Abstract
Endothelial progenitor cells (EPCs) protect kidneys from acute ischemic damage. The aim of this study was to identify “treatment parameters” that optimize an EPC-based therapy of acute ischemic renal failure. Male C57BL/6N mice underwent unilateral nephrectomy with simultaneous contralateral renal artery clamping for 30, 35, and 40 min. Tagged murine EPCs were systemically injected at the time of reperfusion. In some experiments, EPCs were pretreated with the Epac (exchange protein directly activated by cAMP-1) activator 8-pCPT-2′-O-Me-cAMP (Epac-1 Ac) and the integrin binding antagonist cyclic Arg-Gly-Asp peptide (cRGD). Injections of 106 EPCs after 30 and 35 min of renal ischemia protected animals from acute renal failure. The same effect occurred with 0.5×106 EPCs after a 35-min period of ischemia. If ischemia lasted for 40 min, 0.5×106 cells mice did not prevent acute renal failure. To analyze whether EPC integrin receptor activation would modify the cells' renoprotective activity, EPCs were pretreated with Epac-1 Ac. Such animals did not develop acute renal failure, even if ischemia lasted for 40 min. This effect was negated if the cells were pretreated with both Epac-1 Ac and cRGD. In kidneys from those animals medullopapillary EPCs were significantly accumulated. In vitro Epac-1 Ac preactivation of EPCs did not increase the overall expression intensity but induced a redistribution of β1-integrins toward the cell membranes. We conclude that EPC pretreatment with the integrin receptor activator 8-pCPT-2′-O-Me-cAMP augments the anti-ischemic potential of the cells.
Keywords: stem cells, acute renal failure, endothelial progenitor cells, exchange protein directly activated by cAMP
endothelial progenitor cells (EPCs) have been documented to participate in postnatal neovascularization (vasculogenesis) under both physiological and pathological conditions (2, 3, 12, 22, 24, 25). In recent years, the cellular and molecular mechanisms involved in EPC-mediated vasculogenesis and vasoprotection have been elucidated (20). EPCs have been successfully administered in animal models of myocardial and hindlimb ischemia, and the first human studies on the treatment of coronary artery disease with EPC-containing bone marrow cells have been initiated (4).
However, the vast majority of investigations focus on EPCs in ischemic cardiac disease. Recently, we showed for the first time that EPCs are renoprotective in a mouse model of acute ischemic renal failure (16–19). Since EPCs could potentially serve as a therapeutic tool in the treatment of this serious and frequent complication in daily clinical practice, the most important question is related to the therapeutic conditions that help to optimize an EPC-based treatment of acute ischemic renal failure.
In the present study, different populations of either untreated or in vitro pretreated murine EPCs were administered to animals with acute ischemic renal failure of various severity. With respect to ischemia protection we found 1) an inverse correlation between the number of exogenously administered EPCs and the duration of the ischemic episode. 2) EPC pretreatment with the Epac (exchange protein directly activated by cAMP) integrin receptor activator 8-pCPT-2′-O-Me-cAMP (Epac-1 Ac) augmented the anti-ischemic potential of the cells. 3) Combined EPC pretreatment with Epac-1 Ac and the integrin antagonist cRGD (cyclic arginine-glycine-d-aspartic acid) mitigated EPC-mediated renoprotection.
MATERIALS AND METHODS
Animal models.
The animal study protocol was in accordance with the guidelines of the German Institute of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee. C57BL/6N mice were originally obtained from Jackson Laboratory (Bar Harbor, ME) and bred in the local animal facility of the Göttingen University Hospital. For all experiments, male 8–12 wk old C57BL/6N mice were used. All animals were separately caged with a 12:12-h light-dark cycle and had free access to water and chow throughout the study.
Surgical procedures.
Mice were anesthetized (300 μl of 6 mg/100 g ketamine hydrochloride plus 0.77 mg/100 g xylazine hydrochloride) and placed on a heated surgical pad. Rectal temperature was maintained at 37°C. After a 1.5-cm midlaparotomy, the right kidney was removed. The left kidney was exposed and clamping of the renal pedicle was performed with microserrefines (Fine Science Tools, Foster City, CA). After different time periods (30, 35, 40 min) the clamp was released to initiate reperfusion. For induction of acute renal failure contralateral nephrectomy was performed. First, a suture was placed around the renal pedicle. Either without or after the injection of a certain population of mouse-derived EPCs, the suture was closed and the kidney was removed. For injection of cells prior to removal of the kidney, the 29-gauge needle of a cell-containing syringe was inserted into the renal vein directly under the suture. After injection, the suture was rapidly closed to prevent bleeding. EPCs were systemically injected at the time of reperfusion. The abdominal incision was closed with a 4-0 suture and surgical staples. Two days (48 h) after this procedure, animals were euthanized and blood, kidney, and spleen were collected for further analysis. In each experimental group five to six animals were analyzed.
Culture of mouse EPCs.
Mouse mononuclear cells were isolated by density gradient centrifugation with Biocoll solution (Biochrom, Berlin, Germany) from peripheral blood, spleen cell extracts, and bone marrow samples from both femoral bones, respectively. The reason for pooling EPCs was to maximize the total number of cells available for injection. Immediately following isolation, mononuclear cells of the three different sources were mixed and 4×106 cells were plated on 24-well culture dishes coated with human fibronectin (Sigma, St. Louis, MO) and maintained in endothelial cell growth medium-2 (EGM-2; Clonetics, Lonza, Walkersville, MD) supplemented with EGM Single-Quots containing 5% FCS (9). After 4–5 days of culture, EPCs were characterized by the uptake of DiI-labeled acetylated low-density lipoprotein (acLDL) (Invitrogen, Carlsbad, CA) and binding of FITC-labeled BS-1 lectin (BS-1) (Sigma Diagnostics, St. Louis, MO). For this purpose, cells were first incubated with 10 μg/ml DiI-acLDL at 37°C for 1 h and later fixed with 2% formaldehyde for 10 min, followed by incubation with BS-1 lectin at 37°C for 1 h. Cells that demonstrated double-positive immunofluorescence were defined as EPCs. In some experiments, cells were incubated with rat anti-mouse CD29 (1:500 in PBS-BSA 1%) (BD Biosciences, Rockville, MD) for 1 h at room temperature, followed by secondary incubation with Alexa Fluor 647 goat anti-rat IgG (1:200 in PBS-BSA 1%) (Molecular Probes, Eugene, OR), or with mouse-anti-eNOS (NOS type 3 monoclonal antibody) (1:100 in PBS-BSA 1%) (BD Biosciences, San Diego, CA) for 30 min at room temperature, followed by secondary incubation with Alexa Fluor 647 mouse IgG1 (1:500 in PBS-BSA 1%) (Molecular Probes). For eNOS staining cells were fixed with paraformaldehyde 4% (wt/vol) for 10 min, followed by permeabilization with Triton 0.1% (wt/vol) for 10 min. For laser scanning microscopy Dil-acLDL+/FITC BS-1 lectin+ myelomonocytic cells were gated. All double-positive cells were analyzed for CD29 expression. To analyze CD29 expression per individual cell, single cell laser scanning microscopy was performed. Cells were examined by using an inverted fluorescence microscope IX-71 (Olympus Deutschland, Hamburg, Germany) equipped with the appropriate excitation and emission filters (AHF Analysentechnik, Tuebingen, Germany). Images of respective fluorescence channels were recorded as single high-resolution 16-bit black-and-white images using a F-View II extension camera (Olympus Deutschland). The images from every fluorescence channel were then automatically merged using the MFIP-module of the CELL-F software.
EPC in vitro pretreatment.
EPCs employed for systemic injections were detached by trypsinization after the first passage, and, after neutralization of trypsin, incubated with CellTracker [CM-DiI (C7000)-1:1,000 in PBS] (Molecular Probes) for 5 min at 37°C, followed by 15 min of incubation at 4°C. After the cells were washed once with PBS, they were resuspended in 50 μl of EGM-2 for systemic injection or for further in vitro pretreatment. For in vitro pretreatment EPCs were incubated with 8-pCPT-2′-O-Me-cAMP (Epac-1 Ac-100 μmol/l in EGM-2) (Biolog, Bremen, Germany) for 15 min at 37°C. For blocking β1-integrin receptor activity, cells were simultaneously incubated with cRGD (500 nmol/l in EGM-2) (Bachem, Weil am Rhein, Germany) for 15 min at 37°C. After being washed once with EGM-2, the cells were resuspended in 50 μl of EGM-2 for systemic injection.
Western blot analysis.
Untreated or in vitro pretreated murine EPCs were homogenized in lysis buffer [1% (vol/vol) KPO4/EDTA, 5 mM EGTA, 10 mM mgCl2, 50 mM β-glycerolphosphate, 1 mM Na3VaO4, 0.5% (vol/vol) NP4O, 0.1% (vol/vol) Brij-35, 0.1% (vol/vol) PMSF, 0.01% (vol/vol) leupeptin, 0.01% (vol/vol) pepstatin A], followed by 5 min of incubation at 95°C in a mixture of electrophoresis buffer (NuPAGE-NP0009, Invitrogen) and sample buffer (NuPAGE-NP0007, Invitrogen). Equal amounts of protein were electrophoretically separated in 10–20% Tris-glycerine gels (Invitrogen) and transferred to immunoblot membranes (polyvinylidene difluoride membranes, 162-0177, Bio-Rad, Hercules, CA). After being blocked with TBS-Tween 20 (TBS-T) containing nonfat dry milk (5% wt/vol), membranes were incubated with the primary antibody anti-CD29 (550531, 1:500 in TBS-T) (BD Biosciences) overnight at 4°C. After a washing with TBS-T, membranes were incubated with a horseradish peroxidase-conjugated secondary antibody (polyclonal rabbit-anti rat-P0450, 1:2,000, DAKO, Carpinteria, CA) for 60 min at room temperature. Membranes were washed and protein was detected by using a chemiluminescence system (ECL Plus Western Blotting Detection System-RPN2132, GE Healthcare, Amersham, Piscataway, NJ).
Analysis of cell survival.
Trypsinized cells were incubated with Trypan blue (0.4% wt/vol) for 3 min at room temperature. The number of blue cells per 100 cells was evaluated. Blue cells were defined as dead cells.
Morphological evaluation of kidneys.
Formalin-fixated, paraffin-embedded tissue sections were stained with periodic acid-Schiff. Acute tubular damage was semiquantitatively assessed by assigning grade 0 (absent), 1 (minimal), 2 (moderate), and 3 (severe) separately for the following three parameters: tubular epithelial flattening, tubular epithelial necrosis, and epithelial vacuolization.
Immunofluorescence microscopy.
Tissue samples were fixed in a 4% formaldehyde solution for 1 h, followed by incubation in 30% sucrose overnight at 4°C. Embedding was performed in O.C.T. compound (Tissue-Tek, Torrance, CA), and embedded samples were stored at −20°C. Frozen samples were cut into 10-μm-thick sections. Nonspecific protein binding was blocked by 1-h incubation with PBS-BSA (1%). Sections were incubated with FITC-conjugated anti-mouse CD117 (c-Kit, 1:1,000 in PBS-BSA 1%) (BD Biosciences) for 12 h at 4°C. To visualize the nuclei, tissue sections were counterstained with DAPI (1:200 in PBS) (Molecular Probes). Sections were examined as previously described.
Analysis of renal function.
Serum creatinine concentration was measured by using a commercially available kit (Creatinin PAP, Labor und Technik-Eberhard Lehmann, Berlin, Germany) according to the manufacturer's protocol.
Statistical analysis.
The results were expressed as means ± SE. The means of two populations were compared by Student's t-test. Differences were considered significant at P < 0.05.
RESULTS
Expansion of murine acLDL+/BS-1+ EPCs.
Isolation and expansion of EPCs was reproducibly performed from male C57BL/6N mice. After 5 days of culture on fibronectin, mixed mononuclear cells displayed both uptake of acetylated LDL and membrane staining of BS-1 lectin. The cells' viability was 87 ± 0.85% (means ± SD) (Fig. 1). To further characterize the cells, staining of the enzyme endothelial nitric oxide synthase (eNOS) was performed. Both, unstimulated and Epac-1 Ac-prestimulated acLDL+/BS-1+ cells expressed eNOS (Fig. 2).
Fig. 1.
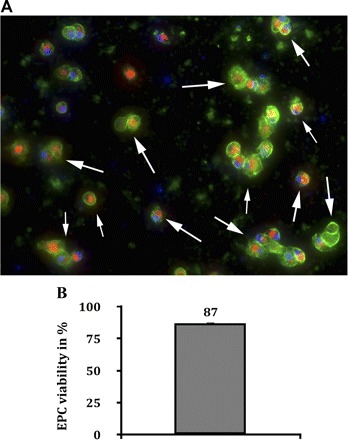
Murine endothelial progenitor cells (EPCs) after 5 days in culture. After 5 days of culture in EBM-2 medium (fibronectin-coated dishes) mixed mononuclear cells, derived from blood, spleen, and bone marrow of donor mice, displayed double-positive staining with both Dil-conjugated acetylated LDL (red) and membrane-bound BS-1 lectin (green). Double-positive staining (indicated by white arrows in A) with acetylated LDL (acLDL) and BS-1 lectin is a major characteristic of EPCs. The cells' viability was 87 ± 0.85% (means ± SD, blue: nuclei, magnification ×100).
Fig. 2.

Expression of the enzyme endothelial nitric oxide synthase (eNOS) in murine EPCs. Murine EPCs cultured from mixed mononuclear cells of blood, spleen, and bone marrow were stained for eNOS expression. Unstimulated as well as Epac (exchange protein directly activated by cAMP)-1 activator 8-pCPT-2′-O-Me-cAMP (Epac-1 Ac)-prestimulated cells displayed eNOS expression [blue: nuclei (DAPI), yellow: Dil-acLDL, green: BS-1 lectin, red: eNOS, magnification ×100].
Effects of different numbers of untreated EPCs on postischemic renal function.
Previous studies proved that EPCs are capable of protecting kidneys from acute ischemic damage (16–19). EPCs migrated into postischemic kidneys after ischemic preconditioning. If EPCs were isolated from the kidneys and systemically administered to animals with acute renal ischemia, renal dysfunction was ameliorated (16, 19). However, treatment conditions that potentially optimize an EPC-based therapeutic regimen in acute ischemic renal failure are unknown so far. To analyze whether different cell numbers and/or the duration of ischemia would influence the therapeutic outcome, mice were exposed to three different periods of renal ischemia (30, 35, and 40 min) and injected with two different populations (0.5×106 and 1×106) of EPCs. Systemic injection of 1×106 EPCs at the end of the ischemic period significantly protected renal function after both a 30-min and a 35-min period of ischemia (Fig. 3). If 0.5×106 EPCs were injected after a 35-min period of ischemia, kidneys were also protected from ischemic damage (Fig. 3). This beneficial effect was absent if ischemia lasted for 40 min. Although creatinine levels were lower in EPC-injected animals, the difference was not significant compared with untreated controls (Fig. 3).
Fig. 3.
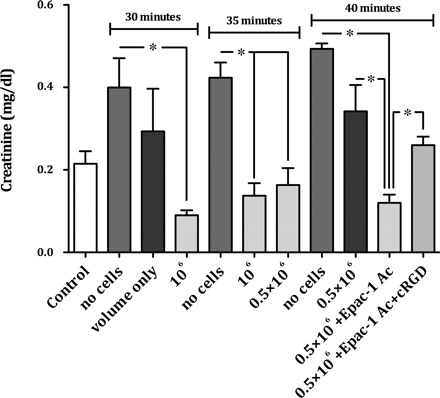
Renal function in mice after 30, 35, and 40 min of renal ischemia with and without systemic injection of untreated or in vitro pretreated murine EPCs. A single injection of 106 untreated EPCs at the end of an ischemic periods of 30 and 35 min protected kidneys from ischemic damage. If 0.5×106 EPCs were injected after a 35-min period of ischemia, kidneys were also protected from ischemic damage. This beneficial effect was absent if ischemia lasted for 40 min. Although creatinine levels were lower in EPC-injected animals, the difference was not significant compared with untreated controls. To test the hypothesis, that a more severe ischemic damage of the kidney, as it occurs after a 40-min period of renal ischemia, can at least in part be prevented by in vitro preactivation of EPCs, the cells were stimulated with Epac-1 Ac. After a 15-min period of in vitro EPC prestimulation, 0.5×106 cells were systemically injected into animals subjected to a 40-min period of renal ischemia. Although the administration of 0.5×106 untreated cells did not protect kidneys from ischemic damage, injection of the same population of Epac-1 Ac-pretreated cells resulted in a significant renoprotection. After combined pretreatment with Epac-1 Ac and cRGD those effects were significantly negated. If animals were injected with volume only (50 μl of cell media), creatinine was lower than in postischemic control animals without volume administration. However, the difference was not significant (data are means ± SE, *P < 0.05).
Consequences of integrin receptor activation on EPC-mediated renoprotection. To test the hypothesis, that a more severe ischemic damage of the kidney, as it occurs after a 40-min period of renal ischemia, can at least in part be prevented by in vitro preactivation of EPCs, the cells were stimulated with the Epac-1 activator Epac-1 Ac. Epac-1 has been documented to increase integrin receptor activity and lateral mobility of β1- and β2-integrins (6). It has also been shown that EPC prestimulation with Epac-1 Ac increases homing of the cells to ischemic muscles in a murine model of hindlimb ischemia (6). Epac-1 and Epac-2 are guanine nucleotide exchange factors for the small GTPases Rap1 and Rap2 (5).
After a 15-min period of in vitro EPC prestimulation, 0.5×106 cells were systemically injected into animals subjected to a 40-min period of renal ischemia. Whereas the administration of 0.5×106 untreated cells did not protect kidneys from ischemic damage, injection of the same population of Epac-1 Ac-pretreated cells resulted in significant renoprotection (Fig. 3). To analyze whether renoprotection in fact resulted from integrin activation, cells were simultaneously incubated with Epac-1 Ac and the α4β1-integrin antagonist cRGD. Renoprotective effects of injected cells were significantly negated (Fig. 3). Conventional histology (epithelial flattening, tubular necrosis, epithelial vacuolizing) of animals in the 40-min group did not show any significant difference between the untreated and treated animals (Fig. 4).
Fig. 4.

Renal histology in the 40-min group. Conventional histology (B) with epithelial flattening (right arrow), tubular necrosis (left arrow), and epithelial vacuolization (double left arrow) as visible in the renal cortex of a mouse treated with 0.5×106 cells [periodic acid-Schiff (PAS), original magnification ×400] did not show any significant difference between the untreated and treated animals (A) (data are means ± SE).
Immunofluorescence analysis of kidneys.
Since EPC pretreatment with Epac-1 Ac alone protected mice from acute renal failure and since this effect could partly be reversed by combined pretreatment with Epac-1 Ac and cRGD, the respective kidneys were analyzed for the presence of c-Kit+/CM-Dil+ cells. Our previous investigations (16) and studies performed by Zhang and colleagues (27) have shown that EPCs express c-Kit. Animals injected with 0.5×106 untreated EPCs showed very mild EPC infiltrates in their respective kidneys. If cells, pretreated with Epac-1 Ac alone were administered, renal c-Kit+/CM-Dil+ cell numbers were moderately elevated. Combined cell pretreatment with Epac-1 Ac and cRGD did not significantly change the number of double-positive cells in the postischemic organs (Fig. 5). None of the analyzed kidneys showed any cells within the cortex. Most of the cells were located in the medullopapillary border zone. This was similar to our previous results (16). Figure 6 shows EPC localization in detail. The cells did not incorporate into the walls of small blood vessels but were localized in close proximity to vessels (Fig. 6). Together, these observations suggested that EPC integrin receptor activation, using Epac-1 Ac as stimulating agent (6), increased EPC homing to the kidney with the subsequent effects on renal function.
Fig. 5.
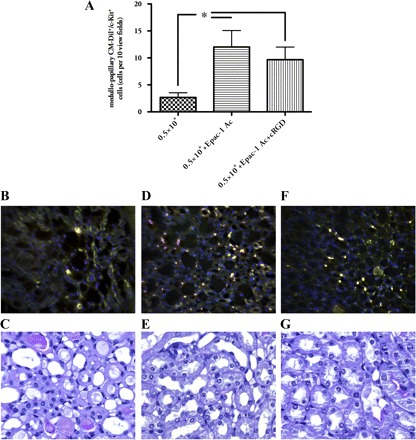
EPCs in postischemic kidneys. Animals injected with 0.5×106 EPCs showed very sporadically EPC infiltrates in their respective kidneys (B). When cells pretreated with Epac-1 Ac alone were administered, renal c-Kit+/CM-Dil+ cell numbers were significantly elevated (D). Combined cell pretreatment with Epac-1 Ac and cRGD did not significantly change the number of double-positive cells in the postischemic organs (A and F). None of the analyzed kidneys showed any injected cells within the cortex. Most of the cells were located in the medullopapillary border zone. The images show representative cross sections of the papilla. A more detailed analysis of the cells' distribution is shown in Fig. 6. C, E, and G show representative areas of kidneys from the respective groups (PAS stained) [yellow: cells, double-positive for CellTracker (red) and FITC-c-Kit (green), magnification ×40 in B, D, and F, ×400 in C, E, and G; data are means ± SE, *P < 0.05].
Fig. 6.

Allogeneic murine EPCs within the medullopapillary area (cross section) of a cell-injected recipient animal. D shows CellTracker-labeled cells only. B and E show the cells after c-Kit (B) and CD29 (E) staining. C displays an overlap of all 4 previous images (including A, nuclear staining with DAPI). In F, CD29-positive EPCs [defined as CellTracker+/c-Kit+ cells (16)] localized next to a distal tubule are magnified. The cells are localized within the interstitial space; there is no direct incorporation into the walls of small blood vessels (indicated by white asterisks) (magnification ×40 in A–E and ×100 in F).
EPC integrin β1-chain expression after in vitro pretreatment with Epac-1 Ac.
Since EPC pretreatment with Epac-1 Ac was followed by an increase in the number of renal (medullopapillary) c-Kit+/CM-Dil+ cells, untreated as well as Epac-1 Ac-pretreated EPCs were analyzed for integrin β1-chain expression. To evaluate whether incubation with Epac-1 Ac would have an influence on the subcellular distribution of β1-integrins, cultivated murine myelomonocytic cells were analyzed for double-positive immunofluorescence of Dil-acLDL and FITC BS-1 lectin by laser scanning microscopy. All double-positive cells were analyzed for CD29 expression. The percentage of CD29+ cells within the Dil-acLDL+/FITC BS-1+ cell population was 58% in untreated vs. 85% in Epac-1 Ac-prestimulated cells. Single cell analysis showed a more intense CD29 staining pattern in Epac-1-prestimulated cells compared with untreated cells (relative expression per individual cell 116 ± 21.5 vs. 507.3 ± 61.5, P < 0.0001, n = 25 per treatment group). Therefore, not only the percentage of Dil-acLDL+/FITC BS-1+ cells expressing CD29, but also the surface expression of CD29 per individual cell was higher in prestimulated than in unstimulated cells. Prestimulated cells showed a more homogenous CD29 expression pattern over the whole cell surface (Fig. 7). In summary, although the total cellular expression of CD29 was not higher in Epac-1 Ac-prestimulated than in untreated cells (see Western blot analysis in Fig. 7), these data clearly show that pretreatment resulted in a redistribution of β1-integrins toward the plasma membrane of EPCs.
Fig. 7.
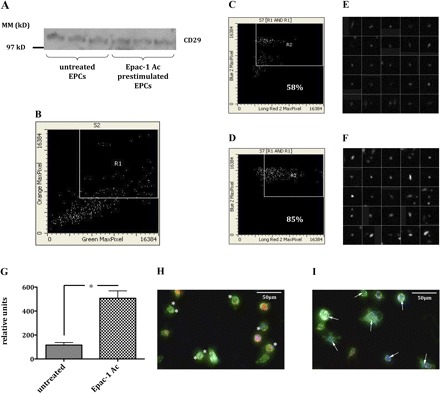
Expression of β-integrins in unstimulated and Epac-1 Ac-prestimulated murine EPCs. First, cultivated murine myelomonocytic cells were analyzed for double-positive immunofluorescence of Dil-acLDL and FITC BS-1 lectin by laser scanning microscopy (area R1 in B). Next, all double-positive cells were analyzed for CD29 expression. The percentage of CD29+ cells within the Dil-acLDL+/FITC BS-1+ cell population was 58% in untreated (C) vs. 85% in Epac-1 Ac-prestimulated cells (D). Single-cell analysis of CD29+/Dil-acLDL+/FITC BS-1+ cells showed a significantly stronger CD29 staining pattern in Epac-1 Ac-prestimulated cells (F and G) compared with untreated cells (E and G). Therefore, not only the percentage of Dil-acLDL+/FITC BS-1+ cells expressing CD29, but also the surface expression of CD29 per individual cell was higher in prestimulated than in unstimulated cells. Prestimulated cells showed a more homogenous CD29 expression pattern over the whole cell surface (white arrows in I). The asterisks in H are located next to β1-integrins (CD29 = blue; Dil-acLDL = red; BS-1 lectin = green) expressed by unstimulated cells. The total expression of CD29 did not differ between unstimulated and Epac-1 Ac-prestimulated cells (A) (S2, section 2; S7, section 7).
DISCUSSION
In this study, conditions that optimize an EPC-based therapy of acute ischemic renal failure have been identified for the first time. Taken together, therapeutic benefit of EPC administration is inversely correlated with the duration of the ischemic period. In longer lasting ischemia, EPC infusion does not sufficiently protect kidneys from ischemic damage, an effect that can be reversed by EPCs in vitro prestimulated with the integrin receptor activator Epac-1 Ac. Since simultaneous Epac-1 Ac/cRGD pretreatment of the cells significantly mitigates Epac-1 Ac-induced EPC-mediated renoprotection, an agonistic role for integrins could be assumed. In which ways Epac-1 Ac possibly activates EPCs besides increasing their homing still needs to be elucidated.
In the past, a number of studies focused on pharmacological/humoral EPC activation. One of the best characterized EPC activators, SDF-1 (24, 25), has been documented to increase cell migration into target tissues if overexpressed by gene transfer (10). Another well-established activator of EPCs is VEGF (12). Recently, uric acid (UA) has been identified as a novel stimulator of EPC mobilization (18). This endogenous metabolite, a degradation product of purines, rapidly (within 3 h) mobilizes murine EPCs. UA-induced EPC mobilization protects mice from acute ischemic renal failure, an effect that is specific for UA, since its precursors, adenosine and inosine, neither activate cell mobilization nor have renoprotective effects.
With respect to integrin receptor activation, the mechanisms involve increased and/or prolonged homing of EPCs to postischemic tissue sites (6). In our series of experiments Epac-1 Ac stimulation led to β1-integrin redistribution and increased the number of renal c-Kit+/CM-Dil+ cells. Combined (Epac-1 Ac + cRGD) pretreatment did not decrease cell numbers, although the renoprotective effects were negated. We therefore conclude that, in addition to increased EPC homing, other mechanisms such as functional activation of the cells must be involved as well. Integrins are known to stimulate the activity of different types of cells independently from their general ability to mediate cell-cell adhesion. The α4β1-integrin VLA-4, for example, is known as an important molecule for T cell costimulation (15).
Histological analysis revealed that the injected cells were located in the medullary part of the kidneys. This observation could reflect the lower postischemic medullary blood flow, which has been attributed to characteristic dysfunctions in acute renal failure (11). The c-Kit+/CM-Dil+ cells had apparently not significantly incorporated into the walls of small vessels. This is, as a matter of fact, in line with newer concepts of biological properties of different EPC subpopulations. According to Case et al. (7) and Yoder et al. (26), there are actually two major EPC populations. The first and by far more analyzed population is most likely represented by cells of myelomonocytic origin. These cells express CD34, CD133, as well as endothelial cell markers. They grow on fibronectin-coated culture dishes in EGM-2 media and, although vasoprotective, only sporadically incorporate into the walls of small vessels (7, 26). The majority of investigations performed over the last years dealt with this colony-forming unit endothelial cell (CFU-EC) subpopulation of EPCs (1–3, 8, 9). With respect to the culture conditions and the procedure of cell isolation and preparation, in this study we dealt with CFU-ECs. It is therefore not surprising to find such low numbers of EPCs in the kidney. CFU-ECs have been show to secrete different substances with paracrine actions on damaged endothelial cells, promoting a faster functional and structural recovery of the microvasculature (12, 21, 24, 25). This mechanism inhibits the so-called “no-reflow phenomenon,” which has been proven to delay the recovery of postischemic organs (14). After secretion of vasoprotective mediators CFU-ECs do not necessarily reside within the organ (20). Tögel and colleagues (13, 23) reported comparable renoprotection mediated by mesenchymal stem cells.
In summary, we herewith presented data on conditions that optimize an EPC-based therapeutic regimen of acute ischemic renal failure. Besides a correlation between the number of exogenously administered EPCs and their renoprotective activity, EPC pretreatment with the integrin receptor activator Epac-1 Ac augmented the anti-ischemic potential of the cells. This was in fact attributable to integrin activation, since combined EPC pretreatment with Epac-1 Ac and the integrin antagonist cRGD negated EPC-mediated renoprotection and since pretreatment increased EPC homing to the kidney and induced integrin β1-chain redistribution in cultured EPCs.
GRANTS
These studies were supported by grants from the Deutsche Forschungsgemeinschaft PA1530/2-1 and PA1530/3-1, and by grants from the Jackstädt-Stiftung (S 134-10.060).
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1. Adams V, Lenk K, Linke A, Lenz D, Erbs S, Sandri M, Tarnok A, Gielen S, Emmrich F, Schuler G, Hambrecht R. Increase of circulating endothelial progenitor cells in patients with coronary artery disease after exercise-induced ischemia. Arterioscler Thromb Vasc Biol 24: 684–690, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 85: 221–228, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964–967, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Assmus B, Honold J, Schachinger V, Britten M, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali N, Tonn T, Dimmeler S, Zeiher A. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med 355: 1222–1232, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci 31: 680–686, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Carmona G, Chavakis E, Koehl U, Zeiher A, Dimmeler S. Activation of Epac stimulates integrin-dependent homing of progenitor cells. Blood 111: 2640–2646, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Case J, Mead LE, Bessler WK, Prater D, White HA, Saadatzadeh MR, Bhavsar JR, Yoder MC, Haneline LS, Ingram DA. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol 35: 1109–1118, 2007 [DOI] [PubMed] [Google Scholar]
- 8. De Groot K, Bahlmann FH, Sowa J, Koenig J, Menne J, Haller H, Fliser D. Uremia causes endothelial progenitor cell deficiency. Kidney Int 66: 641–646, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Heeschen C, Aicher A, Lehmann R, Fichtlscherer S, Vasa M, Urbich C, Mildner-Rihm C, Martin H, Zeiher AM, Dimmeler S. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood 102: 1340–1346, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Hiasa K, Ishibashi M, Ohtani K, Inoue S, Zhao Q, Kitamoto S, Sata M, Ichiki T, Takeshita A, Egashira K. Gene transfer of stromal cell-derived factor-1alpha enhances ischemic vasculogenesis and angiogenesis via vascular endothelial growth factor/endothelial nitric oxide synthase-related pathway: next-generation chemokine therapy for therapeutic neovascularization. Circulation 109: 2454–2461, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Karlberg L, Norlen BJ, Ojteg G, Wolgast M. Impaired medullary circulation in postischemic acute renal failure. Acta Physiol Scand 118: 11–17, 1983 [DOI] [PubMed] [Google Scholar]
- 12. Khakoo AY, Finkel T. Endothelial progenitor cells. Annu Rev Med 56: 79–101, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Lange C, Tögel F, Ittrich H, Clayton F, Nolte-Ernsting C, Zander AR, Westenfelder C. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int 68: 1613–1617, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Leaf A. Cell swelling. A factor in ischemic tissue injury. Circulation 48: 455–458, 1973 [DOI] [PubMed] [Google Scholar]
- 15. Nguyen K, Sylvain NR, Bunnell SC. T cell costimulation via the integrin VLA-4 inhibits the actin-dependent centralization of signaling microclusters containing the adaptor SLP-76. Immunity 28: 810–821, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Patschan D, Krupincza K, Patschan S, Zhang Z, Hamby C, Goligorsky MS. Dynamics of mobilization and homing of endothelial progenitor cells after acute renal ischemia: modulation by ischemic preconditioning. Am J Physiol Renal Physiol 291: F176–F185, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Patschan D, Patschan S, Gobe G, Chintala S, Goligorsky M. Uric acid heralds ischemic tissue injury to mobilize endothelial progenitor cells. J Am Soc Nephrol 18: 1516–1524, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Patschan D, Plotkin M, Goligorsky MS. Therapeutic use of stem and endothelial progenitor cells in acute renal injury: ça ira. Curr Opin Pharmacol 6: 176–183, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med 9: 702–712, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation 107: 1164–1169, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Schuster MD, Kocher AA, Seki T, Martens TP, Xiang G, Homma S, Itescu S. Myocardial neovascularization by bone marrow angioblasts results in cardiomyocyte regeneration. Am J Physiol Heart Circ Physiol 287: H525–H532, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol 289: F31–F42, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Urbich C, Dimmeler S. Endothelial progenitor cells functional characterization. Trends Cardiovasc Med 14: 318–322, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res 95: 343–353, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 109: 1801–1809, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang W, Zhang G, Jin H, Hu R. Characteristics of bone marrow-derived endothelial progenitor cells in aged mice. Biochem Biophys Res Commun 348: 1018–1023, 2006 [DOI] [PubMed] [Google Scholar]


