Abstract
Immune factors are involved in modulating neointimal response to arterial wall injury, but the role of individual immune effectors in this response remains unclear. Using a carotid cuff injury model in mice, we tested the role of immunoglobulin isotypes in modulating intimal thickening by using adoptive transfer of splenocytes from WT mice, or the direct administration of IgG or IgM into immune-deficient Rag-1−/− [Rag-1 knockout (Rag-1KO)] mice. The direct role of complement was also tested by depletion of complement. Splenocytes from WT mice were isolated and adoptively transferred to Rag-1KO mice subjected to carotid cuff arterial injury. Transfer of splenocytes to Rag-1KO mice resulted in increased serum IgM and IgG within 48 h and were comparable to WT levels by 21 days after injury. Splenocyte transfer in Rag-1KO decreased intimal area by 40% compared with Rag-1KO mice without cell transfer. To further differentiate the relative contribution of IgM or IgG in reducing intimal thickening, additional groups of Rag-1KO mice were subjected to injury and given intravenous injections of pooled mouse IgG or IgM. Both IgG and IgM treatment significantly reduced intimal thickening compared with untreated Rag-1KO mice. Immunoglobulin treatments modified serum complement C3 profile and decreased C3 presence in injured arteries. Depletion of C3 using cobra venom factor in Rag-1KO mice significantly decreased intimal thickening. Our results identify the direct role of natural IgG and IgM, and complement in the modulation of neointimal response to arterial injury.
Keywords: immune system, complement system
tissue injury results in the activation of local and systemic repair mechanisms. There is increasing evidence supporting the role of host immune responses in this repair mechanism. Innate immune functions, such as the complement system, appear to contribute to an unfavorable outcome after injury (4, 18, 19). On the other hand, we have reported that T cells and B cells of the adaptive immune system inhibit intimal thickening after arterial injury, independent of each other (5, 6). In these studies, the immune-deficient Rag-1−/− [Rag-1 knockout (Rag-1KO)] mouse (15) provided a model to dissect specific components of the immune system involved in the host response to vascular injury. Since Rag-1KO mice lack both T cells and B cells, one can reconstitute the mice with specific cell types or manipulate immune effectors to test individual roles without the complex interaction among the various immune components. B cell reconstitution of Rag-1KO mice resulted in normalization of serum antibody levels (5). Because the mice were not exposed to any specific immune challenge or vaccination, it is likely that the antibodies produced by the injected B cells were nonimmune, natural antibodies (1, 8).
Natural antibodies are circulating innate antibodies in normal individuals that have been produced in the absence of overt specific antigenic stimulation. They are generally considered to be of the IgM isotype, although autoreactive serum IgG and IgA have also been reported, displaying multireactivity but low affinity for a range of antigens (3). There is growing interest in the role of natural antibodies after injury and trauma (8). This is further highlighted by the interaction between natural antibodies and the complement system (4, 8, 12–14). This is important given that complement inhibition reduced lesion size in injured arteries of apoE−/− mice (18). The normal course of innate immunoglobulin response to vascular injury is not known, nor is it clear whether there is isotype specificity in the response to injury and how this may interact with the complement system.
The present study was designed to extend our previous findings by defining the natural antibody response after injury and determine whether the response is isotype specific. To address the first issue, Rag-1KO mice were given adoptive cell transfer of wild-type (WT) splenocytes and the immunoglobulin response, and intimal thickening was investigated. Isotype specificity was assessed using IgG or IgM from pooled mouse sera injected into Rag-1KO mice, and the effect on intimal thickening was studied. A complement depletion strategy was used to test the role of the complement system. The present report indicates that natural antibodies play a role in attenuating the neointimal response to injury of the vascular wall.
MATERIALS AND METHODS
The Institutional Animal Care and Use Committee of Cedars-Sinai Medical Center approved the experimental protocols used in this study.
Cuff injury.
Mice were housed in a pathogen-free environment monitored by a sentinel program. Injury was performed on the right carotid artery of 25-wk-old male mice (WT and Rag-1KO both on the B6/129 background; Jackson Laboratory) by placing a nonocclusive plastic cuff (length = 2.5 mm; internal diameter = 0.51 mm; Cole-Parmer Instrument) around the artery, and the skin incision was closed. Euthanasia was performed after 1, 3, 7, and 21 days (5, 6). Blood was collected by retroorbital bleed. Carotid arteries were harvested after perfusion with normal saline for 10 min. Arteries were dissected free of connective tissue and were embedded in optical cutting temperature. Spleens were also collected and snap frozen in liquid nitrogen.
Electron microscopy.
Injury was characterized using electron microscopy of carotid arteries of WT mice. Briefly, arteries were perfusion fixed with 1% gluteraldehyde after 0.9% saline solution perfusion and followed by 24-h immersion in 3% gluteraldehyde. The tissues were minced into 1-mm cubes and dehydrated in graded alcohol. Samples were embedded in epon, and one-micron sections were cut with glass knives and stained with methylene blue to select regions for ultra-structural study. Sections were then cut with a diamond knife and stained with uranyl acetate and lead citrate and examined with a JEOL 100 electron microscope.
Splenocyte transfer to Rag-1KO mice.
Forty-eight hours prior to injury, spleen cells from age-matched WT mice were isolated as previously described (5, 6). After lysing the red blood cells, splenocytes were collected by centrifugation, resuspended in PBS, and injected via the tail vein of Rag-1KO mice (7–9 × 107 cells/mouse).
Immunoglobulin treatment of Rag-1KO mice.
Rag-1KO mice were injected with pooled mouse IgG (Jackson Immunolabs) or IgM (Rockland Immunochemicals) at a dose of 0.50 mg/mouse every other day for the first week and twice a week, subsequently until euthanasia. IgM samples were dialyzed overnight against PBS to remove sodium azide. A hybridoma cell line secreting monoclonal antiphosphorylcholine (PC)-IgM with the T15 idiotype [HPCM2 cells (9), a generous gift from Dr. Patricia Gearhart at National Institutes of Health/National Institute on Aging] was maintained and phosphorylcholine-IgM (PC-IgM) was prepared as previously described (7).
Immunoprecipitation and Western blot analysis.
Serum was diluted 1:7 in PBS and incubated with a goat anti-mouse C3 antibody (Cappel) in 4°C for 2 h. Agarose bead-conjugated anti-goat IgG was then added and incubated overnight in 4°C. The beads were then washed twice, resuspended in 2× denaturing loading buffer, and boiled for 5 min, and an aliquot was loaded into a 10% SDS-PAGE gel and transferred to nitrocellulose membrane. The membrane was then incubated with goat anti-C3 overnight. After being washed three times, the membrane was incubated for 30 min with streptavidin-conjugated anti-goat secondary antibody, washed three times, and detected with 3-amino-9-ethylcarbazole (AEC) color development for 4 min. The membrane was scanned as a grey-scale image and analyzed using image analysis software.
Morphometric analysis and immunostaining.
Frozen sections 6- to 8-μm thick were collected from the injured carotid arteries as described previously (2, 5, 6). Sections were stained with hematoxylin and eosin, and the vessel area was measured using computer-assisted analysis (Image Pro Plus).
C3 presence was assessed on acetone-fixed sections using goat anti-mouse C3 antibody (Cappel). Biotin-conjugated donkey anti-goat (Santa Cruz Biotechnologies, Santa Cruz, CA) was used as secondary antibody with streptavidin-horseradish peroxidase (HRP) and AEC as color substrate. Controls included omission of the primary antibody and blocking of the primary antibody with purified mouse complement proteins (Cappel). Stained arteries were analyzed using Image Pro Plus. Immunoglobulin was stained in the injured arteries using biotinylated goat anti-mouse IgG or IgM and detected with streptavidin-HRP or HRP-conjugated goat anti-mouse IgG or IgM and DAB or AEC as color substrate.
Real-time PCR.
Total RNA was isolated from a portion of the spleens using Trizol reagent (Invitrogen). Two micrograms of total RNA was subjected to reverse transcription using Superscript II (Invitrogen). Equal aliquots were then used for real-time PCR using SYBR Green (Bio-Rad) and primer pairs for ubiquitin, interferon-γ (IFN-γ), and interleukin-10 (IL-10). Analysis was performed using the ΔΔCt method with WT splenic RNA as calibrator and expressed as fold change.
Complement depletion.
Rag-1KO mice were treated with cobra venom factor to deplete complement (4) 3 days prior to injury and continued twice weekly until euthanasia (0.025 mg/mouse ip).
ELISA.
Relative serum immunoglobulin levels were determined using an isotyping ELISA kit from Southern Biotechnology Associates as described previously (5). HRP-conjugated IgM (Southern Biotechnology Associates) and IgG (Pierce) antibodies were used for detection. Substrate for color development was 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and optical density was recorded 20 min later on a SpectraMax 190 plate reader (Molecular Devices, Sunnyvale, CA).
Serum PC-IgM levels were detected by coating the ELISA plate with PC-BSA (10 μg/ml) overnight. After washings and blocking with 1% BSA in PBS, diluted serum was added into wells and incubated for 1 h at room temperature. After being washed, biotinylated goat anti-mouse IgM (Pierce) was added for incubation at room temperature for 1 h. HRP-conjugated IgM (Southern Biotechnology Associates ) was used for detection with ABTS, as described above.
Statistical analysis.
Results are presented as means ± SD. ANOVA was used to test for significance followed by Neuman-Keuls for multiple comparisons unless noted otherwise. Significance was set at P < 0.05.
RESULTS
Arterial injury by cuff placement.
Characterization of the injury model was performed using WT mice. Electron microscopy showed endothelial cells sloughing off 1 day after cuffing of the right carotid artery (Fig. 1B), with platelet adhesion by day 3 (Fig. 1C) and a thickened intimal layer by day 21 (Fig. 1E). There was a small increase in IgG levels after arterial injury in WT mice (Table 1). Intimal thickening was significantly increased in Rag-1KO mice compared with WT mice 21 days after injury (Fig. 2, A and B; Table 2).
Fig. 1.
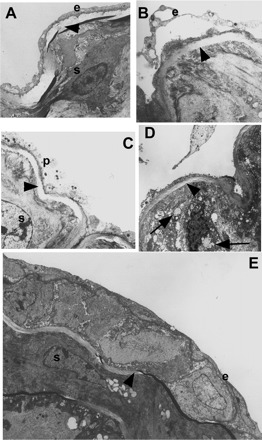
Electron microscopy of the injured arterial segment. A: uninjured carotid artery showing intact endothelial cells (e) and medial smooth muscle cells (s). One day after injury endothelial cells (e) appeared to be sloughing off (B), which was apparent by 3 days where platelet (p) deposition was observed (C). D: at 7 days, highly disorganized medial cells were observed with large vacuoles (arrows). E: by day 21 a neointima was clearly evident with a regenerated endothelial layer (e). C and E: s, smooth muscle cells. A–E: arrowheads indicate internal elastic lamina. Original magnification for A and E: ×6,000, B and C: ×8,000; D: ×10,000.
Table 1.
Relative immunoglobulin levels after arterial injury
| Day 0, n = 7 | Day 3, n = 3 | Day 7, n = 5 | Day 21, n = 7 | |
|---|---|---|---|---|
| Total IgM | 1.89±0.21 | 1.89±0.07 | 2.14±0.10 | 2.13±0.11 |
| Total IgG | 1.64±0.12 | 1.75±0.02 | 1.76±0.02* | 1.81±0.05* |
Values are in optical density at 405 nm.
P < 0.05 vs. day 0.
Fig. 2.

Representative sections showing intimal thickening in arteries 21 days after arterial cuff injury of wild-type (WT) mice (A), immune-deficient Rag-1−/− [Rag-1 knockout (Rag-1KO)] (B), and Rag-1KO mice reconstituted with splenocytes (C) from WT mice. Arrows mark intimal thickening. Bar = 10 microns.
Table 2.
Intimal area measurement 21 days after arterial injury
| Group | No. | Intima, mm2 | I/M |
|---|---|---|---|
| Wild type | 9 | 0.009±0.005* | 0.23±0.09* |
| Rag-1KO | 10 | 0.027±0.012 | 0.71±0.22 |
| Rag-1KO+S | 13 | 0.016±0.009† | 0.38±0.17* |
| Rag-1KO+IgM | 5 | 0.014±0.003† | 0.36±0.08* |
| Rag-1KO+IgG | 8 | 0.012±0.006* | 0.29±0.13* |
| Rag-1KO+CVF | 7 | 0.013±0.006* | 0.29±0.14* |
I/M, intima-to-media ratio; Rag-1KO, immune-deficient Rag-1−/−; KO, knockout; Rag-1KO+S, Rag-1KO mice reconstituted with splenocytes; CVF, cobra venom factor.
P < 0.01 vs. Rag-1KO;
P < 0.05 vs. Rag-1KO.
Whole splenocyte reconstitution of Rag-1KO mice reduces intimal thickening.
Whole splenocyte reconstitution significantly decreased intimal thickening (Fig. 2C) and intima-to-media ratio (I/M; Table 2) in Rag-1KO mice. Previous work in our laboratory showed that reconstitution of Rag-1KO mice with B cells alone resulted in increased immunoglobulin levels (5). However, the normal host immune responses occur in the presence of both B cells and T cells. In the present report, transfer of splenocytes resulted in increased serum IgM and IgG (Fig. 3, A and B; see Supplementary Figure I) within 48 h after transfer (uninjured). Twenty-one days after injury, IgM and IgG levels in recipient Rag-1KO mice were comparable to WT mice also subjected to carotid injury. IgG isotypes 1, 2b, and 3 all increased to levels comparable with WT mice (not shown). Injured arteries of the recipient mice showed presence of both IgM and IgG (Fig. 3, C and D). Splenic cytokine mRNA were also increased by splenocyte transfer, similar to WT levels (not shown). Injected splenocytes were localized both in the spleen and injured arteries (see Supplementary Fig. II).
Fig. 3.

IgM (A) and IgG (B) levels in uninjured (UI) and 21-day injured (D21) WT mice, Rag-1KO mice, and Rag-1KO mice reconstituted with splenocytes (Rag-1KO+S). Splenocytes were transferred to the recipient mice 48 h prior to the UI time point. Values are expressed as optical density at 405 nm (OD 405). *P < 0.01 vs. Rag-1KO; †P < 0.01 vs. WT; ‡P < 0.05 vs. Rag-1KO; n ≥ 4 each. Injured carotid arteries showed the presence of IgM (C) and IgG (D) 21 days after injury in Rag-1KO mice reconstituted with splenocytes, but not in Rag-1KO mice without splenocyte reconstitution (E and F, respectively). Bar = 10 microns.
Effect of specific immunoglobulin isotype on intimal thickening.
After observing that increased immunoglobulin levels in the splenocyte-reconstituted Rag-1KO mice resulted in reduced intimal thickening, we tested the specific role of IgM and IgG in modulating intimal thickening. Administration of pooled serum IgM to Rag-1KO mice resulted in significantly decreased intimal thickening and I/M ratio (Fig. 4B and Table 2). Pooled serum IgG also resulted in significantly reduced intimal thickening and I/M ratio (Fig. 4C and Table 2). IgM and IgG were detected in the serum (Fig. 5, A and B, respectively), and were deposited in the arteries of treated Rag-1KO mice 21 days after injury (Fig. 5, C and D, respectively). These results indicate that both IgM and IgG isotypes can modulate vascular repair in response to injury.
Fig. 4.

Representative sections showing intimal thickening in Rag-1KO mice without immunoglobulin treatment (A), treatment with IgM (B), and IgG (C). Arrows mark intimal thickening. Bar = 10 microns.
Fig. 5.
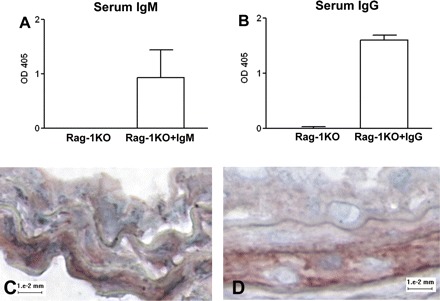
Presence of injected immunoglobulin in Rag-1KO mice. IgM (A) and IgG (B) were detected in serum of mice that received the respective isotype injection. IgM and IgG presence was also detected in the carotid arteries 21 days after injury (C and D, respectively).
Complement component C3.
Previous studies have shown that immunoglobulins regulate complement activation (13, 14, 17). Complement inhibition reduced neointima formation in apoE−/− mice (17). We therefore assessed complement component C3 activation in our study. Serum C3 profile in the experimental groups was assessed by immunoprecipitation and Western blot analysis (4). Rag-1KO mice had increased presence of a ∼115-kDa αC3 fragment compared with WT mice (0.90 ± 0.17 vs. 0.54 ± 0.14 relative densitometric units; P < 0.05; n = 6 and n = 5, respectively; Fig. 6 A, left). The 65-kDa βC3 fragment was similar between the two groups. The 43-kDa C3d band showed a trend for reduced presence in the Rag-1KO mice (0.55 ± 0.45) compared with WT mice (0.96 ± 0.71) but was not statistically significant. IgG treatment of Rag-1KO mice resulted in a significant decrease in the 115-kDa αC3 fragment (0.62 ± 0.21; P < 0.05; n = 6, Fig. 6A, right) compared with untreated Rag-1KO mice. The C3d fragment was trending higher in the IgG-treated group (0.80 ± 0.30), but was again not statistically significant. IgM treatment did not significantly affect the 115-kDa αC3 fragment (0.74 ± 0.10; n = 4) or the 43-kDa C3d fragment (0.26 ± 0.24) compared with untreated Rag-1KO mice. We then assessed the presence of C3 in injured arteries of IgM treated Rag-1KO mice using C3 immunostaining. Rag-1KO mice had increased percentage of C3-stained area compared with WT mice (7.90 ± 2.05% vs. 2.73 ± 1.90%, respectively; n = 4 each; P < 0.05; Fig. 6, C and B). IgM-treated mice had less C3 stain (3.25 ± 2.13%; n = 4; P < 0.05; Fig. 6D) compared with untreated Rag-1KO mice.
Fig. 6.

Immunoblot of C3 immunoprecipitated from serum of 21-day injured WT mice and Rag-1KO mice (A, left). Serum C3 from Rag-1KO mice without treatment (NT; A, right), treated with IgG, or treated with IgM (IgG and IgM, respectively). NT indicates no immunoglobulin treatment. The ∼55-kDa band is a noncomplement band that is an artifact of the immunoprecipitation method and corresponds to the IgG used for immunoprecipitation (4). Representative sections of immunostaining for C3 in injured arteries of WT mice (B), Rag-1KO mice (C), Rag-1KO mice treated with IgM (D), and Rag-1KO mice treated with cobra venom factor (E). N = 4 each. Bar = 20 microns.
Effect of complement depletion on intimal thickening in Rag-1KO mice.
To determine the specific role of complement in arterial wall injury, complement was depleted in injured Rag-1KO mice using cobra venom factor (CVF). Rag-1KO mice treated with CVF had minimal C3 staining (Fig. 6E), significantly reduced intimal thickening, and an I/M ratio compared with Rag-1KO mice without CVF treatment (Table 2). The medial area was not affected by CVF treatment.
Injury and phosphorylcholine IgM.
The role of natural IgM in other diseases is attributed to the presence of phosphorylcholine (PC)-binding antibodies modulating complement activity (11). We therefore determined the relative amount of PC-IgM in uninjured WT mice and at various time points after injury. No significant changes were observed in PC-IgM levels throughout the injury time points (not shown). PC-IgM levels in Rag-1KO mice reconstituted with whole splenocytes was ∼50% of WT levels at day 21 (0.15 ± 0.07 vs. 0.36 ± 0.27 optical density at 405 nm, respectively; P = 0.1; n = 6 each). PC-IgG levels were not affected by injury in WT mice and were not significantly different in Rag-1KO reconstituted with splenocytes compared with WT mice (not shown).
To test the role of PC-specific IgM antibodies, we treated Rag-1KO mice subjected to injury with the same dose of a monoclonal anti-PC IgM. Treatment with PC-IgM did not significantly affect intimal thickening compared with Rag-1KO without anti-PC IgM treatment (0.023 ± 0.021 mm2; n = 5 vs. 0.027 ± 0.012 mm2; n = 10, respectively), suggesting that PC-specific antibody response does not significantly contribute to the inhibitory effect of serum IgM in injury-induced intimal formation.
DISCUSSION
Intravenous Ig preparations have been shown to reduce experimental atherosclerosis and injury-induced intimal thickening (10, 16). These studies used immune-competent animals and high doses of Ig preparations used for treating autoimmune disorders, the possible effect of which is by modulating the host immune system (10, 16). Evidence for the direct role of immunoglobulin in resolving the repair response and limiting arterial intimal thickening, uncoupled from its effect of modulating the adaptive immune response, is lacking. This can only be tested in mice which lack the host immune system. We therefore investigated the role of specific antibody isotypes on intimal thickening induced by arterial injury in immune-deficient Rag-1KO mice.
Although the model of vascular cuff injury we used in our study is peri-adventitial and may have caused adventitial inflammation, it also resulted in the endothelial cells lining the lumen of the artery to slough off within a day, as evidenced by our electron microscopy examination and in agreement with our previous report (2). This was then followed by platelet adhesion at the site. Although the injury itself appears to be relatively acute, the formation of the neointimal layer was not obvious 1 wk later and was observed only at the 21-day time point.
The first part of the investigation used adoptive transfer of WT splenocytes into Rag-1KO mice, which resulted in IgG and IgM isotypes reaching levels comparable with WT mice 21 days after injury. This is consistent with our previous study where Rag-1KO mice were reconstituted with B cells (5). Although we reported that B cells reduced intimal thickening after arterial injury, it was not clear whether the immunoglobulin produced by B cells was directly involved.
To address the question of whether IgG or IgM directly modulates intimal thickening after injury, we injected specific immunoglobulin isotype preparations that were derived from pooled sera of nonimmunized mice. This would strongly support the notion that natural antibodies normally present in the serum are involved in resolving the host response to vascular injury and trauma. The results indicate that presence of natural antibodies in the immunoglobulin preparations limited intimal thickening. As was the case with the splenocyte transfer experiments, there does not appear to be an isotype-specific effect.
One mechanism proposed by which intravenous immunoglobulin may work is through the complement system (12). It was reported that IgG enhanced the sensitivity of C3b-containing complexes to Factor I (13). This has been attributed to an IgG-mediated low-level activation of the classic complement pathway similar to spontaneous C3 tick-over, possibly as a maintenance mechanism to control complement activity (14). Complement is a very plausible candidate through which immunoglobulin mediates its effect in our study due to the lack of mature T and B lymphocytes in Rag-1KO mice. We observed that the profile of serum C3 in Rag-1KO mice was different compared with WT mice. Specifically, the 115-kDa αC3 fragment was increased in the Rag-1KO mice. The increased presence of the 115-kDa αC3 fragment is consistent with a previous report indicating that specific immune-deficient mice, including the Rag−/− genotype, have more serum C3 (14). IgG treatment of Rag-1KO mice resulted in decreased serum αC3, suggesting that IgG increased consumption of the high levels of circulating C3 in the Rag-1KO mice. This is consistent with the reported role of IgG in modulating complement activation (13, 14). IgM treatment on the other hand did not seem to significantly affect serum C3 profile. This in agreement with a previous report that showed more efficient complement fragment generation by IgG compared with IgM (14). However, IgM treatment did reduce the amount of C3 immunostaining in the injured arteries. The results suggested that although IgG and IgM treatments have different mechanisms of action, regulation of complement was a common effect, indicating that the complement pathway may also be involved in intimal thickening. We therefore depleted complement from Rag-1KO mice by administering CVF (4).
CVF treatment significantly reduced intimal thickening in Rag-1KO mice, comparable with WT mice. A report by Cowell et al. (4) showed that, although systemic complement C3 depletion occurred in a model of hypoxic-ischemic brain injury in neonatal rats using CVF, serum and tissue presence of C9 was still detectable. Yet infarct size was still significantly reduced by CVF treatment. Their study further showed that, although CVF did deplete complement in serum, it did not completely eliminate tissue complement expression by specific cells. This suggested that complement expression at the local site, although reduced, was still present. Our results similarly show minimal complement present in the injured artery after CVF treatment. Despite the minimal presence detected, intimal thickening was still significantly reduced, suggesting that similar to the hypoxic-ischemic brain injury model, systemic complement may play a more important role in intimal thickening. Our results identify unregulated systemic complement activation in the Rag-1KO mice as a contributing factor to exuberant intimal thickening after arterial injury. The role of complement is consistent with a recent report that showed decreased lesion formation by complement inhibition in injured apoE−/− mice (18). Given that the Rag-1KO mice are deficient in immunoglobulin, complement activation after injury likely occurred through the alternative pathway. Furthermore, our results suggest that both IgG and IgM regulate complement function.
The protective role of natural IgM antibodies has been described in other disease models. The mechanism is often attributed to the presence of PC-binding IgM (11). Serum levels of PC-IgM in WT mice did not increase after injury, and monoclonal PC-IgM did not significantly affect intimal thickening in Rag-1KO mice, suggesting that PC-IgM does not play a significant role in the inhibitory effect of serum IgM on intimal thickening after injury. In a previous study by our group, host PC-IgM levels increased after syngeneic vein grafting in apoE−/− mice. Accelerated atherosclerosis induced by vein grafting was significantly reduced by PC-IgM treatment (7). These findings suggest important differences in the immune response to different forms of vascular insult.
In conclusion, this study identifies natural antibodies of the IgG and IgM isotypes as factors involved in attenuating the response to vascular injury. In addition, depletion of complement significantly reduced intimal thickening in Rag-1KO mice. Taken together, our results suggest that natural antibodies of the IgG and IgM isotypes and the complement system have a direct role in the response to injury of the arterial wall.
Perspectives and Significance
Immune modulation of the repair response to arterial injury is complex and involves various arms of the immune system. Few studies have attempted to define and characterize these factors with a systematic approach. Our studies on the role of specific arms of the immune system and their interaction with other factors are beginning to provide a clearer picture of the complexities involved. A limitation of the present study is the possible interaction between B cells and T cells in the repair process. Previous studies suggest that this interaction may be important. Another limitation is the possible role of distinct subsets of T cells, including regulatory cells in the repair process. Our laboratory is currently conducting these studies as an extension of our continued work in this field. These findings will enhance the understanding of the process of vascular repair and identify targets or specific pathways of the immune response that can be modulated to result in more favorable outcomes in various vascular diseases.
GRANTS
This work was supported by the Spielberg Family Cardiovascular Research Fund, the Heart Foundation at Cedars-Sinai Medical Center, and a Postdoctoral Fellowship grant (to F. H. Y. Cesena) from The National Council for Scientific and Technological Development (CNPq) of Brazil.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
The authors thank Jonathan Kirzner for technical assistance.
REFERENCES
- 1. Avrameas S. Natural autoantibodies: from “horror autotoxicus” to “gnothi seauton”. Immunol Today 12: 154–159, 1991 [DOI] [PubMed] [Google Scholar]
- 2. Chyu KY, Dimayuga P, Zhu J, Nilsson J, Kaul S, Shah PK, Cercek B. Decreased neointimal thickening after arterial wall injury in inducible nitric oxide synthase knockout mice. Circ Res 85: 1192–1198, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Coutinho A, Kazatchkine MD, Avrameas S. Natural autoantibodies. Curr Opin Immunol 7: 812–818, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Cowell RM, Plane JM, Silverstein FS. Complement activation contributes to hypoxic-ischemic brain injury in neonatal rats. J Neurosci 23: 9459–9468, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dimayuga P, Cercek B, Oguchi S, Fredrikson GN, Yano J, Shah PK, Jovinge S, Nilsson J. Inhibitory effect on arterial injury-induced neointimal formation by adoptive B-cell transfer in Rag-1 knockout mice. Arterioscler Thromb Vasc Biol 22: 644–649, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Dimayuga PC, Li H, Chyu KY, Fredrikson GN, Nilsson J, Fishbein MC, Shah PK, Cercek B. T cell modulation of intimal thickening after vascular injury: the bimodal role of IFN-gamma in immune deficiency. Arterioscler Thromb Vasc Biol 25: 2528–2534, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Faria-Neto JR, Chyu KY, Li X, Dimayuga PC, Ferreira C, Yano J, Cercek B, Shah PK. Passive immunization with monoclonal IgM antibodies against phosphorylcholine reduces accelerated vein graft atherosclerosis in apolipoprotein E-null mice. Atherosclerosis 189: 83–90, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Fleming SD, Tsokos GC. Complement, natural antibodies, autoantibodies and tissue injury. Autoimmun Rev 5: 89–92, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Gearhart PJ, Johnson ND, Douglas R, Hood L. IgG antibodies to phosphorylcholine exhibit more diversity than their IgM counterparts. Nature 291: 29–34, 1981 [DOI] [PubMed] [Google Scholar]
- 10. Keren G, Keren P, Barshack I, Pri-Chen S, George J. The effect of intravenous immunoglobulins on intimal thickening in a mouse model of arterial injury. Atherosclerosis 159: 77–83, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Kim SJ, Gershov D, Ma X, Brot N, Elkon KB. I-PLA2 activation during apoptosis promotes the exposure of membrane lysophosphatidylcholine leading to binding by natural immunoglobulin M antibodies and complement activation. J Exp Med 196: 655–665, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lutz HU, Stammler P, Bianchi V, Trueb RM, Hunziker T, Burger R, Jelezarova E, Spath PJ. Intravenously applied IgG stimulates complement attenuation in a complement-dependent autoimmune disease at the amplifying C3 convertase level. Blood 103: 465–472, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Lutz HU, Stammler P, Jelezarova E, Nater M, Spath PJ. High doses of immunoglobulin G attenuate immune aggregate-mediated complement activation by enhancing physiologic cleavage of C3b in C3bn-IgG complexes. Blood 88: 184–193, 1996 [PubMed] [Google Scholar]
- 14. Manderson AP, Pickering MC, Botto M, Walport MJ, Parish CR. Continual low-level activation of the classical complement pathway. J Exp Med 194: 747–756, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68: 869–877, 1992 [DOI] [PubMed] [Google Scholar]
- 16. Nicoletti A, Kaveri S, Caligiuri G, Bariety J, Hansson GK. Immunoglobulin treatment reduces atherosclerosis in apo E knockout mice. J Clin Invest 102: 910–918, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rieben R, Roos A, Muizert Y, Tinguely C, Gerritsen AF, Daha MR. Immunoglobulin M-enriched human intravenous immunoglobulin prevents complement activation in vitro and in vivo in a rat model of acute inflammation. Blood 93: 942–951, 1999 [PubMed] [Google Scholar]
- 18. Shagdarsuren E, Bidzhekov K, Djalali-Talab Y, Liehn EA, Hristov M, Matthijsen RA, Buurman WA, Zernecke A, Weber C. C1-esterase inhibitor protects against neointima formation after arterial injury in atherosclerosis-prone mice. Circulation 117: 70–78, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Weiser MR, Williams JP, Moore FD, Jr, Kobzik L, Ma M, Hechtman HB, Carroll MC. Reperfusion injury of ischemic skeletal muscle is mediated by natural antibody and complement. J Exp Med 183: 2343–2348, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]


