Abstract
Facilitative UT-B urea transporters enable the passage of urea across cell membranes. Gastrointestinal urea transporters are thought to play a significant role in the urea nitrogen salvaging process that occurs between mammalian hosts and their gut bacteria. This study investigated the expression of UT-B urea transporters in different segments of human colon. Immunoblot analysis showed that human colon expressed a 35-kDa glycosylated UT-B protein in the colonic mucosa. The 35-kDa UT-B transporter was predominantly located in plasma membrane-enriched samples (P < 0.001; n = 6), and its expression was greater in the ascending colon compared with the descending colon (P < 0.01; n = 3). At the cellular level, UT-B transporters were located throughout colonocytes situated in the upper portion of the colonic crypts. Bidirectional trans-epithelial urea transport was significantly greater in the ascending colon than the descending colon (P < 0.05; n = 6). In addition, the facilitative urea transporter inhibitor 1,3,dimethylurea significantly reduced urea transport in the ascending colon (P < 0.05; n = 6) but had no effect in the descending colon (NS; n = 6). These results illustrate differential protein abundance of functional UT-B protein in different sections of the human colon, strongly correlating to regions that contain the largest populations of intestinal bacteria. This study suggests an important role for UT-B urea transporters in maintaining the symbiotic relationship between humans and their gut bacteria.
Keywords: gastrointestinal tract, ascending colon, descending colon
facilitative urea transporters enable the passage of urea across cell membranes down a concentration gradient. Like other mammals, humans possess urea transporters derived from UT-A and UT-B genes (1, 25). The tissue localization of human urea transporters is similar to that reported in other animals. For example, human UT-A1 is located in the renal inner medullary collecting duct (1), while human UT-B1 is found in red blood cells (25). As in other species, human urea transporters have also been localized in the gastrointestinal tract. Initially, human UT-B transcripts were reported in both the small intestine and the colon (26, 28). The presence of UT-B at the protein level has also been reported in human colonic crypts, localized to the epithelial layers, and in the Caco-2 intestinal cell line (11). In terms of UT-A transporters, a novel hUT-A6 isoform has been located in the human gastrointestinal tract at the RNA level (30).
The precise physiological role for gastrointestinal urea transporters remains to be fully determined. In other mammals, particularly ruminants, there is gathering evidence that these transporters allow the passage of blood urea into the lumen of the gastrointestinal tract (20, 22, 31, 33). They therefore play a vital role in the urea nitrogen salvaging (UNS) process in which urea supplied by the host is broken down by bacterial urease to release nitrogen for bacterial growth. In turn, bacterial amino acids and peptides can then be reabsorbed by the mammalian host via epithelial transport systems (32). UNS hence enhances the symbiotic relationship between mammalian host and its gut microbiota, helping maintain nitrogen balance in situations of low protein intake or high protein demand (32).
Although UNS is thought to be less important to human nutrient balance than in ruminants, the maintenance of a healthy gut microbial population is still vital to our health (24). Recent studies have suggested that the microbial populations in our gastrointestinal tract differ greatly between individuals (7) and can have a significant effect on disease states, including diabetes (6), inflammatory bowel disease (14), and obesity (3). Alteration of gut bacterial populations has been shown to effect mammalian capacity to obtain energy from the diet (35) and differences between the intestinal bacterial populations of lean and obese humans have been observed (16). These studies clearly define the importance to human health of understanding our symbiotic relationship with intestinal bacterial populations and in particular the regulation of the UNS process.
It has been well documented that 20–25% of urea produced in humans is hydrolyzed in the colon (8, 36) and that the human colon is permeable to urea (23). As a result, it is important to determine whether urea transporter expression, and the resulting trans-epithelial urea transport, correlate to the varying bacterial populations present in subsections of the human colon. This study therefore investigated the protein abundance of UT-B urea transporters and functional trans-epithelial urea transport in different segments of human colon.
MATERIALS AND METHODS
Tissue source and ethical approval.
Human colon was obtained at surgical resection for colonic carcinoma. The normal histological appearance of tissues was confirmed by routine pathological examination of samples obtained during dissection. Tissues from the resection margins were immediately transferred to the laboratory in preoxygenated Krebs-Henseleit (KH) solution (composition in mM; 118 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, and 11.1 d-glucose, pH 7.4). St. Vincent's University Hospital Institutional Review Board approval (including informed patient consent) was granted for this study. [Note: human colonic protein samples and paraffin-embedded tissue sections were also purchased from AMS Biotechnology.]
Antisera.
To study the distribution of human UT-B transporters, we utilized the previously characterized UT-B antisera BUTB-PAN (33). This antisera was raised in chickens against an immunizing peptide that corresponded to amino acids 366–384 (H2N-EENRIFYLQSRKRTVQGPL-C00H) at the C terminus of bovine UT-B1 (GenBank Accession No. AY624602).
Immunoblotting.
Human gastrointestinal tissue protein samples were obtained (AMS Biotechnology), or alternatively, human colon tissue was homogenized with an automated homogenizer and a specifically prepared buffer (300 mM mannitol and 12 mM HEPES, pH 7.6). Homogenates were spun at 2,500 g at room temperature for 5 min, and the pelleted cellular debris was removed to leave the whole cell homogenate. This sample was further spun at 17,000 g at room temperature for 30 min, producing 1) a pellet of plasma-membrane enriched protein and 2) a supernatant containing intracellular membrane proteins, both of which were retained. Deglycosylation experiments were undertaken by preincubating protein for 1 h at 37°C with and without the presence of PNGaseF enzyme (New England Biolaboratories). For loading onto Western gels, 2× reducing Laemmli sample buffer (5% SDS, 25% glycerol, 0.32 M Tris, pH 6.8, bromophenol blue, and 5% β-mercaptoethanol) was added to protein samples in a ratio of 1:1 and then heated at 70°C for 15 min. SDS-PAGE was performed on minigels of 8 or 12% polyacrylamide by loading ∼20 μg protein per lane. After transfer to nitrocellulose membranes, immunoblots were probed for 16 h at room temperature with a 1:2,000 dilution of the affinity-purified UT-B urea transporter antisera (BUTB-PAN). Immunoblots were then washed and probed with a 1:50,000 dilution of horseradish peroxidase-linked secondary anti-chicken antiserum (AVES Laboratories) for 1 h at room temperature. After further washing, detection of protein was performed using EZ-ECL chemiluminescence detection kit (Geneflow) and ECL film (GE Healthcare). Images of developed film were then captured with Image Reader LAS-4000 package, and densitometry was analyzed with ImageJ software (National Institutes of Health).
Immunolocalization.
Paraffin-embedded 10-μM sections of human colon tissue were purchased for this study (AMS Biotechnology). After xylene treatment and rehydration in a descending series of ethanol concentrations (100–70%), endogenous peroxidase was blocked by incubating sections for 30 min in 3% hydrogen peroxide in methanol. Antigen retrieval was performed by boiling sections for 10 min in a solution containing 25 mM Tris·HCl (pH 8.0), 10 mM EDTA, and 50 mM glucose before overnight incubation at 4°C with 1:1,000 dilution of affinity-purified BUTB-PAN diluted in 0.1% BSA and 0.3% Triton X-100 in PBS. Labeling was visualized with a 1:1,000 dilution of anti-chicken horseradish peroxidase-conjugated secondary antibody (AVES Laboratories), followed by incubation with diaminobenzidine and counterstaining with hematoxylin. Stained sections were then dehydrated in an ascending series of ethanol concentrations (70–100%) and treated with xylene, and coverslips were mounted using Eukitt mounting medium.
Ussing chamber experiments.
Human colonic mucosa was dissected from the overlying muscle by blunt dissection. Mucosal sheets were mounted in Ussing chambers (0.63-cm2 aperture) bathed bilaterally in 5 ml of KH solution and gassed with 95%O2-5%CO2 at 37°C. Short circuit current (ISC, μA/cm2) and voltage (PD, mV) were continuously measured by means of attached Ag-AgCl agar-salt bridge electrodes (3% agar in 3 mol/l of KCl). The transepithelial potential difference generated by the epithelium was continuously short-circuited by passing current across the tissue and was adjusted by a feedback amplifier (World Precision Instruments) to keep the clamp voltage at 0 mV. The amount of current required was recorded using a MacLab data acquisition system (AD Instruments, Hastings, UK). Transepithelial electrical resistance (Ω · cm2) was calculated by applying Ohm's law (R = V/I). Responses to basolateral application of the cholinergic secretagogue carbachol (10 μM) were used to confirm tissue viability at the end of experiments.
Urea flux studies.
Measurement of radiolabeled [14C]urea in the absorptive (lumen to blood) and secretory (blood to lumen) directions was used to determine urea flux. Urea was added to give a total unlabeled concentration of 1 mM. [14C]urea (0.1 μCi/ml) was added to either the apical or basolateral chamber, and in each case an equivalent concentration of unlabeled substrate was present in the contralateral chamber. The tissues were equilibrated for 10 min, when 200-μl samples from both apical and basal chambers were taken. Then, 200-μl samples were then taken every 30 min for up to 120 min. 1,3-Dimethylurea (DMU; 50 mM; Sigma-Aldrich, Dublin, Ireland) was added at 40 min to both apical and basolateral chambers. Vehicle (DMSO) was added to control chambers. Radioactivity was measured using a liquid scintillation analyzer (Packard Tricarb 2900 TR). Fluxes were calculated from the disintegrations/min (dpm) using the apparent permeability coefficient (Papp) equation: Papp = dQ/dt × 1/(A·C0), where Papp is the apparent permeability coefficient (cm/s), dQ/dt is the steady-state flux (mol/s), A is the surface area of membrane (cm2), and C0 is the initial concentration in donor chamber (mol/cm3; Ref. 17).
Statistical analysis.
Data are means ± SE, with n representing the number of samples. Unpaired t-tests or one-way ANOVA were used as appropriate. Groups were deemed statistically significant if P < 0.05, and ANOVA was performed using the Tukey post hoc test (Instat, GraphPad Software).
RESULTS
Initial immunoblot analysis of whole cell homogenates of human colonic protein showed that BUTB-PAN antibody detected strong signals at 30 and 35 kDa (see Fig. 1). Deglycosylation using PNGaseF enzyme treatment reduced the 35-kDa protein signal down to 30 kDa in size. Preincubation of BUTB-PAN with 1 μg per ml of its immunizing peptide completely ablated all these signals.
Fig. 1.
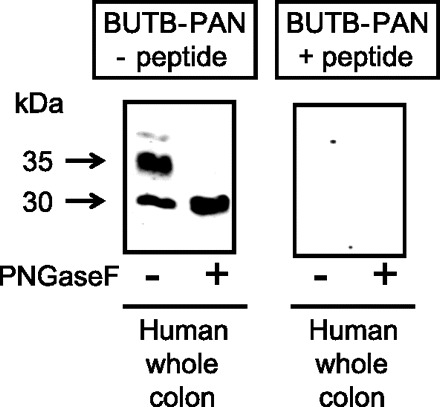
Immunoblots showing whole homogenate protein samples from human colonic tissue probed using the affinity-purified UT-B urea transporter antisera (BUTB-PAN) antibody either with or without 2-h preincubation in 1 μg/ml of specific immunizing peptide. BUTB-PAN detected signals at 30 and 35 kDa within human colon protein. Deglycosylation reduced the 35-kDa band down to 30 kDa, while having no further effect on the 30-kDa signal. These signals were absent when BUTB-PAN was preincubated in its immunizing peptide.
Further immunoblot analysis was undertaken on separated colonic mucosa and colonic muscle following surgical resection (see Fig. 2A). In addition to whole cell homogenates, protein samples enriched for plasma membranes (“17 g”) and intracellular membranes (“SN”) were also analyzed. Colonic mucosal protein contained the majority of 30- and 35-kDa UT-B protein. The 35-kDa signal was located in 17-g protein, while the 30-kDa signal was predominantly within SN protein. In contrast, colonic muscle protein only contained a weak 30-kDa signal. The minimal amount of degraded UT-B protein present at the dye front of this immunoblot illustrates that the protocol was sufficient to prevent considerable breakdown of membrane proteins. Densitometry analysis of further immunoblots of colonic mucosal samples conclusively showed that 35-kDa glycosylated UT-B protein was almost entirely located in 17-g samples (P < 0.001 by ANOVA; n = 6), while the 30-kDa unglycosylated protein was mainly situated within SN samples (P < 0.05 by ANOVA; n = 6; see Fig. 2B).
Fig. 2.
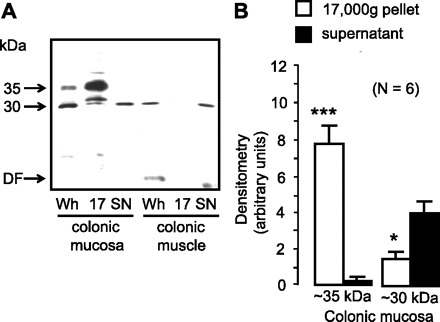
A: immunoblot comparing protein from colonic mucosa and colonic muscle probed with BUTB-PAN antibody. Colonic mucosa contained the vast majority of the 30- and 35-kDa signals, with only the 30-kDa band present in colonic muscle. The 35-kDa protein was predominantly located in the plasma membrane enriched protein samples, while the 30-kDa signal was strongest in the intracellular membrane-enriched protein. There was minimal degraded UT-B protein present at the dye front of the immunoblot. DF, dye front of protein samples; Wh, whole homogenate; 17, 17,000-g pellet containing plasma membrane-enriched protein; SN, 17,000-g supernatant containing intracellular membrane-enriched protein. B: summary of immunoblot densitometry results for UT-B signals within different human colonic mucosal samples. *P < 0.05, ***P < 0.001 vs. supernatant signal by ANOVA (n = 6).
Immunolocalizaton studies were performed using 10-μM sections of paraffin-embedded human colonic tissue and the BUTB-PAN antibody. As expected, BUTB-PAN successfully detected UT-B1 protein on the plasma membrane of red blood cells that were situated within colonic arteries (see Fig. 3A). BUTB-PAN also produced strong staining of colonic mucosal tissue in the upper portion of the colonic crypts (see Fig. 3B). Both longitudinal (see Fig. 3C) and transverse (see Fig. 3D) sections of this upper crypt regions suggested that this BUTB-PAN signal was strongest in the basolateral region of the colonocytes, although staining was also present at the apical membrane (see Fig. 3C). In addition, strong BUTB-PAN staining was present throughout the surface epithelial cells (see Fig. 3E), particularly in the cytoplasm.
Fig. 3.
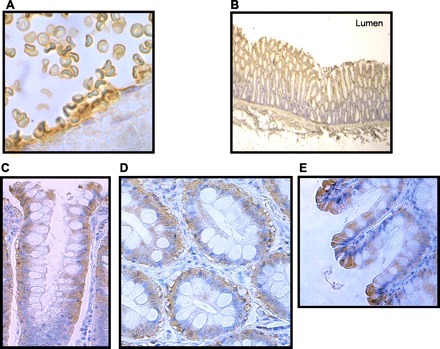
Immunolocalization of UT-B protein within human colon tissue using BUTB-PAN antibody (brown staining) and hematoxylin counterstaining (blue staining). A: BUTB-PAN stains plasma membrane of red blood cells within a human colonic artery (×100 magnification). B: longitudinal section (LS) of colon shows BUTB-PAN only stains the upper portion of the colonic crypts (×3 magnification). C: longitudinal section of colon shows BUTB-PAN signal is predominantly confined to the basolateral region of colonocytes situated in the upper crypt region, although some apical staining is also present (×63 magnification). D: transverse section (TS) of colon confirms BUTB-PAN signal is in the basolateral region of colonocytes (×63 magnification). E: longitudinal section of surface epithelial layer shows BUTB-PAN signal is present throughout the cytoplasm of these cells (×100 magnification).
To investigate UT-B expression in different segments of human colon, we examined whole cell homogenate protein samples from the colonic subsections of an individual subject (purchased from AMS Biotechnology), namely ascending, transverse, descending, and sigmoid colon. Immunoblot analysis suggested that while expression of the 35-kDa UT-B protein varied, the 30-kDa signal was relatively constant (see Fig. 4A). This was not due to differing amount of protein loaded, as shown by the ProtoBlue staining of an identically loaded gel. Differential protein degradation could also be ruled out, due to the minimal signals obtained at the dye front of the immunoblot. Further deglycosylation analysis confirmed that the glycosylated 35 kDa strongly expressed in the ascending colon was largely absent in the descending colon (see Fig. 4B), while the total UT-B protein abundance represented by the deglycosylated 30-kDa signal was unchanged between the two regions.
Fig. 4.
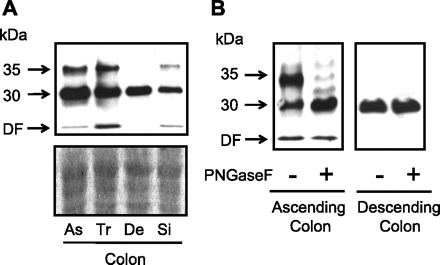
A: comparison of UT-B protein abundance detected by BUTB-PAN in different sections of the human colon against the amount of protein loaded, as shown by ProtoBlue gel staining. Immunoblot suggests that abundance level of glycosylated 35-kDa UT-B protein varies in different sections of the human colon. As, ascending colon; Tr, transverse colon; De, descending colon; Si, sigmoid colon. B: deglycosylation immunoblots confirm differential abundance of the 35-kDa glycosylated UT-B protein between ascending and descending colon.
To confirm this differential abundance of glycosylated UT-B protein in different segments of human colon, we compared surgical tissue samples from the ascending and descending colon from a number of subjects. Immunoblot analysis investigated colonic mucosal abundance of the glycosylated 35-kDa UT-B protein in 17,000-g pellet samples and the unglycosylated 30-kDa UT-B protein in 17,000-g supernatant samples (see Fig. 5A). Once again, minimal degraded UT-B protein was present at the dye front. Densitometry analysis confirmed a significant increase in glycosylated 35-kDa UT-B protein in the ascending colon (P < 0.01 by ANOVA; n = 3). In contrast, the expression of the unglycosylated 30-kDa UT-B protein was significantly reduced in the ascending colon (P < 0.05 by ANOVA; n = 3; see Fig. 5B).
Fig. 5.
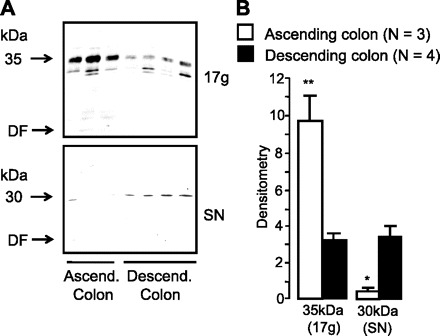
Immunoblots comparing UT-B abundance between ascending and descending colon protein, using surgical tissue samples and the BUTB-PAN antibody. A: immunoblot comparing expression between ascending and descending human colon of 1) glycosylated 35-kDa UT-B protein in plasma membrane-enriched samples and 2) unglycosylated 30-kDa UT-B protein in intracellular membrane-enriched samples. B: summary of the immunoblot densitometry results for UT-B abundance in ascending and descending human colon. Although there was a significant decrease in the levels of unglycosylated 30-kDa UT-B protein, the ascending colon actually contained significantly more glycosylated 35-kDa UT-B protein than the descending colon. *P < 0.05, **P < 0.01 vs. descending colon by ANOVA (n = 3).
To determine functional differences associated with altered UT-B abundance, we performed flux studies in the ascending and descending human colon, using 50 mM DMU to inhibit UT-B function. Initial basal electrophysiological parameters for human colon are shown in Table 1. Importantly, there were no significant differences between the ascending and descending colon in terms of short-circuit current (ISC), transepithelial electrical resistance, or response to 10 μM carbachol (ΔISC). [Note: addition of urea or DMU did not significantly alter basal parameters or responses to carbachol.]
Table 1.
Baseline electrophysiological parameters and responses to carbachol for ascending and descending colon
| Tissue | ISC, μA/cm2 | TEER, Ω·cm2 | ΔISC CCh (10 μM) |
|---|---|---|---|
| Ascending colon (n = 6) | 50.7 ± 4.3 | 110.4 ± 3.9 | 23.9 ± 2.2 |
| Descending colon (n = 6) | 61.9 ± 13.5 | 110.6 ± 8.5 | 28.9 ± 6.3 |
Values are means ± SE; n = number of patients. ISC, short-circuit current; TEER, transepithelial electrical resistance; CCh, carbachol.
In agreement with the regional variation in UT-B protein abundance, the human ascending colon was significantly more permeable to urea compared with the descending colon in both directions (P < 0.05 by unpaired t-test; n = 6; see Fig. 6). This was not due to a paracellular leak flux of urea, as tissue resistances were virtually identical between these two segments of colon. However, both the absorption (apical-to-basolateral) and secretion (basolateral-to-apical) of urea in the ascending colon were significantly inhibited by DMU (P < 0.05 by unpaired t-test; n = 6; see Fig. 7). In direct contrast, the urea flux in the descending colon in either direction was completely unaffected by DMU (NS by unpaired t-test; n = 6).
Fig. 6.
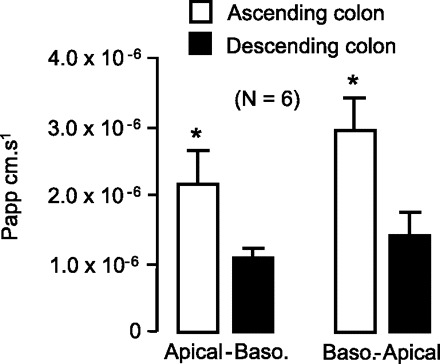
Urea flux was significantly higher in human ascending colon, compared with descending colon, for both apical-basolateral and basolateral-apical directions. Papp, apparent permeability coefficient. *P < 0.05 by unpaired t-tests (n = 6 patients).
Fig. 7.
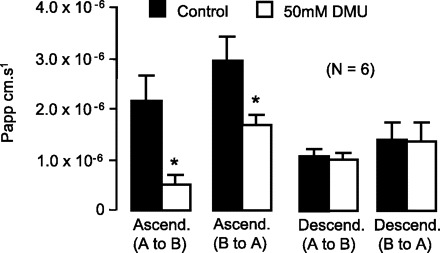
Effect of 50 mM 1,3-dimethylurea (DMU) on urea fluxes in the human colon. Urea flux in both the absorptive and secretory directions were significantly inhibited by DMU in human ascending colon. In contrast, urea flux in human descending colon was completely unaffected by DMU in either direction. *P < 0.05 vs. control data by unpaired t-test (n = 6).
DISCUSSION
In this study, we investigated the expression of UT-B urea transporters in different segments of human colon. Immunoblot analysis using BUTB-PAN antibody detected a 35-kDa protein in whole colon tissue that was deglycosylated to 30 kDa by PNGaseF enzyme activity (see Fig. 1). These signals were confirmed to represent human UT-B transporter proteins, since they were absent in the presence of the original immunizing peptide. Although requiring further investigation, it is most likely that these proteins represent either UT-B1, previously reported in the human colon (11), or a similar sized novel isoform.
Separating colonic tissue into muscle and mucosa revealed that both 30- and 35-kDa proteins were predominantly mucosal in nature (see Fig. 2A). Furthermore, the glycosylated 35-kDa UT-B protein was located in the plasma membrane, while the unglycosylated 30 kDa was mainly intracellular. Further analysis of colonic protein from six different subjects confirmed this pattern (see Fig. 2B), strongly suggesting that glycosylation of the basic 30-kDa UT-B protein to a 35-kDa protein is required for plasma membrane expression to occur. Although the precise mechanisms involved remain to be determined, we have recently described a similar observation for another gastrointestinal urea transporter, bovine UT-B2 (33), which is expressed in the bovine rumen (31) and regulated by dietary intake (29). A previous report (2) has detailed how glycosylation of UT-A1 urea transporters is required to produce trans-epithelial urea transport. The role of glycosylation in the localization and function of gastrointestinal UT-B urea transporters is therefore an important area for future research.
Our immunolocalization studies showed that UT-B protein was localized to the upper regions of the colonic crypts. It appeared weakly expressed in the apical region and strongly expressed in the basolateral region of the crypt cells, while it was present throughout the cytoplasm of surface epithelial cells (see Fig. 3). These findings contrast to the purely apical membrane location within superficial epithelial cells reported in the only previous study of human colonic UT-B urea transporters (11). However, this previous report detected a 50-kDa glycosylated UT-B protein, suggesting that more than one UT-B isoform exists in the human colon. The presence of two UT-B transporters varying in cellular location would explain these differences in immunolocalization. Further investigation is therefore now required to determine the identity of all human colonic UT-B transporters, through the use of multiple N- and C-terminal UT-B antibodies and extensive RT-PCR analysis with UT-B primer sets. Immunofluorescence studies are also required to obtain a more precise location for UT-B protein within different types of colonic cell. Interestingly, a previous report (4) on UT-B staining in rat colon also suggested basolateral and cytoplasmic staining in columnar and goblet cells, particularly in cells nearest the lumen.
After our initial observation that expression of glycosylated 35-kDa UT-B protein varied in different segments of the colon (see Fig. 4), we determined that abundance of this protein was indeed greater in the ascending colon than the descending colon (see Fig. 5). Importantly, there was also the significant decrease in unglycosylated 30-kDa signal within the ascending colon supernatant samples, strongly suggesting the hypothesis that it is the extent of glycosylation that varies between colonic regions. The glycosylated 35-kDa protein represents the functional UT-B urea transporter in the plasma membrane, so this finding has very important implications for trans-epithelial urea transport. Indeed, in agreement with this UT-B abundance profile, bidirectional urea permeability of the ascending colon was greater than that of the descending colon (see Fig. 6). The increased urea transport observed in both directions was due to DMU-sensitive trans-epithelial urea flux (see Fig. 7) and hence must have been mediated through facilitative urea transporters. The resulting higher urea permeability of the ascending colon would allow greater secretion of urea into the gastrointestinal tract lumen, increasing this regions’ ability to facilitate bacterial growth through the UNS process. Indeed, in agreement with this hypothesis, it has previously been reported that there is increased transepithelial urea transport in rat proximal colon compared with distal colon (9).
It is well documented that the ascending or “proximal” colon is the main site of saccharolytic bacterial fermentation (34), due to higher substrate availability (21). We therefore hypothesize that an increased urea transport facility is necessary in this region to provide nitrogen for the increased levels of bacterial growth. Hence, this region requires increased glycosylation of UT-B to produce higher plasma membrane expression levels and therefore greater UT-B-mediated urea transport. It has recently been reported that the amount of urea that is hydrolyzed in the colon increases from 25 to ∼50–60% of total urea production when dietary protein intake is altered (10). It therefore seems highly likely that this important physiological change would occur via regulation of the colonic UT-B transporter function reported in this present study.
Although functional heterogeneity of human colon has previously been reported for fluid secretion (27), little research has been performed looking at the UNS process itself. However, there are reports that expression patterns of the membrane transporters that may be involved are indeed heterologous, including MCT1 (12), NHE3 (5), and UT-A urea transporters (15).
Differential expression of human UT-B may contribute to the variation in colonic bacterial populations. For example, Eubacterium rectale is a gram-positive firmicute bacteria that produces butyric acid (19) and, compared with gram-negative Bacteroidetes bacteria, is known to thrive in the more acidic conditions found in the ascending colon (18). E. rectale has also been reported as having a high urease activity (13) and therefore is ideally suited to utilize the urea that enters the ascending colon through UT-B transporters. Similarly, another butyric acid producing firmicute, Faecalibacterium prausnitzii, is also strongly ureolytic (37) and presumably could also utilise UT-B-mediated urea as a nitrogen source. The fact that F. prausnitzii is reported to be decreased in Crohn's disease (19) illustrates the potential importance to human health of bacteria “fed” with urea by colonic urea transporters.
In conclusion, UT-B urea transporters play an important role in the transport of urea in the human colon and as a result in the maintenance of normal colonic bacterial populations. The differential protein abundance of UT-B transporters at the plasma membrane along the colon helps maintain the symbiotic relationship between humans and their gut bacteria. Regulation of UT-B-mediated urea transport is therefore a potential future site for intervention to influence colonic microbial populations and hence human health.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGEMENTS
This work was funded by The Royal Society and the Irish Research Council for Science, Engineering and Technology postgraduate scholarship scheme.
REFERENCES
- 1. Bagnasco SM, Peng T, Janech MG, Karakashian A, Sands JM. Cloning and characterization of the human urea transporter UT-A1 and mapping of the human Slc14a2 gene. Am J Physiol Renal Physiol 281: F400–F406, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Chen G, Frohlich O, Yang Y, Klein JD, Sands JM. Loss of N-linked glycosylation reduces urea transporter UT-A1 response to vasopressin. J Biol Chem 281: 27436–27442, 2006 [DOI] [PubMed] [Google Scholar]
- 3. DiBaise JK, Zhang H, Crowell MD, Krajmalnik-Brown R, Decker GA, Rittman BE. Gut microbiota and its possible relationship with obesity. Mayo Clin Proc 83: 460–469, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Doran JJ, Klein JD, Kim HY, Smith TD, Kozlowski SD, Gunn RB, Sands JM. Tissue distribution of UT-A and UT-B mRNA and protein in rat. Am J Physiol Regul Integr Comp Physiol 290: R1446–R1459, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Dudeja PK, Rao DD, Syed I, Joshi V, Dahdal RY, Gardner C, Risk MC, Schmidt L, Bavishi D, Kim KE, Harig JM, Goldstein JL, Layden TJ, Ramaswamy K. Intestinal distribution of human Na+/H+ exchanger isoforms NHE-1, NHE-2 and NHE-3 mRNA. Am J Physiol Gastrointest Liver Physiol 271: G483–G493, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Dumas ME, Barton RH, Toye A, Cloarec O, Blancher C, Rothwell A, Fearnside J, Tatoud R, Blanc V, Lindon JC, Mitchell SC, Holmes E, McCarthy MI, Scott J, Gauguier D, Nicholson JK. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Nat Acad Sci USA 103: 12511–12516, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science 308: 1635–1638, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. El-Khoury AE, Fugakawa NF, Sanchez M, Tsay RH, Gleason RE, Chapman TE, Young VR. Validation of the tracer-balance concept with reference to leucine: 24-h intravenous tracer studies with l-[1-13C]leucine and [15N-15N]urea. Am J Clin Nutr 59: 1000–1011, 1994 [DOI] [PubMed] [Google Scholar]
- 9. Fihn BM, Jodal M. Permeability of the proximal and distal rat colon crypt and surface epithelium to hydrophilic molecules. Pflügers Arch 441: 656–662, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Fouillet H, Juillet B, Bos C, Mariotti F, Gaudichon C, Benamouzig R, Tome D. Urea-nitrogen production and salvage are modulated by protein intake in fed humans: results of an oral stable-isotope-tracer protocol and compartmental modelling. Am J Clin Nutr 87: 1701–1714, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Inoue H, Jackson SD, Vikulina T, Klein JD, Tomita K, Bagnasco SM. Identification and characterization of a Kidd antigen/UT-B urea transporter expressed in human colon. Am J Physiol Cell Physiol 287: C30–C35, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Iwanga T, Takebe K, Kato I, Karaki S, Kuwahara A. Cellular expression of monocarboxylate transporters (MCT) in the digestive tract of the mouse, rat and humans, with special reference to slc5a8. Biomed Res 27: 243–254, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Kamiya S, Taniguchi I, Yamamoto T, Sawamura S, Kai M, Ohnishi N, Tsuda M, Yamamura M, Nakasaki H, Yokoyama S. Analysis of intestinal flora of a patient with congenital absence of the portal vein. FEMS Immunol Med Microbiol 7: 73–80, 1993 [DOI] [PubMed] [Google Scholar]
- 14. Kinross JM, von Roon AC, Holmes E, Darzi A, Nicholson JK. The human gut microbiome: implications for future health care. Curr Gastroenterol Rep 10: 396–403, 2008 [DOI] [PubMed] [Google Scholar]
- 15. LaPointe LC, Dunne R, Brown GS, Worthley DL, Molloy PL, Wattchow D, Young GP. Map of differential transcript expression in the normal human large intestine. Physiol Genomics 33: 50–64, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Human gut microbes associated with obesity. Nature 444: 1022–1023, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Lindmark T, Nikkila T, Artursson P. Mechanisms of absorption enhancement by medium chain fatty acids in intestinal epithelial Caco-2 cell monolayers. J Pharmacol Exp Ther 275: 958–964, 1995 [PubMed] [Google Scholar]
- 18. Louis P, Scott KP, Duncan SH, Flinh HJ. Understanding the effects of diet on bacterial metabolism in the large intestine. J Appl Microbiol 102: 1197–1208, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett 294: 1–8, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Ludden PA, Stohrer RM, Austin KJ, Atkinson RL, Belden EL, Harlow HJ. Effect of protein supplementation on expression and distribution of urea transporter B in lambs fed low-quality forage. J Anim Sci 87: 1354–1365, 2009 [DOI] [PubMed] [Google Scholar]
- 21. MacFarlane S, MacFarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc 62: 67–72, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Marini JC, Klein JD, Sands JM, Van Amburgh ME. Effect of nitrogen intake on nitrogen recycling and urea transporter abundance in lambs. J Anim Sci 82: 1157–1164, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Moran BJ, Jackson AA. 15N-urea metabolism in the functioning human colon: luminal hydrolysis and mucosal permeability. Gut 31: 454–457, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mountzouris KC, McCartney AL, Gibson GR. Intestinal microflora of human infants and current trends for its nutritional modulation. Br J Nutr 87: 405–420, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Olives B, Neau P, Bailly P, Hediger M, Rousselet G, Cartron J, Ripoche P. Cloning and functional expression of a urea transporter from human bone marrow cells. J Biol Chem 269: 31649–31652, 1994 [PubMed] [Google Scholar]
- 26. Olives B, Martial S, Mattei MG, Matassi G, Rousselet G, Ripoche P, Catron JP, Bailly P. Molecular characterization of a new urea transporter in the human kidney. FEBS Lett 386: 156–160, 1996 [DOI] [PubMed] [Google Scholar]
- 27. Park JH, Rhee PL, Lee JH, Kim JJ, Rhee JC, Kim SJ, Lee J. Segmental heterogeneity of electrogenic secretions in human ascending colon and rectum. Int J Colorectal Dis 21: 357–364, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Ritzhaupt A, Wood IS, Jackson AA, Moran BJ, Shirazi-Beechey SP. Isolation of a RT-PCR fragment from human colon and sheep rumen RNA with nucleotide sequence similarity to human and rat urea transporter isoforms. Biochem Soc Trans 26: S40, 1998 [DOI] [PubMed] [Google Scholar]
- 29. Simmons NL, Chaudhry AS, Graham C, Scriven ES, Thistlethwaite A, Smith CP, Stewart GS. Dietary regulation of ruminal bUT-B urea transporter expression and localization. J Anim Sci 87: 3288–3299, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Smith CP, Potter EA, Fenton RA, Stewart GS. Characterization of a human colonic cDNA encoding a structurally novel urea transporter, hUT-A6. Am J Physiol Cell Physiol 287: C1087–C1093, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Stewart GS, Graham C, Cattell S, Smith TPL, Simmons NL, Smith CP. UT-B expressed in bovine rumen: potential role in ruminal urea transport. Am J Physiol Regul Integr Comp Physiol 289: R605–R612, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Stewart GS, Smith CP. Urea nitrogen salvage mechanisms and their relevance to ruminants, non-ruminants and man. Nutr Res Rev 18: 49–62, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Tickle P, Thistlethwaite A, Smith CP, Stewart GS. Novel bUT-B2 urea transporter isoform is constitutively activated. Am J Physiol Regul Integr Comp Physiol 297: R323–R329, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev 81: 1031–1064, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444: 1027–1031, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Walser M, Bondelos LJ. Urea metabolism in man. J Clin Invest 38: 1617–1626, 1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wozny MA, Bryant MP, Holdeman LV, Moore WE. Urease assay and urease-producing species of anaerobes in the bovine rumen and human faeces. Appl Environ Microbiol 33: 1097–1104, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]


