Abstract
Physiological disturbances, including temporary hypoxia, are expected to drive angiogenesis during bone repair. Evidence suggests that the angiogenic ligand vascular endothelial growth factor (VEGF)-A plays an important role in this process. We characterized the expression of two receptors that are essential for mediating VEGF signaling, VEGFR1/Flt-1 and VEGFR2/Flk-1/KDR, in a mouse rib fracture model. Their mRNA and protein levels were assessed in four healing phases, which were characterized histologically as hemorrhage formation on postfracture day (PFD) 1, inflammatory response on PFD 3, initiation of callus development on PFD 7, and the presence of a mature callus on PFD 14. Transcript was detected for VEGFR1 and VEGFR2, as well as VEGF. While mRNA expression of VEGFR1 was monophasic throughout all healing phases, VEGFR2 showed a biphasic profile with significantly increased mRNA expression during callus formation and maturation. Expression of VEGF mRNA was characterized by a more gradual increase during callus formation. The protein level for VEGFR1 was below detection sensitivity during the initial healing phase. It was then restored to a stable level, detectable through the subsequent healing phases. Hence, the VEGFR1 protein levels partially mirrored the transcript expression profile. In comparison, the protein level of VEGFR2 increased gradually during the healing phases and peaked at callus maturation. This correlated well with the transcriptional expression of VEGFR2. Intact bone from age-matched male mice had considerable protein levels of VEGFR1 and VEGF, but no detectable VEGFR2. Together, these findings uncovered expression signatures of the VEGF-VEGFR axis in endochondral bone repair.
Keywords: fracture model, damage, vascularization, angiogenesis
in landmark contributions, Rhinelander and colleagues (43–46) demonstrated greatly increased medullary and periosteal vascular circulation during fracture repair. This augmented circulation was found to be driven by new blood vessel formation. Although these observations were made more than 40 yr ago, the molecular mechanisms governing angiogenesis during bone healing only began to unfold after the identification of vascular endothelial growth factor (VEGF) and its receptors (VEGFRs) as principle pathways of the vascular endothelial cell response to pathological and damage stimuli (15). In 1994, the VEGF-VEGFR axis was first recognized as an angiogenic pathway in osteoblasts and bone tissue by Midy and Plouet (34) and Harada et al. (19), respectively. Later, a number of investigations described an important role for VEGF in experimental models of bone repair after fracture or drill hole injury (11, 14, 25, 28–32, 42, 47, 49, 57, 58). It appeared that the amount of detectable VEGF was subject to temporal changes, thereby suggesting that modulations of VEGF expression might serve as a crucial mechanism by which signaling through the VEGF-VEGFR axis is regulated.
In contrast to VEGF, data on the role of the VEGFRs are sparse. As VEGF elicits cellular signaling through homo- and heterodimerization of VEGFR1 and VEGFR2, it can be speculated that the availability of the receptors is likely to be a second essential regulatory component of the VEGF-VEGFR axis in bone healing. The level of VEGFR1, which displays a high affinity for VEGF, has additional potential to modulate the VEGF-VEGFR axis because of the receptor's capacity for VEGF sequestration. Indeed, Street et al. (53) have demonstrated that augmentation of VEGFR1 levels through administration of exogenous receptor reduced vascularity and bone formation in both fracture and injury models. Related observations were made in models of postnatal bone growth (16) and skeletal gene therapy (41), but little is known about the endogenous mRNA and protein levels of the VEGFRs.
Previous immunohistological studies have identified VEGFRs on osteoblast-linage cells (4, 35, 58), osteoclasts (35, 42), fibroblast-like cells (4), osteocytes (4), and endothelial cells (35, 42, 58) during endochondral or intramembranous bone healing. In addition, the VEGFRs and VEGF have been identified on cultured osteoblasts (9, 10, 19, 47, 48), osteoclasts (36), hypertrophic chondrocytes (5, 8), and endothelial cells (3). Based on these existing data and before the initiation of any comprehensive functional studies, it is crucial to establish the detailed expression pattern of the VEGFRs during endochondral bone repair, which is clinically an important skeletal repair mechanism. For this study, we hypothesized that the two VEGFRs display synchronized expressions, which may recapitulate VEGF expression. Furthermore, we postulated a dynamic behavior of VEGFR expression that manifests in distinct protein expression signatures corresponding to defined bone-healing phases.
We utilized a mouse rib fracture model for the assessment of VEGFR expression at the transcriptional and protein levels during endochondral bone healing. First, we characterized this model histologically to define distinct healing phases. Based on this assessment, we confined our VEGFR analysis to postfracture days (PFDs) 1, 3, 7, and 14, which were characterized by hemorrhage formation, inflammatory response, initiation of callus development, and callus maturation, respectively. For comparison, we analyzed intact ribs from unfractured mice at the same age. Quantitative gene expression and qualitative protein expression analysis using quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and Western blotting, respectively, revealed discrete expression patterns for the VEGFRs at both the transcriptional and protein level, thus rejecting our original hypothesis. As postulated, we discovered distinct protein expression signatures for the VEGF-VEGFR axis during the individual healing phases.
MATERIALS AND METHODS
Mouse rib fracture model.
Male C57BL/6N mice (Taconic, Hudson, NY, or The Jackson Laboratories, Bar Harbor, ME), ranging in age from 10 to 12 wk (24 g ± 2 g body wt), were studied as outlined in Table 1. All procedures were performed in accordance with an animal protocol approved by the Institutional Animal Care and Use Committee. The right 8th rib was subjected to an osteotomy, as first described by Hashimoto et al. (20). Briefly, each mouse was anesthetized with ketamine (50 mg/kg ip) and 2% isoflurane supplemented with 1% oxygen. A longitudinal incision was made along the thoracic spine, and the superficial back muscles were retracted carefully to expose the dorsal aspect of the right ribs. Counting the ribs from caudal to cranial starting with the 13th rib, the 8th rib was identified and fixed mechanically with forceps. A ruler was used to place the osteotomy 1 cm lateral of the spine. The 8th rib was cut with scissors vertically to the axis of the ribs. The skin incision was closed with wound clips; no internal sutures were needed. On designated PFDs, fractured or intact 8th ribs were removed en bloc, together with the neighboring 7th and 9th ribs as a rectangular specimen (∼8 mm horizontal and 6 mm vertical). Subsequent tissue processing is outlined in the following paragraphs. Other tissues harvested from unfractured mice included skeletal muscle from the rib cage, as well as isolated rib bone and bone marrow.
Table 1.
Experimental design
| Day of Tissue Harvest | No. of Animals | Methods |
|---|---|---|
| Postfracture day | ||
| 1 | 8 | Histology and IHC |
| 5 | Protein extraction | |
| 5 | RNA extraction | |
| 3 | 8 | Histology and IHC |
| 5 | Protein extraction | |
| 5 | RNA extraction | |
| 5 | 8 | Histology and IHC |
| 7 | 8 | Histology and IHC |
| 5 | Protein extraction | |
| 5 | RNA extraction | |
| 14 | 8 | Histology and IHC |
| 5 | Protein extraction | |
| 4 | RNA extraction | |
| 21 | 8 | Histology and IHC |
| Intact bone | 4 | Histology and IHC |
| 5 | Protein extraction | |
| 5 | RNA extraction | |
| 1 | Protein extraction for musculoskeletal tissue analysis |
IHC, immunohistochemistry.
Histology and immunohistochemistry.
All dissected rib samples were fixed in 10% formalin at 4°C. For hematoxylin and eosin (H&E) and Alcian blue staining, as well as immunohistochemistry, samples were fixed for 24 h, while short 2- to 4-h fixations were chosen before stainings for tartrate-resistant acid phosphatase and alkaline phosphatase (AlkPhos). Samples were decalcified in 10% EDTA at pH 7.4 and embedded in paraffin. Serial, longitudinal sections of 6- to 7-μm thickness were cut from each sample. Sections were deparaffinized in xylene and rehydrated in an ethanol gradient. Stainings for H&E, Alcian blue, tartrate-resistant acid phosphatase, and AlkPhos were carried out using standard protocols. For pro-collagen-1 immunohistochemistry, an antibody from the Developmental Studies Hybridoma Bank (University of Iowa) was used. All subsequent immunohistochemical incubations and washes were carried out in PBS at room temperature (RT). Antigens were retrieved through sequential treatments in hot citrate buffer (20 min), 2% hydrogen peroxide in PBS (5 min), 2.5% periodic acid (5 min), and 0.02% sodium borohydride (5 min). After a 20-min protein block (Protein Block, Dako, Carpinteria, CA), sections were incubated at RT overnight with primary antibody (1:100). Subsequently, sections were washed three times and incubated with biotinylated secondary anti-mouse IgG (1:200, Vectastain ABC System, Vector Laboratories, Burlingame, CA) for 1 h at RT. After three additional washes, slides were incubated in ABC solution (Vectastain ABC System) for 1 h, followed by another three washes and antibody visualized using diaminobenzidine reagent (Sigma Aldrich, St. Louis, MO). Specificity of the above immunoreactivity was confirmed by staining sections of rat tibial and femoral growth plates for pro-collagen-1 (data not shown). Digital bright-field images from slides were captured using the Mirax Scan system (Carl Zeiss, Thornwood, NY).
RNA extraction and protein preparation.
Immediately after harvesting, the rib specimens were snap frozen on dry ice and stored at −80°C. Before extraction, neighboring ribs and surrounding soft tissues were removed carefully from the frozen ribs using a dissection microscope (Stemi 2000, Carl Zeiss) at ×2 magnification. During dissection, samples were kept on dry ice to prevent thawing. For RNA extractions, a mortar and pestle were cooled in liquid nitrogen. The frozen bone samples were crushed on a bed of 50-μl frozen TRIZOL (Invitrogen, Carlsbad, CA). Then 950 μl of TRIZOL were added, and samples were grinded until fully melted and pulverized. This was followed by a phenol/chloroform purification. To extract RNA from the aqueous phase, the RNeasy Mini kit (Qiagen, Hilden, Germany), including a RNase-Free DNase set, was used according to the manufacturers' instructions. All RNA concentrations were determined using a NanoDrop spectrophotometer (Thermo Fisher Scientific). For quality assessments, the RNA integrity number (RIN) was measured using an Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA), and samples with an RIN < 5.6 were excluded from further analysis.
For the preparation of protein extracts, all bones were individually homogenized in liquid nitrogen with a Bessman Tissue Pulverizer (Spectrum Laboratories, Greensboro, NC). The tissue samples were pooled for each PFD, as well as intact bone. Subsequent protein extraction was carried out at 4°C. Samples were preincubated in RIPA buffer (Santa Cruz Biotechnologies, Santa Cruz, CA) for 15 min, sonicated (Sonicator 3000, Ultrasonic liquid processor, Misonix, Farmingdale, NY) three times for 10 s, and then incubated for an additional 10 min. Soluble protein was recovered by centrifugation (Microcentrifuge 5415R, Eppendorf, Hauppauge, NY) for 10 min at 16,000 relative centrifugal force, and total protein concentration was determined using a colorimetric assay (BCA protein assay kit, Thermo Fisher Scientific, Waltham, MA).
qRT-PCR.
A total of 10 μg RNA (RIN > 5.6) was used for qRT-PCR analysis. qRT-PCR was carried out using the One-Step QuantiTect SYBR Green RT PCR kit (Qiagen) on the Opticon-2 Real-Time PCR Detector System (Bio-Rad Laboratories, Hercules, CA). Targets were amplified through a single cycle of 15 min at 95°C, followed by 40 cycles of 15 s of denaturation at 94°C, 30 s of annealing at 55°C, and 30 s of extension at 72°C. The QuantiTect Primer Assay (Qiagen) was used to amplify GAPDH (Mm_Gapdh_3_SG, no. QT01658692), VEGF-A (Mm_Vegfa_1_SG, no. QT00160769), and VEGFR2 (Mm_Kdr_1_SG, no. QT00097020). Sense (5′-TTC TGT CCT CCA GAA AGT GC-3′) and antisense (5′-ATC CAT TTT AGG GGA AGT CG-3′) primers for VEGFR1 amplification were obtained from a different source (Real Time Primers, Elkins Park, PA). Each qRT-PCR consisted of duplicates for intact bone and PFD 1, 3, 7, and 14 for each gene of interest (VEGFR1, VEGFR2, and VEGF) and GAPDH. Expression of the latter was found to be stable at different time PFDs (Supplemental Fig. S1; the online version of this article contains supplemental data). To correct for variations in amplification efficiency, the standard curve method was employed. Briefly, every qRT-PCR was accompanied by amplifications of cDNA dilutions, which were synthesized from a mix of equal amounts of RNA derived from PFDs and intact bone samples. The cycle threshold values for the dilutions were plotted against concentrations to generate the standard curve. Concentrations for the genes of interest (VEGFR1, VEGFR2, or VEGF) and the housekeeping gene (GAPDH) were expressed relative to the mean value of the intact bones and normalized to their corresponding GAPDH values. Thus gene expression relative to intact bone was measured. Gene expression levels between PFDs were compared using an exact version of the Wilcoxon rank-sum test. Significance was defined as P < 0.05.
Western blot analysis.
For Western blotting, equal amounts of protein were fractionated by SDS-PAGE gel electrophoresis on 10% or 7.5% Tris·HCl gels (Ready Gels, Bio-Rad Laboratories) at 200 V for 40 min, utilizing a Mini PROTEAN Tetra System (Bio-Rad Laboratories). Proteins were transferred to a Protran nitrocellulose membrane (Whatman, Dassel, Germany) using a semi-dry blotting unit (SCIE-PLAS, Southam, UK). After reversible Ponceau S staining (Ponceau S solution, Sigma Aldrich), the membrane was blocked with 5% nonfat dry milk (Santa Cruz Biotechnologies, Santa Cruz, CA) in Tris-buffered saline containing 0.1% Tween-20 (TBST) for 1 h at RT. Primary antibodies for VEGF-A [ab1316 (VG-1) Abcam, Cambridge, MA, 1:1,000 dilution, recognizing protein at ∼25, ∼40, and ∼65 kDa], VEGFR1 [sc-316 (C-17), Santa Cruz Biotechnologies, 1:200 dilution, recognizing protein at ∼100 kDa], and β-actin [A3854 (AC-15), Sigma Aldrich, 1:1,000 dilution, recognizing protein at ∼40 kDa] were incubated in blocking buffer for 1–2 h at RT, while primary antibodies for VEGFR2 [no. 2479 (55B11), Cell Signaling Technology, Danvers, MA, 1:200 dilution, recognizing protein at ∼250 kDa] and GAPDH [no. 2118 (14C10), Cell Signaling Technology, 1:1,000 dilution, recognizing protein at ∼35 kDa] demanded overnight incubations in blocking buffer at 4°C. Before secondary antibody exposure, blots were washed in blocking buffer three times for 5 min at RT. Then, horseradish peroxidase-conjugated goat anti-rabbit or goat anti-mouse antibodies (Santa Cruz Biotechnologies, 1:5,000 dilution) were incubated in blocking buffer for 45 min at RT. Subsequently, membranes were washed three times for 5 min in TBST. Antibody binding was detected using Western Blotting Luminol Reagent (Santa Cruz Biotechnologies). If needed, membranes were stripped with stripping buffer (Restore Western Blot Stripping Buffer, Thermo Scientific, Rockford, IL) for 40 min, followed by a single 10-min wash in PBS, and three 10-min washes in blocking buffer. To confirm antibody specificity, VEGFR1 was detected in A10 cell extracts (Santa Cruz Biotechnologies), while VEGFR2 and VEGF were recognized in protein extracts from healing mouse skin harvested 14 days after injury, as previously described by Wang et al. (60).
RESULTS
Mouse rib osteotomy healing recapitulates endochondral bone repair.
A mouse rib osteotomy was utilized as fracture model. Bone healing after osteotomy occurred in distinct healing phases (Fig. 1, B–O, compared with intact bone, Fig. 1A). On PFD 1, the area between the damaged bones presented morphological features typical of a hematoma formation, including absence of cohesive cellularity, and matrix embedded cells of hematopoietic appearance (Fig. 1B). Distant from the hemorrhage, the periosteum showed an early osteoblastic response (Fig. 1C), which developed into a visibly enlarged periosteum along living cortical bone on PFD 3 (Fig. 1D). Adjacent to the disrupted bones, empty lacunae in the cortices and the absence of cellularity in the bone marrow space were noted (Fig. 1, D and E). With the onset of the inflammatory response between PFD 1 and 3, the hematoma largely resolved, giving way to fibroblast infiltration on PFD 5 (Fig. 1F). Concurrently, cartilage deposition started along the intact cortical bone (Fig. 1G). The vast majority of cartilage-associated chondrocytes acquired a hypertrophic morphology over the following 2 days (Fig. 1, H and I). The onset of callus formation was seen on PFD 7 and was discerned by a dense cellularity of fibroblast-like cells around the damage site that defined the periphery of the developing callus, as well as large areas of chondrocytes within the fracture gap (Fig. 1, H and I). Mature callus was detected on PFD 14. A cartilage bridge was seen between the edges of the bone ends, and a strong osteoblastic response resulted in new bone formation between the distant cortical bones (Fig. 1, J, K, and L). Mononucleated cells of hematopoietic appearance populated the callus, and new vessel formation was pronounced (Fig. 1J). The callus remained at PFD 21 during the onset of bone remodeling (Fig. 1, M–O). Thus hallmarks of endochondral bone repair were observed in this mouse fracture model. Distinct healing phases were found on PFDs 1, 3, 7, and 14 and were characterized by hemorrhage, inflammatory response, callus development, and callus maturation. These healing phases were the focus of subsequent analyses.
Fig. 1.
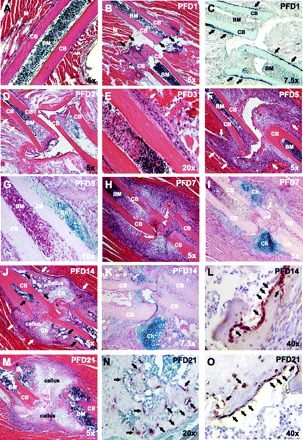
Mouse rib fracture model. B–O: histological and immunohistochemical analysis of the healing time course postfracture day (PFD) 1 to PFD 21. A: hematoxylin and eosin (H&E) staining of an intact rib capturing cortical bone (CB), intramedullar bone marrow space (BM), and surrounding skeletal muscle (M). B: H&E staining after rib osteotomy on PFD 1. Black arrows indicate the disconnected bone ends and the fracture gap. C: higher magnification alkaline phosphatase staining on PFD 1 shows strong osteoblastic activity in the periosteal areas distant from the damage site (black arrows). D: H&E staining on PFD 3. The thickening of the periosteum along CB is visible (white arrows). E: high-magnification view of this area shows that the periosteal response (white arrows) extends along viable into nonviable CB. F: H&E staining on PFD 5 reveals influx of predominantly fibroblast-like cells into the damage site from surrounding tissues (white arrows). G: higher magnification Alcian blue staining on PFD 5 shows chondrocytes (Ch) in areas neighboring the thickened periosteum. H&E (H) and Alcian blue (I) stainings of adjacent sections on PFD 7 prove increasing organization of cells around the damage site and demonstrate extended areas populated by Ch. J: H&E staining on PFD 14 shows that cartilage starts to bridge the bone ends in the enlarged callus. White arrows mark the perimeter of the callus. Black arrows indicate mononucleated cells of hematopoietic appearance populating the callus and pronounced new vessel formation. K: higher magnification view in Alcian blue staining. L: high magnification view of pro-collagen-1 immunohistochemistry on PFD 14 reveals new bone formation in the callus areas close to the disrupted bone ends. M: H&E staining of the remaining callus on PFD 21. N and O: high-magnification views on newly formed bone in the callus area reflecting remodeling by osteoclasts (N, black arrows) and osteoblasts (O, black arrows), as detected by tartrate-resistant acid phosphatase and pro-collagen-1 staining, respectively.
Disparity in mRNA expression of VEGFR during endochondral bone repair.
Expression of VEGFR1, VEGFR2, and VEGF transcript during endochondral bone formation was measured by qRT-PCR on PFDs 1, 3, 7, and 14 (Fig. 2). A constant expression of VEGFR1 was observed (Fig. 2A), and the level of expression was similar to that of intact bone at all PFDs. In clear contrast, VEGFR2 was found to be expressed in a biphasic manner. Gene expression levels were significantly higher on PFDs 7 and 14 compared with PFDs 1 and 3 (Fig. 2B). The measured level of mean increase did not exceed twofold. The VEGF transcript showed a more gradual increase, reflected by significantly higher levels on PFD 14 compared with PFDs 1 and 3 (Fig. 2C).
Fig. 2.
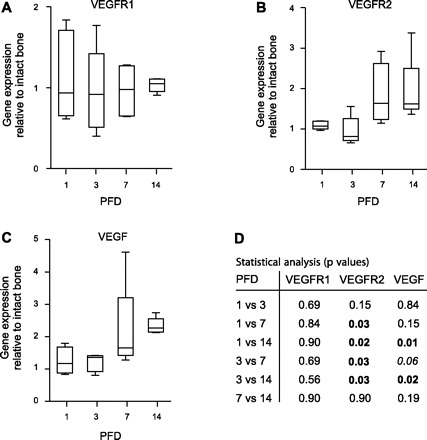
Analysis of vascular endothelial growth factor (VEGF) receptor 1 (VEGFR1), VEGFR2, and VEGF transcript expression. The time course of mRNA expression of VEGFR1 (A), VEGFR2 (B), and VEGF (C) was determined by quantitative RT-PCR, as detailed in materials and methods. Box plots with whiskers represent gene expression relative to intact bone (IB), with error bars indicating median, minimum, and maximum of all individual animals for each PFD. D: the table shows a detailed statistical analysis, presenting P values for PFD comparisons for each target of interest (numbers in bold denote statistical significance; number in italics indicates value that is close to a statistical significant difference).
Differential VEGFR protein levels during endochondral bone repair.
Protein expression of VEGFR1, VEGFR2, and VEGF at PFDs 1, 3, 7, and 14, as well as in intact bone, was assessed by conventional Western blotting (Fig. 3A). No VEGFR1 was detected on PFD 1. Considerable levels of VEGFR1 first appeared on PFD 3. A modest increase in VEGFR1 levels was detected between PFDs 7 and 14. The maximum VEGFR1 expression occurred on PFD 14 and was comparable to the VEGFR1 levels seen in intact bone. Similar to VEGFR1, no VEGFR2 was detected on PFD 1. In sharp contrast to VEGFR1, however, a steady and robust increase in VEGFR2 levels over the following three time points was observed. Notably, VEGFR2 levels in intact bone were below detection sensitivity, suggesting a distinct role for VEGFR2 in endochondral bone healing. Compared with the VEGFRs, VEGF was readily detected on PFD 1. It appeared as three distinct proteins at 25, 40, and 65 kDa, which may correspond to the VEGF isoforms VEGF120, VEGF164, and VEGF188. Between PFDs 1 and 14, 40- and 65-kDa VEGF were seen. The low-molecular-weight VEGF band was observed only on PFD 1 and in intact bone; expression was most pronounced on PFD 1. Interestingly, the high-molecular-weight isoform of VEGF was markedly decreased in intact bone.
Fig. 3.
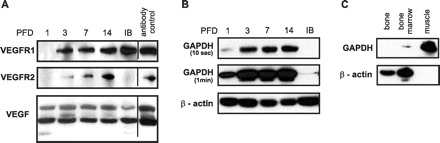
Analysis of protein levels of VEGFR1, VEGFR2, and VEGF. A: temporal protein expression of VEGFR1, VEGFR2, and VEGF was determined by Western blotting, as detailed in materials and methods. Analyses included PFDs 1, 3, 7, and 14, which corresponded to hemorrhage formation, inflammatory response, initiation of callus development, and the presence of a mature callus, respectively, as well as IB. Antibody specificity was verified carefully using appropriate positive controls (antibody control). B: equal amounts of protein extracted from PFDs 1, 3, 7, and 14 and IB were probed against housekeeping proteins GAPDH and β-actin. The Western blot for GAPDH is shown at two exposure times (10 s and 1 min). C: equal amounts of protein extracted from bone, bone marrow, and muscle derived from the 8th rib were probed against GAPDH and β-actin.
Figure 3B shows that the protein expression of the housekeeping genes GAPDH and β-actin, which are often used as added protein loading controls in Western blotting, varied appreciably between PFDs. Similar observations were made for the housekeeping gene α-tubulin (data not shown). Further analysis revealed that levels of GAPDH and β-actin differed in tissues that constitute the bone-healing site (Fig. 3C); hence it is likely that the dynamic tissue composition of the healing site (Fig. 1) resulted in the observed variability in housekeeping protein levels between PFDs. In contrast to the differences in housekeeping protein levels, the corresponding transcripts were expressed at a stable level (Supplemental Fig. S1).
DISCUSSION
In this study, we examined the expression of angiogenic genes, specifically VEGFR1, VEGFR2, and VEGF, during endochondral bone repair at the transcriptional and protein level. A rib osteotomy in male mice was used as a fracture model (37). Figure 1 demonstrates that this fracture model heals predominantly through endochondral bone formation in a fashion observed previously in rodents after stabilized femur or tibia fracture (2, 13, 21). The rib fracture model provokes endochondral healing without the need for invasive fracture fixation, such as an intramedullary pin. Hence the bone marrow compartment remains preserved. This may be of significance due to documentation of VEGFR expression on a variety of bone marrow-derived cells and the close biological interaction between the bone and bone marrow compartments that has been acknowledged previously (7, 12, 40). Our practical experience with the rib fracture model revealed advantages, including simple and time-efficient surgery with limited surgery-related tissue damage other than the osteotomy, fast recovery, no mortality, and consistent PFD morphology among mice. The smaller size of the rib compared with, for example, the femur enabled capturing of healing morphology with a limited number of sections, and it provided sufficient mRNA and protein for molecular analysis. In addition, the superficial nature of the rib osteotomy might be well suited for administration of agents to the damaged bone site or the application of live imaging approaches. As with every fracture model, disadvantages exist. Tissue damage in the osteotomy model is largely restricted to the periosteum, bone, and bone marrow (Fig. 1), and damage to the skeletal muscle is limited. Furthermore, despite the semistabilization of the rib osteotomy through the surrounding muscle, the breathing motion of the animal may influence long-term healing. We collected histological data on mouse rib fracture healing for up to 6 mo and noted that, upon fracture union, the final remodeling phases progressed slower compared with reported femur or tibia fractures (Reumann M, Mayer-Kuckuk P, unpublished data). Thus the use of the presented model for studies beyond 21 PFDs warrants further in-depth validation. In this study, we used the rib fracture model to recover tissue from hemorrhage formation, inflammatory response, initiation of the callus, and mature callus, which corresponded to PFD 1, 3, 7, and 14, respectively.
Four principle observations were made. First, expression of transcript was detected for both VEGFRs and VEGF. While expression of VEGFR1 was monophasic at the four healing phases, VEGFR2 showed a biphasic profile with significant increased expression during callus formation and maturation. Expression of VEGF showed a more gradual increase during callus formation. Sample size precluded statistical analysis using multiple comparisons, which is a limitation of the study. Second, the tissue specimens obtained from the four healing phases showed significant variation in the levels of standard housekeeping proteins. This observation indicates that they are generally of limited value for protein loading control in Western blotting, if a temporal analysis of bone healing tissue is performed. Instead of housekeeping protein loading controls, we recommend for Western blotting at different PFDs the use of a combination of equal protein loading, reversible protein staining after membrane transfer, and multiple replicates. Third, VEGFR1 protein was first detected on PFD 3 and was found to be stable throughout the later healing phases, largely consistent with the transcript expression profile. In contrast, levels of VEGFR2 increased gradually during the healing phases and peaked in the mature callus on PFD 14. Protein levels correlated well with the transcriptional expression of VEGFR2. The VEGFR ligand VEGF was expressed in an isoform-dependent fashion. A limited correlation between VEGF transcript expression and VEGF protein levels was observed. Fourth, intact bone from age-matched male mice expressed high-protein levels of VEGFR1 and VEGF, while VEGFR2 was not highly expressed in intact bone.
This study is the first to analyze the expression of VEGFR1 and VEGFR2, as well as VEGF, during endochondral bone repair at both the transcriptional and protein level. Previous studies in rodents on the expression of the VEGF-VEGFR axis during fracture repair included a total of eight reports on VEGF (11, 25, 28–30, 32, 42, 47) and three investigations that included fragmentary data on the VEGFRs as part of a larger study (30, 32, 42). These studies employed male (25, 28, 30, 47), as well as female (11, 29, 42) rat (11, 28, 42, 47) and mouse (25, 29, 30, 32) models of different genetic backgrounds, in combination with a variety of stabilized (11, 25, 28, 30, 42, 47) and unstabilized (29, 32) fractures, harvested uni- (29, 42, 47) or bilaterally (11, 28, 32) from the femur (11, 28), tibia (25, 29, 30, 32), tibia plus fibula (42), and mandible (47).
Angiogenic gene expression analyses in the studies outlined above were based on the examination of one or more PFDs. Because the temporal initiation of the conserved endochondral bone healing phases adapts to the specific characteristics of a fracture, including species (37), genetic background (33), and type and rigidity of stabilization (6, 17, 27), PFDs are not sufficient as single reference points for the comparison between different fracture repair models without morphological or molecular correlation to a specific endochondral bone-healing phase. This report is the first to provide such a correlation based on detailed histological analysis of the PFDs. This improves the accuracy of angiogenic gene expression measurements during healing and will enable meaningful cross-study comparisons in the future. In addition, it allows us to develop a hypothetical working model of the role of the VEGF-VEGFR axis in endochondral bone fracture repair, as detailed below.
Previous work in rodent endochondral fracture models has resulted in the immunolocalization of VEGF and its receptors on osteoblasts and their precursor (28, 42, 47, 49), osteoclasts (42, 47), endothelial cells (42), proliferating chondrocytes (28), and hypertrophic chondrocytes (49). Because the present investigation provides data on the protein expression levels of VEGFR1 and VEGFR2 as well as VEGF, we have assigned the protein expression levels to the predominant cell types at the four morphologically distinct healing phases that we investigated (Fig. 4). Clearly, the suggested model is speculative and includes substantial assumptions with regard to the cell types present at a particular healing phase, their contribution to the total detectable protein amount, and their individual protein expression levels. However, the model provides a comprehensive molecular-morphological concept and leads to several appealing interpretations.
Fig. 4.
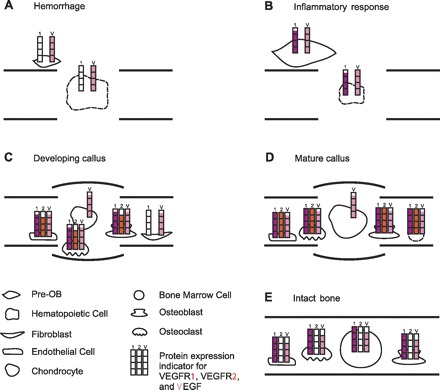
Morphological-molecular correlation model. The cartoon depicts the four healing phases (A–D) and the intact control bone (E), which were investigated in this study. For each healing phase (A: hemorrhage formation on PFD 1; B: inflammatory response on PFD 3; C: initiation of callus development on PFD 7; and D: the presence of a mature callus on PFD 14) and IB, the protein expression signature for VEGF, VEGFR1, and VEGFR2, as determined in this study (Fig. 3), was visualized semiquantitatively using color-coded protein expression indicators for VEGFR1 (red), VEGFR2 (orange), and VEGF (pink). Assignment of the protein expression indicators to the cell types present in a particular healing phase or IB was based on previous immunohistological studies that defined presentation of the components of the VEGF-VEGFR axis on bone-healing cells. These studies included investigations on endochondral (28, 42, 47, 49) and intramembranous (4, 35, 39, 58) bone healing. If the presence of a component of the VEGF-VEGFR axis has previously not been validated for a particular cell type, the corresponding bar of the protein expression indicator was excluded. Hematopoietic and bone marrow cells are marked with dashed lines to indicate that expression of the VEGF-VEGFR axis, specifically during bone healing, has yet to be confirmed. The hematopoietic cell composition during fracture healing is largely unknown. Involvement of neutrophils and macrophages, which express VEGF and VEGFR1, respectively (38, 51), has been suggested (1). Expression of the VEGFR-VEGFR axis on bone marrow cells has been reported (24), but protein assignments to bone marrow cells are limited due to cell heterogeneity. Cell types dominant at a healing phase are shown enlarged. This model allows for relative comparisons between the healing phases, but does not permit quantitative comparisons between VEGFR1, VEGFR2, and VEGF. Pre-OB, osteoblast precursor cell.
The model depicted in Fig. 4 offers the following insights. First, it illustrates that the four distinct healing phases, as well as intact bone, are characterized by distinct protein expression signatures for the VEGF-VEGFR axis. Second, the healing phases we assessed show a gradual increase in expression of the VEGF-VEGFR axis, with high-protein levels in the calluses, which indicates a central role for VEGF signaling in cartilage ossification. In agreement, recent functional studies by Wan et al. (59) and Jacobsen et al. (22) on distraction osteogenesis have shown decreased angiogenesis and bone formation after antibody blockage of the VEGFRs. Furthermore, Jacobsen found that receptor obstruction halted hypertrophic chondrocyte progression (22). However, a direct comparison between fracture healing and distraction osteogenesis is limited due to differences in healing biology (1). Third, the presence of VEGF, including a low-molecular-weight isoform, but absence of VEGFRs on PFD 1 could suggest that VEGF functions as the primer of the subsequent activity of the VEGF-VEGFR axis. Fourth, the lack of VEGFR1 protein expression on PFD 1 was followed by a considerably stable expression of the receptor. This raises the possibility that VEGFR1 is an indispensible component of the VEGF-VEGFR axis in bone healing. In support of this view, gene expression data from distraction osteogenesis showed coexpression of VEGFR1 and VEGFR2 and revealed a relatively stable level of VEGFR1 concomitantly with VEGFR2 presentation similar to our findings, suggesting a functional interplay (22). The functions of VEGFR1 during endochondral bone repair are presumed to be complex. Already established is that VEGFR1 mediates placental growth factor signaling essential for accurate cartilage turnover (32). The initial expression of VEGFR1 during the inflammatory response is likely associated with cell activation/migration (52). As VEGFR1 exhibits a relatively constant protein level from an early time point on, we further speculate that VEGFR1 promotes accurate spatial formation of the vascular network, especially during the initial stages of healing. Supporting this concept is previous in vitro work showing that both, a membrane-bound VEGFR1 truncated by its intracellular tail, as well as soluble VEGFR1, which was not investigated in our study, are sufficient to stimulate endothelial proliferation, but that the soluble isoform is needed for vessel branching (23, 26). However, vascular network formation during endochondral bone repair is likely to be more sophisticated and may include other key regulators, including VEGFR3 (56). Lastly, the most dynamic change in protein expression levels was seen for VEGFR2, including a lack of detectable VEGFR2 protein in intact bone. This observation may suggest considerable specificity of VEGFR2 action during bone repair and is in line with the fact that VEGFR2 is a highly active kinase that stimulates a variety of signaling pathways and broad biological responses in endothelial cells, among others.
At present, the assessment of the clinical relevance of the presented data, including the model described in Fig. 4, is limited. To our knowledge, no data on the expression of the VEGFRs in human fracture tissue are available. However, studies have demonstrated that the human fracture hematoma produces VEGF (18, 54, 55). This observation is consistent with our model. In addition, studies in patients that measured systemic VEGF levels after fracture indicated increased levels of VEGF immediately postfracture, on PFD 7 as well as PFD 14 (50, 61), a time point characterized by vascularization of the cartilage callus (61). Our model suggests elevated VEGF protein expression at similar time points, despite potential limitations in the direct comparison between systemic measurements in humans and healing site-specific assessments in mice. All together, the presented data may help to redefine hypotheses for future investigations addressing 1) the molecular mechanisms that govern individual healing phases, 2) the identity of all cell types participating in bone repair, and 3) their respective expression of the VEGF-VEGFR axis.
GRANTS
This work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) Grant AR055294 (P. Mayer-Kuckuk), the Orthopaedic Trauma Association (P. Mayer-Kuckuk), and NIAMS Core Center Grant AR046121.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Steven B. Doty for invaluable advice on histology; Elyn Riedel for expert statistical analysis; Dr. Miguel Otero for help with the qRT-PCR; Dr. Britt Wildemann for helpful comments on the manuscript; and Orla O'Shea, Rachel Sibson, as well as Janane Nejjar Diouri, for outstanding technical assistance.
REFERENCES
- 1. Ai-Aql ZS, Alagl AS, Graves DT, Gerstenfeld LC, Einhorn TA. Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. J Dent Res 87: 107–118, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bonnarens F, Einhorn TA. Production of a standard closed fracture in laboratory animal bone. J Orthop Res 2: 97–101, 1984 [DOI] [PubMed] [Google Scholar]
- 3. Bouletreau PJ, Warren SM, Spector JA, Peled ZM, Gerrets RP, Greenwald JA, Longaker MT. Hypoxia and VEGF up-regulate BMP-2 mRNA and protein expression in microvascular endothelial cells: implications for fracture healing. Plast Reconstr Surg 109: 2384–2397, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Byun JH, Park BW, Kim JR, Lee JH. Expression of vascular endothelial growth factor and its receptors after mandibular distraction osteogenesis. Int J Oral Maxillofac Surg 36: 338–344, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Carlevaro MF, Cermelli S, Cancedda R, Descalzi Cancedda F. Vascular endothelial growth factor (VEGF) in cartilage neovascularization and chondrocyte differentiation: auto-paracrine role during endochondral bone formation. J Cell Sci 113: 59–69, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Claes L, Augat P, Suger G, Wilke HJ. Influence of size and stability of the osteotomy gap on the success of fracture healing. J Orthop Res 15: 577–584, 1997 [DOI] [PubMed] [Google Scholar]
- 7. Colnot C, Huang S, Helms J. Analyzing the cellular contribution of bone marrow to fracture healing using bone marrow transplantation in mice. Biochem Biophys Res Commun 350: 557–561, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Colnot CI, Helms JA. A molecular analysis of matrix remodeling and angiogenesis during long bone development. Mech Dev 100: 245–250, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Deckers MM, Karperien M, van der Bent C, Yamashita T, Papapoulos SE, Lowik CW. Expression of vascular endothelial growth factors and their receptors during osteoblast differentiation. Endocrinology 141: 1667–1674, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Deckers MM, van Bezooijen RL, van der Horst G, Hoogendam J, van Der Bent C, Papapoulos SE, Lowik CW. Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A. Endocrinology 143: 1545–1553, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Desai BJ, Meyer MH, Porter S, Kellam JF, Meyer RA., Jr The effect of age on gene expression in adult and juvenile rats following femoral fracture. J Orthop Trauma 17: 689–698, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Devine MJ, Mierisch CM, Jang E, Anderson PC, Balian G. Transplanted bone marrow cells localize to fracture callus in a mouse model. J Orthop Res 20: 1232–1239, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Einhorn TA. The cell and molecular biology of fracture healing. Clin Orthop Relat Res 355, Suppl: S7–S21, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Ferguson C, Alpern E, Miclau T, Helms JA. Does adult fracture repair recapitulate embryonic skeletal formation? Mech Dev 87: 57–66, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 9: 669–676, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med 5: 623–628, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Goodship AE, Kenwright J. The influence of induced micromovement upon the healing of experimental tibial fractures. J Bone Joint Surg Br 67: 650–655, 1985 [DOI] [PubMed] [Google Scholar]
- 18. Groothuis A, Duda GN, Wilson CJ, Thompson MS, Hunter MR, Simon P, Bail HJ, van Scherpenzeel KM, Kasper G. Mechanical stimulation of the pro-angiogenic capacity of human fracture haematoma: involvement of VEGF mechano-regulation. Bone 47: 438–444, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Harada S, Nagy JA, Sullivan KA, Thomas KA, Endo N, Rodan GA, Rodan SB. Induction of vascular endothelial growth factor expression by prostaglandin E2 and E1 in osteoblasts. J Clin Invest 93: 2490–2496, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hashimoto J, Yoshikawa H, Takaoka K, Shimizu N, Masuhara K, Tsuda T, Miyamoto S, Ono K. Inhibitory effects of tumor necrosis factor alpha on fracture healing in rats. Bone 10: 453–457, 1989 [DOI] [PubMed] [Google Scholar]
- 21. Hiltunen A, Vuorio E, Aro HT. A standardized experimental fracture in the mouse tibia. J Orthop Res 11: 305–312, 1993 [DOI] [PubMed] [Google Scholar]
- 22. Jacobsen KA, Al-Aql ZS, Wan C, Fitch JL, Stapleton SN, Mason ZD, Cole RM, Gilbert SR, Clemens TL, Morgan EF, Einhorn TA, Gerstenfeld LC. Bone formation during distraction osteogenesis is dependent on both VEGFR1 and VEGFR2 signaling. J Bone Miner Res 23: 596–609, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kappas NC, Zeng G, Chappell JC, Kearney JB, Hazarika S, Kallianos KG, Patterson C, Annex BH, Bautch VL. The VEGF receptor Flt-1 spatially modulates Flk-1 signaling and blood vessel branching. J Cell Biol 181: 847–858, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Katoh O, Tauchi H, Kawaishi K, Kimura A, Satow Y. Expression of the vascular endothelial growth factor (VEGF) receptor gene, KDR, in hematopoietic cells and inhibitory effect of VEGF on apoptotic cell death caused by ionizing radiation. Cancer Res 55: 5687–5692, 1995 [PubMed] [Google Scholar]
- 25. Kayal RA, Tsatsas D, Bauer MA, Allen B, Al-Sebaei MO, Kakar S, Leone CW, Morgan EF, Gerstenfeld LC, Einhorn TA, Graves DT. Diminished bone formation during diabetic fracture healing is related to the premature resorption of cartilage associated with increased osteoclast activity. J Bone Miner Res 22: 560–568, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kearney JB, Kappas NC, Ellerstrom C, DiPaola FW, Bautch VL. The VEGF receptor flt-1 (VEGFR-1) is a positive modulator of vascular sprout formation and branching morphogenesis. Blood 103: 4527–4535, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Kenwright J, Goodship AE. Controlled mechanical stimulation in the treatment of tibial fractures. Clin Orthop Relat Res 241: 36–47, 1989 [PubMed] [Google Scholar]
- 28. Komatsu DE, Hadjiargyrou M. Activation of the transcription factor HIF-1 and its target genes, VEGF, HO-1, iNOS, during fracture repair. Bone 34: 680–688, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Lee DY, Cho TJ, Kim JA, Lee HR, Yoo WJ, Chung CY, Choi IH. Mobilization of endothelial progenitor cells in fracture healing and distraction osteogenesis. Bone 42: 932–941, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Lehmann W, Edgar CM, Wang K, Cho TJ, Barnes GL, Kakar S, Graves DT, Rueger JM, Gerstenfeld LC, Einhorn TA. Tumor necrosis factor alpha (TNF-alpha) coordinately regulates the expression of specific matrix metalloproteinases (MMPs) and angiogenic factors during fracture healing. Bone 36: 300–310, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Lienau J, Schmidt-Bleek K, Peters A, Haschke F, Duda GN, Perka C, Bail HJ, Schutze N, Jakob F, Schell H. Differential regulation of blood vessel formation between standard and delayed bone healing. J Orthop Res 27: 1133–1140, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Maes C, Coenegrachts L, Stockmans I, Daci E, Luttun A, Petryk A, Gopalakrishnan R, Moermans K, Smets N, Verfaillie CM, Carmeliet P, Bouillon R, Carmeliet G. Placental growth factor mediates mesenchymal cell development, cartilage turnover, and bone remodeling during fracture repair. J Clin Invest 116: 1230–1242, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Manigrasso MB, O'Connor JP. Comparison of fracture healing among different inbred mouse strains. Calcif Tissue Int 82: 465–474, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Midy V, Plouet J. Vasculotropin/vascular endothelial growth factor induces differentiation in cultured osteoblasts. Biochem Biophys Res Commun 199: 380–386, 1994 [DOI] [PubMed] [Google Scholar]
- 35. Mori S, Akagi M, Kikuyama A, Yasuda Y, Hamanishi C. Axial shortening during distraction osteogenesis leads to enhanced bone formation in a rabbit model through the HIF-1alpha/vascular endothelial growth factor system. J Orthop Res 24: 653–663, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Niida S, Kaku M, Amano H, Yoshida H, Kataoka H, Nishikawa S, Tanne K, Maeda N, Kodama H. Vascular endothelial growth factor can substitute for macrophage colony-stimulating factor in the support of osteoclastic bone resorption. J Exp Med 190: 293–298, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nunamaker DM. Experimental models of fracture repair. Clin Orthop Relat Res 355, Suppl: S56–S65, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signaling–in control of vascular function. Nat Rev Mol Cell Biol 7: 359–371, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Pacicca DM, Patel N, Lee C, Salisbury K, Lehmann W, Carvalho R, Gerstenfeld LC, Einhorn TA. Expression of angiogenic factors during distraction osteogenesis. Bone 33: 889–898, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Paley D, Young MC, Wiley AM, Fornasier VL, Jackson RW. Percutaneous bone marrow grafting of fractures and bony defects. An experimental study in rabbits. Clin Orthop Relat Res 208: 300–312, 1986 [PubMed] [Google Scholar]
- 41. Peng H, Usas A, Olshanski A, Ho AM, Gearhart B, Cooper GM, Huard J. VEGF improves, whereas sFlt1 inhibits, BMP2-induced bone formation and bone healing through modulation of angiogenesis. J Bone Miner Res 20: 2017–2027, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Pufe T, Wildemann B, Petersen W, Mentlein R, Raschke M, Schmidmaier G. Quantitative measurement of the splice variants 120 and 164 of the angiogenic peptide vascular endothelial growth factor in the time flow of fracture healing: a study in the rat. Cell Tissue Res 309: 387–392, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Rhinelander FW. The normal microcirculation of diaphyseal cortex and its response to fracture. J Bone Joint Surg Am 50: 784–800, 1968 [DOI] [PubMed] [Google Scholar]
- 44. Rhinelander FW. Tibial blood supply in relation to fracture healing. Clin Orthop Relat Res 105: 34–81, 1974 [PubMed] [Google Scholar]
- 45. Rhinelander FW, Baragry R. Microangiography in bone healing. I. Undisplaced closed fractures. J Bone Joint Surg Am 44-A: 1273–1298, 1962 [PubMed] [Google Scholar]
- 46. Rhinelander FW, Phillips RS, Steel WM, Beer JC. Microangiography in bone healing. II. Displaced closed fractures. J Bone Joint Surg Am 50: 643–662, 1968 [DOI] [PubMed] [Google Scholar]
- 47. Saadeh PB, Mehrara BJ, Steinbrech DS, Dudziak ME, Greenwald JA, Luchs JS, Spector JA, Ueno H, Gittes GK, Longaker MT. Transforming growth factor-beta1 modulates the expression of vascular endothelial growth factor by osteoblasts. Am J Physiol Cell Physiol 277: C628–C637, 1999 [DOI] [PubMed] [Google Scholar]
- 48. Saadeh PB, Mehrara BJ, Steinbrech DS, Spector JA, Greenwald JA, Chin GS, Ueno H, Gittes GK, Longaker MT. Mechanisms of fibroblast growth factor-2 modulation of vascular endothelial growth factor expression by osteoblastic cells. Endocrinology 141: 2075–2083, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Saijo M, Kitazawa R, Nakajima M, Kurosaka M, Maeda S, Kitazawa S. Heparanase mRNA expression during fracture repair in mice. Histochem Cell Biol 120: 493–503, 2003 [DOI] [PubMed] [Google Scholar]
- 50. Sarahrudi K, Thomas A, Braunsteiner T, Wolf H, Vecsei V, Aharinejad S. VEGF serum concentrations in patients with long bone fractures: a comparison between impaired and normal fracture healing. J Orthop Res 27: 1293–1297, 2009 [DOI] [PubMed] [Google Scholar]
- 51. Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA. The neutrophil as a cellular source of chemokines. Immunol Rev 177: 195–203, 2000 [DOI] [PubMed] [Google Scholar]
- 52. Shibuya M. Vascular endothelial growth factor receptor-1 (VEGFR-1/Flt-1): a dual regulator for angiogenesis. Angiogenesis 9: 225–230; discussion 231, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Street J, Bao M, deGuzman L, Bunting S, Peale FV, Jr, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL, Daugherty A, van Bruggen N, Redmond HP, Carano RA, Filvaroff EH. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A 99: 9656–9661, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Street J, Winter D, Wang JH, Wakai A, McGuinness A, Redmond HP. Is human fracture hematoma inherently angiogenic? Clin Orthop Relat Res 378: 224–237, 2000 [DOI] [PubMed] [Google Scholar]
- 55. Street JT, Wang JH, Wu QD, Wakai A, McGuinness A, Redmond HP. The angiogenic response to skeletal injury is preserved in the elderly. J Orthop Res 19: 1057–1066, 2001 [DOI] [PubMed] [Google Scholar]
- 56. Tammela T, Zarkada G, Wallgard E, Murtomaki A, Suchting S, Wirzenius M, Waltari M, Hellstrom M, Schomber T, Peltonen R, Freitas C, Duarte A, Isoniemi H, Laakkonen P, Christofori G, Yla-Herttuala S, Shibuya M, Pytowski B, Eichmann A, Betsholtz C, Alitalo K. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature 454: 656–660, 2008 [DOI] [PubMed] [Google Scholar]
- 57. Tatsuyama K, Maezawa Y, Baba H, Imamura Y, Fukuda M. Expression of various growth factors for cell proliferation and cytodifferentiation during fracture repair of bone. Eur J Histochem 44: 269–278, 2000 [PubMed] [Google Scholar]
- 58. Uchida S, Sakai A, Kudo H, Otomo H, Watanuki M, Tanaka M, Nagashima M, Nakamura T. Vascular endothelial growth factor is expressed along with its receptors during the healing process of bone and bone marrow after drill-hole injury in rats. Bone 32: 491–501, 2003 [DOI] [PubMed] [Google Scholar]
- 59. Wan C, Gilbert SR, Wang Y, Cao X, Shen X, Ramaswamy G, Jacobsen KA, Alaql ZS, Eberhardt AW, Gerstenfeld LC, Einhorn TA, Deng L, Clemens TL. Activation of the hypoxia-inducible factor-1alpha pathway accelerates bone regeneration. Proc Natl Acad Sci U S A 105: 686–691, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang Y, Iyer M, Annala A, Wu L, Carey M, Gambhir SS. Noninvasive indirect imaging of vascular endothelial growth factor gene expression using bioluminescence imaging in living transgenic mice. Physiol Genomics 24: 173–180, 2006 [DOI] [PubMed] [Google Scholar]
- 61. Weiss S, Zimmermann G, Pufe T, Varoga D, Henle P. The systemic angiogenic response during bone healing. Arch Orthop Trauma Surg 129: 989–997, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


