Abstract
To keep pace with progressing urbanization organisms must cope with extensive habitat change. Anthropogenic light and noise have modified differences between day and night, and may thereby interfere with circadian clocks. Urbanized species, such as birds, are known to advance their activity to early morning and night hours. We hypothesized that such modified activity patterns are reflected by properties of the endogenous circadian clock. Using automatic radio-telemetry, we tested this idea by comparing activity patterns of free-living forest and city European blackbirds (Turdus merula). We then recaptured the same individuals and recorded their activity under constant conditions. City birds started their activity earlier and had faster but less robust circadian oscillation of locomotor activity than forest conspecifics. Circadian period length predicted start of activity in the field, and this relationship was mainly explained by fast-paced and early-rising city birds. Although based on only two populations, our findings point to links between city life, chronotype and circadian phenotype in songbirds, and potentially in other organisms that colonize urban habitats, and highlight that urban environments can significantly modify biologically important rhythms in wild organisms.
Keywords: urbanization, circadian rhythms, birds, radio-telemetry, chronotype, light at night
1. Introduction
We live in the urban millennium, when global change and rapid expansion of cities hasten the loss of animal and plant species [1]. Yet despite dramatic alteration of the environment by urban sprawl, many wild organisms have succeeded to colonize man-made habitats. Their surprising ability to thrive under novel environmental conditions has sparked increasing biological interest in urbanization [2]. The vast majority of studies on effects of urbanization on wild species has focused on the response to spatial habitat change, but urbanization also modifies temporal niches. For example, artificial light at night has been suggested to alter temporal activity patterns and physiology in different species among reptiles, birds and mammals [3–6]. Moreover, noise in urban areas has been suggested to promote nocturnal activity in diurnal species, at least in songbirds [7].
Daily cycles of behaviour and body function are ubiquitous phenomena and are thought to be evolutionary adaptations to the Earth's 24 h rotation [8]. The daily alternation of activity and rest is not a mere response but an entrainment of the endogenous circadian clock to cyclic environmental signals. Organisms benefit from the synchronization of the circadian clock with environmental cycles, mainly the light–dark cycle, for maximizing their fitness [9]. In humans, the shift to the 24 h society typical of urban areas in developed countries can disrupt synchronization between circadian physiological functions and daily activities such as sleep and food intake, with serious health implications [10]. Such a change in human lifestyle also affects other species through shared urban habitats, but it is currently unclear whether endogenous circadian clocks are implicated in responses to urbanization.
To address temporal implications of city life we compared common songbirds (European blackbirds, Turdus merula) from a city and forest population. Blackbirds have long been associated with humans in Europe, and are an efficient model species because they thrive in cities, where they display nocturnal behaviour, especially during early morning hours [11]. Our main objective was to examine variation in circadian traits between the two populations, and to relate this variation, if present, to variation in chronotype in the wild. Chronotype is defined as the timing of an individual relative to an external synchronizing cue (Zeitgeber), and results from interactions between the endogenous circadian clock and environmental factors [12]. Assignment of a particular chronotype to an individual requires within-individual consistency in timing. Across a population, variation in chronotype correlates with circadian properties in many species, including humans [13]. Early chronotypes typically have shorter circadian period length (tau, τ) than late chronotypes [14]. In view of the nightly and early morning activities exhibited by European blackbirds, we hypothesized that individuals from urban areas had faster free-running circadian clocks than their forest conspecifics. We furthermore proposed that in urban habitats, where Zeitgeber information is compromised by anthropogenic light and noise, organisms may fare better with relatively labile circadian clocks.
2. Material and methods
(a). Activity recording of free-living animals
During the breeding season of 2010, we captured 12 adult male European blackbirds (forest, n = 6; city, n = 6) in various locations in the city of Munich (Germany, 48°07′ N, 11°34′ E; 518 m.a.s.l.) and in a rural forest near the village of Raisting (47°53′ N, 11°04′ E, 553 m.a.s.l.), 40 km southwest of Munich (see the electronic supplementary material, figure S1). Birds were equipped with 2.2-gram pulse radio-transmitters (Sparrow Systems, IL) and immediately released at their capture site. Daily activity was recorded using automated recording units (ARUs, Sparrow Systems). The ARU was placed close to the territory of a bird and connected to an H-antenna (Sparrow Systems). The unit was programmed to scan every minute for the corresponding frequency of each bird and to record the signal strength of the radio-transmitter pulse. We used the data from birds that spent at least a week within the range of the antennas, and thus provided continuous and high-quality recordings (mean ± s.d. = 21 ± 8 days). We used the change in signal strength over time to infer the time of start of activity, as previously described [15]. Briefly, the time of start and end of activity was estimated to the minute by comparing consecutive data points and scoring the minute when a change in signal strength was greater than 4 db. We used start of activity as a proxy for chronotype because of evidence that the morning hours are of particular ecological and evolutionary relevance for songbirds [4,16–18], but we also examined end of activity and total duration of daily activity, and related timing to day-length information from astronomical charts. For every day of recording, we standardized start, end and duration of activity by subtracting each value from the start of morning civil twilight, end of evening civil twilight, and duration of daylight for Raisting and Munich, as available at the United States Naval Observatory database (www.usno.navy.mil/USNO).
(b). Recording of circadian rhythms in captivity
Tagged birds were recaptured within the same breeding season between the end of April and end of June 2010, and were transported in cloth cages to our facilities in Andechs, Germany (47°58′ N, 11°11′ E, 690 m.a.s.l.). In addition, we captured additional adult male birds for which activity was not recorded in the wild (forest, n = 8; city, n = 8). In total, we captured 14 birds from each population. Birds were placed in individual cages (width × height × length: 45 × 70 × 80 cm), each of them located in a light-proofed, sound-insulated chamber. Once transported to our facilities, birds were immediately placed under constant dim light (LLdim; 0.3 lux, tungsten warm bulb, Osram, Germany). Food (mealworms and mixed dry insects, Aleckwa, Germany) was provided at random times during day and night to prevent birds from synchronizing to feeding cycles. Drinking water was available ad libitum. Activity was recorded in LLdim for an average of 10 days per bird (10 ± 2 days). Afterwards, birds were put under light/dark cycles (LD; 500 lux, cool white fluorescent bulb, Osram, Germany), which followed the natural seasonal variation of photoperiod in Andechs. Locomotor activity was recorded over the entire duration of the experiment through a passive infrared sensor mounted on each cage (Intellisense, CK Systems, Eindhoven, The Netherlands). Movements were counted and stored as 2 min bins into a computer. All the experimental procedures were carried out in accordance with the guidelines of the relevant German agencies.
(c). Analysis of circadian traits
We employed methods of digital signal analysis to extract relevant circadian information from the activity recorded in constant dim light conditions. These techniques are reliable and have been used extensively [19,20]. To run the analysis, we used the libraries written by Harold Dowse and described in [21]. We removed the first day of activity recording in all analyses to limit possible bias in the data owing to birds habituating to the new environment.
First, we used autocorrelation analysis to assess the strength of the periodicity in LLdim as described by Levine et al. [19]. Briefly, we calculated the coefficient of temporal autocorrelation at each time lag and plotted it over time. We then assessed the strength of the rhythm as the height of the third peak in the autocorrelation plot, a measure that has been named rhythmicity index [19]. The third peak was chosen because its amplitude, relative to that of the first peak, is considered a reliable measure for the strength of the rhythmicity [19]. To assess whether a bird was rhythmic at all, we calculated the 95 per cent acceptance region for the null hypothesis of no correlation. If the third peak in the autocorrelation plot exceeded the acceptance region, we defined this bird as rhythmic and used its data in maximum entropy spectral analysis (MESA) to estimate the circadian period length. MESA works by fitting an autoregressive model to the time-series in order to compute a spectrum. We referred to [22] for details and validation tests of this technique. Graphic examples for two representative city and forest birds are shown in the electronic supplementary material, figure S4. After release from LLdim into LD, the birds showed an unexpected pattern of daily activity: most birds shifted their active phase into the dark period (see the electronic supplementary material, figure S5). This behaviour is hard to interpret and could, for example, represent nocturnal restlessness that typically occurs during migration or following captivity [23]. Because the biological interpretation would have been unclear, we did not include this aspect of the data into analyses.
(d). Statistical analysis
All statistical tests were computed with R v. 2.13.0 software [24]. We applied a significance level of α = 0.05.
To test for differences in the start, end and duration of daily activity between forest and city birds, we used linear mixed models (LMMs) from the R package nlme [25]. In all models, we included origin and τ as fixed factors and date as covariate. We modelled random intercept dependent on individual to correct for repeated measures. Heteroscedasticity was present in all models since variance in the residuals was higher in city than forest birds. We corrected for this by including weights on the residuals by origin. We removed one outlier from the urban population in the analysis of end of activity, because its mean exceeded two standard deviations of the mean of all other individuals pooled together. Within-individual variation and between-individual variation in start, end and duration of daily activity in the field were extracted from the model outputs and used to calculate repeatability (i.e. the proportion of phenotypic variance explained by individual [26]) following Lessells & Boag [27]. The difference between city and forest birds in the between-individual variation in start of daily activity was assessed by Levene's test from the R package lawstat (http://cran.r-project.org/package=lawstat).
Circadian traits were tested by independent two-sample test. We used a t-test to compare the rhythmicity index between forest and city birds. To compare τ in the two populations, we switched to a non-parametric Mann–Whitney test because assumptions of normality were not met. Furthermore, since the photoperiod experienced by an animal prior to recording under constant conditions can affect its circadian period length [28], we tested for this potential effect with a linear model (LM) including τ as a response variable and the date of recapture from the field (activity recording under LLdim started on the same day) as explanatory variable.
3. Results
City birds started their activity on average 29 ± 17 minutes (mean ± s.d.) before civil twilight, while forest birds differed by synchronizing activity to the onset of twilight (mean onset ± s.d. = 0 ± 3 min, LMM, d.f. = 9, p = 0.0093; figure 1 and table 1). Between-individual variation in start of activity was higher in city than in forest blackbirds (Levene's test: test statistic = 7.44, p < 0.001; electronic supplementary material, table S1). Within-individual variation was also higher in city than in forest individuals (variance forest = 80.2, variance city = 351.04; electronic supplementary material, table S1). Since the difference between city and forest birds in both between- and within-individual variation was very similar, repeatability estimates for the two populations were very close (forest R = 0.51, city R = 0.50; electronic supplementary material, table S1). At the end of the day, city birds extended their activity on average 6 ± 9 min later into the evening than forest birds, although the difference was not significant (mean ± s.d., LMM, d.f. = 9, p = 0.52; electronic supplementary material, figure S2; table 1). Therefore, city birds were active for longer than forest birds, on a daily average by 40 ± 14 min per day (mean ± s.d., LMM, d.f. = 9, p = 0.0043; electronic supplementary material, figure S3; table 1). All investigated aspects of timing in the wild showed high repeatability (see the electronic supplementary material, table S1), indicating that activity patterns were consistent properties of individuals within the study.
Figure 1.
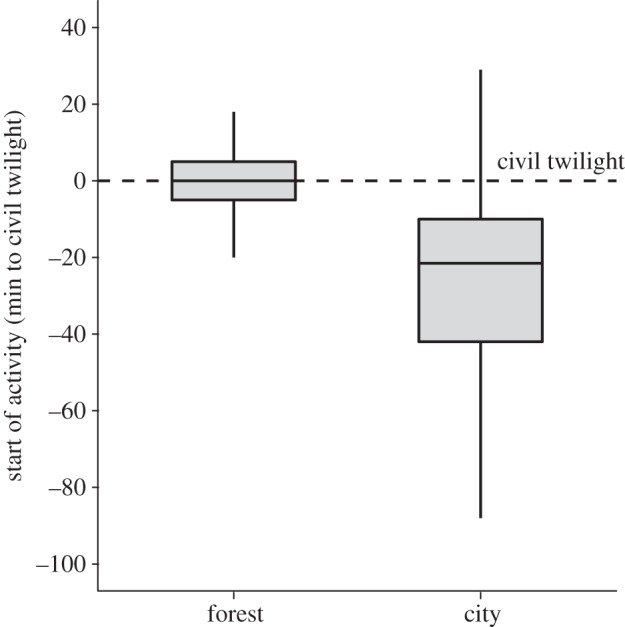
Differences in daily start of activity between forest and city European blackbirds in the wild. Daily activity was continuously recorded on free-living forest (n = 6) and city (n = 6) birds by an automated telemetry system. Start of activity was standardized to the onset of civil twilight (dashed horizontal line) to correct for daily changes in photoperiod. Values indicate minutes before (negative values) or after (positive values) the morning onset of civil twilight. Box plots represent, from bottom to top: one standard deviation (s.d.) below the mean, lower quartile, median, upper quartile and one s.d. above the mean.
Table 1.
Differences between city and forest birds in start, end and duration of daily activity in the field. Each response variable was standardized, respectively, on start of morning twilight, end of evening twilight and daylight hours to correct for the seasonal change in day length. Models are LMMs with date, origin and τ as fixed effects. Subjects were used as random factor to correct for repeated measurements.
| trait | factors | estimates | s.e.m. | d.f. | t-value | p-value |
|---|---|---|---|---|---|---|
| start of activity | intercept | −115.06 | 13.86 | 243 | −8.30 | <0.001 |
| date | −0.42 | 0.08 | 243 | 5.94 | <0.001 | |
| τ | 9.91 | 5.11 | 9 | 4.10 | 0.0027 | |
| origin | 16.83 | 2.42 | 9 | 3.29 | 0.0093 | |
| end of activity | intercept | 37.99 | 11.25 | 226 | 3.38 | <0.001 |
| date | −0.49 | 0.08 | 226 | −6.35 | <0.001 | |
| τ | 1.37 | 5.69 | 9 | 0.24 | 0.8100 | |
| origin | 6.58 | 9.88 | 9 | 0.67 | 0.5200 | |
| duration of diurnal activity | intercept | 87.84 | 27.80 | 220 | 3.16 | 0.0018 |
| date | −0.97 | 0.12 | 220 | −7.80 | <0.001 | |
| τ | 1.99 | 5.01 | 9 | 0.40 | 0.6900 | |
| origin | 40.19 | 10.61 | 9 | 3.79 | 0.0043 |
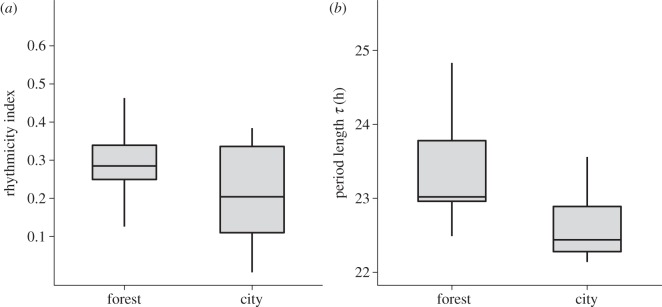
City birds showed clearly reduced circadian rhythmicity compared with forest birds, as measured by the rhythmicity index (t-test: t = 2.08, d.f. = 25.67, p = 0.048, figure 2a), supporting the idea of more labile circadian clocks in the city. For analyses of period length, the birds that were found to be arrhythmic (n = 0 for forest; n = 5 for city) were excluded because periodicity could not be determined. In the remaining city birds circadian period length was on average shorter by 50 min than in forest birds (mean ± s.d., forest birds = 23 h 45 min ± 37 min, city birds = 22 h 55 min ± 35 min; Mann–Whitney test: U = 26, p = 0.021; figure 2b). The start date of recording under LLdim (as a proxy for the day length experienced by the birds prior to constant conditions) did not affect τ (LM: t = 0.66, p = 0.52; electronic supplementary material, figure S5).
Figure 2.
Differences in rhythmicity index and circadian period length between forest and city blackbirds. Forest (n = 14) and city (n = 14) birds were held in constant dim light of 0.3 lux and their activity was continuously recorded for at least a week (10 ± 2 days). From these recordings, we estimated (a) robustness of the rhythmicity and (b) period length τ. Since five city birds showed a statistically non-significant rhythmicity index, they were excluded from the analysis of period length. For box plot specification see figure 1.
We found a positive linear relationship between τ and onset of activity. Indeed, fast-paced individuals with shorter free-running periodicities were the birds waking up earlier in the wild (LMM, d.f. = 9, p = 0.0027; figure 3 and table 1). However, this relationship was absent in birds with slower clocks, in which start of activity was closely linked to onset of civil twilight. Thus, because blackbirds from urban and rural sites differed in period length, the relationship between τ and chronotype was influenced by site (LMM, d.f. = 9, p = 0.009; figure 3 and table 1). In the fast-paced city birds, early rising was related to the pace of the circadian clock, whereas forest birds showed variation in τ but consistently synchronized their morning activity to civil twilight (figures 1, 3, 4). In contrast, neither end nor total duration of daily activity were related to τ (LMMs: end of activity: d.f. = 9, p = 0.81; duration of activity: d.f. = 9, p = 0.69; table 1). In all these models, date was included as a covariate, so that we considered effects of τ and origin to be independent of date.
Figure 3.
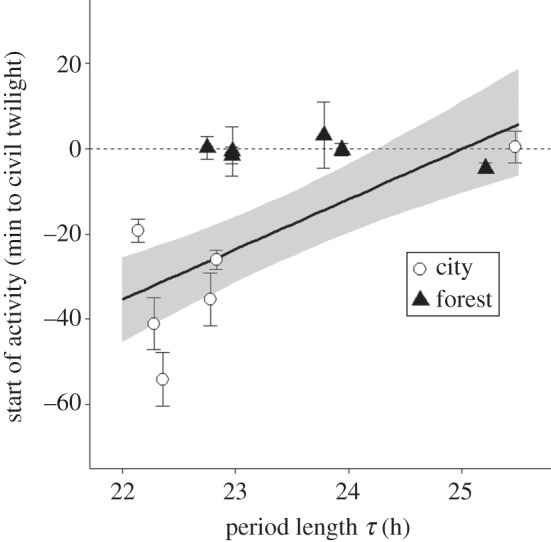
Relationship between τ and chronotype. Circadian period length predicted the time of start of morning activity in the field, relative to morning twilight. Most of the relationship is explained by city birds (white dots, n = 6), while forest birds (black triangles, n = 6) seem to be closely synchronized with dawn. Values indicate minutes before (negative values) or after (positive values) the onset of morning civil twilight (dashed horizontal line). Error bars represent mean of raw data ± s.e.m. for each individual.
Figure 4.
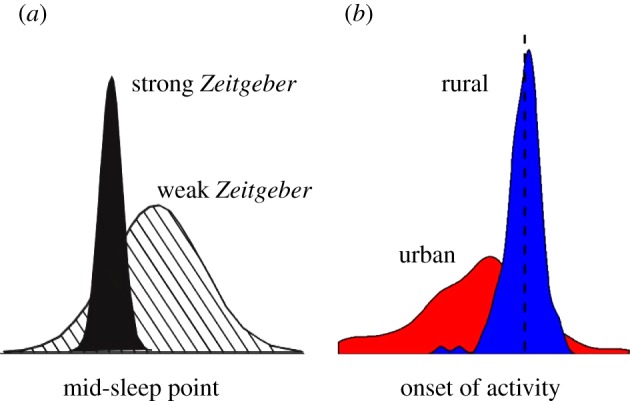
Chronotype distribution in (a) humans and (b) European blackbirds in relation to Zeitgeber strength and city or forest environmental conditions. (a) Analysis of empirical data from human beings and application of classical oscillator theory led Roenneberg et al. [12] to postulate that chronotype distribution should depend on the Zeitgeber conditions a population experiences. Under strong Zeitgebers chronotypes should be less variable and more closely synchronized than chronotypes under weak Zeitgebers (graph adapted from original manuscript). x-axis shows time of mid sleep point, y-axis shows the relative frequency of chronotype. (b) Chronotype distributions for free-roaming city and forest blackbirds based on recordings by an automated telemetry system resemble those theorized by Roenneberg et al. [12]. City birds showed much higher variation and a lower peak in frequency of timing of start of activity than forest birds, which in turn appeared to be highly synchronized to the onset of morning twilight (dashed vertical line). x-axis shows time of start of activity, y-axis shows relative frequency of chronotype. (Online version in colour.)
4. Discussion
The causes and consequences of variation in circadian period length between natural populations of the same species are crucial for the understanding of how organisms adapt to changes in their temporal environment [29,30]. Here, we show that two populations of city and forest European blackbirds differ in both chronotype and circadian traits. The shift towards shorter circadian period length in the urban population is mirrored by behaviour in the wild, because city birds started their activity earlier than forest birds. Furthermore, chronotype of city birds was correlated with the endogenous periodicity of their circadian clock under constant conditions, whereas the timing of onset and end of daily activity in forest birds was more closely related to civil twilight. Overall, we conclude that urbanization may not only modify daily organization of activities, but may also alter endogenous circadian rhythmicity and its interaction with the Zeitgeber in wild animals. However, our conclusion is based on only one forest and one city population. Therefore, in order to consolidate an effect of urbanization on circadian timing, as distinguished from other processes, data from additional rural and urban sites will be necessary.
Given our current data, we can merely speculate about the origin of these observed differences in chronotype and circadian traits between city and forest blackbirds. The causes could be environmentally induced and/or intrinsic. For instance, city and forest habitats differ in several environmental characteristics that could influence temporal patterns of activity, such as daily noise patterns and artificial light at night. In particular, recent work, including our own studies on seasonal physiology of urban and forest blackbirds [6], suggested that the detection of day length might be altered by the artificial illumination at night [4,6]. In our study, city birds could have perceived the ‘lights on’ signal earlier than forest birds, which may have advanced chronotype (i.e. onset of activity in the field) [6] and promoted after-effects on τ [28]. The 24 h light profiles for our two study sites are shown in the electronic supplementary material, figure S6. Our measurements suggest that patterns of light intensity differed between the urban and rural habitat, but more detailed data on the individual exposure to light would be necessary to directly link light–dark cycles to chronotype and circadian rhythms. If altered day length perception seems a plausible reason to explain the difference in chronotypes between the two populations, circadian period lengths were surprisingly robust with respect to after-effects of prior photoperiodic condition, since τ was independent of date (see the electronic supplementary material, figure S5). Another process known to affect both the duration of daily activity and τ is seasonal change in reproductive physiology [31,32]. In order to minimize this potentially confounding effect, we sampled blackbirds during May and June when both study populations exhibit their peak breeding stage during the reproductive season [33].
A fascinating hypothesis is that these observed differences are the result of micro-evolutionary changes to the new life in cities of this originally forest-dwelling species. Possible evolutionary implications of a link between circadian traits and chronotypes are suggested by two recent studies and by general evidence. For songbirds, performance during early morning hours is particularly important and may promote selection for early start of activity. Early birds increased reproductive success by greater extra-pair copulation opportunities in several studies [4,16] and were more efficient at territorial defence (e.g. by early dawn song [17,18]). Kempenaers et al. [4] have shown that male blue tits (Cyanistes caeruleus), which occupied territories near streetlamps, sang earlier in the morning, even before civil twilight. These males had also the highest rate of extra-pair paternity in the population, suggesting that early awakening as a response to artificial light at night could confer fitness benefits [4]. Hence, one potential scenario may be conceivable: colonization of urban areas may increase selection for early chronotypes, which in turn would ease the exploitation of new temporal niches into the night. To the extent that chronotype depends on circadian properties, selection should also affect the circadian clock. A further recent study pointed directly to reproductive benefits of fast circadian clocks. In wild-derived great tits (Parus major), chicks from extra-pair fathers had shorter circadian period length than the within-pair offspring. The same study also showed that circadian period length was highly heritable [34]. High heritability of a trait is one of the prerequisites for natural selection to act on this trait. Thus, assuming similar heritability of timing in blackbirds, selection in favour of early chronotypes in urban environment could lead to micro-evolutionary adjustments of both chronotype and underlying circadian traits.
Although the hypothesis of natural selection favouring faster clocks and early risers in urban habitats seems conceivable and exciting, results from our wild-caught subjects do not allow distinguishing between genetic difference, developmental plasticity or effects of previous exposure to a particular environment. These three mechanisms are not mutually exclusive. All three processes are known to have the potential to alter circadian rhythmicity. For instance, Tauber et al. have shown that a recent mutation in the circadian clock gene timeless has altered diapause in Drosophila melanogaster [35]. In addition, epigenetic and specifically maternal effects can alter endogenous rhythmicity. For example, in quails the circadian phenotype of the mothers can predict that of their young [36]. Finally, as already mentioned above, after-effects of previous environmental exposure may affect circadian period length τ [28], for example, depending on the amplitude and length of the photoperiod an animal experiences [37–40]. For the future, we suggest possible experiments in order to better understand the mechanism behind the observed differences between city and forest-dwelling animals. We need experiments in which we record τ not only in wild-caught, but also in hand-reared forest and city birds. If differences in τ are not found in hand-reared animals, this would strongly argue against genetic difference between forest and city populations, and either developmental plasticity or environmental effects might be considered. In order to test for developmental effects, eggs could be cross-fostered between the two populations and subsequently τ could be recorded in nestlings raised from either their natural mother or a female in the opposite environment. Finally, to test for after-effects of artificial light at night, we would suggest recording τ after exposing forest and city birds to the same photoperiod. Effects of additional light at night could then be tested by separate treatments of both groups.
The distribution of chronotypes in our two populations closely fits theoretical predictions by Roenneberg et al. [12] based on oscillatory theory. The authors used empirical data of circadian periodicity in human beings for modelling possible chronotype distributions in relation to Zeitgeber strength. They suggested that with increasing Zeitgeber strength of light, chronotypes should be less variable and more closely synchronized, implying that chronotype distribution should depend on the Zeitgeber conditions a population experiences (figure 4a). The distributions of chronotypes in city and forest blackbirds are in line with this theory (figure 4b). Birds living in the forest habitat showed a distribution with a sharp and high peak, and low variance around the mean. Conversely, the chronotype of city birds showed a lower peak and higher between-individual variation than that of forest birds, mirroring the distribution of humans subjected to weak Zeitgebers. In addition, city birds were less consistent in their timing of morning activity than forest birds. Analysis of underlying rhythms suggests two circadian features that may affect activity patterns in the city, one being a possibly closer link of chronotype to period length in city birds than in their rural counterparts, as discussed above. The second feature is the higher heterogeneity in timing of onset of morning activity, which is mirrored by the weakness of the circadian rhythmicity under constant laboratory conditions. City birds were clearly less rhythmic than forest conspecifics, as suggested by the index of robustness. Interestingly, the five city birds that were found to be arrhythmic were all sampled at the same downtown location in the central business district of Munich, whereas the remaining city birds originated from other urban locations including parks, cemeteries and botanical gardens. Although anecdotal, this observation supports the idea that loss of circadian rhythmicity is related to inhabiting highly urbanized areas where environmental time cues may be less precise. We propose that urban environments could be seen as habitats where the time information is noisy and weak, thereby giving greater room for alternative temporal activity patterns if a potential advantage is present.
The consequences of urbanization on daily and seasonal organization and wellbeing are currently raising strong interest among both scientists and the general public [8,41], especially with respect to light at night and circadian biology [42,43]. Our study is novel in showing that changed activity patterns are associated with altered circadian rhythmicity not just in humans [12,44], but also in wild animals thriving in urban areas. We compared city and forest European blackbirds, but we suggest that our framework and findings may be relevant for scientists looking for a link between circadian rhythms, environmental change and daily activity in other species [3]. One fascinating explanation for the observed differences in circadian traits between forest and city birds could be selection for early chronotypes in urban habitats, possibly owing to potential reproductive advantages for early birds. The environmental pressures which could have promoted the shift in both endogenous and overt rhythmicity remain to be elucidated, but we suggest light pollution as a potential candidate. We believe that given the fitness and health implications of circadian disruption [45], there is an urgent need for scientists to understand the costs and benefits of altered circadian rhythmicity in urban areas.
Acknowledgements
This study was funded by the Volkswagen Stiftung (‘Initiative of Evolutionary Biology’) to J.P. Additional funding to D.M.D. and M.L. was provided by the International Max Planck Research School for Organismal Biology at the University of Konstanz. B.H. is indebted to the Baden-Württemberg Stiftung for financial support by the Eliteprogramme for Postdocs. Willi Jensen provided invaluable technical support. We are thankful to T. Roenneberg, who allowed us to reproduce his figure. S. Brown, P. Grant and three anonymous referees read and commented on previous drafts of the manuscript.
References
- 1.Grimm NB, Faeth SH, Golubiewski NE, Redman CL, Wu J, Bai X, Briggs JM. 2008. Global change and the ecology of cities. Science 319, 756–760. 10.1126/science.1150195 (doi:10.1126/science.1150195) [DOI] [PubMed] [Google Scholar]
- 2.Shochat E, Warren PS, Faeth SH, McIntyre NE, Hope D. 2006. From patterns to emerging processes in mechanistic urban ecology. Trends Ecol. Evol. 21, 186–191. 10.1016/j.tree.2005.11.019 (doi:10.1016/j.tree.2005.11.019) [DOI] [PubMed] [Google Scholar]
- 3.Longcore T, Rich C. 2006. Ecological consequences of artificial night lighting. Washington, DC: Island Press. [Google Scholar]
- 4.Kempenaers B, Borgström P, Loës P, Schlicht E, Valcu M. 2010. Artificial night lighting affects dawn song, extra-pair siring success, and lay date in songbirds. Curr. Biol. 20, 1735–1739. 10.1016/j.cub.2010.08.028 (doi:10.1016/j.cub.2010.08.028) [DOI] [PubMed] [Google Scholar]
- 5.Rotics S, Dayan T, Kronfeld-Schor N. 2011. Effect of artificial night lighting on temporally partitioned spiny mice. J. Mammol. 92, 159–168. 10.1644/10-MAMM-A-112.1 (doi:10.1644/10-MAMM-A-112.1) [DOI] [Google Scholar]
- 6.Dominoni D, Quetting M, Partecke J. 2013. Artificial light at night advances avian reproductive physiology. Proc. R. Soc. B 280, 20123017. 10.1098/rspb.2012.3017 (doi:10.1098/rspb.2012.3017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuller RA, Warren PH, Gaston KJ. 2007. Daytime noise predicts nocturnal singing in urban robins. Biol. Lett. 3, 368–370. 10.1098/rsbl.2007.0134 (doi:10.1098/rsbl.2007.0134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foster RG, Kreitzmann L. 2004. Rhythms of life: the biological clocks that control the daily lives of every living thing. New Haven, CT: Yale University Press. [Google Scholar]
- 9.Dunlap JC, Loros JJ, DeCoursey PJ. 2004. Chronobiology: biological timekeeping. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 10.Klerman EB. 2005. Clinical aspects of human circadian rhythms. J. Biol. Rhythms 20, 375–386. 10.1177/0748730405278353 (doi:10.1177/0748730405278353) [DOI] [PubMed] [Google Scholar]
- 11.Nemeth E, Brumm H. 2009. Blackbirds sing higher-pitched songs in cities: adaptation to habitat acoustics or side-effect of urbanization?. Anim. Behav. 78, 637–641. 10.1016/j.anbehav.2009.06.016 (doi:10.1016/j.anbehav.2009.06.016) [DOI] [Google Scholar]
- 12.Roenneberg T, Wirz-Justice A, Merrow M. 2003. Life between clocks: daily temporal patterns of human chronotypes. J. Biol. Rhythms 18, 80–90. 10.1177/0748730402239679 (doi:10.1177/0748730402239679) [DOI] [PubMed] [Google Scholar]
- 13.Kyriacou CP, Peixoto AA, Sandrelli F, Costa R, Tauber E. 2008. Clines in clock genes: fine-tuning circadian rhythms to the environment. Trends Genet. 24, 124–132. 10.1016/j.tig.2007.12.003 (doi:10.1016/j.tig.2007.12.003) [DOI] [PubMed] [Google Scholar]
- 14.Brown SA, Kunz D, Dumas A, Westermark PO, Vanselow K, Tilmann-Wahnschaffe A, Herzel H, Kramer A. 2008. Molecular insights into human daily behavior. Proc. Natl Acad. Sci. USA 105, 1602–1607. 10.1073/pnas.0707772105 (doi:10.1073/pnas.0707772105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bisson IA, Butler LK, Hayden TJ, Romero LM, Wikelski MC. 2009. No energetic cost of anthropogenic disturbance in a songbird. Proc. R. Soc. B 276, 961–969. 10.1098/rspb.2008.1277 (doi:10.1098/rspb.2008.1277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poesel A, Kunc HP, Foerster K, Johnsen A, Kempenaers B. 2006. Early birds are sexy: male age, dawn song and extrapair paternity in blue tits, Cyanistes (formerly Parus) caeruleus. Anim. Behav. 72, 531–538. 10.1016/j.anbehav.2005.10.022 (doi:10.1016/j.anbehav.2005.10.022) [DOI] [Google Scholar]
- 17.Amrhein V, Erne N. 2006. Dawn singing reflects past territorial challenges in the winter wren. Anim. Behav. 71, 1075–1080. 10.1016/j.anbehav.2005.07.023 (doi:10.1016/j.anbehav.2005.07.023) [DOI] [Google Scholar]
- 18.Kacelnik A, Krebs JR. 1983. The dawn chorus in the great tit (Parus major): proximate and ultimate causes. Behaviour 3, 287–308. 10.1163/156853983X00200 (doi:10.1163/156853983X00200) [DOI] [Google Scholar]
- 19.Levine JD, Funes P, Dowse HB, Hall JC. 2002. Signal analysis of behavioral and molecular cycles. BMC Neurosci. 3, 1–25. 10.1186/1471-2202-3-1 (doi:10.1186/1471-2202-3-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krishnan B, Levine JD, Lynch MK, Dowse HB, Funes P, Hall JC, Hardin PE, Dryer SE. 2001. A new role for cryptochrome in a Drosophila circadian oscillator. Nature 411, 313–317. 10.1038/35077094 (doi:10.1038/35077094) [DOI] [PubMed] [Google Scholar]
- 21.Dowse HB. 2007. Statistical analysis of biological rhythm data. In Circadian rhythms: methods and protocols (ed. Rosato E.), pp. 29–45. Totowa, NJ: Humana Press. [DOI] [PubMed] [Google Scholar]
- 22.Dowse HB, Ringo JM. 1991. Comparisons between ‘periodograms’ and spectral analysis: apples are apples after all. J. Theor. Biol. 148, 139–144. 10.1016/S0022-5193(05)80468-0 (doi:10.1016/S0022-5193(05)80468-0) [DOI] [PubMed] [Google Scholar]
- 23.Mukhin A, Grinkevich V, Helm B. 2009. Under cover of darkness: nocturnal life of diurnal birds. J. Biol. Rhythms 24, 225–231. 10.1177/0748730409335349 (doi:10.1177/0748730409335349) [DOI] [PubMed] [Google Scholar]
- 24.R Development Core Team 2011. R: a language and environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 25.Pinheiro JC, Bates DM. 2000. Mixed-effect models in S and S-PLUS. New York, NY: Springer. [Google Scholar]
- 26.Falconer DS, Mackay TFC. 1996. Introduction to quantitative genetics. New York, NY: Longman. [Google Scholar]
- 27.Lessells C, Boag P. 1987. Unrepeatable repeatabilities: a common mistake. The Auk 104, 116–121. 10.2307/4087240 (doi:10.2307/4087240) [DOI] [Google Scholar]
- 28.Pittendrigh C. 1960. Circadian rhythms and the circadian organization of living systems. Cold Spring Harb. Symp. Quant. Biol. 25, 159–184. 10.1101/SQB.1960.025.01.015 (doi:10.1101/SQB.1960.025.01.015) [DOI] [PubMed] [Google Scholar]
- 29.Sawyer LA, Hennessy JM, Peixoto AA, Rosato E, Parkinson H, Costa R, Kyriacou CP. 1997. Natural variation in a Drosophila clock gene and temperature compensation. Science 278, 2117–2120. 10.1126/science.278.5346.2117 (doi:10.1126/science.278.5346.2117) [DOI] [PubMed] [Google Scholar]
- 30.Michael TP, Salomé PA, Yu HJ, Spencer TR, Sharp EL, McPeek MA, Alonso JM, Ecker JR, McClung CR. 2003. Enhanced fitness conferred by naturally occurring variation in the circadian clock. Science 302, 1049–1053. 10.1126/science.1082971 (doi:10.1126/science.1082971) [DOI] [PubMed] [Google Scholar]
- 31.Gwinner E. 1967. Circannuale Periodik der Mauser und der Zugunruhe bei einem Vogel. Naturwissenschaften 54, 447. 10.1007/BF00603157 (doi:10.1007/BF00603157) [DOI] [PubMed] [Google Scholar]
- 32.Gwinner E. 1974. Testosterone induces splitting of circadian locomotor activity in birds. Science 185, 72–74. 10.1126/science.185.4145.72 (doi:10.1126/science.185.4145.72) [DOI] [PubMed] [Google Scholar]
- 33.Partecke J, Van't Hof TJ, Gwinner E. 2005. Underlying physiological control of reproduction in urban and forest-dwelling European blackbirds Turdus merula. J. Avian Biol. 36, 295–305. 10.1111/j.0908-8857.2005.03344.x (doi:10.1111/j.0908-8857.2005.03344.x) [DOI] [Google Scholar]
- 34.Helm B, Visser ME. 2010. Heritable circadian period length in a wild bird population. Proc. R. Soc. B 277, 3335–3343. 10.1098/rspb.2010.0871 (doi:10.1098/rspb.2010.0871) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tauber E, et al. 2007. Natural selection favors a newly derived timeless allele in Drosophila melanogaster. Science 316, 1895–1898. 10.1126/science.1138412 (doi:10.1126/science.1138412) [DOI] [PubMed] [Google Scholar]
- 36.Formanek L, Richard-Yris M-A, Houdelier C, Lumineau S. 2009. Epigenetic maternal effects on endogenous rhythms in precocial birds. Chronobiol. Int. 26, 396–414. 10.1080/07420520902892433 (doi:10.1080/07420520902892433) [DOI] [PubMed] [Google Scholar]
- 37.Pittendrigh C, Daan S. 1976. A functional analysis of circadian pacemakers in nocturnal rodents. I. The stability and lability of spontaneous frequency. J. Comp. Physiol. A 252, 223–252. 10.1007/BF01417856 (doi:10.1007/BF01417856) [DOI] [Google Scholar]
- 38.Aschoff J. 1979. Circadian rhythms: influences of internal and external factors on the period measured in constant conditions. Z. Tierpsychol. 49, 225–249. 10.1111/j.1439-0310.1979.tb00290.x (doi:10.1111/j.1439-0310.1979.tb00290.x) [DOI] [PubMed] [Google Scholar]
- 39.Eskin A. 1971. Some properties of the system controlling the circadian activity rhythm of sparrows. In Biochronometry (ed. Menaker M.), pp. 55–80. Washington, DC: National Academy of Sciences. [Google Scholar]
- 40.Diegmann J, Stück A, Madeti C, Roenneberg T. 2010. Entrainment elicits period aftereffects in Neurospora crassa. Chronobiol. Int. 27, 1335–1347. 10.3109/07420528.2010.504316 (doi:10.3109/07420528.2010.504316) [DOI] [PubMed] [Google Scholar]
- 41.Lederbogen F, et al. 2011. City living and urban upbringing affect neural social stress processing in humans. Nature 474, 498–501. 10.1038/nature10190 (doi:10.1038/nature10190) [DOI] [PubMed] [Google Scholar]
- 42.Kantermann T, Roenneberg T. 2009. Is light-at-night a health risk factor or a health risk predictor? Chronobiol. Int. 26, 1069–1074. [DOI] [PubMed] [Google Scholar]
- 43.Navara KJ, Nelson RJ. 2007. The dark side of light at night: physiological, epidemiological, and ecological consequences. J. Pineal Res. 43, 215–224. 10.1111/j.1600-079X.2007.00473.x (doi:10.1111/j.1600-079X.2007.00473.x) [DOI] [PubMed] [Google Scholar]
- 44.Vollmer C, Michel U, Randler C. 2012. Outdoor light at night (LAN) is correlated with eveningness in adolescents. Chronobiol. Int. 29, 502–508. 10.3109/07420528.2011.635232 (doi:10.3109/07420528.2011.635232) [DOI] [PubMed] [Google Scholar]
- 45.Emerson KJ, Bradshaw WE, Holzapfel CM. 2008. Concordance of the circadian clock with the environment is necessary to maximize fitness in natural populations. Evolution 62, 979–983. 10.1111/j.1558-5646.2008.00324.x (doi:10.1111/j.1558-5646.2008.00324.x) [DOI] [PMC free article] [PubMed] [Google Scholar]