Abstract
Obesity and hypertension are risk factors for the development of chronic kidney disease. The mechanisms by which elevated blood pressure and fatty acids lead to the development of renal injury are incompletely understood. Here, we investigated the contributions of cholesterol and oxidative stress to renal endothelial dysfunction and glomerular injury in a model of obesity and hypertension. Male Wistar-Kyoto (WKY) rats and spontaneously hypertensive rats (SHR) were fed a normal diet, a high-fat diet, a high-fat diet with tempol, or a high-fat diet with simvastatin for up to 10 wk. Blood pressure was not altered by a high-fat diet or treatments. After 3 wk, renal afferent dilatory responses to acetylcholine were impaired in WKY rats and SHR fed a high-fat diet. Tempol treatment prevented this vascular dysfunction in both strains; however, simvastatin treatment demonstrated greater beneficial effects in the SHR. Albuminuria was observed in the SHR and was exacerbated by a high-fat diet. Tempol and simvastatin treatment significantly ameliorated albuminuria in the SHR fed a high-fat diet. Ten weeks on a high-fat resulted in an increase in urinary 8-isoprostane in WKY rats and SHR, and tempol and simvastatin treatment prevented this increase, indicating a reduction in renal oxidative stress. Monocyte chemoattractant protein-1 (MCP-1) excretion was significantly elevated by a high-fat diet in both strains, and tempol prevented this increase. Interestingly, simvastatin treatment had no effect on MCP-1 levels. These data indicate that tempol and simvastatin treatment via a reduction in oxidative stress improve renal endothelial function and decrease glomerular injury in a model of obesity and hypertension.
Keywords: kidney, oxidative stress, metabolic syndrome, inflammation
obesity has reached epidemic proportions in the developed world, with 50% of the US population now classified as overweight or obese, and the numbers are rising (National Health and Nutrition Examination Survey, available at http://www.cdc.gov/nchs/nhanes.htm). Obesity is associated with increased risk of developing diabetes, hypercholesterolemia, and hypertension, which together are described as the metabolic syndrome (18, 29). Independently, obesity and hypertension increase the risk of renal dysfunction; obese patients are four times more likely to develop renal disease than nonobese patients, and hypertension accounts for 25% of renal dysfunction cases (27). Obese patients are at a higher risk of developing hypertension, therefore combining risk factors and compounding the negative effect on cardiovascular health. In particular, renal injury associated with obesity and hypertension has been shown to be more severe than renal disease observed as a result of each risk factor alone. This additive effect has also been observed in animal models of obesity with hypertension such as the SHR/NDmcr-cp, a rat model of metabolic syndrome and the spontaneously hypertensive rat (SHR) fed a high-fat diet (26, 32).
Obesity and hypertension have both been associated with elevated levels of circulating oxidative metabolites, such as superoxide. Increased levels of oxidative stress in both obese and hypertensive patients are thought to contribute to chronic kidney disease; however, the extent to which blood pressure and oxidative stress contribute to the progression of renal vascular dysfunction, inflammation, and injury remains unclear (12, 34). In hypertensive animal models such as the SHR and angiotensin hypertension, elevated levels of superoxide have been detected (24). These elevated oxidative molecules may contribute to some of the characteristic pathological changes that occur in the SHR, such as renal morphological alterations as hypertension develops (1, 28). Because hypertension is associated with increased superoxide production, we were interested in whether hypertension combined with a high-fat diet increases the susceptibility or severity of renal injury and the contribution of superoxide and cholesterol to this injury. This is the first time that the effect of tempol and simvastatin on endothelial function and kidney injury has been studied in an animal model of essential hypertension with obesity. Increased production of superoxide can overpower endogenous scavenging molecules such as superoxide dismutase and induce renal dysfunction by way of a number of mechanisms, for example, reducing the bioavailability of vasodilators such as nitric oxide (35, 36). We have previously observed no increase in blood pressure in WKY rats as a result of 10-wk high-fat feeding; however, in a clinical setting hypercholesterolemia is often present in combination with hypertension (26). Therefore, in this study we use the rat model of essential hypertension, the SHR fed a-high fat diet, to explore the contributions of obesity and hypertension to renal injury. High levels of renal vascular oxidative stress have been identified in animal models of obesity such as the leptin-deficient mouse, the db/db and the leptin receptor-deficient rat model, the obese Zucker rat, as well as in obese humans (2, 4, 16). One reason for the increased production of superoxide in obesity could be as a result of elevated levels of cholesterol, which has also been associated with obesity and increased risk of developing cardiovascular disease (20) (http://www.americanheart.org/presenter.jhtml?identifier=4639). High cholesterol levels have been shown to contribute to elevated superoxide generation; therefore, high cholesterol levels could contribute to the renal dysfunction frequently observed in obesity (10).
We hypothesized that in an animal model of obesity and hypertension, the increased severity of renal injury observed in SHR as a result of a high-fat diet is a result of elevated cholesterol levels combined with preexisting oxidative stress as a result of the hypertension. Elevated levels of circulating cholesterol give rise to increased production of superoxide, which could contribute to endothelial dysfunction and renal injury that are characteristic of this model of obesity. 3-Hydroxy-3-methylglutaryl-coenzyme A (HMG -CoA) reductase inhibitors or statins are hypolipidemic drugs used for the treatment of high cholesterol in those at risk for the development of cardiovascular disease. HMG -CoA reductase is the rate-limiting step in the production of cholesterol, and statins have been shown to reduce “bad” LDL cholesterol levels from up to 30–50% (3). In addition to the cholesterol-lowering effects of statins, other non-lipid-mediated beneficial pleiotropic effects have been observed, such as improved endothelial function and antioxidative effects (5, 13, 23). Therefore, in this study we used simvastatin, an HMG-CoA reductase inhibitor, and 4-hydroxy-tempol (tempol), a superoxide dismutase mimetic, to explore the contribution of cholesterol and oxidative stress to afferent arteriole endothelial dysfunction and renal injury, in a model of obesity and hypertension.
MATERIALS AND METHODS
All animal studies were approved by the Medical College of Georgia institutional review committee according to the National Institutes of Health guidelines for the care and use of laboratory animals. Eight-week-old male Wistar-Kyoto (WKY) rats and SHR were purchased from Charles River Laboratories (Wilmington, MA) and were divided into four groups: A–D (n = 5–6). Group A were fed normal rat chow containing 7% fat (Teklad), and the remaining groups, groups B–D, received a high-fat diet containing 36% fat (no. F2685, BioServ, Frenchtown, NJ) ad libitum for 10 wk. Group C received 1 mmol tempol in their drinking water, and group D received simvastatin (10 mg·kg−1·day−1) in their drinking water throughout the 10-wk study period. After an initial training period, rats were weighed and systolic blood pressure was measured by tail-cuff plethysmography every 7 days, as previously demonstrated (26).
In Vitro Perfused Juxtamedullary Nephron Experiments
Experiments were conducted in vitro using the perfused juxtamedullary nephron technique, as previously described (26). Male WKY rats and SHR (n = 4) were anesthetized with pentobarbital sodium (40 mg/kg body wt ip). The right renal artery was cannulated and perfused with a Tyrode buffer solution containing 5.2% BSA and a complement of l-amino acids. The kidney was removed and sectioned along the longitudinal axis, with care taken to leave the papilla intact on the dorsal two-thirds of the kidney. The vasculature was isolated as previously described (26). The perfusate was consistently perfused with 95% O2-5% CO2. Perfusion pressure was set at 110 mmHg and monitored continuously. The inner cortical surface of the kidney was superfused with warmed (37°C) Tyrode buffer containing 1% BSA. Vessel inner diameters were viewed by video microscopy and measured using an image-shearing monitor. An afferent arteriole was selected, and after a 20-min equilibration the vessel was constricted using 1 mmol/l phenylephrine added to the superfusate. The vessel diameter was measured and recorded as the baseline. Acetylcholine was added to the perfusate to make a final concentration of 1 × 10−8 mmol/l; then, the concentration of acetylcholine was increased to 1 × 10−7 mmol, 1 × 10−6, and finally 1 × 10−5 mmol/l. Mean vessel diameter was recorded for 15 min at each concentration of acetylcholine. The vessel was perfused with 1% BSA for 15 min in the absence of acetylcholine, followed by a 15-min incubation with sodium nitroprusside to exclude the contribution of the smooth muscle to any differences in dilatory response to acetylcholine.
In Vitro Assays and Enzyme-Linked Immunoassays
Rats were housed in metabolic cages for 24 h at the end of the 10-wk experiment to collect urine for analysis. Plasma-free cholesterol levels were measured using a commercially available kit from Wako Diagnostics (Richmond VA). Urinary microalbumin levels were measured by enzyme-linked immunoassay (ELISA) using a commercial kit from SPI-bio (Paris, France). Urinary monocyte chemoattractant protein-1 (MCP-1) and 8-isoprostane levels were also measured by commercial ELISA (BD Biosciences, San Jose, CA and Cayman, Ann Arbor, MI).
CD68 Immunohistochemistry
Five-micrometer frozen kidney sections were cut and incubated overnight at room temperature with mouse anti-rat CD68 primary antibody (1:100, Serotec, Raleigh, NC) followed by the secondary antibody goat anti-mouse IgG HRP (1:50, Serotec) for 1 h at room temperature. Slides were incubated with AEC substrate chromogen (DAKO, Carpinteria, CA) for 20 min, rinsed, and counterstained with Mayers hematoxylin for 30 s. Photographs were taken at ×400 magnification. Eight randomly selected glomeruli were photographed from each of 6 kidneys per treatment group, totaling 48 images. A blinded reviewer counted the number of CD68-positive cells. Group means and SEs of positively stained cells were calculated per square millimeter.
Nephrin Immunofluorescence
Five-micrometer frozen kidney sections were incubated overnight at room temperature with goat anti-human nephrin primary antibody (1:50, sc-19000, Santa Cruz Biotechnology, Santa Cruz, CA) followed by rabbit anti-goat Cy-3 fluorescent-tagged secondary antibody (1:400, for 1 h, Zymed). Slides were mounted using Prolong Gold anti-fade (Invitrogen) and glass coverslips. Photographs were taken at ×400 magnification. Ten randomly selected glomeruli were photographed from each of 6 kidneys per treatment group, totaling 60 images/group. Fluorescent intensity of glomeruli was measured using Metamorph software (Molecular Devices), correcting for background fluorescence, and means and SEs were calculated.
Statistical Analysis
All statistical analysis of data was performed using 2-way ANOVA and the Bonferroni posttest. Differences are considered statistically significant if P values are 0.05 or below.
RESULTS
Body Weight and Blood Pressure
We measured mean body weight in all groups at baseline and throughout the study. Figure 1, A and B, displays the mean body weight of the WKY and SHR fed a normal diet, a high-fat diet, a high-fat diet with tempol in their drinking water, or a high-fat diet with simvastatin in their drinking water. We observed that after 10 wk of high-fat feeding, the WKY rats had a slightly higher body weight than the SHR; however, this did not reach significance. Interestingly, the WKY rats receiving a high-fat diet with tempol or simvastatin treatment displayed a significant increase in body weight compared with those fed a normal diet (P < 0.05). Figure 1B shows data for the SHR where 10 wk on a high-fat diet induced a significant increase in body weight compared with SHR fed a normal diet (P < 0.05). Treatment with tempol or simvastatin in the drinking water with a high-fat diet did not effect this increase in body weight, which was also significantly higher than those fed a normal diet (P < 0.05).
Fig. 1.
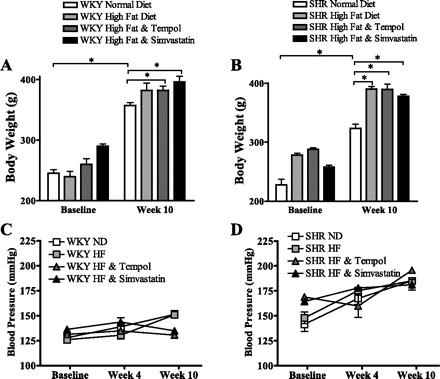
Body weight in Wistar-Kyoto (WKY; A) rats and spontaneously hypertensive rats (SHR; B) is increased after 10 wk compared with baseline (*P < 0.05). Ten weeks of a high-fat diet caused a significantly greater increase in body weight compared with a normal diet (*P < 0.05). Tempol and simvastatin had no effect on body weight. Blood pressure was unaffected by a 10-wk high-fat diet, tempol, or simvastatin treatment in both WKY (C) rats and SHR (D).
The SHR developed a higher systolic blood pressure compared with the WKY rats after the 10-wk study period typical of the SHR strain. No significant differences were observed in systolic blood pressure in either rat strain between treatment groups throughout the 10-wk study period (Fig. 1, C and D).
Plasma Cholesterol
Significantly lower levels of plasma cholesterol were apparent in all SHR groups compared with the WKY rats, independent of diet (P < 0.05) (Fig. 2A). Ten weeks of high-fat diet feeding produced a significant increase in plasma cholesterol levels in both rat strains (P < 0.05). Treatment with tempol did not affect this elevation in cholesterol in either rat strain; however, 10 wk of simvastatin treatment significantly reduced plasma cholesterol levels in both WKY and SHR compared with groups receiving a high-fat diet alone for 10 wk (P < 0.05).
Fig. 2.
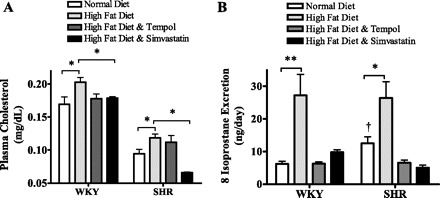
Plasma cholesterol levels were significantly increased by a 10-wk high-fat diet compared with a normal diet in both WKY rats and SHR (A). This increase was absent in both WKY and SHR strains treated with simvastatin (*P < 0.05). Urinary 8-isoprostane levels were significantly higher in SHR compared with WKY rats fed a normal diet (†P < 0.05; B). Ten weeks of a high-fat diet resulted in a significant increase in 8-isoprostane levels compared with a normal diet in both rat strains, which was ameliorated by both tempol and simvastatin treatment (*P < 0.05).
Oxidative Stress
To measure the effect of a 10-wk high-fat diet with hypertension on renal oxidative stress, we measured urinary levels of 8-isoprostane from WKY rats and SHR fed a normal diet, a high-fat diet, a high-fat diet with tempol, or a high-fat diet with simvastatin treatment (Fig. 2B). We observed a marked increase in 8-isoprostane excretion in both WKY rats and SHR fed a high-fat diet for 10 wk compared with those fed a normal diet throughout the study period (P < 0.01 and P < 0.05, respectively). In addition, treatment with tempol or simvastatin in the drinking water for 10 wk prevented this increase in 8-isoprostane levels, which remained at levels similar to those observed in rats fed a normal diet for 10 wk.
Renal Endothelial Function
We have previously shown that just 3 wk of a high-fat diet can induce renal endothelial dysfunction in both the WKY rats and SHR in the absence of changes in systolic blood pressure (26). Three weeks of a high-fat diet impaired the endothelial response to acetylcholine in the WKY rats and SHR compared with those fed a normal diet (Fig. 3, A and B). Figure 3A shows that a 3-wk high-fat diet impairs endothelial dilation in response to acetylcholine, resulting in vessels reaching just 51% of baseline in high fat-fed WKY rats (P < 0.05). Tempol treatment prevents the impaired response to acetylcholine of the renal endothelium, and mean vessel diameter reached 96% of baseline. The SHR fed a high-fat diet also displayed a significantly reduced endothelial relaxation to acetylcholine, reaching just 47% of basal diameter, compared with rats fed a normal diet that reached 75% of baseline. The increased blood pressure of the SHR induced endothelial dysfunction compared with the WKY rats, even when fed a normal diet. Three weeks of a high-fat diet exacerbated this dysfunction in the SHR, resulting in vessel diameters reaching just 47% of baseline. Tempol treatment ameliorated this dysfunction, and vessels reached 79% of baseline, levels similar to those observed in SHR fed a normal diet.
Fig. 3.
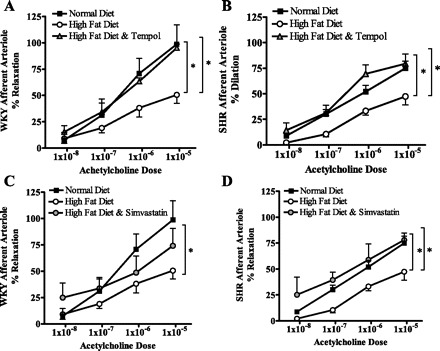
Afferent arteriole dilatory response to acetylcholine was significantly reduced in WKY (A) rats and SHR (B) fed a high-fat diet for 3 wk (*P < 0.05). Tempol treatment prevented this reduction in endothelial function in both rat strains. Normal and high-fat data are duplicated in C and D and compared with simvastatin-treated rats. Three weeks of simvastatin treatment prevented the injurious effect of a high-fat diet in SHR; however, this did not have a significant effect on the WKY rats.
Simvastatin treatment also appeared to have a beneficial effect on the endothelial response to acetylcholine in WKY rats and SHR fed a high-fat diet for 3 wk (Fig. 3, C and D). Maximum dilation to acetylcholine in WKY rats fed a high-fat diet with simvastatin in the drinking water reached 74% of baseline, which was higher than the 51% observed without simvastatin treatment; however, this increase did not reach statistical significance. On the other hand, in the SHR, simvastatin treatment improved the vascular dilation to 78% of baseline (P < 0.05) (Fig. 3D). There appeared to be little contribution of the smooth muscle to the vascular dysfunction observed in this study. Vasodilatation in response to sodium nitroprusside administration in the WKY rats fed a normal and high-fat diet reached 86 ± 31 and 74 ± 25% of baseline diameters, respectively, and 92 ± 10 and 79 ± 25% in the tempol- and simvastatin-treated mice, which were not significantly different from baseline. Also, in the SHR vessel diameters reached 89 ± 8, 89 ± 6, 89 ± 15, and 86 ± 10% of baseline diameters in normal diet-, high fat-, high fat with tempol-, and high fat with simvastatin-fed animals, respectively. Again, the vessel diameters were not significantly reduced compared with baseline values, indicating that the dilatory dysfunction in these vessels was specifically endothelial.
Renal Injury
Glomerular nephrin expression.
Nephrin is expressed on the slit diaphragm, which is a major component of the filtration barrier in the renal glomerulus. Downregulation of renal nephrin expression indicates that there is a reduction in the ability of the glomerulus to filter appropriately. A reduction in renal nephrin expression is associated with forms of renal injury such as glomerulersclerosis and diabetic nephropathy (30, 46). In this study, we observed a significant reduction in nephrin expression measured by immunofluorescence (Fig. 4, A and B) (P < 0.05). Treatment with both tempol and simvastatin prevented a significant reduction in glomerular nephrin expression in both WKY rats and SHR compared with WKY rats and SHR fed a normal diet.
Fig. 4.
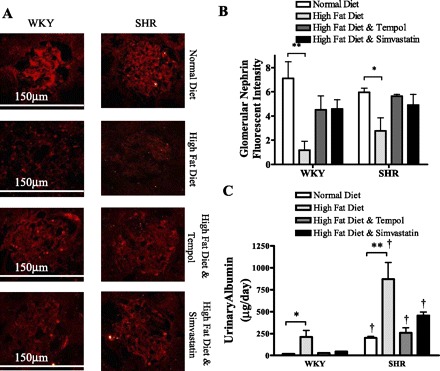
Nephrin expression is significantly reduced in kidneys from WKY rats and SHR fed a high-fat diet compared with those fed a normal diet for 10 wk (*P < 0.05, **P < 0.001) shown in fluorescent micrographs (A) and quantified (B). Means and SE are calculated from 60 images/treatment group. Baseline urinary excretion in higher in the SHR compared with WKY rats (†P < 0.05). Albumin excretion was significantly increased in both rat strains fed a high-fat diet for 10 wk compared with rats fed a normal diet (*P < 0.05, **P < 0.001). Rats treated with tempol or simvastatin with a high-fat diet exhibited reduced levels of albumin excretion compared with those fed a high-fat diet alone. All SHR had higher albumin levels than similarly treated WKY rats (†P < 0.05).
Urinary albumin excretion.
We measured urinary albumin levels as a marker of damage to the filtration barrier in WKY rats and SHR fed a normal diet, a high-fat diet, a high-fat diet with tempol, and a high-fat diet with simvastatin (Fig. 4C). We observed a significant increase in albumin excretion in all groups of SHR compared with WKY rats (P < 0.05). Elevated urinary albumin indicates renal filter dysfunction in the SHR, as a result of the increased blood pressure characteristic of SHR at 18 wk of age. Ten weeks of a high-fat diet caused a significant increase in urinary albumin excretion in both the WKY rats and SHR compared with those fed a normal diet (P < 0.05). This data indicates that a 10-wk high-fat diet increased albumin leakage in the WKY rats and exacerbated renal injury in SHR, in the absence of a change in blood pressure. Treatment with tempol and simvastatin in combination with the high-fat diet prevented this elevation in albumin excretion in both the WKY rats and SHR (P < 0.05). These data indicate that both antioxidant treatment and the inhibition of free fatty acid generation protected the kidney from the damage incurred by a 10-wk high-fat diet.
Renal inflammation.
Increased cytokine release and renal macrophage infiltration have been shown to contribute to renal injury in models of obesity (14). An increase in renal macrophage-specific CD68-positive staining was observed in the SHR fed a high-fat diet compared with those fed a normal diet (P < 0.05) (Fig. 5A). There was also an increase in CD68-positive-stained cells in the WKY rats fed a high-fat diet compared with those fed a normal diet; however, this did not reach significance. In the WKY rats on simvastatin treatment with a high-fat diet, a statistically significant increase in CD68-positive cells was recorded, whereas those receiving tempol did not present a significant change in macrophage number compared with the normal diet-fed WKY rats. The high-fat diet induced a greater number of macrophages to infiltrate the renal tissue in the SHR compared with those receiving a normal diet (P < 0.05). In the SHR, however, tempol treatment resulted in a significant reduction in CD68-positive-stained cells; SHR displayed a slightly different pattern than the WKY rats. Ten weeks of a high-fat diet increased macrophage infiltration into the kidney of the SHR (P < 0.05). In contrast, tempol treatment in the WKY rats prevented this elevation in macrophage infiltration in response to a high-fat diet whereas simvastatin treatment did not (P < 0.05).
Fig. 5.
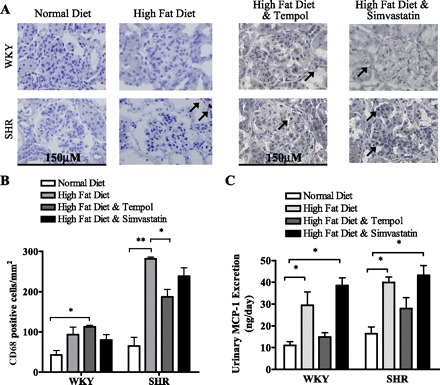
Macrophage infiltration into the kidney was measured by CD68 immunohistochemistry (A) and quantified (B). Photographs were taken on a light microscope using ×400 magnification. Positive CD68 cells are indicated by an arrow. Means and SE are calculated from 48 images/treatment group. B: there was an increase in renal infiltration of CD68-positive cells in WKY rats fed a high-fat diet compared with WKY rats fed a normal diet; however, this only reached significance in WKY rats fed a high-fat diet with tempol (*P < 0.05). CD68-positive cell infiltration is markedly increased in SHR fed a high-fat diet compared with those fed a normal diet (**P < 0.001). Tempol treatment significantly reduced renal macrophage infiltration in SHR compared with those fed a high-fat diet (*P < 0.05). C: urinary monocyte chemoattractant protein-1 (MCP-1) excretion is significantly increased in WKY rats and SHR fed a high-fat diet compared with those fed a normal diet (*P < 0.05). Tempol treatment reduced MCP-1 excretion to levels comparable with controls, whereas simvastatin treatment had no effect.
Inflammation can play a key role in the development of renal injury, and MCP-1 excretion is often used as an indicator of renal inflammatory status (42). We observed a significant increase in urinary MCP-1 excretion in both WKY rats and SHR fed a high-fat diet for 10 wk compared with those fed a normal diet (Fig. 5C) (P < 0.05). With high fat feeding, treatment with tempol prevented this increase in MCP-1 excretion in both normotensive WKY rats and hypertensive SHR compared with those fed a high-fat diet alone. Interestingly, groups of both rat strains fed a high-fat diet and receiving simvastatin in their drinking water excreted similar MCP-1 levels to those fed a high-fat diet alone (P < 0.05). Together, the CD68-positive cell infiltration and MCP-1 excretion data suggest that simvastatin treatment does not prevent the inflammatory effect of a high-fat diet, but 10 wk of tempol treatment alleviated the inflammatory response induced by a high-fat diet.
DISCUSSION
Obesity and hypertension have been shown to contribute to the development of renal injury and endothelial dysfunction; however, the mechanism by which obesity exacerbates renal injury in the presence of hypertension remains to be determined (38). We hypothesized that a 10-wk high-fat diet, which increased circulating cholesterol levels, combined with hypertension played a crucial role in the development of renal injury in this model via the elevation of oxidative metabolite production. Supporting this hypothesis using a rat model of obesity with hypertension, we demonstrate that lowering cholesterol with simvastatin and scavenging superoxide with tempol treatment result in the amelioration of both afferent arterial endothelial dysfunction and renal injury. However, the presence of hypertension prevented the complete protection from renal injury due to a high-fat diet. Albumin and MCP-1 excretion and CD68-positive cell infiltration remained elevated in the SHR despite tempol or simvastatin treatment.
In this study, we observed no effect on systolic blood pressure with 1 mmol tempol administration for 10 wk. These data are in accord with some previous studies using the hypertensive rat strain, the SHR, where tempol has not altered blood pressure (25). In contrast, however, some other investigators have reported a hypotensive effect of tempol administration in the SHR (47). Disparities in reports on the effect of tempol on blood pressure in the SHR may be attributable to the specific doses used, administration route, time course, and device used for blood pressure measurement. Simvastatin and other statins have also been reported to have variable effects on blood pressure in the SHR (6, 19). In this study, WKY rats and SHR fed a high-fat diet underwent no significant change in blood pressure with simvastatin treatment as measured by tail-cuff plethysmography. However, any change in blood pressure that may have occurred as a result of tempol or simvastatin administration may have been masked by the effect of 10 wk of high-fat feeding in this study. We observed no change in blood pressure despite an increase in body weight in both rat strains, which indicates that the insult of 10 wk of feeding a 36% fat diet was not great enough to impact blood pressure in these rat strains despite the effect on renal function. Therefore, the beneficial effects of tempol and simvastatin treatment may be the direct result of a reduction in superoxide and a downregulation of injurious inflammatory proteins.
Ten weeks of high-fat feeding resulted in an increase in plasma cholesterol levels in both the normotensive WKY rats and hypertensive SHR that was prevented by simvastatin treatment, via its inhibition of HMG-CoA reductase. Superoxide scavenging with tempol in animals fed a high-fat diet did nothing to alter their circulating cholesterol levels. Evidence of substantial renal oxidative stress was observed, indicated by significantly elevated 8-isoprostane excretion as a result of a 10-wk high-fat diet in both rat strains. Elevated 8-isoprostane levels had also been reported in other models of obesity such as the Zucker diabetic fatty rat (31). High levels of urinary 8-isoprostane reflect prolonged elevations in renal oxidative metabolites, resulting in peroxidation of lipoproteins. This high-fat diet-induced elevation in oxidative stress was independent of a change in systolic blood pressure. Increases in oxidative metabolite excretion indicate elevations in renal oxidative stress in this model as a result of high-fat feeding. Both tempol and simvastatin treatment ameliorated this increase in 8-isoprostane excretion, indicating reduced levels of renal oxidative stress. This reduction in oxidative stress in the kidney correlated with a reduction in high-fat diet-induced endothelial dysfunction and renal injury and implicates oxidative stress as a potential mechanism for injury in this model. Interestingly simvastatin treatment reduced 8-isoprostane levels with equal efficiency as the SOD mimetic tempol. There is evidence that simvastatin can reduce oxidative stress through two mechanisms: a reduction in free fatty acid levels and a cholesterol-independent pathway (22). In this study, the reduction in oxidative stress caused by simvastatin treatment may have been through both cholesterol-lowering and direct antioxidant activity.
We observed afferent arterial endothelial dysfunction after just 3 wk of a high-fat diet in both rat strains studied, 7 wk before any evidence of reduced renal function. These data are in accordance with results we have previously reported where we recorded marked endothelial dysfunction in WKY rats and SHR after just 3 wk high-fat feeding (26). Previous studies using alternative models of hypercholesterolemia, such as those carried out by Chade et al. (11), have reported endothelial dysfunction as a result of prolonged high-fat feeding and implicate oxidative stress in the development of renal injury in their model. Supporting our hypothesis that reactive oxygen species play a role in the development of renal endothelial dysfunction, tempol administration in the drinking water prevented endothelial dysfunction in both strains, independently of blood pressure changes. These data suggest that a 3-wk high-fat diet increases renal superoxide generation, which in turn impairs the afferent arteriole dilatory response to acetylcholine. The reduced vasodilatory response to acetylcholine in the renal vasculature is most likely a result of the inhibitory effect of superoxide on nitric oxide-dependent vasodilation, which has been previously reported by other investigators (39, 41). Simvastatin treatment also prevented the endothelial dysfunction observed in the SHR as a result of a 3-wk high-fat diet. Interestingly, no significant amelioration of endothelial function was observed in the WKY rats treated with simvastatin, despite a trend at the higher doses of acetylcholine. This strain disparity indicates that the endothelial dysfunction we observed as a result of high-fat diet in the WKY rats may be less attributable to increased oxidative stress or fatty acid generation than in the SHR.
After an additional 7 wk of high-fat feeding following the development of endothelial dysfunction, we observed significant increases in urinary albumin in both rat strains. This albuminuria was independent of changes in blood pressure. The SHR displayed evidence of extreme renal injury with high levels of albumin excretion reaching almost 1 mg/day after a 10-wk high-fat diet. In comparison, the WKY rats experienced a far milder level of renal injury as a result of high-fat feeding. These data correlate with our previous study, where we observed severe renal injury in SHR as a result of a 10-wk high-fat diet (26). Despite significant albuminuria, there was not an obvious increase in fibrosis as a result of the high-fat diet. Both tempol and simvastatin treatment prevented the albuminuria that was observed in both rat strains after a 10-wk high-fat diet. A reduction in free fatty acids in vivo has been associated with a fall in reactive oxygen species bioavailability (14). There is evidence that reductions in reactive oxygen species generation can reduce blood pressure in models of hypertension and renal injury (45). However, we observed that tempol treatment reduced 8-isoprostane levels in the absence of changes in blood pressure but ameliorated renal injury. Therefore, we hypothesize that the protective effect on renal filtration barrier integrity was likely, at least in part, to be a result of the reduction in free radical generation independently of a change in blood pressure. A reduction in free radical production by tempol or simvastatin treatment, directly or via a reduction in fatty acid levels, prevented the leakage of albumin that was observed in the high fat-fed groups. Previous studies have shown that statin treatment reduces glomerular podocyte injury by way of activation of the phosphatidylinositol 3-kinase/AKT signaling pathway (9). Although we cannot rule out possible effects on glomerular hydrostatic pressure, the data provide convincing evidence that simvastatin or tempol treatment decreased oxidative stress and improved endothelial dysfunction that most likely contributed to decreased renal inflammation and injury.
Increased oxidative stress is involved in the development of endothelial dysfunction by way of reductions in nitric oxide bioavailability and therefore reduced vasodilatory ability of the vessel (39). We hypothesize that the ensuing development of albuminuria in this model may be as a result of a number of mechanisms. It is possible that glomerular endothelial dysfunction developed, therefore increasing glomerular filtration pressure, leading to protein leakage (17). Reactive oxygen species may have also caused a loss of negatively charged surface of the capillary wall, therefore allowing negatively charged albumin to freely pass through the filtration barrier. A third possible mechanism is by way of reactive oxygen species, either directly or indirectly via increased inflammatory cytokines, disrupting the glomerular podocyte barrier by podocyte flattening, again resulting in albuminuria (7).
Renal nephrin expression has been linked to podocyte function, and a reduction in nephrin expression is a characteristic of a number of renal injury models (21). Obesity has been associated with podocyte injury, and Nagase et al. (32) showed in an obese SHR/NDmcr-cp rat model that nephrin gene expression was reduced compared with SHR. In this study, we observed a reduction in nephrin expression after a 10-wk high-fat diet but preservation of expression of the podocyte-associated protein nephrin by both simvastatin and tempol treatment. Normal nephrin expression in kidneys from tempol-treated mice indicates reactive oxygen species play a role in the downregulation of nephrin protein expression and therefore altered podocyte function. Statin treatment of a diabetic mouse model has been shown to have a beneficial effect on podocyte number and nephrin levels in the kidney via a reduction in oxidative metabolite availability (48). Here, we show that simvastatin treatment prevented the downregulation of nephrin expression in the presence of a high-fat diet. In addition, as mentioned previously, simvastatin treatment resulted in a fall in oxidative metabolites; therefore, we believe that the protective effect on podocyte number and nephrin expression may be via a reduction in free fatty acids and a fall in oxidative damage. This protective effect of simvastatin may be a result of the ability of statins to prevent NADPH oxidase activation and therefore reduce reactive oxygen species generation (44).
Renal inflammation has been identified as a characteristic of obesity and hypertension, and there is evidence that inflammatory cytokines contribute to the development of renal injury (43). We have reported previously that high fat-fed SHR undergo a significant increase in glomerular macrophage infiltration, MCP-1 excretion, and an elevation in renal inflammatory gene expression (26). Interestingly, this inflammatory response was a result of the combination of a 10-wk high-fat diet with hypertension and was not observed in the normotensive WKY strain fed a high-fat diet for 10 wk. In the present study, we demonstrate that scavenging superoxide with tempol treatment prevented an increase in excretion of the inflammatory cytokine MCP-1 and reduced macrophage infiltration into the kidney in both SHR and WKY rats. There is a positive relationship between tubulointerstitial macrophage infiltration and oxidative stress in hypertension and animal models such as the SHR (37, 40). Increases in superoxide can induce NF-κB activation, which acts to increase expression of leukocyte attractants ICAM and VCAM (37). Tempol treatment could prevent NF-κB activation in the high fat-fed SHR, prevent renal macrophage infiltration, and therefore protect the kidney from inflammatory cell damage brought on by increased oxidative stress. Despite the apparent protective effect of treatment with 10 mg·kg−1·day−1 simvastatin on renal excretory function, no reduction in macrophage infiltration or MCP-1 levels in high fat-fed rats was recorded in either rat strain. Our data suggest that the reduction in reactive oxygen species measured by 8-isoprostane levels with simvastatin treatment was sufficient to have a significant protective effect on endothelial and renal function. This beneficial antioxidant effect of simvastatin treatment was observed despite increased inflammatory protein expression after a 10-wk high fat feeding. These data indicate that in this model of obesity with hypertension, the contribution of inflammation to the development of renal injury is important but independent of the contribution of oxidative stress. In human and animal studies, statin treatment has been shown to have a complex relationship with inflammation. There are studies in which statin treatment reduced inflammatory protein expression, which has been associated with the protective effect on renal injury in obesity (8, 15). However, in human mesangial cells simvastatin treatment has acted to increase expression of inflammatory cytokines and proinflammatory cyclooxygenase-2 (33). In this model of obesity and hypertension, we observed no renal anti-inflammatory effect with 10 mg·kg−1·day−1 simvastatin treatment. Simvastatin treatment protected renal endothelial function and prevented albuminuria in the absence of a change in renal inflammatory status. This would indicate that in this model of essential hypertension with obesity, increases in inflammatory proteins are not solely responsible for the development of endothelial dysfunction and renal injury. Thus the relationship between simvastatin and inflammatory protein expression is a complex one and not controlled completely by its antioxidant properties.
Conclusion
In this study, we demonstrate in a rat model of essential hypertension with a high-fat diet that afferent arterial endothelial dysfunction occurs followed by renal injury accompanied by renal inflammation. Treatment with both the SOD mimetic tempol and HMG-CoA inhibitor simvastatin prevented the development of endothelial dysfunction and preserved renal function in this model. These data provide evidence for the involvement of increased free radical generation as a potential mechanism of renal injury in obesity. Interestingly, the protective effect of simvastatin treatment was independent of any change in inflammatory response observed as a result of the high-fat diet and hypertension.
GRANTS
This work was supported by National Institutes of Health Grants HL59699 and DK38266.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1. Arendshorst WJ, Beierwaltes WH. Renal and nephron hemodynamics in spontaneously hypertensive rats. Am J Physiol Renal Fluid Electrolyte Physiol 236: F246–F251, 1979 [DOI] [PubMed] [Google Scholar]
- 2. Armutcu F, Ataymen M, Atmaca H, Gurel A. Oxidative stress markers, C-reactive protein and heat shock protein 70 levels in subjects with metabolic syndrome. Clin Chem Lab Med 46: 785–790, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Ballantyne CM, Raichlen JS, Cain VA. Statin therapy alters the relationship between apolipoprotein B and low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol targets in high-risk patients: the MERCURY II (Measuring Effective Reductions in Cholesterol Using Rosuvastatin) trial. J Am Coll Cardiol 52: 626–632, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Barati MT, Merchant ML, Kain AB, Jevans AW, McLeish KR, Klein JB. Proteomic analysis defines altered cellular redox pathways and advanced glycation end-product metabolism in glomeruli of db/db diabetic mice. Am J Physiol Renal Physiol 293: F1157–F1165, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Bayorh MA, Ganafa AA, Eatman D, Walton M, Feuerstein GZ. Simvastatin and losartan enhance nitric oxide and reduce oxidative stress in salt-induced hypertension. Am J Hypertens 18: 1496–1502, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Bezerra DG, Mandarim-de-Lacerda CA. Beneficial effect of simvastatin and pravastatin treatment on adverse cardiac remodelling and glomeruli loss in spontaneously hypertensive rats. Clin Sci (Lond) 108: 349–355, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Binder CJ, Weiher H, Exner M, Kerjaschki D. Glomerular overproduction of oxygen radicals in Mpv17 gene-inactivated mice causes podocyte foot process flattening and proteinuria: a model of steroid-resistant nephrosis sensitive to radical scavenger therapy. Am J Pathol 154: 1067–1075, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blanco S, Vaquero M, Gomez-Guerrero C, Lopez D, Egido J, Romero R. Potential role of angiotensin-converting enzyme inhibitors and statins on early podocyte damage in a model of type 2 diabetes mellitus, obesity, and mild hypertension. Am J Hypertens 18: 557–565, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Bussolati B, Deregibus MC, Fonsato V, Doublier S, Spatola T, Procida S, Di Carlo F, Camussi G. Statins prevent oxidized LDL-induced injury of glomerular podocytes by activating the phosphatidylinositol 3-kinase/AKT-signaling pathway. J Am Soc Nephrol 16: 1936–1947, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol 24: 816–823, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Chade AR, Rodriguez-Porcel M, Herrmann J, Zhu X, Grande JP, Napoli C, Lerman A, Lerman LO. Antioxidant intervention blunts renal injury in experimental renovascular disease. J Am Soc Nephrol 15: 958–966, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Chalmers L, Kaskel FJ, Bamgbola O. The role of obesity and its bioclinical correlates in the progression of chronic kidney disease. Adv Chronic Kidney Dis 13: 352–364, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Chinen I, Shimabukuro M, Yamakawa K, Higa N, Matsuzaki T, Noguchi K, Ueda S, Sakanashi M, Takasu N. Vascular lipotoxicity: endothelial dysfunction via fatty-acid-induced reactive oxygen species overproduction in obese Zucker diabetic fatty rats. Endocrinology 148: 160–165, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Chow FY, Nikolic-Paterson DJ, Ma FY, Ozols E, Rollins BJ, Tesch GH. Monocyte chemoattractant protein-1-induced tissue inflammation is critical for the development of renal injury but not type 2 diabetes in obese db/db mice. Diabetologia 50: 471–480, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Di Renzo L, Noce A, De Angelis S, Miani N, Di Daniele N, Tozzo C, De Lorenzo A. Anti-inflammatory effects of combined treatment with acetyl salicylic acid and atorvastatin in haemodialysis patients affected by normal weight obese syndrome. Pharmacol Res 57: 93–99, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Du X, Edelstein D, Obici S, Higham N, Zou MH, Brownlee M. Insulin resistance reduces arterial prostacyclin synthase and eNOS activities by increasing endothelial fatty acid oxidation. J Clin Invest 116: 1071–1080, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Futrakul N, Sridama V, Futrakul P. Microalbuminuria—a biomarker of renal microvascular disease. Ren Fail 31: 140–143, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Garrison RJ, Kannel WB, Stokes J, 3rd, Castelli WP. Incidence and precursors of hypertension in young adults: the Framingham Offspring Study. Prev Med 16: 235–251, 1987 [DOI] [PubMed] [Google Scholar]
- 19. Gianella A, Nobili E, Abbate M, Zoja C, Gelosa P, Mussoni L, Bellosta S, Canavesi M, Rottoli D, Guerrini U, Brioschi M, Banfi C, Tremoli E, Remuzzi G, Sironi L. Rosuvastatin treatment prevents progressive kidney inflammation and fibrosis in stroke-prone rats. Am J Pathol 170: 1165–1177, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang J, Parish R, Mansi I, Yu H, Kennen EM, Davis T, Carden D. Non-high-density lipoprotein cholesterol in patients with metabolic syndrome. J Investig Med 56: 931–936, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Ichinose K, Maeshima Y, Yamamoto Y, Kinomura M, Hirokoshi K, Kitayama H, Takazawa Y, Sugiyama H, Yamasaki Y, Agata N, Makino H. 2-(8-Hydroxy-6-methoxy-1-oxo-1h-2-benzopyran-3-yl) propionic acid, an inhibitor of angiogenesis, ameliorates renal alterations in obese type 2 diabetic mice. Diabetes 55: 1232–1242, 2006 [PubMed] [Google Scholar]
- 22. Ivanovski O, Szumilak D, Nguyen-Khoa T, Nikolov IG, Joki N, Mothu N, Maizel J, Westenfeld R, Ketteler M, Lacour B, Drueke TB, Massy ZA. Effect of simvastatin in apolipoprotein E deficient mice with surgically induced chronic renal failure. J Urol 179: 1631–1636, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Jasinska M, Owczarek J, Orszulak-Michalak D. Statins: a new insight into their mechanisms of action and consequent pleiotropic effects. Pharmacol Rep 59: 483–499, 2007 [PubMed] [Google Scholar]
- 24. Just A, Olson AJ, Whitten CL, Arendshorst WJ. Superoxide mediates acute renal vasoconstriction produced by angiotensin II and catecholamines by a mechanism independent of nitric oxide. Am J Physiol Heart Circ Physiol 292: H83–H92, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Kagiyama S, Tsuchihashi T, Abe I, Matsumura K, Fujishima M. Central infusion of l-arginine or superoxide dismutase does not alter arterial pressure in SHR. Hypertens Res 23: 339–343, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Knight SF, Quigley JE, Yuan J, Roy SS, Elmarakby A, Imig JD. Endothelial dysfunction and the development of renal injury in spontaneously hypertensive rats fed a high-fat diet. Hypertension 51: 352–359, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kramer H, Luke A, Bidani A, Cao G, Cooper R, McGee D. Obesity and prevalent and incident CKD: the Hypertension Detection and Follow-Up Program. Am J Kidney Dis 46: 587–594, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Lazaro A, Gallego-Delgado J, Justo P, Esteban V, Osende J, Mezzano S, Ortiz A, Egido J. Long-term blood pressure control prevents oxidative renal injury. Antioxid Redox Signal 7: 1285–1293, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Lind L, Berne C, Lithell H. Prevalence of insulin resistance in essential hypertension. J Hypertens 13: 1457–1462, 1995 [PubMed] [Google Scholar]
- 30. Macconi D, Bonomelli M, Benigni A, Plati T, Sangalli F, Longaretti L, Conti S, Kawachi H, Hill P, Remuzzi G, Remuzzi A. Pathophysiologic implications of reduced podocyte number in a rat model of progressive glomerular injury. Am J Pathol 168: 42–54, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miyata K, Ohashi N, Suzaki Y, Katsurada A, Kobori H. Sequential activation of the reactive oxygen species/angiotensinogen/renin-angiotensin system axis in renal injury of type 2 diabetic rats. Clin Exp Pharmacol Physiol 35: 922–927, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nagase M, Yoshida S, Shibata S, Nagase T, Gotoda T, Ando K, Fujita T. Enhanced aldosterone signaling in the early nephropathy of rats with metabolic syndrome: possible contribution of fat-derived factors. J Am Soc Nephrol 17: 3438–3446, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Naito M, Shenoy A, Aoyama I, Koopmeiners JS, Komers R, Schnaper HW, Bomsztyk K. High ambient glucose augments angiotensin ii-induced proinflammatory gene mRNA expression in human mesangial cells: effects of valsartan and simvastatin. Am J Nephrol 30: 99–111, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nangaku M, Izuhara Y, Usuda N, Inagi R, Shibata T, Sugiyama S, Kurokawa K, van Ypersele de Strihou C, Miyata T. In a type 2 diabetic nephropathy rat model, the improvement of obesity by a low calorie diet reduces oxidative/carbonyl stress and prevents diabetic nephropathy. Nephrol Dial Transplant 20: 2661–2669, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Peixoto EB, Pessoa BS, Biswas SK, Lopes de Faria JB. Antioxidant SOD mimetic prevents NADPH oxidase-induced oxidative stress and renal damage in the early stage of experimental diabetes and hypertension. Am J Nephrol 29: 309–318, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Rafikova O, Salah EM, Tofovic SP. Renal and metabolic effects of tempol in obese ZSF1 rats—distinct role for superoxide and hydrogen peroxide in diabetic renal injury. Metabolism 57: 1434–1444, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Rodriguez-Iturbe B, Vaziri ND, Herrera-Acosta J, Johnson RJ. Oxidative stress, renal infiltration of immune cells, and salt-sensitive hypertension: all for one and one for all. Am J Physiol Renal Physiol 286: F606–F616, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Sarafidis PA. Obesity, insulin resistance and kidney disease risk: insights into the relationship. Curr Opin Nephrol Hypertens 17: 450–456, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Schnackenberg CG, Welch WJ, Wilcox CS. Normalization of blood pressure and renal vascular resistance in SHR with a membrane-permeable superoxide dismutase mimetic: role of nitric oxide. Hypertension 32: 59–64, 1998 [DOI] [PubMed] [Google Scholar]
- 40. Stewart T, Jung FF, Manning J, Vehaskari VM. Kidney immune cell infiltration and oxidative stress contribute to prenatally programmed hypertension. Kidney Int 68: 2180–2188, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Wang D, Chen Y, Chabrashvili T, Aslam S, Borrego Conde LJ, Umans JG, Wilcox CS. Role of oxidative stress in endothelial dysfunction and enhanced responses to angiotensin II of afferent arterioles from rabbits infused with angiotensin II. J Am Soc Nephrol 14: 2783–2789, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Wang J, Zou H, Li Q, Wang Y, Xu Q. The expression of monocyte chemoattractant protein-1 and C-C chemokine receptor 2 in post-kidney transplant patients and the influence of simvastatin treatment. Clin Chim Acta 373: 44–48, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Whaley-Connell A, Habibi J, Nistala R, Cooper SA, Karuparthi PR, Hayden MR, Rehmer N, DeMarco VG, Andresen BT, Wei Y, Ferrario C, Sowers JR. Attenuation of NADPH oxidase activation and glomerular filtration barrier remodeling with statin treatment. Hypertension 51: 474–480, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wilcox CS, Pearlman A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol Rev 60: 418–469, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wu Y, Dong J, Yuan L, Liang C, Ren K, Zhang W, Fang F, Shen J. Nephrin and podocin loss is prevented by mycophenolate mofetil in early experimental diabetic nephropathy. Cytokine 44: 85–91, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Yanes L, Romero D, Iliescu R, Cucchiarelli VE, Fortepiani LA, Santacruz F, Bell W, Zhang H, Reckelhoff JF. Systemic arterial pressure response to two weeks of Tempol therapy in SHR: involvement of NO, the RAS, and oxidative stress. Am J Physiol Regul Integr Comp Physiol 288: R903–R908, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Zhang H, Saha J, Byun J, Schin M, Lorenz M, Kennedy RT, Kretzler M, Feldman EL, Pennathur S, Brosius FC., III Rosiglitazone reduces renal and plasma markers of oxidative injury and reverses urinary metabolite abnormalities in the amelioration of diabetic nephropathy. Am J Physiol Renal Physiol 295: F1071–F1081, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]


