Abstract
The stress-induced initiation of proapoptotic signaling in Leydig cells is relatively well defined, but the duration of this signaling and the mechanism(s) involved in opposing the stress responses have not been addressed. In this study, immobilization stress (IMO) was applied for 2 h daily, and animals were euthanized immediately after the first (IMO1), second (IMO2), and 10th (IMO10) sessions. In IMO1 and IMO2 rats, serum corticosterone and adrenaline were elevated, whereas serum androgens and mRNA transcription of insulin-like factor-3 in Leydig cells were inhibited. Reduced oxygen consumption and the mitochondrial membrane potential coupled with a leak of cytochrome c from mitochondria and increased caspase-9 expression, caspase-3 activity, and number of apoptotic Leydig cells was also observed. Corticosterone and adrenaline were also elevated in IMO10 rats but were accompanied with a partial recovery of androgen secretion and normalization of insulin-like factor-3 transcription coupled with increased cytochrome c expression, abolition of proapoptotic signaling, and normalization of the apoptotic events. Blockade of intratesticular glucocorticoid receptors diminished proapoptotic effects without affecting antiapoptotic effects, whereas blockade of intratesticular α1-adrenergic receptors diminished the antiapoptotic effects without affecting proapoptotic effects. These results confirmed a critical role of glucocorticoids in mitochondria-dependent apoptosis and showed for the first time the relevance of stress-induced upregulation of α1-adrenergic receptor expression in cell apoptotic resistance to repetitive IMOs. The opposite role of two hormones in control of the apoptotic rate in Leydig cells also provides a rationale for a partial recovery of androgen production in chronically stressed animals.
Keywords: Leydig cells, immobilization stress, corticosterone, adrenaline, testis, apoptosis
the levels of testosterone (T) in circulation reflect the steroidogenic capacity of individual testicular Leydig cells and the total number of these cells per testes (2, 3, 10, 16). Because Leydig cells from adult animals are fully differentiated and rarely proliferate or die under normal physiological conditions, their steroidogenic capacity is controlled predominantly by the status of the hypothalamo-pituitary-gonadal axis via GnRH-LH secretory pathway (10, 23). The sustained stress lowers circulating LH and androgen levels (13, 40, 42), and acute stress also lowers T levels without changing circulating LH levels (35, 42). Furthermore, the reciprocal changes in plasma corticosterone (CORT) and T in stressed rats (45) and increase in steroidogenic capacity of Leydig cells induced by suppression of CORT levels (19) suggested that this stress hormone directly inhibits T biosynthesis.
Several hypotheses have also been introduced to explain the mechanism by which glucocorticoids directly inhibit androgenesis. The CORT-induced decline in steroidogenic capacity could reflect inhibition of the expression (48) and activity (47) of T-biosynthetic enzymes. Repetitive immobilization stress (IMO)-induced elevation in endogenous CORT levels is also accompanied by changes in the expression of several steroidogenic enzyme RNAs (52). Exogenous glucocorticoids also increase apoptotic events in numerous cell types (32), including Leydig cells (20), which raised the possibility that a decrease in the total number of Leydig cells could contribute to or accounts for LH-independent decline in circulating T levels in stressed animals. In further support of this hypothesis, an increase in apoptotic events was observed in stressed animals (11). In addition, it has been suggested that stress-induced glucocorticoids suppress T biosynthesis through a nongenomic mechanism by activating plasma membrane glucocorticoid receptors (GRs) negatively coupled to the adenylyl cyclase signaling pathway (15). We recently showed inhibition of basal adenylyl cyclase activity in IMO rats coupled with sustained upregulation of mRNA expression for several adenylyl cyclase and phosphodiesterase subtypes. Our study also revealed the lack of strong correlation between cAMP and androgen levels in Leydig cells stimulated with epinephrine, suggesting that other factor(s) negatively influence(s) androgen production in vivo in stressed animals (52).
Stress also accelerates turnover of brain noradrenaline and adrenaline, and the resulting changes in their blood concentrations depend on the nature, intensity, and duration of a stressor. It has also been documented that stress affects the expression of receptors for these neurotransmitters, called adrenergic receptors (ADRs). There are five groups of these receptors (α1- α2-, β1-, β2-, and β3-ADRs), and it is well established that stress affects the expression of α2-, β1-, and β2-ADRs (17). More recently, it has also been reported that stress and glucocorticoids rapidly increase α1d-ADR mRNA in the rat brain (8). Repeated stress also increased transcripts for all ADRs expressed in Leydig cells (52), and catecholamines stimulated androgen production in rat (2), golden hamster (44), and Siberian hamster (43) cells. Because it is well known that ADRs play an important antiapoptotic role in breast cancer cells (28), human umbilical vascular endothelial cells (39), and cardiac myocytes (54), we speculated that this signaling pathway may also contribute to the control of apoptotic and/or antiapoptotic signaling and androgenesis in stressed animals.
Here, we studied the role of CORT- and adrenaline-mediated signaling in stress-induced testicular androgenesis and apoptosis. IMO was chosen as a typical and frequently used model of psychophysiological stress (33–36, 42, 47, 52). The IMO sessions, established and justified previously (34–36, 52), include the acute (IMO1) and repeated stress without (IMO2) and with (IMO10) partial recovery of circulating T levels. The focus in our study was on the mechanism by which endogenous CORT triggers apoptosis and duration of proapoptotic signaling and whether and through which signaling pathway this process could be stopped during repetitive IMO sessions. To do this, we studied serum hormonal profiles and the status of pro- and antiapoptotic markers and the number of apoptotic Leydig cells after IMO was applied once (IMO1), twice (IMO2), and 10 times (IMO10). To evaluate roles of CORT and adrenaline in stress response, RU-486 (mifepristone), a GR antagonist (7), and prazosin (minipress), a potent blocker of α1-ADR (14), were also applied in vivo.
MATERIALS AND METHODS
Materials.
The anti-T-11-BSA serum no. 250 was kindly supplied by Gordon D. Niswender. The anti-mouse and anti-rabbit secondary antibodies linked to the horseradish peroxidase were obtained from Kirkegaard & Perry Laboratories (Gaithersburg, MD). The [1,2,6,73H(N)]-labeled T was from Perkin-Elmer Life Sciences (Waltham, MA), superscript III kit for cDNA preparation was from Invitrogen (Grand Island, NY), and primers for real-time quantitative PCR were from Integrated DNA Technologist (Munich, Germany). Medium 199 containing Earle's salt and l-glutamine (M199), DMEM-nutrient mixture F-12 Ham with l-glutamine and 15 mM HEPES (DMEM-F-12), HEPES, penicillin, streptomycin, EDTA, Percoll, BSA fraction V, collagenase type IA, RU-486, prazosin, acid β-glycerophosphate, tergitol (type 4), dithiothreitol, leupeptin, and aprotinin were from Sigma-Aldrich (St. Louis, MO).
Animals.
Three-month-old (250–270 g) male Wistar rats, bred and raised in the Animal Facility of Faculty of Sciences, University of Novi Sad, Serbia were used for the experiments. Animals were raised in controlled environmental conditions (22 ± 2°C, 12:12-h light-dark cycle, lights on at 0700) with food and water ad libitum. All the experimental protocols were approved by the local Ethics Committee on Animal Care and Use at the University of Novi Sad, operating under the rules of National Council for Animal Welfare and the National Law for Animal Welfare (March 2009), and in accordance with the National Research Council publication Guide for the Care and Use of Laboratory Animals (National Academy of Sciences, Washington, DC, 1996) and National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH publication no. 80-23, revised 1996, 7th ed.).
Experimental models.
IMO stress was performed daily, as described previously (36). Briefly, rats were handled daily during a 3-wk period of acclimation before experiments. After that, animals were bound in the supine position for 2 h (from 0800 to 1000) by fixing their limbs to the wooden board with thread and with head motion not limited and euthanized immediately after the first, second, and 10th sessions. Freely moving rats served as controls. Groups of animals also received 20 μg·20 μl−1·testis−1 RU-486 or solvent (20 μl of sterile DMSO) 12 h before IMO stress and were euthanized immediately after the first, second, or 10th session, whereas control animals were injected only with RU-486 or solvent once, twice, or 10 times and euthanized at the same times as stressed animals. Another group of controls and stressed rats was injected with 7.5 μg·20 μl−1·testis−1 prazosin or solvent (20 μl of sterile distillated water) 30 min before IMO stress was applied. All animals were quickly decapitated without anesthesia, and the trunk blood was collected. Individual serum samples were stored at −80°C until they were assayed for hormone levels. All groups consisted of four animals. All experiments were repeated three times.
Preparation of purified Leydig cells and ex vivo androgen production.
The primary cultures of purified Leydig cells were obtained from all experimental groups, as described previously (34, 35, 52). The proportion of Leydig cells present in culture was 95.3 ± 1.7%, as determined by staining for HSD3B activity. Purified Leydig cells were plated in 90-mm Petri dishes (5 × 106 cells in 5 ml/dish culture medium) for analysis or the ex vivo T production and the expression of pro/antiapoptotic markers. For mitochondrial membrane potential and caspase-3 activity measurements, Leydig cells were plated in 96-well plates (5 × 104 cells·0.2 ml−1·well−1).
Hormone measurements.
For serum LH level determination, all samples were measured in duplicate in a single assay (sensitivity <1 ng/ml; intra-assay coefficient of variation of 4.2% and a minimum detectable concentration of 0.14 ng/ml) according to the manufacturer's protocol (ALPCO, Salem, NH). Levels of androgens in serum, extracellular medium, or cell extracts were estimated by RIA and are referred to as T + dihydrotestosterone (DHT) because the anti-T serum no. 250 showed 100% cross-reactivity with DHT. All samples were measured in duplicate in one assay (sensitivity: 6 pg/tube; intra-assay coefficient of variation: 5–8%; interassay coefficient of variation: 7.5%), as described previously (34). Serum CORT measurements were done in duplicates in one assay (the IC50 value of ∼150 pg/ml and detection limit of ∼30 pg/ml) using an EIA Kit (Cayman, Ann Arbor, MI). Serum adrenaline levels were also determined in duplicate (standard range of 0.45–45 ng/ml and detection limit of 3.9 pg/ml) using the adrenaline research ELISA Kit (Labor Diagnostika Nord, Nordhorn, Germany).
TUNEL assay.
Apoptotic cells were quantified by labeling DNA breaks using APO-BrdU TUNEL Assay Kit according to the the manufacturer's instructions (Invitrogen). At the end of procedures, cells were stained with Alexa Fluor 488 dye-labeled anti-5-bromo-2′-deoxyuridine 5′-triphosphate antibody for 30 min at room temperature, followed by propidium iodide staining for an additional 30 min. Cells were then centrifuged, and pellets were resuspended in 100 μl of of PBS; drops of cell suspensions were deposited onto slides and analyzed under the Olympus IX70 fluorescent microscope. The apoptotic-stained (green fluorescence) and total number of cells (red fluorescence) were counted randomly in 30 fields of each sample. Data are expressed as a percent of apoptotic cells in total cell number.
Measurement of mitochondrial membrane potential.
To monitor mitochondrial membrane potential of Leydig cells, the procedure with the tetramethylrhodamine ethylester was applied as described previously (1). The fluorescence readings were taken on a fluorimeter, with excitation wavelength at 550 nm and emission wavelength at 590 nm on a fluorimeter (Fluoroskan, Ascent, FL, and Thermo Labsystems, Waltham, MA).
Measurement of oxygen consumption by Leydig cells.
The oxygen consumption by suspension of Leydig cells was measured at 34°C in a close chamber containing a Clark type oxygen electrode connected to a YSI model 5300 monitor (YSI, Yellow Springs, OH), as previously described (33). For calculation of oxygen uptake and presentation of oxygraphic curves, Digital Multimeter VC 820 (Conrad Electronic) and software Digiscope for Windows (version 2.06) were used.
Measurement of caspase-3 activity in Leydig cells.
The caspase-3 colorimetric assay kit was used, and measurements were performed in triplicate (3 × 106 Leydig cells in each tube, 150 μg/50 μl), following a protocol recommended by the manufacturer (Enzo, Farmingdale, NY). The cleavage of a tetrapeptide substrate was monitored colorimetrically by increased absorption at 405 nm in a microtiter plate reader. The fold increase in caspase-3 activity was determined by comparing with the level of the uninduced control.
RNA isolation and cDNA synthesis.
Total RNA from purified rat Leydig cells were isolated using an RNeasy kit reagent and protocol recommended by the manufacturer (Qiagen, Valencia, CA). Following the DNase I treatment, the first-strand cDNA was synthesized according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). An aliquot of 5 μl of the RT reaction product (25 ng of RNA calculatedon starting RNA) was amplified with the PCR reagent system. Negative controls consisting of non-reverse transcribed samples were included in each set of reactions. Quality of RNA and DNA integrity was checked as described previously (52).
Real-time polymerase chain reaction and relative quantification.
The relative expression of the genes was quantified by PCR using the SYBR Green-based chemistry from Applied Biosystems (Foster City, CA) in the presence of an aliquot of 5 μl of the RT reaction product (25 ng of RNA calculated on starting RNA) and specific primers. The primer sequences used for real-time PCR analysis, including GenBank accession codes for full gene sequences, are shown in Table 1. GAPDH was also measured in the same samples and used to correct variations in RNA content among samples. Relative quantification of each gene was done in duplicate three times for each gene and twice for each of three independent in vivo experiments.
Table 1.
The primer sequences used for real-time PCR analysis of Insl3, Cytc, and Hsd11b2 in Leydig cells isolated from control and treated animals
| Gene | GeneBank Accession Code | Primer Sequence | CT Values |
|---|---|---|---|
| Insl3 | NM_053680 | F: 5′-CGCCAAGCTCTGTGGTCA-3′ | 22.15 |
| R: 5′-CTGAGAAGCCTGGTGAGGA-3′ | |||
| Cytc | NM_012839 | F: 5′-GCAAGCATAAGACTGGACCAAA-3′ | 20.85 |
| R: 5′-TTGTTGGCATCTGTGTAAGAGAATC-3′ | |||
| Hsd11b2 | NM_017081 | F: 5′-CAAACCCTTCCCCCACAG-3′ | 27.36 |
| R: 5′-GGCTGGGCTTTTCTTAACAG-3′ | |||
| Gapdh | NM_017008 | F: 5′-TGCCAAGTATGATGACATCAAGAAG-3′ | |
| R: 5′-AGCCCAGGATGCCCTTTAGT-3′ | 19.81 |
Cytc, cytochrome c; Hsd11b2, 11β-hydroxysteroid dehydrogenase 2; F, forward; R, reverse. Primers were design by using Primer Express 3.0 software (Applied Biosystems) and full gene sequences from the NCBI Entrez Nucleotide database (www.ncbi.nlm.nih.gov/sites/entrez).
Protein extraction and Western blot analysis.
After incubation, Leydig cells (5 × 106/well) were washed twice with ice-cold PBS and lysed, and Western blot analysis was performed as described previously (34). The immunodetection of the Akt, p-Akt, p-ERK1/2, and ERK1/2 was detected using the commercial antibodies from Cell Signaling Technology (Beverly, MA), and the caspase-9 and cytochrome were detected using the antibodies purchased from Assay Design (Farmingdale, NY). The β-actin (ACTB) was detected using a detection kit developed by EMB Bioscience (La Jolla, CA). The immunoreactive bands were analyzed as two-dimensional images using Image J (version 1.32). The optical density of images is expressed as volume adjusted for the background, which gives arbitrary units of adjusted volume group.
Statistical analysis.
For in vivo studies, the results represents group means ± SE values of individual variation from three to five independent experiments (4 rats per group per experiment). For ex vivo measurements, the data represent means ± SE from three to five independent replicates. The results from each experiment were analyzed by Mann-Whitney's unpaired nonparametric two-tailed test (for 2-point data experiments) or by one-way ANOVA for group comparison, followed by Student-Newman-Keuls multiple range test.
RESULTS
Stress-induced Leydig cell dysfunction and apoptosis.
CORT, adrenaline, LH, and androgens (T + DHT) were measured in serum from controls and stressed animals. Figure 1, A and B, shows that IMO was effective as a stressor, elevating serum CORT (Fig. 1A) and adrenaline levels (Fig. 1B) in all stressed groups compared with undisturbed controls. Serum androgens were reduced in IMO1 and IMO2 rats, whereas a partial recovery of androgenesis was observed after IMO10 (Fig. 1D). Insl3 transcription was also attenuated after IMO1 and IMO2 but was normalized after IMO10 (Fig. 1E). Finally, the TUNEL assay analysis revealed that controls and IMO10 had negligible numbers of positively labeled cells (controls = 2.2 ± 0.8; IMO10 = 2.5 ± 0.9 out of 100 cells), which was in contrast to IMO1 and IMO2 animals exhibiting increased apoptosis incidence of Leydig cells (Fig. 1F).
Fig. 1.
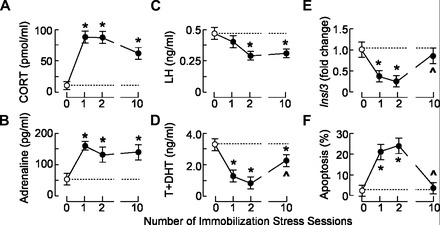
Effects of stress on circulating levels of stress hormones, LH, androgens, transcription of the Insl3 gene, and apoptosis of Leydig cells. A–D: stress-induced increase in circulating corticosterone (CORT; A) and adrenaline (B) levels was accompanied with a decrease in LH (C) and androgen [testosterone + dehydrotestosterone (T + DHT)] levels (D). Note that there is a difference in LH and androgen levels after immobilization stress (IMO)1 and IMO10 (1st and 10th sessions of IMO, respectively). E and F: IMO affected Insl3 transcription (E), and changes in apoptotic rates (F) mirrored androgen and Insl3 profiles. In this and the following figures, rats were subjected to IMO1, IMO2, or IMO10 or left undisturbed (controls; 0), trunk blood was collected for hormonal analysis, and Leydig cells were isolated (for details, see materials and methods). Data bars are means ± SE of 3–5 independent in vivo experiments. Statistical significance was at level P < 0.05: *vs. control group; ^vs. IMO1 group.
Effects of IMO stress on Leydig cell mitochondria.
Because mitochondria are critical for the regulation of steroid hormone biosynthesis (1) and play key roles in activating apoptosis (53), we examined the physiological status of these organelles in Leydig cells from IMO and control rats by measuring the oxygen consumption and mitochondrial membrane potential. In the absence of ADP, the oxygen consumption was reduced in Leydig cells from IMO1 and IMO2 groups and was restored to normal levels in the IMO10 group (Fig. 2A). In contrast, stimulation of Leydig cell respiration by ADP was comparable in IMO and controls (data not shown). The mitochondrial membrane potential was also reduced after IMO1 and IMO2 and was restored in Leydig cells from IMO10 rats (Fig. 2A). These results indicate the potential relevance of mitochondria in initiation of apoptosis in stressed animals.
Fig. 2.
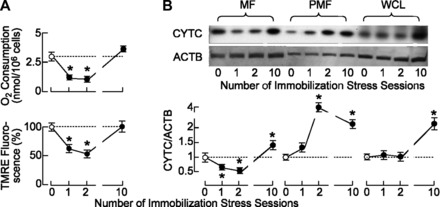
Stress disturbed mitochondrial function and distribution of cytochrome c (CYTC) in Leydig cells. A: IMO1 and IMO2 transiently inhibited oxygen consumption by Leydig cells (top) and the mitochondrial membrane potential (bottom). B: distribution of CYTC in Leydig cell mitochondrial fraction (MF), postmitochondrial fraction (PMF), and whole cell lysate (WCL) was also affected by IMO stress. Representative blots of CYTC and β-actin (ACTB; used as an internal control) are shown at top, and pooled data from scanning densitometry normalized on ACTB values are shown at bottom as means ± SE from 3 independent experiments. Statistical significance at level P < 0.05: *vs. control group.
Cytochrome c is a component of the electron transport chain mitochondria, which is also involved in initiation of apoptosis (50). Consistent with this, we observed a significant decrease in cytochrome c levels in mitochondrial fractions obtained from IMO1 and IMO2 Leydig cells (Fig. 2B). However, the expression of cytochrome c mRNA transcripts in Leydig cells from IMO10 but not IMO1 and IMO2 rats increased significantly (in relative units: controls = 1 ± 0.05, IMO1 = 1.1 ± 0.06, IMO2 = 1.2 ± 0.04, and IMO10 = 1.82 ± 0.12). The level of cytochrome c protein transcripts in whole cell lysate, mitochondrial, and postmitochondrial fractions from IMO10 rats also paralleled the gene expression (Fig. 2B). These results indicate that de novo cytochrome c synthesis could serve as an indicator of gradual restoration of Leydig cell function in stressed animals.
Status of pro- and antiapoptotic signaling in Leydig cells.
In general, caspases are critical intracellular signal transducers and executioners of apoptosis (29), prompting us to examine their status in Leydig cells. Figure 3A shows that there was a significant increase in expression of caspase-9 proteins in Leydig cells from IMO1 and IMO2 rats, but not IMO10 rats. The caspase-3 activity was also elevated in Leydig cells from IMO1 and IMO2 rats but was back to the control level in Leydig cells from IMO10 rats (Fig. 3B), confirming the transient nature of Leydig cell apoptosis during repetitive IMO sessions.
Fig. 3.
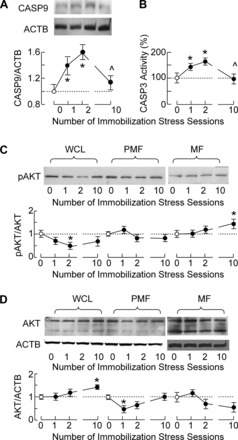
Stress triggered changes in caspase-9 (CASP9) protein transcription, caspase-3 (CASP3) activity, and phospho (p)-Akt/Akt distribution in Leydig cells. A: IMO-induced changes in the expression of CASP9 protein. Representative blots are shown at top, and pooled data from scanning densitometry normalized on ACTB values are shown at bottom as means ± SE from 3 independent experiments. B: IMO-induced changes in CASP3 activity. C and D: distribution of p-Akt/Akt in Leydig cells in WCL, MF, and PMF fractions. Top: representative blots. Bottom: pooled data from scanning densitometry normalized on Akt and ACTB values. Normalized data shown are means ± SE from triplicate determination. Statistical significance at level P < 0.05: *vs. control group; ^vs. IMO1 group.
Concomitant with the activation of apoptotic pathways, the survival phosphatidylinositol 3-kinase protein kinase B (Akt) and ERK1/2 signaling pathways play a critical role in balancing apoptosis in Leydig cells (12). In our experiments, there were significant decreases in the p-Akt/Akt ratio in whole cell lysate from IMO2 rats (Fig. 3C) and total Akt in postmitochondrial fraction (Fig. 3D), whereas IMO10 treatment increased Akt significantly in the whole cell lysate (Fig. 3D) and p-Akt/Akt ratio in the mitochondrial fraction of Leydig cells (Fig. 3C).
Role of testicular GRs in stress-induced Leydig cell apoptosis.
To estimate the possible contribution of glucocorticoids in IMO-induced Leydig cell apoptosis, the intratesticular treatment with RU-486 (20 μg·20 μl−1·testis−1), the potent blocker of classic GRs (7), was performed 12 h before IMO. Such a treatment did not affect serum LH levels in controls and IMO animals (Fig. 4A). However, serum androgen levels were affected significantly; intratesticular application of RU-486 increased circulating androgens in IMO1 and IMO2 rats compared with the corresponding untreated IMO animals (Fig. 4B, right). Furthermore, RU-486 injection prevented IMO1/IMO2-induced decrease in mitochondrial membrane potential without affecting its recovery in Leydig cells from IMO10 rats (Fig. 4C). In addition, RU-486 attenuated IMO1/IMO2-induced apoptosis of Leydig cells (Fig. 4D). The increase in Leydig cell caspase-9 level induced by IMO1/IMO2 was also abolished after intratesticular application of this GR blocker (Fig. 4E). Finally, blockade of intratesticular GRs diminished the IMO10-induced increase in mRNA transcript for Hsd11b2 (Fig. 4F), confirming that this transcription is related to GR activation (18, 21).
Fig. 4.
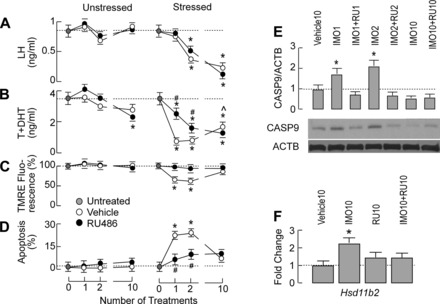
Time course effects of RU-486, a glucocorticoid receptor antagonist, on hormonal profile and apoptotic signaling in unstressed and stressed animals. RU-486 (20 μg in 20 μl/testis) or vehicle (20 μl/testis) was injected intratesticularly to unstressed and stressed rats 12 h before the IMO session; animals were euthanized at the end of the IMO session, blood was collected, and Leydig cells were prepared (for details, see materials and methods). A–D, left: in unstressed animals, RU-486 alone did not affect serum LH (A), but it decreased serum androgens (B), the mitochondrial membrane potential (C), and the rate of Leydig cell apoptosis (D). A–D, right: in stressed animals, RU-486 attenuated IMO-induced inhibition of serum androgens (B) without affecting serum LH levels (A). It also inhibited IMO-induced decrease in mitochondrial membrane potential (C) and reduced the percentage of apoptotic cells (D). Data shown are means ± SE with 4 animals/group. Statistical significance at level P < 0.05: *vs. untreated group; ^vs. IMO1 group; #vs. corresponding IMO group. E: RU-486 inhibited the IMO-induced caspase-9 expression. Bottom: representative blots for caspase-9 and ACTB. Top: mean ± SE values from 3 experiments. Statistical significance at level P < 0.05: *vs. vehicle. F: RU-486 abolished the IMO10-induced expression of Hsd11b2 gene in Leydig cells. Bars illustrate mean ± SE values from 3 experiments. *P < 0.05 vs. vehicle.
Role of testicular α1-ADRs in Leydig cell functional recovery.
To study the potential involvement of adrenergic components of the stress response in Leydig cell apoptosis, the intratesticular treatment with prazosin (7.5 μg in 20 μl/testis), the potent blocker of α1-ADRs, was performed 30 min before IMO stress. Such a treatment did not change circulating LH levels in controls and IMO rats (Fig. 5A). However, intratesticular application of prazosin two and 10 times decreased circulating androgen levels significantly in unstressed rats (Fig. 5B, left). In stressed rats, prazosin did not alter the IMO1/IMO2-induced drop in androgenesis but protected the partial recovery of androgen secretion in IMO10 rats (Fig. 5B, right). It also prolonged the duration of reduced mitochondrial membrane potential compared with values registered in Leydig cells from IMO10 rats (Fig. 5C). Furthermore, blockade of testicular α1-ADRs prolonged the duration of increased apoptotic rate in stressed animals and increased Leydig cell apoptosis significantly in unstressed animals compared with control/vehicle groups not treated with prazosin (Fig. 5D).
Fig. 5.
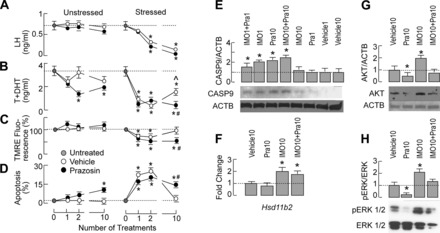
Time-dependent effects of prazosin (Pra), an α1-adrenergic receptor antagonist, on hormonal profile and apoptotic signaling in unstressed and stressed animals. Pra (7.5 μg in 20 μl/testis) or vehicle (20 μl/testis) was injected intratesticularly to unstressed and stressed rats 30 min before the stress session; animals were euthanized at the end of the stress session, blood was collected, and Leydig cells were prepared (for details, see materials and methods). A–D, left: in unstressed animals, Pra decreased serum androgen levels (B) without affecting serum LH levels (A). It also increased the percentage of apoptotic cells during repetitive application (D) without affecting mitochondrial membrane potential (C). A–D, right: in stressed animals, Pra did not affect LH levels (A) or the IMO1- and IMO2-induced drop in androgenesis, but it protected the partial recovery of androgenesis in IMO10 rats (B). It also protected recovery of mitochondrial membrane potential (C) and sustained apoptosis (D) in IMO10 rats. E: Pra alone stimulated CASP9 expression and enhanced IMO10-induced expression of this enzyme. Bottom: representative blots for CASP9 and ACTB. Top: mean ± SE values from 3 experiments. *P < 0.05 vs. vehicle; #P < 0.05 vs. corresponding IMO group. F: Pra did not affect the expression of the Hsd11b2 gene in Leydig cells. G and H: the level of Akt (G) and p-ERK/ERK ratio (H) in Leydig cells. Representative blots are shown at bottom, and pooled data from scanning densitometry normalized on ACTB or ERK values are shown as means ± SE for 3 independent experiments. Data bars are means ± SE values of 3 independent experiments. Statistical significance at level P < 0.05: *vs. control group. TMRE, tetramethylrhodamine ethylester.
Application of prazosin 10 times in controls significantly increased the level of caspase-9 in Leydig cells compared with untreated/vehicle-treated groups. In addition, prazosin increased caspase-9 expression in Leydig cells from IMO10 rats (Fig. 5E). Blockade of intratesticular α1-ADRs did not change the IMO10-induced Hsd11b2 increase (Fig. 5F), confirming that this transcription reflects activation of GRs (18). It also did not abolish the increased levels of transcripts for ADRs in Leydig cells from IMO10 rats (data not shown). Finally, the α1-ADR blockade diminished IMO10-induced stimulation of the expression of so-called recovery kinases by increasing Akt and p-ERK/ERK ratio in Leydig cells, whereas 10 injections of prazosin decreased these ratios significantly compared with control/vehicle-treated groups (Fig. 5, G and H).
DISCUSSION
It is well established that LH is the main factor regulating the biosynthesis and secretion of T (16). Earlier studies showed that sustained stress lowers circulating LH and androgen levels (13, 40, 42), a finding consistent with data presented in this study. In further agreements with the literature (9, 42), we also observed that acute stress lowers T levels without changing LH secretion. In addition, partial recovery of androgenesis occurs at lower serum LH levels. Along with androgens, Insl3 transcription was also attenuated after IMO1 and IMO2 but was normalized after IMO10. INSL3 is a major secretory product of testicular Leydig cells, and although released in a constitutive manner, it is considered in pair with androgens in the evaluation of testicular function and dysfunction (5). These observations clearly indicate that the residual circulating LH is sufficient for Leydig cell steroidogenesis and that other factors cause down- and upregulation of Leydig cell secretion.
Because the levels of T in circulation reflect the steroidogenic capacity of individual testicular Leydig cells and the total number of these cells per testis, it was reasonable to suggest that stress decreases the number of responsive cells, leading to decrease in the secretory output (20). Negative correlation between the numbers of apoptotic Leydig cells vs. serum androgen concentration and expression of Insl3 shown here supports this hypothesis. Furthermore, there is a general agreement in the field that stress causes Leydig cell apoptosis in a CORT-dependent manner, which in turn affects a balance between proliferation and cell death. Here, we show that IMO stress causes rapid and sustained increases in circulating CORT levels. Finally, our study with blockade of testicular GRs confirmed that IMO-elicited CORT is sufficient to trigger apoptosis of Leydig cells.
It has been suggested that glucocorticoid-induced Leydig cell apoptosis is mediated by GRs, which translocate from cytoplasm to nucleus (11). In addition to the nuclear mechanisms of glucocorticoid action, these receptors could directly or indirectly affect the mitochondrial function (41). In several cell lines, GRs translocation to the mitochondria was observed in correlation with susceptibility to glucocorticoid-induced apoptosis via the ligand-induced mitochondrial pathway rather than nuclear translocation of the GRs. The same study also showed that mitochondrial GRs control respiration and oxidative phosphorylation through transcriptional regulation of the mitochondrial genes cytochrome oxidase 1 and cytochrome oxidase 3 (51). Both are catalytic subunits of cytochrome c oxidase, the last enzyme in the respiratory electron transport chain of mitochondria (38, 49).
In parallel with serum androgen profiles, we show here that stress also transiently affected the Leydig cell mitochondrial function, including lowering of basal oxygen consumption and mitochondrial membrane potential, formation of transition pores and cytochrome c liking from mitochondria, and activation of caspase-9 and -3 pathways, leading to induction of apoptosis in IMO1 and IMO2 rats. Caspase-3 activation is also required for Leydig cell apoptosis induced by ethane dimethanesulfonate (31). Appearance of proapoptotic markers in Leydig cell IMO1 rats points to the central role of mitochondria in IMO-triggered cell death. In general, the mitochondria enclose a potent cocktail of proapoptotic proteins. The reduction in mitochondrial membrane potential and leakage of cytochrome c from them that we observed in Leydig cells from IMO1 and IMO2 rats have also been observed in a number of models of apoptosis and are considered to be the first signs of initiation of the mitochondrial death pathway (4, 25, 55). Thus, the presence of proapoptotic markers in Leydig cells from IMO1/IMO2 rats fits into this scenario.
The most important finding present in this study is that duration of proapoptotic signaling is limited and that after 10 IMO sessions the ratio in normal/apoptotic cells was normalized at elevated serum CORT levels. This clearly indicates the existence of an adaptive mechanism to repetitive stress response. In general, downregulation of GR gene activity and protein expression or facilitated degradation of CORT in Leydig cells could provide such a mechanism. However, our earlier quantitative RT-PCR analysis showed no changes in mRNA transcripts for GRs (52), arguing against the first hypothesis. The occupancy of GRs in Leydig cells is modulated by 11β-hydroxysteroid dehydrogenase (HSD11B)-mediated oxidative inactivation of CORT. Leydig cells express two types of this enzyme: HSD11B1, a low-affinity and high-capacity bidirectional oxidoreductase with the oxidative activity prevailed over reduction, and HSD11B2, an unidirectional oxidase exhibiting high affinity in inactivation of glucocorticoids (24, 27). It has been shown previously that the single and repeated IMO stress increased the level of transcripts for Hsd11b2 but did not change transcripts for Hsd11b1 in Leydig cells (24, 52). Here, we show that the stress-induced upregulation of Hsd11b2 transcription was abolished in Leydig cells from stressed animals with blocked testicular GRs, indicating that CORT could regulate its local concentration by facilitating the expression of this enzyme. Further studies are needed to clarify whether the expression of HSD11B2 protein is enhanced at this time point, what the level of CORT in Leydig cells from stressed animals is, and whether HSD11B is a significant determinant of CORT steroidogenic and apoptotic capacities.
In addition to glucocorticosteroids, catecholamines are elicited by stress and play a central role in the control of metabolic function and energy homeostasis. Specifically, adrenaline and noradrenaline stimulate lipolysis and glycogenolysis by interacting with β-ADRs coupled to the Gs signaling pathway, leading to facilitation of cAMP production (41). Leydig cells also express β-ADRs (3), and the activated receptors stimulate cAMP and androgen production in rats (2). Thus, elevated serum adrenaline should compensate for lower LH level in stimulating cAMP de novo production and androgenesis. However, stress causes a decrease rather than an increase in cAMP production (15, 52). Inhibition of cAMP production in stressed animals could reflect an action mediated by plasma membrane GRs signaling through the Gi/o pathway (15) and/or a switch from Gs to Gi/o signaling of β-ADRs mediated by phosphodiesterase-4 (52). Our previous study also showed a clear dissociation between cAMP and androgen levels in adrenaline-stimulated Leydig cells (52), indicating that this stress hormone influences androgenesis through other signaling pathways. In accord with this, daily injection of propranolol, a β-ADR antagonist, did not affect recovery of androgenesis in IMO10 rats (Andric SA, Bjelic MM, Mihajlovic AI, Baburski AZ, Sokanovic SJ, Janjic MM, Stojkov NJ, and Kostic TS, unpublished observations).
Leydig cells also express all three subtypes of α1-ADRs, and IMO stress increases the expression of their mRNAs after IMO10 sessions (52). This provides a rationale for delayed action of these receptors through the Gq/11 signaling pathway, leading to activation of phospholipase C and activation of ERK1/2 (6, 56). Here, we also show that blockade of testicular α1-ADRs initiated Leydig cell apoptosis and canceled development of apoptosis resistance in Leydig cells from IMO10 animals. This was associated with a low level of total Akt and p-ERK1/2 in Leydig cells. Furthermore, sustained IMO repetition normalized Leydig cell respiration and mitochondrial membrane potential, increased ERK1/2, total Akt, and p-Akt/Akt ratio in mitochondria, and stimulated cytochrome c expression, probably accounting for Leydig cell adaptation to IMO.
Blockade of α1-ADR with prazosin or doxazosin in other cell types also introduced apoptosis (28, 39). Previous reports also suggest that α1-ADR survival signaling could be mediated by a mechanism downstream of ERK1/2, including the phosphorylation and inactivation of the Bad (54). These observations suggest that the phosphatidylinositol 3-kinase/Akt and ERK1/2 pathways might be activated in connection with α1-ADR signaling in Leydig cells from stressed animals. In support of this hypothesis, there are numerous data indicating an antiapoptotic role of the phosphatidylinositol 3-kinase and Akt pathways in preservation of mitochondria function in different testicular and other cell models (26, 30, 37). Also, in denervated testes the rate of apoptosis increased 14 and 21 days after operation, suggesting that this pathway may exert the antiapoptotic action in normal physiological conditions (22).
In conclusion, the results obtained in this study supported the opposite role of GRs and α1-ADRs in crosstalk with mitochondrial Leydig cell death pathways triggered by IMO stress and might provide new insight into the relationship between stress and the mammalian reproductive function.
GRANTS
This work was supported by the Autonomic Province of Vojvodina Grant (no. 2570), the Serbian Ministry of Science and Technological Development Grant (no. 173057), and the Intramural Research Program of the National Institute of Child Health and Human Development.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.A.A., S.S.S., and T.S.K. did the conception and design of the research; S.A.A., Z.K., M.M.B., A.I.M., A.Z.B., S.J.S., M.M.J., N.J.S., and T.S.K. performed the experiments; S.A.A., Z.K., M.M.B., A.I.M., A.Z.B., S.J.S., M.M.J., N.J.S., S.S.S., and T.S.K. analyzed the data; S.A.A., S.S.S., and T.S.K. interpreted results of experiments; S.A.A., S.S.S., and T.S.K. prepared the figures; S.A.A., S.S.S., and T.S.K. drafted the manuscript; S.A.A., Z.K., M.M.B., A.I.M., A.Z.B., S.J.S., M.M.J., N.J.S., S.S.S., and T.S.K. edited and revised the manuscript; S.A.A., Z.K., M.M.B., A.I.M., A.Z.B., S.J.S., M.M.J., N.J.S., S.S.S., and T.S.K. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We are thankful to Gordon Niswender (Colorado State University) for supplying antibodies for RIAs.
REFERENCES
- 1. Allen JA, Shankara T, Janus P, Buck S, Diemer T, Hales KH, Hales DB. Energized, polarized, and actively respiring mitochondria are required for acute Leydig cell steroidogenesis. Endocrinology 147: 3924– 3935, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Anakwe OO, Moger WH. Catecholamine stimulation of androgen production by rat Leydig cells. Interactions with luteinizing hormone and luteinizing hormone-releasing hormone. Biol Reprod 35: 806– 814, 1986 [DOI] [PubMed] [Google Scholar]
- 3. Anakwe OO, Murphy PR, Moger WH. Characterization of beta-adrenergic binding sites on rodent Leydig cells. Biol Reprod 33: 815– 826, 1985 [DOI] [PubMed] [Google Scholar]
- 4. Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron 15: 961– 973, 1995 [DOI] [PubMed] [Google Scholar]
- 5. Bay K, Andersson AM. Human testicular insulin-like factor 3: in relation to development, reproductive hormones and andrological disorders. Int J Androl 34: 97– 109, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Bruchas MR, Toews ML, Bockman CS, Abel PW. Characterization of the alpha1-adrenoceptor subtype activating extracellular signal-regulated kinase in submandibular gland acinar cells. Eur J Pharmacol 578: 349– 358, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cadepond F, Ulmann A, Baulieu EE. RU486 (mifepristone): mechanisms of action and clinical uses. Annu Rev Med 48: 129– 156, 1997 [DOI] [PubMed] [Google Scholar]
- 8. Campeau S, Nyhuis TJ, Kryskow EM, Masini CV, Babb JA, Sasse SK, Greenwood BN, Fleshner M, Day HE. Stress rapidly increases alpha 1d adrenergic receptor mRNA in the rat dentate gyrus. Brain Res 1323: 109– 118, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Charpenet G, Tache Y, Forest MG, Haour F, Saez JM, Bernier M, Ducharme JR, Collu R. Effects of chronic intermittent immobilization stress on rat testicular androgenic function. Endocrinology 109: 1254– 1258, 1981 [DOI] [PubMed] [Google Scholar]
- 10. Chen H, Ge RS, Zirkin BR. Leydig cells: From stem cells to aging. Mol Cell Endocrinol 306: 9– 16, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen Y, Wang Q, Wang FF, Gao HB, Zhang P. Stress induces glucocorticoid-mediated apoptosis of rat Leydig cells in vivo. Stress 15: 74– 84, 2012 [DOI] [PubMed] [Google Scholar]
- 12. Cross TG, Scheel-Toellner D, Henriquez NV, Deacon E, Salmon M, Lord JM. Serine/threonine protein kinases and apoptosis. Exp Cell Res 256: 34– 41, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Demura R, Suzuki T, Nakamura S, Komatsu H, Odagiri E, Demura H. Effect of immobilization stress on testosterone and inhibin in male rats. J Androl 10: 210– 213, 1989 [DOI] [PubMed] [Google Scholar]
- 14. Desiniotis A, Kyprianou N. Advances in the design and synthesis of prazosin derivatives over the last ten years. Expert Opin Ther Targets 15: 1405– 1418, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dong Q, Salva A, Sottas CM, Niu E, Holmes M, Hardy MP. Rapid glucocorticoid mediation of suppressed testosterone biosynthesis in male mice subjected to immobilization stress. J Androl 25: 973– 981, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Dufau ML, Winters CA, Hattori M, Aquilano D, Baranao JL, Nozu K, Baukal A, Catt KJ. Hormonal regulation of androgen production by the Leydig cell. J Steroid Biochem 20: 161– 173, 1984 [DOI] [PubMed] [Google Scholar]
- 17. Flugge G, Van Kampen M, Mijnster MJ. Perturbations in brain monoamine systems during stress. Cell Tissue Res 315: 1– 14, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Gao HB, Ge RS, Lakshmi V, Marandici A, Hardy MP. Hormonal regulation of oxidative and reductive activities of 11 beta-hydroxysteroid dehydrogenase in rat Leydig cells. Endocrinology 138: 156– 161, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Gao HB, Shan LX, Monder C, Hardy MP. Suppression of endogenous corticosterone levels in vivo increases the steroidogenic capacity of purified rat Leydig cells in vitro. Endocrinology 137: 1714– 1718, 1996 [DOI] [PubMed] [Google Scholar]
- 20. Gao HB, Tong MH, Hu YQ, Guo QS, Ge R, Hardy MP. Glucocorticoid induces apoptosis in rat leydig cells. Endocrinology 143: 130– 138, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Ge RS, Gao HB, Nacharaju VL, Gunsalus GL, Hardy MP. Identification of a kinetically distinct activity of 11beta-hydroxysteroid dehydrogenase in rat Leydig cells. Endocrinology 138: 2435– 2442, 1997 [DOI] [PubMed] [Google Scholar]
- 22. Gong YG, Wang YQ, Gu M, Feng MM, Zhang W, Ge RS. Deprival of testicular innervation induces apoptosis of Leydig cells via caspase-8-dependent signaling: a novel survival pathway revealed. Biochem Biophys Res Commun 382: 165– 170, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Habert R, Lejeune H, Saez JM. Origin, differentiation and regulation of fetal and adult Leydig cells. Mol Cell Endocrinol 179: 47– 74, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Hardy MP, Gao HB, Dong Q, Ge R, Wang Q, Chai WR, Feng X, Sottas C. Stress hormone and male reproductive function. Cell Tissue Res 322: 147– 153, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Heiskanen KM, Bhat MB, Wang HW, Ma J, Nieminen AL. Mitochondrial depolarization accompanies cytochrome c release during apoptosis in PC6 cells. J Biol Chem 274: 5654– 5658, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Hixon ML, Boekelheide K. Expression and localization of total Akt1 and phosphorylated Akt1 in the rat seminiferous epithelium. J Androl 24: 891– 898, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Hu GX, Lian QQ, Lin H, Latif SA, Morris DJ, Hardy MP, Ge RS. Rapid mechanisms of glucocorticoid signaling in the Leydig cell. Steroids 73: 1018– 1024, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hui H, Fernando MA, Heaney AP. The alpha1-adrenergic receptor antagonist doxazosin inhibits EGFR and NF-kappaB signalling to induce breast cancer cell apoptosis. Eur J Cancer 44: 160– 166, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Hyman BT, Yuan J. Apoptotic and non-apoptotic roles of caspases in neuronal physiology and pathophysiology. Nat Rev Neurosci 13: 395– 406, 2012 [DOI] [PubMed] [Google Scholar]
- 30. Khan SA, Ndjountche L, Pratchard L, Spicer LJ, Davis JS. Follicle-stimulating hormone amplifies insulin-like growth factor I-mediated activation of AKT/protein kinase B signaling in immature rat Sertoli cells. Endocrinology 143: 2259– 2267, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Kim JM, Luo L, Zirkin BR. Caspase-3 activation is required for Leydig cell apoptosis induced by ethane dimethanesulfonate. Endocrinology 141: 1846– 1853, 2000 [DOI] [PubMed] [Google Scholar]
- 32. King KL, Cidlowski JA. Cell cycle regulation and apoptosis. Annu Rev Physiol 60: 601– 617, 1998 [DOI] [PubMed] [Google Scholar]
- 33. Kojic Z, Scepanovic LJ, Kostic T. Immobilization stress reduces oxygen consumption of the isolated interstitial rats' testes cells. Acta Physiol Hung 98: 45– 50, 2011 [DOI] [PubMed] [Google Scholar]
- 34. Kostic TS, Stojkov NJ, Janjic MM, Andric SA. Structural complexity of the testis and PKG I / StAR interaction regulate the Leydig cell adaptive response to repeated immobilization stress. Int J Androl 33: 717– 729, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Kostic TS, Stojkov NJ, Janjic MM, Maric D, Andric SA. The adaptive response of adult rat Leydig cells to repeated immobilization stress: the role of protein kinase A and steroidogenic acute regulatory protein. Stress 11: 370– 380, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Kvetnansky R, Weise VK, Kopin IJ. Elevation of adrenal tyrosine hydroxylase and phenylethanolamine-N-methyl transferase by repeated immobilization of rats. Endocrinology 87: 744– 749, 1970 [DOI] [PubMed] [Google Scholar]
- 37. Lee J, Kanatsu-Shinohara M, Inoue K, Ogonuki N, Miki H, Toyokuni S, Kimura T, Nakano T, Ogura A, Shinohara T. Akt mediates self-renewal division of mouse spermatogonial stem cells. Development 134: 1853– 1859, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Liang HL, Ongwijitwat S, Wong-Riley MT. Bigenomic functional regulation of all 13 cytochrome c oxidase subunit transcripts in rat neurons in vitro and in vivo. Neuroscience 140: 177– 190, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Liao CH, Guh JH, Chueh SC, Yu HJ. Anti-angiogenic effects and mechanism of prazosin. Prostate 71: 976– 984, 2011 [DOI] [PubMed] [Google Scholar]
- 40. Lopez-Calderon A, Ariznavarreta C, Gonzalez-Quijano MI, Tresguerres JA, Calderon MD. Stress induced changes in testis function. J Steroid Biochem Mol Biol 40: 473– 479, 1991 [DOI] [PubMed] [Google Scholar]
- 41. Manoli I, Alesci S, Blackman MR, Su YA, Rennert OM, Chrousos GP. Mitochondria as key components of the stress response. Trends Endocrinol Metab 18: 190– 198, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Maric D, Kostic T, Kovacevic R. Effects of acute and chronic immobilization stress on rat Leydig cell steroidogenesis. J Steroid Biochem Mol Biol 58: 351– 355, 1996 [DOI] [PubMed] [Google Scholar]
- 43. Mayerhofer A, Bartke A, Began T. Catecholamines stimulate testicular steroidogenesis in vitro in the Siberian hamster, Phodopus sungorus. Biol Reprod 48: 883– 888, 1993 [DOI] [PubMed] [Google Scholar]
- 44. Mayerhofer A, Bartke A, Steger RW. Catecholamine effects on testicular testosterone production in the gonadally active and the gonadally regressed adult golden hamster. Biol Reprod 40: 752– 761, 1989 [DOI] [PubMed] [Google Scholar]
- 45. Monder C, Sakai RR, Miroff Y, Blanchard DC, Blanchard RJ. Reciprocal changes in plasma corticosterone and testosterone in stressed male rats maintained in a visible burrow system: evidence for a mediating role of testicular 11 beta-hydroxysteroid dehydrogenase. Endocrinology 134: 1193– 1198, 1994 [DOI] [PubMed] [Google Scholar]
- 46. Orr TE, Mann DR. Role of glucocorticoids in the stress-induced suppression of testicular steroidogenesis in adult male rats. Horm Behav 26: 350– 363, 1992 [DOI] [PubMed] [Google Scholar]
- 47. Orr TE, Taylor MF, Bhattacharyya AK, Collins DC, Mann DR. Acute immobilization stress disrupts testicular steroidogenesis in adult male rats by inhibiting the activities of 17 alpha-hydroxylase and 17,20-lyase without affecting the binding of LH/hCG receptors. J Androl 15: 302– 308, 1994 [PubMed] [Google Scholar]
- 48. Payne AH, Sha LL. Multiple mechanisms for regulation of 3 beta-hydroxysteroid dehydrogenase/delta 5—-delta 4-isomerase, 17 alpha-hydroxylase/C17–20 lyase cytochrome P450, and cholesterol side-chain cleavage cytochrome P450 messenger ribonucleic acid levels in primary cultures of mouse Leydig cells. Endocrinology 129: 1429– 1435, 1991 [DOI] [PubMed] [Google Scholar]
- 49. Psarra AM, Sekeris CE. Glucocorticoids induce mitochondrial gene transcription in HepG2 cells: role of the mitochondrial glucocorticoid receptor. Biochim Biophys Acta 1813: 1814– 1821, 2011 [DOI] [PubMed] [Google Scholar]
- 50. Reubold TF, Eschenburg S. A molecular view on signal transduction by the apoptosome. Cell Signal 24: 1420– 1425, 2012 [DOI] [PubMed] [Google Scholar]
- 51. Sionov RV, Cohen O, Kfir S, Zilberman Y, Yefenof E. Role of mitochondrial glucocorticoid receptor in glucocorticoid-induced apoptosis. J Exp Med 203: 189– 201, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stojkov NJ, Janjic MM, Bjelic MM, Mihajlovic AI, Kostic TS, Andric SA. Repeated immobilization stress disturbed steroidogenic machinery and stimulated the expression of cAMP signaling elements and adrenergic receptors in Leydig cells. Am J Physiol Endocrinol Metab 302: E1239– E1251, 2012 [DOI] [PubMed] [Google Scholar]
- 53. Tucci P, Cione E, Perri M, Genchi G. All-trans-retinoic acid induces apoptosis in Leydig cells via activation of the mitochondrial death pathway and antioxidant enzyme regulation. J Bioenerg Biomembr 40: 315– 323, 2008 [DOI] [PubMed] [Google Scholar]
- 54. Valks DM, Cook SA, Pham FH, Morrison PR, Clerk A, Sugden PH. Phenylephrine promotes phosphorylation of Bad in cardiac myocytes through the extracellular signal-regulated kinases 1/2 and protein kinase A. J Mol Cell Cardiol 34: 749– 763, 2002 [DOI] [PubMed] [Google Scholar]
- 55. Wadia JS, Chalmers-Redman RM, Ju WJ, Carlile GW, Phillips JL, Fraser AD, Tatton WG. Mitochondrial membrane potential and nuclear changes in apoptosis caused by serum and nerve growth factor withdrawal: time course and modification by (−)-deprenyl. J Neurosci 18: 932– 947, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xiao L, Pimental DR, Amin JK, Singh K, Sawyer DB, Colucci WS. MEK1/2-ERK1/2 mediates alpha1-adrenergic receptor-stimulated hypertrophy in adult rat ventricular myocytes. J Mol Cell Cardiol 33: 779– 787, 2001 [DOI] [PubMed] [Google Scholar]


