Abstract
Ventricular arrhythmias in the setting of a healed myocardial infarction have been studied to a much lesser degree than acute and subacute infarction, due to the pericardial scarring, which results from the traditional open-chest techniques used for myocardial infarction (MI) induction. We sought to develop a segmental MI with low perioperative mortality in the rabbit that allows optimal visualization and therefore improved study of the infarction borderzone. Rabbits underwent MI using endovascular coil occlusion of the first obtuse marginal artery. Three weeks postprocedure, we evaluated our model by echocardiography and electrophysiology studies, optical mapping of isolated hearts, and histological studies. Seventeen rabbits underwent the protocol (12 MI and 5 sham) with a 92% survival to completion of the study (11 MI and 5 sham). MI rabbits demonstrated wall motion abnormalities on echocardiography while shams did not. At electrophysiological study, two MI rabbits had inducible ventricular tachycardia and one had inducible ventricular fibrillation. Isolated hearts demonstrated no pericardial scarring with a smooth, easily identifiable infarct borderzone. Optical mapping of the borderzone region showed successful mapping of peri-infarct reentry formation, with ventricular fibrillation inducible in 11 of 11 MI hearts and 1 of 5 sham hearts. We demonstrate successful high resolution mapping in the borderzone, showing delayed conduction in this region corresponding to late deflections in the QRS on ECG. We report the successful development of a minimally invasive MI via targeted coil delivery to the obtuse marginal artery with an exceptionally high rate of procedural survival and an arrhythmogenic phenotype. This model mimics human post-MI on echocardiography, gross pathology, histology, and electrophysiology.
Keywords: arrhythmia, optical mapping, ventricular tachycardia
sudden cardiac death (SCD) remains a major public health issue constituting an estimated 20% of deaths in industrialized countries (31, 40, 49). In autopsy series, nearly 50% of SCD victims have had healed myocardial infarction (MI; Refs. 24, 69). The majority of sudden deaths after MI occur due to ventricular tachyarrhythmias (26). Post-MI ventricular arrhythmias have been studied extensively in animal models. The animal studies suggest the epicardial borderzone as a key player in the formation of reentrant ventricular tachyarrhythmias due to changes in tissue structure, ion channels, and gap junctions that slow conduction and generate anisotropy (6, 21, 22, 35, 47, 65, 67). While these studies have greatly enhanced our understanding of post-MI arrhythmias, our understanding of the arrhythmia mechanisms in healed infarct remains limited because most of studies used 5 days postinfarction, not a fully healed post-MI heart. In addition, the use of pericardial incision and coronary ligation in these models may result in a healing process that deviates from post-MI healing in humans (17). With larger animal models, this concern has been addressed by development of closed-chest models (7, 37).
Investigations of postinfarction remodeling specifically using the rabbit may further our understanding of action potential (AP) dynamics related to arrhythmia formation. The rabbit heart shares with the human heart ion currents responsible for depolarization and repolarization, demonstrates an AP shape similar to that of the human, and has been successfully used in transgenic models (3, 42). Furthermore, the rabbit heart appears to most resemble the human heart with respect to wave dynamics during complex arrhythmia such as ventricular fibrillation (46, 64). Complex wave dynamics of ventricular fibrillation are most suitably investigated with high resolution optical mapping techniques using charged-coupled device cameras or photodiode arrays (9–11, 34, 44, 56).
Previous studies of arrhythmia formation in rabbit models of healed MI have used an open-chest method of infarction using a thorocotomy for epicardial access followed by ligation of one of the three coronary arteries. This technique, however, has several drawbacks; 1) the use of coronary ligation in an open-chest model results in pericardial fibrosis that may limit the use of optical mapping of electrical activation and repolarization due to signal attenuation (12, 18, 19); 2) perioperative mortality with this model has traditionally been high, ranging from 30% to 35% during the perioperative period (41, 50, 51); and 3) epicardial visualization of the left circumflex is difficult, with 10–20% failures to induce infarction (39). These limitations reduce the efficiency of generating the MI model, increasing the costs of the MI model and potentially introducing a selection bias. A minimally invasive, closed-chest model in the rabbit, using endovascular techniques that target a segmental artery, would therefore theoretically reduce perioperative mortality and avoid limitations listed above.
Given the purported importance of the borderzone and the potential usefulness of the rabbit postinfarction model, our laboratory set out to develop a novel, minimally invasive, closed-chest model of chronic healed myocardial infarction in the rabbit with improved periprocedural survival. We developed a coil embolization technique with a two-catheter system that allows for targeted coil embolization into the obtuse marginal coronary circulation. Our two objectives were to obtain a consistent segmental myocardial infarction with low perioperative mortality and to generate a model that allows optimal visualization and therefore improved study of the infarction borderzone and periborderzone regions.
METHODS
This investigation conformed to the current Guide for Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85–23, revised 1996), as well as the standards recently delineated by the American Physiological Society (16), and was approved by the Animal Welfare Committee at Rhode Island Hospital. Adult male rabbits (3.5–5.5 kg) ages 6–12 mo were used. Rabbits were anesthetized with intramuscular ketamine/xylazine (25 mg/kg/3.75 mg/kg) and buprenorphine (0.03 mg/kg), intubated, and ventilated with isoflurane (1–2%, FiO2 0.5). Blood pressure and peripheral oxygen saturation were measured continuously. A neck cut down was performed, and the right carotid artery was canalized. A 4-Fr guide catheter (Cook, Slip-Cath Beacon Tip with custom alteration for use in a rabbit) was advanced retrogradely to the ascending aorta using fluoroscopy and angiography of the left main coronary artery was performed. A continuous 12-lead ECG was recorded during the induction of myocardial infarction. Lidocaine (1 mg/kg load followed by 20 mcg·kg−1·min infusion−1) was infused during the procedure to reduce periinfarct ventricular arrhythmias. Heparin was administered to achieve a target activation clotting time of 300 s. With the use of fluoroscopic guidance with the guide at the left main coronary ostium, a 0.014-in. floppy wire was advanced into the coronary. Based on the angiogram performed, the wire was advanced into the left circumflex artery and then distally into the obtuse marginal branch coursing toward the apex. Over the wire, a 2.5-Fr delivery catheter (Boston Scientific, FastTracker18) was tracked into the obtuse marginal until the tip reached two-thirds the distance from the left main coronary ostium to the left ventricular (LV) apex. After removal of the 0.014-in. floppy wire, a platinum 0.018-in. Hilal microcoil (Cook) cut to a length of 1.5–2 mm was pushed out the tip of the delivery catheter using an 0.018-in. floppy wire. The microcoil has a synthetic thrombogenic fiber (30), which induces clot in the targeted artery during the first 2–5 min of coil delivery. MI was confirmed postcoil embolization using ST elevations on two consecutive ECG leads. The rabbits were continued on a Lidocaine infusion and monitored for 1-h postcoil embolization. Sustained ventricular arrhythmias during this period were treated with external defibrillation (Medtronic Biphasic Defibrillator) using 6 J.
Sham rabbits underwent the exact same protocol, but the procedure was terminated after coronary angiography. Two rabbits undergoing MI were killed 2 h after coil embolization for measurement of infarct size. These hearts were cut in the coronal plane into four disks from apex to base and placed in 1% triphenyltetrazolium chloride for 15 min. Surfaces were scanned, and percentage of infarct was measured by calculating percentage of area not stained brick red.
Minimally Invasive In Vivo Electrophysiological Study
The protocol was modified from a previously published protocol from our laboratory (45). Rabbits were anesthetized and monitored as described for MI induction. A steerable 4-Fr decapolar EP catheter (Irvine Biomedical, Irvine, CA) was inserted in the right femoral vein through a 4-Fr sheath and placed in the right ventricle (RV), guided by fluoroscopy and pacing thresholds (Fig. 1A). Signals from the His-bundle were obtained with the basal electrodes on the ventricular EP catheter (RV base; Fig. 1B). During the procedure, 12-lead surface, 5 intracardiac ECG signals were recorded continuously using the EP-Bard-System Software OS2/warp (kindly provided by Bard, Lowell, MA), filtered with a bandwidth of 30–250 Hz (intracardiac signals) and 0.01–100 Hz (surface ECG).
Fig. 1.
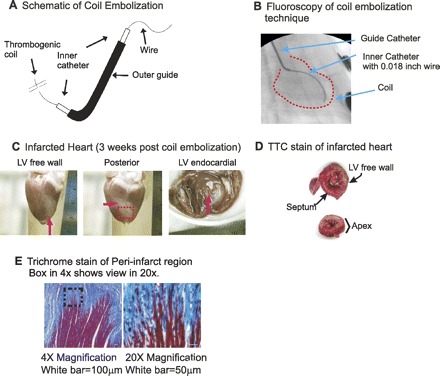
Technique of targeted coil embolization. A: schematic of coil, not to scale- see dimensions in methods. Coil length is 1. B: fluoroscopic image of coil embolization. Right anterior oblique caudal projection view, showing the inner catheter is successfully inserted in mid-region of the first obtuse marginal branch of the left circumflex artery. C: myocardial infarction (MI) image from Langendorff perfused heart. Note that MI region can be identified as white fibrosis region, and the surface of heart including MI regions is clean without blood clots or scars from surgery. D: triphenyltetrazolium chloride (TTC) staining to verify survival tissue and fibrotic tissue. Infarct size was measured from TTC staining. E: histological staining with Masson's trichrome in postoptical mapping hearts of the periphery zone (PZ) demonstrated areas of scar with strands of surviving myocardial tissue invading into this region of scar. LV, left ventricular.
Electrophysiological study (EPS) was performed at a stimulation cycle length (CL) of 240 and 200 ms. The following parameters were analyzed as previously described (36). Atrium to His (AH) and His to ventricle (HV) intervals were measured. Programmed ventricular stimulation was performed with one, two, and three extra stimuli in apical and basal positions to examine inducibility of monomorphic ventricular tachycardia or ventricular fibrillation (45). We strictly defined all ventricular tachycardia or ventricular fibrillation as sustained ventricular arrhythmias requiring cardioversion or defibrillation respectively.
Echocardiography
Transthoracic echocardiography was performed in sedated animals immediately before EPS. Two-dimensional ECG images (Hewlett Packard 5500) were obtained with a 7.5-mHz probe, and long axis and short axis views were used mimicking those used in human ECGs. Analysis included dimensions of the LV, left atrium, wall thickness, valve function, and LV ejection fraction (calculated using Simpson's Rule) and was performed by an experienced echocardiographer blinded to the protocol used and animal group.
Isolated Heart Preparations
Rabbits were injected with buprenorphene (0.03 mg/kg im), acepromazine (0.5 mg/kg im), xylazene (15 mg/kg im), ketamine (60 mg/kg im), pentothal (35 mg/kg iv), and heparin (200 U/kg). After the appropriate level of anesthesia was obtained as determined by corneal reflex and response to painful stimuli, rabbits were euthanized via beating heart harvest. The hearts were retrogradely perfused through the aorta with the following (in mmol/l): 130 NaCl, 24 NaHCO3, 1.0 MgCl2, 4.0 KCl, 1.2 NaH2PO4, 5 dextrose, 25 mannitol, and 1.25 CaCl2, at pH 7.4, gassed with 95% O2-5% CO2. Temperature was maintained at 37.0 ± 0.2°C, and perfusion pressure was adjusted to ∼60 mmHg with a peristaltic pump (Radnoti Glass Technology, Monrovia, CA). Hearts were placed in a chamber to maintain temperature and to reduce movement artifact, and 5 μmol/l blebbistatin were added to the perfusate (25). At the end of optical mapping, the LV free wall was dissected and fixed with a 10% solution of formalin in PBS for 24 h, embedded in paraffin, and then cut serially from endocardium to epicardium. Sections were stained with Masson's trichrome stain for histopathological analysis.
Optical Mapping
The optical apparatus has been previously described (10). Fluorescence images from the anterior surface and LV free wall of heart were focused on a CMOS camera (100 × 100 pixels, Ultima-L; Scimedia) with a 50-mm Nikon f/1.2 lens. Excitation light was illuminated through a dichroic box located between the camera lens and the camera. Sampling rate was set to 1,000 frames/s, and data were analyzed with a custom built software using Interactive Data Language (ITT Visual Information Solutions, Boulder, CO). Hearts were monitored for adequate perfusion throughout the study by visual inspection for pink hue, homogeneous fluorescence, and AP shape (with prominent plateau phase).
We used two fields of view. For studies of arrhythmia initiation, a 1.5 × 1.5 cm2 (with a spatial resolution of 150 × 150 μm2) field of view was used. For high resolution mapping of the borderzone region, a 1.5 × 1.5 mm2 (with a spatial resolution of 15×15 μm2) field of view was used. Hearts were stained with the voltage-sensitive dye di-4 ANEPPS (Invitrogen, Carlsbad, CA) using 25 μl of stock solution (1 mg/ml DMSO) delivered through a bubble trap above the aortic cannula. ECG and perfusion pressure were continuously monitored (Powerlab, ADinstrument, Colorado Springs, CO). All hearts were initially challenged with our standard protocol (2, 3, 29) of decrement in pacing CL consisting of 10-ms steps with progressively shorter CL until loss of 1:1 capture or ventricular fibrillation induction. A second protocol was also used mimicking the extra stimuli at in vivo EPS (above). Ventricular fibrillation was terminated by injection of KCL solution (0.3 mM, 100 μl to the bubble trap). As with in vivo studies, during whole heart experiments we defined ventricular tachycardia and ventricular fibrillation as sustained ventricular arrhythmia requiring intervention for termination.
Data Analysis
The activation and repolarization time-points at each site were determined from fluorescence (F) signals by calculating (dF/dt)max and (d2F/dt2)max. Data were filtered using a spatial Gaussian filter (3 × 3 pixel) and first/second derivatives (dF/dt, d2F/dt2) were calculated using polynomial filter (3rd order, 13 points). Pixels with low signal-to-noise ratio determined by (dF/dt)max (<3×σ of baseline) and outliers of pixels determined by Grubbs' test were removed from the analysis (typically <1% of total pixels). Action potential duration (APD) dispersion was defined as APD max-min across the field of view. Reproducibility of infarct size was analyzed further by calculating the surface area of the infarct on the epicardium of the LV free wall. Digital photographs of the LV free wall were analyzed using ImageJ (1) to report average infarct surface area.
Statistical Analysis
Normally distributed continuous variables were compared using a Student unpaired t-test. Categorical values were compared using a Fisher's exact test. Statistical significance was set at P < 0.05.
RESULTS
A total of 17 rabbits entered the study with 5 receiving sham interventions and 12 receiving true coil embolization. Angiograms of the left coronary artery were successfully performed in all rabbits studied (Fig. 1). In all angiograms performed, the left circumflex coronary was dominant, supplying the left free wall and apex via obtuse marginal branches. In two animals, postembolization angiogram of the left coronary artery was performed, both demonstrating total occlusion of the first obtuse marginal branch. All sham animals received angiograms successfully. No complications were seen with angiograms alone.
Twelve rabbits underwent coil embolization (Fig. 1, A and B). Eleven survived the procedure (92%; Table 1). The single death occurred during attempted coil deployment. Failure to successfully load the coil into the delivery catheter resulted in several passes of the delivery catheter and wire. During these attempts, ventricular fibrillation storm developed, with electrical-mechanical dissociation seen after defibrillation attempts. The rabbit died likely from left main spasm. With the 11 rabbits that survived the procedure, ventricular fibrillation occurred within 15 min of coil deployment in 3 of 11 (27%). In each of these three cases, successful defibrillation and circulatory stabilization was achieved with a single application of external defibrillation. All 11 rabbits that survived the remainder of the 3 wk protocol and 5 sham rabbits underwent ECG, EPS, and isolated heart preparation optical mapping. These results are presented below. When inspected before optical mapping, hearts demonstrated a healed infarct appearing white with a pink rim borderzone region over the apex and apical lateral walls (Fig. 1C). With perfusion, akinesis was visually seen in this region. Additionally, fluorescence was absent from this region with optical mapping. Two additional rabbits that underwent the protocol were killed acutely for triphenyltetrazolium chloride staining, which revealed a segmental infarction that included the lateral and posterior aspect of the apex through the mid LV free wall (Fig. 1D). The infarct size was 20 ± 2% (n = 2) of the entire LV. All infarcts had a transmural section. Transmurality of the infarct was reduced at the borderzones. A pink rim of surviving myocardium was seen at the epicardial borderzone as with other MI models (14, 15, 20, 22, 23, 48, 66, 68) (Fig. 1D). We also noted a well-defined junction of normal myocardium basal to the scar comprising a basal periphery of the scar (see Fig. 4). Histological staining with Masson's trichrome in postoptical mapping hearts of this periphery zone (PZ) demonstrated areas of scar with strands of surviving myocardial tissue invading into regions of scar (Fig. 1E).
Table 1.
Coil embolization procedure results
| Total | Successful Deployment | Acute VF | Acute Death | 3-wk Survival | |
|---|---|---|---|---|---|
| MI | 12 | 12 | 4 | 1 | 11 |
| Sham | 5 | 0 | 0 | 0 | 5 |
VF, ventricular fibrillation; MI, myocardial infarction.
Fig. 4.
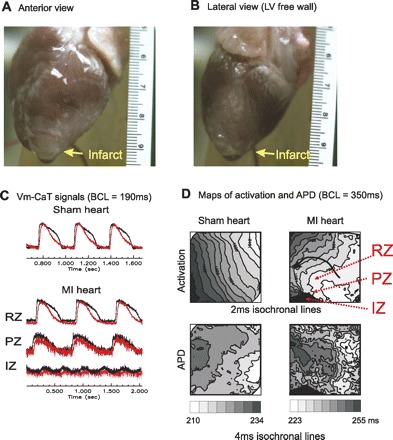
Simultaneous optical mapping of voltage and calcium transients. A and B: isolated heart MI seen in anterior and lateral view (LV free wall). C: fluorescence signals from the LV free wall of sham hearts and post-MI hearts. Vm-CaT, voltage and calcium; BCL, pacing basic cycle length of 190 ms. D: corresponding activation and action potential duration (APD) maps from C. Note that APD dispersion in MI heart increased dramatically and the amplitude of fluorescence signals are diminished in the MI region. RZ, remote zone; IZ, infarct zone.
Echocardiograms
All hearts with MI demonstrated wall motion abnormality (Fig. 2), while no sham demonstrated wall motion abnormality (11/11 vs. 0/5; P < 0.05). Significantly lower LV ejection fraction was seen in post-MI rabbits compared with shams (37 ± 9 vs. 59 ± 7%; P < 0.05). All other measurements were not significantly different between the sham and MI groups (Table 2).
Fig. 2.
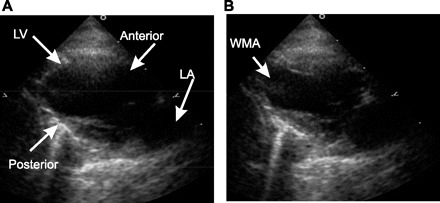
ECG 2-chamber view demonstrating systole and diastole in a sham animal (A) and MI animal (B). Note the apical wall motion abnormality (WMA in yellow) seen in the MI rabbit. LA, left arterial.
Table 2.
Inducibility of ventricular arrhythmias
| In Vivo EP Study |
Ex Vivo Extra Stimuli Protocol |
Ex Vivo Ramp Protocol* |
||||
|---|---|---|---|---|---|---|
| VT/VF | No VT/VF | VT/VF | No VT/VF | VT/VF | No VT/VF | |
| Sham | 0 | 5 | 0 | 5 | 1 | 4 |
| MI | 3 | 8 | 4 | 7 | 11 | 0 |
EP, electrophysiological; VT, ventricular tachycardia.
P < 0.05 for sham vs. MI.
Electrophysiology Study
EPS was successfully performed with survival of the rabbit postprocedure in all 11 MI rabbits and 5 sham rabbits. Programmed stimulation with up to three extra stimuli induced sustained ventricular arrhythmias in 3 of 11 MI rabbits but none of the shams (P = NS). Sustained ventricular fibrillation with two extra stimuli was induced in one MI rabbit and sustained monomorphic ventricular tachycardia was induced in two rabbits, each requiring triple extra stimuli for induction. Ventricular tachycardia was terminated with over-driving pacing in one rabbit and with delivery of external shock in another. One-to-one ventricular arrhythmias conduction was seen in both cases (Table 2). Ventricular tachycardia was verified in each instance with His activation onset subsequent to the initial deflection of the QRS and altered QRS morphology and axis from baseline (Fig. 3). Ventricular tachycardia morphology was right-bundle with a left-superior axis and a CL of 157 ms in one case and right-bundle with a right-superior axis and a CL of 172 ms in the other.
Fig. 3.
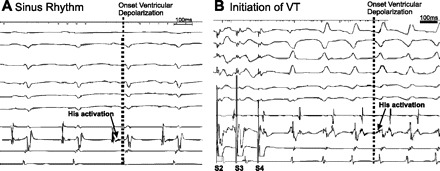
Induction of monomorphic ventricular tachycardia (VT) from programmed stimulation in in vivo electrophysiological study. A: sinus rhythm. His activation (arrow) indicates His activation followed by ventricular activation. B: programmed stimulation caused VT. VT was induced by S1-S2-S3-S4 protocol. Note that the ventricular depolarization on ECGs (dotted line) precedes His activation (arrow), demonstrating a ventricular origin to the wide complex monomorphic tachycardia.
Mapping Activation and Repolarization in MI Region
Hearts were visually inspected after isolation from the chest. In both sham and MI hearts, the pericardium appeared pristine upon isolation and was easily removed from the epicardium (Fig. 4, A and B). No pericardial adhesions or fibrosis was seen in either group. In all 11 postinfarction rabbits, MI at the apical-lateral region of the LV free wall was easily identifiable. The infarct size was consistent with a mean infarct surface area of 4.3 ± 0.94 cm2 (n = 8) and a minimum and maximum infarct area of 3.4 and 5.9 cm2, respectively. Infarction was absent in all sham hearts. The periphery of the infarct was easily identifiable in all infarct hearts and demonstrated an irregular pink margin along the LV free wall between the infarct region and the remote, preserved region. We defined this region as the PZ in our optical mapping studies. This PZ is distinct from the epicardial border zone seen in this model and traditional models. We focused on the periphery of the infarct and our defined PZ since in our optical mapping studies described below, this region demonstrated conduction abnormality and reentry formation that was of interest.
Optical mapping of voltage and calcium transients using a 1.5 × 1.5 cm field of view showed excellent signal quality due to the clean surface of the epicardium (Fig. 4). The remote zone signals have normal appearing upstroke and calcium transient similar to the signals seen in the LV free wall of the sham hearts. However, PZ signals demonstrate lower amplitude and lower signal to noise ratio, with slower rise in activation upstroke. Signals were absent in the infarct zone (Fig. 4C). Examples of epicardial activation maps are shown in Fig. 4D. The infarct zone introduces new curvature to the activation wavefront as demonstrated in the activation map (Fig. 5B, black arrow). The extra-stimuli protocol mimicking in vivo programmed stimulation was used and 4 of 11 hearts were inducible for sustained ventricular arrhythmias. The arrhythmias began with single reentry beats that then typically lead to ventricular fibrillation that was sustained. Three of the four hearts were also inducible during in vivo EPS. With the ramp protocol, all 11 of the post-MI hearts were inducible for ventricular fibrillation. Only one of the five sham hearts was inducible with the ramp protocol and none of the five were inducible by the extra-stimuli protocol (Table 2). Reentry formation appeared to occur at the basal border between the normal LV and the infarct territory. We therefore focused on this region in optical mapping studies rather than the traditional epicardial rim borderzone. Figure 5 demonstrates reentry induction in a postinfarct heart. The map in Fig. 5A demonstrates a peri-infarct region where a third extra stimulus generates reentry formation with activation proceeding from region 1 to region 4, with conduction block occurring between region 1 and 4. Optical signals in Fig. 5, right, show APs in positions 1–4 during the train of S1-S2-S3-S4 stimuli (stimulus artifact at the Fig. 5, top). Traces in Fig. 5, bottom right, are optical signal from positions 1–4. S4 correlates to the activation map in Fig. 5, left. S4 in traces is the fourth upstroke seen, indicated by the gray arrow on traces. Activation progresses from position 1 to position 4 and reenters position 1 again. The following beat is a beat of ventricular tachycardia, followed quickly by degeneration to ventricular fibrillation in the following beats in the traces. In Fig. 5B, the activation and AP duration maps for the stimulation are shown. Activation between regions 1 and 4 are initially smooth, without block. During S3, a large repolarization gradient occurs which allows for functioning line of block to develop between position 1 and 4 and reentry. Notably, maps of the complex activation and repolarization dynamics in the peri-infarct zone are clearly delineated.
Fig. 5.
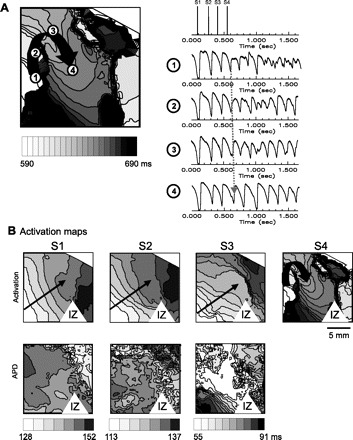
Activation maps of reentry formation in post-MI heart at the MI PZ. A, left: activation map of functional reentry formation in the PZ within the region of optical mapping. A, right, top: we demonstrate the S1-S2-S3-S4 protocol used to induce this arrhythmia. Traces at right are optical signal from positions 1–4. S4 correlates to the activation map at left. S4 in traces is the fourth upstroke seen, indicated by the gray arrow on traces. Activation progresses from position 1 to position 4 and reenters position 1 again. The following beat is a beat of VT, followed quickly by degeneration to ventricular fibrillation in the following beats in the traces. B: activation (top) and APD (bottom) maps of premature stimulation followed leading up to the functional reentry seen in the activation map in A. Changes in activation and APD in this PZ of the MI with introduction of extra stimuli is seen. APD dispersion increased dramatically in S3 premature beat, followed by functional conduction block in S4 that causes reentry formation.
High resolution field of view optical mapping of the borderzone was performed to investigate detail activation patterns along the heterogeneous cell population shown in the histology images (see Fig. 1E) at the MI periphery. Due to amplification of motion artifact, this mapping was performed with higher concentration of blebbistatin (10 μM). An example is shown in Fig. 6 of borderzone activation from epicardial pacing at BCL = 350 ms from 3 × 3 mm2 field of view. ECG recordings (Fig. 6A) near this region of borderzone demonstrated a late fractionation in the QRS. A local activation map of the region is shown in Fig. 6B. Activation in the region appears to have two components, an initial rapid (estimated conduction velocity of 0.75 mm/ms) component (labeled 1 in yellow) from top right to bottom left, aspect of the map (red to orange) and a second late component (labeled 2 and 3 in yellow) at the center of the map (white to green) progressing right to left after a large delay initially (40 ms) requiring 38 ms to completely traverse the 2-mm central area (estimated 0.05 mm/ms). Fluorescence recordings (Fig. 6C) from the regions 1–4 exhibit a multiple component upstroke, as shown in two peaks in the first derivatives (red). Since the fluorescence signals are the sum of multiple cells from the spatial resolution of 30 × 30 μm2 and depth resolution of ∼120 μm, multiple peaks suggest multiple activations in different depth of heterogeneous tissue in the border zone of MI. Figure 6D shows overlapped first derivative of fluorescence signal in the region. Activation wavefronts labeled 1, 2, and 3 are labeled demonstrating the time lag between each activation wavefront.
Fig. 6.
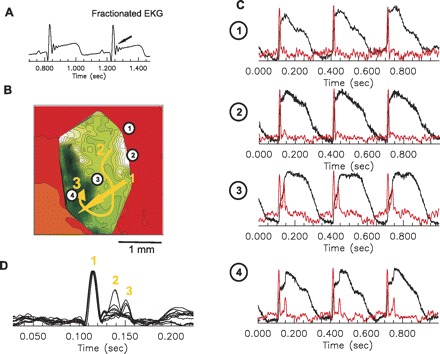
High resolution mapping of late activation from the MI. A: fractionated ECG indicating multiple late activations through MI region. B: activation maps of late activation in MI border zone. Field of view was set to 3 × 3 mm2, 30 × 30 μm2 pixel resolution, at 5,000 f/s. C: fluorescence recordings from single pixel shows multiple component of depolarization indicating multiple activation through the border zone (see text for details). First derivatives of fluorescence recordings are shown in red. D: superimposed first derivatives along the activation pathways exhibit rapid, normal activation initially through the region followed by 2 distinct late activations, which correlates with timing of deflections in the corresponding ECG recordings in the A. E: histology images of MI border zone. Finger like fibrosis into ventricular tissue may be responsible for complex activation along the border zone.
Figure 1E shows histology images from an MI border zone. The heterogeneous MI borderzone seen may be responsible for the complex optical signals and the fractionated wave form in ECG. Understanding the activation and repolarization dynamics of these borderzone late activation sites may greatly enhance our understanding of post-MI arrhythmias.
DISCUSSION
We report the successful development of a minimally invasive MI via targeted coil delivery to the obtuse marginal artery with an exceptionally high rate of procedural survival and an arrhythmogenic phenotype. Furthermore, we have documented that this model successfully mimics echocardiographic, gross pathology, histology, and electrophysiology of human postinfarction heart. Of specific interest, is the fact that using the same stimulation protocol and definitions of arrhythmia initiation used in human trials, our cohort demonstrated similar results as seen human trials of postinfarction risk stratification for SCD (5). The simultaneous use of a 12-lead ECG and a decapolar intracardiac catheter, with the ability to capture the His, allows us to demonstrate without question the induction of monomorphic ventricular tachycardia despite the 1:1 ventricular arrhythmias activation in these post-MI rabbits using the same stimulation protocol used in humans. Finally, our technique leaves the pericardium untouched, which enables access to an easily identifiable infarct borderzone. The clean epicardium in our model would allow for high quality optical images and high resolution borderzone optical mapping. Much of the previous work on post-MI arrhythmias focuses on the epicardial rim of healthy tissue remaining after a total occlusion of a coronary artery (15, 21, 23, 48, 68). This same rim is present in human transmural infarcts (57) as well. Still, from catheter ablation experience in humans, substrate allowing for ventricular tachyarrhythmias is highly complex and likely involve endocardial as well as epicardial rim borderzones (53, 54, 59, 61, 63). The MI border with normal tissue at the periphery of the infarct territory may also play an important role in these arrhythmias. Noncontact mapping in human hearts has suggested that functional reentry at the periphery of the MI may play a role in early reentry formation and the initiation of these arrhythmias (13, 58). The electrophysiology at this junction of normal and infarcted tissue at this periphery is not as well studied. Our model is well suited to study this region where noninfarcted tissue meets a border with scar and surviving muscle fibers and may therefore increase the knowledge base of ventricular arrhythmias post-MI. Further detailed analysis of the endocardial surface needs to be performed in this model along with transmural investigations, as these surfaces may play a significant role in arrhythmogenesis in this model as well.
Comparison to Previous Studies
We found one other report of coil embolization into a large coronary artery in a closed-chest model of MI in a rabbit (17). This prior study attempted to deliver the coil with a catheter sitting at the ostium of the left main artery. In our hands, reproduction of this protocol yielded a large perioperative mortality and difficulty in delivering the coil into the left coronary. Of the four animals attempted, two died during the procedure, one animal failed two attempts at coil embolization (coil embolized into peripheral circulation rather than the coronary), and one rabbit received a successful embolization. This coil lodged in a small obtuse marginal branch that resulted in a small linear infarction at the lateral border of the LV. We surmised that the prior closed-chest model, not currently used by any group to our knowledge, lacks dependable results. In our experience, full catheter engagement into the left main ostium predisposed the animal to left main artery spasm and occlusion during the procedure and an intra-procedure death. On the other hand, when the catheter sits just outside the ostium, the coil can fail to embolize into the coronary. Finally, even if the coil embolizes into the artery, its final position is left up to chance. Our targeted delivery method is a novel method that targets segmental artery. In this procedure, a guide catheter sits outside the left main ostium and a floppy wire is advanced into a branch of the left circumflex artery that is chosen to induce a reproducible size infarction. A delivery inner catheter is then advanced to the location of coil embolization, and there, the coil is delivered. This method is associated with low perioperative mortality and ease of coil delivery, potentially making this method of targeted coil delivery more reproducible by multiple labs.
We also found one previous description of induction of ventricular tachycardia in vivo in the rabbit postepicardial coronary ligation MI (43). However, this study used up to five extra stimuli in the inductions that resulted in ventricular tachycardia with significantly shorter CL. Compared with human ventricular tachycardias (5, 33, 36, 52, 60, 62), this model more closely resembles ventricular flutter. Here we report monomorphic ventricular tachycardia with a CL similar to that of humans post-MI with induction method that exactly duplicate human EPS. Therefore, this model of healed MI in the rabbit is more arrhythmogenic than other healed MI rabbit models. Furthermore, our rate of induction of ventricular arrhythmias is similar to rates seen in humans, ranging from 20–30% with EPS using this same protocol (4). Finally, the pericardium in previous investigations has had demonstrable effects on hemodynamics and therefore its instrumentation may result in ventricular remodeling (27, 28). Such remodeling would add a confounder to investigations of effects of remodeling on arrhythmias in the healed MI. Thus we believe that the study of arrhythmias seen in our model is more likely applicable to the human postinfarct condition compared with the open-chest models.
Previous optical mapping studies of post-MI rabbit hearts have been published (12, 38, 55, 56) all using epicardial coronary ligation open-chest techniques. However, high resolution detailed analyses of activation and repolarization at the site of reentry formation and high resolution optical mapping of late activation correlating with fractionated ECGs have not been reported,. While earlier studies have clearly improved our understanding of post-MI arrhythmias, we believe that these previous reports may have been limited in their insight into borderzone dynamics due to pericardial adhesions and fibrosis in the borderzone after the pericardium was opened during the coronary ligation procedure. Furthermore, while the role of individual ion channels in formation of arrhythmias has been studied (14, 32, 48) effects of manipulation of individual currents has been limited as a result of the lack of a transgenic infarct model (8). We suggest that use of a reproducible closed-chest model MI in the rabbit, with available transgenically modified repolarization currents (3), may significantly enhance the investigator's ability to image and thus study the important peri-infarct zone in a model that allows for manipulation of repolarization currents.
Many models of post-MI ventricular arrhythmias have been generated in the past number of decades (6, 21, 22, 35, 47, 65, 67). These have all improved our understanding of these arrhythmias and aided in the treatment and prevention of sudden cardiac death post-MI. We believe our model has a niche among these previous models in further enhancing this body of knowledge. Our model is ideally for optical mapping and may be best suited for studying ventricular fibrillation in the setting of a healed MI due to the high ex vivo yield of ventricular fibrillation in our studies.
CONCLUSION
We present here a novel technique of targeted coil embolization closed-chest MI in the rabbit, which demonstrates low mortality periprocedure, applicability to human post-MI arrhythmias, and is ideal for high resolution optical mapping techniques due to the pristine nature of the epicardium after MI healing. Use of this technique by this group and other investigators may further our understanding of the crucial activation and repolarization dynamics leading to post-MI arrhythmias and SCD Table 3.
Table 3.
Echocardiographic findings
| LV Septal Wall Thickness in Diastole | LV Posterior Wall Thickness in Diastole | LV End-Diastole Diameter | LV End-Systole Diameter | LV EF, %* | |
|---|---|---|---|---|---|
| Sham | 2.7 ± 0.1 | 2.6 ± 0.2 | 15 ± 2.2 | 11 ± 1.7 | 59 ± 7 |
| MI | 2.7 ± 0.1 | 2.5 ± 0.7 | 16 ± 2.2 | 12 ± 1.7 | 37 ± 9 |
Values are means ± SE. EF, ejection fraction.
P < 0.05 for sham vs. MI.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: O.Z., B.-R.C., and G.K. conception and design of research; O.Z., L.S., E.L., L.C., D.P., P.J., X.P., and B.-R.C. performed experiments; O.Z., L.S., E.L., L.C., D.P., P.J., and B.-R.C. analyzed data; O.Z., E.L., B.-R.C., and G.K. interpreted results of experiments; O.Z., L.C., P.J., and B.-R.C. prepared figures; O.Z. drafted manuscript; O.Z., B.-R.C., and G.K. edited and revised manuscript; O.Z., B.-R.C., and G.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Zachary Pfeiffer for work on data gathering and analysis for this manuscript.
Present address for O. Ziv: Case Western Reserve, MetroHealth Medical Center, Cleveland, Ohio.
REFERENCES
- 1. Abramoff MD, Magalhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int 11: 34–42, 2004 [Google Scholar]
- 2. Banville I, Gray RA. Effect of action potential duration and conduction velocity restitution and their spatial dispersion on alternans and the stability of arrhythmias. J Cardiovasc Electrophysiol 13: 1141–1149, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Brunner M, Peng X, Liu GX, Ren XQ, Ziv O, Choi BR, Mathur R, Hajjiri M, Odening KE, Steinberg E, Folco EJ, Pringa E, Centracchio J, Macharzina RR, Donahay T, Schofield L, Rana N, Kirk M, Mitchell GF, Poppas A, Zehender M, Koren G. Mechanisms of cardiac arrhythmias and sudden death in transgenic rabbits with long QT syndrome. J Clin Invest 118: 2246–2259, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buxton AE. Risk stratification for sudden death in patients with coronary artery disease. Heart Rhythm 6: 836–847, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med 341: 1882–1890, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Cabo C, Yao J, Boyden PA, Chen S, Hussain W, Duffy HS, Ciaccio EJ, Peters NS, Wit AL. Heterogeneous gap junction remodeling in reentrant circuits in the epicardial border zone of the healing canine infarct. Cardiovasc Res 72: 241–249, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Capone RJ, Most AS, Sydik PA. Precordial ST segment mapping. A sensitive technique for the evaluation of myocardial injury? Chest 67: 577–582, 1975 [DOI] [PubMed] [Google Scholar]
- 8. Chang MG, Zhang Y, Chang CY, Xu L, Emokpae R, Tung L, Marban E, Abraham MR. Spiral waves and reentry dynamics in an in vitro model of the healed infarct border zone. Circ Res 105: 1062–1071, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen J, Mandapati R, Berenfeld O, Skanes AC, Jalife J. High-frequency periodic sources underlie ventricular fibrillation in the isolated rabbit heart. Circ Res 86: 86–93, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Choi BR, Jang W, Salama G. Spatially discordant voltage alternans cause wavebreaks in ventricular fibrillation. Heart Rhythm 4: 1057–1068, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choi BR, Liu T, Salama G. Ventricular fibrillation: mother rotor or multiple wavelets? Circ Res 89: E30, 2001 [PubMed] [Google Scholar]
- 12. Chou CC, Zhou S, Hayashi H, Nihei M, Liu YB, Wen MS, Yeh SJ, Fishbein MC, Weiss JN, Lin SF, Wu D, Chen PS. Remodelling of action potential and intracellular calcium cycling dynamics during subacute myocardial infarction promotes ventricular arrhythmias in Langendorff-perfused rabbit hearts. J Physiol 580: 895–906, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chow AW, Segal OR, Davies DW, Peters NS. Mechanism of pacing-induced ventricular fibrillation in the infarcted human heart. Circulation 110: 1725–1730, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Christensen MD, Dun W, Boyden PA, Anderson ME, Mohler PJ, Hund TJ. Oxidized calmodulin kinase II regulates conduction following myocardial infarction: a computational analysis. PLoS Comput Biol 5: e1000583, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Davidoff AW, Boyden PA, Schwartz K, Michel JB, Zhang YM, Obayashi M, Crabbe D, ter Keurs HE. Congestive heart failure after myocardial infarction in the rat: cardiac force and spontaneous sarcomere activity. Ann NY Acad Sci 1015: 84–95, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Drummond GB. Reporting ethical matters in the Journal of Physiology: standards and advice. J Physiol 587: 713–719, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Edwards R, Yousef Z, Rakhit R, Wright M, Rosenthal E, Redwood S, Marber M. A model of closed chest regional myocardial infarction in the rabbit: a clinically relevant in vivo assay system of post-infarction remodelling. Basic Res Cardiol 97: 374–383, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Efimov IR, Huang DT, Rendt JM, Salama G. Optical mapping of repolarization and refractoriness from intact hearts. Circulation 90: 1469–1480, 1994 [DOI] [PubMed] [Google Scholar]
- 19. Efimov IR, Nikolski VP, Salama G. Optical imaging of the heart. Circ Res 95: 21–33, 2004 [DOI] [PubMed] [Google Scholar]
- 20. El-Sherif N, Gough WB, Zeiler RH, Hariman R. Reentrant ventricular arrhythmias in the late myocardial infarction period. 12. Spontaneous versus induced reentry and intramural versus epicardial circuits. J Am Coll Cardiol 6: 124–132, 1985 [DOI] [PubMed] [Google Scholar]
- 21. El-Sherif N, Hope RR, Scherlag BJ, Lazzara R. Re-entrant ventricular arrhythmias in the late myocardial infarction period. 2. Patterns of initiation and termination of re-entry. Circulation 55: 702–719, 1977 [DOI] [PubMed] [Google Scholar]
- 22. El-Sherif N, Scherlag BJ, Lazzara R, Hope RR. Re-entrant ventricular arrhythmias in the late myocardial infarction period. 1. Conduction characteristics in the infarction zone. Circulation 55: 686–702, 1977 [DOI] [PubMed] [Google Scholar]
- 23. El-Sherif N, Smith RA, Evans K. Canine ventricular arrhythmias in the late myocardial infarction period. 8. Epicardial mapping of reentrant circuits. Circ Res 49: 255–265, 1981 [DOI] [PubMed] [Google Scholar]
- 24. Farb A, Tang AL, Burke AP, Sessums L, Liang Y, Virmani R. Sudden coronary death. Frequency of active coronary lesions, inactive coronary lesions, and myocardial infarction. Circulation 92: 1701–1709, 1995 [DOI] [PubMed] [Google Scholar]
- 25. Fedorov V, Lozinsky I, Sosunov E, Anyukhovsky E, Rosen M, Balke C, Efimov I. Application of blebbistatin as an excitation-contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Heart Rhythm 4: 619–626, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Gang UJ, Jons C, Jorgensen RM, Abildstrom SZ, Haarbo J, Messier MD, Huikuri HV, Thomsen PE. Heart rhythm at the time of death documented by an implantable loop recorder. Europace 12: 254–260 [DOI] [PubMed] [Google Scholar]
- 27. Hamilton DR, Dani RS, Semlacher RA, Smith ER, Kieser TM, Tyberg JV. Right atrial and right ventricular transmural pressures in dogs and humans. Effects of the pericardium. Circulation 90: 2492–2500, 1994 [DOI] [PubMed] [Google Scholar]
- 28. Hammond HK, White FC, Bhargava V, Shabetai R. Heart size and maximal cardiac output are limited by the pericardium. Am J Physiol Heart Circ Physiol 263: H1675–H1681, 1992 [DOI] [PubMed] [Google Scholar]
- 29. Hayashi H, Shiferaw Y, Sato D, Nihei M, Lin SF, Chen PS, Garfinkel A, Weiss JN, Qu Z. Dynamic origin of spatially discordant alternans in cardiac tissue. Biophys J 92: 448–460, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hilal SK, Solomon RA. Endovascular treatment of aneurysms with coils. J Neurosurg 76: 337–339, 1992 [DOI] [PubMed] [Google Scholar]
- 31. Huikuri HV, Makikallio TH, Raatikainen MJ, Perkiomaki J, Castellanos A, Myerburg RJ. Prediction of sudden cardiac death: appraisal of the studies and methods assessing the risk of sudden arrhythmic death. Circulation 108: 110–115, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Hund TJ, Decker KF, Kanter E, Mohler PJ, Boyden PA, Schuessler RB, Yamada KA, Rudy Y. Role of activated CaMKII in abnormal calcium homeostasis and I(Na) remodeling after myocardial infarction: insights from mathematical modeling. J Mol Cell Cardiol 45: 420–428, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Inada K, Seiler J, Roberts-Thomson KC, Steven D, Rosman J, John RM, Sobieszczyk P, Stevenson WG, Tedrow UB. Substrate characterization and catheter ablation for monomorphic ventricular tachycardia in patients with apical hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol 22: 41–48, 2011 [DOI] [PubMed] [Google Scholar]
- 34. Jalife J, Gray R. Drifting vortices of electrical waves underlie ventricular fibrillation in the rabbit heart. Acta Physiol Scand 157: 123–131, 1996 [DOI] [PubMed] [Google Scholar]
- 35. Janse MJ, Wit AL. Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol Rev 69: 1049–1169, 1989 [DOI] [PubMed] [Google Scholar]
- 36. Josephson ME. Clinical Cardiac Electrophysiology: Techniques and Interpretation. Philadelphia, PA: Lea & Febiger, 1993, p. xi [Google Scholar]
- 37. Lappin HA, Botvinick EH, Parmley WW, Tyberg JV. Myocardial infarction in closed-chest dogs: a simplified method for production. J Appl Physiol 39: 831–833, 1975 [DOI] [PubMed] [Google Scholar]
- 38. Li L, Nikolski V, Wallick DW, Efimov IR, Cheng Y. Mechanisms of enhanced shock-induced arrhythmogenesis in the rabbit heart with healed myocardial infarction. Am J Physiol Heart Circ Physiol 289: H1054–H1068, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Litwin SE, Bridge JH. Enhanced Na(+)-Ca2+ exchange in the infarcted heart. Implications for excitation-contraction coupling. Circ Res 81: 1083–1093, 1997 [DOI] [PubMed] [Google Scholar]
- 40. Lopshire JC, Zipes DP. Sudden cardiac death: better understanding of risks, mechanisms, and treatment. Circulation 114: 1134–1136, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Mahaffey KW, Raya TE, Pennock GD, Morkin E, Goldman S. Left ventricular performance and remodeling in rabbits after myocardial infarction. Effects of a thyroid hormone analogue. Circulation 91: 794–801, 1995 [DOI] [PubMed] [Google Scholar]
- 42. Marian AJ, Wu Y, Lim DS, McCluggage M, Youker K, Yu QT, Brugada R, DeMayo F, Quinones M, Roberts R. A transgenic rabbit model for human hypertrophic cardiomyopathy. J Clin Invest 104: 1683–1692, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McLachlan CS, McGuire MA. Characterization and incidence of inducible monomorphic ventricular tachycardia in a postinfarction rabbit model. J Electrocardiol 40: 89–93, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Mironov S, Jalife J, Tolkacheva EG. Role of conduction velocity restitution and short-term memory in the development of action potential duration alternans in isolated rabbit hearts. Circulation 118: 17–25, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Odening KE, Kirk M, Brunner M, Ziv O, Lorvidhaya P, Liu GX, Schofield L, Chaves L, Peng X, Zehender M, Choi BR, Koren G. Electrophysiological studies of transgenic long QT type 1 and type 2 rabbits reveal genotype-specific differences in ventricular refractoriness and His conduction. Am J Physiol Heart Circ Physiol 299: H643–H655, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Panfilov AV. Is heart size a factor in ventricular fibrillation? Or how close are rabbit and human hearts? Heart Rhythm 3: 862–864, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Peters NS. Myocardial gap junction organization in ischemia and infarction. Microsc Res Tech 31: 375–386, 1995 [DOI] [PubMed] [Google Scholar]
- 48. Pinto JM, Boyden PA. Electrical remodeling in ischemia and infarction. Cardiovasc Res 42: 284–297, 1999 [DOI] [PubMed] [Google Scholar]
- 49. Podrid PJ, Myerburg RJ. Epidemiology and stratification of risk for sudden cardiac death. Clin Cardiol 28: I3–11, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Pye MP, Black M, Cobbe SM. Comparison of in vivo and in vitro haemodynamic function in experimental heart failure: use of echocardiography. Cardiovasc Res 31: 873–881, 1996 [PubMed] [Google Scholar]
- 51. Pye MP, Cobbe SM. Arrhythmogenesis in experimental models of heart failure: the role of increased load. Cardiovasc Res 32: 248–257, 1996 [DOI] [PubMed] [Google Scholar]
- 52. Richardson AW, Callans DJ, Josephson ME. Electrophysiology of postinfarction ventricular tachycardia: a paradigm of stable reentry. J Cardiovasc Electrophysiol 10: 1288–1292, 1999 [DOI] [PubMed] [Google Scholar]
- 53. Richardson AW, Josephson ME. Ablation of ventricular tachycardia in the setting of coronary artery disease. Curr Cardiol Rep 1: 157–164, 1999 [DOI] [PubMed] [Google Scholar]
- 54. Rothman SA, Hsia HH, Cossu SF, Chmielewski IL, Buxton AE, Miller JM. Radiofrequency catheter ablation of postinfarction ventricular tachycardia: long-term success and the significance of inducible nonclinical arrhythmias. Circulation 96: 3499–3508, 1997 [DOI] [PubMed] [Google Scholar]
- 55. Saba S, Mathier MA, Mehdi H, Gursoy E, Liu T, Choi BR, Salama G, London B. Prevention of adverse electrical and mechanical remodeling with biventricular pacing in a rabbit model of myocardial infarction. Heart Rhythm 5: 124–130, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Saba S, Mathier MA, Mehdi H, Liu T, Choi BR, London B, Salama G. Dual-dye optical mapping after myocardial infarction: does the site of ventricular stimulation alter the properties of electrical propagation? J Cardiovasc Electrophysiol 19: 197–202, 2008 [DOI] [PubMed] [Google Scholar]
- 57. Schuijf JD, Kaandorp TA, Lamb HJ, van der Geest RJ, Viergever EP, van der Wall EE, de Roos A, Bax JJ. Quantification of myocardial infarct size and transmurality by contrast-enhanced magnetic resonance imaging in men. Am J Cardiol 94: 284–288, 2004 [DOI] [PubMed] [Google Scholar]
- 58. Segal OR, Chow AW, Peters NS, Davies DW. Mechanisms that initiate ventricular tachycardia in the infarcted human heart. Heart Rhythm 7: 57–64, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Stevenson WG. Catheter ablation of monomorphic ventricular tachycardia. Curr Opin Cardiol 20: 42–47, 2005 [PubMed] [Google Scholar]
- 60. Stevenson WG. Ventricular scars and ventricular tachycardia. Trans Am Clin Climatol Assoc 120: 403–412, 2009 [PMC free article] [PubMed] [Google Scholar]
- 61. Stevenson WG, Delacretaz E, Friedman PL, Ellison KE. Identification and ablation of macroreentrant ventricular tachycardia with the CARTO electroanatomical mapping system. Pacing Clin Electrophysiol 21: 1448–1456, 1998 [DOI] [PubMed] [Google Scholar]
- 62. Stevenson WG, Ellison KE, Sweeney MO, Epstein LM, Maisel WH. Management of arrhythmias in heart failure. Cardiol Rev 10: 8–14, 2002 [DOI] [PubMed] [Google Scholar]
- 63. stevenson WG, Friedman PL, Ganz LI. Radiofrequency catheter ablation of ventricular tachycardia late after myocardial infarction. J Cardiovasc Electrophysiol 8: 1309–1319, 1997 [DOI] [PubMed] [Google Scholar]
- 64. ten Tusscher KH, Mourad A, Nash MP, Clayton RH, Bradley CP, Paterson DJ, Hren R, Hayward M, Panfilov AV, Taggart P. Organization of ventricular fibrillation in the human heart: experiments and models. Exp Physiol 94: 553–562, 2009 [DOI] [PubMed] [Google Scholar]
- 65. Ursell PC, Gardner PI, Albala A, Fenoglio JJ, Jr, Wit AL. Structural and electrophysiological changes in the epicardial border zone of canine myocardial infarcts during infarct healing. Circ Res 56: 436–451, 1985 [DOI] [PubMed] [Google Scholar]
- 66. Wit AL, Bigger JT., Jr Electrophysiology of ventricular arrhythmias accompanying myocardial ischaemia and infarction. Postgrad Med J 53, Suppl 1: 98–113, 1977 [PubMed] [Google Scholar]
- 67. Wit AL, Dillon SM, Coromilas J, Saltman AE, Waldecker B. Anisotropic reentry in the epicardial border zone of myocardial infarcts. Ann NY Acad Sci 591: 86–108, 1990 [DOI] [PubMed] [Google Scholar]
- 68. Wit AL, Hoffman BF, Cranefield PF. Slow conduction, reentry, and the mechanism of ventricular arrhythmias in myocardial infarction. Bull NY Acad Med 47: 1233–1234, 1971 [PubMed] [Google Scholar]
- 69. Zipes DP, Wellens HJ. Sudden cardiac death. Circulation 98: 2334–2351, 1998 [DOI] [PubMed] [Google Scholar]


