Summary
In sexual conflict, aggressive males frequently diminish the long-term reproductive success of females in efforts to gain a short-term advantage over rival males. This short-term advantage can selectively favour high-exploitation males. However, just as the over-exploitation of resources can lead to local extinction, the over-exploitation of females in the form of harassment by aggressive males can yield similar consequences resulting in reduced female fecundity, increased female mortality and overall decline in mating activity. This outcome may often be prevented by selection acting at multiple levels of biological organization. Directional selection favouring aggressive exploitation within groups can be balanced by directional selection amongst groups opposing exploitation. Such between-group selection has recently been demonstrated in laboratory studies of water striders, where the conditional dispersal of individuals increased variation amongst groups and influenced the balance of selection toward reduced male aggression. This multilevel selection (MLS) framework also provides predictive value when investigating natural populations differing in their relative strength of selection within versus among groups. For water striders, the consequences of local exploitation cause fitness differences between groups, favouring less aggressive males. Inconsistently flowing ephemeral streams consist of isolated pools that prevent aggressive male water striders from escaping the consequences of local exploitation. We, therefore, predicted that inconsistently flowing ephemeral streams would favour the evolution of less aggressive males than would perennial streams, which allow aggressive males to move more freely and to escape the group-level costs of their aggression. Comparing two neighbouring streams during the mating season, we found that males dispersed naturally between pools at much higher rates in the perennial stream than in the ephemeral stream. As predicted, we found that males from the perennial stream were significantly more aggressive than those from the ephemeral stream. We also found that dispersers were significantly more aggressive than non-dispersers within each stream. These field results illustrate the relevance of the MLS framework in our understanding of the evolution of sexual conflict.
Keywords: multilevel selection, group selection, tragedy of the commons, water strider, sexual conflict, altruism, Aquarius remigis
Introduction
Sexual conflict occurs when males and females have separate and conflicting routes to increased fitness. Males frequently diminish the long-term reproductive success of females in an effort to outcompete rivals in the short-term (Chapman et al., 2003). Selection favouring individual self-interest in the form of the overexploitation of a shared resource, such as the overgrazing of a common pasture, can lead to the exhaustion of the resource and detrimental consequences for the group (Hardin, 1968; Rankin et al., 2007). In regards to sexual conflict, overexploitation in the form of the excessive harassment of females by aggressive males can also yield similar consequences resulting in decreased female fecundity, as well as increased female mortality (Bauer et al., 2005; Le Galliard et al., 2005; Rankin & López-Sepulcre, 2005; Sih &Watters, 2005; Rankin & Kokko, 2006; Eldakar et al., 2009a,b). The local advantage of exploitative males makes it difficult to explain how less exploitative — or more prudent — males persist in populations. One potential solution involves selection acting at multiple levels of biological organization (Wilson & Wilson, 2007).
In multi-group populations, the local advantages of exploitation may be countered by selection beyond the local scale (Wilson & Wilson, 2007). If groups vary in the proportion of high-exploitation males, then the differential contribution of groups to the total gene pool can favor prudent males despite their selective disadvantage within each group. This possibility was recently supported in both Drosophila melanogaster (Wigby & Chapman, 2005) and the water strider Aquarius remigis (Sih & Watters, 2005; Eldakar et al., 2009b) where groups exhibiting less sexual conflict were more productive than higher-conflict groups. Conversely, if the variation between groups was eliminated, selection favoured exploitation based on its local advantage (Eldakar et al., 2009a). Therefore, the balance of these conflicting selection forces is based on the population structure partitioning variance within and between groups. As the population structure changes, so does the resulting phenotypic distributions of exploitative males.
Although the partitioning of variance within and between groups was artificially imposed in most laboratory studies, the structures of natural populations are influenced by a variety of factors, including the dispersal of individuals. If movement is conditional, then migration between groups can increase group heterogeneity and strengthen selection at the scale of groups (Pepper, 1997; Pepper & Smuts, 2002; Aktipis, 2004). This is especially salient in the case of sexual conflict, as the distribution of females amongst groups can be greatly influenced by the distribution of highly aggressive males (Wilcox, 1984; Krupa et al., 1990; Haskins et al., 1997; Bauer et al., 2005; Sih &Watters, 2005; Darden & Croft, 2008; Eldakar et al., 2009a, 2010; Turlure & Van Dyck, 2009).
The A. remigis system is ideal for studying these consequences of sexual conflict in multi-group populations as striders are well known to engage in mating aggression and live in a multi-group population structure imposed by the alternating pools and riffles of moderately flowing streams. Aggressive males actively pursue females, leaping onto their backs and initiating an energetic battle in which the female attempts to escape (Arnqvist, 1997). During these attempts, mating females expend 200% more energy than solitary females (Watson et al., 1998), suffer increased predation risks (Fairbairn, 1993), and solely bear the locomotion costs for the mated pair, carrying males from a few minutes to up to 12 h (Rubenstein, 1984; Wilcox, 1984; Fairbairn, 1988; Weigensberg & Fairbairn, 1994). Therefore, as male mating aggression increases in local frequency, mating activity, presence of females, female foraging/fecundity have all been shown to decline (Sih & Watters, 2005; Eldakar et al., 2009b). In efforts to avoid these costly interactions, harassed females seek refuge off of the water surface, flee to higher flowing marginal habitats suffering reduced foraging opportunities or otherwise disperse to alternative pools within the stream (Sih & Watters, 2005). This movement contributes to influencing the distribution of females and aggressive males, altering the costs and benefits associated with aggressive mating.
Recently, we demonstrated in A. remigis, that this free movement of individuals strongly influences variation amongst groups (Eldakar et al., 2009a, 2010). In these studies,male aggression strongly correlated with male dispersal (Figure 1), indicating males that degrade local mating environments frequently leave these regions, essentially looking for greener pastures. In populations comprised of generally isolated groups with brief periods of mixing, however, harmful males are unable to escape the consequences of local exploitation (as per Sih & Watters, 2005; Wigby & Chapman, 2005; Eldakar et al., 2009b). Under this rigid group structure, any natural variation occurring amongst groups becomes paramount to natural selection. During periods of mixing, the differential productivity of groups based on their proportion of aggressive males favours reduced aggression in the population (lower on the regression line in Figure 1). However when dispersal was unrestricted, the free movement of individuals created a more dynamic group structure, whereby the variation amongst groups was mediated by the conflicting dispersal patterns of males and females. Females clustered around less aggressive males, leaving more aggressive males with male-biased sex ratios. These female dispersal patterns created sex-ratio heterogeneity amongst groups favouring reduced aggression. Conversely, aggressive males also dispersed amongst groups, disrupting female driven heterogeneity, yet did not entirely counteract female movement in its effect on local sex-ratio. The net-effect of these opposing dispersal patterns selected for the full spectrum of males (illustrated in Figure 1).
Figure 1.
Aggression correlated with dispersal tendency in males in laboratory populations. Data of solid circles from Eldakar et al. (2009a). Data of open circles from Eldakar et al. (2010). Dispersal tendency was considered as the average number of pool switches per 5 minutes. Aggression scores were measured as per Eldakar et al. (2009a).
This framework explains how populations are expected to evolve under certain population structures. Although to our knowledge, it has yet to be demonstrated how natural populations under these alternative conditions actually have evolved. Here we continue our investigation of the A. remigis mating system and observe whether different population structures in nature select for different phenotypic distributions of aggressive males. Inconsistently flowing ephemeral streams alternate between a continuous habitat and isolated pools with little opportunity for dispersal, compared to perennial streams that always allow free movement. The relative genetic isolation of streams (Preziosi & Fairbairn, 1992) combined with heterogeneous water flow regimes amongst different streams provides an ideal natural experiment to explore the effect of population structure on the evolution of sexual conflict.
In this report, we investigate differences in male mating aggression and dispersal across a consistently flowing perennial stream and two predominantly ephemeral streams. We predict that (1) during the mating season ephemeral streams will have less mixing among naturally occurring pools than the perennial stream. (2) The consistently flowing perennial stream will be greater in average male aggression than dispersal-limited ephemeral streams and (3) within streams, dispersing males will be more aggressive than non-dispersing males. In addition, we predict (4) that the free movement of individuals in the perennial stream will produce the same sex specific dispersal patterns as observed in the laboratory.
Material and methods
Study populations
In our survey, we selected 3 neighbouring streams within 9.5 km proximity of one another located on Mount Lemmon in the Coronado National Forest in southern Arizona (Figure 2). Although there is little to no gene flow amongst streams, their close proximity allows for similar ecological conditions aside from water flow. The populations of our sample region follow similar life history patterns as those in the bivoltine populations of the Eastern United States (Blanckenhorn, 1994). Adult striders emerge from winter diapause in the early spring and immediately mate producing the first summer generation. These striders then come to maturity (approx. 30–40 days development) as adults and mate in the early summer (June) unless extended winter conditions delay adult emergence. The resulting first summer generation matings produce the second summer generation which refrains from mating once reaching maturity. Instead, the second generation striders prioritize their efforts towards overwintering survival and comprise the adults to begin the cycle again the following spring. Our current study is focused on the behaviours of the first summer generation during the summer mating season.
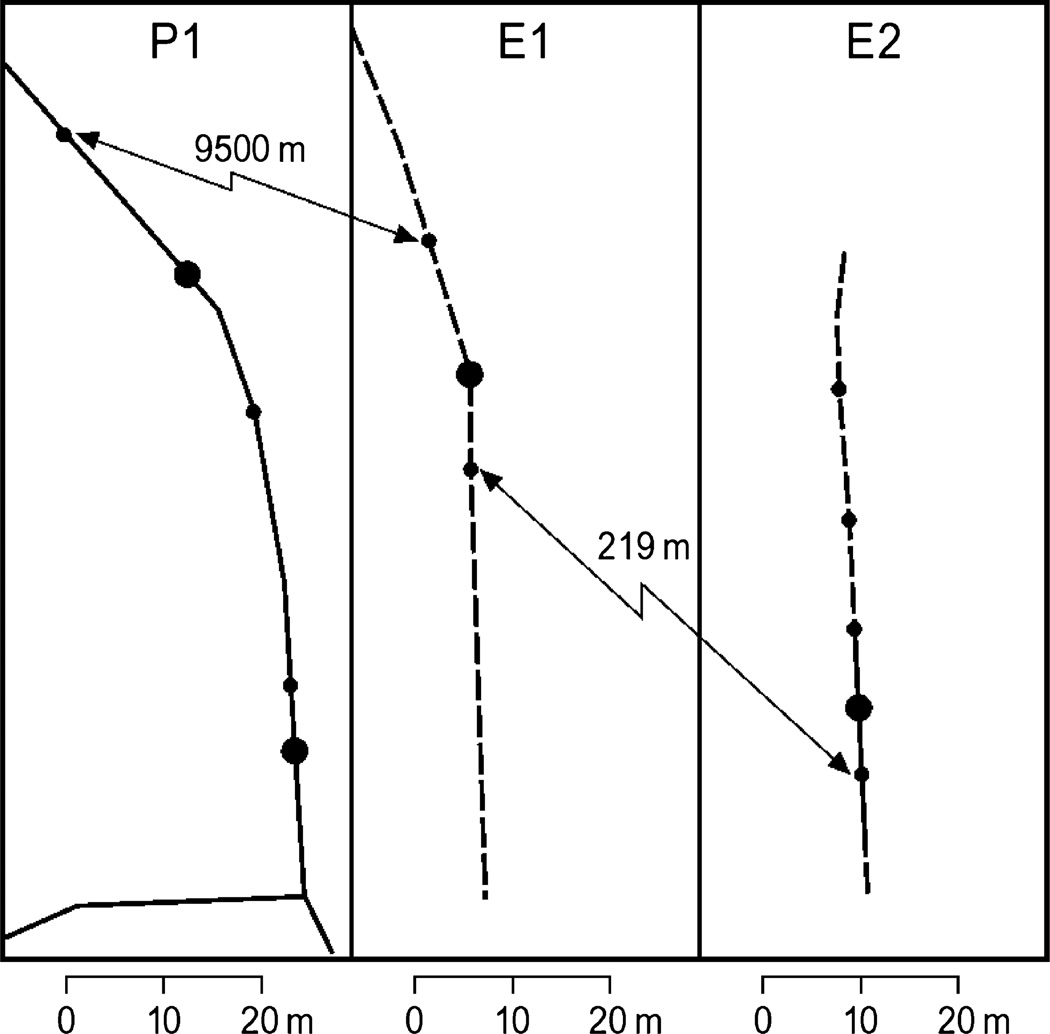
Figure 2.
Sample streams of study populations. P1 represents the perennial flowing stream while E1 and E2 represents the emphemeral flowing streams as described in the methods. Solid circles indicate study pools, solid and dashed lines indicate flowing and non-flowing regions of the stream respectively. Distances are based on GPS coordinates and pool sizes are scaled to indicate water surface area.
Our perennial stream site consisted of a 60-m section of the Sabino Canyon stream referred to as P1, which flows perennially without periods of interrupted flow through the year. Due to an elevation of approx. 2345 m, striders inhabiting this stream undergo the bivoltine patterns as described above (Blanckenhorn, 1994). The first summer generation striders sexually mature in June and continue to mate until the onset of the summer monsoons typically beginning in mid July. The consistent flow of P1 allows for the free mixing of the population throughout the summer mating season; therefore, first summer generation water striders remain unrestricted in dispersal within the stream through their entire life cycle.
The first ephemeral stream site, referred to as E1, consists of a 46-m section of stream that remains largely comprised of perennial pools throughout the year eventually draining into the larger Bear Canyon downstream during the summer monsoons. Also, due to an elevation of approx. 1840 m, first summer generation also sexually mature in June. In contrast to P1, E1 does not experience surface water flow between pools until the summer monsoons, confining first summer generation striders to isolated pools through the mating season however dispersal over dry land is possible but relatively uncommon.
The second of our ephemeral stream sites, referred to as E2, consists of a 50-m section residing within 219 m of E1 and drains into Bear Canyon in the summer monsoons. E2 exhibits much of the features as E1; however, E2 maintains a slight flow between pools throughout the summer mating season, potentially permitting for slightly greater dispersal between pools than E1.
Tracking dispersal within streams
Dispersal was tracked to support expectations that striders of the perennial stream disperse more between pools throughout the mating season than striders of ephemeral streams. To track the movement of striders, we marked newly eclosed adult water striders on their dorsal tergum with enamel paint. All visible adult striders were captured and marked with a pool specific colour as well as an additional marking indicating sex, such that all striders could be easily discriminated by pool of origin and sex. In total 514 (275 males, 239 females) striders were marked amongst the 3 streams (for breakdown of streams see Table 1). Although there was a clear distinction between pools in E1 and E2, the consistent flowing nature of P1 was problematic for classifying distinct pools. In response, we considered a major inhabited pool (N = 5), as well as the immediately connected minor pools (N = 7) as a single pool region. Movement within regions was not considered dispersal in this study; however, differences amongst these pools were considered in behavioural analysis.
Table 1.
Breakdown of the 3 stream sites.
| Stream | N of pools |
N total (males, females) |
N/pool | Closest pool (m) |
Pool density (striders/m2) |
|---|---|---|---|---|---|
| E1 | 3 | 177 (87, 90) | 59 ± 43.3 | 14.9 ± 6.1 | 14.2 ± 6.5 |
| E2 | 5 | 155 (77, 78) | 31 ± 11.1 | 9.8 ± 6.0 | 12.8 ± 5.7 |
| P1 | 5 | 182 (111, 71) | 36.4 ± 20.6 | 11.7 ± 9.4 | 13.2 ± 6.9 |
N represents number of, and closest pool is the average measured distance between the centres of neighbouring pools. Data represent average values and standard deviation, except for columns of stream and number of pools.
Tracking the dispersal of striders amongst pools consisted of surveys conducted every other day for E1 and E2, and daily for P1. Surveys for E1 and E2 were conducted from June 23 to July 3, with E1 and E2 surveyed on alternate days. P1 was surveyed daily in identical fashion to E1 and E2 from June 29 to July 3. Striders disperse both up and down streams however not amongst streams. Surveys consisted of 2 researchers walking opposite sides of the stream sites recording instances of individuals residing in pools other than their originally marked home pools. Once individual dispersers were identified, they were captured and marked with an additional paint mark to avoid recounting in subsequent surveys. Surveys were repeated consecutively for fidelity.
Behavioural observations
Individuals were observed randomly with replacement for 5-min focal sampling periods from June 23 to July 3 for E1 and E2, and June 29–July 3 for P1. Overall we performed 176 observation periods (880 min) for site E2 and 169 observation periods (845 min) for site P1. Behavioural observations from E1 were disqualified as only a small minority of the population engaged in summer mating. Behavioural data taken for males are described in Table 2. Lunge-ats, lunge-jump-ons and jump-ons were considered to represent increasing levels of aggression (as per Wilcox & Ruckdeschel, 1982; Eldakar et al., 2009b). Using the average occurrences of lunge-ats, jump-ons on both males and females as well as mating attempts, we calculated an aggression index for focal males as by Eldakar et al. (2009b). Male aggression towards both males and females were combined in the overall aggression score. We have previously demonstrated that male aggression towards other males is highly correlated with aggression towards females, such that males scoring highly in male–male conflict also scored high in female harassment (Eldakar et al., 2009b). Observations were performed in the proportions of males within the dispersal categories such that if dispersing males comprised 11 of the 77 males in E2 then observations of dispersers comprised 14% of the 176 observations for E2. Aggression scores were log-transformed for normality. These scores were used to investigate differences in aggression among streams, among pools within streams and between dispersing males and non-dispersing males.
Table 2.
Ethogram of observed behaviours during focal observations.
| Classification | Definition |
|---|---|
| Lunge-at | Donor strider quickly propels itself forward towards recipient strider, possibly making contact, but not ending up on top of the recipient strider’s body. |
| Lunge-jump-on | Donor strider quickly propels itself forward towards recipient strider, ending up partially on top of the recipient’s body. |
| Jump-on | Donor strider jumps off the water surface to land on top of the recipients body without traversing the space between the two striders. |
| Mating attempt | A male strider lunges or jumps on a female strider and attempts to hold himself on the female’s back while trying to insert his penis into the female’s spermatheca. The female eventually shakes the male off during her pre-copulatory struggle. |
Sex ratio surveys
To test whether dispersal influenced sex ratio heterogeneity within streams, we surveyed the sex ratio of inhabited pools in extended regions at each site. Surveys consisted of collecting all visible striders and recording the sex ratio and total number of striders for each pools within a total 1 km stretch of P1 and 1 km stretched each for E1 and E2. In addition, to avoid influencing behaviour prior to periods of behavioural observations, we determined pool sex ratio based on paint marking.
Results
Differences in dispersal amongst streams
As expected, both ephemeral streams E1 and E2 had less dispersal amongst pools than perennial stream P1, despite greater overall distances between P1 pools. Overall, only 1.5 ± 1.6% of the marked E1 and 1.4 ± 2.7% of E2 striders changed pools per every 48 h, while 10.7±5.9% of striders changed pools every 24 h (21.7%/48 h) in P1. There was no overall sex difference in dispersal, with females comprising 46% and males 54% of total migrants amongst pools within the streams.
Differences in aggression and sex-ratio
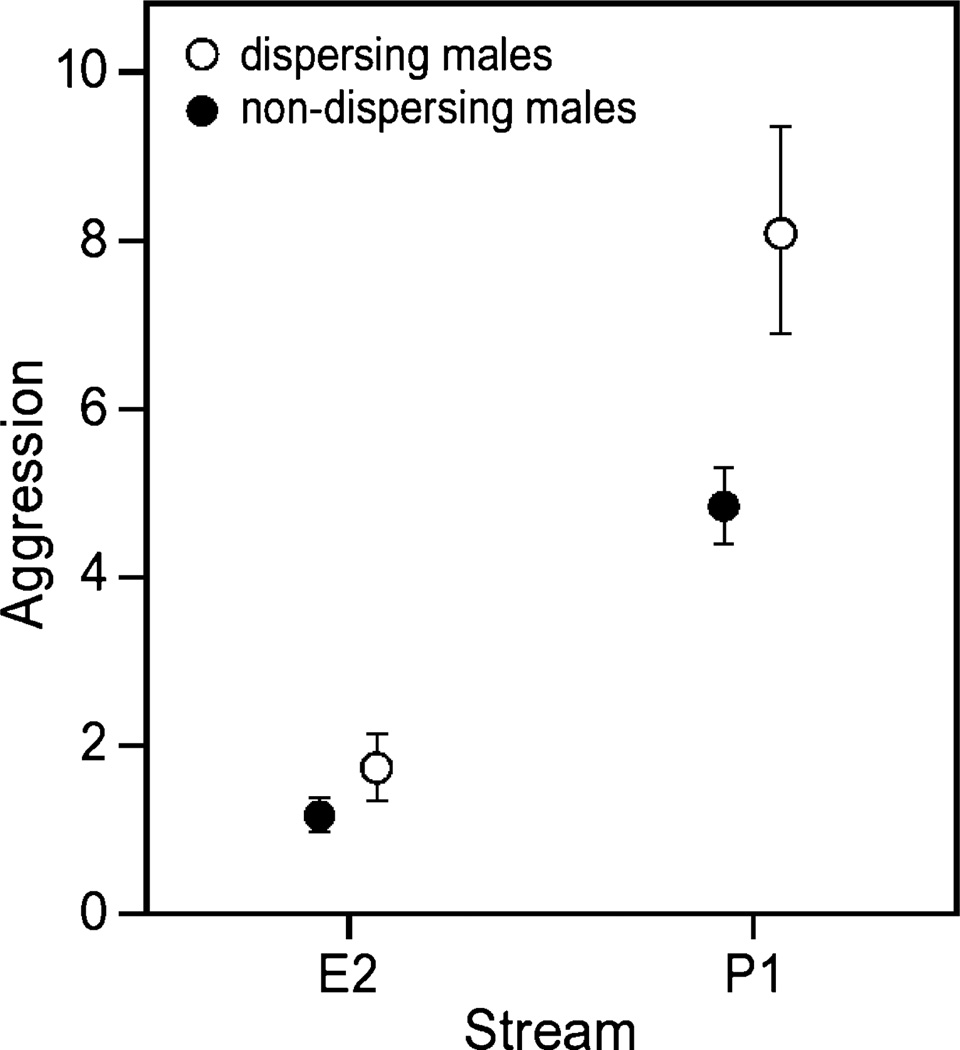
As predicted, P1 male striders were significantly more aggressive than E2 males (ANOVA, F1,344 = 75.311, p < 0.001) (Figure 3). Furthermore, dispersing males were significantly more aggressive than non-dispersing males (ANOVA, F1,344 = 10.811, p = 0.001) (Figure 3). Within E2 there was no significant difference in male aggression amongst the 5 pools for E2 (ANOVA, F4,172 = 1.501, p = 0.204). On the other hand, the pools of P1 varied considerably in sex ratio and male aggression.
Figure 3.
Male striders of P1 were significantly more aggressive than males of E2. Furthermore, within streams, dispersing males (open circles) were significantly more aggressive than their non-dispersing male counterparts (solid circles). Bars indicate standard error.
We predicted that the free movement conditions of P1 will produce the sex-specific distribution patterns as per (Eldakar et al., 2010), whereas the limited dispersal of E2 will not produce these trends. Comparing behavioural observations and sex ratios for all pools within P1 (major and minor), males within female-biased pools were significantly less aggressive (aggression = 4.58 ± 5.67) than males of male-biased pools (aggression = 5.82 ± 5.97) (ANOVA, F1,168 = 3.898, p = 0.05). However, there was no such trend amongst the pools of E2 (mean aggression in male-biased pools = 1.20±2.18, mean aggression in female-biased pools = 1.39±1.98, ANOVA, F1,176 = 0.538, p = 0.464). Additional sex ratio surveys revealed that the frequency of females had a negative relationship with group size in extended regions of P1 (Figure 4) (logarithmic R2 = 0.294, F43 = 17.073, p < 0.001), but not for the combined regions of E1 and E2 (logarithmic R2 = 0.087, F36 = 3.226, p = 0.081). This supports previous field observations by Krupa & Sih (1993) describing naturally occurring ‘hot spots’ as densely populated male biased regions of high harassment within streams. In contrast, the lack of movement amongst pools prevented the formation of these heterogeneous pool environments in ephemeral streams.
Figure 4.
Group size correlates with the frequency of females within the perennial stream P1. As group size increases, the frequency of females decreases within pools.
Discussion
The multilevel selection framework is crucial to the understanding of social adaptations. Non-social traits, such as the hydrophobic properties of water striders legs (Hu et al., 2003) and the ability of striders to decipher information in surface water waves (Wilcox, 1979), provide a local advantage to their bearers. Individuals possessing these traits outcompete individuals that do not, explaining the existence of these traits in the population. However, social adaptations that are advantageous to individuals locally are not always adaptive in the population and vice versa. Behaviours such as altruism increase the fitness of the group yet are outcompeted locally by selfishness to the detriment of the group. In regards to sexual conflict, aggressive males outcompete prudent males locally, despite the deleterious side-effects to female and group fitness (Chapman et al., 2003; Lessells, 2006). Explaining the persistence of these adaptations in the population depends on the understanding the interrelationship of opposing selection pressures at multiple levels of biological organization.
Lab research has demonstrated that sexual conflict is mediated by the structure of populations. In these populations, the local advantage of male sexual exploitation was countered by selection beyond the local scale (Wigby & Chapman, 2005; Eldakar et al., 2009b). Such between-group selection has recently been demonstrated in laboratory studies of water striders, where the conditional dispersal of individuals increased variation amongst groups and influenced the balance of selection toward reduced male aggression. In these laboratory studies, when movement was restricted between groups, the differential productivity of groups based on their proportion of aggressive males favoured reduced aggression in the population, despite the selective disadvantage of reduced male aggression within each group (Eldakar et al., 2009b). Conversely, as dispersal was added, the contingent movement of individuals balanced within- and between-group forces, selecting for intermediate level of aggression (Eldakar et al., 2009a).
These studies support the multilevel selection framework suggesting that alternatively structured populations are expected to evolve predictable behavioural differences. In this report, we have shown that populations inhabiting the limited dispersal regimes of ephemeral streams were significantly less aggressive than those of the free-flowing perennial stream. These preliminary findings support the relevance of relating the behavioural differences of natural populations to corresponding differences in their population structures.
The inconsistent flow of the ephemeral streams subjected populations to isolated pools during the mating season, alternated with periods of continuous habitat as breeding ceased. These conditions provide the ideal ingredients for selection at the group level, whereby the lifespan of groups and the reproductive periods of the organisms are synchronized (Wilson, 1975; Okasha, 2006). If populations are exposed to these conditions, traits reducing group productivity will decrease in the population over evolutionary time. In regards to A. remigis, mating activity, presence of females, female foraging and fecundity all decline in groups with increasing frequencies of aggressive males (Sih & Watters, 2005; Eldakar et al., 2009b). As subsequent generations are populated from the relative contributions of groups, it is expected that aggression will decrease over time. This does not contradict the concept of individual selection in terms of fitness averaged across groups. Phenotypes are still selected on the basis of the highest per capita fitness in the total population. It is simply that when considering the differential productivity across groups, the overall winner is not always the phenotype that confers the local advantage within groups (Wilson, 1975).
Conversely, it has also been demonstrated that California populations of A. remigis disperse away from ephemeral streams as they dry, in preference for streams with more stable flow (Kaitala & Dingle, 1992). However, our striders remained confined not only to their stream, but also to their respective pools within the stream. It is likely that the extreme scarcity of available habitat in our study region prevents striders from emigrating as flow regimes change. The few males that did disperse amongst pools were significantly more aggressive than non-dispersers. This indicates that the presence of aggressive males is dependent on the dispersal regime of the population. Therefore, we would not expect all ephemeral stream populations to conform to the same behavioural trends, only those populations with similar dispersal patterns.
In contrast to the rigid structure of the ephemeral streams, the continuous habitat of the perennial stream produced more dynamic dispersal patterns. The distribution of males and females in the perennial stream population strongly resembled those of our previous laboratory studies allowing the free movement of individuals (Eldakar et al., 2009a, 2010). In these laboratory studies, males and females demonstrated conflicting dispersal patterns. Therefore, we assert that within natural streams the movement of females imposed sex-ratio heterogeneity amongst pools, creating the range of pool environments from female biased, low-aggression pools to male biased, high-aggression pools or ‘hot spots’ as previously described by Krupa & Sih (2003). Under these conditions, when only considering the dispersal patterns of females, selection favours reduced male aggression on the merit of its advantage among groups. However, the movement of aggressive males disrupts female imposed heterogeneity, homogenizing groups and favouring male aggression on the basis of its local advantage. This was evident in the correlation between aggression and dispersal in the laboratory (Eldakar et al., 2009a, 2010) and was further demonstrated here in the differences in aggression between dispersing and non-dispersing males within streams.
The balance of these conflicting dispersal patterns creates an intriguing form of stabilizing selection on male aggression. Unlike negative frequency dependent selection within groups, stabilizing selection is produced by the balance of opposing selection at alternate levels. Directional selection favouring aggression at the local scale is balanced by directional selection favouring non-aggression at the larger scale of groups, maintaining individual differences in aggression in the total population. This suggests that studies using striders from streams allowing dispersal should also permit dispersal in the lab. Evolution within free flowing streams is the net result of the heterogeneous group environment created from the conditional movement of individuals. Disrupting this structure creates an evolutionary mismatch between adaptations and the corresponding environments for which they have evolved.
Although the behavioural distributions of these streams conform to expectations of the multilevel selection framework, these populations also face various unrelated selection pressures that may lead to evolved behavioural differences. It has been previously demonstrated that predators decrease overall strider activity (Sih & Krupa, 1995; Krupa & Sih, 1998) and that striders attempting to mate are especially vulnerable to predation (Fairbairn, 1993). Furthermore, striders respond differently to different types of predators (Krupa & Sih, 1998). Both of our ephemeral and perennial stream environments lacked fish predators which are the major predation risk on the water striders. However, ephemeral and perennial streams do host a myriad of different species which may produce varying predation regimes potentially contributing to the behavioural differences amongst streams.
Population differences in sexual conflict may also be influenced by density-dependent factors (Kokko & Rankin, 2006; Rankin, 2007). A spatial model by Rankin (2007) suggests that the density of conspecifics strongly influences the cost benefit trade-off of maintaining competitive traits in males. As population densities decrease and local competition is reduced, the relative costs of expressing competitive traits outweigh the benefits conferred to the individual. Therefore, if densities vary amongst populations, it is likely so will the magnitude of sexual conflict.
The multilevel selection framework helps to explain conflicts in many species (Wilson & Wilson, 2007). Although species vary considerably in their population structure and, thus, evolutionary trajectories, the same can be said for populations within species. These preliminary findings illustrate the relevance of the multilevel selection framework to field behavioural ecology. Just as predation, climate and other environmental factors influence populations so can differences in the social environment.
Acknowledgements
We thank Z. Aidala, N. Prince, W. Driscoll, A. Gallup, S. Wilcox, E. Art, B. Dadayeva and D. Shender for the helpful comments. We would also like to thank Hanna Kokko and an anonymous reviewer for providing valuable comments. Funding was provided by PERT of the Center for Insect Science, University of Arizona, NIH grant 5 K12 GM000708.
References
- Aktipis CA. Know when to walk away: contingent movement and the evolution of cooperation. J. Theor. Biol. 2004;231:249–260. doi: 10.1016/j.jtbi.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Arnqvist G. The evolution of water strider mating systems: causes and consequences of sexual conflicts. In: Choe JC, Crespi BJ, editors. Social competition and cooperation in insects and arachnids. Vol. I. Cambridge: Cambridge Univ. Press; 1997. pp. 146–163. [Google Scholar]
- Bauer S, Samietz J, Berger U. Sexual harassment in heterogeneous landscapes can mediate population regulation in a grasshopper. Behav. Ecol. 2005;16:239–246. [Google Scholar]
- Blanckenhorn WV. Fitness consequences of alternative life histories in water striders, Aquarius remigis (Heteroptera: Gerridae) Oecologia. 1994;97:354–365. doi: 10.1007/BF00317325. [DOI] [PubMed] [Google Scholar]
- Chapman T, Arnqvist G, Bangham J, Rowe L. Sexual conflict. Trends Ecol. Evol. 2003;18:41–47. [Google Scholar]
- Darden SK, Croft DP. Male harassment drives females to alter habitat use and leads to segregation of the sexes. Biol. Lett. 2008;4:449–451. doi: 10.1098/rsbl.2008.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldakar OT, Dlugos MJ, Pepper JW, Wilson DS. Population structure mediates sexual conflict in water striders. Science. 2009a;326:816. doi: 10.1126/science.1180183. [DOI] [PubMed] [Google Scholar]
- Eldakar OT, Dlugos MJ, Wilcox RS, Wilson DS. Aggressive mating as a tragedy of the commons in the water strider Aquarius remigis. Behav. Ecol. Sociobiol. 2009b;64:25–33. [Google Scholar]
- Eldakar OT, Wilson DS, Dlugos MJ, Pepper JW. The role of multilevel selection in the evolution of sexual conflict in the water strider Aquarius remigis. Evolution. 2010 doi: 10.1111/j.1558-5646.2010.01087.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbairn DJ. The costs of loading associated with mate-carrying in the waterstrider, Aquarius remigis. Behav. Ecol. 1993;4:224–231. [Google Scholar]
- Hardin G. The tragedy of the commons. Science. 1968;162:1243–1248. [PubMed] [Google Scholar]
- Haskins KE, Sih A, Krupa JJ. Predation risk and social interference as factors influencing habitat selection in two species of stream-dwelling waterstriders. Behav. Ecol. 1997;8:351–363. [Google Scholar]
- Hu DL, Chan B, Bush JWM. The hydrodynamics of water strider locomotion. Nature. 2003;424:663–666. doi: 10.1038/nature01793. [DOI] [PubMed] [Google Scholar]
- Kaitala A, Dingle H. Spatial and temporal variation in wing dimorphism of California populations of the water strider Aquarius remigis (Heteroptera: Gerridae) Ann. Entomol. Soc. Am. 1992;85:590–595. [Google Scholar]
- Kokko H, Rankin DJ. Lonely hearts or sex in the city? Density-dependent effects in mating systems? Phil. Trans. Roy. Soc. B. 2006;361:319–334. doi: 10.1098/rstb.2005.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupa JJ, Leopold WR, Sih A. Avoidance of male giant water striders by females. Behaviour. 1990;115:247–253. [Google Scholar]
- Krupa JJ, Sih A. Experimental studies on water strider mating dynamics: spatial variation in density and sex ratio. Behav. Ecol. Sociobiol. 1993;33:107–120. [Google Scholar]
- Krupa JJ, Sih A. Fishing spiders, green sunfish, and a stream-dwelling water strider: male–female conflict and prey responses to single versus multiple predator environments. Oecologia. 1998;117:258–265. doi: 10.1007/s004420050656. [DOI] [PubMed] [Google Scholar]
- Krupa JJ, Sih A. 2003 [Google Scholar]
- Le Galliard JF, Fitze PS, Ferriere R, Clobert J. Sex ratio bias, male aggression, and population collapse in lizards. Proc. Natl. Acad. Sci. USA. 2005;102:18231–18236. doi: 10.1073/pnas.0505172102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessells CM. The evolutionary outcome of sexual conflict. Phil. Trans. Roy. Soc. B. 2006;361:301–317. doi: 10.1098/rstb.2005.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okasha S. Evolution and the levels of selection. Oxford: Oxford University Press; 2006. [Google Scholar]
- Pepper JW. Simple models of assortment through environmental feedback. Artif. Life. 1997;13:1–9. doi: 10.1162/artl.2007.13.1.1. [DOI] [PubMed] [Google Scholar]
- Pepper JW, Smuts BBB. A mechanism for the evolution of altruism among non-kin: positive assortment through environmental feedback. Am. Nat. 2002;160:205–213. doi: 10.1086/341018. [DOI] [PubMed] [Google Scholar]
- Preziosi RF, Fairbairn DJ. Genetic population structure and levels of gene flow in the stream-dwelling waterstrider, Aquarius (= Gerris) remigis (Hemiptera: Gerridae) Evolution. 1992;46:430–444. doi: 10.1111/j.1558-5646.1992.tb02049.x. [DOI] [PubMed] [Google Scholar]
- Rankin DJ. Resolving the tragedy of the commons: the feedback between intraspecific conflict and population density. J. Evol. Biol. 2007;20:173–180. doi: 10.1111/j.1420-9101.2006.01211.x. [DOI] [PubMed] [Google Scholar]
- Rankin DJ, Bargum K, Kokko H. The tragedy of the commons in evolutionary biology. Trends Ecol. Evol. 2007;22:643–651. doi: 10.1016/j.tree.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Rankin DJ, Kokko H. Sex, death and tragedy. Trends Ecol. Evol. 2006;21:225–226. doi: 10.1016/j.tree.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Rankin DJ, López-Sepulcre A. Can adaptation lead to extinction? Oikos. 2005;111:616–619. [Google Scholar]
- Rubenstein DI. Resource acquisition and alternative mating strategies in water striders. Am. Zool. 1984;24:345–353. [Google Scholar]
- Sih A, Krupa JJ. Interacting effects of predation risk, sex ratio and density on male/female conflicts and mating dynamics of stream water striders. Behav. Ecol. 1995;6:316–325. [Google Scholar]
- Sih A, Watters J. The mix matters: behavioural types and group dynamics in water striders. Behaviour. 2005;142:1423–1443. [Google Scholar]
- Turlure C, Van Dyck H. On the consequences of aggressive male mate-locating behaviour and micro-climate for female host plant use in the butterfly Lycaena hippothoe. Behav. Ecol. Sociobiol. 2009;64:1–11. [Google Scholar]
- Watson PJ, Arnqvist G, Stallmann R. Sexual conflict and the energetic costs of mating and mate choice in water striders. Am. Nat. 1998;15:46–58. doi: 10.1086/286101. [DOI] [PubMed] [Google Scholar]
- Weigensberg I, Fairbairn DJ. Conflicts of interest between the sexes: a study of mating interactions in a semiaquatic bug. Anim. Behav. 1994;48:893–901. [Google Scholar]
- Wigby S, Chapman T. Sex peptide causes mating costs in female Drosophila melanogaster. Curr. Biol. 2005;15:316–321. doi: 10.1016/j.cub.2005.01.051. [DOI] [PubMed] [Google Scholar]
- Wilcox RS. Sex discrimination in Gerris remigis: role of a surface wave signal. Science. 1979;206:1325–1327. doi: 10.1126/science.206.4424.1325. [DOI] [PubMed] [Google Scholar]
- Wilcox RS. Male copulatory guarding enhances female foraging in a water strider. Behav. Ecol. Sociobiol. 1984;15:1–174. [Google Scholar]
- Wilcox RS, Ruckdeschel R. Food threshold territoriality in a water strider (Gerris remigis) Behav. Ecol. Sociobiol. 1982;11:85–90. [Google Scholar]
- Wilson DS. A theory of group selection. Proc. Natl. Acad. Sci. USA. 1975;72:143–146. doi: 10.1073/pnas.72.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DS, Wilson EO. Rethinking the theoretical foundation of sociobiology. Q. Rev. Biol. 2007;82:327–348. doi: 10.1086/522809. [DOI] [PubMed] [Google Scholar]