Abstract
Graft-versus-host disease (GVHD) is the immune response of donor T lymphocytes responding to the recipient’s alloantigens. The cellular and cytokine mechanisms driving GVHD are now well defined and have led to several prophylactic approaches. Selective allodepletion techniques promise to prevent GVHD without causing immune deficiency provoked by global T-cell depletion. Targeted dosing other (non-T-cells) cells in the graft – such as CD34+ progenitors, regulatory T cells, natural killer cells and mesenchymal stromal cells – can also lead to transplants designed to retain immune capability without causing GVHD. Immunosuppressive drugs such as methotrexate, cyclosporine and anti-lymphocyte antibodies are the mainstay in the prevention of GVHD and can be used in conjunction with engineered grafts to eliminate GVHD. In future it is anticipated that further refinements in targeting the elimination or suppression of GVHD reacting T cells should be selective enough to preserve the important graft-versus-leukemia effect which contributes to the cure of malignant diseases by allogeneic stem-cell transplantation.
Keywords: selective T-cell depletion, regulatory T cells, natural killer cells, mesenchymal stromal cells, calcineurin inhibitors, graft-versus-leukemia
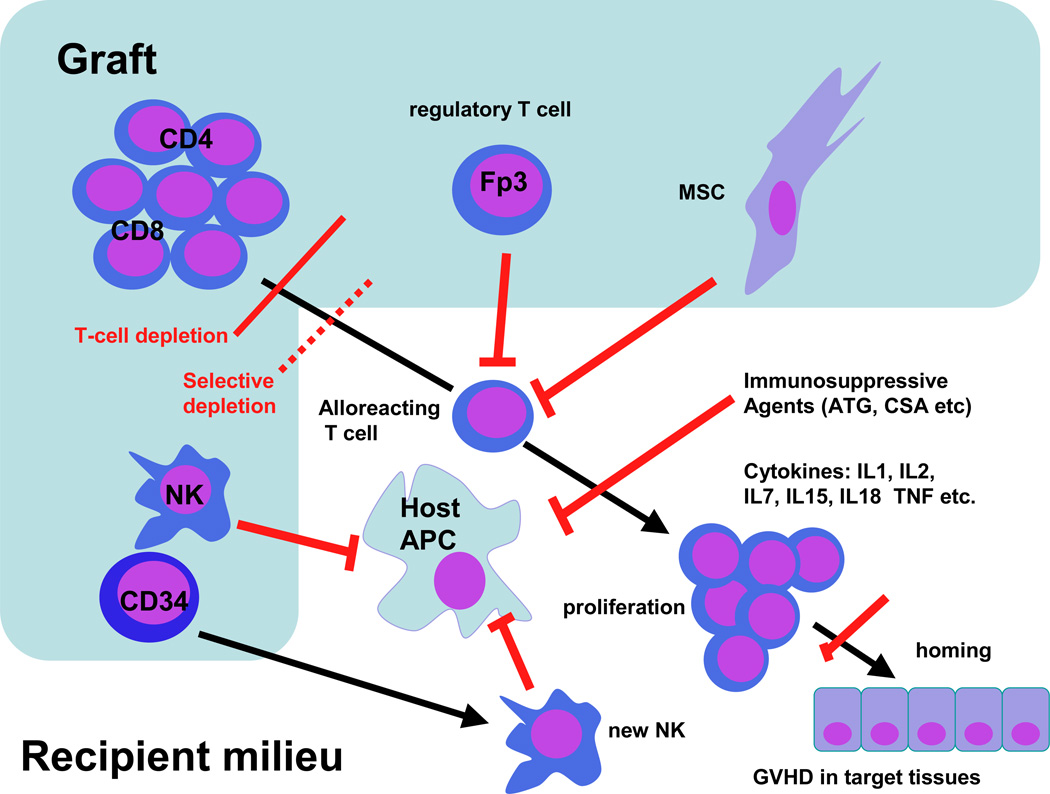
While the classic rules of the conditions necessary for GVHD development proposed by Billingham remain as valid today as they did when he proposed them, we have since greatly refined our understanding of the positive and negative factors influencing the development of GVHD.1 Figure 1 summarizes factors regulating the development of acute GVHD. This schema identifies key events leading to GVHD which are susceptible to therapeutic intervention to prevent GVHD. GVHD development can be interrupted at two distinct stages: (1) selection of the cells to be engrafted, and (2) modulation of the post-transplant milieu.
Figure 1.
Immune pathways in graft-versus-host disease (GVHD) and sites where therapy is used to block GVHD development (red bars). APC, antigen-presenting cell; MSC, mesenchymal stromal cell; ATG, antithymocyte globulin; CSA, cyclosporine A; IL1, IL2 etc, interleukin 1, 2 etc; TNF, tumor necrosis factor; NK, natural killer cell.
The cellular contents of the graft include T, B and NK lymphocytes, CD34 cells, and, in bone-marrow transplants, non-hematopoietic cells, including mesenchymal stromal cells (MSCs). Since they are central to the GVHD alloresponse, the predominant cells determining GVHD are T lymphocytes, although – with the exception of B lymphocytes – all these cell types can influence GVHD. On engraftment, donor T cells encounter recipient antigen-presenting cells (APCs) and respond to alloantigens, viral antigens and tumor antigens, leading in the case of alloantigenic stimuli to clinically significant GVHD. Lymphocytes activated by APCs home to target organs under the control of specific selectin-receptor binding. Lymphocytes (both T and NK cells) are subjected to powerful activating stimuli from cytokines released following lympho- and myeloablative preparative regimens as well as proliferative stimuli from lymphopoietic cytokines released in response to lymphopenia. In this chapter we will focus on practical ways currently in use or in development to prevent GVHD by modifying the graft contents and by modulating T-cell recovery after stem-cell transplant (SCT).
TRANSPLANTED CELLS AND GVHD
Manipulation of T lymphocytes
Depending on the degree of histocompatibility disparity, it is estimated that between about 1/100,000 and 1/1000 donor helper CD4 T cells and similar frequencies of CD8 T cells can clonally expand in the recipient and cause GVHD alloreactions.2 Two strategies have been used to modify this T-cell-mediated GVHD: T-cell depletion, and selective removal of functional T-cell subsets.
T cell depletion has been used to prevent GVHD for over 25 years.3 Physical separation techniques can achieve >4-log depletion of T cells such that as few as 103 CD3 cells/kg remain in the stem-cell product. Antibody-based depletion ‘in the bag’ also causes profound depletion of T cells, but the technique used makes it impossible to quantify the actual dose of functional T cells delivered. At NIH we explored several approaches to T-cell depletion in protocols delivering targeted T-cell doses that have ranged from 5 × 105 CD3/kg to 2 × 104 CD3/kg. These approaches resulted in a greatly reduced incidence and severity of acute GVHD (aGVHD), but it is important to point out that even doses as low as 104 CD3/kg are associated with a small risk of aGVHD.4 It is generally accepted that T-cell depletion significantly reduces both the risk and the severity of aGVHD. However, the procedure increases the risk of leukemic relapse, graft failure, and in some studies mortality from infection. Nevertheless, partial T-cell depletion may avoid the need for immunosuppression, thus conserving the graft-versus-leukemia (GVL) effect. Elhasid et al used a partial T-cell depletion and no post-transplantation GVHD prophylaxis to treat 16 patients receiving a myeloablative SCT from matched related donors; all patients survived at a median of 3 years post-transplantation, and only one developed chronic GVHD.5 An alternative means of conserving GVL effects is to follow a T-cell-depleted SCT with a T-cell add-back. It is generally clear that T-cell doses of 106–107 /kg given 2–3 months after transplant confer a low risk of severe of GVHD. Some studies report favorably reduced GVHD and outcomes comparable to those of unmanipulated SCT.4,6
Selective depletion of functional T-cell subsets
Many investigators have sought to prevent GVHD while permitting immune reconstitution by selective removal of alloreacting lymphocytes, and various approaches are under evaluation or in development (see below).
CD8 depletion
Two groups report some benefit in reduced GVHD after CD8 depletion. The Munich group added back CD8-depleted T cells, after day 60, to 11 reduced-intensity transplant recipients effectively T-cell-depleted by administration of the CD52 monoclonal alemtuzemab. These prophylactic CD8-depleted donor leukocyte infusions (DLIs) accelerated CD4-T-cell immune reconstitution – e.g. to cytomegalovirus (CMV) – with only a low risk of inducing severe GVHD.7,8
Bias to Th2 subsets
Fowler and colleagues demonstrated in murine experiments that the T-cytotoxic-2 (Tc2) and T-helper-2 (Th2) lymphocyte subsets supported engraftment with minimal GVHD in murine models. Subsequently, by adjusting the culture conditions, they were able to generate T-cell add-backs to the transplant that were predominantly Tc2 Th2. More recently they showed that addition of sirolimus to lymphocyte cultures achieves the same skewing to the Th2 Tc2 phenotype. Ongoing studies with this improved selection approach are promising.9
Selective allodepletion
The selective removal of the T cells responsible for mediating GVHD while conserving cells mediating GVL and antimicrobial immune responses has been a long-term goal of allogeneic SCT. By eliminating the need for immunosuppressive agents, such a strategy could be used to enhance GVL and prevent GVHD by permitting the safe transfusion of large numbers of GVL-reactive, GVHD-non-reactive lymphocytes. We and others have succeeded at separating GVHD from GVL effects in vitro by co-incubating donor lymphocytes with allogeneic stimulator cells.10 Under these conditions, alloreactive donor cells can be selectively identified by their surface phenotype (e.g. CD25, CD69) proliferative potential, or preferential retention of photoactive dyes, and can be subsequently targeted for elimination using an immunotoxin, immunomagnetic bead separation, fluorescence-activated cell sorting, or photodynamic purging. Using standard proliferation assays, such as the mixed lymphocyte reaction (MLR) and the helper T-lymphocyte precursor (HTLp) frequency assay, alloreactivity can be substantially depleted in both the HLA-mismatched and HLA-matched setting, while third-party responses are maintained. Responder cells obtained after allodepletion also maintain antitumor and antiviral activity. This promising approach has been validated in animal models in a number of centers, and three clinical trials using a CD25 immunotoxin to selectively deplete alloreactivity (two in haploidentical peripheral-blood stem-cell transplantations in pediatric patients, and one in adults with hematological malignancies in HLA-identical sibling transplants) indicate that the concept is feasible, reducing GVHD while promoting rapid immune reconstitution.11–13 We used a photodepletion (PD) technique which works by preferential killing of allo-activated cells which retain the photosensitive dye TH9402. To restrict the allostimulation and conserve GVL reactions, expanded T lymphocytes from the patients were co-cultured with the responder cells. In mismatched stimulator–responder pairs, the median reduction of allo-reactivity was 500-fold when compared with the unmanipulated responder, while third-party responses were maintained. In matched pairs, alloreactive helper T-lymphocyte precursors (HTLp) were reduced below 1/100,000, while third-party responses, CMV-specific T cells and responses to staphylococcal enterotoxin B (SEB) were preserved. This technique is now being evaluated in a clinical trial.14
Suicide gene insertion
The principle of this approach is to transplant engineered donor T cells expressing the suicide gene thymidine kinase of herpes simplex virus (TK). Severe GVHD reactions can then be aborted by administering the anti-HSV viral drug ganciclovir. The antitumor effect of donor lymphocytes infused after SCT can thus be preserved right up to the point where the risk from GVHD outweighs the benefit of the alloresponse. In a recent feasibility and safety study, 17 of 23 patients with high-risk or relapsed leukemia evaluable for engraftment and GVL had circulating TK(+) cells. There were six (35%) complete remissions and five (29%) partial responses. Seven patients required ganciclovir with elimination of TK(+) cells and control of GVHD. These promising results suggest that the approach is safe and effective in selectively preventing GVHD. Several phase-I/II studies using this technology have been completed and thus provide proof of principle.15,16
CD34 cell dose
The role of CD34 cells and the impact of the dose on SCT outcome are complicated by the fact that the higher CD34 cell doses are found in peripheral-blood stem-cell transplants, which also contain ten-fold greater lymphocyte numbers. Several reports link an increased risk of aGVHD with CD34 doses >10×106/kg, but notably the effect of higher CD34 dose on GVHD was not seen in T-cell-depleted SCT.17 As a source of newly generated NK cells, higher doses of CD34 cells could affect GVHD favorably through NK-mediated destruction of residual host APCs. In the absence of any clear data, it seems reasonable to deliver CD34 doses adequate for engraftment and optimal for outcome. This appears to be in the range 5–10 × 106/kg.18
Alloreacting NK cells
Unlike T lymphocytes, NK cells have a restricted interaction with hematopoietic lineages. As a consequence, alloreacting NK cells are not directly cytotoxic to the tissues normally involved in GVHD. Furthermore, because they can attack and destroy recipient hematopoietic cells they can improve engraftment, and by removing recipient APCs can actually reduce the chance of aGVHD. Velardi and colleagues in the Perugia SCT group have indeed demonstrated that in transplants between KIR-mismatched donor–recipient pairs the incidence of aGVHD is significantly reduced.19 Interestingly, a reduced incidence of aGVHD is also seen in HLA-identical SCT when NK recovery is prompt after SCT.20 These observations support the idea of using donor NK cells to prevent GVHD. Techniques to select alloreacting NK cells from donor apheresis are now available, but no studies using NK cells deliberately to prevent GVHD have yet been reported. It is now feasible to select circulating NK cells by apheresis of healthy donors for transfusion to recipients. Preliminary clinical trials suggest that these alloreactive NK cells have antitumor reactivity.21 Because NK cells are the first lymphocytes to recover after SCT, they may exert very early GVL effects. Little is known about the role of NK cells in HLA-matched SCT. We studied NK cells on day 30 post-transplant (NK30) in 54 SCT recipients with leukemia, and correlated NK recovery with donor and recipient killer immunoglobulin-like receptor (KIR) genotype. In multivariate analysis the NK30 emerged as the single independent determinant of transplant outcome. Patients with NK30 >150/µL had less relapse and less acute graft-versus-host disease, a lower transplant-related mortality and improved survival.20 These findings emphasize an important role for NK-cell function in the early post-graft period in HLA-identical SCT and indicate that the GVL effect might be enhanced by selecting donors with a favorable KIR genotype, and by optimizing CD34 and CD3 doses.
Mesenchymal stromal cells
In addition to hematopoietic stem cells (HSCs), bone marrow also contains MSCs capable of giving rise to adipose, bone, cartilage and other mesenchymal tissues. MSCs secrete cytokines that are important for supporting hematopoiesis. The stroma, damaged by chemo-irradiation therapy during SCT, reconstitutes slowly to provide the optimal environment for hematopoietic regeneration. Few donor MSCs are transferred during the allograft procedure, particularly when PBSCs are used as cell source. When infused together with HSCs, MSCs cultured ex vivo to achieve a several-fold expansion promote engraftment of HSCs in experimental animal models. The enhancing effect is most prominent when the dose of HSCs is limiting. Whether co-transplantation of MSCs and HSCs reduces cytopenia after SCT in humans is less clear.22–24 After haploidentical SCT, co-transplantation with MSCs enhanced NK-cell but not neutrophil or platelet recovery.25 In vitro, MSCs have anti-proliferative properties that target virtually all types of immune cells. Accordingly, human MSCs suppress lymphocyte alloreactivity when added in MLRs. After infusion to experimental animals, MSCs improve the outcome of renal, neural and lung injury and prolong survival of skin allografts. MSCs may also be immunosuppressive in vivo in humans as they have been reported to reverse severe steroid-resistant acute GVHD of the gut and liver.26,27 Co-infusion of MSCs reduced the incidence of graft failure and rejection in preliminary clinical trials.24,25 In a phase-I study, the incidence of acute GVHD grade II–IV was observed in 28% of patients co-administered with culture-expanded MSCs and HLA-identical sibling-matched HSCs.23 However, whether MSCs exert preventive effects on the development of GVHD as well as therapeutic effects in established GVHD needs to be evaluated in prospective clinical trials.
MANIPULATING THE POST-TRANSPLANT ENVIRONMENT
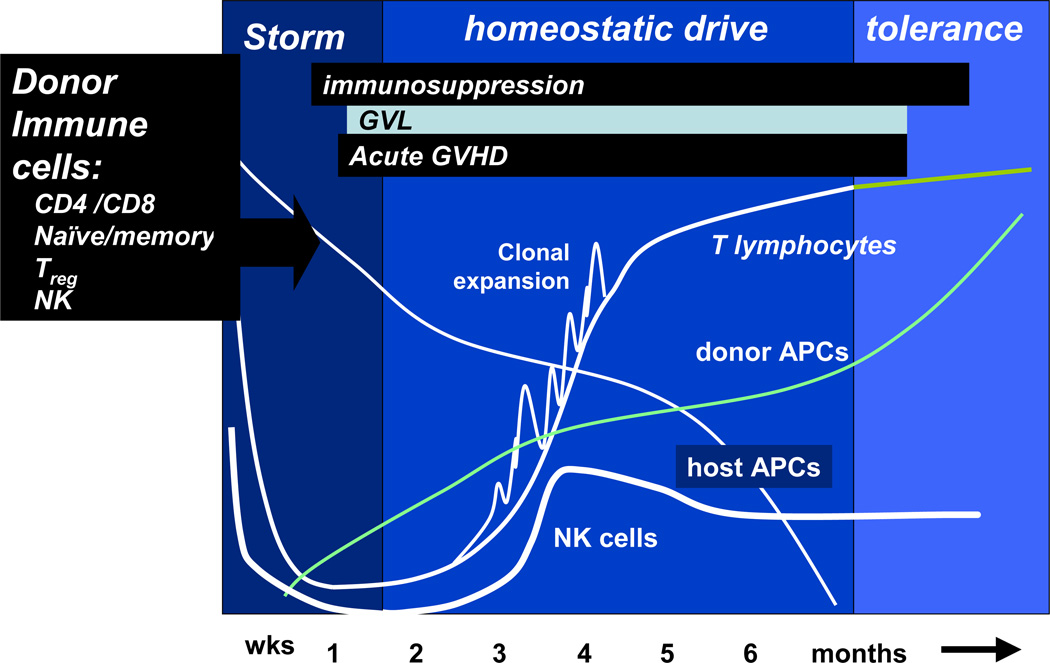
The post-transplant immune environment represents a unique period where GVHD is facilitated by the cytokine storm following the conditioning regimen, and then by a period of immune recovery driven by release of lymphocyte growth factors stimulating rapid proliferation of engrafted lymphocytes (the so-called homeostatic drive). At the same time persisting host APCs can efficiently present host alloantigens to the donor and trigger GVHD. Only much later, when a new repertoire of stem-cell-derived lymphocyte precursors becomes established by thymic processing, does a period of immune tolerance occur. The kinetics of early transplant immune recovery and the timing of aGVHD are illustrated in Figure 2.
Figure 2.
Early immune reconstitution following allogeneic stem-cell transplantation. GVL, graft-versus-leukemia effect; GVHD, graft-versus-host disease; APCs, antigen-presenting cells; NK cells, natural killer cells.
Immunosuppression
An immunosuppressive treatment is necessary post-SCT to prevent GVHD. Cyclosporine (CsA) and methotrexate (MTX) are equally effective in preventing GVHD, but a combination of the two significantly decreases the incidence and severity of both acute and chronic GVHD and improves survival.28,29 This therapy remains the conventional form of GVHD prevention both in HLA-identical as well as unrelated-donor transplants. Higher doses of CSA (3 mg/kg or 5 mg/kg) may be used in non-malignant disorders, but low-dose CSA (1 mg/kg) during the first 10 days post-transplant protects both adult and pediatric patients with leukemia from relapse.30,31 Tacrolimus (FK506), a calcineurin inhibitor like CSA, was used in combination with methotrexate and found to significantly reduce incidence of GVHD II–IV compared to CSA + MTX, but with no reduction in the risk of chronic GVHD and no benefit in survival.32 Prednisolone may be substituted for methotrexate, but a beneficial effect is not clear. Mycophenylate mofetil (MMF) may also substitute MTX and reduce incidence of severe mucositis. However, a retrospective study indicated an increased risk of aGVHD in patients treated with reduced-intensity conditioning (RIC) who were given a MMF instead of MTX in combination with CSA.33
Sirolimus is an inhibitor of mTOR, central to a complex intracellular signaling pathway involved in cell growth, proliferation, angiogenesis and metabolism.34 Recently, sirolimus was combined with tacrolimus for GVHD prevention.35 With either HLA-identical sibling donors and unrelated donors, the combination was shown to give a low incidence of GVHD and good survival. In contrast to the calcineurin inhibitors, sirolimus may not only be immunosuppressive by interfering with T-cell proliferation but also by selectively expanding CD4+, CD25+, Fox P3+ regulatory T cells and via inhibition of a dendritic cell activity.34 Many groups have combined antibody treatment in addition to drug-based immunosuppression; antilymphocyte/antithymocyte globulins (ALG/ATG) or the CD52 monoclonal antibody alemtuzumab are frequently incorporated around the time of the transplant. The timing of administration is critical: earlier administration of alemtuzumab, for example, favors suppression of host immunity and facilitates engraftment, whereas schedules in which the monoclonal is given after SCT cause profound persisting immunosuppression. Nevertheless with additional DLI alemtuzumab-based RIC regimens can be designed to produce favorable results with a low risk of GVHD.36 ATG is usually associated with shorter post-transplant half-life, making it a better agent for early protection against GVHD, facilitating engraftment and avoiding prolonged post-transplant immunosuppression.37
Reduced-intensity conditioning (RIC) regimens
One of the potential benefits of RIC regimens is that the conditioning should release fewer inflammatory cytokines, therefore reducing the risk of GVHD. Indeed, in a recent report Ogawa et al describe only 5/26 patients developing grade-II aGVHD after a 2–3 antigen-mismatched haploidentical SCTs and a fludarabine/busulfan RIC regimen and post-transplant tacrolimus and methylprednisolone.38 However, a large comparative study from the Seattle group failed to identify differences in frequency of acute and chronic GVHD between RIC and standard regimens.39 Indeed, the persistence of host APCs may exacerbate GVHD after RIC transplants,40 and overall the contribution of the regimen intensity to GVHD risk is only one of many factors.41
New developments
Removal of naïve T cells
Based on observations in murine SCT models showing that GVHD reactivity is conserved within the naive T-cell compartment and that depletion of naïve (CD62L-positive) T cells from the transplant prevents GVHD in a mismatched model, Chao and colleagues are developing a naïve T-cell-depleted SCT.42
Regulatory T cells
One of the most promising new avenues for GVHD research is the possibility of manipulating immune recovery with regulatory T lymphocytes (Tregs). These natural regulators of alloreactivity are characterized as CD4+ CD25+ intracytoplasmic FoxP3+ T cells. Animal experiments clearly demonstrate that post-transplant transfer of Tregs can reduce GVHD, and depleting them from the graft increases GVHD.43 Tregs appear to recover rapidly after SCT (even following T-cell-depleted or selectively depleted SCT), and the risk of GVHD is related both to the degree of Treg recovery and to the resting frequency of Treg in the donor.44,45 Whether Treg recovery can be controlled to prevent GVHD has yet to be determined.
Eliminating host APCs
Experiments in transgenic mice, where transplants were performed in the presence or absence of allostimulatory recipient APCs, highlighted the critical need for host APCs to initiate aGVHD mediated by MHC class-I mismatching.46 Alloreacting NK-cell populations in haploidentical SCT are thought to reduce GVHD by a donor NK-mediated elimination of host APCs.19
Controlling T-cell homing
In pioneering work, Sackstein demonstrated that alloactivated T cells home to tissue sites to cause GVHD according to specific ‘addressins’ expressed on their surface. T cells expressing E-selectin home to the gut, whereas those expressing P-selectin home to skin. Using appropriate antibodies, or by modification of the selectin glycochemistry, it might be possible to block GVHD by preventing activated T cells reaching their tissue target.47
Improving thymic function
The acquisition of a new T-cell repertoire derived from thymic-processed donor-derived prethymic T cells ultimately established full donor–recipient tolerance, but the process may take many months and remains incomplete in older recipients who lack a functioning thymus. An attractive strategy to prevent GVHD is therefore to boost recipient thymic function either by administration of cytokines such as IL-7 or by the use of epithelial growth factors to stimulate growth of thymic stroma.48
FUTURE PROSPECTS: COMBINING STRATEGIES FOR THE ‘PERFECT TRANSPLANT’
The so-called Holy Grail of allogeneic SCT has been to separate GVHD from GVL reactivity so that prevention of GVHD is not achieved at a compromise to the acquisition of full donor immunity. Of the many approaches described in this chapter, none so far has been proven unequivocally to selectively eliminate GVHD, although selective allodepletion or T-cell suicide-gene technologies appear to hold that promise. In the future, the provision of a selectively depleted SCT without requirement for post-transplant immunosuppression should be a platform for transplant approaches where immunity to leukemia and infectious agents is further boosted. The demonstration that leukemia-antigen-specific T-cell clones from the donor can transfer and expand in the recipient supports the development of new strategies for the adoptive transfer of GVL immunity.49 First, vaccination of a donor not yet tolerized to the leukemia could increase the frequency of leukemia-specific T cells conveyed in the graft. Second, the cytokine storm and the lymphopenia that follows the conditioning regimen may be favorable to the expansion of these leukemia-specific cytotoxic T cells, which could be further boosted by early vaccination of the transplant recipient. Third, leukemia-antigen-specific peptides could be used to induce antigen-specific T cells in donor lymphocytes in vitro for adoptive transfer to the recipient. Although the careful construction of post-transplant immunity is likely to remain technically challenging and expensive, the effort taken to create the ‘perfect transplant’ should be justified economically if it is demonstrated that most patients have a complication-free post-transplant course, without disease recurrence.
Practice points.
standard GVHD prophylaxis is a combination of low-dose methotrexate with a calcineurin inhibitor
T-cell depletion of the graft can prevent GVHD but will be replaced in future by selective allodepletion techniques which preserve immune function and graft-versus-leukemia reactivity
Research agenda.
optimizing the graft content to provide a GVHD-free transplant not requiring post-transplant immunosuppression yet capable of initiating rapid immune recovery
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
John Barrett, Hematology Branch, National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, MD, USA.
Katarina Le Blanc, Division of Clinical Immunology and Transfusional Medicine, F79, Karolinska Institutet, Karolinska University Hospital Huddinge, SE-141 86 Stockholm, Sweden.
REFERENCES
- 1.Billingham RE. The biology of graft-versus-host reactions. Harvey Lect. 1966–1967;62:21–78. [PubMed] [Google Scholar]
- 2.Hensel N, Agarwala V, Jiang YZ, Mavroudis D, Molldrem J, Barrett AJ. A technique for dual determination of cytotoxic and helper lymphocyte precursor frequency by a miniaturized dye release method. Bone Marrow Transplant. 1999;23:71–78. doi: 10.1038/sj.bmt.1701528. [DOI] [PubMed] [Google Scholar]
- 3.Holler E. Risk assessment in haematopoietic stem cell transplantation: GVHD prevention and treatment. Best Pract Res Clin Haematol. 2007;20:281–294. doi: 10.1016/j.beha.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Barrett AJ, Mavroudis D, Tisdale J, Molldrem J, Clave E, Dunbar C, Cottler-Fox M, Phang S, Carter C, Okunnieff P, Young NS, Read EJ. T cell-depleted bone marrow transplantation and delayed T cell add-back to control acute GVHD and conserve a graft-versus-leukemia effect. Bone Marrow Transplant. 1998;21:543–545. doi: 10.1038/sj.bmt.1701131. [DOI] [PubMed] [Google Scholar]
- 5.Elhasid R, Arush MB, Zaidman I, Leiba R, Barak AB, Postovsky S, Haddad N, Katz T, Pollack S, Sami I, Gidoni O, Rubin D, Mandel H, Attias D, Reisner Y, Etzioni A, Rowe JM. Safe and efficacious allogeneic bone marrow transplantation for nonmalignant disorders using partial T cell depletion and no posttransplantation graft-versus-host-disease prophylaxis. BBMT. 2007;13:329–338. doi: 10.1016/j.bbmt.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 6.Drobyski WR, Hessner MJ, Klein JP, Kabler-Babbitt C, Vesole DH, Margolis DA, Keever-Taylor CA. T-cell depletion plus salvage immunotherapy with donor leukocyte infusions as a strategy to treat chronic-phase chronic myelogenous leukemia patients undergoing HLA-identical sibling marrow transplantation. Blood. 1999;94:434–441. [PubMed] [Google Scholar]
- 7.Kalaycio M, Rybicki L, Pohlman B, Sobecks R, Ball E, Cook D, Andresen S, Kuczkowski E, Bolwell B. CD8+ T-cell-depleted, matched unrelated donor, allogeneic bone marrow transplantation for advanced AML using busulfan-based preparative regimens. Bone Marrow Transplant. 2005 Feb;35(3):247–252. doi: 10.1038/sj.bmt.1704736. [DOI] [PubMed] [Google Scholar]
- 8.Meyer RG, Britten CM, Wehler D, Bender K, Hess G, Konur A, Hartwig UF, Wehler TC, Ullmann AJ, Gentilini C, Uharek L, Huber C, Kolbe K, Herr W. Prophylactic transfer of CD8-depleted donor lymphocytes after T-cell-depleted reduced-intensity transplantation. Blood. 2007 Jan 1;109(1):374–382. doi: 10.1182/blood-2006-03-005769. [DOI] [PubMed] [Google Scholar]
- 9.Bishop MR, Steinberg SM, Gress RE, Hardy NM, Marchigiani D, Kasten-Sportes C, Dean R, Pavletic SZ, Gea-Banacloche J, Castro K, Hakim F, Krumlauf M, Read EJ, Carter C, Leitman SF, Fowler DH. Targeted pretransplant host lymphocyte depletion prior to T-cell depleted reduced-intensity allogeneic stem cell transplantation. Br J Haematol. 2004;126:837–843. doi: 10.1111/j.1365-2141.2004.05133.x. [DOI] [PubMed] [Google Scholar]
- 10.Mielke S, Solomon SR, Barrett AJ. Selective depletion strategies in allogeneic stem cell transplantation. Cytotherapy. 2005;7:109–115. doi: 10.1080/14653240510018172. [DOI] [PubMed] [Google Scholar]
- 11.André-Schmutz I, Le Deist F, Hacein-Bey-Abina S, Vitetta E, Schindler J, Chedeville G, Vilmer E, Fischer A, Cavazzana-Calvo M. Immune reconstitution without graft-versus-host disease after haemopoietic stem-cell transplantation: a phase 1/2 study. Lancet. 2002;360:130–137. doi: 10.1016/S0140-6736(02)09413-8. [DOI] [PubMed] [Google Scholar]
- 12.Solomon SR, Mielke S, Savani BN, Montero A, Wisch L, Childs R, Hensel N, Schindler J, Ghetie V, Leitman SF, Mai T, Carter CS, Kurlander R, Read EJ, Vitetta ES, Barrett AJ. Selective depletion of alloreactive donor lymphocytes: a novel method to reduce the severity of graft-versus-host disease in older patients undergoing matched sibling donor stem cell transplantation. Blood. 2005;106:1123–1129. doi: 10.1182/blood-2005-01-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amrolia PJ, Muccioli-Casadei G, Yvon E, Huls H, Sili U, Wieder ED, Bollard C, Michalek J, Ghetie V, Heslop HE, Molldrem JJ, Rooney CM, Schlinder J, Vitetta E, Brenner MK. Selective depletion of donor alloreactive T cells without loss of antiviral or antileukemic responses. Blood. 2003 Sep 15;102(6):2292–2299. doi: 10.1182/blood-2002-11-3516. [DOI] [PubMed] [Google Scholar]
- 14.Mielke S, Nunes R, Rezvani K, Fellowes VS, Venne A, Solomon SR, Fan Y, Gostick E, Price DA, Scotto C, Read EJ, Barrett AJ. A clinical scale selective allodepletion approach for the treatment of HLA-mismatched and matched donor-recipient pairs using expanded T lymphocytes as antigen-presenting cells and a TH9402-based photodepletion technique. Blood. 2007 Sep 24; doi: 10.1182/blood-2007-08-104471. E pub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bondanza A, Valtolina V, Magnani Z, Ponzoni M, Fleischhauer K, Bonyhadi M, Traversari C, Sanvito F, Toma S, Radrizzani M, La Seta-Catamancio S, Ciceri F, Bordignon C, Bonini C. Suicide gene therapy of graft-versus-host disease induced by central memory human T lymphocytes. Blood. 2006;107:1828–1836. doi: 10.1182/blood-2005-09-3716. [DOI] [PubMed] [Google Scholar]
- 16.Bonini C, Bondanza A, Perna SK. The suicide gene therapy challenge: how to improve a successful gene therapy approach. Molecular Therapy. 2007;157:1248–1252. doi: 10.1038/sj.mt.6300190. [DOI] [PubMed] [Google Scholar]
- 17.Schmitz N, Barrett J. Optimizing engraftment--source and dose of stem cells. Semin Hematol. 2002;39:3–14. doi: 10.1053/shem.2002.29245. [DOI] [PubMed] [Google Scholar]
- 18.Heimfeld S. HLA-identical stem cell transplantation: is there an optimal CD34 cell dose? Bone Marrow Transplant. 2003;31:839–845. doi: 10.1038/sj.bmt.1704019. [DOI] [PubMed] [Google Scholar]
- 19.Ruggeri L, Mancusi A, Burchielli E, Aversa F, Martelli MF, Velardi A. Natural killer cell alloreactivity and haplo-identical hematopoietic transplantation. Cytotherapy. 2006;8(6):554–558. doi: 10.1080/14653240601078721. [DOI] [PubMed] [Google Scholar]
- 20.Savani BN, Mielke S, Adams S, Uribe M, Rezvani K, Yong AS, Zeilah J, Kurlander R, Srinivasan R, Childs R, Hensel N, Barrett AJ. Rapid natural killer cell recovery determines outcome after T-cell-depleted HLA-identical stem cell transplantation in patients with myeloid leukemias but not with acute lymphoblastic leukemia. Leukemia. 2007 Oct;21(10):2145–2152. doi: 10.1038/sj.leu.2404892. [DOI] [PubMed] [Google Scholar]
- 21.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, Orchard PJ, Blazar BR, Wagner JE, Slungaard A, Weisdorf DJ, Okazaki IJ, McGlave PB. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005 Apr 15;105(8):3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 22.Koç ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, Lazarus HM. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18(2):307–316. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- 23.Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK, Shpall EJ, McCarthy P, Atkinson K, Cooper BW, Gerson S, Laughlin M, Loberiza F, Moseley A, Bacigalupo A. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant. 2005;11(5):389–398. doi: 10.1016/j.bbmt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Le Blanc K, Samuelsson H, Gustafsson B, Remberger M, Sundberg B, Arvidson J, Ljungman P, Lonnies H, Nava S, Ringden O. Transplantation of mesenchymal stem cells to enhance engraftment of hematopoietic stem cells. Leukemia. 2007;21(8):1733–1738. doi: 10.1038/sj.leu.2404777. [DOI] [PubMed] [Google Scholar]
- 25.Ball LM, Bernardo ME, Roelofs H, Lankester A, Cometa A, Egeler RM, Locatelli F, Fibbe WE. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood. 2007;110(7):2764–2767. doi: 10.1182/blood-2007-04-087056. [DOI] [PubMed] [Google Scholar]
- 26.Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 27.Ringden O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lonnies H, Marschall HU, Dlugosz A, Szakos A, Hassan Z, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81(10):1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 28.Storb R, Deeg HJ, Pepe M, Appelbaum F, Anasetti C, Beatty P, Bensinger W, Berenson R, Buckner CD, Clift R, et al. Methotrexate and cyclosporine versus cyclosporine alone for prophylaxis of graft-versus-host disease in patients given HLA-identical marrow grafts for leukemia: long-term follow-up of a controlled trial. Blood. 1989;73(6):1729–1734. [PubMed] [Google Scholar]
- 29.Ringden O, Horowitz MM, Sondel P, Gale RP, Biggs JC, Champlin RE, Deeg HJ, Dicke K, Masaoka T, Powles RL, et al. Methotrexate, cyclosporine, or both to prevent graft-versus-host disease after HLA-identical sibling bone marrow transplants for early leukemia? Blood. 1993;81(4):1094–1101. [PubMed] [Google Scholar]
- 30.Bacigalupo A, Van Lint MT, Occhini D, Gualandi F, Lamparelli T, Sogno G, Tedone E, Frassoni F, Tong J, Marmont AM. Increased risk of leukemia relapse with high-dose cyclosporine A after allogeneic marrow transplantation for acute leukemia. Blood. 1991;77(7):1423–1428. [PubMed] [Google Scholar]
- 31.Locatelli F, Zecca M, Rondelli R, Bonetti F, Dini G, Prete A, Messina C, Uderzo C, Ripaldi M, Porta F, et al. Graft versus host disease prophylaxis with low-dose cyclosporine-A reduces the risk of relapse in children with acute leukemia given HLA-identical sibling bone marrow transplantation: results of a randomized trial. Blood. 2000;95(5):1572–1579. [PubMed] [Google Scholar]
- 32.Nash RA, Antin JH, Karanes C, Fay JW, Avalos BR, Yeager AM, Przepiorka D, Davies S, Petersen FB, Bartels P, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96(6):2062–2068. [PubMed] [Google Scholar]
- 33.Le Blanc K, Remberger M, Uzunel M, Mattsson J, Barkholt L, Ringden O. A comparison of nonmyeloablative and reduced-intensity conditioning for allogeneic stem-cell transplantation. Transplantation. 2004;78(7):1014–1020. doi: 10.1097/01.tp.0000129809.09718.7e. [DOI] [PubMed] [Google Scholar]
- 34.Hartford CM, Ratain MJ. Rapamycin: something old, something new, sometimes borrowed and now renewed. Clin Pharmacol Ther. 2007;82(4):381–388. doi: 10.1038/sj.clpt.6100317. [DOI] [PubMed] [Google Scholar]
- 35.Cutler C, Li S, Ho VT, Koreth J, Alyea E, Soiffer RJ, Antin JH. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood. 2007;109(7):3108–3114. doi: 10.1182/blood-2006-09-046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peggs KS, Sureda A, Qian W, Caballero D, Hunter A, Urbano-Ispizua A, Cavet J, Ribera JM, Parker A, Canales M, Mahendra P, Garcia-Conde J, Milligan D, Sanz G, Thomson K, Arranz R, Goldstone AH, Alvarez I, Linch DC, Sierra J, Mackinnon S. Reduced-intensity conditioning for allogeneic haematopoietic stem cell transplantation in relapsed and refractory Hodgkin lymphoma: impact of alemtuzumab and donor lymphocyte infusions on long-term outcomes. Br J Haematol. 2007;139:70–80. doi: 10.1111/j.1365-2141.2007.06759.x. [DOI] [PubMed] [Google Scholar]
- 37.Mohty M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia. 2007;21:1387–1394. doi: 10.1038/sj.leu.2404683. [DOI] [PubMed] [Google Scholar]
- 38.Ogawa H, Ikegame K, Yoshihara S, Kawakami M, Fujioka T, Masuda T, Taniguchi Y, Hasei H, Kaida K, Inoue T, Kim EH, Kawase I. Unmanipulated HLA 2–3 antigen-mismatched (haploidentical) stem cell transplantation using nonmyeloablative conditioning. Biol Blood Marrow Transplant. 2006 Oct;12(10):1073–1084. doi: 10.1016/j.bbmt.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Mielcarek M, Storer BE, Sandmaier BM, Sorror ML, Maloney DG, Petersdorf E, Martin PJ, Storb R. Comparable outcomes after nonmyeloablative hematopoietic cell transplantation with unrelated and related donors. Biol Blood Marrow Transplant. 2007;13:1499–1507. doi: 10.1016/j.bbmt.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Remberger M, Mattsson J, Hassan Z, Karlsson N, Leblanc K, Omazic B, Okas M, Sairafi D, Ringdén O. Risk factors for acute graft-versus-host disease grades II–IV after reduced intensity conditioning allogeneic stem cell transplantation with unrelated donors-a single centre study. Bone Marrow Transplant. 2007 Nov 5; doi: 10.1038/sj.bmt.1705913. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 41.Hirshfeld E, Weiss L, Kasir J, Zeira M, Slavin S, Shapira MY. Post transplant persistence of host cells augments the intensity of acute graft-versus-host disease and level of donor chimerism, an explanation for graft-versus-host disease and rapid displacement of host cells seen following non-myeloablative stem cell transplantation? Bone Marrow Transplant. 2006;38:359–364. doi: 10.1038/sj.bmt.1705449. [DOI] [PubMed] [Google Scholar]
- 42.Chen BJ, Deoliveira D, Cui X, Le NT, Son J, Whitesides JF, Chao NJ. Inability of memory T cells to induce graft-versus-host disease is a result of an abortive alloresponse. Blood. 2007 Apr 1;109(7):3115–3123. doi: 10.1182/blood-2006-04-016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salomon BL, Sudres M, Cohen JL. Regulatory T cells in graft-versus-host disease. Springer Semin Immunopathol. 2006;28:25–29. doi: 10.1007/s00281-006-0020-9. Review. [DOI] [PubMed] [Google Scholar]
- 44.Mielke S, Rezvani K, Savani BN, Nunes R, Yong AS, Schindler J, Kurlander R, Ghetie V, Read EJ, Solomon SR, Vitetta ES, Barrett AJ. Reconstitution of FOXP3+ regulatory T cells (Tregs) after CD25-depleted allotransplantation in elderly patients and association with acute graft-versus-host disease. Blood. 2007 Sep 1;110(5):1689–1697. doi: 10.1182/blood-2007-03-079160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rezvani K, Mielke S, Ahmadzadeh M, Kilical Y, Savani BN, Zeilah J, Keyvanfar K, Montero A, Hensel N, Kurlander R, Barrett AJ. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood. 2006;15(108):1291–1297. doi: 10.1182/blood-2006-02-003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson BE, McNiff JM, Jain D, Blazar BR, Shlomchik WD, Shlomchik MJ. Distinct roles for donor- and host-derived antigen-presenting cells and costimulatory molecules in murine chronic graft-versus-host disease: requirements depend on target organ. Blood. 2005;105:2227–2234. doi: 10.1182/blood-2004-08-3032. [DOI] [PubMed] [Google Scholar]
- 47.Dimitroff CJ, Kupper TS, Sackstein R. Prevention of leukocyte migration to inflamed skin with a novel fluorosugar modifier of cutaneous lymphocyte-associated antigen. J Clin Invest. 2003;112:980–983. doi: 10.1172/JCI19220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fry TJ, Mackall CL. Immune reconstitution following hematopoietic progenitor cell transplantation: challenges for the future. Bone Marrow Transplant. 2005 Mar;35(Suppl 1):S53–S57. doi: 10.1038/sj.bmt.1704848. [DOI] [PubMed] [Google Scholar]
- 49.Barrett J. Improving outcome of allogeneic stem cell transplantation by immunomodulation of the early post-transplant environment. Curr Opin Immunol. 2006;18:592–598. doi: 10.1016/j.coi.2006.06.002. [DOI] [PubMed] [Google Scholar]