Abstract
Matrix metalloprotease (MMP) activity is increased in the postpartum vagina of wild-type (WT) animals. This degradative activity is also accompanied by a burst in elastic fiber synthesis and assembly. The mechanisms that precipitate these changes are unclear. The goals of this study were to determine how vaginal distention (such as in parturition) affects elastic fiber homeostasis in the vaginal wall and the potential significance of these changes in the pathogenesis of pelvic organ prolapse. Vaginal distention with a balloon simulating parturition resulted in increased MMP-2 and MMP-9 activity in the vaginal wall of nonpregnant and pregnant animals. This was accompanied by visible fragmented and disrupted elastic fibers in the vaginal wall. In nonpregnant animals, the abundant amounts of tropoelastin and fibulin-5 in the vagina were not increased further by distention. In contrast, in pregnant animals, the suppressed levels of both proteins were increased 3-fold after vaginal distention. Distention performed in fibulin-5-deficient (Fbln5−/−) mice with defective elastic fiber synthesis and assembly induced accelerated pelvic organ prolapse, which never recovered. We conclude that, in pregnant mice, vaginal distention results in increased protease activity in the vaginal wall but also increased synthesis of proteins important for elastic fiber assembly. Distention may thereby contribute to the burst of elastic fiber synthesis in the postpartum vagina. The finding that distention results in accelerated pelvic organ prolapse in Fbln5−/− animals, but not in WT, indicates that elastic fiber synthesis is crucial for recovery of the vaginal wall from distention-induced increases in vaginal protease activity.
Keywords: childbirth trauma, MMP9, protease, pregnancy
pelvic organ prolapse is a common problem for women with significant psychological, social, and financial implications (12, 26, 29). Whereas many factors appear to incite or promote the progression of prolapse, the two major risk factors for pelvic organ prolapse are history of vaginal delivery and aging. Although cesarean delivery does not completely protect the pelvic floor from the adverse effects of pregnancy, it is universally agreed that vaginal delivery has a deleterious impact on the development of pelvic organ prolapse in women, and this impact may not be of clinical significance until decades after delivery. The mechanisms by which childbirth leads to failure of pelvic organ support, however, are not well understood (3, 26).
Mice with null mutations in genes encoding lysyl oxidase like 1 (LOXL1) (15, 16) or fibulin-5 (FBLN5) (11) develop pelvic organ prolapse. Since both proteins play important roles in elastic fiber synthesis and assembly (19, 30), these findings indicate that failure of elastic fiber synthesis may be involved in the pathophysiology of pelvic organ prolapse. Previously, we demonstrated that a burst in elastic fiber synthesis and cross-linking occurs in the vaginal wall postpartum (11). This burst in elastic fiber synthesis, however, was also accompanied by upregulation of matrix metalloproteinase-2 (MMP-2), MMP-9, and an unidentified casein protease in the postpartum vaginal wall (28). These results, together with the phenotype of postpartum prolapse in LOXL1 knockout mice (15), suggest that synthesis and assembly of elastic fibers is crucial for recovery of pelvic organ support after vaginal delivery and that increased protease activity in the postpartum vagina may lead to pelvic organ prolapse in the absence of the timely initiation of elastic fiber synthesis in connective tissues of the pelvic floor.
The mechanisms by which pregnancy and parturition incite the postpartum burst in elastic fiber synthesis are not known. It seems logical, however, that the trauma of physical stretching and vaginal distention involved in vaginal delivery may play a role in turnover of connective tissue components of the pelvic floor. Thus, the objective of this study was to determine the effect of vaginal distention on changes in elastic fiber homeostasis in the fibromuscular connective tissue of the vaginal wall. Further, we tested the hypothesis that, in the absence of elastic fiber synthesis, vaginal distention induces pelvic organ prolapse.
MATERIALS AND METHODS
Mice.
Female mice (n = 68) were studied and killed in accordance with the standards of humane animal care described by the National Institutes of Health Guide for the Care and Use of Laboratory Animals, using protocols approved by the Institutional Animal Care and Use Committee of University of Texas Southwestern Medical Center. Animals were housed under a 12:12-h light-dark cycle (lights on, 6:00 AM, lights off 6:00 PM) at 22°C. All wild-type (WT) mice used in these studies were C3BL/6J. Fbln5−/− mice were of a similar mixed strain (C57BL/6 × 129SvEv). To obtain timed pregnant animals, nulliparous females were housed with males for 4 to 6 h and checked at midday for vaginal plugs. Plug day was considered day 0. At death, after disarticulation of the pubic symphysis, uterine horns together with the bladder, cervix, and vagina were dissected down to the perineal skin. The vaginal dissection extended down to the connective tissue, suspending the vaginal wall to the pubocaudalis. Using microinstruments and a dissection microscope, we removed the uterine horns at the cervicovaginal junction. Perineal skin was removed, and the bladder and urethra were dissected from the anterior vaginal wall. The wet weight of the vagina and cervix was determined. Thereafter, the cervix was removed and weighed. The vaginas were cut into two equally sized pieces, and all tissues were stored at −80°C.
Eighteen nonpregnant and 20 pregnant (day 14) WT mice, after anesthesia, had a latex balloon inserted into the vagina. Half of the animals in each group underwent vaginal distention for 40 min by inflating the balloon with 750 μl of saline, while the remaining animals had the uninflated balloon alone. Forty minutes is the approximate time required for delivery of a litter, and 750 μl is the volume of an average pup. Because previous experiments had demonstrated a dramatic increase in FBLN5 and tropoelastin expression in the vaginal wall of nonpregnant animals by 48 h postpartum, mice were killed 24 and 48 h after balloon distention or sham procedure, as described above. Vaginal tissues were homogenized for immunoblot analysis and zymography. A subset of vaginal tissue was fixed in neutral buffered formalin (10%) for 24 h. Five-micrometer cross sections of the formalin-fixed, paraffin-embedded tissues were obtained at 0.1-mm intervals throughout the specimen. Tissues were stained with hematoxylin and eosin or were stained for elastic fibers using Hart's stain and analyzed with a Nikon Eclipse E1000N microscope.
Vaginal distention was also performed after anesthesia in 30 virginal Fbln5−/−, Fbln5 heterozygotes, and WT mice at 6 wk of age. A similar distention protocol was used as above, except 400 μl of saline was injected in the balloon due to the animals' smaller size. The previously validated Mouse Pelvic Organ Quantification (MOPQ) exam was used to measure the degree of vaginal, perineal, and anal prolapse in all animals preoperatively and then on postoperative days 3, 6, 10, 14, and continued weekly until postoperative day 98 (i.e., 14 wk after the distention, or 20 wk of age). The MOPQ was completed by having one investigator hold the animal by the scruff of the neck, which resulted in a prolonged, reflex valsalva (evidenced by defecation), while the other investigator performed the measurements using a caliper with the precision of 1/100th of a millimeter. Six assessments were performed in each animal: 1) stage of perineal bulge; 2) height of perineal bulge; 3) cervical descent; 4) anal prolapse; 5) perineal body length, and 6) vaginal diameter (27).
Immunoblot analysis.
Frozen pieces of vagina were weighed and then pulverized with a liquid nitrogen-chilled mortar and pestle. Tissue powder was then homogenized in buffer (16 mmol/l potassium phosphate, pH 7.8, 0.12 mol/l NaCl, 1 mmol/l ethylenediaminetetraacetic acid) containing a protease inhibitor cocktail (Complete Mini, product number 11836153001, Roche Diagnostics, Penzberg, Germany), and then centrifuged at 10,000 g. The supernatant was removed, and the previous homogenization step was repeated after resuspending the remaining tissue pellet in basic buffer. After removal of the second supernatant, the remaining tissue pellet was suspended in urea buffer (6.0 mol/l in above buffer), homogenized, and placed on a rotating rack for overnight extraction at 4°C. Thereafter, the samples were centrifuged (13,000 g for 30 min), and the supernatant was removed. Protein concentrations were determined using a bicinchoninic acid protein assay (Pierce, Rockford, IL) and standard curves of BSA in appropriate buffers.
Total protein (10 μg/lane) was applied to 4 to 20% Criterion gradient polyacrylamide gels (Bio-Rad, Hercules, CA), separated by electrophoresis, and transferred to nitrocellulose membranes overnight at 4°C. To ensure equal protein loading, identical gels were run side-by-side for Coomassie brilliant blue staining. Nitrocellulose membranes were placed in blocking buffer (10 mmol/l Tris·HCl, pH 7.5, 0.15 mol/l NaCl, 0.1% Tween 20, 2% nonfat powdered milk) for 1 h at 37°C and incubated with primary antibody for 1 h at 30°C. Primary antibodies used were rabbit anti-mouse FBLN5 (BSYN1923) (30) at 1:250 dilution and rabbit anti-mouse tropoelastin (Elastin Products, Owensville, MO) also at 1:250 dilution. Membranes were then washed with Tris-buffered saline solution (TBST) (10 mmol/l Tris·HCl, pH 7.5, 0.15 mol/l NaCl, and 0.1% Tween 20) for 5 min × 1, an enhanced detergent wash (TBST, Nonidet P-40 0.05%, 3 mmol/l sodium deoxycholate, and 0.1% SDS) for 7 min × 2, and again with TBST for 5 min × 3. Thereafter, the blot was incubated with goat-anti-rabbit horseradish peroxidase conjugate (Bio-Rad) at room temperature for 1 h. The membrane wash protocol was repeated, followed by incubation with Western Lighting Chemiluminescence Reagent Plus (Perkin-Elmer, Boston, MA) for 2 min. Chemiluminescence images were obtained on a Fuji LAS 3000 image analysis system (Fujifilm Life Science, Stamford, CT). The relative signal strength per microgram urea-extracted protein was calculated.
Gelatin zymography.
Vaginal specimens were thawed on ice, minced, and washed in PBS. Tissues were then homogenized in MMP-2 assay buffer (ANASPEC EnzoLyte 520 MMP-2 Assay Kit, San Jose, CA) containing 0.1% Triton-X 100 95 × volume:tissue wet weight. Thereafter, the homogenates were centrifuged at 10,000 g for 15 min at 4°C. The supernatant was used for determination of protease activity. Protein concentrations were determined using a bicinchoninic acid protein assay and standard curves of BSA in appropriate buffers. Samples (5 μg per lane) were applied to gelatin polyacrylamide minigels (Invitrogen, Carlsbad, CA) (10%) in standard SDS loading buffer containing 0.1% SDS with no β-mercaptoethanol; the samples were not boiled before loading. Gels were run at room temperature at 125 V. After electrophoresis, gels were soaked in renaturing buffer [2.7% (vol/vol) Triton X-100 in distilled water] in a shaker for 30 min with one change after 30 min to remove SDS. Next, gels were soaked in assay buffer (50 mmol/l Tris, 200 mmol/l NaCl, 10 mmol/l CaCl2, 0.05% Brij 35, pH 7.5) overnight at 37°C and then stained with Coomassie brilliant blue-R 250 in 50% methanol and 10% acetic acid followed by washing with distilled water for 1 min. Clear zones of lysis against a dark background indicated enzyme activity. Areas of lysis were quantified using the Fuji LAS 3000 image analysis system.
Histology.
Mice were anesthetized and perfused with 10% neutral buffered formalin, pH 7.4 48 h after sham or vaginal distention. Thereafter, the female urogenital tract was dissected en bloc. Serial transverse sections (5 μm) were obtained in 100-μm increments throughout the specimen, stained with hematoxylin and eosin or Hart's stain, and analyzed with a Nikon Eclipse E1000N microscope.
Statistical analysis.
Protease activity and amounts of fibulin-5 and tropoelastin were compared in balloon-distended vs. sham-operated animals using Student's t-tests with SigmaStat software version 2.03 (Jandel Scientific, San Rafael, CA). MOPQ score differences between vaginally distended Fbln5−/−, Fbln5 heterozygotes, and WT controls were assessed using two-way ANOVA with time as a repeated factor and analyzed as a random-effects model. Multiple comparisons were adjusted for using a Tukey-Kramer approach. P values less than 0.05 were considered statistically significant; this portion of the analysis was accomplished using SAS Version 9.1 (SAS Institute, Cary, NC).
RESULTS
Effect of vaginal distention on matrix degradation.
A number of different proteases have been implicated in the proteolytic degradation of elastic fibers and collagens, most prominent among which are members of the MMP family. Although MMPs can cleave virtually all protein components of the extracellular matrix, we focused on the effect of vaginal distention on two MMPs that have been shown to be upregulated in the postpartum vaginal wall (28) and have well-known elastase activity. Gelatin zymography was conducted on vaginal tissues from both pregnant (day 14) and nonpregnant WT mice 24 and 48 h after vaginal distention or sham operation. In nonpregnant animals, distention resulted in marked increases in MMP-2 and MMP-9: pro-MMP9, 5.6-fold; active MMP9, 3.5-fold; pro-MMP2, 2.4-fold; and active MMP2, 3.7-fold (all P < 0.05 compared with sham-operated animals, Fig. 1). In pregnant animals, vaginal distention also resulted in significant increases in vaginal MMP2 and MMP9, but the magnitude of increase was less than that in vaginal tissues from nonpregnant animals (i.e., 1.7- to 2.3-fold, all P < 0.05 compared with shams) (Fig. 2).
Fig. 1.
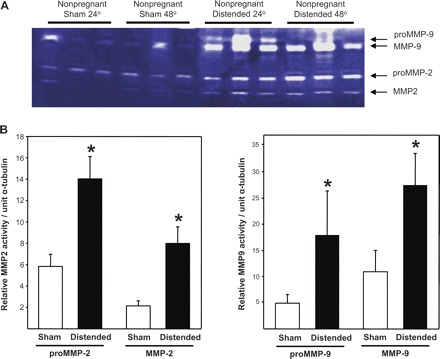
Effect of vaginal distention on protease activity in the vaginal wall of nonpregnant animals. A: gelatin zymograms of vaginal homogenates (5 μg per lane) from nonpregnant wild-type mice 24 or 48 h after distention or sham operation. B: quantification of MMP-2 and MMP-9 enzyme activity (sham, n = 6; distended, n = 6). *P ≤ 0.05 compared with sham.
Fig. 2.
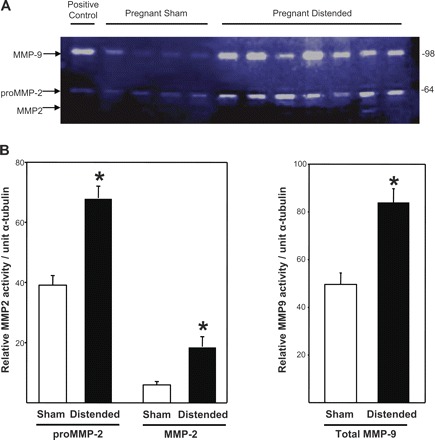
Effect of vaginal distention on protease activity in the vaginal wall of pregnant animals. A: gelatin zymograms of vaginal homogenates (5 μg per lane) from pregnant wild-type mice 24 or 48 h after distention or sham operation on D14. B: quantification of MMP-2 and MMP-9 enzyme activity (sham, n = 6; distended, n = 6). *P ≤ 0.05 compared with sham.
To examine the effect of vaginal distention on morphology of elastic fibers, tissues from nonpregnant animals were fixed in formalin and stained for elastic fibers using Hart's stain. In transverse sections of midvagina in sham-operated animals, elastic fibers were thin, long, and extended radially from the muscularis to branch in the subepithelium beneath the basement membrane. Some fibers were also oriented circumferentially in the muscularis (Fig. 3A). After vaginal distention, elastic fibers appeared fragmented and disrupted compared with sham-operated mice (Fig. 3B). Infiltrating inflammatory cells with morphologic features of both monocytes and neutrophils were also noted, and the collagenous matrix of the lamina propria and vaginal muscularis appeared loose and disorganized (Fig. 3B).
Fig. 3.
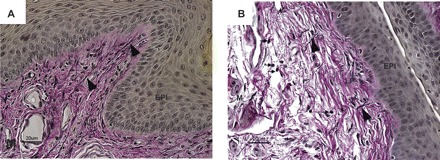
Effect of vaginal distention on elastic fiber morphology in the vaginal wall. Transverse sections of midvagina from nonpregnant mice obtained 48 h after sham operation (A) or balloon distention (B). Elastic fibers are noted by arrowheads, and monocytes are noted by small arrows. Experimental results represent those of four wild-type animals in each group. EPI, epithelium; LP, lamina propria; M, muscularis.
Effect of vaginal distention on elastic fiber synthesis.
Previously, we reported that FBLN5 and tropoelastin are highly expressed in the vaginal wall of nonpregnant animals, decrease to a nadir at mid-to-late pregnancy, and increase dramatically 48 h postpartum (11). To determine whether physical distention of the vaginal wall may precipitate this burst in elastic fiber synthesis activity, immunoblot analysis was used to quantify FBLN5 and tropoelastin in urea-extracted proteins from the vaginal wall of sham-operated and distended animals. In nonpregnant animals, baseline FBLN5 and tropoeleastin expression was robust in the vaginal wall. Vaginal distention did not result in further increases or decreases in FBLN5 (Fig. 4, A and C) or tropoelastin (Fig. 5, A and C). In pregnant animals, however, baseline FBLN5 expression was suppressed and vaginal distention resulted in significant upregulation of FBLN5 (Fig. 4, B and C) and tropoelastin in the vaginal wall (3-fold, P < 0.05, Fig. 5, B and C). Collectively, these data indicate that distention of the pregnant vagina during parturition may contribute to the burst of elastic fiber synthesis that occurs in the postpartum vagina.
Fig. 4.
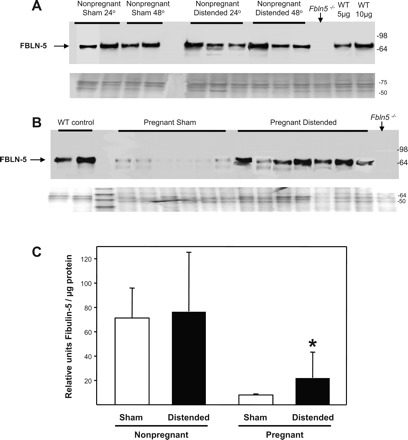
Effect of vaginal distention on FBLN5 protein expression in the vaginal wall of nonpregnant and pregnant animals. Immunoblots for fibulin-5 protein in nonpregnant (A) and pregnant (B) wild-type mice vaginal homogenates after sham operation and balloon distention (n = 6 in each group). Coomassie-stained gels are presented beneath the immunoblots. Fbln5−/−, negative control. WT, wild-type positive controls that underwent neither sham nor balloon procedures. C: relative amounts of fibulin-5 in sham-operated and balloon-distended vaginal tissues from nonpregnant and pregnant animals (n = 6 in each group). *P < 0.05.
Fig. 5.
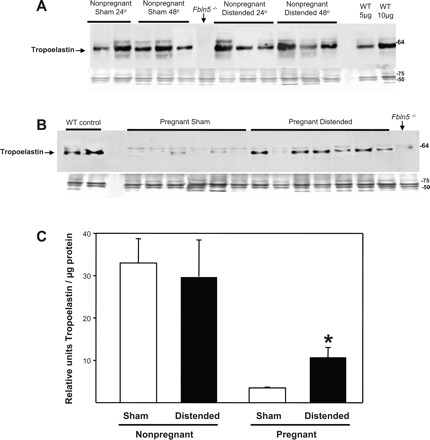
Effect of vaginal distention on tropoelastin protein expression in the vaginal wall of nonpregnant and pregnant animals. Immunoblots for tropoelastin protein in nonpregnant (A) and pregnant (B) wild-type mice vaginal homogenates after sham operation and balloon distention. Coomassie-stained gels are presented beneath the immunoblots. WT, wild-type positive controls that underwent neither sham nor balloon procedures. C: relative amounts of tropoelastin in sham-operated and balloon-distended vaginas from nonpregnant and pregnant animals (n = 6 in each group); *P < 0.05.
Effect of vaginal distention in Fbln5−/− mice.
Although vaginal distention resulted in increased protease activity in the vaginal wall of WT animals, pelvic organ prolapse was not observed, and prolapse is not observed in WT animals after parturition despite an increase in vaginal wall protease activity postpartum. To test the hypothesis that concomitant elastic fiber synthesis is necessary to maintain pelvic organ support after vaginal distention, vaginal distention was conducted in animals deficient in elastic fiber assembly (Fbln5−/−), and pelvic organ support was monitored using the MOPQ scoring system (27) as a function of time. Historically, by 6 mo of age, 91% of virginal Fbln5−/− mice demonstrate stage 3 prolapse (27). Nonetheless, pelvic organ support is normal until 10–12 wk of age when small, but significant, increases in perineal bulge and perineal body length are detectable (27). To determine whether vaginal distention affects the rate or severity of vaginal prolapse in Fbln5−/− mice, distention was performed in 6 WT, 11 Fbln5+/−, and 13 Fbln5−/− animals at 6 wk of age (i.e., several weeks prior to the normal onset of prolapse in Fbln5−/− mice). Examiners blinded to genotype conducted distention and monitoring. Genotype was confirmed in all animals at death, in which emphysematous lungs and loose or tortuous great vessels could be seen in knockout animals.
Vaginal distention resulted in marked acceleration of prolapse development in Fbln5−/− animals compared with heterozygotes or WT controls (Fig. 6). Normally, in Fbln5−/− mice, prolapse of the perineum (i.e., a perineal bulge of stage 1) does not emerge until 10–12 wk of age. In distended knockouts, however, greater degrees of bulge (stage 1 to 2) were observed just 3 days postoperatively (i.e., aged 6–7 wk). The difference, over time, between Fbln5−/−, heterozygote, and WT mice remained significant (P < 0.001) (Fig. 6, A and B). Further, the magnitude of perineal bulge, stage of cervical descent, and vaginal diameter increased significantly in knockout animals over time compared with Fbln5+/− or WT animals (Fig. 6). Acceleration of pelvic organ prolapse in knockout, but not WT, mice suggests that vaginal distention results in loss of pelvic organ support if elastic fiber synthesis and assembly is compromised.
Fig. 6.
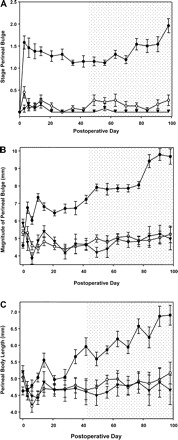
Effect of vaginal distention on pelvic organ support in young WT, Fbln5+/−, and Fbln5−/− mice. Vaginal distention or sham operation was conducted at 6 wk of age (i.e., ≥ 6 wk before the onset of prolapse in Fbln5−/− mice). Perineal bulge stage (A), magnitude of perineal bulge (B), and change in perineal body length (C) in Fbln5 −/− (•, n = 13); Fbln5+/− (○, n = 11), and WT (▾, n = 6) animals. Shading indicates the time at which measurements begin to increase during the natural history of prolapse in Fbln5−/− animals. Data are expressed as means ± SE.
Distended Fbln5−/−, heterozygote, and WT mice were killed at 20 wk of age and changes in pelvic anatomy recorded. Although intact in all animals, the vaginal wall of some knockout mice appeared thick, short, and scarred. Vaginas from most Fbln5−/− mice were thin and patulous, and the connective tissue paravaginal and apical attachments (e.g., the uterosacral ligaments) were either absent or attenuated. Vaginal weight was increased in Fbln5−/− animals compared with heterozygotes (70.3 ± 8.9 compared with 52.8 ± 5.9 mg, P = 0.06).
DISCUSSION
Vaginal delivery confers a 4- to 7-fold increase in the incidence of pelvic organ prolapse. It has been suggested that childbirth causes direct muscular trauma or denervation injury of the striated muscles of the pelvic floor (termed the levator ani) and thereby leads to failure of muscular support of pelvic organs (10). Nevertheless, several studies demonstrate that defects in the levator ani do not universally result in or correlate with the degree of pelvic organ prolapse (13, 14), and levator injury is not found in primate models of pelvic organ prolapse (20). A potential role of fibromuscular connective tissue in the pathophysiology of prolapse has been proposed by us (1, 2) and others (18). It has been difficult, however, to determine whether changes in connective tissue support precede the development of prolapse or occur as a consequence of prolonged tissue stretching, hypoxia, and mechanical stress of the prolapsed organs. Recent evidence in mice with null mutations in two genes important in elastic fiber assembly (fibulin-5) and synthesis (lysyl oxidase like 1), however, suggest that lack of elastic fibers in connective tissues plays an important role in the pathogenesis of pelvic organ prolapse (11, 16).
Vaginal wall support tissues include the cardinal-uterosacral ligament apical attachments and the fibromuscular layer of the vaginal wall that interdigitates with the arcus tendineous fasciae pelvis suspending the vaginal wall to the pelvic musculature. In this investigation, we used the vaginal wall to represent pelvic floor connective tissue. The major findings of this study were that, in pregnant animals, vaginal distention results in significant increases in vaginal wall protease activity and, simultaneous, increased expression of two proteins involved in elastic fiber assembly (fibulin-5 and tropoelastin). Further, we demonstrated that increases in elastic fiber synthesis are crucial for pelvic organ support after vaginal distention.
Others have examined how vaginal distention may be one of the inciting factors that ultimately results in pelvic floor disorders. Damaser et al. (9) and Sievert et al. (25) have shown that vaginal distention results in significantly reduced urethral leak point pressures in female rats, and, thus, may play a role in urinary incontinence. This finding was echoed by Cannon et al. (6), who showed that increased duration of vaginal distention and multiple vaginal deliveries also decreased leak point pressures and increased anatomical injury. It may be that the distention causes ischemia to the urethra or that there is reperfusion injury after the vaginal distention has resolved (8). A similar mechanism of injury may be operative in the connective tissues of the vaginal wall.
A number of different proteases have been implicated in the pathophysiology of pelvic organ prolapse (7, 18), and several proteases have been linked to the remodeling of pelvic support tissues after parturition (28) and during aging (27). Collagen and elastin are substrates for a number of proteases, most prominent among which are members of the MMP family. MMPs form a subfamily of Ca2+-dependent zinc enzymes expressed predominantly in connective tissue and bone marrow cells and include collagenases, gelatinases, and stromelysins, as well as membrane-type MMPs (21, 24). MMPs can cleave virtually all protein components of the extracellular matrix. In this study, we focused on the gelatinases, MMP2 and MMP9, because these enzymes are prominent elastases and are increased significantly in vaginal tissues from Fbln5−/− mice with prolapse and from wild-type mice after parturition (28). Herein, we found that vaginal distention induced increases in both MMP-2 and MMP-9 in vaginal tissues from nonpregnant and pregnant mice, although the magnitude of distention-induced increases in protease activity was decreased in pregnant animals compared with nonpregnant animals.
There are several possible explanations for the blunted upregulation of MMPs in response to vaginal distention in pregnant animals. First, estrogen- and relaxin-induced increases in vaginal size (4, 5) and distensibility (17, 23) during pregnancy may facilitate less pressure-induced trauma to the vaginal wall. Further, the increased vascularity of the vaginal wall during pregnancy may decrease hypoxia induced by prolonged vaginal distention. Nevertheless, the decreased content of fibulin-5 and tropoelastin in the vaginal wall during pregnancy appears to make the organ particularly susceptible to loss of elastic fibers (11). Vaginal distention and parturition, however, result in increased synthesis of tropoelastin and fibulin-5, suggesting that these adaptations are important in recovery of elasticity and resilience in pelvic floor connective tissues after childbirth.
In this study, vaginal distention in Fbln5−/− animals at 6 wk of age resulted in a rapid acceleration in the development of prolapse. Knockout animals had stage 1 to 2 prolapse within days of surgery and eventually progressed to an average perineal bulge of stage 2 to 2.5 (Fig. 6A). Interestingly, while the rate of prolapse progression was increased, the ultimate degree of decensus did not appear as severe as is seen in animals that naturally progress to prolapse. This may be due to vaginal lacerations from distention that resulted in scarring of the vaginal wall that then could not completely progress to overt stage 3 prolapse. Indeed, some of the vaginal specimens observed upon dissection of the Fbln5−/− mice were dimpled and thickened. Pelvic adhesions, however, were not observed at dissection. Resplande et al. (22) studied the vaginal walls of rats after distention injury and found marked vaginal mucosal atrophy, an increase in collagen content, and a decrease in the muscular layer. The vaginal specimens also were contracted, appearing with many folds in the subepithelial layer (22). This increased collagen content between stromal cells of the vaginal wall perhaps protects the vagina from progression to its most severe degree of decensus.
Perspectives and Significance
In summary, this study underscores the importance of vaginal distention in the pathophysiology of pelvic organ prolapse and the need for mature elastic fiber synthesis and assembly to maintain connective tissue support of the pelvic floor. Distention injury results in increased elastic fiber degradation and a burst in the proteins necessary for new elastic fiber synthesis in the pregnant vagina. In the absence of this elastic fiber synthesis, rapid progression of prolapse of the vaginal wall ensues. Taken together, the results indicate that vaginal wall distention induces increased protease activity and that elastic fiber synthesis is crucial for recovery of the vaginal wall from distention-induced injury.
GRANTS
This work is supported by National Institutes of Health Grants AG028048.
Acknowledgments
We thank Mr. Patrick Keller for expert technical assistance. This article was presented as a paper at the American Urogynecologic Society 28th Annual Scientific Meeting, Hollywood, FL, September 27–29, 2007.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Boreham MK, Miller RT, Schaffer JI, Word RA. Smooth muscle myosin heavy chain and caldesmon expression in the anterior vaginal wall of women with and without pelvic organ prolapse. Am J Obstet Gynecol 185: 944–952, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Boreham MK, Wai CY, Miller RT, Schaffer JI, Word RA. Morphometric analysis of smooth muscle in the anterior vaginal wall of women with pelvic organ prolapse. Am J Obstet Gynecol 187: 56–63, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Bump R, Norton P. Epidemiology and natural history of pelvic floor dysfunction. Obstet Gynecol Clin North Am 25: 723–746, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Burger LL, Sherwood OD. Evidence that cellular proliferation contributes to relaxin-induced growth of both the vagina and the cervix in the pregnant rat. Endocrinology 136: 4820–4826, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Burger LL, Sherwood OD. Relaxin increases the accumulation of new epithelial and stromal cells in the rat cervix during the second half of pregnancy. Endocrinology 139: 3984–3995, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Cannon TW, Wojcik EM, Ferguson CL, Saraga S, Thomas C, Damaser MS. Effects of vaginal distension on urethral anatomy and function. BJU Int 90: 403–407, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Chen BH, Wen Y, Li H, Polan ML. Collagen metabolism and turnover in women with stress urinary incontinence and pelvic prolapse. Int Urogynecol J Pelvic Floor Dysfunct 13: 80–87; discussion 87, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Damaser M, Whitbeck C, Chichester P, Levin RM. Effect of vaginal distension on blood flow and hypoxia of urogenital organs of the female rat. J Appl Physiol 98: 1884–1890, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Damaser MS, Broxton-King C, Ferguson C, Kim FJ, Kerns JM. Functional and neuroanatomical effects of vaginal distention and pudendal nerve crush in the female rat. J Urol 170: 1027–1031, 2003 [DOI] [PubMed] [Google Scholar]
- 10.DeLancey JO, Morgan DM, Fenner DE, Kearney R, Guire K, Miller JM, Hussain H, Umek W, Hsu Y, Ashton-Miller JA. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol 109: 295–302, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Drewes PG, Yanagisawa H, Starcher B, Hornstra IK, Csiszar K, Marinis SI, Keller P, Word RA. Pelvic organ prolapse in fibulin-5 knockout mice: pregnancy changes in elastic fiber homeostasis in mouse vagina. Am J Pathol 170: 578–589, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jelovsek JE, Barber MD, Paraiso MFR, Walters MD. Functional bowel and anorectal disorders in patients with pelvic organ prolapse and incontinence. Am J Obstet Gynecol 193: 2105–2111, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Jundt K, Kiening M, Fischer P, Bergauer F, Rauch E, Janni W, Peschers U, Dimpfl T. Is the histomorphological concept of the female pelvic floor and its changes due to age and vaginal delivery correct? Neurourol Urodyn 24: 44–50, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Koelbl H, Strassegger H, Riss PA, Gruber H. Morphologic and functional aspects of pelvic floor muscles in patients with pelvic relaxation and genuine stress incontinence. Obstet Gynecol 74: 789–795, 1989 [PubMed] [Google Scholar]
- 15.Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA, Yanagisawa H, Zuo J, Li T. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet 36: 178–182, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Zhao Y, Pawlyk B, Damaser M, Li T. Failure of elastic fiber homeostasis leads to pelvic floor disorders. Am J Pathol 168: 519–528, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowder JLDK, Moon DK, Howden N, Abramowitch SD, Moalli PA. Biomechanical adaptations of the rat vagina and supportive tissues in pregnancy to accommodate delivery. Obstet Gynecol 109: 136–143, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Moalli PA, Klingensmith WL, Meyn LA, Zyczynski HM. Regulation of matrix metalloproteinase expression by estrogen in fibroblasts that are derived from the pelvic floor. Am J Obstet Gynecol 187: 72–79, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Nakamura T, Lozano PR, Ikeda Y, Iwanaga Y, Hinek A, Minamisawa S, Cheng CF, Kobuke K, Dalton N, Takada Y, Tashiro K, Ross J Jr, Honjo T, Chien KR. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature 415: 171–175, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Pierce LM, Baumann S, Rankin MR, Wasserman RM, Biaggi A, Kuehl TJ, Coates KW. Levator ani muscle and connective tissue changes associated with pelvic organ prolapse, parity, and aging in the squirrel monkey: a histologic study. Am J Obstet Gynecol 197: 60 e61–e69, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Rawlings ND, Barrett AJ. Evolutionary families of metallopeptidases. Methods Enzymol 248: 183–228, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Resplande J, Gholami SS, Graziottin TM, Rogers R, Lin CS, Leng W, Lue TF. Long-term effect of ovariectomy and simulated birth trauma on the lower urinary tract of female rats. J Urol 168: 323–330, 2002 [PubMed] [Google Scholar]
- 23.Rundgren A. Physical properties of connective tissue as influenced by single and repeated pregnancies in the rat. Acta Physiol Scand Suppl 417: 1–138, 1974 [PubMed] [Google Scholar]
- 24.Shapiro SD. A concise yet informative stroll through matrix metalloproteinases and TIMPs. J Cell Sci 113: 3355–3356, 2000 [Google Scholar]
- 25.Sievert KD, Emre Bakircioglu M, Tsai T, Dahms SE, Nunes L, Lue TF. The effect of simulated birth trauma and/or ovariectomy on rodent continence mechanism. Part I: functional and structural change. J Urol 166: 311–317, 2001 [PubMed] [Google Scholar]
- 26.Weber AM, Richter HE. Pelvic organ prolapse. Obstet Gynecol 106: 615–634, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Wieslander CK, Acevedo JF, Drewes PG, Yanagisawa HK, Word RA. Pelvic organ prolapse severity increases with age in fibulin-5 knockout mice. Int Urogynecol J 17: S371–S372, 2006 [Google Scholar]
- 28.Wieslander CK, Marinis SI, Drewes PG, Keller P, Acevedo J, Word RA. Regulation of elastolytic activity in the vagina during pregnancy, parturition, and the puerperium in wild type mice and nonpregnant Fibulin-5 knockout mice. Int Urogynecol J 17: 378–379, 2006 [Google Scholar]
- 29.Woodman PJ, Swift SE, O'Boyle AL, Valley MT, Bland DR, Kahn MA, Schaffer JI. Prevalence of severe pelvic organ prolapse in relation to job description and socioeconomic status: a multicenter cross-sectional study. Int Urogynecol J 17: 340–345, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Yanagisawa H, Davis EC, Starcher BC, Ouchi T, Yanagisawa M, Richardson JA, Olson EN. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature 415: 168–171, 2002 [DOI] [PubMed] [Google Scholar]


