Abstract
Acute β-blockade with metoprolol has been associated with increased mortality by undefined mechanisms. Since metoprolol is a relatively high affinity blocker of β2-adrenoreceptors, we hypothesized that some of the increased mortality associated with its use may be due to its abrogation of β2-adrenoreceptor-mediated vasodilation of microvessels in different vascular beds. Cardiac output (CO; pressure volume loops), mean arterial pressure (MAP), relative cerebral blood flow (rCBF; laser Doppler), and microvascular brain tissue Po2 (G2 oxyphor) were measured in anesthetized mice before and after acute treatment with metoprolol (3 mg/kg iv). The vasodilatory dose responses to β-adrenergic agonists (isoproterenol and clenbuterol), and the myogenic response, were assessed in isolated mesenteric resistance arteries (MRAs; ∼200-μm diameter) and posterior cerebral arteries (PCAs ∼150-μm diameter). Data are presented as means ± SE with statistical significance applied at P < 0.05. Metoprolol treatment did not effect MAP but reduced heart rate and stroke volume, CO, rCBF, and brain microvascular Po2, while concurrently increasing systemic vascular resistance (P < 0.05 for all). In isolated MRAs, metoprolol did not affect basal artery tone or the myogenic response, but it did cause a dose-dependent impairment of isoproterenol- and clenbuterol-induced vasodilation. In isolated PCAs, metoprolol (50 μM) impaired maximal vasodilation in response to isoproterenol. These data support the hypothesis that acute administration of metoprolol can reduce tissue oxygen delivery by impairing the vasodilatory response to β2-adrenergic agonists. This mechanism may contribute to the observed increase in mortality associated with acute administration of metoprolol in perioperative patients.
Keywords: cerebral hypoxia, β-adrenergic antagonist, isoproterenol, clenbuterol
the efficacy of β-adrenergic antagonists to reduce mortality and ischemia following an acute myocardial infarction (1, 2) contributed to the subsequent widespread use of this class of medication to treat a broad spectrum of cardiovascular diseases, including hypertension, heart failure, and coronary artery disease (24). In addition, data from two early clinical studies (22, 25) in perioperative medicine suggested that cardioselective β-blockade (atenolol and bisoprolol) reduced the incidence of myocardial infarction and mortality in patients with cardiovascular risk undergoing surgery. As a result of these studies, “cardioselective” β-adrenergic antagonists (metoprolol, atenolol, and bisoprolol) have become one of the most prescribed medications in North America (19).
These apparently favorable trials have lead to the broad application of perioperative β-blocker therapy (metoprolol, atenolol, and bisoprolol) as a strategy to reduce the risk of adverse cardiac events (14, 20). Recently, the enthusiasm toward this approach has been tempered by evidence that acute perioperative β-blockade with metoprolol has been associated with an increased incidence of bradycardia, hypotension, stroke, major adverse cardiac events, and mortality (4–6, 10, 28, 34).
This evidence has prompted a number of clinical studies designed to reassess the efficacy of β-adrenergic antagonism in perioperative medicine. While most studies (26) demonstrated a clear cardioprotective effect, these benefits did not come without significant risk to the patient population. Specifically, the risk for several other adverse cardiovascular events including stroke (4, 10), major adverse cardiac events (6), and mortality (4–6, 10, 28) were increased with perioperative β-blockade in specific patient populations. These data have lead some authors (4) to advocate against the widespread use of β-blockers in the perioperative period. The increased morbidity and mortality have been most strongly associated with 1) acute administration of the β-antagonists (11), 2) use of the relatively poorly β1-selective drug metoprolol (3, 10, 28, 34), and 3) use of β-adrenergic antagonists in the setting of increased cardiovascular demand, including acute blood loss and fluid resuscitation (6, 33). We chose to investigate whether β-blockade alters vascular reactivity, as a potential mechanism underlying the increase in mortality; we focused on metoprolol as it is one of the most frequently prescribed β-blockers in North America (www.imshealth.com) (5, 6, 19, 34).
In the current experimental study, we tested the hypothesis that acute administration of metoprolol impairs resistance artery dilation in response to β-adrenergic agonists and thereby worsens vital organ perfusion. We first assessed the impact of acute metoprolol administration in a whole animal model with a focus on cerebral oxygen delivery. Based on evidence of increased vascular resistance and reduced brain perfusion, we then assessed the effect of metoprolol on ex vivo preparations of isolated mesenteric resistance arteries (MRAs) and posterior cerebral arteries (PCAs).
MATERIALS AND METHODS
Animal model.
All animal protocols were approved by the Animal Care Committee at St. Michael's Hospital and the University of Toronto (Toronto, Ontario, Canada). Two- to three-month-old C57BL6/J mice were purchased from either The Jackson Laboratory (Bar Harbor, ME) or Charles River Laboratories (Montreal, QC, Canada) and housed under standard conditions with food and water ad libitum. All animals were anesthetized with 2% isoflurane for all in vivo experiments. For the studies utilizing isolated resistance arteries, all mice were euthanized under isoflurane anesthesia by cervical dislocation before vessel isolation.
The following drugs were used for in vitro experiments on isolated MRAs and PCAs: l-phenylephrine hydrochloride (P6126), ±-metoprolol (+)-tartrate salt (M5391), dl-isoproterenol hydrochloride (I5627), and clenbuterol hydrochloride (C5423; Sigma-Aldrich, St. Louis, MO). Metoprolol tartrate solution was used in whole animal studies [Betaloc (1 mg/ml); prod. no. 1332; AstraZeneca Canada, Mississauga, ON, Canada].
Effect of metoprolol on mean arterial pressure, relative cerebral blood flow, and microvascular brain oxygen tension.
In a separate group of anesthetized mice, femoral artery and vein cannulations were performed to measure mean arterial pressure (MAP) and heart rate (HR) following administration of saline vehicle or metoprolol (3 mg/kg). This dose was chosen based on previous pharmacokinetic studies in rodents (16). HR was measured using EKG electrodes. MAP was measured by direct femoral artery cannulation. In two separate groups of mice, either relative changes in cerebral blood flow (rCBF; laser Doppler, OxyFlo; Oxford Optronix) or quantitative brain microvascular Po2 were measured (phosphorescence quenching) as previously described (27). Briefly, anesthetized (2% isoflurane) spontaneously breathing animals were placed in a stereotaxic frame. For rCBF, two bilateral laser-Doppler flow probes were placed directly over the temporal aspect of the skull after exposure via a central skin incision. For brain Po2 measurements, the skull was exposed by a sagittal skin incision and excitation and detection light guides were placed over the parieto-temporal cerebral cortex and positioned such that the light path crossed through the cerebral cortex and deeper brain structures. Mice were injected with Oyxphor G2-phosphorescent dye [0.1 mg in 20 μl, Pd-tetra-(4-carboxyphenyl) tetrabenzoporphyrin dendrimer] allowing measurements of phosphorescence quenching by oxygen through the PMOD 5000 probes (Oxygen Enterprises, Philadelphia, PA). HR, femoral MAP, rCBF, and microvascular brain oxygen tension (PBrmvO2) were measured before and within 1 h after saline vehicle or metoprolol 3 mg/kg administration, and data were recorded electronically (PowerLabs).
Effect of metoprolol on cardiac physiology.
Cardiac catheterization was performed as previously published (37). In brief, animals (n = 6) were placed on a warming pad (37°C), intubated, and ventilated using positive pressure ventilation using 2% isoflurane in 100% O2. Mice were secured in a recumbent position and the right jugular vein was cannulated. Pressure was calibrated after the catheter was warmed in 0.9% NaCl at 37°C for 30 min (#FT112B; Scisense, London, Canada). The right internal carotid was then identified and ligated cranially. A 1.2-F miniaturized combined conductance catheter-micro-manometer (Scisense, London, ON, Canada) was inserted into the right carotid artery and advanced into the left ventricle until stable pressure volume loops were obtained. After 10 min, 0.9% heparinized saline was injected by the right jugular vein and a steady state was achieved. Following saline vehicle or metoprolol (3 mg/kg) intravenous injection, another steady state was reached after ∼10 min. All steady-state loops (pre- and postmetoprolol) were obtained with the ventilator turned off for 5–10 s with the animal apnoeic. With the use of the pressure conductance data, a range of real-time functional parameters were then calculated using the ADVantage system (Scisence). These included end diastolic pressure, end systolic pressure, end diastolic volume, end systolic volume (SV), cardiac output (CO), and systemic vascular resistance (SVR).
Pressure myography in isolated resistance arteries.
Pressure myography experiments were conducted as previously described (7, 18, 21). For MRA and PCA isolation, the mesentery or brain was removed following cervical dislocation under anesthesia (2% isoflurane) and placed in a dish containing ice-cold MOPS-buffered saline solution [MOPS pH 7.4: (in mmol/l) 145 NaCl, 4.7 KCl, 1.5 CaCl2·2H2O, 1.17 MgSO4·7H2O, 1.2 NaH2PO4.2H2O, 2.0 pyruvate, 0.02 EDTA, 3.0 MOPS, and 5.0 glucose]. Second order MRAs (∼200 μm in diameter) were dissected under ice-cold conditions and mounted onto a pressure myography system (Living Systems Instrumentation; Burlington, VT, USA). For PCAs, the brain was rapidly removed from the cranium and placed in ice-cold MOPS. First order PCA segments (∼150-μm diameter) were isolated and mounted onto a pressure myography system (Living Systems Instrumentation). Both artery types were warmed to 37°C at a transmural pressure (TMP) of 45 mmHg for 30 min; after warming, MRA TMP was raised to 60 mmHg. All vasomotor responses were assessed at 60 mmHg in MRAs and at 45 mmHg in PCAs (in both cases, with no flow through the vessel lumen).
Vessel viability was assessed at the start of the experiment by stimulating vasoconstriction with 1 μM (MRAs) or 10 μM (PCAs) phenylephrine (α1-adrenergic agonist); viable vessels maintained ≥30–50% constriction from baseline. All data are presented as tone, which represents normalized acute diameter measurements. Tone is computed as follows: tone (%diamax) = (diamax − diameasured)/diamax × 100, where diameasured is the diameter measured under a given condition and diamax is the maximal diameter (observed in calcium-free MOPS solution). Dilation is presented as a proportion of a preconstriction level: %dilation = (diameasured − diamax)/(diamax − diamin) × 100, where diamin is the preconstricted diameter. Pilot experiments determined an optimal phenylephrine preconstriction dose of 1 μM for MRAs and 3 μM for PCAs.
Myogenic responses in MRAs were initiated by a rapid elevation of TMP from 60 to 110 mmHg (7, 21). The passive distension following the pressure step is rapidly reversed by a continuous, active constriction, which was measured as the percent constriction compared with the initial distension: reversal of initial distension (%) = (diadist − diat=7)/(diadist − diat=0) × 100, where diat=0 is the diameter immediately preceding the pressure step, diadist is the distended diameter measured immediately following the pressure step, and diat=7 is the diameter measured 7 min following the pressure step, an arbitrary time point where the constriction is normally stable. Paired comparisons where made before and following exposure to 50 μM metoprolol (30-min treatment, with subsequent assessment in the presence of metoprolol).
Statistical analyses and calculations of vascular tone and dilation.
All data are presented as means ± SE; n values indicated number of animals or number of vessels tested. For in vivo experiments, analysis of differences in HR, MAP, rCBF, PBrmvO2 was performed by two-way repeated measures ANOVA. For rCBF experiments, all data (HR, MAP, and rCBF) were normalized to baseline for each animal as the rCBF values are reported in relative, nonquantitative, units. Analysis of cardiac responsiveness and left ventricular function were performed by paired t-test. Statistical analyses were done in SigmaPlot 11 (Systat Software, Chicago, IL).
An increase in percent dilation is indicative of increased vasodilation. Dose-response curves of agonist before and after incubation with 0, 5, 10, or 50 μM metoprolol were tested by two-way repeated measures ANOVA. The concentration of agonist that elicited half the maximal response of a dose-response curve is expressed as the EC50 (mol/l) value and is represented here as the logEC50, which is unitless. Mean logEC50 values are tested by paired t-test when both curves were generated in the same artery otherwise they were tested by unpaired student's t-test. Dose-response curves were also compared by the percent response at the top of the curve (Emax), which corresponds to maximal response of the vessel to the agonist. Vasoconstrictor (tone) and vasodilator (%dilation) responses to any one dose of agonist before and after metoprolol (or MOPS buffer) incubation were tested by paired t-tests. Statistical analyses were performed in Prism 5 (GraphPad Software, La Jolla, CA). Differences were significant at alpha P value <0.05.
RESULTS
Effect of acute metoprolol treatment on cardiovascular physiology in mice.
In isoflurane anesthetized mice, metoprolol caused a reduction in HR, SV, and CO, while SVR increased (Fig. 1; P < 0.05 for all). On average, changes in stroke volume (26 ± 4 vs. 20 ± 4 μl) and ejection fraction (39 ± 9 vs. 26 ± 4%) achieved statistical significance (n = 6; P < 0.05). No differences in end systolic or diastolic pressures or volumes were observed. An example of a pressure volume loops, before and after metoprolol, is demonstrated in Fig. 2. In a second group of anesthetized mice, metoprolol caused a decrease in HR and rCBF by ∼20% after 60 min (P < 0.05; Fig. 3), while femoral MAP remained unchanged. In a third group of anesthetized mice, metoprolol caused a comparable reduction in HR while femoral MAP was maintained (Fig. 4). In these mice, PBrmvO2 was decreased by ∼15% relative to baseline and control saline-treated mice (Fig. 4; PBrmvO2, 60.8 ± 2.5 vs. 69.7 ± 2.1 mmHg; P < 0.05).
Fig. 1.
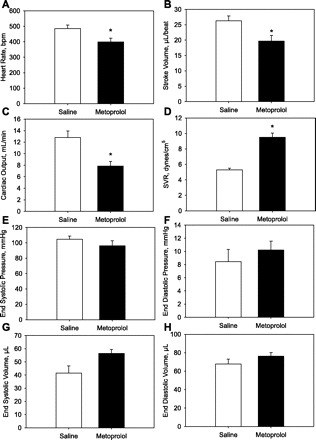
Changes in cardiovascular function after metoprolol administration in mice. Heart rate (A), stroke volume (B), and cardiac output (C) are significantly reduced while systemic vascular resistance (SVR; D) is increased following metoprolol injection (3 mg/kg). Average end-systolic and diastolic pressures and volumes (E–H) were not affected by metoprolol treatment (n = 6; *P < 0.05). bpm, Beats/min.
Fig. 2.
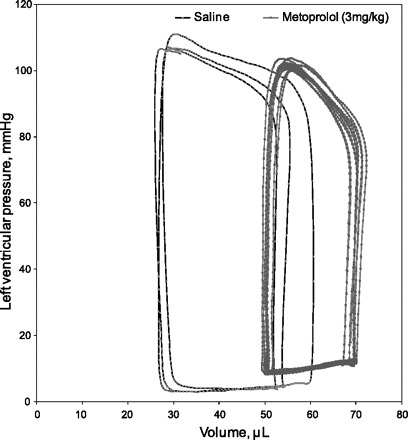
Representative pressure-volume loop before and after metoprolol administration in mice. An example of a tracing of steady state pressure-volume loops recorded in a representative mouse (n = 6). Steady-state loops were acquired 10 min after intravenous saline bolus and 10 min after intravenous metoprolol bolus. In this case, there is a clear increase in the end diastolic and systolic volumes and a reduction in stroke volume and ejection fraction after metoprolol administration.
Fig. 3.
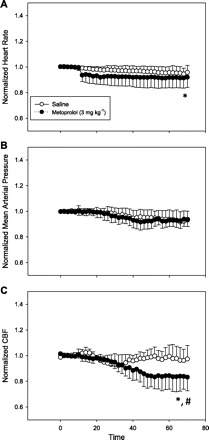
Relative changes in heart rate (HR), mean arterial pressure (MAP), and relative cerebral blood flow (rCBF) before and after metoprolol administration in mice. Metoprolol resulted in a ∼10% reduction in HR (A) from baseline, without affecting MAP (B). C: rCBF was decreased by ∼20% within the first hour of treatment with metoprolol (n = 6; *P < 0.05 vs. baseline; #P < 0.05 between groups).
Fig. 4.
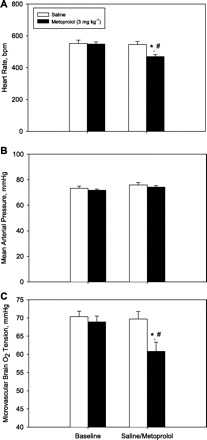
Mean HR, MAP, and microvascular brain oxygen tension (PBrmvO2) before and after metoprolol administration in mice. Within 60 min, administration of metoprolol 3 mg/kg resulted in a decrease in HR (A) and microvascular brain Po2 (C), while MAP remained unchanged (B). *P < 0.05 vs. baseline; #P < 0.05 between groups; n = 6.
Metoprolol impairs β-adrenergic-mediated dilation in MRAs.
Metoprolol did not cause a change in vessel diameter after drug administration (Table 1). Metoprolol (50 μM) did not alter the magnitude of the myogenic response or kinetics (assessed as the time to half-maximal response, control: 163 ± 18 s, metoprolol: 153 ± 24 s; n = 5; P > 0.05) of the myogenic response in isolated MRAs (Fig. 5).
Table 1.
Baseline vessel diameters
| Control, μM | Metoprolol, μM | |
|---|---|---|
| Mesenteric arteries | ||
| Isoproterenol ± 5 μM metoprolol (n = 7) | 199 ± 27 | 201 ± 25 |
| Isoproterenol ± 10 μM metoprolol (n = 7) | 217 ± 36 | 216 ± 36 |
| Isoproterenol ± 50 μM metoprolol (n = 6) | 224 ± 14 | 230 ± 18 |
| Posterior cerebral arteries | ||
| Isoproterenol ± 50 μM metoprolol (n = 7) | 148 ± 7.6 | 145 ± 9 |
Values are means ± SE.
Fig. 5.
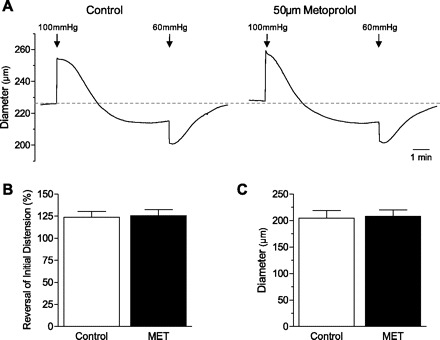
Effect of metoprolol on the myogenic response in isolated mesenteric arteries. There is no effect of metoprolol (50 μM) on the myogenic response kinetic (A), reversal of distension (B), or vessel diameter (C).
The nonselective β-adrenergic agonist isoproterenol was used to generate dose-response curves before and after incubation with metoprolol. Data for each metoprolol-treated vessel were compared with its own isoproterenol control response. A typical experiment (vessel diameter) is depicted in Fig. 6A. There was no effect of time on the isoproterenol dose-response curve as shown before and after incubation with 0 μM metoprolol (Fig. 6B). Similarly, there was no effect with 5 or 10 μM metoprolol (Fig. 6, C and D). A significant right shift in the isoproterenol dose-response curve was observed at a metoprolol concentration of 50 μM (Fig. 6E). The logEC50 of isoproterenol at 50 μM metoprolol was significantly higher than at 0 μM metoprolol (−4.1 ± 0.082 vs −4.6 ± 0.084; n = 6–7; P < 0.05). In addition, the percent dilation at the control EC50 dose of isoproterenol was significantly attenuated by metoprolol at 50 μM (17 ± 2.3 vs. 34 ± 2.0%; n = 6; P < 0.05). At all concentrations of metoprolol, dose-response curves reached equivalent Emax plateau values. The relative β2-specific agonist clenbuterol dose-response curve was significantly right shifted by 50 μM metoprolol, relative to its control curve (Fig. 6F; P < 0.05). The percent dilation at the control EC50 dose (10 μM or logEC50=−5) was significantly attenuated at 50 μM metoprolol (25 ± 1.6 vs. 34 ± 1.0; n = 5; P < 0.05). Comparable Emax plateaus were achieved in both groups (Fig. 6).
Fig. 6.
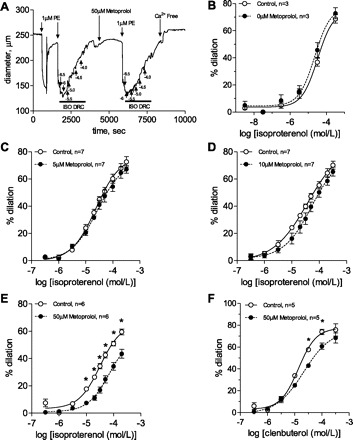
Effect of metoprolol (MET) on isoproterenol dose-response curves in mesenteric resistance arteries. Isoproterenol dose-response curves were generated before (control) and after incubation with metoprolol in mesenteric resistance arteries (A). Isoproterenol dose-response curve was unaffected by both time (0 μM metoprolol; B) and 5 or 10 μM of metoprolol (C and D). There is a significant rightward shift in the isoproterenol (β1/2-nonspecific agonist) and clenbuterol (β2-specific agonist) dose-response curves with 50-μM metoprolol treatment (E and F). *P < 0.05; n = 3 to 7.
Metoprolol impairs PCA dilation in vitro.
Preliminary experiments demonstrated that 3 μM of phenylephrine was optimal for preconstriction in PCAs. At this dose, there were no significant changes in tone or dilation over time before or after administration of 50 μM metoprolol. Isoproterenol dose-response curves were generated before and after metoprolol incubation (0 μM metoprolol), which represented a time control (Fig. 7, A and B). In the control experiment, 0 μM metoprolol had no significant treatment or interaction effect on the isoproterenol curve. However, there was a significant treatment and interaction effect before and after incubation with 50 μM metoprolol (Fig. 7B; n = 7; P < 0.05). There was no effect of metoprolol on the logEC50, but the Emax of isoproterenol was significantly reduced at 50 μM metoprolol (61 ± 5.5 vs. 79 ± 4.0%; n = 7; P < 0.05).
Fig. 7.
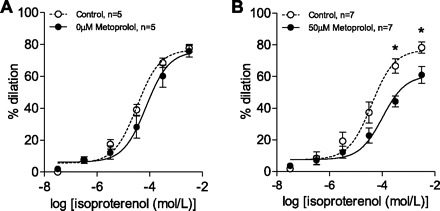
Effect of metoprolol on isoproterenol dose response in posterior cerebral arteries. Isoproterenol dose-response curve was unaffected by time in control vessels (A). As with MRAs, there is a significant rightward shift in the isoproterenol dose-response curve with 50 μM metoprolol treatment (B). *P < 0.05; n = 7.
DISCUSSION
These experimental results support the hypothesis that metoprolol may impair vital organ perfusion by attenuating β2-adrenergic-mediated vasodilation at the level of the small resistance artery. When metoprolol was administered to anesthetized mice in vivo, MAP was maintained while HR, SV, CO, rCBF, and PBrmvO2 were all reduced. This occurred in the context of increased SVR. Thus metoprolol exerted the expected negative chronotropic effect by inhibiting β1-adrenoreceptors at the level of the cardiac myocyte (9). The concurrent reduction in stroke volume suggested that, in addition to its negative chronotropic effect, metoprolol may have also exerted a negative lusitropic effect. The combined impact on HR and stroke volume led to a 40% reduction in CO with acute metoprolol treatment. However, mean femoral arterial pressure and end-systolic and end-diastolic left ventricular pressures were maintained by an increase in SVR. This increase in SVR may have been due to a reflex increase in efferent sympathetic output to maintain microvascular tone and/or due to a direct effect of metoprolol at the level of the β2-adrenoreceptor on the vascular smooth muscle of resistance arteries. If the increase in SVR was mediated by an increase in sympathetic tone, then cerebral perfusion would have been expected to be maintained by endogenous mechanisms including cerebral blood flow autoregulation. The observed reduction in microvascular rCBF and brain tissue Po2 provide evidence for a direct effect of metoprolol on brain perfusion. This effect was not likely influenced by changes in the cerebral metabolic rate for oxygen, as this parameter was not acutely affected by metoprolol in anesthetized humans (12). Subsequent experiments were performed in isolated arteries to demonstrate a direct effect of metoprolol on the vasculature.
Evidence in favor of a direct effect of metoprolol on β2-adrenoreceptor-mediated vasodilation was obtained in ex vivo MRAs and PCAs. We first performed studies in the most widely used model of resistance artery function: the MRA. Results demonstrated that metoprolol inhibited both isoproterenol (β1/2-nonspecific agonist)- and clenbuterol (β2-specific agonist)-mediated vasodilation. A similar effect for isoproterenol was observed in isolated PCAs demonstrating that the impact of metoprolol was conserved across different vascular beds. The similar effect of metoprolol on two different vascular beds suggests that β-adrenergic-mediated vasodilation may be generally impaired by metoprolol.
By contrast, incubation with metoprolol did not have a direct effect on resting tone in either mesenteric or brain vessels in vitro. This may reflect the lack of neural inputs and/or the lack of endogenous adrenergic agonist activity relative to vessels in vivo. In addition, metoprolol did not affect the myogenic response in mesenteric arteries in vitro. This suggests that the impact of metoprolol was limited to adrenergic-mediated vasodilation, as supported by the ability of metoprolol to inhibit isoproterenol- and clenbuterol-mediated vasodilation. This may occur at the level of the β1- and β2-adrenoreceptor since both receptor types have been identified on vascular smooth muscle on mesenteric arteries in the rat (8). Metoprolol has a relatively high affinity for the β2-adrenoreceptor, which may explain its effect on inhibition of clenbuterol-mediated vasodilation.
β-Blockers are one of the most frequently prescribed medications in North America, with prescriptions for metoprolol exceeding 70 million in 2010 (www.imshealth.com). We chose to study metoprolol because it remains one of the predominant β-blockers in current clinical use in North America with published concerns regarding increased morbidity and mortality (5, 10, 28, 34). An increasing number of clinical reports have suggested that metoprolol is associated with increased ischemic organ injury (brain and heart; Refs. 6, 10) and mortality (6, 10, 28, 34), relative to placebo or other more β1-selective drugs (atenolol and bisoprolol; Refs. 5, 28, 34). Our experimental data support the clinical hypothesis that metoprolol may impair adrenergic-mediated resistance artery dilation by a direct inhibitory effect at the β2-receptor.
The clinical settings in which metoprolol administration has been implicated as a risk for increased morbidity and mortality include patients undergoing surgery. Maintenance of vital organ perfusion is required at all times but is of particular importance during times of increased oxygen demand (surgery and exercise; Ref. 13) and/or reduced oxygen supply (blood loss and anemia; Ref. 23). The additional stress of acute blood loss and fluid resuscitation is known to elicit a strong adrenergic response (27). Under these conditions of increased adrenergic stress and reduced blood oxygen content (acute hemodilutional anemia), β-adrenergic mechanisms have been demonstrated to maintain microvascular perfusion in experimental models (15, 27). Adrenergic mechanisms are also required to increase cardiac output and maintain optimal oxygen delivery to vital organs under these conditions (13, 27, 30, 31), in part by diverting an increased proportion of cardiac output toward vital organs with high oxygen demand such as the heart and brain (13, 15, 32). Thus the heart and brain receive increased blood flow by mechanisms that tightly couple oxygen supply and demand. Inhibition of these mechanisms by β-blockers may contribute to the pathophysiology of organ injury and increased mortality in perioperative patients treated with this class of drug (5, 33). These findings are supported by clinical studies that demonstrate an increased incidence of stroke (10), myocardial infarction (6, 33), and mortality (5) in acutely anemic patients treated with metoprolol. The current study supports the hypothesis that metoprolol may impair organ perfusion by limiting active cerebral vasodilation.
In a recent meta-analysis (3), hypotheses were explored to determine why metoprolol was associated with worse clinical outcomes. The authors propose that metoprolol's relatively poor β1-selectivity, compared with the other clinically available β-blockers (bisoprolol and atenolol), may result in direct vascular cross-reactivity and impairment of β-adrenergic-mediated vasodilation. Our data provide direct evidence for this hypothesis. Indeed, recent clinical trials with bisoprolol did not report an increased incidence of stroke (25, 26), suggesting that more cardioselective β-blockers may be preferential when used to reduced the risk of myocardial ischemia in the perioperative setting (3, 33).
There are several limitations to our study: 1) we assessed the impact of metoprolol in a mouse model that has a much higher intrinsic HR than humans. Attempts were made to use a clinically relevant dose of metoprolol that reduced HR by ∼10–20%. 2) In addition, the dose of metoprolol was assessed as pharmacologically comparable to appropriate clinical doses in humans (16, 17). The isolated arteries studied are denervated and therefore lack the input from autonomic and other perivascular nerves. Although this shortcoming cannot be overcome by this model, our in vivo data are in agreement with the isolated vascular data and provide a closer tie to data obtained from clinical studies. 3) We did not study the effect of metoprolol in a stroke model, although such experimental models may provide further important insight into the impact of metoprolol on ischemic stroke. Our data remain relevant since many patients who are reported to have a stroke after metoprolol treatment did no have a past history of cerebral-vascular disease. 4) The dose of metoprolol required to produce similar pharmacodynamic responses in rats is higher that required in humans (17, 29, 35, 36). In human studies, oral doses of metoprolol (25–100 mg po) result in peak plasma levels near 136 ng/ml. After intravenous administration of metoprolol in rats, a corresponding dose of 3 mg/kg results in similar HR responses but at a higher plasma concentration (∼1,200 ng/ml). However, pharmacokinetic and pharmacodynamic studies (16, 17) have demonstrated that an intravenous dose of 3 mg/kg is the lowest dose that provided a clinically relevant reduction in HR ∼20%. The concentration of metoprolol, which impaired adrenergic-mediated vasodilation in vitro (50 uM), corresponded to an ∼10-fold higher drug concentration. This higher dose may be required in vitro as the isolated vessels lack extrinsic innervation and the drug was applied by superfusion to the adventitial side of the vessel. 5) We have not measured the effect of metoprolol on other vasodilatory mechanism (i.e., nitric oxide).
In conclusion, we have provided new evidence that metoprolol can limit cardiac output and increase systemic vascular resistance in a mouse model in vivo. This was associated with a reduction in rCBF and microvascular brain tissue Po2 in anesthetized mice suggesting that brain perfusion can be impaired by metoprolol. Isolated mesenteric and cerebral arteries were utilized to demonstrate a direct effect of metoprolol to inhibit β-adrenergic-mediated vasodilation. These data support a vascular mechanism for the observed reduction in brain perfusion in vivo and may help to understand the observed increase in stroke and mortality in patients treated with metoprolol. These data could be utilized to develop treatment strategies with newer β-adrenergic antagonists that may have less impact on the systemic and cerebral vasculature.
GRANTS
Funding was provided by the Department of Anesthesia, St. Michael's Hospital, University of Toronto, Canadian Anesthesiologists' Society, and Physicians' Services, Toronto, Ontario, Canada. K. Connelly was supported by an HSF Canada Phase 1 Clinician Scientist. D. F. Wilson was supported in part by US Publilc Health Services Grants NS-31465 and HL-081273. W. S. Beattie received funding from the R. Fraser Elliot Endowment. S.S. Bolz was supported by a Heart and Stroke Foundation of Ontario (HSFO) Grant-in-Aid (T6652) and a HSFO New Investigator Award.
DISCLOSURES
G.M.T. Hare has received funding from Forest Laboratories inc.
ACKNOWLEDGMENTS
This research is attributed to the Department of Anesthesia, Keenan Research Centre of the Li Ka Shing Knowledge Institute, Centre for Translational Microvascular Research, St. Michael's Hospital, and the Department of Physiology, University of Toronto. This study was presented in part at the meeting of the Canadian Anesthesiologists' Society, June 2010, Montreal PQ; the International Society for Tissue Transport to Tissue, Cleveland, July 2009; and the Canadian Critical Cardiovascular Congress, Edmonton, October 2009.
REFERENCES
- 1. Anonymous Metoprolol in acute myocardial infarction (MIAMI). A randomised placebo-controlled international trial. The MIAMI Trial Research Group. Eur Heart J 6: 199–226, 1985 [PubMed] [Google Scholar]
- 2. Anonymous Randomized.trial of intravenous atenolol among 16 027 cases of suspected acute myocardial infarction: ISIS-1. First International Study of Infarct Survival Collaborative Group. Lancet 2: 57–66, 1986 [PubMed] [Google Scholar]
- 3. Badgett RG, Lawrence VA, Cohn SL. Variations in pharmacology of beta-blockers may contribute to heterogeneous results in trials of perioperative beta-blockade. Anesthesiology 113: 585–592, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Bangalore S, Wetterslev J, Pranesh S, Sawhney S, Gluud C, Messerli FH. Perioperative beta blockers in patients having non-cardiac surgery: a meta-analysis. Lancet 372: 1962–1976, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Beattie WS, Karkouti K, Wijeysundera DN, Tait G. Risk associated with preoperative anemia in noncardiac surgery: a single-center cohort study. Anesthesiology 110: 574–581, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Beattie WS, Wijeysundera DN, Karkouti K, McCluskey S, Tait G, Mitsakakis N, Hare GM. Acute surgical anemia influences the cardioprotective effects of beta-blockade: a single-center, propensity-matched cohort study. Anesthesiology 112: 25–33, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Bolz SS, Vogel L, Sollinger D, Derwand R, Boer C, Pitson SM, Spiegel S, Pohl U. Sphingosine kinase modulates microvascular tone and myogenic responses through activation of RhoA/Rho kinase. Circulation 108: 342–347, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Briones AM, Daly CJ, Jimenez-Altayo F, Martinez-Revelles S, Gonzalez JM, McGrath JC, Vila E. Direct demonstration of beta1- and evidence against beta2- and beta3-adrenoceptors, in smooth muscle cells of rat small mesenteric arteries. Br J Pharmacol 146: 679–691, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brixius K, Bundkirchen A, Bolck B, Mehlhorn U, Schwinger RH. Nebivolol, bucindolol, metoprolol and carvedilol are devoid of intrinsic sympathomimetic activity in human myocardium. Br J Pharmacol 133: 1330–1338, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Devereaux PJ, Yang H, Yusuf S, Guyatt G, Leslie K, Villar JC, Xavier D, Chrolavicius S, Greenspan L, Pogue J, Pais P, Liu L, Xu S, Malaga G, Avezum A, Chan M, Montori VM, Jacka M, Choi P. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 371: 1839–1847, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Ellenberger C, Tait G, Beattie WS. Chronic beta blockade is associated with a better outcome after elective noncardiac surgery than acute beta blockade: a single-center propensity-matched cohort study. Anesthesiology 114: 817–823, 2011 [DOI] [PubMed] [Google Scholar]
- 12. Felding M, Jakobsen CJ, Cold GE, Davidsen B, Jensen K. The effect of metoprolol upon blood pressure, cerebral blood flow and oxygen consumption in patients subjected to craniotomy for cerebral tumours. Acta Anaesthesiol Scand 38: 271–275, 1994 [DOI] [PubMed] [Google Scholar]
- 13. Gao F, de Beer VJ, Hoekstra M, Xiao C, Duncker DJ, Merkus D. Both β1- and β2-adrenoceptors contribute to feedforward coronary resistance vessel dilation during exercise. Am J Physiol Heart Circ Physiol 298: H921–H929, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Grayburn PA, Hillis LD. Cardiac events in patients undergoing noncardiac surgery: shifting the paradigm from noninvasive risk stratification to therapy. Ann Intern Med 138: 506–511, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Hare GMT, Worrall JMA, Baker AJ, Liu E, Sikich N, Mazer CD. Beta 2 adrenergic antagonist inhibits cerebral cortical oxygen delivery after severe hemodilution in rats. Br J Anaesth 97: 617–623, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Hocht C, Di VC, Opezzo JA, Bramuglia GF, Taira CA. Pharmacokinetic-pharmacodynamic (PK-PD) modeling of cardiovascular effects of metoprolol in spontaneously hypertensive rats: a microdialysis study. Naunyn Schmiedebergs Arch Pharmacol 373: 310–318, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Hocht C, DiVerniero C, Opezzo JA, Taira CA. Applicability of microdialysis as a technique for pharmacokinetic-pharmacodynamic (PK-PD) modeling of antihypertensive beta-blockers. J Pharmacol Toxicol Methods 52: 244–250, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Hoefer J, Azam MA, Kroetsch JT, Poi HL, Momen MA, Voigtlaender-Bolz J, Scherer EQ, Meissner A, Bolz SS, Husain M. Sphingosine-1-phosphate-dependent activation of p38 MAPK maintains elevated peripheral resistance in heart failure through increased myogenic vasoconstriction. Circ Res 107:923–33, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Jackevicius CA, Tu K, Filate WA, Brien SE, Tu JV. Trends in cardiovascular drug utilization and drug expenditures in Canada between 1996 and 2001. Can J Cardiol 19: 1359–1366, 2003 [PubMed] [Google Scholar]
- 20. Leape LL, Berwick DM, Bates DW. What practices will most improve safety? Evidence-based medicine meets patient safety. JAMA 288: 501–507, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Lidington D, Peter BF, Meissner A, Kroetsch JT, Pitson SM, Pohl U, Bolz SS. The phosphorylation motif at serine 225 governs the localization and function of sphingosine kinase 1 in resistance arteries. Arterioscler Thromb Vasc Biol 29: 1916–1922, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Mangano DT, Goldman L. Preoperative assessment of patients with known or suspected coronary disease. N Engl J Med 333: 1750–1756, 1995 [DOI] [PubMed] [Google Scholar]
- 23. McLaren AT, Marsden PA, Mazer CD, Baker AJ, Stewart DJ, Tsui AK, Li X, Yucel Y, Robb M, Boyd SR, Liu E, Yu J, Hare GM. Increased expression of HIF-1α, nNOS, and VEGF in the cerebral cortex of anemic rats. Am J Physiol Regul Integr Comp Physiol 292: R403–R414, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Messerli FH, Bangalore S, Yao SS, Steinberg JS. Cardioprotection with beta-blockers: myths, facts and Pascal's wager. J Intern Med 266: 232–241, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Poldermans D, Boersma E, Bax JJ, Thomson IR, van d V, Blankensteijn JD, Baars HF, Yo TI, Trocino G, Vigna C, Roelandt JR, van UH. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med 341: 1789–1794, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Poldermans D, Schouten O, van LF, Hoeks SE, van d V, Stolker RJ, Fleisher LA. Perioperative strokes and beta-blockade. Anesthesiology 111: 940–945, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Ragoonanan TE, Beattie WS, Mazer CD, Tsui AK, Leong-Poi H, Wilson DF, Tait G, Yu J, Liu E, Noronha M, Dattani ND, Mitsakakis N, Hare GM. Metoprolol reduces cerebral tissue oxygen tension after acute hemodilution in rats. Anesthesiology 111: 988–1000, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Redelmeier D, Scales D, Kopp A. Beta blockers for elective surgery in elderly patients: population based, retrospective cohort study. BMJ 331: 932, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sharp CF, Gardiner SJ, Jensen BP, Roberts RL, Troughton RW, Lainchbury JG, Begg EJ. CYP2D6 genotype and its relationship with metoprolol dose, concentrations and effect in patients with systolic heart failure. Pharmacogenomics J 9: 175–184, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Smith JC, Paulson ES, Cook DB, Verber MD, Tian Q. Detecting changes in human cerebral blood flow after acute exercise using arterial spin labeling: implications for fMRI. J Neurosci Methods 191: 258–262, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Tune JD, Gorman MW, Feigl EO. Matching coronary blood flow to myocardial oxygen consumption. J Appl Physiol 97: 404–415, 2004 [DOI] [PubMed] [Google Scholar]
- 32. van Bommel J, Trouwborst A, Schwarte L, Siegemund M, Ince C, Henny C. Intestinal and cerebral oxygenation during severe isovolemic hemodilution and subsequent hyperoxic ventilation in a pig model. Anesthesiology 97: 660–670, 2002 [DOI] [PubMed] [Google Scholar]
- 33. van Klei WA, Bryson GL, Yang H, Forster AJ. Effect of beta-blocker prescription on the incidence of postoperative myocardial infarction after hip and knee arthroplasty. Anesthesiology 111: 717–724, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Wallace AW, Au S, Cason BA. Perioperative beta-blockade: atenolol is associated with reduced mortality when compared to metoprolol. Anesthesiology 114: 824–836, 2011 [DOI] [PubMed] [Google Scholar]
- 35. Yilmaz B, Asci A, Arslan S. Determination of metoprolol in human plasma and urine by high-performance liquid chromatography with fluorescence detection. J Sep Sci 33: 1904–1908, 2010 [DOI] [PubMed] [Google Scholar]
- 36. Yoon IS, Choi MK, Kim JS, Shim CK, Chung SJ, Kim DD. Pharmacokinetics and first-pass elimination of metoprolol in rats: contribution of intestinal first-pass extraction to low bioavailability of metoprolol. Xenobiotica 41: 243–251, 2011 [DOI] [PubMed] [Google Scholar]
- 37. Yuen DA, Connelly KA, Advani A, Liao C, Kuliszewski MA, Trogadis J, Thai K, Advani SL, Zhang Y, Kelly DJ, Leong-Poi H, Keating A, Marsden PA, Stewart DJ, Gilbert RE. Culture-modified bone marrow cells attenuate cardiac and renal injury in a chronic kidney disease rat model via a novel antifibrotic mechanism. PLos One 5: e9543, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]


