Abstract
The avian magnetic compass works in a fairly narrow functional window around the intensity of the local geomagnetic field, but adjusts to intensities outside this range when birds experience these new intensities for a certain time. In the past, the geomagnetic field has often been much weaker than at present. To find out whether birds can obtain directional information from a weak magnetic field, we studied spontaneous orientation preferences of migratory robins in a 4 µT field (i.e. a field of less than 10 per cent of the local intensity of 47 µT). Birds can adjust to this low intensity: they turned out to be disoriented under 4 µT after a pre-exposure time of 8 h to 4 µT, but were able to orient in this field after a total exposure time of 17 h. This demonstrates a considerable plasticity of the avian magnetic compass. Orientation in the 4 µT field was not affected by local anaesthesia of the upper beak, but was disrupted by a radiofrequency magnetic field of 1.315 MHz, 480 nT, suggesting that a radical-pair mechanism still provides the directional information in the low magnetic field. This is in agreement with the idea that the avian magnetic compass may have developed already in the Mesozoic in the common ancestor of modern birds.
Keywords: magnetic compass, functional window, ability to adjust, radical-pair mechanism, radiofrequency fields
1. Introduction
One of the characteristic properties of the magnetic inclination compass of birds is that it spontaneously works only in a fairly narrow functional window. Decreasing or increasing the local intensity by about 25–30 per cent was shown to lead to disorientation in European robins, Erithacus rubecula (Turdidae), and garden warblers, Sylvia borin, two species of small passerine migrants, and in domestic chickens, Gallus gallus [1–3]. This functional window is not fixed, however, but can adjust to intensities outside this range when the birds experience these other intensities for a certain time. European robins caught in Frankfurt am Main in a local field of 47 µT and housed for at least 3 days at 16 µT were able to orient in 16 µT and continued to be oriented in 46 µT. The same was true for birds pre-exposed in a 150 µT field [4].
For migratory birds, the ability to adjust their magnetic compass to new intensities is important, because the intensity of the geomagnetic field varies worldwide between 23 and 66 µT, generally with high values in polar regions and low values near the equator [5]. For example, robins that grow up in central Scandinavia in intensities above 50 µT migrate to the Mediterranean region with intensities of about 40 µT. Long-distance migrants such as garden warblers from the same region of origin winter in Africa south of the Sahara desert, where intensities are as low as 32 µT. With southward migration lasting about three months, the intensity changes encountered en route are obviously small enough for the magnetic compass to gradually adjust.
The geomagnetic intensity does not only vary across the globe, but also in time. Since the first absolute measurements by C. F. Gauss in 1833, the geomagnetic dipole strength has decreased by about 10 per cent. However, the present-day magnetic field is still 30 per cent larger compared with the temporal average over the last 800 000 years [6], and at times it may have been as low as 4 µT [7].
This raises the question of whether the avian magnetic compass can still perform in such a strongly diminished field, and, if so, how much pre-exposure time birds would need to adjust. A radical pair process, which forms the physical basis of the avian inclination compass [8,9], was theoretically predicted to be effective in present-day Earth-strength fields, but whether it could cope with fields below 5 µT is unclear [10]. Apart from the radical-pair mechanism, birds have a second type of magnetoreception pathway, which is based on magnetite and linked to the trigeminal nerve. Because it seemed possible that this magnetite-based mechanism takes over under low field conditions, we pre-exposed and tested robins in a low field of 4 µT and applied specific treatments targeting either mechanism.
2. Material and methods
The experiments were performed in Frankfurt am Main, Germany (50°08′ N, 8°40′ E) in spring and autumn 2010.
(a). Test birds
The test birds were European robins, a small passerine species that breeds in most parts of Europe and spends the winter season in the Mediterranean countries; the northern and eastern populations are nocturnal migrants. Juvenile transmigrants, probably of Scandinavian origin, were caught in September 2009 in the Botanical Garden in Frankfurt. They were kept in individual cages in a photoperiod simulating the natural one during autumn until beginning of December, when it was reduced to 8 L : 16 D cycle. At the end of December, the photoperiod was increased in two steps to 13 L : 10 D to induce premature Zugunruhe (migratory restlessness) that allowed us to test the birds in spring migratory state from early January to mid-February 2010. A second group of robins was caught in September 2010, kept under the same photoperiod as the first group and was tested during autumn migration from end of September until mid-October 2010.
(b). Test conditions
The test rooms were wooden buildings where the local geomagnetic field of 47 µT with 66° inclination was largely undisturbed; it was used for control tests. For the critical tests in the 4 µT field with 66° inclination, the geomagnetic field was partly compensated with the help of Helmholtz coils. Magnetic field homogeneity at the test sites was controlled with a triaxial flux gate magnetometer (FL3-BT, Stefan Maier Instruments).
The 12 birds to be tested in spring 2010 were exposed to the 4 µT field in December 2009 in another room for 3 days, because earlier experiments had shown that an exposure of at least 3 days enables robins to orient in the 16 µT field [1,4]. Prior to each test in the 4 µT field, the birds to be tested that night were transferred to a second set of housing cages 8 h before the tests began. These cages stood in an exposure room, a wooden building similar to the test huts, where the birds were pre-exposed in a 4 µT field identical with the test field. In autumn, the 16 birds of the second group did not receive a 3-day pre-exposure before testing began, but were tested in the 4 µT field after a 8 h pre-exposure to this field.
Additional tests in spring 2010 were performed to analyse the mechanism providing the information on magnetic directions in the 4 µT field:
— To disturb an underlying radical-pair mechanism (see previous studies [8,11–13]), but not a magnetite-based mechanism (see [8]), the birds were exposed to a radiofrequency magnetic field of 480 nT, applied at an angle of 24° to the vector of the 4 µT static field. We could have used any frequency above 0.650 MHz (see [9]); for practical reasons, we chose 1.315 MHz. The radiofrequency field was produced by a magnetic loop antenna consisting of a single winding of coaxial cable with 2 cm of the screening removed opposite to the feed (for details, see [8]). Both radiofrequency generator and power amplifier were put up outside the test building and switched on 1 h before the start of the actual experiment for control measurements and to avoid drift. The spectral purity of the radiofrequency field and its spatial homogeneity in the test sites were controlled with a passive H-field probe (6 cm diameter, Rhode & Schwarz, probe set HZ-11), connected to a spectrum analyser with 1 Hz resolution (HP 89410A).
— To temporarily deactivate a putative magnetite-based magnetoreception pathway associated with the ophthalmic branch of the trigeminal nerve [14–16] (for review, see [17]), the upper beak of the birds was locally anaesthetized with Xylocaine 2 per cent (Astra Zeneca GmbH, Wedel, Germany; active substance: lydocainhydrochlorid 1 H2O). The anaesthetic was applied externally by gently rubbing a soaked cotton plug along the edges of the upper beak about 5 min before tests began, a procedure that had been shown to disrupt responses that do not originate from the radical pair processes in the eye; yet it does not affect normal compass orientation (see earlier studies [18,19] for details).
(c). Test procedures
Testing followed standard procedures: the robins were tested individually once per day in funnel-shaped cage lines with thermo paper (Blumberg Systempapiere; see [20]). The test cages were lit with 565 nm green light at an intensity of 1.9 mW m−2—birds have always shown excellent orientation using their inclination compass in this condition [18,21]. The activity was recorded for 60 min. Each bird was tested three times in each condition; if a bird failed to produce at least 35 scratches, the activity was considered insufficient and the test was repeated.
(d). Data analysis and statistics
For data analysis, the paper was removed from the cages, divided into 24 sectors and the scratches were counted blind. From the distribution of the scratches, the heading of the test was calculated. The three headings of each bird in each condition were added to produce a vector with the heading αb and the length rb for that bird. From the mean headings αb we calculated second-order grand mean vectors which were tested for significant directional preference using the Rayleigh test. The data obtained in the 4 µT fields are compared with the control data in the geomagnetic field for differences in scatter by the Mann–Whitney U-test applied to the deviations of the individual mean headings from the grand mean [22]. From the birds’ vectors lengths rb, medians were calculated and they were compared with the corresponding control data by the Wilcoxon test for paired samples.
3. Results
Table 1 summarizes the numerical data of our experiments and indicates significant differences between experimental and control conditions; the data for the individual birds are given in the electronic supplementary material, tables S1 and S2.
Table 1.
Orientation of European Robins in the various test conditions. Condition: C, control tests in the geomagnetic field; 8pe4µT, after 8 h pre-exposure in a 4 µT field tested in the 4 µT field; 8pe4µTRF, as before, but with a radiofrequency field of 1.315 MHz, 480 nT added; 8pe4µTXy, after 8 h pre-exposure tested in the 4 µT field with the beak locally anaesthetized. N, number of birds tested; n, number of tests per bird; med rb, median vector length per bird based on three recordings, with symbols indicating significant differences to control by the Wilcoxon test; αN and rN, direction and length, respectively, of the grand mean vector (in parentheses if not significant), with symbols indicating significance by the Rayleigh test; ΔC and Δ8pe4µT, angular difference to control and difference to 8pe4µT, respectively, with symbols indicating statistically significant differences by the Mann Whitney U-test. Significance levels: ***p < 0.001; **p < 0.01; *p < 0.05; n.s., not significant.
| season | condition | N | n | med rb | αN | rN | ΔC | Δ8pe4µT |
|---|---|---|---|---|---|---|---|---|
| spring | C | 12 | 3 | 0.94 | 11° | 0.95*** | C1 | |
| 8pe4µT | 12 | 3 | 0.68** | 23° | 0.81*** | +12°(n.s.) | ||
| 8pe4µTRF | 12 | 3 | 0.54** | (127°) | 0.11 (n.s.) | +116° *** | +104° ** | |
| 8pe4µTXy | 12 | 3 | 0.81n.s. | 7° | 0.87*** | −4°(n.s.) | −16° (n.s.) | |
| autumn | C | 16 | 3 | 0.56 | 199° | 0.54** | C2 | |
| 8pe4µT | 16 | 4 | 0.59n.s. | 197° | 0.54** | −2°(n.s.) |
(a). Can birds adjust to a 4 µT field?
The aim of the experiments in spring 2010 was to find out whether birds could in principle adjust to a magnetic intensity as low as 4 µT, and possibly to identify the mechanism providing the directional information in this low field. The test birds had experienced the 4 µT field already for 3 days before the experimental season and they were pre-exposed to this field for 8 h before each test in this intensity. Their orientation is shown in figure 1: they were oriented in their northerly migratory direction in control tests in the geomagnetic field and also in the 4 µT field (upper diagrams), with the two distributions not being significantly different. A 1.315 MHz radiofrequency field led to disorientation, whereas anaesthesia of the upper beak had no effect (lower diagrams). This indicates that the directional information in the 4 µT field was still provided by the radical-pair mechanism.
Figure 1.
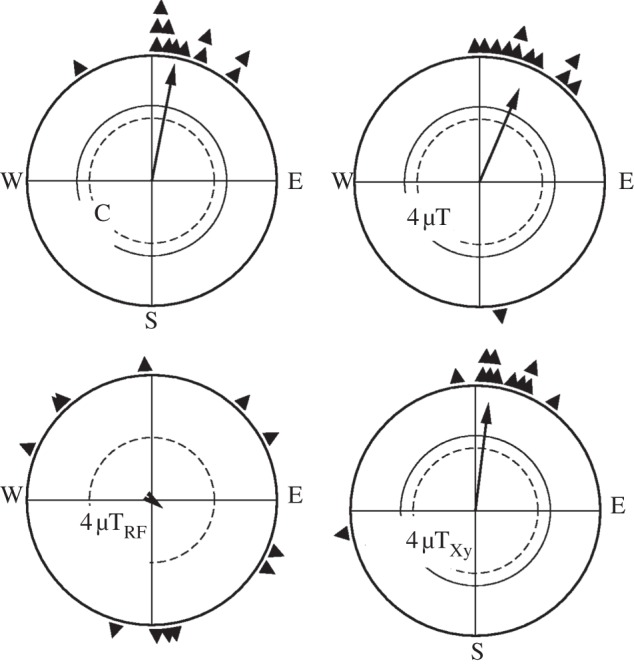
Orientation of European robins during spring migration in the local geomagnetic field (control, C) and, after pre-exposure, in the 4 µT field (4 µT), in this field with a radiofrequency field of 1.315 MHz, 480 nT added (4 µTRF), and with the skin of the upper beak locally anaesthetized by Xylocaine (4 µTXy). The triangles at the periphery of the circle mark the mean headings of the individual birds, the arrows represent the grand mean vector in relation to the radius of the circle = 1, and the two inner circles are the 5% (dashed) and the 1% border of the Rayleigh test [22].
While the distributions of the birds’ mean headings in the three oriented samples do not differ from each other (table 1), the comparison of the intra-individual variance represented by the individual vector length rb is inconsistent: the rb values in the 4 µT field were significantly shorter than the ones of the control tests in the geomagnetic field, but they were not different when the birds additionally had their beaks anaesthetized. In the tests with the radiofrequency field added, the birds’ mean headings were more scattered, and their individual mean vectors were shorter (table 1).
(b). How much time is required for the adjustment?
The experiments in autumn 2010 were conducted primarily to obtain a rough estimate of the exposure time required to adjust to the 4 µT field. We tested new birds, this time without prior exposure to the 4 µT field before the testing began; the birds were exposed to the low field only for 8 h before each test in this field.
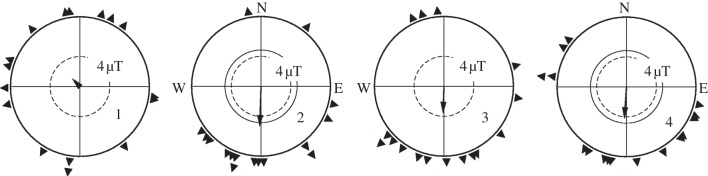
In the control tests, the robins were significantly oriented in their seasonally appropriate southerly migratory direction (figure 2a). In the 4 µT field, however, their behaviour changed as testing progressed. The birds were tested four times under this condition. Their individual headings in each round of tests are given in figure 3: their first recordings after just 8 h pre-exposure were still disoriented (N = 16, 316°, 0.16 n.s., Rayleigh test), but in the second round of tests after another pre-exposure of 8 h, the lengths of the resulting vector increased and indicated a significant southerly directional preference (N = 16, 182°, 0.58, p < 0.01). They continued to appear oriented in the following two tests (figure 3). Summarizing the four tests per birds (including the first ones) resulted in oriented behaviour in the migratory direction, which was statistically indistinguishable from the direction observed in the local geomagnetic field (figure 2b). The individual vector lengths rb did not differ either.
Figure 2.
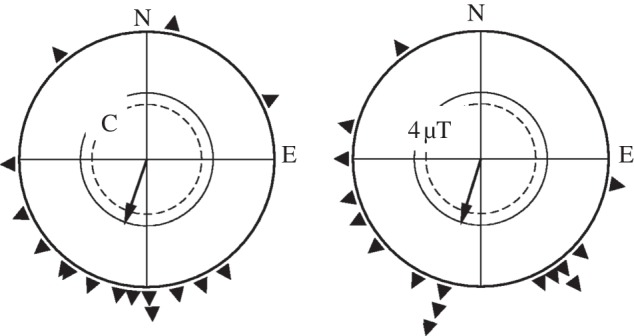
Orientation of robins in autumn in the local geomagnetic field (control, C) and, after pre-exposure, in the 4 µT field (4 µT). Symbols as in figure 1.
Figure 3.
Orientation of robins in autumn tested in the 4 μT field after 8 h pre-exposure in the 4 μT field before each test. In the four diagrams, the triangles at the periphery of the circle indicate the individual headings of the 16 test birds during the 1st, 2nd, 3rd and 4th test. Symbols as in figure 1.
Obviously, birds require a certain amount of time to adjust their magnetic compass to an intensity as low as 4 µT. Eight hours had not been sufficient, but after a total exposure time of 17 h, they were able to orient in the low field.
4. Discussion
Our findings demonstrate great plasticity of the avian magnetic compass: it was able to adjust to a magnetic field of intensity as low as 4 µT, which is less than 10 per cent of the geomagnetic field at the test site. The adjustment to this low intensity required a certain time. In previous experiments [9,23], a pre-exposure of only 1 h had been sufficient to enable robins to orient in a field of 92 µT (i.e. about twice the local intensity); in the 4 µT field, they needed an exposure time of more than 8 h to be able to obtain directional information.
This raises a question about the underlying mechanism. The inclination compass normally used for direction finding is provided by the radical-pair mechanism in the eye. Theoretical considerations [24,25] indicate that this mechanism may indeed be able to convey directional information also in rather low magnetic fields. However, under experimental conditions that prevented the radical-pair mechanism from working properly, such as total darkness or certain bichromatic illumination conditions, birds show so-called ‘fixed direction’ responses, which were found to be based on directional information that was probably derived from a magnetite-based mechanism in the beak (see [17] for review). Hence, it seemed possible that this mechanism took over in the low field situation. The results of the tests with the radiofrequency field added and with the upper beak anaesthetized speak against this possibility: even in the 4 µT field, the directional information appears to be still mediated by the radical-pair mechanism. This is also supported by the observation that the birds in the low field preferred their seasonally appropriate migratory direction in both seasons—they headed south in autumn and north in spring—whereas ‘fixed direction’ responses are characterized by not showing this seasonal change.
We can only speculate as to how the adjustment of the radical-pair mechanism to higher or lower intensities works. According to the radical pair model [10], birds derive magnetic directions from an activity pattern produced by radical pair processes on their retina. Changes in magnetic intensity alter this pattern, but retain its symmetry about the magnetic field axis. Within the functional window, these changes seem to be so small that the birds can still spontaneously interpret a slightly altered pattern. Yet, when faced with higher or lower intensities, birds may at first be confused by the novel pattern, but with increasing exposure time may learn to interpret the unfamiliar pattern and derive directional information from its axis of symmetry. Interestingly, birds pre-exposed to 150 µT were oriented in a 150 µT field and also in the local geomagnetic field of 46 µT, but not in an intermediate intensity of 81 µT, suggesting the formation of an additional window rather than a shift or enlargement of the original window [4]. This observation is in agreement with the idea that birds must learn to interpret new patterns by experience. The adjustment of the magnetic compass to an intensity as low as 4 µT requires considerably more time than that to 92 µT, for example [23]. Possibly, in the weak magnetic field, the differences in activation of the pattern are less pronounced so that it is generally harder to interpret, resulting in the birds needing more time until their magnetic compass is ready to cope with it.
The ability of the avian magnetic compass to adjust to very low magnetic intensities has important implications in an evolutionary context. The magnetic compass of European robins and domestic chickens was shown to function in the same way [3], and cryptochrome, the most likely receptor molecule, is found at the discs of the UV/V cones in the retina of both species [26]. Chickens and passerine birds are only distantly related—their lineages branched off in the late Cretaceous, around 95 Ma [27]. Hence, the similarities in their magnetic compass mechanism (which differs, for example, from that of mammals [28–31]) appear to suggest a common origin—a compass mechanism developed already by the ancestors of all modern birds in the Mesozoic age [32].
Such considerations must take into account past variations in the geomagnetic field. The most dramatic magnetic field changes of global extent are polarity reversals, during which the normally predominating dipolar part of the field drops to anomalously low values before changing sign. According to a mathematical model of the last reversal at about 775 000 years ago [33], surface fields of 4 µT and less were common in most of Europe and Africa for at least 100 years (see [7]). Since the last reversal, there have been several geomagnetic excursions, during which the dipolar field significantly weakened without undergoing a complete reversal [6,34]. For example, during the Laschamp excursion 40 000 years ago, the surface field over most of Europe and northwest Africa was as weak as 4 µT (see [7], on the basis of the model by Leonhardt et al. [35]). Our present data suggest that the avian magnetic compass based on radical-pair processes would have been able to cope with such low fields. We cannot exclude that the ancestors of our birds may have developed additional mechanisms to handle the very low intensity effectively—mechanisms that became too costly to maintain when no longer needed. On the other hand, since birds accustomed to the strong present-day field can orient in a field as low as 4 µT after a while of exposure, there appears to be no need to invoke additional mechanisms in their ancestors. This prompts the interesting question of whether the avian inclination compass can adjust to yet weaker magnetic fields and ultimately reach the limit intensity dictated by the Heisenberg uncertainty principle.
Acknowledgements
Our work was supported by the Deutsche Forschungsgemeinschaft (grant to R.W.). We sincerely thank S. Denzau and D. Gehring for their valuable help with conducting the experiments. The experiments were performed in accordance with the rules and regulations of animal welfare in Germany.
References
- 1.Wiltschko W. 1968. Über den Einfluß statischer Magnetfelder auf die Zugorientierung der Rotkehlchen (Erithacus rubecula). Z. Tierpsychol. 25, 536–558. 10.1111/j.1439-0310.1968.tb00028.x (doi:10.1111/j.1439-0310.1968.tb00028.x) [DOI] [PubMed] [Google Scholar]
- 2.Wiltschko W. 1974. Der Magnetkompaß der Gartengrasmücke (Sylvia borin). J. Ornithol. 115, 1–7. 10.1007/BF01647313 (doi:10.1007/BF01647313) [DOI] [Google Scholar]
- 3.Wiltschko W, Freire R, Munro U, Ritz T, Rogers L, Thalau P, Wiltschko R. 2007. The magnetic compass of domestic chickens, Gallus gallus. J. Exp. Biol. 210, 2300–2310. 10.1242/jeb.004853 (doi:10.1242/jeb.004853) [DOI] [PubMed] [Google Scholar]
- 4.Wiltschko W. 1978. Further analysis of the magnetic compass of migratory birds. In Animal migration, navigation, and homing (eds Schmidt-Koenig K, Keeton WT.), pp. 302–310. Berlin, Germany: Springer. [Google Scholar]
- 5.Finlay CC, et al. 2010. International geomagnetic reference field: the eleventh generation. Geophys. J. Int. 183, 1216–1230. [Google Scholar]
- 6.Guyodo Y, Valet J-P. 1999. Global changes in the intensity of the Earth's magnetic field during the past 888 kyr. Nature 399, 249–252. 10.1038/20420 (doi:10.1038/20420) [DOI] [Google Scholar]
- 7.Kirschvink JL, Winklhofer M, Walker MM. 2010. Biophysics of magnetic orientation: strengthening the interface between theory and experimental design. J. R. Soc. Interface 7, S179–S191. 10.1098/rsif.2009.0491.focus (doi:10.1098/rsif.2009.0491.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ritz T, Thalau P, Philllips JB, Wiltschko R, Wiltschko W. 2004. Resonance effects indicate a radical-pair mechanism for avian magnetic compass. Nature 429, 177–180. 10.1038/nature02534 (doi:10.1038/nature02534) [DOI] [PubMed] [Google Scholar]
- 9.Ritz T, Wiltschko R, Hore PJ, Rodgers CT, Stapput K, Thalau P, Timmel CR, Wiltschko W. 2009. Magnetic compass of birds is based on a molecule with optimal directional sensitivity. Biophys. J. 96, 3451–3457. 10.1016/j.bpj.2008.11.072 (doi:10.1016/j.bpj.2008.11.072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ritz T, Adem S, Schulten K. 2000. A model for photoreceptor-based magnetoreception in birds. Biophys. J. 78, 707–718. 10.1016/S0006-3495(00)76629-X (doi:10.1016/S0006-3495(00)76629-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ritz T. 2001. Disrupting magnetic compass orientation with radio frequency oscillating fields. In Orientation and navigation—birds, humans and other animals (ed. Royal Institute of Navigation) , paper no. 4. Oxford, UK: Royal Institute of Navigation.
- 12.Henbest KB, Kukura P, Rodgers CT, Hore PJ, Timmel CR. 2004. Radio frequency magnetic field effects on a radical recombination reaction: a diagnostic test for the radical pair mechanism. J. Am. Chem. Soc. 126, 8102–8103. 10.1021/ja048220q (doi:10.1021/ja048220q) [DOI] [PubMed] [Google Scholar]
- 13.Thalau P, Ritz T, Stapput K, Wiltschko R, Wiltschko W. 2005. Magnetic compass orientation of migratory birds in the presence of a 1.315 MHz oscillating field. Naturwissenschaften 92, 86–90. 10.1007/s00114-004-0595-8 (doi:10.1007/s00114-004-0595-8) [DOI] [PubMed] [Google Scholar]
- 14.Semm P, Beason RC. 1990. Responses to small magnetic variations by the trigeminal system of the bobolink. Brain Res. Bull. 25, 735–740. 10.1016/0361-9230(90)90051-Z (doi:10.1016/0361-9230(90)90051-Z) [DOI] [PubMed] [Google Scholar]
- 15.Wiltschko W, Munro U, Ford H, Wiltschko R. 2009. Avian orientation: the pulse effect is mediated by the magnetite receptors in the upper beak. Proc. R. Soc. B. 276, 2227–2232. 10.1098/rspb.2009.0050 (doi:10.1098/rspb.2009.0050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heyers D, Zapka M, Hoffmeister M, Wild JM, Mouritsen H. 2010. Magnetic field changes activate the trigeminal brainstem complex in a migratory bird. Proc. Natl Acad. Sci. USA 197, 9394–9399. 10.1073/pnas.0907068107 (doi:10.1073/pnas.0907068107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiltschko R, Wiltschko W. 2013. The magnetite-based receptors in the beak of birds and their role in avian navigation. J. Comp. Physiol. A 199, 89–98. 10.1007/s00359-012-0769-3 (doi:10.1007/s00359-012-0769-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiltschko R, Stapput K, Thalau P, Wiltschko W. 2010. Directional orientation of birds by the magnetic field under different light conditions. J. R. Soc. Interface 7, S163–S177. 10.1098/rsif.2009.0367.focus (doi:10.1098/rsif.2009.0367.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiltschko R, Dehe L, Gehring D, Thalau P, Wiltschko W. 2013. Interactions between the visual and the magnetoreception system: different effects of bichromatic light regimes on the directional behavior of migratory birds. J. Physiol. Paris 107, 137–146. 10.1016/j.jphysparis.2012.03.003 (doi:10.1016/j.jphysparis.2012.03.003) [DOI] [PubMed] [Google Scholar]
- 20.Mouritsen H, Feenders G, Hegemann A, Liedvogel M. 2009. Thermal paper can replace typewriter correction paper in Emlen funnels. J. Ornithol. 150, 713–715. 10.1007/s10336-009-0421-3 (doi:10.1007/s10336-009-0421-3) [DOI] [Google Scholar]
- 21.Wiltschko W, Gesson M, Wiltschko R. 2001. Magnetic compass orientation of European robins under 565 nm green light. Naturwissenschaften 88, 387–390. 10.1007/s001140100248 (doi:10.1007/s001140100248) [DOI] [PubMed] [Google Scholar]
- 22.Batschelet E. 1981. Circular statistics in biology. London, UK: Academic Press. [Google Scholar]
- 23.Wiltschko W, Stapput K, Thalau P, Wiltschko R. 2006. Avian magnetic compass: fast adjustment to intensities outside the normal functional window. Naturwissenschaften 93, 300–304. 10.1007/s00114-006-0102-5 (doi:10.1007/s00114-006-0102-5) [DOI] [PubMed] [Google Scholar]
- 24.Cintolesi F, Ritz T, Kay CWM, Timmel CR, Hore PJ. 2003. Anisotropic recombination of an immobilized radical pair in a 50-µT magnetic field: a model avian photomagnetoreceptor. Chem. Phys. 294, 385–399. 10.1016/S0301-0104(03)00320-3 (doi:10.1016/S0301-0104(03)00320-3) [DOI] [Google Scholar]
- 25.Hogben H, Biskup T, Hore PJ. 2012. Entanglement and sources of magnetic anisotropy in radical pair-based avian magnetoreceptors. Phys. Rev. Lett. 109, 220501. 10.1103/PhysRevLett.109.220501 (doi:10.1103/PhysRevLett.109.220501) [DOI] [PubMed] [Google Scholar]
- 26.Nießner C, Denzau S, Gross J, Peichl L, Bischof HJ, Fleissner G, Wiltschko W, Wiltschko R. 2011. Avian ultraviolet/violet cones identified as probable magnetoreceptors. PLoS ONE 6, e20091. 10.1371/journal.pone.0020091 (doi:10.1371/journal.pone.0020091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ericson PGP, et al. 2006. Diversification of Neoaves: integration of molecular sequence data and fossils. Biol. Lett. 2, 543–547. 10.1098/rsbl.2006.0523 (doi:10.1098/rsbl.2006.0523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marhold S, Wiltschko W, Burda H. 1997. A magnetic polarity compass for direction finding in a subterranean mammal. Naturwissenschaften 84, 421–423. 10.1007/s001140050422 (doi:10.1007/s001140050422) [DOI] [Google Scholar]
- 29.Thalau P, Ritz T, Burda H, Wegner RE, Wiltschko R. 2006. The magnetic compass mechanisms of birds and rodents are based on different physical principles. J. R. Soc. Interface 3, 583–587. 10.1098/rsif.2006.0130 (doi:10.1098/rsif.2006.0130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Pan Y, Parsons S, Walker M, Zhang S. 2007. Bats respond to polarity of a magnetic field. Proc. R. Soc. B 274, 2901–2905. 10.1098/rspb.2007.0904 (doi:10.1098/rspb.2007.0904) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holland RA, Kirschvink JL, Doak TG, Wikelski M. 2008. Bats use magnetite to detect the earth's magnetic field. PLoS ONE 3, e1576. 10.1371/journal.pone.0001676 (doi:10.1371/journal.pone.0001676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiltschko R, Wiltschko W. 2010. Avian magnetic compass: its functional properties and physical basis. Curr. Zool. 56, 265–276. [Google Scholar]
- 33.Leonhardt R, Fabian K. 2007. Paleomagnetic reconstruction of the global geomagnetic field evolution during the Matuyama/Brunhes transition: iterative Bayesian inversion and independent verification. Earth Planet. Sci. Lett. 253, 172–195. 10.1016/j.epsl.2006.10.025 (doi:10.1016/j.epsl.2006.10.025) [DOI] [Google Scholar]
- 34.Roberts AP. 2008. Geomagnetic excursions: known and unknowns. Geophys. Res. Lett. 35, L17307. 10.1029/2008GL034719 (doi:10.1029/2008GL034719) [DOI] [Google Scholar]
- 35.Leonhardt R, Fabian K, Winklhofer M, Ferk A, Laj C, Kissel C. 2009. Geomaganetic field evolution during the Laschamp excursion. Earth Planet. Sci. Lett. 278, 87–95. 10.1016/j.epsl.2008.11.028 (doi:10.1016/j.epsl.2008.11.028) [DOI] [Google Scholar]