoclonal. Fifteen oocytes for each channel construct (CH) examined by TEVC in D were loaded on the gel. F: time course of representative current traces of chimeras activated by pH 6.5.
The structures underlying the differences in desensitization between rat and lamprey AISC1 are not known; however, the extensive work of substituting residues in the ECD of lamprey did not identify any residue that accounts for this observation. Therefore, we next examined TM1 because this segment differs from rat in several amino acids (Fig. 1A). Among them, W64 in lamprey corresponds to R64 in rat as in other ASIC1 and ASIC3 channels sequenced to date. The mutant lamprey ASIC1-W64R desensitized rapidly, with a time constant for the decay of the current of 0.4 s (Fig. 4B). It also exhibited a fivefold increase in the rate of activation; time constant of the rising current was 0.2 s compared with 1 s of ASIC1-W64 measured at pH 6.5.
The mutation also induced desensitization from the closed state; the proton concentration for half-maximal desensitization was calculated to be pH 7.3 (Fig. 4C). The graph in Fig. 4C also shows that W64R displaces the apparent affinity for proton activation to the acid range: pH50A 5.9. These changes were not due solely to the introduction of a positively charged residue because lysine did not induce desensitization or shift the pH50A but alanine did (data not shown), implying that titration of the ionizable side chain of arginine is not the underlying mechanism. It is worth noticing that the substitution W64R illustrates an instance where marked shifts in the pH50A, 7.2 to 5.9, arise from changes in channel kinetics and not from changes in the actual affinity of the proton sensor.
The analysis was extended to the whole TM1 segment by constructing chimeras between rat and lamprey as shown in Fig. 4D. The number of the chimeras follows the position of the residue in TM1 where the switch between lamprey and rat was made: gray indicates sequences from rat and black indicates sequences from lamprey. The chimera containing the whole TM1 from rat, rTM1-Lamp-ASIC1, was functional but it exhibited faster desensitization than wild-type rat ASIC1 (τd 0.8 ± 0.09 s vs. 2.6 ± 0.2 s). As lamprey residues were reintroduced, channels regained slow desensitization, but the transition was neither abrupt nor gradual as several constructs were nonfunctional (CH-62, CH-60, CH-59, CH-58) despite the fact that the substitutions belong to functional channels and that all constructs expressed at the cell surface with similar levels (Fig. 4E). Moreover, residues close to the cytoplasmic side of TM1 also modified the desensitization properties, as illustrated by construct CH-47, which exhibited an intermediate rate and partial desensitization (Fig. 4F). Thus, the whole TM1 segment contributes to the desensitization process, with the outer segment having a more prominent role.
DISCUSSION
This work examined ASIC1 from two evolutionary distant species, lamprey and rat, to identify structural elements and residues that account for the functional differences of these channels. Despite high amino acid sequence conservation, the two proteins exhibit markedly different properties. We started with a non-proton-sensitive lamprey ASIC1, but as few as four residue substitutions from rat ASIC1 conferred to the lamprey protein most of the typical features of the mammalian channel: proton-induced currents with desensitization from the open and closed states. These results differ from previous studies in two fundamental ways. First, other works have identified potentially important residues by selecting for residues that eliminate channel function. This type of result sheds little light on the underlying structural or functional role of the identified amino acid. Substitutions of most highly conserved residues usually impair function as expected by the fact that they have been selected and are conserved under positive evolutionary pressure. Findings of residues that confer or enhance function are not only rare but are of higher value in predicting functional significance. The other difference is that here substitutions were made not with randomly selected amino acids but with amino acids present in two evolutionarily distant species. Therefore, the substitutions reflect natural selection of a residue to optimize and/or adapt properties of the channel.
Because the absence of proton-evoked currents in the parent lamprey ASIC1 was not due to lack of protein expression at the cell surface, we sought alternative explanations. Restoration of the putative proton sensor and other negatively charged residues present in the ECD of rat ASIC1 did not confer proton activation to the lamprey, whereas two nonionizable residues Q77L and T85L were sufficient and necessary to confer response to protons. This indicates that the parent lamprey ASIC1 has a proton sensor(s) but channel openings are not evident owing to a fast transduction mechanism given by Q77 and T85. In an independent study we previously showed that replacement of a methionine in position 85 in fish ASIC1, M85L, reduces the rate of desensitization of the channel (6). Here, the substitutions Q77L and T85L together with C73H slowed the kinetics, making it possible to detect channel openings; indeed, these substitutions turned lamprey AISC1 into a channel with slow opening and closing.
It is noticeable that the three residues crucial for proton sensitivity are located in a contiguous segment that encompasses the β1-strand and the β1-β2-linker. By connecting TM1 with the ECD, this segment is poised to transmit conformational changes from the ECD to the pore. As expected from an element that participates in transduction of conformational changes there is already some evidence of movement of the distal end of the β1-repositioning of residue E79, while the channel transitions from the closed to desensitized state (9). The functional importance of this region has been independently noticed in studies that looked for the proton sensor or residues binding Zn2+ and led to mutations of charged residues such as H73 in rat ASIC1 (18) and ASIC2 (20) and E79 in rat ASIC3 (9). We identified here an additional important residue in the β1-, Q77, whose side chain faces the inner cavity above the channel pore.
The results also make evident the importance of TM1 in modulating gating. The crystal structure of chicken ASIC1 provided by Gonzales et al. (11) places the “gate” of the pore to residue D433 in TM2. The side chain of D433 from each subunit points to the lumen of the ion pathway occluding the pore in the desensitized state. Adjacent to D433 is residue 64 in TM1, close enough to interact or interfere with the side chain of D433 whether TM2 undergoes rotation or tilting during gating. Furthermore, the results indicate that modulation of channel kinetics by TM1 is not restricted to the outer segment of TM1 where residue 64 is located but extends to most of the length of TM1, supporting the notion of substantial rearrangement of the transmembrane segments during gating. Because the amino acid sequence of TM1 is not highly conserved, we expect that the variability in kinetics observed among ASICs is attributed in part to TM1, whereas the constant pore properties (conductance, ion selectivity, amiloride affinity) are attributed to the highly conserved TM2.
The stark interspecies differences in functional properties of the ASICs are not understood yet but they most likely reflect adaptations to the environments and conditions in which these channels operate, including interspecies differences in temperature and external pH and in the ways that vertebrate species control proton concentration in confined microdomains of the nervous system.
In summary, we show that the absence of proton-evoked current in the parent lamprey ASIC1 channel stems from fast kinetics and not from lack of a “proton sensor” and that the segment made by the β1-β2-linker, the β1-strand and the outer segment of TM1 play a major role in determining the variability of interspecies ASIC kinetics.
GRANTS
The work was supported by National Institutes of Health Grant RO1-DK-054062.06A1 (to C. M. Canessa).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Alvarez de la Rosa D, Krueger SR, Kolar A, Shao D, Fitzsimonds RM, Canessa CM. Distribution, subcellular localization and ontogeny of ASIC1 in the mammalian central nervous system. J Physiol 546: 77–87, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Askwith CC, Cheng C, Ikuma M, Benson C, Price MP, Welsh MJ. Neuropeptide FF and FMRFamide potentiate acid-evoked currents from sensory neurons and proton-gated DEG/ENaC channels. Neuron 26: 133–141, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Babini E, Paukert M, Geisler HS, Grunder S. Alternative splicing and interaction with di- and polyvalent cations control the dynamic range of acid-sensing ion channel 1 (ASIC1). J Biol Chem 277: 41597–41603, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Baron A, Schaefer L, Lingueglia E, Champigny G, Lazdunski M. Zn2+ and H+ are coactivators of acid-sensing ion channels. J Biol Chem 276: 35361–35367, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Chen CC, England S, Akopian AN, Wood JN. A sensory neuron-specific, proton-gated ion channel. Proc Natl Acad Sci USA 95: 10240–10245, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coric T, Zhang P, Todorovic N, Canessa CM. The extracellular domain determines the kinetics of desensitization in acid-sensitive ion channel 1. J Biol Chem 278: 45240–45247, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Coric T, Zheng D, Gerstein M, Canessa CM. Proton sensitivity of ASIC1 appeared with the rise of fishes by changes of residues in the region that follows TM1 in the ectodomain of the channel. J Physiol 568: 725–735, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coric T, Passamaneck YJ, Zhang P, Di Gregorio A, Canessa CM. Simple chordates exhibit a proton-independent function of acid-sensing ion channels. FASEB J 22: 1914–1923, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Cushman KA, Marsh-Haffner J, Adelman JP, McCleskey EW. A conformation change in the extracellular domain that accompanies desensitization of acid-sensing ion channel (ASIC) 3. J Gen Physiol 129: 345–350, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Diochot S, Baron A, Rash LD, Deval E, Escoubas P, Scarzello S, Salinas M, Lazdunski M. A new sea anemone peptide, APETx2, inhibits ASIC3, a major acid-sensitive channel in sensory neurons. EMBO J 23: 1516–1525, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gonzales EB, Kawate T, Gouaux E. Pore architecture and ion sites in acid-sensing ion channels and P2X receptors. Nature 460: 599–604, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hille B. Selective permeability: independence. In: Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer, 1992, p. 337–361 [Google Scholar]
- 13. Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature 449: 316–323, 2007. [DOI] [PubMed] [Google Scholar]
- 14. Kapoor N, Bartoszewski R, Qadri YJ, Bebok Z, Bubien JK, Fuller CM, Benos DJ. Knockdown of ASIC1 and epithelial sodium channel subunits inhibits glioblastoma whole cell current and cell migration. J Biol Chem 284: 24526–24541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li T, Yang Y, Canessa CM. Interaction of the aromatics Y72/W288 in the interface of the extracellular and transmembrane domains is essential for proton-gating of ASIC. J Biol Chem 284: 4689–4694, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lingueglia E, De Weille JR, Bassilana F, Heurteaux C, Sakai H, Waldmann R, Lazdunski M. A modulatory subunit of acid sensing ion channels in brain and dorsal root ganglion cells. J Biol Chem 272: 29778–29783, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Molliver DC, Immke DC, Fierro L, Paré M, Rice FL, McCleskey EW. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Mol Pain 1: 35, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Paukert M, Chen X, Polleichtner G, Schindelin H, Gründer S. Candidate amino acids involved in H+ gating of acid-sensing ion channel 1a. J Biol Chem 283: 572–581, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Salinas M, Rash LD, Baron A, Lambeau G, Escoubas P, Lazdunski M. The receptor site of the spider toxin PcTx1 on the proton-gated cation channel ASIC1a. J Physiol 570: 339–354, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smith EJ, Zhang X, Cadiou H, McNaughton PA. Proton binding sites involved in the activation of acid-sensing ion channel ASIC2a. Neurosci Lett 426: 12–17, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski MA. A proton-gated cation channel involved in acid-sensing. Nature 386: 173–717, 1997 [DOI] [PubMed] [Google Scholar]
- 22. Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, Yoder PG, Lamani E, Hoshi T, Freeman JH, Jr, Welsh MJ. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron 34: 463–477, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Xie J, Price MP, Wemmie JA, Askwith CC, Welsh MJ. ASIC3 and ASIC1 mediate FMRFamide-related peptide enhancement of H+-gated currents in cultured dorsal root ganglion neurons. J Neurophysiol 89: 2459–2465, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, MacDonald JF, Wemmie JA, Price MP, Welsh MJ, Simon RP. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell 118: 687–698, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, 3rd, Howard MA, Welsh MJ, Wemmie JA. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell 139: 1012–1021, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


