Abstract
Spatial variation in lighting environments frequently leads to population variation in colour patterns, colour preferences and visual systems. Yet lighting conditions also vary diurnally, and many aspects of visual systems and behaviour vary over this time scale. Here, we use the bluefin killifish (Lucania goodei) to compare how diurnal variation and habitat variation (clear versus tannin-stained water) affect opsin expression and the preference to peck at different-coloured objects. Opsin expression was generally lowest at midnight and dawn, and highest at midday and dusk, and this diurnal variation was many times greater than variation between habitats. Pecking preference was affected by both diurnal and habitat variation but did not correlate with opsin expression. Rather, pecking preference matched lighting conditions, with higher preferences for blue at noon and for red at dawn/dusk, when these wavelengths are comparatively scarce. Similarly, blue pecking preference was higher in tannin-stained water where blue wavelengths are reduced. In conclusion, L. goodei exhibits strong diurnal cycles of opsin expression, but these are not tightly correlated with light intensity or colour. Temporally variable pecking preferences probably result from lighting environment rather than from opsin production. These results may have implications for the colour pattern diversity observed in these fish.
Keywords: opsin, colour vision, light, diurnal effects, behavioural preference, contrast
1. Introduction
Our visual world is extremely dynamic. From high noon to the depth of night, the amount of light available for vision can vary by a factor of over a billion [1]. The ability to see food, mates and predators across this vast range of conditions requires an incredibly plastic visual system that can respond to abrupt changes in light. The visual system that has evolved in vertebrates to deal with this complex process uses specialized photoreceptor cells—rods and cones. Rods are specialized for low-light vision, whereas cones are specialized for light or colour vision. The outer segments of these cells are composed of stacks of discs filled with a protein (opsin) linked to a light-absorbing chromophore (retinal or 3-dehydroretinal). There are multiple classes of cone opsins, each of which has a wavelength range to which it is most sensitive (λmax). By comparing the quantum catch of the different cone classes, the brain is able to deduce colour [2].
Species evolve visual systems suitable for the lighting environment of their respective habitats to facilitate activities such as foraging and mate choice [3–5]. This can happen through altering the number of different photoreceptor types found in the retina, or through mutations in the amino acid residues of an opsin that change the opsin's λmax [6–9]. However, even over the course of an individual's lifetime, the visual system can be altered to match a changing environment. Simply switching the form of the chromophore can shift the λmax of the photoreceptor by several nanometres, a process seen in many anadromous and catadromous fishes [10]. Many animals use various types of filters to affect the wavelengths of light that reach their photoreceptors. Some birds, reptiles and lungfish have oil droplets in their cones that are filled with coloured carotenoids. The oil absorbs shorter wavelengths and transmits only longer wavelengths [2]. By positively correlating the density of carotenoids in the oil droplets with light levels, these animals can increase sensitivity in dim environments and colour discrimination in bright environments [11]. Opsin expression can also change to match the lighting environment. For example, salmon switch the opsins their cones express as they move from shallow to deeper waters [12], and the black bream (Acanthopagrus butcheri) shifts opsin expression as it makes habitat changes from clear to deeper, tannin-stained water [13,14].
Clearly, many organisms have the ability to alter opsin expression as they transition between different habitats (i.e. lighting environments). However, organisms also experience huge (yet predictable) fluctuations in lighting environment over the course of each day. As the sun changes position in the sky, both the absolute irradiance and the relative amounts of each wavelength of light change. During the day, higher wavelengths of visual light are represented in roughly equal proportions, with a gradual drop-off as wavelength values fall under 450 nm. At dawn and dusk, however, the available light becomes blue-shifted as the amount of orange light drops much faster than the amount of blue light [15–17].
Organisms can alter their behaviour to manage the effects of temporal variation in a visual environment. For example, guppies display closer to females in dim light [18] and preferentially court/mate early and late in the day, when they are most conspicuous to conspecifics but less likely to be seen by predators [19]. Some birds choose to perform courting displays during times when the lighting conditions optimize contrast between their plumage and background [20]. These studies assume a constant visual system and behavioural modifications to ambient light conditions. Yet, the visual system itself might also vary with these changes in lighting environment and, in turn, also affect these behaviours. Cone cells shed the part of their photopigment-containing outer segments every night and subsequently rebuild them during the day [21]. The extent to which this cycle correlates with light levels and alters visually based behaviours is unclear.
We sought to determine whether organisms deal with diurnal visual variation by manipulating the overall or relative amount of opsin they express in correspondence with the wavelengths of light available. In zebrafish, expression of the long-wavelength-sensitive (LWS) opsin varies over the course of a day, and increased LWS expression leads to increased opsin protein density within the cone and a subsequent increase in behavioural sensitivity to red light [22]. Therefore, we reasoned that short-term differences in opsin expression in response to the diel pattern of light might occur and have a subsequent functional significance. In order to address this question, we used a fish that exhibits dynamic opsin expression to match its visual environment. The bluefin killifish, Lucania goodei, is an abundant diurnal freshwater fish living throughout the southeastern United States. This species is found across a wide variety of habitats that vary in lighting conditions. Populations in habitats with more dissolved humic materials, which inhibit the transmission of short wavelengths of light, have fewer short-wavelength cones [23] and express lower relative amounts of short-wavelength opsins [24]. Raising fry in different lighting conditions (tannin-stained versus clear water) can induce a developmental change in the proportional amount of short-wavelength-sensitive (SWS) opsin expression [25], as can simply switching adult fish from one water condition to another [26]. This change in opsin expression is extremely rapid, happening in less than 3 days.
It is clear that these killifish can respond over the course of a just a few days to different lighting conditions, but whether the fish adjust their opsins over shorter time periods to match the daily fluctuations inherent to their lighting environment is unknown. In this study, we addressed the following four questions. First, are there diurnal patterns in opsin expression that follow the daily patterns in light abundance where light (and opsin expression) are lowest at midnight, highest at midday and intermediate at dawn and dusk? Second, we asked whether the proportional expression (i.e. the amount of each opsin relative to the total pool of opsins) varied over time or whether all of the opsins varied in a similar manner over the course of the day. Changes in proportional opsin expression are theoretically consistent with changes in colour sensitivity [27]. Third, we asked whether diurnal opsin expression varied among lighting habitats (i.e. clear versus tannin-stained water) and whether diurnal fluctuations or habitat changes had larger effects on opsin expression. Finally, we asked whether visually based behaviour (preference to peck at different-coloured dots) varied as a function of time, lighting habitat or their interaction and whether there was a strong relationship between shifts in opsin expression and behaviour.
2. Methods
The killifish, L. goodei, used for this experiment were collected in early October 2011 using dip nets and seines from Rum Island Park, Florida. This site occurs on the Santa Fe River where two springs (Rum Island Springs and Blue Springs) join. The site is known for being highly variable both spatially and temporally in the amount of dissolved humic material. Thus, the source population is exposed to widely different visual environments, from clear to darkly humic water. The fish were transported to the University of Illinois and placed in six tanks (114 l) at a density of 14 fish per tank. Lipton instant decaffeinated tea was added to three tanks to match the visual environment to a tannin-stained habitat, whereas the other three tanks remained clear. The fish were housed in a greenhouse with siding that transmitted wavelengths from 385 to 800+ nm, ensuring that they were exposed to natural light cycles. At that time of year, sunrise and sunset are approximately 10.5 h apart with some light visible (daylight plus nautical twilight) for approximately 12.5 h d−1. The fish lighting environment was supplemented with broad spectrum xenon lamps on a 12 L : 12 D cycle, which supplemented the ultraviolet (UV) light that the greenhouse siding filtered out. Representative irradiance spectra of light conditions at noon and dawn/dusk are available in the electronic supplementary material, figure S1. Fish were allowed to acclimate to their tanks for 10 days before behavioural trials began.
To determine how time of day affected pecking preference, the fish were given a colour choice test. A plastic overhead sheet was painted with different colours of acrylic paint: red, orange, yellow, green, blue, black and white. Fuller et al. [28] described the reflectance spectra of each colour, except orange, which is included in the electronic supplementary material, figure S2. A standard hole-punch (6 mm) was used to punch out coloured circles, and the circles were glued to a clear Petri dish in a random order. The dish was placed in the centre of the tank against the pale blue background underneath the glass tank, and the number of times the fish pecked at each colour was recorded for 2 min after the first peck. The fish generally peck at these dots similar to the manner in which they peck at their food. We assume that these pecking preferences are reflective of foraging preferences.
If no fish pecked at the dish for any time during the first 10 min of being exposed to the Petri dish, then the trial was discarded. Trials were conducted at three separate times: within an hour of dawn, midday and dusk. We did not measure pecking behaviour at midnight because the fish are not active at this time, and we would have had to disrupt the natural light cycle in order to measure the behaviour. Each tank was measured at least once at all three times; however, the fish in one tank failed to peck at any circles during repeated trials for two of the time points. For each tank, no more than one measurement was taken per day, with at least 24 h between each tank's successive measurements. Trials occurred over 10 days.
Following the behavioural trials, one fish from each of the six tanks was harvested for three successive days at four separate time points: within an hour of dawn, midday, dusk and midnight (72 fish total). For the midnight collection, a red-light headlamp was used as a visual aid. Each fish was euthanized with an overdose of MS-222. Both eyes were removed, punctured and stored in RNA later until RNA could be extracted. RNA was extracted from the eyes with trizol using the protocol described in earlier studies [26,29,30] and stored at −80°C until we performed cDNA synthesis using SuperScript III.
Five distinct cone classes have been identified in L. goodei via microspectrophotometry: UV (λmax = 359 nm), violet (λmax = 405 nm), blue (λmax = 455 nm), yellow (λmax = 539 nm) and red (λmax = 573 nm) [23]. Yokoyama et al. [31] cloned the SWS opsins and expressed them in vitro to determine their λmax when combined with 11-cis retinal (SWS1 λmax = 354, SWS2B λmax = 397, SWS2A λmax = 448 nm). Because these in vitro λmax values are similar to those observed by microspectrophotometry, Fuller and co-workers assumed that the five cone classes observed in L. goodei were SWS1 (UV), SWS2B (violet), SWS2A (blue), RH2-1 (yellow) and LWS (red). However, Fuller & Claricoates [26] recently discovered an RH2-2 sequence also existed, and its expression was much higher than expression measures of SWS2A. Thus, the blue cones observed in L. goodei are probably RH2-2 rather than SWS2A, whereas SWS2A is believed to be co-expressed at low levels with SWS2B in the violet cones [26]. In this study, the transcription of these six separate opsins (LWS, RH2-1, RH2-2, SWS2A, SWS2B and SWS1) was measured using six specifically designed Taqman primers and probes. There are two distinct LWS opsins, but they are identical at the exon–exon boundary for which the primers and probes were designed, and so a single set of primers and probes was used for the LWS opsin. Elongation factor 1-α (hereafter EF1-α) was also included as a housekeeping control (see [26] for sequences and accession numbers).
For each of the six opsins and EF1-α, three technical replicates were measured on an ABI Prism 7700 sequence detection system. The average critical threshold for each individual, after removing outliers, was used to estimate both proportional opsin expression (i.e. each opsin proportional to the total pool of all the fish's opsins) and relative opsin expression (i.e. each opsin relative to that fish's EF1-α expression). One of 72 individuals did not run and was removed from further analysis.
To calculate the proportion of the total quantity of opsins that each individual opsin contributed, the following formula was used:
In this case, Ti/Tall is the amount of each individual opsin for each fish divided by the sum of all opsins. The amount of each opsin was calculated using Ei as the efficiency of each opsin and Cti as the critical threshold obtained for each individual gene. Efficiencies are from Fuller & Claricoates [26], who used the same primers and probes and detection system, and listed their efficiencies as one plus their calculated values.
The amount of each opsin relative to housekeeping gene EF1-α was also calculated using the following equation:
In this case, Ti/Tef is the amount of the individual opsin for that fish relative to the amount of EF1-α, and the amount of each opsin is independent of the amount of the other opsins. The opsin amounts relative to EF1-α were log-transformed to normalize the data.
All data were analysed using SAS v. 9.3 (SAS Institute, Cary, NC). Proc Mixed was used to analyse the opsin data. Time of day, water and sex were treated as fixed effects. The day on which each individual was euthanized, and the qRT-PCR plate on which each individual's cDNA was run were treated as random effects for all opsins. Sex had no effect on any opsin and was removed from the models. Similarly, no interactions were significant, and all were removed.
To analyse the behavioural data, all trials within each time of day were summed so that each tank had three separate values, one for each time. The number of pecks each colour received out of the total number of pecks was modelled with Proc Genmod using water and time as fixed effects (see the electronic supplementary material, table S1). The model used a binomial distribution and the logit link function and was corrected for overdispersion. Because of low numbers, models using the proportion of yellow, black and white pecks failed to converge. To determine whether there was an overall preference for pecking at a certain time, the number of pecks for each tank at each time were averaged and then square-root-transformed to normalize the data. An ANOVA in Proc Mixed with tank as a random factor was used to see whether there was an overall preference for time. Raw data for both the opsin and pecking preferences analyses have been deposited in Dryad (doi:10.5061/dryad.3fp5b) [32].
3. Results
(a). Opsin data
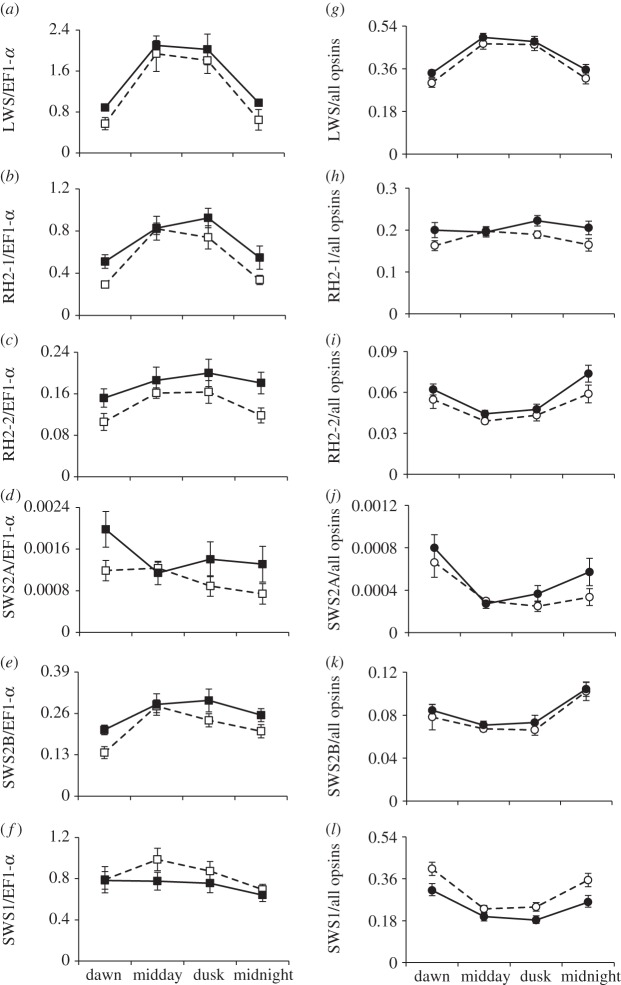
Opsin expression varied dramatically over the course of the day. There was no effect of time of day or water colour on our control gene EF1-α (p = 0.80 and p = 0.56, respectively), allowing us to use it to standardize the amount of each opsin and the total amount of opsin expression summed. Total opsin levels were not strongly affected by habitat (F1,57 = 3.43, p = 0.069), but they were affected by time (F3,57 = 21.06, p < 0.0001), with midday and dusk having significantly higher expression values than midnight and dawn (F1,57 = 62.74, p < 0.0001). Most of the opsins followed this overall expression pattern: expression was low from midnight to dawn and high from midday to dusk (table 1 and figure 1a–f). LWS had the largest increase, with relative expression more than two times higher at midday/dusk than at midnight/dawn (figure 1a). Expression of RH2-1, RH2-2 and SWS2B showed similar patterns, though the magnitude of change was not as great (figure 1b,c,e), whereas SWS1 and SWS2A showed no significant effect of time (table 1 and figure 1d,f).
Table 1.
Analysis of variance results on opsin values relative to EF1-α and on proportional opsin values. (Day and the qRT-PCR plate each individual was run on are included as random effects. Values relative to EF1-α were log-transformed. λmax values are from previous studies [22,25,30]. Values in italics are considered significant.)
| relative to EF1-α (opsin/EF1-α) |
proportional (opsin/all opsins) |
|||||||
|---|---|---|---|---|---|---|---|---|
| time |
water |
time |
water |
|||||
| F3,57 | p | F1,57 | p | F3,57 | p | F1,57 | p | |
| LWS (λmax = 573) | 31.72 | <0.0001 | 5.09 | 0.028 | 33.16 | <0.0001 | 4.05 | 0.049 |
| RH2-1 (λmax = 539) | 21.61 | <0.0001 | 13.63 | 0.0005 | 1.89 | 0.14 | 15.73 | 0.0002 |
| RH2-2 (λmax = 455) | 3.21 | 0.030 | 7.98 | 0.0065 | 13.06 | <0.0001 | 4.41 | 0.040 |
| SWS2A (λmax ∼ 448) | 1.78 | 0.16 | 4.42 | 0.040 | 11.53 | <0.0001 | 3.7 | 0.059 |
| SWS2B (λmax = 405) | 8.43 | <0.0001 | 8.45 | 0.0052 | 11.97 | <0.0001 | 0.93 | 0.34 |
| SWS1 (λmax = 359) | 2.06 | 0.12 | 2.08 | 0.15 | 21.46 | <0.0001 | 23.03 | <0.0001 |
Figure 1.
(a–f) Opsin expression relative to housekeeping gene EF1-α, and (g–l) proportional to all opsins. Open symbols are means (±s.e.) of individuals from clear tanks. Closed symbols are means (±s.e.) of individuals from tea-stained tanks.
Proportional opsin expression values (the proportion of total opsin expression contributed by each individual opsin) were greatly influenced by the sizable change through time in LWS opsin expression. LWS proportional expression climbed after dawn, stabilized from midday to dusk and fell back to starting levels overnight (figure 1g). Although RH2-1 also increased at midday and dusk relative to EF1-α, because the magnitude of this change was not as great at that seen in LWS, proportional expression of RH2-1 was flat through time (figure 1h). Similarly, though RH2-2 and SWS2B were higher at midday and dusk relative to EF1-α, they actually decreased proportionally at those times (figure 1i,k). SWS1, while flat relative to EF1-α, showed a large decrease in proportional expression at midday/dusk (figure 1l). Thus, proportional opsin expression varied through time, with the most striking differences occurring on the opsins at the extreme ends of the visible light spectrum (table 1 and figure 1g,l).
Water colour significantly affected opsin expression. LWS, RH2-1 and RH2-2 all had significantly higher expression in tea-stained water, irrespective of time by both proportional and relative measures. SWS2A and SWS2B showed significantly more expression in tea-stained water only in the data relative to EF1-α (table 1 and figure 1). Relative to EF1-α, SWS1 expression did not vary between clear and tea-stained water; however, because of the pattern of the other opsins, proportional expression of SWS1 was significantly lower in tea-stained versus clear water (table 1 and figure 1f,l).
(b). Behavioural data
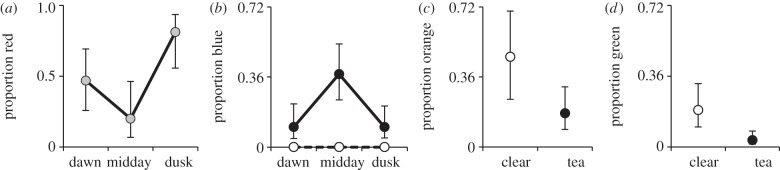
Fish pecked at the coloured circles three times more frequently in tea-stained water. Of the 271 pecks observed, 50 per cent were red. The average numbers of pecks per each observation (mean ± s.e.) were red (4.0 ± 1.5), orange (2.0 ± 0.4), blue (1.5 ± 0.5), green (0.5 ± 0.2), yellow (0.2 ± 0.1), white (0.09 ± 0.06) and black (0.03 ± 0.03). There was no significant difference in the amount of pecking observed at different times of day (F2,8 = 1.78, p = 0.23). However, time of day affected the proportion of pecks that the blue and red circles received (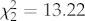 , p = 0.0013;
, p = 0.0013; 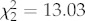 , p = 0.0015, respectively). The proportion of blue pecks was significantly increased at noon (figure 2b), whereas the red proportion was significantly higher at dusk than midday (figure 2a). Water colour strongly affected fish preference for blue. The number of trials recording blue pecks was significantly higher in tea-stained tanks (
, p = 0.0015, respectively). The proportion of blue pecks was significantly increased at noon (figure 2b), whereas the red proportion was significantly higher at dusk than midday (figure 2a). Water colour strongly affected fish preference for blue. The number of trials recording blue pecks was significantly higher in tea-stained tanks (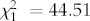 , p < 0.0001). In fact, of 36 blue pecks, all were in tea-stained tanks (figure 2b). Water colour also had a significant effect on green (
, p < 0.0001). In fact, of 36 blue pecks, all were in tea-stained tanks (figure 2b). Water colour also had a significant effect on green (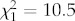 , p = 0.001) and orange (
, p = 0.001) and orange (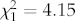 , p = 0.042), where fish in clear tanks pecked significantly more at these colours than fish in tea-stained tanks (figure 2c,d).
, p = 0.042), where fish in clear tanks pecked significantly more at these colours than fish in tea-stained tanks (figure 2c,d).
Figure 2.
Proportion of pecks that were (a) red, (b) blue, (c) orange, or (d) green as a function of either time of day or water colour. Six tanks of 14 fish were given the opportunity to peck at red, orange, yellow, green, blue, black or white spots. Black symbols are for tea-stained water, open symbols are for clear water and grey symbols are from combined data. Error bars represent 95% confidence intervals.
4. Discussion
(a). Opsin expression is dynamic
Our study shows that there are strong diurnal patterns of opsin expression in L. goodei. Opsin expression in both clear and tea-stained tanks rises substantially from morning to afternoon and then falls back to base levels after sunset. However, contrary to our expectations, opsin expression does not precisely match light levels, with highest expression at midday. Rather, the patterns of expression might be better described as a delayed bimodal reaction to changes in light levels, with upregulation and downregulation occurring only after prolonged exposure to rising or falling light levels. Of course, there is an inherent time delay between opsin expression and a change in cone phenotype. In rods, mRNA moves from the nucleus to the myoid region, where it forms opsin, within an hour [33], and Bok & Young [34] found that it took from 30 min to 2 h for radioactively labelled proteins to be incorporated into cone outer segments. That means the mRNA expression pattern that we observe here is probably related to outer segment growth approximately 2 h subsequent. Nonetheless, our results suggest that opsin abundance, and therefore sensitivity, in the cones is highest in the late afternoon/early evening and lowest in the pre-dawn and early morning hours. This matches previous work on diurnal rhythms found in other fishes; both zebrafish and the cichlid Haplochromis burtoni have cone opsin expression that peaks in the late afternoon [22,35]. In zebrafish, the peak in expression correlates with a peak in sensitivity to visual stimuli, with fish most sensitive to visual stimuli in the late afternoon and least sensitive in the early morning hours [22,36]. This suggests that we have observed a general cross-taxa pattern where opsin expression does not march in tune with light availability.
Throughout the day, the relative amounts of each wavelength of light also change in addition to changes in overall light levels, with light becoming blue-shifted at dawn and dusk. We sought to determine whether proportional expression of the cone opsins matches these shifts. While proportional expression of SWS1 was highest in the morning, it was not matched by a corresponding proportional increase in the evening (figure 1l), which would be indicative of increased sensitivity to the relatively abundant blue/UV wavelengths available at those times. The same pattern was found with the LWS opsin. Proportional LWS expression was highest at noon, when long wavelengths are particularly abundant, but proportional expression remained high through the evening, after long-wavelength light had fallen proportionally. Therefore, our results do not indicate proportional opsin expression matches relative wavelength abundance.
Interestingly, we did find the temporal effects on expression were most pronounced in those opsins with λmaxs that correspond to the most striking overall light shifts (i.e. the longer wavelengths). LWS and RH2-1, which maximally absorb yellow and green light, had very pronounced circadian rhythms, increasing from their low points (midnight and dawn) to high points (noon and dusk) on an average of 2.6-fold and twofold, respectively, when calculated relative to EF1-α (figure 1a,b). These increases largely drove the patterns observed in the proportional data. On the other hand, RH2-2, SWS2A, SWS2B and SWS1, which absorb maximally in the blue to UV range, increased only 1.1- to 1.3-fold (figure 1c–f), and the increase was not significant in SWS1 or SWS2A (table 1). Work on the cichlid H. burtoni also found smaller diurnal changes in expression in the SWS opsin (SWS2A) than in middle (RH2) and LWS opsins. Considering that available yellow light rises and falls much faster than blue light at dawn and dusk, it could be that cones sensitive to longer wavelengths are therefore much more affected by diurnal rhythms than shorter wavelength cones. On the other hand, the invariant production of SWS1 might also serve a purpose. Given that previous work has indicated that there are fewer SWS1 cones than LWS cones in L. goodei [23], but SWS1 expression was actually higher than LWS expression in the morning, it seems likely that SWS1 cones maintain a consistently high (rather than consistently low) production of opsin throughout the day. There might be some advantage to maintaining a constant high level of opsin production in the shorter wavelengths if the ability to see predators, food or mates during the transitionary times of dawn and dusk is especially important. For example, Munz & McFarland [17] have suggested that the rhodopsins of reef fishes are attuned to evening twilight conditions, because predation reaches a maximum during this transitionary time, although Endler's [19] study on guppies, a freshwater species, indicated the opposite pattern of predation. We have very little data concerning temporal differences in predation, or mating or foraging behaviours, making it difficult to say whether dawn and dusk are key times of day for L. goodei. Fuller [37] examined male mating behaviours in the field and found a small effect of time on male–male aggression, with aggression peaking a couple hours after dawn, but the author attributed this peak to local boating activity suppressing behaviours in the afternoon.
Our results on opsin expression in tea-stained and clear water also point to the idea that SWS1 is less labile than the other opsins. All opsins, with the exception of SWS1, demonstrated increased expression in tea-stained water (table 1 and figure 1) across all the time points measured. Humic L. goodei populations have fewer SWS1 cones than clear populations [23], which is caused in part by static expression of SWS1 opsin, whereas the longer wavelength-sensitive opsins upregulate [26]. Thus, SWS opsins, especially SWS1, seem to be less influenced than other opsins by lighting environment as a whole, whether considering temporal or habitat factors. The reasons for this are unclear, but UV vision in fishes is important for a variety of things such as predator and prey detection, navigation, identification of conspecifics and avoidance of excessive UV exposure [38].
(b). Behavioural pecking preference
We were interested in whether the opsin expression patterns that we observed were related to a measurable behavioural phenotype, in this case, a pecking preference for different-coloured objects. On a general level, expression of the LWS opsin was highest, and this corresponded to a high preference for red circles (and orange circles to a lesser extent) overall. Lucania goodei can be added to a long list of organisms that exhibit a preference for long-wavelength colours [39–41]. This bias could be the result of selection for increased consumption of carotenoids, selection for detecting red-coloured food items such as chironomid larvae, or related to sexual selection (L. goodei males have red, yellow and orange coloration while females do not).
The temporal opsin expression data indicate that the fish should be most attracted to red in the evening, and the fish did peck at red significantly more at dusk than at midday, whereas dawn was intermediate (figure 2a). However, based solely on expression data, the fish should also have been more attracted to blue in the morning, which we did not observe. Rather, we see a peak attraction to blue at midday (figure 2b). These results suggest that attraction to colours in killifish is not based on opsin production, but rather on lighting conditions. The fish prefer red at dusk, and to a lesser extent dawn, times when less long-wavelength light is available. At noon, when short-wavelength light is at a relative minimum, the fish have a higher preference for blue. Irrespective of time of day, animals in tea-stained water, which transmits less short-wavelength light, also peck significantly more at the blue discs, perhaps at a cost of green and orange (figure 2b–d).
The L. goodei preference for blue in tea-stained waters has been found previously [28], and when combined with the temporal data suggest that contrast is highly important to these fish. When there are low levels of short-wavelength light, as in either tea-stained waters or waters at midday, blue colours appear high in contrast, and the fish demonstrate a high preference for blue. Colour contrast such as this is highly important to many organisms [42–46]. The link between pecking preferences and mating preferences (males come in blue, yellow and red morphs) is unclear. However, the fact that (i) we have observed increased blue pecking preference in tannin-stained waters in two separate studies, and (ii) blue males are more abundant in tannin-stained waters in nature [47], suggests that the attendance to contrast behaviour being detected by the pecking assays may also be important to sexual selection in L. goodei. Our results also suggest a mechanism of maintenance of this polymorphism. Red and yellow males may have higher mating success at dawn and dusk, when they are most conspicuous to females, whereas blue males may have higher success at midday. This is an exciting avenue of research to continue. Nonetheless, the behavioural study shows that attraction to colours as measured by pecking preference does not match opsin expression, but rather correlates negatively with available light colour.
Our conjecture concerning contrast and preference obviously requires actual measurements of colour contrast. Sophisticated visual detection models have been developed that allow for the calculation of achromatic (i.e. brightness) and chromatic (i.e. colour) contrasts for species whose visual properties differ from that of humans [48]. These models have been applied to studies of both mating [46,49] and foraging preferences [50] and have been used to estimate the effects of altering the lighting environment [44,51] and the visual system properties of the receivers [52,53] on colour perception. The application of these models to the L. goodei system may resolve both (i) the pattern of blue males being more abundant in tannin-stained waters, and (ii) the maintenance of multiple colour morphs within populations.
This work supports previous results that found inter-individual differences in opsin expression were not correlated with pecking preference [28]. However, pecking preferences must be a function of opsin production and cone abundance at some level. Colour perception relies on the differential stimulation of different cone classes. Presumably, deleting (or adding) an entire opsin class would alter colour perception dramatically. Such wholesale shifts in opsin expression are seen over the course of development in many animals [12,14,54–56]. Closely related species can use different templates of opsins [57]. Hence, it is not appropriate to say that opsin expression has no effect on pecking preferences. Still, our data here are consistent with the idea that short-term plastic shifts in opsin expression do not drive pecking preferences.
5. Conclusions
We have demonstrated that there are very large diurnal effects on cone opsin expression, but that the variation in expression does not precisely match lighting levels. It is striking how large these effects are, especially in comparison with known effects of habitat lighting environments. For example, LWS opsin increases 260 per cent from midnight/dawn to noon/dusk. However, switching the fish's habitat to that of a tea-stained swamp elicits a much smaller increase (25%) in our study. This suggests that animals might be able to use standing temporal variation to easily alter their visual system to match the environment. Given the large effect of time on opsin expression, our results also illustrate the dangers of comparing opsin expression across populations or individuals without controlling for time of day. Our results indicate that lighting environment is far more important than diurnal opsin expression patterns in L. goodei in regard to behavioural impact. Pecking preference is highly influenced by natural variation in lighting conditions. When short wavelengths are abundant (dawn and dusk) red is preferred, when they are proportionally scarce (noon) blue is a preferred colour. In these fish, colour contrast is highly important.
Acknowledgements
Treatment of animals was approved by the University of Illinois Animal Care and Use Committee (no. 11143).
We thank the Robinson laboratory for use of their qRT-PCR machine. We thank E. Berdan for help collecting and transporting the fish and G. Kozak for comments on the manuscript. Research was supported by the National Science Foundation and the University of Illinois (NSF DEB nos. 0953716, 1011369).
References
- 1.Davies WIL, Collin SP, Hunt DM. 2012. Molecular ecology and adaptation of visual photopigments in craniates . Mol. Ecol. 21, 3121–3158. 10.1111/j.1365-294X.2012.05617.x (doi:10.1111/j.1365-294X.2012.05617.x) [DOI] [PubMed] [Google Scholar]
- 2.Bowmaker JK. 2008. Evolution of vertebrate visual pigments . Vision Res. 48, 2022–2041. 10.1016/j.visres.2008.03.025 (doi:10.1016/j.visres.2008.03.025) [DOI] [PubMed] [Google Scholar]
- 3.Horth L. 2007. Sensory genes and mate choice: evidence that duplications, mutations, and adaptive evolution alter variation in mating cue genes and their receptors . Genomics 90, 159–175. 10.1016/j.ygeno.2007.03.021 (doi:10.1016/j.ygeno.2007.03.021) [DOI] [PubMed] [Google Scholar]
- 4.Temple S, Hart NS, Marshall NJ, Collin SP. 2010. A spitting image: specializations in archerfish eyes for vision at the interface between air and water . Proc. R. Soc. B 277, 2607–2615. 10.1098/rspb.2010.0345 (doi:10.1098/rspb.2010.0345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings M, Partridge J. 2001. Visual pigments and optical habitats of surfperch (Embiotocidae) in the California kelp forest . J. Comp. Physiol. A 187, 875–889. 10.1007/s00359-001-0258-6 (doi:10.1007/s00359-001-0258-6) [DOI] [PubMed] [Google Scholar]
- 6.Osorio D, Vorobyev M. 2005. Photoreceptor spectral sensitivities in terrestrial animals: adaptations for luminance and colour vision . Proc. R. Soc. B 272, 1745–1752. 10.1098/rspb.2005.3156 (doi:10.1098/rspb.2005.3156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carleton K. 2009. Cichlid fish visual systems: mechanisms of spectral tuning . Integr. Zool. 4, 75–86. 10.1111/j.1749-4877.2008.00137.x (doi:10.1111/j.1749-4877.2008.00137.x) [DOI] [PubMed] [Google Scholar]
- 8.Kelber A, Vorobyev M, Osorio D. 2003. Animal colour vision: behavioural tests and physiological concepts . Biol. Rev. 78, 81–118. 10.1017/S1464793102005985 (doi:10.1017/S1464793102005985) [DOI] [PubMed] [Google Scholar]
- 9.Frentiu FD, Bernard GD, Cuevas CI, Sison-Mangus MP, Prudic KL, Briscoe AD. 2007. Adaptive evolution of color vision as seen through the eyes of butterflies . Proc. Natl Acad. Sci. USA 104, 8634–8640. 10.1073/pnas.0701447104 (doi:10.1073/pnas.0701447104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beatty D. 1984. Visual pigments and the labile scotopic visual-system of fish . Vision Res. 24, 1563–1573. 10.1016/0042-6989(84)90314-6 (doi:10.1016/0042-6989(84)90314-6) [DOI] [PubMed] [Google Scholar]
- 11.Hart NS, Lisney TJ, Collin SP. 2006. Cone photoreceptor oil droplet pigmentation is affected by ambient light intensity . J. Exp. Biol. 209, 4776–4787. 10.1242/jeb.02568 (doi:10.1242/jeb.02568) [DOI] [PubMed] [Google Scholar]
- 12.Cheng C, Flamarique I. 2004. Opsin expression: new mechanism for modulating colour vision: single cones start making a different opsin as young salmon move to deeper waters . Nature 428, 279. 10.1038/428279a (doi:10.1038/428279a) [DOI] [PubMed] [Google Scholar]
- 13.Shand J, Hart N, Thomas N, Partridge J. 2002. Developmental changes in the cone visual pigments of black bream Acanthopagrus butcheri . J. Exp. Biol. 205, 3661–3667. 10.1242/jeb.012047 (doi:10.1242/jeb.012047) [DOI] [PubMed] [Google Scholar]
- 14.Shand J, et al. 2008. The influence of ontogeny and light environment on the expression of visual pigment opsins in the retina of the black bream, Acanthopagrus butcheri . J. Exp. Biol. 211, 1495–1503. 10.1242/jeb.012047 (doi:10.1242/jeb.012047) [DOI] [PubMed] [Google Scholar]
- 15.Johnsen S, Kelber A, Warrant E, Sweeney A, Widder E, Lee R, Hernandez-Andres J. 2006. Crepuscular and nocturnal illumination and its effects on color perception by the nocturnal hawkmoth Deilephila elpenor . J. Exp. Biol. 209, 789–800. 10.1242/jeb.02053 (doi:10.1242/jeb.02053) [DOI] [PubMed] [Google Scholar]
- 16.Endler JA. 1991. Variation in the appearance of guppy color patterns to guppies and their predators under different visual conditions . Vision Res. 31, 587–608. 10.1016/0042-6989(91)90109-I (doi:10.1016/0042-6989(91)90109-I) [DOI] [PubMed] [Google Scholar]
- 17.Munz FW, McFarland WN. 1973. Significance of spectral position in rhodopsins of tropical marine fishes . Vision Res. 13, 1829–1874. 10.1016/0042-6989(73 (doi:10.1016/0042-6989(73)90060-6) [DOI] [PubMed] [Google Scholar]
- 18.Long K, Rosenqvist G. 1998. Changes in male guppy courting distance in response to a fluctuating light environment . Behav. Ecol. Sociobiol. 44, 77–83. 10.1007/s002650050518 (doi:10.1007/s002650050518) [DOI] [Google Scholar]
- 19.Endler JA. 1987. Predation, light-intensity and courtship behavior in Poecilia reticulate (Pisces: Poeciliidae) . Anim. Behav. 35, 1376–1385. 10.1016/S0003-3472(87)80010-6 (doi:10.1016/S0003-3472(87)80010-6) [DOI] [Google Scholar]
- 20.Endler J, Thery M. 1996. Interacting effects of lek placement, display behavior, ambient light, and color patterns in three neotropical forest-dwelling birds . Am. Nat. 148, 421–452. 10.1086/285934 (doi:10.1086/285934) [DOI] [Google Scholar]
- 21.Eckmiller M. 1997. Morphogenesis and renewal of cone outer segments . Prog. Retin. Eye Res. 16, 401–441. 10.1016/S1350-9462(96)00026-2 (doi:10.1016/S1350-9462(96)00026-2) [DOI] [Google Scholar]
- 22.Li P, Temple S, Gao Y, Haimberger T, Hawryshyn C, Li L. 2005. Circadian rhythms of behavioral cone sensitivity and long wavelength opsin mRNA expression: a correlation study in zebrafish . J. Exp. Biol. 208, 497–504. 10.1242/jeb.01424 (doi:10.1242/jeb.01424) [DOI] [PubMed] [Google Scholar]
- 23.Fuller RC, Fleishman LJ, Leal M, Travis J, Loew E. 2003. Intraspecific variation in retinal cone distribution in the bluefin killifish, Lucania goodei . J. Comp. Physiol. A 189, 609–616. 10.1007/s00359-003-0435-x (doi:10.1007/s00359-003-0435-x) [DOI] [PubMed] [Google Scholar]
- 24.Fuller RC, Carleton KL, Fadool JM, Spady TC, Travis J. 2004. Population variation in opsin expression in the bluefin killifish, Lucania goodei: a real-time PCR study . J. Comp. Physiol. A 190, 147–154. 10.1007/s00359-003-0478-z (doi:10.1007/s00359-003-0478-z) [DOI] [PubMed] [Google Scholar]
- 25.Fuller RC, Carleton KL, Fadool JM, Spady TC, Travis J. 2005. Genetic and environmental variation in the visual properties of bluefin killifish, Lucania goodei . J. Evol. Biol. 18, 516–523. 10.1111/j.1420-9101.2005.00886.x (doi:10.1111/j.1420-9101.2005.00886.x) [DOI] [PubMed] [Google Scholar]
- 26.Fuller RC, Claricoates KM. 2011. Rapid light-induced shifts in opsin expression: finding new opsins, discerning mechanisms of change, and implications for visual sensitivity . Mol. Ecol. 20, 3321–3335. 10.1111/j.1365-294X.2011.05180.x (doi:10.1111/j.1365-294X.2011.05180.x) [DOI] [PubMed] [Google Scholar]
- 27.Endler JA. 1992. Signals, signal conditions, and the direction of evolution . Am. Nat. 139, S125–S153. 10.1086/285308 (doi:10.1086/285308) [DOI] [Google Scholar]
- 28.Fuller RC, Noa LA, Strellner RS. 2010. Teasing apart the many effects of lighting environment on opsin expression and foraging preference in bluefin killifish . Am. Nat. 176, 1–13. 10.1086/652994 (doi:10.1086/652994) [DOI] [PubMed] [Google Scholar]
- 29.Fuller RC, Travis J. 2004. Genetics, lighting environment, and heritable responses to lighting environment affect male color morph expression in bluefin killifish, Lucania goodei . Evolution 58, 1086–1098. 10.1554/03-561 (doi:10.1554/03-561) [DOI] [PubMed] [Google Scholar]
- 30.Fuller RC, Noa LA. 2010. Female mating preferences, lighting environment, and a test of the sensory bias hypothesis in the bluefin killifish . Anim. Behav. 80, 23–35. 10.1016/j.anbehav.2010.03.017 (doi:10.1016/j.anbehav.2010.03.017) [DOI] [Google Scholar]
- 31.Yokoyama S, Takenaka N, Blow N. 2007. A novel spectral tuning in the short wavelength-sensitive (SWS1 and SWS2) pigments of bluefin killifish (Lucania goodei) . Gene 396, 196–202. 10.1016/j.gene.2007.03.019 (doi:10.1016/j.gene.2007.03.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson AM, Stanis S, Fuller RC. 2013. Data from: diurnal lighting patterns and habitat alter opsin expression and colour preferences in a killifish . Dryad Digital Repository. 10.5061/dryad.7tg4h (doi:10.5061/dryad.7tg4h) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bok D. 1970. Distribution and renewal of RNA in retinal rods . Invest. Ophthalmol. 9, 516–523. 10.1016/0042-6989(72)90108-3 (doi:10.1016/0042-6989(72)90108-3) [DOI] [PubMed] [Google Scholar]
- 34.Bok D, Young RW. 1972. Renewal of diffusely distributed protein in outer segments of rods and cones . Vision Res. 12, 161–168. 10.1016/0042-6989(72)90108-3 (doi:10.1016/0042-6989(72)90108-3) [DOI] [PubMed] [Google Scholar]
- 35.Halstenberg S, Lindgren K, Samagh S, Nadal-Vicens M, Balt S, Fernald R. 2005. Diurnal rhythm of cone opsin expression in the teleost fish Haplochromis burtoni . Vis. Neurosci. 22, 135–141. 10.1017/S09522523805222022 (doi:10.1017/S09522523805222022) [DOI] [PubMed] [Google Scholar]
- 36.Li L, Dowling J. 1998. Zebrafish visual sensitivity is regulated by a circadian clock . Vis. Neurosci. 15, 851–857. 10.1017/S0952523898155050 (doi:10.1017/S0952523898155050) [DOI] [PubMed] [Google Scholar]
- 37.Fuller RC. 2001. Patterns in male breeding behaviors in the bluefin killifish, Lucania goodei: a field study (Cyprinodontiformes: Fundulidae) . Copeia 2001, 823–828. 10.1643/0045-8511(2001)001[0823:PIMBBI]2.0.CO;2 (doi:10.1643/0045-8511(2001)001[0823:PIMBBI]2.0.CO;2) [DOI] [Google Scholar]
- 38.Losey G, Cronin T, Goldsmith T, Hyde D, Marshall N, McFarland W. 1999. The UV visual world of fishes: a review . J. Fish Biol. 54, 921–943. 10.1111/j.1095-8649.1999.tb00848.x (doi:10.1111/j.1095-8649.1999.tb00848.x) [DOI] [Google Scholar]
- 39.Rodd FH, Hughes KA, Grether GF, Baril CT. 2002. A possible non-sexual origin of mate preference: are male guppies mimicking fruit? Proc. R. Soc. Lond. B 269, 475–481. 10.1098/rspb.2001.1891 (doi:10.1098/rspb.2001.1891) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith C, Barber I, Wootton R, Chittka L. 2004. A receiver bias in the origin of three-spined stickleback mate choice . Proc. R. Soc. Lond. B 271, 949–955. 10.1098/rspb.2004.2690 (doi:10.1098/rspb.2004.2690) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ham AD, Osorio D. 2007. Colour preferences and colour vision in poultry chicks . Proc. R. Soc. B 274, 1941–1948. 10.1098/rspb.2007.0538 (doi:10.1098/rspb.2007.0538) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leal M, Fleishman L. 2002. Evidence for habitat partitioning based on adaptation to environmental light in a pair of sympatric lizard species . Proc. R. Soc. Lond. B 269, 351–359. 10.1098/rspb.2001.1904 (doi:10.1098/rspb.2001.1904) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thery M, Pincebourde S, Feer F. 2008. Dusk light environment optimizes visual perception of conspecifics in a crepuscular horned beetle . Behav. Ecol. 19, 627–634. 10.1093/beheco/arn024 (doi:10.1093/beheco/arn024) [DOI] [Google Scholar]
- 44.Gray SM, Dill LM, Tantu FY, Loew ER, Herder F, McKinnon JS. 2008. Environment-contingent sexual selection in a colour polymorphic fish . Proc. R. Soc. B 275, 1785–1791. 10.1098/rspb.2008.0283 (doi:10.1098/rspb.2008.0283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uy J, Endler J. 2004. Modification of the visual background increases the conspicuousness of golden-collared manakin displays . Behav. Ecol. 15, 1003–1010. 10.1093/beheco/arh106 (doi:10.1093/beheco/arh106) [DOI] [Google Scholar]
- 46.Dalton BE, Cronin TW, Marshall NJ, Carleton KL. 2010. The fish eye view: are cichlids conspicuous? J. Exp. Biol. 213, 2243–2255. 10.1242/jeb.037671 (doi:10.1242/jeb.037671) [DOI] [PubMed] [Google Scholar]
- 47.Fuller RC. 2002. Lighting environment predicts the relative abundance of male colour morphs in bluefin killifish (Lucania goodei) populations . Proc. R. Soc. Lond. B 269, 1457–1465. 10.1098/rspb.2002.2042 (doi:10.1098/rspb.2002.2042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vorobyev M, Osorio D. 1998. Receptor noise as a determinant of colour thresholds . Proc. R. Soc. Lond. B 265, 351–358. 10.1098/rspb.1998.0302 (doi:10.1098/rspb.1998.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baldwin J, Johnsen S. 2012. The male blue crab, Callinectes sapidus, uses both chromatic and achromatic cues during mate choice . J. Exp. Biol. 215, 1184–1191. 10.1242/jeb.067512 (doi:10.1242/jeb.067512) [DOI] [PubMed] [Google Scholar]
- 50.Osorio D, Vorobyev M. 1996. Colour vision as an adaptation to frugivory in primates . Proc. R. Soc. Lond. B 263, 593–599. 10.1098/rspb.1996.0089 (doi:10.1098/rspb.1996.0089) [DOI] [PubMed] [Google Scholar]
- 51.Uy JAC, Stein AC. 2007. Variable visual habitats may influence the spread of colourful plumage across an avian hybrid zone . J. Evol. Biol. 20, 1847–1858. 10.1111/j.1420-9101.2007.01378.x (doi:10.1111/j.1420-9101.2007.01378.x) [DOI] [PubMed] [Google Scholar]
- 52.Cummings ME. 2004. Modelling divergence in luminance and chromatic detection performance across measured divergence in surfperch (Embiotocidae) habitats . Vision Res. 44, 1127–1145. 10.1016/j.visres.2003.12.013 (doi:10.1016/j.visres.2003.12.013) [DOI] [PubMed] [Google Scholar]
- 53.Vorobyev M, Osorio D, Bennett ATD, Marshall NJ, Cuthill IC. 1998. Tetrachromacy, oil droplets and bird plumage colours . J. Comp. Physiol A 183, 621–633. 10.1007/s003590050286 (doi:10.1007/s003590050286) [DOI] [PubMed] [Google Scholar]
- 54.Cheng CL, Flamarique IN. 2007. Chromatic organization of cone photoreceptors in the retina of rainbow trout: single cones irreversibly switch from UV (SWS1) to blue (SWS2) light sensitive opsin during natural development . J. Exp. Biol. 210, 4123–4135. 10.1242/jeb.009217 (doi:10.1242/jeb.009217) [DOI] [PubMed] [Google Scholar]
- 55.Evans BI, Fernald RD. 1993. Retinal transformation at metamorphosis in the winter flounder (Pseudopleuronectes americanus) . Vis. Neurosci. 10, 1055–1064. 10.1017/S0952523800010166 (doi:10.1017/S0952523800010166) [DOI] [PubMed] [Google Scholar]
- 56.Taylor SM, Loew ER, Grace MS. 2011. Developmental shifts in functional morphology of the retina in Atlantic tarpon, Megalops atlanticus (Elopomorpha: Teleostei) between four ecologically distinct life-history stages . Vis. Neurosci. 28, 309–323. 10.1017/S0952523810000362 (doi:10.1017/S0952523810000362) [DOI] [PubMed] [Google Scholar]
- 57.Carleton KL, Kocher TD. 2001. Cone opsin genes of African cichlid fishes: tuning spectral sensitivity by differential gene expression . Mol. Biol. Evol. 18, 1540–1550. 10.1093/oxfordjournals.molbev.a003940 (doi:10.1093/oxfordjournals.molbev.a003940) [DOI] [PubMed] [Google Scholar]