Abstract
Ecological and evolutionary mechanisms are increasingly thought to shape local community dynamics. Here, I evaluate if the local adaptation of a meso-predator to an apex predator alters local food webs. The marbled salamander (Ambystoma opacum) is an apex predator that consumes both the spotted salamander (Ambystoma maculatum) and shared zooplankton prey. Common garden experiments reveal that spotted salamander populations which co-occur with marbled salamanders forage more intensely than those that face other predator species. These foraging differences, in turn, alter the diversity, abundance and composition of zooplankton communities in common garden experiments and natural ponds. Locally adapted spotted salamanders exacerbate prey biomass declines associated with apex predation, but dampen the top-down effects of apex predation on prey diversity. Countergradient selection on foraging explains why locally adapted spotted salamanders exacerbate prey biomass declines. The two salamander species prefer different prey species, which explains why adapted spotted salamanders buffer changes in prey composition owing to apex predation. Results suggest that local adaptation can strongly mediate effects from apex predation on local food webs. Community ecologists might often need to consider the evolutionary history of populations to understand local diversity patterns, food web dynamics, resource gradients and their responses to disturbance.
Keywords: eco-evolutionary dynamics, community ecology, species interactions, evolutionary ecology, food web, top-down effects
1. Introduction
Ecologists have long sought to develop general predictions about community structure and dynamics based on local abiotic and biotic factors. Despite important successes (e.g. keystone predation [1]), purely ecological factors often have proved unsatisfying in their generality [2]. As a result, ecological surprises and failed predictions are common [3]. Some of these failed predictions might be explained by the local adaptation of key species in these communities.
Ecologists increasingly recognize the value of understanding evolutionary as well as ecological factors underlying community dynamics [4–12]. Evolutionary differentiation has been shown to alter pairwise species interactions [13], communities [14–17] and ecosystems [16,18–20]. We can state with increasing certainty that evolution can affect ecology. However, we still do not understand when and how evolution will affect ecology [8,12]. In particular, we do not know when evolution will dampen or exaggerate the top-down effects of apex predators on lower trophic levels. Here, I evaluate if local adaptation of prey to apex predators can alter local food webs by mediating top-down (predator-dependent) ecological effects.
I hypothesize that the covariance of genotypic and environmental contributions to phenotypic variation across heterogeneous environments will determine how locally adapted populations alter ecological dynamics. The sign of this covariance determines if local adaptation will exaggerate trait differences among heterogeneous selection environments or buffer it. A negative covariance produces countergradient variation, and a positive covariance produces co-gradient variation [21]. Countergradient variation occurs when the covariance of genotypic and environmental effects on the phenotype vary inversely across populations because genetic differences compensate for environmental effects on the phenotype and thereby dampen phenotypic differences among populations distributed across heterogeneous environments [21]. For instance, populations from cold habitats sometimes evolve to grow faster than those in warm habitats such that observed growth rates become similar despite underlying genetic differences [22,23]. Countergradient variation has the potential to buffer ecological properties across heterogeneous environments by producing similar phenotypes in populations of important species regardless of environmental heterogeneity. This buffering effect could lead to cryptic community dynamics (sensu [24]), where evolution masks ongoing ecological changes. Co-gradient variation occurs when genotypic and environmental effects operate in concert and exaggerate phenotypic differences. This exaggerated phenotypic differentiation, in turn, could amplify differences in emergent ecological properties between habitats.
Here, I investigate a set of spotted salamander (Ambystoma maculatum) populations that inhabit neighbouring ponds and encounter varying levels of risk from the apex predator, the marbled salamander (Ambystoma opacum; see the electronic supplementary material, figure S1). Marbled salamanders impose strong natural selection on spotted salamander traits through both direct predation and indirect consumption of shared zooplankton [25]. The apex predator's reduction of resources, in particular, might favour compensatory and countergradient selection for intense foraging in the meso-predator. Common garden experiments indicate that populations which co-occur with marbled salamanders forage more intensely than low-risk populations across microgeographical scales [26]. These differences in spotted salamander foraging intensity among populations, in turn, could potentially alter the ecological properties of aquatic food webs via effects on prey biomass and diversity.
I predict that countergradient selection for higher growth in resource-poor environments should generally exacerbate existing resource gradients because fast growers will consume more resources where resources are already scarce. Countergradient selection and the resulting genetic response of the meso-predator populations would thereby exacerbate the negative effects of apex predation on temporary pond prey community density, biomass and diversity (see predicted ecological effects of adapted spotted salamanders; table 1). Further, I predict that compositional effects will depend on how marbled and spotted salamanders partition resources. If the two salamanders prefer the same prey species, then rapidly foraging spotted salamanders will exaggerate compositional changes. If the two salamanders prefer different species, then spotted salamander adaptation will dampen compositional effects. I first evaluate how the marbled salamander affects zooplankton biomass, density, diversity and composition. I then evaluate how differently adapted spotted salamander populations alter zooplankton density, biomass, richness and composition in laboratory experiments, more realistic outdoor mesocosm experiments and natural ponds.
Table 1.
Does predation by marbled salamanders and intense foraging by high-risk populations of spotted salamanders increase (↑) or decrease (↓) zooplankton density, biomass and diversity? (*p < 0.05; **p < 0.10; ***p > 0.10.)
| zooplankton response | ecological effects of apex predator | predicted ecological effects of adapted salamanders | ecological effects of adapted spotted salamanders |
ecological effects of apex predator + adapted salamanders | |
|---|---|---|---|---|---|
| mesocosm | laboratory | mesocosm | field | ||
| density | ↓* | ↓ | ↓* | *** | ↓** |
| biomass | ↓* | ↓ | ↓* | ↓* | ↓* |
| Simpson's diversity | ↓* | ↓ | ↑** | ↑*a | ↑* |
aSignificant (p < 0.05) interaction with marbled salamander treatment—direction of effect corresponds to response with marbled salamanders.
2. Material and methods
(a). Natural history and study site
The spotted salamander (A. maculatum) inhabits eastern North America. Each spring, adults move from terrestrial habitat into temporary ponds to mate and to lay eggs. Small (approx. 15 mg) aquatic larvae hatch from eggs after eight to 10 weeks. Spotted salamander larvae undergo metamorphosis into terrestrial juveniles by late summer when most temporary ponds dry.
The larval marbled salamander (A. opacum) is one of the most important predators of spotted salamander larvae in southern New England temporary ponds [27]. The marbled salamander eats spotted salamander larvae and competes with them for zooplankton prey. Marbled salamanders breed in the autumn, their larvae grow under the ice, and, as a result, reach a size large enough to prey on spotted salamander larvae. By foraging on invertebrate prey beginning in the autumn, the marbled salamander has a strong potential to affect prey communities in spring when spotted salamanders breed.
For the past 11 years, I have observed spotted and marbled salamander population dynamics in 14 temporary ponds on an isolated forested ridge (area = 2 km2) on Totoket Mountain in Northford, Connecticut, USA (see the electronic supplementary material, figure S1). Across the study region, ponds differ in the annual probability of marbled salamander prevalence and mean abundance based on standardized dipnet surveys performed each spring in each pond using the same methods [27]. Marbled salamanders occur every year and at high densities in some ponds (e.g. B-9; electronic supplementary material, figure S1) and rarely and at low densities in others (e.g. B-15). These local differences in marbled salamander occurrence and mean abundance remain consistent across years [25] and reflect, in part, the probability that a pond freezes solid in a given winter, and that this ice kills overwintering marbled salamander larvae [28].
Spotted salamander populations face a mosaic of antagonistic selection at the study site because marbled salamanders dominate in some ponds, whereas other predator species such as dragonflies and beetle larvae dominate in others. Marbled salamanders impose strong selection on spotted salamander growth and foraging behaviours [25,26]. This selection is based in part on the marbled salamander's strong gape-limitation that allows spotted salamander larvae to grow into a size refuge [26,29]. Other predators are not gape-limited on spotted salamanders, such as the diving beetle Dytiscus verticalis, and produce opposing selection on foraging traits [25]. Most other predator species select for reduced foraging in spotted salamanders because rapid foraging produces higher instantaneous predation risk for gape-unconstrained and visually oriented predators [30]. In response to this antagonistic selection, research suggests that spotted salamander populations that co-occur with marbled salamanders forage more intensely than those in ponds dominated by other predator species [26].
(b). Laboratory experiment
Spotted salamanders from eight populations that differed in marbled salamander predation risk fed for 24 h on four naturally co-occurring zooplankton taxa that I provided in the same initial quantities under simulated marbled salamander predation risk (i.e. chemical cues [31]). To limit environmental trait induction, I collected egg masses from the field directly after fertilization and raised them in a common garden in 38 l outdoor containers. One week after salamanders hatched, two individuals from each family were placed individually into one of eighty 900 ml containers. Each container was placed randomly in an incubator set to 13.2°C, the average pond temperature for this developmental stage.
The zooplankton in choice experiments originated from tow net samples from the natal ponds of the eight salamander populations in the experiment. I hand-counted 83 sets of the four numerically dominant zooplankton taxa in natural ponds: 10 Cyclopoid copepods, 10 large Cladocerans (mostly Scapholeberis mucronata), and 80 small Cladocerans (Bosmina longirostris and Chydorus sphaericus). The zooplankton samples were randomized and added to containers. Three randomly selected samples were preserved to validate the initial distribution of zooplankton taxonomic abundances. After 24 h, uneaten zooplankton were collected with 150 µm mesh and preserved for enumeration and identification. After 24 h, I evaluated the change in proportion of each taxon and prey preference using standardized Chesson's alpha, which varies from 1.0 (strong preference) to −1.0 (strong avoidance) [32,33].
(c). Mesocosm experiment
I created a temporary pond food web including the apex marbled salamander predator, the spotted salamander, their jointly shared zooplankton prey, and algae in thirty six 1100 l outdoor mesocosms (see the electronic supplementary material, for complete methodological details). Half the mesocosms received four marbled salamanders, which corresponds to natural densities. I chose six spotted salamander populations that differed in foraging rate based on previous research [26]. I raised 40 spotted salamander larvae in each of six replicated mesocosms from each of the six populations for a total of 36 experimental units. Each population×treatment combination was replicated three times in a randomized complete block experimental design.
I added 25 g of dried deciduous leaves and 50 g of pellet-based rabbit food to provide basal food web resources following standard practice [34]. I twice inoculated each mesocosm with natural zooplankton and phytoplankton collected from all six study ponds. I added field-collected marbled salamanders on 21st April 2009. I collected spotted salamander eggs from ponds as soon as possible after breeding and raised them in a common garden environment to limit trait induction. I placed eggs into floating mesh rearing containers in each mesocosm to mimic natural conditions. I collected 40 hatchlings from each rearing container after a majority had hatched and released them into the corresponding mesocosm on 8th May.
Before releasing hatched salamanders into mesocosms, I sampled the zooplankton communities to understand top-down effects of marbled salamander predation on zooplankton. This set-up is consistent with the marbled salamander's early consumption of zooplankton before spotted salamanders hatch in late spring. I estimated salamander and zooplankton densities at two, four, six and 10 weeks (metamorphosis) after spotted salamander hatching. On each sampling date, I estimated salamander densities with dipnet surveys and collected three zooplankton samples using a 15.6 cm diameter vertical pipe sampler [35]. Zooplankton samples were combined, filtered, and preserved in 70 per cent ethanol [36]. For each sample, all individuals were identified to the finest taxonomic scale possible [36–38]. I measured the length of each identifiable taxonomic group in a sample to a maximum of 100 and applied length–mass regressions [39] to estimate biomass.
(d). Field studies
I evaluated marbled salamander occurrence and abundance and zooplankton communities in 13 temporary ponds on Totoket Mountain in Northford, Connecticut, USA (see the electronic supplementary material, figure S1). I measured marbled salamander predation risk as the proportion of years out of seven in which marbled salamanders were observed during annual area-standardized dipnet surveys performed using the same methods. I use marbled salamander prevalence to estimate selection rather than density because we have better long-term data on occurrence and because density estimates require standardization by pond area, which varies greatly within and among years depending on recent rainfall. These long-term occurrence patterns better characterize natural selection on the long-lived spotted salamanders [26] and correlate well with the sparser dataset on mean marbled salamander density (ρ = 0.69).
I collected zooplankton samples with a vertical tube zooplankton sampler at maximum depth and at each cardinal direction midway between the point of maximum depth and the shoreline. Zooplankton were filtered through 150 μm mesh, pooled, and preserved in 70 per cent ethanol [36]. I identified and enumerated samples and calculated biomass corrected for the volume of water sampled using length–mass regressions for a maximum of 100 individuals in each taxon [39]. I assessed the relative contributions of marbled salamander predation versus locally adapted spotted salamanders by analysing how zooplankton communities vary among ponds based on long-term marbled predation risk. I included marbled salamander densities at the particular time of sampling as an additional covariate. Long-term marbled salamander predation risk correlates most strongly with spotted salamander foraging adaptations that have evolved over many generations, whereas marbled salamander numbers at the time of sampling are associated with stochastic seasonal variation that affects shorter-term zooplankton dynamics.
(e). Statistical analyses
For all analyses, I first estimated the full model and then found a parsimonious model by iteratively removing non-significant random effects via log-likelihood ratio tests, interaction terms, and then non-significant quadratic terms [40]. The exception is that I retained random effects that reflected the appropriate hierarchical experimental design. I tested for normality of residual errors using the Shapiro–Wilk normality test and used non-parametric rank tests when a transformation could not be found to normalize data. For each model, I evaluated model fit versus residuals and explored nonlinear models when structure remained in the residuals. I applied the protected-ANOVA approach of first testing for an omnibus significant effect in a multivariate analysis of variance (MANOVA) and then tested for univariate effects upon finding a significant multivariate effect.
I analysed generalized linear models for field results and mixed-effects models for laboratory and mesocosm results in R (v. 2.13). Mixed-effects models were used to account for correlated errors including population, repeated measures on replicate containers through time and detected spatial autocorrelation in field arrays (see the electronic supplementary material for details). Assessing the significance of fixed effects in mixed-effects models remains controversial [41]. Instead of assuming specific degrees of freedom, I generated confidence intervals from the posterior distribution of 10 000 parameter estimates obtained by Markov chain Monte Carlo (MCMC) simulations performed in pvals (univariate response) or MCMCglmm (multivariate) in R. No method exists to determine overall significance of a treatment in a mixed-effect MANOVA. In these cases, I used the model deviance information criterion (DIC), a parameter-adjusted likelihood statistic appropriate for Bayesian model comparisons. I retained a factor when ΔDIC > 4 between models with and without the factor, which indicates substantial support [42]. For mesocosm experiments, I included a random effect of spatial location (numbered rows and columns) after finding significant spatial structure in the data unrelated to treatment. I assumed binomial errors for models of proportional salamander survival and species abundances. If a high residual deviance indicated overdispersion in binomial tests, I modelled this overdispersion directly using an individual random effect by assigning each mesocosm at each time period a unique identifier, as advocated by [43]. p-values based on z-values were used for binomial models, for which MCMC methods were unavailable. To evaluate the overall relationship between treatments and community composition, I evaluated effects on the major axes of zooplankton community composition via redundancy analysis (RDA; vegan package in R) of Hellinger-transformed community composition data. All data analysed in this study are available at Dryad (http://datadryad.org/) with accession number (doi:10.5061/dryad.1q9d0).
3. Results
(a). Ecological effects of apex predation
I evaluated the top-down ecological effects of marbled salamander in outdoor mesocosms. Marbled salamanders significantly reduced zooplankton density by 40 per cent and invertebrate biomass by 87 per cent (see table 1 and electronic supplementary material, figure S2 and table S1; generalized linear model (GLM); p < 0.001, p = 0.005, respectively) relative to controls prior to spotted salamander hatching. Marbled salamanders also altered zooplankton community composition (see the electronic supplementary material, table S1; RDA; p = 0.011) and reduced zooplankton (inverse-Simpson's) diversity by 30 per cent (GLM; p = 0.050). In particular, marbled salamanders significantly decreased the average proportion of Cyclopoid copepods by 31 per cent and increased S. mucronata, and Daphnia ambigua by 500 per cent and 3000 per cent, respectively (see the electronic supplementary material, figure S3 and table S2; GLM; p = 0.001, p = 0.022, p = 0.005). Of the species that established late, marbled salamanders significantly increased C. sphaericus by 250% (p = 0.040), but did not significantly affect Ceriodaphnia dubia (p = 0.843).
(b). Effects of foraging evolution on prey communities in laboratory experiments
I next evaluated mean foraging rates among populations along a gradient of increasing marbled salamander predation risk in the spotted salamander's natal habitat. Based on expected values from a polynomial regression, spotted salamanders from populations experiencing the highest marbled salamander predation risk (i.e. 1.0) consumed 13 more zooplankton on average per day than those from the lowest (i.e. 0.286) marbled salamander risk population (figure 1; MCMC randomization test; p = 0.023). A polynomial line was fitted to the data after detecting remaining curvature in residuals. Overall, the evolutionary history of marbled salamander predation risk explained 76 per cent of the variation in mean foraging rates. This intense foraging also resulted in declines in both zooplankton density and biomass as expected (table 1).
Figure 1.
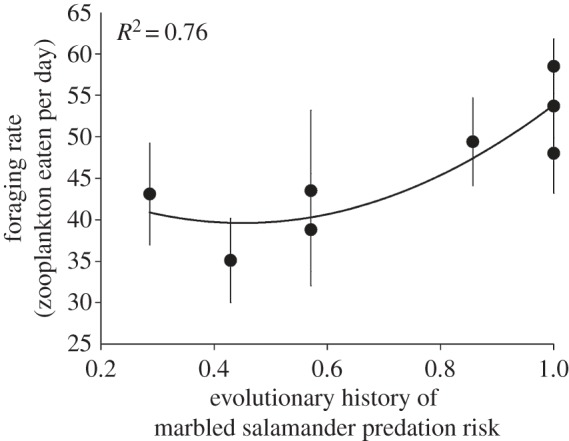
Common garden variation in spotted salamander foraging rate in relation to evolutionary history of marbled salamander predation risk (long-term prevalence) in each population's natal pond. The significant relationship was fit with a polynomial regression. Each point represents a different population's foraging rate in a common garden experiment. Error bars indicate s.e.m.
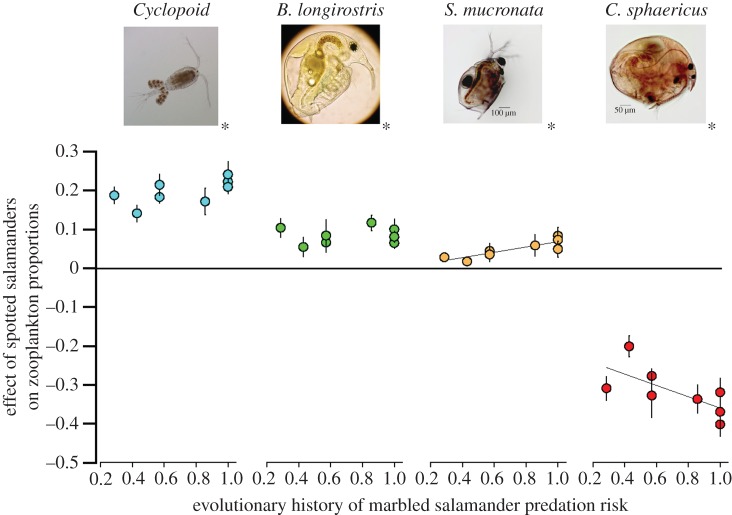
Across all populations, spotted salamanders in this region preferred to eat the Cladocerans C. sphaericus, S. mucronata, B. longirostris and avoided Cyclopoid copepods (see the electronic supplementary material, figure S4; mixed-effects multivariate analysis of variance (m-MANOVA); ΔDIC = 22.2, DIC > 10 indicates strong model support, p < 0.005 for each taxon). However, populations did not shift their prey preference based on their evolutionary history of predation risk (ΔDIC = 3.6), rejecting the possible local evolution of prey preference.
Adaptive differences in foraging rate among populations altered zooplankton composition in laboratory experiments after 24 h of feeding (m-MANOVA; ΔDIC = 12.6) because the more intense foraging by some populations depleted preferred species to a greater extent. In particular, high-risk spotted salamander populations reduced C. sphaericus populations, their preferred prey species (figure 2; m-GLM; p = 0.034).
Figure 2.
Effect of evolutionary history of predation risk on the spotted salamander's impact on zooplankton prey proportions in laboratory experiments. Each symbol indicates a spotted salamander population arranged along an axis of increasing predation risk. An asterisk next to each taxon's picture indicates a significant difference from zero across all populations. Regression lines indicate a significant effect of evolutionary history. Error bars indicate s.e.m. (Online version in colour.)
(c). Effects of foraging evolution on prey communities in mesocosm experiments
I next evaluated eco-evolutionary impacts under more natural conditions in thirty six 1100 l outdoor mesocosms with more diverse food webs. Marbled salamanders decreased spotted salamanders by 80 per cent (m-GLM; p < 0.001), but evolutionary history of marbled salamander predation was not a significant predictor of survival (p = 0.712).
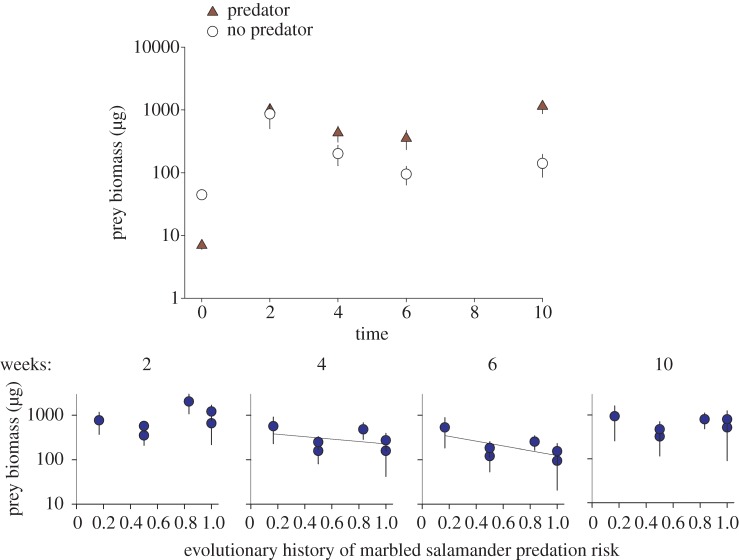
As predicted from laboratory experiments, high-risk spotted salamander populations significantly decreased prey biomass through time (see figure 3; electronic supplementary material, table S3; m-GLM; p < 0.040). In particular, prey biomass decreased in mesocosms with high-risk spotted salamander populations in weeks 4 and 6. High-risk spotted salamanders decreased zooplankton density, but the trend was not significant (see the electronic supplementary material, table S4). Contrary to predictions, high-risk spotted salamander populations increased prey diversity (measured as inverse-Simpson's) when exposed to marbled salamander predation, but decreased diversity in the apex predator's absence (see figure 4a; electronic supplementary material, table S5; m-GLM; p = 0.010).
Figure 3.
Changes in prey invertebrate biomass through time with and without marbled salamander predation (a) and depending on spotted salamander evolutionary history of predation (b). Means of back-transformed fitted values from mixed-effect models are depicted ± s.e.m. The interaction between evolutionary history and time was significant in the full model. For presentation purposes, linear regression lines are plotted in the bottom graph for r2 values > 0.2. (Online version in colour.)
Figure 4.
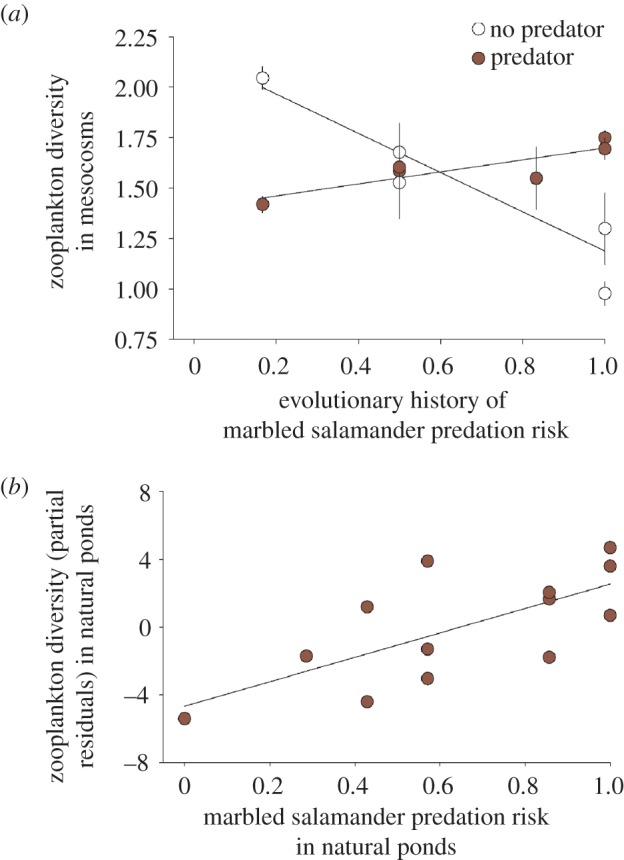
Zooplankton diversity in experimental mesocosms and natural ponds. (a) Zooplankton diversity with (filled circles) and without (open circles) marbled salamanders in experimental mesocosms versus the evolutionary history of marbled salamander predation for each spotted salamander population. Each symbol indicates the mean (±s.e.m.) fitted value for each spotted salamander population. (b) Zooplankton diversity in relation to predation risk from marbled salamanders in natural ponds, after statistically controlling for marbled salamander densities at the time of sampling. Partial residuals of significant multiple regression are plotted. (Online version in colour.)
Spotted salamander adaptations significantly altered zooplankton community composition overall as determined by a multivariate RDA (p < 0.001). Given this significant multivariate effect, I next explored effects on the three most dominant zooplankton taxa: Cyclopoid copepods, S. mucronata and C. sphaericus, representing 96 per cent of the individuals identified from experiments. High-risk spotted salamander populations were associated with a significant increase in the proportion of Cyclopoid copepods and decreases in S. mucronata and C. sphaericus relative to low-risk populations (see figure 5 and electronic supplementary material, figure S5 and table S6; m-GLM; p = 0.027; p = 0.026; p = 0.006). The effect on C. sphaericus depended on an interaction with marbled salamanders, with steep decreases when the predator was present (see the electronic supplementary material, figure S5B). The effect on S. mucronata varied through time with the strongest effects at weeks 2 and 4 (see the electronic supplementary material, figure S5C).
Figure 5.
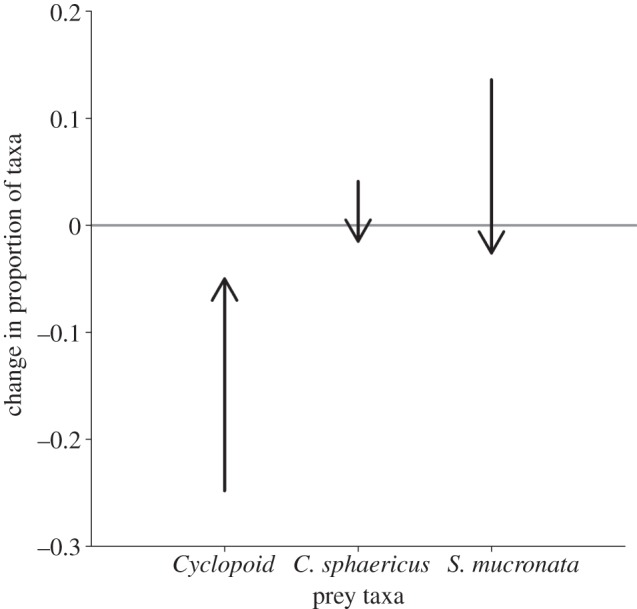
Proportional changes in three dominant zooplankton taxa. The position of the arrow tail indicates the effect of marbled salamander predation on the proportion of each prey taxon. So, for example, marbled salamanders decrease Cyclopoid copepods by 25%. The arrow direction and length indicates the mean change in the proportion of each taxon between mesocosms with the spotted salamander populations from ponds with the highest predation risk versus those with the weakest predation risk. For Cylopoid copepods, adapted spotted salamander populations increase their proportion by 20%, which would produce a smaller overall effect of marbled salamander predation of 5% (indicated as the position of the arrow head). In each case, the evolutionary effect of spotted salamanders counteracts the ecological effect of apex predation, dampening differences between habitats with and without the apex predator.
The numerical effects on each taxon by spotted salamanders matched their overall preference for them in laboratory preference trials (see the electronic supplementary material, figure S4). The rapidly foraging spotted salamander populations depleted their favoured prey items more so than the slow foraging populations, allowing the less preferred taxa to dominate. Interestingly, the preference for these individual taxa differed between marbled and spotted salamanders such that spotted salamanders preferred the taxa that the marbled salamanders avoided. As a result, the direction of the evolutionary effect on prey numbers counteracted the top-down ecological effect of the apex predator for each of the three dominant prey taxa (figure 5).
(d). Natural zooplankton communities
Zooplankton biomass declined in natural ponds characterized by high long-term marbled salamander predation risk (table 1; GLM; n = 13; p = 0.009), after accounting for marbled salamander densities at the time of sampling. Zooplankton density also declined but was only marginally significant (p = 0.098). These patterns are consistent with the complementary impacts from both marbled salamanders and locally adapted spotted salamander populations.
Zooplankton diversity increased in natural ponds with higher long-term marbled salamander predation risk (figure 4b; GLM; n = 13; p = 0.024). This pattern is consistent with the effect of locally adapted spotted salamanders rather than the ecological effect of apex predation, which instead tends to diminish diversity (table 1).
4. Discussion
The evolutionary divergence of populations offers a potentially general source of context dependency in community ecology [4,5,8,12]. Apex predators, in particular, often disproportionately affect prey community diversity and composition [1] while simultaneously imposing strong natural selection on prey populations [44]. Here, I explore the ecological and evolutionary effects of marbled salamanders on temporary pond communities. I show that marbled salamanders are apex predators in temporary ponds and can shape community abundance, diversity and composition. When I added marbled salamanders to experimental tanks, they altered the proportion of some zooplankton species by as much as 30-fold.
Marbled salamanders not only affect communities ecologically, but impose strong natural selection on members of the food web. Spotted salamanders that coexist with marbled salamanders foraged more intensely than those from other populations which face different predator species [26]. These differences in foraging rate among populations have now been demonstrated in three different common garden experiments, including [26] and the laboratory and mesocosm experiments presented here. Common garden experiments isolate genetically determined phenotypic variation and indicate local adaptation [45]. Most studies of evolutionary effects on communities use wild-caught individuals [16–19,46], rather than individuals raised in a common garden. Using wild-caught individuals potentially confounds genetic and plastic contributions to phenotypic variation and subsequent ecological effects of that variation [20].
Maternal effects also can contribute to phenotypic variation. I can reject four of the most common sources of maternal effects [47,48] in this system. First, I raised eggs in a common environment to limit effects from habitat choice. Second, spotted salamanders do not care for offspring. Third, spotted salamander egg size, one of the most common forms of maternal effects in amphibians, does not vary significantly among populations or with marbled salamander predation risk [26]. Fourth, I find no evidence of maternal effects arising from the environmental conditioning of females or eggs. If the pond environment induces mothers or egg phenotypes during mating and egg laying, then the presence of marbled salamanders during breeding should correlate better with foraging differences among populations than long-term marbled salamander selection. The year I performed the experiments in this study was abnormally warm, and marbled salamanders colonized many ponds from which they are usually absent. The density of marbled salamanders in this abnormal year did not significantly explain foraging differences (p = 0.331), but long-term marbled salamander prevalence did, indicating no significant effect of marbled salamander presence per se on trait variation. Previous research also demonstrates that chemical cues from marbled salamanders during the egg stage do not significantly affect hatching date [31], initial hatchling size [26], and survival with marbled salamanders [25]. However, I cannot conclusively eliminate all maternal effects without raising this long-lived and late-maturing salamander for multiple generations in the laboratory. Therefore conclusions about genetic determination should be treated with caution.
Local adaptation requires both strong antagonistic selection and limited gene flow [49]. The spotted salamander fulfils both requirements. Antagonistic selection between ponds with low and high marbled salamander predation risk is strong [25]. Gene flow is limited because spotted salamanders disperse short distances [26], demonstrate high breeding site philopatry [50], avoid outbreeding [51], and marbled salamander predation is spatially autocorrelated such that gene flow is less disruptive than in a landscape of randomly distributed selection [26,52,53]. Furthermore, we detected significant neutral genetic differentiation between populations that face selection from different predator communities, indicating that strong selective barriers or habitat selection decrease gene flow among ponds that differ in selection and thereby allow for fine-scale adaptive differentiation [53]. Strong antagonistic selection, low dispersal, high philopatry, and outbreeding avoidance probably allow for local adaptation in spotted salamanders.
Thus far, evidence suggests that the repeated evolution of high foraging rate in multiple spotted salamander populations constitutes an adaptive response to local selection from marbled salamander predation. Six alternative selection regimes, including two different predator species, total predator density, pond temperature, canopy cover and pond area did not significantly explain interpopulation differentiation in foraging rate [26,53]. Previous research suggests that intense foraging evolves to support growth into a size refuge from gape-limited predators like marbled salamanders [26,29]. The strong depletion of shared invertebrate prey by marbled salamanders revealed in this study suggests an additional reason based on countergradient selection on growth rates in resource-poor environments [21]. Spotted salamanders must reach a critical body size to undergo metamorphosis before ponds dry. In ponds with high marbled salamander predation and consequently low zooplankton resources, spotted salamander populations probably evolve higher foraging rates both to compensate for lower food resources and to reduce the time window of predation from the gape-limited marbled salamander [26,29].
(a). Ecological effects of spotted salamander evolution
Community ecologists often assume that only ecological factors such as the abundance of marbled salamanders are necessary to predict community patterns. However, this ecological approach would be wrong in this case. To understand how marbled salamanders structure communities, we must understand both its direct ecological effects as a predator and its indirect effects via the natural selection it imposes on the spotted salamander.
The evolution of increased foraging by spotted salamanders in resource-poor ponds exacerbated the effects of apex predators on prey resources and the resulting resource differences among temporary ponds. Spotted salamanders from marbled salamander ponds foraged more intensely, and as a result, decreased prey density and biomass in both laboratory and mesocosm experiments. Spotted salamander populations did not differ in predation rates from marbled salamanders, which means that any community differences associated with differently adapted populations arise from differences in traits rather than density. In another example, guppies coexisting with Rivulus consumed more shared invertebrate prey than in experiments when both species originated from different sites [18]. The evolution of higher foraging in resource-poor, competitive conditions offers one explanation for this pattern. Countergradient selection on traits linked with resource consumption might commonly support trait evolution that exacerbates top-down effects from apex predation and differences in resource levels between habitats.
Contrary to my predictions, evolution dampened top-down effects for community diversity and composition. I incorrectly assumed that marbled and spotted salamanders preferred the same prey species. However, spotted salamanders prefer the prey taxa that marbled salamanders avoid. As a result, spotted salamander populations from marbled salamander ponds increased zooplankton diversity by feeding on the most common taxa and facilitating a more even distribution of abundances among community members. As a result, the spotted salamander populations adapted to life with marbled salamanders dampen this apex predator's top-down ecological effect on local community composition. In another example, a stickleback population co-occurs with another intraguild predator, the sculpin, which prefers benthic invertebrates. Wild-caught stickleback from sculpin lakes ate more pelagic zooplankton than low-predation populations in experiments [17], and thus could potentially dampen effects of predation on benthic versus pelagic prey biomass by evolving to prey on zooplankton when living with sculpin. Whenever niche partitioning occurs along with adaptation, evolution might instead homogenize spatial patterns of diversity by dampening the top-down effects of predation on local prey communities.
Importantly, results observed in artificial experiments reflect those observed in natural, unmanipulated ponds. Ponds with high marbled salamander predation risk had lower zooplankton density and biomass as expected. However, these ponds supported greater zooplankton diversity in contrast to what would be expected based on the ecological impacts of marbled salamander predation alone. Instead, field diversity patterns are more consistent with a prevailing indirect effect of spotted salamander foraging differences. An alternative explanation is that marbled salamanders reduce spotted salamander densities, and reduced spotted salamander densities increase prey diversity. However, this explanation is less likely because other predator species occur in high densities in non-marbled salamander ponds and thus spotted salamander mortality is expected to be similar in ponds with and without marbled salamanders. Also, marbled salamanders decreased spotted salamanders by 80 per cent versus controls in the mesocosm experiment, and prey diversity remained the same or even declined slightly (figure 4a). Overall, evidence suggests that the evolution of foraging in spotted salamander larvae explains an apparent ecological surprise in natural diversity patterns that could not be understood based on ecological principles alone.
5. Conclusions
Usually, only ecological explanations are proposed to explain ecological patterns. Yet, populations might often adapt locally. Evolutionary divergence among antagonistic selection regimes within the same region can create and maintain trait differences sufficient to alter ecological patterns [7–9,11,12]. Here, I show how the adaptation of the spotted salamander can mediate the top-down effects of an apex predator. High-risk spotted salamander populations exacerbated declines in prey biomass initiated by the apex predator because they feed more in the resource-poor environments created by the apex predator. However, spotted salamanders facilitated greater prey diversity, which counteracted the effect of apex predation. This diversity effect occurs because predators partition resources such that the rapidly foraging spotted salamanders dampen top-down effects on individual taxa, leading to relative stability in prey community composition between habitats with different apex predator species (figure 5). Such effects might occur frequently for intraguild predators, which both induce selection on meso-predators and simultaneously deplete shared prey [17].
Results suggest that just knowing the abundance of an apex predator in local habitats is not sufficient to understand the true strength of its top-down effects on food webs. In some cases, observed top-down effects might be weak not because the apex predator has little effect on lower trophic levels, but rather because its interactions are so strong that they select for compensatory evolutionary responses in other species. In many food webs, we might be concluding weak interactions because local adaptive evolution disguises strong top-down ecological effects. A wide range of additional ecological predictions could prove to be unreliable because we ignore how evolution affects community dynamics.
Acknowledgements
Research was conducted in accordance with IACUC permit A09-003.
Research was supported by NSF award DEB-1119877, the James S. McDonnell Foundation, and a University of Connecticut large faculty grant. Special thanks to the South Central Connecticut Regional Water Authority for access to field sites and to E. Herstoff and L. Didan for research assistance. J. Haney provided the zooplankton pictures in figure 2. S. Alonzo, N. Freidenfelds, J. Richardson, J. Shurin, P. Zarnetske, and several anonymous reviewers provided insightful comments.
References
- 1.Paine RT. 1966. Food web complexity and species diversity. Am. Nat. 100, 65–75. 10.1086/282400 (doi:10.1086/282400) [DOI] [Google Scholar]
- 2.Lawton JH. 1999. Are there general laws in ecology? Oikos 84, 177–192. 10.2307/3546712 (doi:10.2307/3546712) [DOI] [Google Scholar]
- 3.Doak DF, et al. 2008. Understanding and predicting ecological dynamics: are major surprises inevitable? Ecology 89, 952–961. 10.1890/07-0965.1 (doi:10.1890/07-0965.1) [DOI] [PubMed] [Google Scholar]
- 4.Antonovics J. 1992. Toward community genetics. In Plant resistance to herbivores and pathogens: ecology, evolution, and genetics (eds Frite RS, Simms EL.), pp. 426–449. Chicago, IL: University of Chicago Press. [Google Scholar]
- 5.Thompson JN. 1999. The evolution of species interactions. Science 284, 2116–2118. 10.1126/science.284.5423.2116 (doi:10.1126/science.284.5423.2116) [DOI] [PubMed] [Google Scholar]
- 6.Whitham TG, et al. 2006. A framework for community and ecosystem genetics: from genes to ecosystems. Nat. Rev. Genet. 7, 510–523. 10.1038/nrg1877 (doi:10.1038/nrg1877) [DOI] [PubMed] [Google Scholar]
- 7.Ellner SP, Geber MA, Hairston NG., Jr 2011. Does rapid evolution matter? Measuring the rate of contemporary evolution and its impacts on ecological dynamics. Ecol. Lett. 14, 603–614. 10.1111/j.1461-0248.2011.01616.x (doi:10.1111/j.1461-0248.2011.01616.x) [DOI] [PubMed] [Google Scholar]
- 8.Schoener TW. 2011. The newest synthesis: understanding the interplay of evolutionary and ecological dynamics. Science 331, 426–429. 10.1126/science.1193954 (doi:10.1126/science.1193954) [DOI] [PubMed] [Google Scholar]
- 9.Fussmann GF, Loreau M, Abrams PA. 2007. Eco-evolutionary dynamics of communities and ecosystems. Funct. Ecol. 21, 465–477. 10.1111/j.1365-2435.2007.01275.x (doi:10.1111/j.1365-2435.2007.01275.x) [DOI] [Google Scholar]
- 10.Crutsinger GM, Collins MD, Fordyce JA, Gompert Z, Nice CC, Sanders NJ. 2006. Plant genotypic diversity predicts community structure and governs ecosystem process. Science 313, 966–968. 10.1126/science.1128326 (doi:10.1126/science.1128326) [DOI] [PubMed] [Google Scholar]
- 11.Post DM, Palkovacs EP. 2009. Eco-evolutionary feedbacks in community and ecosystem ecology: interactions between the ecological theatre and the evolutionary play. Phil. Trans. R. Soc. B 364, 1629–1640. 10.1098/rstb.2009.0012 (doi:10.1098/rstb.2009.0012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pelletier F, Garant D, Hendry AP. 2009. Eco-evolutionary dynamics. Phil. Trans. R. Soc. B 364, 1483–1489. 10.1098/rstb.2009.0027 (doi:10.1098/rstb.2009.0027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshida T, Jones LE, Ellner SP, Fussmann GF, Hairston NG. 2003. Rapid evolution drives ecological dynamics in a predator-prey system. Nature 424, 303–306. 10.1038/nature01767 (doi:10.1038/nature01767) [DOI] [PubMed] [Google Scholar]
- 14.Wimp GM, Martinsen GD, Floate KD, Bangert RK, Whitham TG. 2005. Plant genetic determinants of arthropod community structure and diversity. Evolution 59, 61–69. [PubMed] [Google Scholar]
- 15.De Meester L, Louette G, Duvivier C, Van Damme C, Michels E. 2007. Genetic composition of resident populations influences establishment success of immigrant species. Oecology 153, 431–440. 10.1007/s00442-007-0721-3 (doi:10.1007/s00442-007-0721-3) [DOI] [PubMed] [Google Scholar]
- 16.Harmon LJ, Matthews B, Des Roches S, Chase JM, Shurin JB, Schluter D. 2009. Evolutionary diversification in stickleback affects ecosystem functioning. Nature 458, 1167–1170. 10.1038/nature07974 (doi:10.1038/nature07974) [DOI] [PubMed] [Google Scholar]
- 17.Ingram T, Svanback R, Kraft NJB, Kratina P, Southcott L, Schluter D. 2012. Intraguild predation drives evolutionary niche shift in threespine stickleback. Evolution 66, 1819–1832. 10.1111/j.1558-5646.2011.01545.x (doi:10.1111/j.1558-5646.2011.01545.x) [DOI] [PubMed] [Google Scholar]
- 18.Palkovacs EP, Marshall MC, Lamphere BA, Lynch BR, Weese DJ, Fraser DF, Reznick DN, Pringle CM, Kinnison MT. 2009. Experimental evaluation of evolution and coevolution as agents of ecosystem change in Trinidadian streams. Phil. Trans. R. Soc. B 364, 1617–1628. 10.1098/rstb.2009.0016 (doi:10.1098/rstb.2009.0016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bassar RD, et al. 2010. Local adaptation in Trinidadian guppies alters ecosystem processes. Proc. Natl Acad. Sci. USA 107, 3616–3621. 10.1073/pnas.0908023107 (doi:10.1073/pnas.0908023107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walsh MR, DeLong JP, Hanley TC, Post DM. 2012. A cascade of evolutionary change alters consumer-resource dynamics and ecosystem function. Proc. R. Soc. B 279, 3184–3192. 10.1098/rspb.2012.0496 (doi:10.1098/rspb.2012.0496) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conover DO, Schultz ET. 1995. Phenotypic similarity and the evolutionary significance of countergradient variation. Trends Ecol. Evol. 10, 248–252. 10.1016/S0169-5347(00)89081-3 (doi:10.1016/S0169-5347(00)89081-3) [DOI] [PubMed] [Google Scholar]
- 22.Lankford TE, Billerbeck JM, Conover DO. 2001. Evolution of intrinsic growth and energy acquisition rates. II. Trade-offs with vulnerability to predation in Menidia menidia. Evolution 55, 1873–1881. [DOI] [PubMed] [Google Scholar]
- 23.Walsh MR, Post DM. 2011. Interpopulation variation in a fish predator drives evolutionary divergence in prey in lakes. Proc. R. Soc. B 278, 2628–2637. 10.1098/rspb.2010.2634 (doi:10.1098/rspb.2010.2634) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshida T, Ellner SP, Jones LE, Bohannan BJM, Lenski RE, Hairston NG., Jr 2007. Cryptic population dynamics: rapid evolution masks trophic interactions. PLoS Biol. 5, e235. 10.1371/journal.pbio.0050235 (doi:10.1371/journal.pbio.0050235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urban MC. 2010. Microgeographic adaptations of spotted salamander morphological defenses in response to a predaceous salamander and beetle. Oikos 119, 646–658. 10.1111/j.1600-0706.2009.17970.x (doi:10.1111/j.1600-0706.2009.17970.x) [DOI] [Google Scholar]
- 26.Urban MC. 2007. Risky prey behavior evolves in risky habitats. Proc. Natl Acad. Sci. USA 104, 14 377–14 382. 10.1073/pnas.0704645104 (doi:10.1073/pnas.0704645104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Urban MC. 2007. Predator size and phenology shape prey survival in temporary ponds. Oecology 154, 571–580. 10.1007/s00442-007-0856-2 (doi:10.1007/s00442-007-0856-2) [DOI] [PubMed] [Google Scholar]
- 28.Herstoff E, Urban MC. In press Will pre-adaptation buffer the impacts of climate change on novel species interactions? Ecography. [Google Scholar]
- 29.Urban MC. 2007. The growth-predation risk tradeoff under a growing gape-limited predation threat. Ecology 88, 2587–2597. 10.1890/06-1946.1 (doi:10.1890/06-1946.1) [DOI] [PubMed] [Google Scholar]
- 30.Lima SL, Dill LM. 1990. Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68, 619–640. 10.1139/z90-092 (doi:10.1139/z90-092) [DOI] [Google Scholar]
- 31.Urban MC. 2008. The evolution of prey body size reaction norms in diverse communities. J. Anim. Ecol. 77, 346–355. 10.1111/j.1365-2656.2007.01337.x (doi:10.1111/j.1365-2656.2007.01337.x) [DOI] [PubMed] [Google Scholar]
- 32.Vanderploeg HA, Scavia D. 1979. Calculation and use of selectivity coefficients of feeding: zooplankton grazing. Ecol. Model. 7, 135–149. 10.1016/0304-3800(79)90004-8 (doi:10.1016/0304-3800(79)90004-8) [DOI] [Google Scholar]
- 33.Chesson J. 1983. The estimation and analysis of preference and its relationship to foraging models. Ecology 64, 1297–1304. 10.2307/1937838 (doi:10.2307/1937838) [DOI] [Google Scholar]
- 34.Relyea RA. 2002. Local population differences in phenotypic plasticity: predator-induced changes in wood frog tadpoles. Ecol. Monogr. 72, 77–93. 10.1890/0012-9615(2002)072[0077:LPDIPP]2.0.CO;2 (doi:10.1890/0012-9615(2002)072[0077:LPDIPP]2.0.CO;2) [DOI] [Google Scholar]
- 35.Paggi JC, Mendoza RO, Debonis CJ, Jose de Paggi SB. 2001. A simple and inexpensive trap-tube sampler for zooplankton collection in shallow waters. Hydrobiologia 464, 45–49. 10.1023/A:1013951431394 (doi:10.1023/A:1013951431394) [DOI] [Google Scholar]
- 36.Williamson CE, Reid JW. 2001. Copepoda. In Ecology and classification of North American freshwater invertebrates (eds Thorp JH, Covich AP.), pp. 915–953. Boston, FL: Academic Press. [Google Scholar]
- 37.Dodson SI, Frey DG. 2001. Cladocera and other Branchipoda. In Ecology and classification of North American freshwater invertebrates (eds Thorp JH, Covich AP.), pp. 849–913. Boston, FL: Academic Press. [Google Scholar]
- 38.Haney JF. 2010. An image-based key to the zooplankton of the northeast, USA version 4.0 released 2010. Durham, NH: University of New Hampshire Center for Freshwater Biology. See http://cfb.unh.edu/cfbkey/html/. [Google Scholar]
- 39.McCauley DE. 1984. The estimation of the abundance and biomass of zooplankton in samples. In A manual on methods for assessment of secondary productivity in fresh waters (eds Downing JA, Rigler FH.), pp. 228–265. Oxford, UK: Blackwell Scientific Publications. [Google Scholar]
- 40.Crawley MJ. 2007. The R book. Chichester, UK: John Wiley & Sons. [Google Scholar]
- 41.Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White J-SS. 2009. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135. 10.1016/j.tree.2008.10.008 (doi:10.1016/j.tree.2008.10.008) [DOI] [PubMed] [Google Scholar]
- 42.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach. New York, NY: Springer. [Google Scholar]
- 43.Warton DI, Hui FKC. 2011. The arcsine is asinine: the analysis of proportions in ecology. Ecology 92, 3–10. 10.1890/10-0340.1 (doi:10.1890/10-0340.1) [DOI] [PubMed] [Google Scholar]
- 44.Reznick D, Butler MJ, Rodd FH, Ross P. 1996. Life history evolution in guppies (Poecilia reticulata). 6. Differential mortality as a mechanism for natural selection. Evolution 50, 1651–1660. 10.2307/2410901 (doi:10.2307/2410901) [DOI] [PubMed] [Google Scholar]
- 45.Kawecki TJ, Ebert D. 2004. Conceptual issues in local adaptation. Ecol. Lett. 7, 1225–1241. 10.1111/j.1461-0248.2004.00684.x (doi:10.1111/j.1461-0248.2004.00684.x) [DOI] [Google Scholar]
- 46.Palkovacs EP, Post DM. 2008. Eco-evolutionary interactions between predators and prey: can predator-induced changes to prey communities feed back to shape predator foraging traits? Evol. Ecol. Res. 10, 699–720. [Google Scholar]
- 47.Lynch M, Walsh B. 1998. Genetics and analysis of quantitative traits. Sunderland, UK: Sinauer Associates, Inc. [Google Scholar]
- 48.Mousseau TA, Fox CW. 1998. The adaptive significance of maternal effects. Trends Ecol. Evol. 13, 403–407. 10.1016/S0169-5347(98)01472-4 (doi:10.1016/S0169-5347(98)01472-4) [DOI] [PubMed] [Google Scholar]
- 49.Wright S. 1969. Evolution and the genetics of populations, vol. 2: the theory of gene frequencies. Chicago, IL: University of Chicago Press. [Google Scholar]
- 50.Petranka JW. 1998. Salamanders of the US and Canada. Washington, DC: Smithsonian Institution. [Google Scholar]
- 51.Chandler CH, Zamudio KR. 2008. Reproductive success by large, closely related males facilitated by sperm storage in an aggregate breeding amphibian. Mol. Ecol. 17, 1564–1576. 10.1111/j.1365-294X.2007.03614.x (doi:10.1111/j.1365-294X.2007.03614.x) [DOI] [PubMed] [Google Scholar]
- 52.Urban MC. 2011. The evolution of species interactions across natural landscapes. Ecol. Lett. 14, 723–732. 10.1111/j.1461-0248.2011.01632.x (doi:10.1111/j.1461-0248.2011.01632.x) [DOI] [PubMed] [Google Scholar]
- 53.Richardson JL, Urban MC. In press Strong selection barriers explain microgeographic adaptation in wild salamander populations. Evolution. 10.1111/evo.12052 (doi:10.1111/evo.12052) [DOI] [PubMed] [Google Scholar]