Abstract
Background aims
The immunomodulatory and anti-inflammatory effects of mesenchymal stromal cells (MSC) could prove to be a potential therapeutic approach for prolongation of survival of cell xenotransplantation. Adipose (Ad) MSC from genetically modified pigs could be an abundant source of pig donor-specific MSC.
Methods
Pig (p) MSC were isolated from adipose tissue of α1,3-galactosyltransferase gene knock-out pigs transgenic for human (h) CD46 (GTKO/ hCD46), a potential source of islets. After characterization with differentiation and flow cytometry (FCM), AdMSC were compared with bone marrow (BM) MSC of the same pig and human adipose-derived (hAd) MSC. The modulation of human peripheral blood mononuclear cell (hPBMC) responses to GTKO pig aortic endothelial cells (pAEC) by different MSC was compared by measuring 3H-thymidine uptake. The supernatants from the AdMSC cultures were used to determine the role of soluble factors.
Results
GTKO/hCD46 pAdMSC (i) did not express galactose-α1,3-galactose (Gal) but expressed hCD46, (ii) differentiated into chondroblasts, osteocytes and adipocytes, (iii) expressed stem cell markers, (iv) expressed lower levels of Swine Leucocyte Antigen I (SLAI), Swine Leucocyte Antigen II DR (SLAIIDR) and CD80 than pAEC before and after pig interferon (IFN)-γ stimulation. The proliferative responses of hPBMC to GTKO/hCD46 pAdMSC and hAdMSC stimulators were similar, and both were significantly lower than to GTKO pAEC (P < 0.05). The proliferation of hPBMC to GTKO pAEC was equally suppressed by GTKO/hCD46 pAdMSC and hAdMSC (P > 0.05). The supernatant from GTKO/hCD46 pAdMSC did not suppress the human xenoresponse to GTKO pAEC, which was cell–cell contact-dependent.
Conclusions
Initial evidence suggests that genetically modified pAdMSC function across the xenogeneic barrier and may have a role in cellular xenotransplantation.
Keywords: α1,3-galactosyl transferase knock-out; adipose-derived; mesenchymal stromal cells; pig; xenotransplantation
Introduction
Currently, mesenchymal stromal cells (MSC) from humans are defined based upon three minimal criteria: (i) plastic adherence, (ii) trilineage differentiation and (iii) surface expression of CD73, CD90 and CD105 and absence of expression of CD45, CD34, CD14 or CD11b, CD79α or CD19 and HLA-DR. MSC from animal origin have been defined as cells that fulfill the first two criteria (1). The successful management of severe graft-versus-host disease (GvHD) in a patient with MSC (2) has received considerable attention, and led to the investigation of their potential role in the fields of autoimmune disorders and organ transplantation.
The role of MSC in immunomodulation has been studied and confirmed in vivo in various pre-clinical (3) and clinical (2) models. MSC suppress the proliferation of CD4 + T cells (3), prevent maturation of dendritic cells (4), induce T-regulatory cells (5) and produce soluble factors such as prostaglandin (PG) E2 (6), Human Leucocyte Antigen (HLA)-G5, interleukin (IL)-10, transforming growth factor (TGF)-β (5) and indolamine 2,3-dioxygenase (IDO) (7), all of which have immunomodulatory effects. Hence MSC may prove to be a potent cytotherapeutic agent in clinical practice in the not-too-distant future. One of the problems in establishing MSC therapy clinically would be an inadequate supply of human (h) MSC; many regimens under study require multiple dosing of the cells. Although MSC numbers can be expanded in vitro, MSC should not be passaged too often as there may be a risk of malignant transformation (8,9) and/ or loss of function because of replicative senescence.
Cross-species transplantation (xenotransplantation) is being explored as an alternative source of organs and cells, for example islets, for clinical transplantation. Pigs offer the prospect of providing an unlimited supply of organs and cells for this purpose, but there are several immune barriers that need to be overcome before success can be assured. These barriers are steadily being resolved by genetic modification of the source pigs. The hyperacute rejection of a pig organ transplanted into a human or non-human primate results from anti-pig antibody binding to the pig vascular endothelial cells followed by activation of the complement. The major target antigen for anti-pig antibodies is galactose-α1,3-galactose (Gal). Pigs are now available in which the gene for the enzyme responsible for production of Gal has been deleted [Gal-knockout (GTKO) pigs], and this modification reduces the incidence of hyperacute rejection. A transgene for a human complement regulatory protein, for example CD46, has been introduced into GTKO pigs, reducing the incidence of rejection further. Nevertheless, other barriers remain, such as the adaptive (T-cell mediated) immune response, and these might be overcome or minimized by the concomitant transplantation of MSC. This approach may be indicated particularly when pig islets are transplanted into humans or non-human primates.
For purposes of xenotransplantation, genetically engineered pig MSC may be superior to human MSC for the effects we are trying to obtain (10). For example, the expression of human CD46 on endothelial cells from transgenic pigs is significantly higher than human CD46 on human endothelial cells, thus probably providing greater protection to the human immune response. We therefore believe that, with further genetic engineering, pig cells, including MSC, will have significantly greater protection from the human immune response than human cells. Furthermore, in view of the very large number of MSC that can be obtained from a single large pig, we believe that there will be advantages both in the larger number of MSC available from a single source and in the reduced number of passages that will be required for expansion of the number. With regard to xenotransplantation, the organ or cells (e.g. islets) and the MSC will be obtained from the same source pig, and therefore the MSC will be donor-specific. In clinical transplantation, MSC are almost invariably third-party (allogeneic) MSC, which may complicate the immunologic picture.
Genetically modified pigs are a potential source of MSC, which have been shown to inhibit the hT-cell xenoresponse (10,11). As adipose tissue has a high frequency of MSC (12), and is present in ample amount in pigs, it may provide very large numbers of minimally passaged MSC. We have been able to isolate the MSC efficiently from the adipose tissue of genetically modified pigs, which may become sources of organs and cells for xenotransplantation. We have characterized and functionally evaluated adipose (Ad) MSC from α1,3-galactosyltransferase gene-knockout pigs transgenic for the human complement-regulatory protein CD46.
Methods
Pigs
Adipose tissue and bone marrow (BM) were harvested from GTKO pigs transgenic for hCD46 (GTKO/ hCD46 pigs; Revivicor, Blacksburg, VA, USA). Pig (p) aortic endothelial cells (pAEC) were harvested from wild-type (WT) and GTKO pigs. Three pigs from each type were used for these experiments. The WT, GTKO and GTKO/hCD46 pigs were of a Large White/Landrace/Duroc cross-breed.
Isolation of Ad- and BM-derived MSC
Pig Ad-derived MSC (pAdMSC)
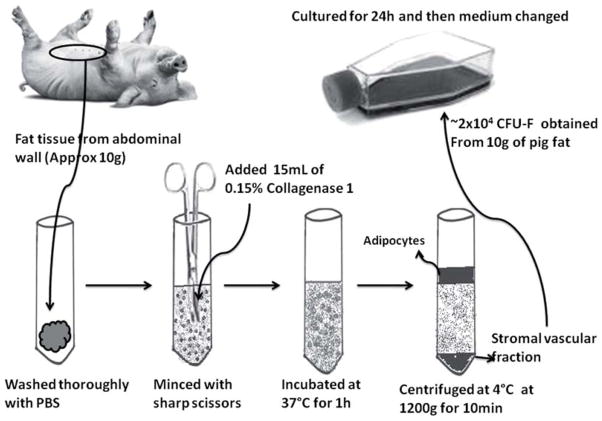
Adipose tissue from the anterior abdominal wall of the pigs was collected and transferred to the laboratory in Dulbecco’s modified Eagle medium (DMEM; Invitrogen, Grand Island, NY, USA) with antibiotics (see below). About 10 g of pig adipose tissue was minced into 2–4-mm pieces with sterile scissors and digested with 15 mL 0.15% collagenase type 1 (Worthington, Lakewood, NJ, USA) for 1 h at 37°C (Figure 1). The lysate was then centrifuged at 1200 g at 4°C for 5 min, and the pellet (stromal vascular fraction) washed with phosphate-buffered saline (PBS) and seeded in 75-cm2 collagen-coated flasks (BD [Becton Dickinson] Lab-ware, Franklin Lakes, NJ, USA). MSC medium contained DMEM (low glucose with L-glutamine), 10% fetal bovine serum (FBS; Sigma-Aldrich, St Louis, MO, USA), 1% 4-(2-hydroxycthl)-1-piperazincethane sulfonic acid (HEPES) buffer, penicillin G (10 000 U/mL), streptomycin (10 000 μg/mL) and amphotericin B (25 μg/m) (Invitrogen). Cells not adherent to the bottom of the culture flask after 24 h of culture were removed. The medium was changed every 2–3 days. At 90–95% confluence, adherent cells were harvested using trypsin–ethylene diamine tetra acetic acid (EDTA; Invitrogen), and further passaged at a concentration of 3000 cells/cm2. All experiments were performed using MSC after 3–7 passages.
Figure 1.
Stepwise-isolation of MSC from the pig abdominal wall adipose tissue.
Pig BM-derived MSC (pBMMSC)
BM was scraped and flushed from the femur under aseptic conditions, and collected in a Petri dish. After filtering through a 70-μm cell strainer (BD Biosciences, Bedford, MA, USA), the diluted BM was layered over Ficoll-paque (GE Lifesciences, Piscataway, NJ, USA) for density centrifugation at 600 g for 20 min at 24°C. The buffy coat collected was washed twice in cold PBS and centrifuged at 700 g for 5 min at 4°C and cultured in 75-cm2 collagen-coated flasks. The medium was changed every 2–3 days. At 90–95% confluence, adherent cells were harvested using trypsin–EDTA, and further passaged at 3000 cells/cm2.
Characterization of pAdMSC
Surface phenotyping of isolated GTKO/hCD46 pAdMSC and pBMMSC was carried out using flow cytometry. Antibodies used were (i) anti-CD29 and anti-CD105 (both from Novus-Biologicals, Littleton, CO, USA); (ii) anti-pig CD45 (a marker for hematopoietic cells), anti-pig Swine Leucocyte Antigen I (SLAI), anti-pig Swine Leucocyte Antigen II DR (SLAIIDR), anti-human CD46 and hamster anti-mouse CD80 (all from Serotec, Raleigh, NC, USA); (iii) anti-human CD73, anti-pig CD31 (a marker for endothelial cells) and anti-human CD90 (all from BD Pharmingen, San Diego, CA, USA); and (iv) BSI-B4 lectin (Sigma-Aldrich).
Surface expression of SLAI, SLAIIDR and CD80 on GTKO/hCD46 pAdMSC was compared with GTKO pAEC before and after pig interferon (IFN)-γ (40 ng/mL for 48 h; R&D Systems, Minneapolis, MN, USA). In order to measure the expression of SLAI and Swine Leucocyte Antigen II (SLAII) before and after stimulation, a high concentration of pIFN-γ was used to stimulate the pAEC and pAdMSC. (In preliminary studies, we had tested different concentrations and different time-periods of exposure to pIFN-γ. The minimum concentration of pIFN-γ that resulted in maximum up-regulation of SLAI and II expression was selected.) Pig CD80 is an important co-stimulation molecule in the human T-cell response to pig antigens.
Induction of trilineage differentiation
For adipocyte differentiation, 2.5 × 104 cells were cultured in six-well collagen-coated plates (Becton Dickinson [BD]). At 95–100% confluence, adipogenic differentiation medium [1 μM dexamethasone, 10 μg/mL human recombinant insulin, 0.5 mM 3-isobutyl-1-methylxanthine (IBMX) and 10 mM indomethacin] was added and changed every 3 days. Adipogenic differentiation was confirmed by Oil Red staining on days 7 and 14. All reagents were purchased from Sigma-Aldrich.
For osteoblast differentiation, 2.5 ×104 cells were cultured in six-well collagen-coated plates. At 95–100% confluence, osteogenic differentiation medium (100 μM dexamethasone, 50 mM ascorbic acid and 10 mM β-glycerol phosphate in DMEM) was added and changed every 3 days. Osteogenic differentiation was confirmed by Von Kossa staining (ScyTek Laboratories, Logan, UT, USA) for calcium deposition on days 7 and 14.
For chondroblast differentiation, GTKO/hCD46 pAdMSC were trypsinized, and aliquots of 25 × 104 cells in 0.5 mL of chemically defined medium [10 ng/mL pTGF-1β (Merck KGaA, Darmstadt, Germany) and 100 nM dexamethasone] were pelleted at the bottom of the conical tubes and incubated at 37°C with 5% CO2 for 21 days. After 2 days, the pellets condensed into a cohesive spherical body at the bottom of the conical tubes. Medium was changed carefully every 3 days without disturbing the pellet at the bottom. At 21 days, the condensed cells were spread on a slide, air dried, fixed with absolute alcohol, and stained with Alcian Blue (ScyTek) to confirm the presence of acidic mucosubstances suggestive of chondrogenic differentiation.
Measurement of IgM and IgG antibody binding
Heat-inactivated naive human serum and serum from baboons sensitized to GTKO pig antigens (non-Gal antigens) were incubated with GTKO pAEC and GTKO pAdMSC. IgM and IgG binding was determined by using fluorescein isothiocyanate (FITC)-conjugated goat anti-human IgM (μ-chain) (Cat#62-7511) and IgG (γ-chain) (Cat#62-8411) antibodies (Invitrogen, Carlsbad, CA, USA) after blocking with 10% goat serum. Indirect mean immunofluorescence intensity (MFI) was measured with a BD-FACSDiva™. Relative MFI was calculated by dividing the sample MFI by non-serum control MFI (secondary antibody only).
Lymphocyte proliferation assay
The immune response to GTKO/hCD46 pAdMSC was tested by measuring the proliferative response of hPBMC, and comparing it with the response to GTKO pAEC, WT pAdMSC and hAdMSC (as stimulators), where GTKO pAEC were used as positive control. hPBMC, used as responders, were isolated from fresh blood using Ficoll-paque density-gradient centrifugation (as described above for BMMSC isolation). The stimulators were harvested following serial culture, resuspended in AIM-V medium (Invitrogen), irradiated (2500 rads) and plated at 2 × 104/well in flat-bottomed 96-well plates (Corning, Lowell, MA, USA). hPBMC were added (2 × 105 /well) and cultured in an incubator at 37°C and 5% CO2/95% air. The proliferation was measured using 3H-thymidine uptake after 5 days of culture, and 16 h after addition of thymidine, using a beta-scintillation counter (PerkinElmer, Waltham, MA, USA).
The immunomodulation was measured by the suppression of the hPBMC proliferative response to GTKO pAEC (at a fixed ratio of hPBMC to GTKO pAEC of 10:1) in the presence of irradiated GTKO/ hCD46 pAdMSC or allogeneic hAdMSC (Lonza, Walkersville, MD, USA) at different ratios (1:2, 1:1 and 2:1) with respect to GTKO pAEC. The proliferation was measured using 3H-thymidine uptake (as above). GTKO pAEC were selected as stimulators because of the stronger hPBMC proliferative response to them compared with GTKO/hCD46 pAEC, which was very weak; this allowed us to determine more adequately the suppressive effect of the addition of MSC.
Supernatant harvested from GTKO/hCD46 pAdMSC cultured in pIFN-γ containing AIM-V medium for 24 h was used as conditioned medium to study the role of soluble factors in hPBMC proliferation against GTKO pAEC and compared with the presence of irradiated GTKO/hCD46 pAdMSC in similar conditions. The conditioned medium was added to a 96-well plate at different volumes (25–150 μL) along with GTKO pAEC (stimulators) and hPBMC (responders) at a ratio of 1:10. The proliferation was measured using 3H-thymidine uptake (as above).
Statistical analyzes
All data are shown as mean ± SEM. The significance of differences in the mean was determined using an unpaired Student’s t-test. The software used for the statistical analysis was Graphpad prism version 5. A P < 0.05 was considered significant.
Results
pAdMSC can be isolated efficiently from genetically modified pigs
The most important technical difficulty in the isolation of AdMSC from GTKO/hCD46 pigs was the satisfactory digestion of the fat tissue, which was achieved by fine mincing followed by adequate utilization of collagenase I. Once the fat tissue was adequately digested, the stromal vascular fraction was centrifuged and cultured in a 75T flask (Figure 1). Fibroblast-like cells (Figure 2A) were found to have adhered to the flask after 24 h. Each of these cells represented a fibroblastoid colony-forming unit (CFU-F). Ten grams of fat tissue yielded approximately 2 × 104 CFU-F initially. After an initial lag time of 5–7 days, these cells expanded with a doubling time of about 1.5–2 days. The morphology of GTKO/hCD46 pAdMSC was similar to that of GTKO/hCD46 pBMMSC and hAdMSC. Adipose tissue has the highest frequency of CFU-F (12). The CFU-F frequency from BM has been found to be approximately 0.01% (13), suggesting that approximately 2 × 108 BM cells are equivalent to 10 g of pig fat tissue.
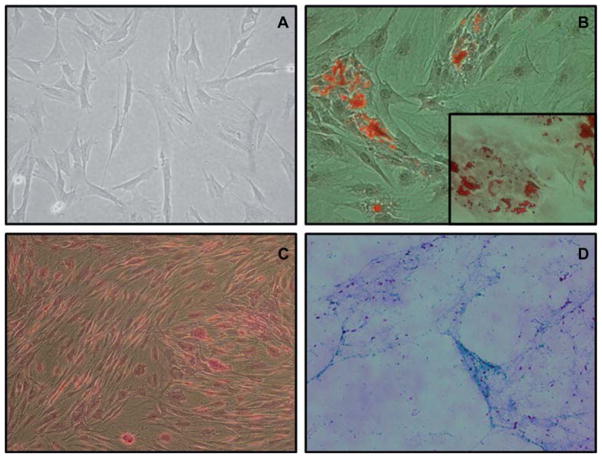
Figure 2.
(A) Fibroblast-like morphology of GTKO/hCD46 pAdMSC (light microscopy 10 ×). (B) Adipogenic differentiation of GTKO/ hCD46 pAdMSC: Oil Red staining (20 ×). (Insert: fat droplets stained with Oil Red, 40 ×). (C) Osteogenic differentiation of GTKO/ hCD46 pAdMSC: Von Kossa staining (20 ×), showing morphologic changes in pAdMSC and linear calcium deposition. (D) Chondrogenic differentiation of GTKO/hCD46 pAdMSC: Alcian Blue staining (10 ×), showing strongly acidic mucosubstance (blue) and goblet cells (red nuclei).
Successful induction of trilineage differentiation of pAdMSC from genetically engineered pigs
On exposure to the respective differentiation media, as a proof of confirmation, these cells differentiated into adipocytes, osteoblasts and chondroblasts (Figure 2B–D).
pAdMSC expressed surface stem cell markers and human CD46
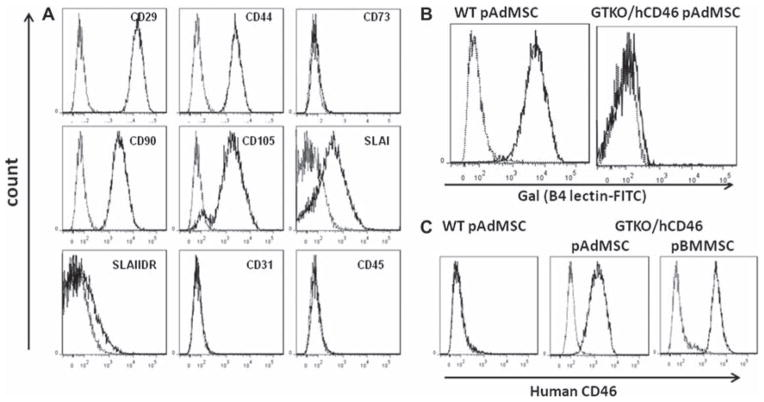
Upon surface phenotypic characterization, GTKO/ hCD46 pAdMSC showed expression of the stem cell markers CD29, CD44, CD90 and CD105, and SLAI, were minimally positive for SLAIIDR, and were negative for CD31 and CD45 (Figure 3A). The expression of CD73 could not be ascertained (Figure 3A), possibly because of the non-cross-reactive nature of anti-human antibody (14). The genetic modifications were expressed effectively on the cell surface of GTKO/hCD46 pAdMSC, with the absence of expression of Gal and the presence of human CD46 (Figure 3B,C).
Figure 3.
(A) Flow cytometry characterization of GTKO/hCD46 pAdMSC, showing positive expression of CD29, CD44, CD90 and CD105, with negative expression of CD31, CD45, CD73 and SLAIIDR, and weakly positive expression of SLAI. (B) No surface expression of Gal was detected on GTKO/hCD46 pAdMSC compared with WT pAdMSC. (C) Expression of hCD46 on GTKO/hCD46 pAdMSC compared with expression on WT pAdMSC and on GTKO/hCD46 pBMMSC.
Relative paucity of expression of SLA and co-stimulatory molecules on GTKO/hCD46 pAdMSC
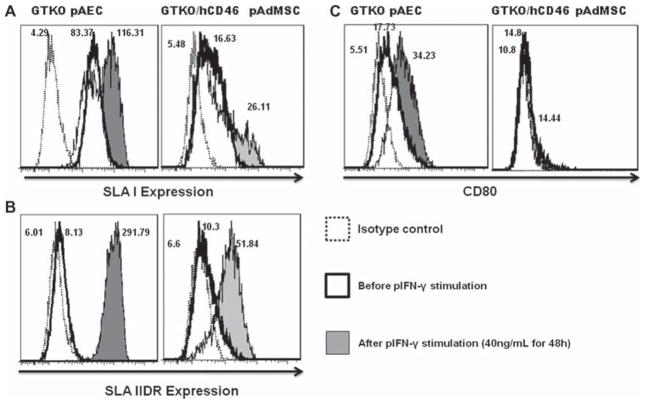
The immunologic characterization of GTKO/hCD46 pAdMSC was carried out by determining the expression of SLAI, SLAIIDR and the co-stimulatory molecule CD80, before and after pIFN-γ stimulation (maximal stimulation achieved with 40 ng/mL pIFN-γ). SLAI was present on GTKO/hCD46 pAdMSC, but not as strongly expressed as on GTKO pAEC (34% versus 100%) (Figure 4A). Although expression of SLAI could be up-regulated by pIFN-γ stimulation (34–43%), it still remained less than with GTKO pAEC (100%). Expression of SLAIIDR was minimal (0–5%) on the surface of GTKO/hCD46 pAdMSC and was less up-regulated by pIFN-γ activation (to 11–70%) than on GTKO pAEC (to 59–100%) (Figure 4B). The expression of SLAIIDR on GTKO/hCD46 pAdMSC was similar to that on GTKO pBMMSC (11). There was no difference in expression of CD80, before and after pIFN-γ stimulation (40 ng/mL for 48 h; 14% both before and after stimulation), compared with GTKO pAEC (17% before and 34% after stimulation; Figure 4C).
Figure 4.
Flow cytometry comparing expression of SLAI, SLAIIDR and CD80 between GTKO pAEC and GTKO/hCD46 pAdMSC following pIFN-γ stimulation. (A) Both cells show a slight increase in SLA class I expression. (B) There is greater expression of SLA class IIDR on GTKO pAEC than on GTKO/hCD45 pAdMSC. (C) There was no change in expression of CD80 on GTKO/hCD46 pAdMSC. MFI values are indicated.
Binding of anti-non-Gal antibodies to pAEC and pAdMSC
The total IgM and IgG binding to GTKO pAEC and GTKO/hCD46 pAdMSC, when exposed to human (preformed anti-non-Gal antibodies) and sensitized baboon sera (induced anti-non-Gal antibodies), was measured using flow cytometry. Both IgM and IgG antibody binding were lower with GTKO/ hCD46 pAdMSC than with GTKO pAEC (P < 0.01; Figure 5). [We have previously demonstrated that binding to pig cells equates with the cytotoxicity of the cells (15).]
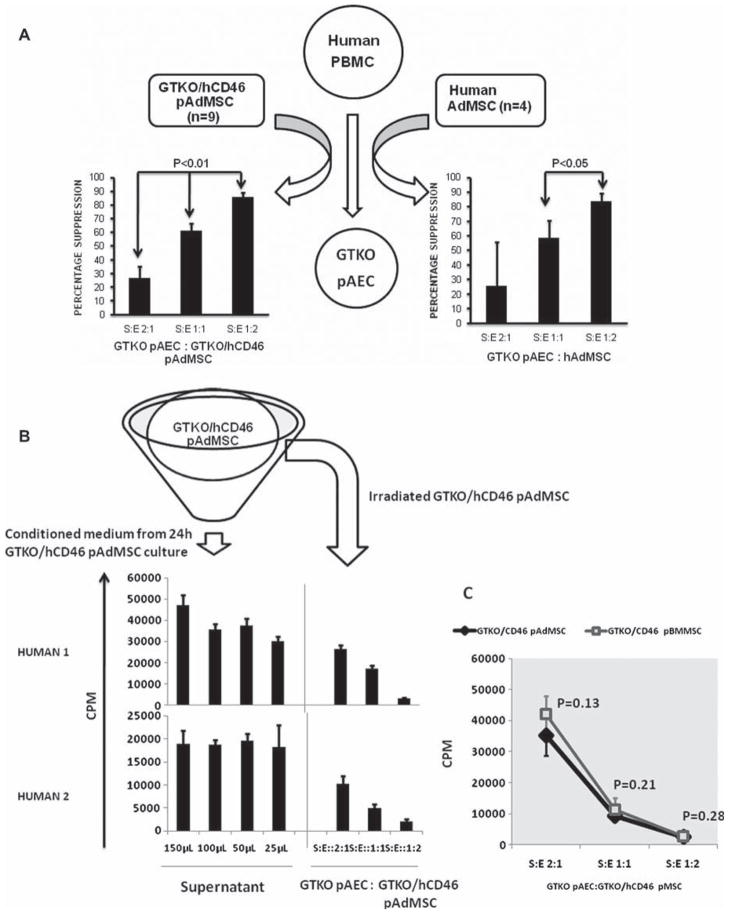
Figure 5.
Binding of IgM and IgG antibodies from naive human serum and sensitized baboon serum (following exposure to GTKO pig antigens) to GTKO pAEC and GTKO/hCD46 pAdMSC measured by flow cytometry. The relative MFI was calculated using the MFI of the sample divided by the MFI of the negative control. IgM and IgG binding to GTKO pAEC was significantly greater than to GTKO/ hCD46 pAdMSC. [Baboon antibody binding, particularly of IgG, to pig cells is significantly greater after sensitization (10).]
Human lymphocyte proliferation against GTKO/ hCD46 pAdMSC was weak and comparable with that against hAdMSC
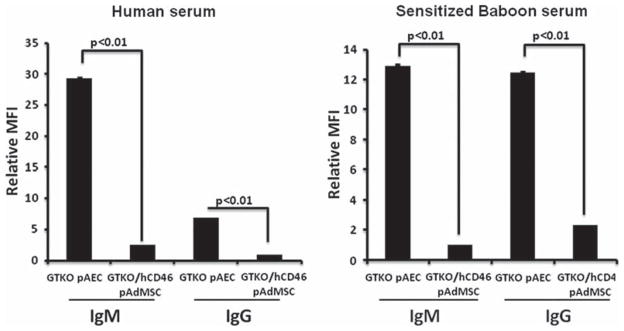
The proliferation of hPBMC against GTKO/hCD46 pAdMSC was not significantly different from that against allogeneic hAdMSC (P = 0.43) (Figure 6A). There was significantly less proliferation of hPBMC cells against GTKO/hCD46 pAdMSC than against GTKO pAEC (positive control) (P < 0.05; Figure 6A). There was significantly less proliferation of hPBMC against GTKO/hCD46 pAdMSC compared with WT pAdMSC (P < 0.05; Figure 6B), indicating a role of the genetic modification in reducing the immunogenicity of these cells. The kinetics of proliferation of hCD4 + T cells when co-cultured with GTKO/hCD46 pAdMSC or hAdMSC showed no significant differences (P = 0.58); maximum proliferation was at 7 days in both cases (Figure 6C).
Figure 6.
Proliferation of hPBMC measured by 3H-thymidine uptake against GTKO/hCD46 pAdMSC in comparison with (A) hAdMSC and GTKO pAEC (n = 5), and (B) WT pAdMSC and GTKO pAEC (n = 3). The stimulation index (SI) was calculated by dividing sample counts per minute (c.p.m.) with autologous c.p.m. (C) Kinetics of proliferation of hCD4 + T cells (measured by 3H-thymidine uptake) against GTKO/hCD46 pAdMSC and hAdMSC. Although the difference in maximum proliferation between the two was not significant, the proliferation of CD4 + T to hAdMSC was much more rapid than to GTKO/hCD46 pAdMSC.
The presence of GTKO/hCD46 pAdMSC in co-culture inhibited the proliferation of hPBMC against GTKO pAEC
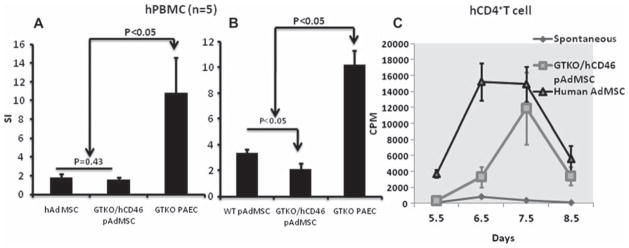
When co-cultured with GTKO pAEC and hPBMC, both GTKO/hCD46 pAdMSC and hAdMSC suppressed the xenogeneic response of hPBMC to GTKO pAEC. As the concentration of MSC with respect to GTKO pAEC/hPBMC increased, the extent of suppression increased linearly (Figure 7A). At a high concentration (stimulator:responder ratios of 1:1 or 1:2), GTKO/hCD46 pAdMSC could almost completely suppress the response of hPBMC to GTKO pAEC. There was no significant difference between the suppressive actions of GTKO/hCD46 pAdMSC and hAdMSC at different concentrations [stimulator:responder ratios of 2:1 (P = 0.49), 1:1 (P = 0.42) and 1:2 (P = 0.39)]. The variability of suppressive function at low concentrations may possibly have been because of interspecies differences in the functional molecules, which could be surmounted by increasing the numbers of GTKO/ hCD46 pAdMSC.
Figure 7.
(A) Mean percentage suppression of proliferation of hPBMC (responders) against GTKO pAEC (stimulators) (with a fixed responder:stimulator ratio of 10:1) when co-cultured with increasing concentrations of GTKO/hCD46 pAdMSC (n = 9) or hAdMSC (n = 4) (effectors) at ratios of 2:1, 1:1 and 1:2 with respect to GTKO pAEC. The mean percentage suppression was calculated by dividing the differences between mean c.p.m. against GTKO pAEC alone and mean c.p.m. against MSC + GTKO pAEC co-culture by the mean c.p.m. against GTKO pAEC. Mean percentage suppression by GTKO/hCD46 pAdMSC was comparable with hAdMSC and increased in a linear fashion as the concentration of MSC increased. (B) Comparison of the proliferation of hPBMC (responders) against GTKO pAEC (stimulators) (with a fixed responder:stimulator ratio of 10:1) in the presence of either conditioned medium (in different volumes, 25, 50, 100 and 150 μL) from GTKO/hCD46 pAdMSC culture (i.e. supernatant) or irradiated GTKO/hCD46 pAdMSC (in different ratios) (i.e. cell contact) using a 3H-thymidine uptake assay. The presence of conditioned medium did not suppress the proliferation of hPBMC, compared with the addition of GTKO/hCD46 pAdMSC in different concentrations. (C) Comparison between pAdMSC and pBMMSC with respect to suppression of hPBMC proliferation against GTKO pAEC using a 3H-thymidine uptake assay. There was no significant difference in suppression of mean c.p.m. by pAdMSC and pBMMSC (S:E, stimulator:effector ratio).
Supernatant from GTKO/hCD46 pAdMSC cultures could not suppress hPBMC proliferation against GTKO pAEC
The presence of conditioned medium obtained following stimulation with pIFN-γ (10 ng/mL) from GTKO/hCD46 pAdMSC cultures did not decrease the proliferation of hPBMC against GTKO pAEC. Furthermore, the presence of irradiated pAdMSC in similar settings suppressed the proliferation of lymphocytes (Figure 7B), suggesting cell–cell contact is an important mode of action. The mechanisms involved require further elucidation.
Comparison of pMSC from adipose tissue and BM showed a similar phenotype and immunomodulatory function
GTKO/hCD46 pAdMSC and pBMMSC were compared with respect to phenotypic characteristics, immunogenicity and immunomodulatory function in vitro. The relative MFI for the surface expression of transgenic hCD46 was comparable between these cells. Although surface expression of CD29, CD44, CD90 and CD105 was seen on both pAdMSC and pBMMSC, the relative MFI was slightly higher in pAdMSC than pBMMSC (data not shown). The suppression of the proliferative response of hPBMC against GTKO pAEC in vitro was comparable between pAdMSC and pBMMSC (Figure 7C; P = 0.13, 0.21 and 0.28 at different ratios).
Discussion
In this study, we found that GTKO/hCD46 pAdMSC can be obtained in large numbers. Genetically modified pAdMSC are no more immunogenic than hAdMSC, and the immunomodulatory function of pAdMSC is comparable with that of hAdMSC and pBMMSC and is contact-dependent; they inhibited the human-to-pig xenoresponses in a dose-dependent manner in vitro. Genetically modified pAdMSC may provide a potential therapy in cell xenotransplantation because they are equally effective as hAdMSC with respect to immune modulation of human xenoresponses. They may have an important role in islet xenotransplantation, as they can be obtained from the same donor pig.
MSC have been studied extensively, largely because of their potential in cytotherapy, evidence for which is gradually accumulating. If successful, there would be numerous potential indications for MSC therapy. Encouraging results have been obtained in animal models of ischemic cardiac injury (16), pulmonary hypertension (17), sepsis (18), renal ischemia-reperfusion (19), spinal injury (20) and diabetes (21). Their role in the treatment of autoimmune disorders (22) and GvHD (2) is under investigation. Most of the beneficial effects of MSC have been ascribed to their anti-inflammatory, anti-proliferative, angiogenic and immunomodulatory functions. Additionally, MSC may have a role as vehicles for gene therapy and drug delivery (23).
Effective cytotherapy, however, may require multiple administrations of MSC. Large numbers of MSC can be obtained by multiple serial passages in vitro, but there is a risk of chromosomal instability (8,9), reduction in cytokine production and loss of multipotentiality (24) with each passage. Until reliable data regarding the absence or otherwise of malignant transformation of cultured MSC are available, a logical option would be to use low-passage MSC for cytotherapy. Human MSC usually reach replicative senescence following 25 cell divisions, potentially limiting their expansion for therapeutic purposes. There is considerable potential in obtaining the MSC from animal tissues. MSC from pig adipose tissue are an attractive option as (i) pigs provide an ample source of adipose tissue, (ii) MSC are obtained from them in large numbers because of a high frequency of CFU-F (12), and (iii) AdMSC have similar immunomodulatory functions as BMMSC (25).
With the advent of somatic cell transfer technology, genetically modified pigs that can be considered for the aforementioned purposes are increasingly becoming available. These genetically modified pigs are being investigated extensively as a source of islets, hepatocytes and, eventually, solid vascularized organs. In addition, they could provide an abundant supply of donor-specific MSC (both AdMSC and BMMSC) for therapeutic purposes in xenotransplantation.
Before genetically modified pMSC can be used clinically, some fundamental questions need to be investigated. (i) Will pMSC survive in a human host? (ii) How efficiently will pMSC suppress the human immune response? (iii) What adverse effects of pMSC can be anticipated?
Survival of xenogeneic MSC is contingent upon overcoming the xenogeneic barrier. The human innate immune system launches an attack with preformed antibodies and complement activation against pig surface Gal and other ‘non-Gal’ antigens. This overwhelming attack has been reduced by eliminating the surface expression of Gal on pig cells by knocking out the α1,3-galactosyltransferase gene (GTKO pigs) (26) and by the expression of one or more human complement-regulatory proteins, for example CD46, on the pig cells (27). Furthermore, the absence of Gal expression and the expression of hCD46 have both been documented to reduce the human T-cell response to pig cells (28–30). In the present study, human cellular immunity against GTKO/hCD46 pAdMSC was not found to be greater than to allogeneic hAdMSC, possibly because of the relative paucity of some surface immunologic determinants on MSC in addition to the genetic modifications mentioned above.
Allogeneic hMSC are known to be hypo-immunogenic, but are usually eliminated by natural killer (NK) cells in a major histocompatibility complex (MHC)-unrestricted manner (31); NK cytotoxicity is also likely to play a role in the destruction of pMSC. However, genetic modifications in pigs, for example expression of HLA-E/β2 microglobulin and/ or of HLA-G molecules, decrease the cytotoxicity of human NK cells (32,33) and may therefore protect the MSC from NK cytotoxicity. As progress in the field of xenotransplantation research continues, long-term survival of pMSC is likely to be possible.
Regarding the functionality of GTKO/hCD46 pMSC, we limited the present study to an investigation of the immunomodulatory aspects of MSC. GTKO/hCD46 AdMSC were found to decrease the proliferation of hPBMC in vitro almost as efficiently as hAdMSC, suggesting that they functioned across the species barrier. Although the mechanisms of immunomodulation by MSC are yet to be understood completely, a role for soluble factors, for example PGE2, IL-10, TGF-β1 and IDO (5,6), has been suggested. Contact-dependent mechanisms, which may have a more important role in MSC-based immunomodulation, are largely unknown.
Finally, if pig-derived MSC are to be therapeutically useful, they will need to be administered safely without significant adverse effects. Following encouraging results seen in preclinical pig-to-non-human primate studies using WT and genetically modified pigs as sources of islets (34–36), pig islet xenotransplantation is drawing close to clinical trials. However, transplanted islets face several insults, for example from the instant blood-mediated inflammatory reaction (IBMIR), hypoxia and host immunity. Co-transplantation of MSC and islets improves islet graft survival in both small animal (37) and Non-human primate (NHP) (38) models. This beneficial effect has been attributed to the immunomodulatory, anti-inflammatory, angiogenic and trophic effects of MSC. Allogeneic donor MSC are considered to be better in inducing T-cell anergy when compared with autologous MSC (39,40). Whether pig MSC will induce anergy in a xenotransplantation model is currently being investigated in our laboratory. Genetically modified pigs could serve as sources of both islets and MSC, and could provide a logistical and logical solution to the inadequate supply of human islets. In conclusion, GTKO/hCD46 pAdMSC have the potential to become a new therapeutic option, and need to be explored further.
Acknowledgments
The authors thank Drs Agnes Azimzadeh (University of Maryland) and Muhammad Mohiuddin (NHLB1) for providing pig adipose tissue and BM. Studies on xenotransplantation at the Thomas E. Starzl Transplantation Institute of the University of Pittsburgh are supported in part by the Competitive Medical Research Fund of the UPMC Health System, NIH grant number 1U19AI090959-01 and by Sponsored Research Agreements between the University of Pittsburgh and Revivicor Inc., Blacksburg, VA.
Footnotes
Disclosure of conflict of interest: Dr Ayares owns stock in Revivicor Inc. No other author has a conflict of interest.
References
- 1.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–17. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 2.Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–41. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 3.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–8. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W, Ge W, Li C, You S, Liao L, Han Q, et al. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 2004;13:263–71. doi: 10.1089/154732804323099190. [DOI] [PubMed] [Google Scholar]
- 5.Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, et al. Human leukocyte antigen-G5 secretion by human mesenchy-mal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4 + CD25high FOXP3 + regulatory T cells. Stem Cells. 2008;26:212–22. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 6.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 7.Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–98. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 8.Miura M, Miura Y, Padilla-Nash HM, Molinolo AA, Fu B, Patel V, et al. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells. 200;24:1095–103. doi: 10.1634/stemcells.2005-0403. [DOI] [PubMed] [Google Scholar]
- 9.Røsland GV, Svendsen A, Torsvik A, Sobala E, McCormack E, Immervoll H, et al. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 2009;69:5331–9. doi: 10.1158/0008-5472.CAN-08-4630. [DOI] [PubMed] [Google Scholar]
- 10.Ezzelarab M, Ayares D, Cooper DK. The potential for genetically-modified pig mesenchymal stromal cells in xenotrans-plantation. Xenotransplantation. 2010;17:3–5. doi: 10.1111/j.1399-3089.2009.00567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ezzelarab M, Ezzelarab C, Wilhite T, Kumar G, Hara H, Ayares D, et al. Genetically-modified pig mesenchymal stro-mal cells: xenoantigenicity and effect on human T-cell xeno-responses. Xenotransplantation. 2011;18:183–95. doi: 10.1111/j.1399-3089.2011.00635.x. [DOI] [PubMed] [Google Scholar]
- 12.Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 13.Jones EA, Kinsey SE, English A, Jones RA, Straszynski L, Meredith DM, et al. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002;46:3349–60. doi: 10.1002/art.10696. [DOI] [PubMed] [Google Scholar]
- 14.Kilroy GE, Foster SJ, Wu X, Ruiz J, Sherwood S, Heifetz A, et al. Cytokine profile of human adipose-derived stem cells: expression of angiogenic, hematopoetic, and pro-inflammatory factors. J Cell Physiol. 2007;212:702–9. doi: 10.1002/jcp.21068. [DOI] [PubMed] [Google Scholar]
- 15.Hara H, Long C, Lin YJ, Tai HC, Ezzelarab M, Ayares D, Cooper DKC. In vitro investigation of pig cells for resistance to human antibody-mediated rejection. Transplant Int. 2008;21:1163–74. doi: 10.1111/j.1432-2277.2008.00736.x. [DOI] [PubMed] [Google Scholar]
- 16.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baber SR, Deng W, Master RG, Bunnell BA, Taylor BK, Murthy SN, et al. Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H1120–28. doi: 10.1152/ajpheart.00173.2006. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Rey E, Anderson P, González MA, Rico L, Büscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–39. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]
- 19.Lin F, Cordes K, Li L, Hood L, Couser WG, Shankland SJ, et al. Hematopoietic stem cells contribute to the regeneration of renal tubules after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol. 2003;14:1188–99. doi: 10.1097/01.asn.0000061595.28546.a0. [DOI] [PubMed] [Google Scholar]
- 20.McDonald JW, Liu XZ, Qu Y, Liu S, Mickey SK, Turetsky D, et al. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med. 1999;5:1410–12. doi: 10.1038/70986. [DOI] [PubMed] [Google Scholar]
- 21.Itakura S, Asari S, Rawson J, Ito T, Todorov I, Liu CP, et al. Mesenchymal stem cells facilitate the induction of mixed hematopoietic chimerism and islet allograft tolerance without GVHD in the rat. Am J Transplant. 2007;7:336–46. doi: 10.1111/j.1600-6143.2006.01643.x. [DOI] [PubMed] [Google Scholar]
- 22.MacDonald GI, Augello A, De Bari C. Role of Mesenchymal stem cells: re-establishing immunological tolerance in autoimmune rheumatic diseases. Arthritis Rheum. 2011;63:2547–57. doi: 10.1002/art.30474. [DOI] [PubMed] [Google Scholar]
- 23.Prockop DJ. Marrow stromal cells as stem cells for nonhe-matopoietic tissues. Science. 1997;276:71–4. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 24.Vacanti V, Kong E, Suzuki G, Sato K, Canty JM, Lee T. Phenotypic changes of adult porcine mesenchymal stem cells induced by prolonged passaging in culture. J Cell Physiol. 2005;205:194–201. doi: 10.1002/jcp.20376. [DOI] [PubMed] [Google Scholar]
- 25.Puissant B, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129:118–29. doi: 10.1111/j.1365-2141.2005.05409.x. [DOI] [PubMed] [Google Scholar]
- 26.Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299:411–14. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Truscott SM, Abate G, Price JD, Kemper C, Atkinson JP, Hoft DF. CD46 engagement on human CD4 + T cells produces T regulatory type 1-like regulation of antimycobacterial T cell responses. Infect Immun. 2010;78:5295–306. doi: 10.1128/IAI.00513-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin Y, Hara H, Tai H, Long C, Tokita D, Yeh P, et al. Suppressive efficacy and proliferative capacity of human regulatory T cells in allogeneic and xenogeneic responses. Transplantation. 2008;86:1452–62. doi: 10.1097/TP.0b013e318188acb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ezzelarab M, Welchons D, Torres C, Hara H, Long C, Yeh P, et al. Atorvastatin down-regulates the primate cellular response to porcine aortic endothelial cells in vitro. Transplantation. 2008;86:733–7. doi: 10.1097/TP.0b013e3181821cad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hara H, Koike N, Long C, Piluek J, Roh DS, Sundarraj N, et al. Initial in vitro investigation of the human immune response to corneal cells from genetically engineered pigs. Invest Ophthalmol Vis Sci. 2011;52:5278–86. doi: 10.1167/iovs.10-6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–36. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 32.Weiss EH, Lilienfeld BG, Müller S, Müller E, Herbach N, Kessler B, et al. HLA-E/human beta2-microglobulin transgenic pigs: protection against xenogeneic human anti-pig natural killer cell cytotoxicity. Transplantation. 2009;87:35–43. doi: 10.1097/TP.0b013e318191c784. [DOI] [PubMed] [Google Scholar]
- 33.Forte P, Pazmany L, Matter-Reissmann UB, Stussi G, Schneider MK, Seebach JD. HLA-G inhibits rolling adhesion of activated human NK cells on porcine endothelial cells. J Immunol. 2001;167:6002–8. doi: 10.4049/jimmunol.167.10.6002. [DOI] [PubMed] [Google Scholar]
- 34.Hering BJ, Wijkstrom M, Graham ML, Hårdstedt M, Aasheim TC, Jie T, et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med. 2006;12:301–3. doi: 10.1038/nm1369. [DOI] [PubMed] [Google Scholar]
- 35.Cardona K, Korbutt GS, Milas Z, Lyon J, Cano J, Jiang W, et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006;12:304–6. doi: 10.1038/nm1375. [DOI] [PubMed] [Google Scholar]
- 36.van der Windt DJ, Bottino R, Casu A, Campanile N, Smetanka C, He J, et al. Long-term controlled normoglycemia in diabetic non-human primates after transplantation with hCD46 transgenic porcine islets. Am J Transplant. 2009;9:2716–26. doi: 10.1111/j.1600-6143.2009.02850.x. [DOI] [PubMed] [Google Scholar]
- 37.Solari MG, Srinivasan S, Boumaza I, Unadkat J, Harb G, Garcia-Ocana A, et al. Marginal mass islet transplantation with autologous mesenchymal stem cells promotes long-term islet allograft survival and sustained normoglycemia. J Autoimmun. 2009;32:116–24. doi: 10.1016/j.jaut.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Berman DM, Willman MA, Han D, Kleiner G, Kenyon NM, Cabrera O, et al. Mesenchymal stem cells enhance allogeneic islet engraftment in nonhuman primates. Diabetes. 2010;59:2558–68. doi: 10.2337/db10-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deng W, Han Q, Liao L, Li C, Ge W, Zhao Z, et al. Allo-geneic bone marrow-derived flk-1 + Sca-1-mesenchymal stem cells leads to stable mixed chimerism and donor-specific tolerance. Exp Hematol. 2004;32:861–7. doi: 10.1016/j.exphem.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 40.Maccario R, Podestà M, Moretta A, Cometa A, Comoli P, Montagna D, et al. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4 + T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005;90:516–25. [PubMed] [Google Scholar]