Abstract
It is hypothesized that the primary function of permanent social relationships among female sperm whales (Physeter macrocephalus) is to provide allomothers for calves at the surface while mothers make foraging dives. In order to investigate how reciprocity of allocare within units of sperm whales facilitates group living, we constructed weighted social networks based on yearly matrices of associations (2005–2010) and correlated them across years, through changes in age and social role, to study changes in social relationships within seven sperm whale units. Pairs of association matrices from sequential years showed a greater positive correlation than expected by chance, but as the time lag increased, the correlation coefficients decreased. Over all units considered, calves had high values for all measured network statistics, while mothers had intermediate values for most of the measures, but high values for connectedness and affinity. Mothers showed sharp drops in strength and connectedness in the first year of their new calves' lives. These broad patterns appear to be consistent across units. Calves appeared to be significant nodes in the network of the social unit, and thus provide quantitative support for the theory in which communal care acts as the evolutionary force behind group formation in this species.
Keywords: social structure, group formation, reciprocity, network, social role, alloparental care
1. Introduction
Among mammals, group formation is thought to provide increased access to resources or improved protection from predators [1]. For the cetaceans, it is believed that the latter is the primary factor promoting groups [2]. By living in groups, individuals reduce the chances of being preyed upon through increased vigilance, dilution, predator mobbing or predator confusion [2]. In our study species, the sperm whale (Physeter macrocephalus, Linnaeus 1758), the sexes have different patterns of gregariousness, presumably because of differing selective pressures. Female and immature sperm whales live in stable social groupings, called units, characterized by stable long-term social relationships between individuals [3–5], which are often, but not always, matrilineally related [6–8]. However, males disperse from their natal units in their early teens and live relatively solitary lives at higher latitudes [5]. Based on these contrasting patterns and other evidence, several scientists have suggested that the primary function of permanent social relationships among female sperm whales in social units is to increase offspring survival by providing babysitters for calves at the surface while mothers make long (approx. 40 min), deep (approx. 500 m) foraging dives [9–12]. In contrast, adult males are solitary or form ephemeral groups, presumably because there is no benefit to permanent grouping [12]. Thus, it can be hypothesized that the evolution of communal care for calves was the driving force for sociality in sperm whales [9,11,13].
Should this evolutionary framework hold, one would expect that calves play a central role in the social relationships within a unit. Social network analysis has been used to study a variety of aspects of animal interactions, including information transfer [14], cooperative behaviours [15] and social role [16]. However, many have used binary or filtered networks that are static in time; here, we construct weighted social networks based on yearly matrices of associations and correlate them across years to study changes in the animals' social network and examine why these changes have occurred. Gero et al. [17] showed that individuals within a particularly well-studied social unit of sperm whales have preferred associates and avoidances among their unit-members, and that these associations are correlated with genetic relatedness. Changes in composition within this unit over the course of this study allowed us to compare changes in relationships and network statistics with changes in age and social role, and to investigate reciprocity of allocare. We then compared the patterns observed in this unit with those in six other units for which we had sufficient data to conduct similar analyses. In particular, we test whether calves are significant social connection nodes in the network of sperm whale social units, and thus provide support for the hypothesis of the link between communal care and sociality.
2. Material and methods
(a). Field methods
Social units of female and immature sperm whales were located and followed in an area that covered approximately 2000 km2 along the entire west (leeward) coast of the island of Dominica (15.30° N, 61.40° W). Research was conducted from one of three platforms (a dedicated auxiliary sailing vessel, a dedicated outboard skiff or a whale-watch vessel) during the winters of 2005–2010 for a total of 2549 h with whales across 320 days of effort (2005: 14 January to 13 April 13, 58 days effort, sailing vessel only; 2006: 17 January to 11 February, 21 days effort, whale watch only; 2007: 28 January to 28 February, 30 days effort, skiff and whale watch; 2008: 8 February to 8 May, 75 days effort, all platforms; 2009: 11 January to 29 March, 64 days effort, skiff and whale watch; 2010: 20 January to 18 April, 72 days effort, sailing vessel only). During outboard skiff seasons, on heavier weather days, when the small (5 m, 88 hp) skiff was unable to operate, the research team operated from a larger (60 ft, twin 420 hp) whale-watch vessel. Whale-watch tours focused their search effort on sperm whales. As a result, methods remained the same across all three platforms, with the work on those days being restricted only by the length of time spent at sea on the whale-watch vessel.
During daylight hours, clusters of individuals visible at the surface were approached and photographs were taken to identify individuals. If calves were present, priority was given to taking dorsal fin pictures of the calf from alongside the animals, before moving behind the adults in the cluster in order to photograph distinct markings on the trailing edge of their flukes for individual identification purposes [18]. In an attempt to minimize the impact of our vessel's presence on the animals and their behaviour, we used small research vessels (less than 11 m) and our protocols maximized approach distance while not impacting our ability to undertake the intended data collection. After the individuals had dived, sloughed skin samples were collected in the slicks of individuals for genetic determination of sex, haplotype and pairwise relatedness [8,19–21]. Relatedness of the individuals in this study was determined as by Gero et al. [17].
Additional data had been collected, using similar methods, by the International Fund for Animal Welfare (IFAW) during the winters of 1995 and 1996 (dedicated sailboat, 59 days effort; see [22]). These data were used to provide a long-term comparison of association patterns over more than a decade.
(b). Analyses
(i). Identifications
A quality rating (Q) between 1 and 5 was designated to each photograph, where 1 indicated a very poor photograph, and 5 indicated a very high-quality photograph [18,23]. Only pictures with Q ≥ 3 were used for the analyses. The best picture for each individual within encounters was assigned a temporary identification code then matched between encounters using a computer-based matching program [24]. In a few cases (less than 5% of identifications), well-known individuals which could not be photographed when multiple animals fluked synchronously but whose flukes were observed by S.G. were recorded as identified and given a Q-rating of 6. Calves, which do not fluke, were individually identified using the shape of their dorsal fin and distinct markings on the dorsal fin and body. The best picture for each individual calf within each encounter was then matched between encounters by eye.
(ii). Defining associations
While previous work in this species [3] used a 30-day minimum duration of association to delineate unit membership, we used a more stringent minimum duration of across years. That unit members were associated across years suggests constant companionship as originally defined by Whitehead et al. [25] and increases the level of certainly of long-term, stable relationships between unit members. Therefore, in this study, a unit was defined as a set of individuals for which each pair was observed to be associated during at least two different years.
To examine social relationships within units, individuals were deemed to be associating if they were within the same cluster at the surface. Spatial and temporal synchronization through social cohesion at the surface is costly to individuals [26] such that these clusters should be representative of individual social preferences. Furthermore, the assumption that membership in the same spatio-temporal cluster indicates probability of behavioural interaction [27] is also supported, in this case, as individuals clustered together at the surface often interact vocally by matching or echoing codas (a social vocalization) upon initiating dives [28]. An individual was considered part of a cluster if it was within approximately three adult body lengths of any other cluster member (approx. 40 m ‘chain rule’) and their behaviours were coordinated [12]. A 2 h sampling period was used (such that individuals observed in the same cluster during a 2 h sampling period are said to be associated within the sampling period) along with the ‘half-weight index’ (HWI), as this measure of association accounts best for observer biases that are usually inherent in photo-identification techniques [29]. The HWI estimates the proportion of time when a whale is at the surface that it is clustered with the other whale.
(iii). Calculation of network statistics
We constructed weighted social networks based on yearly matrices of association and calculated five nodal network measures: strength (a measure of gregariousness), eigenvector centrality (a measure of how well an individual is connected), reach (a measure of indirect connectedness), clustering coefficient (a measure of how well one's associates are connected with each other) and affinity (a measure of the average weighted strength of associates). All measures are defined and calculated as described previously [30–32], and standard errors around measures were based on 1000 bootstrap replicates [30].
(iv). Between-year comparisons
Mantel Z-tests [33,34] and matrix correlation coefficients between matrices of associations calculated between the adults within each year indicated whether the association indices were correlated between years or if patterns of association change through time. A test variant, the Rr-test, was also used as it controls for individual gregariousness by replacing the values of association with their within-row ranks (i.e. within-individual ranks [35]). Correlations between studies separated by the same number of years were averaged in order to get an average correlation coefficient for a given time lag.
The calculation of the HWI and network statistics, as well as the Rr-tests described above, were carried out using Socprog v. 2.3 [32] in Matlab v. 2006b (The Mathworks, Inc., Natick, MA).
3. Results
(a). Unit F: the Group of Seven
Unit F, or ‘The Group of Seven’ (GOS), has at its base five adult females, who have been consistent associates since at least 1995 [36]. This unit has been observed every year from 2005 to 2010 and its members are the most sighted individuals during our work in Dominica (average 182 clusters per individual whale; range 91–262). There have been several changes in the composition of the unit over the six seasons of study (figure 1). One female (‘Fingers’ #5722) lost her calf (‘Thumb’ #5703) after the 2005 season; ‘Puzzle Piece’ (#5130) disappeared between the 2005 and 2006 seasons, and has not been sighted since; and two new calves have been born: ‘Enigma’ (#6068) to ‘Mysterio’ (#5561) in 2005 and ‘Tweak’ (#6070) to ‘Pinchy’ (#5560) in 2007 (figure 1). The two young calves were born late in the year and so do not appear in data until the following year's research (e.g. Tweak was born late 2007 after the 2007 fieldwork, so the first year he is included is 2008). Lastly, there is an immature male, ‘Scar' (#5727), who, based on his length, was estimated to be between 8 and 10 years of age in 2005 [37], making him between 13 and 15 years old in 2010.
Figure 1.
The Group of Seven from 2005 to 2010 laid out in a probable pedigree (S. Gero & C. Herbinger 2007, unpublished data) based on 13 microsatellite markers [17]. Males are represented as rectangles, females as ellipses and deceased animals are crossed out.
(i). Relationships across Years
Matrix correlations between years suggest that social relationships progressively change over time and are not constant. Mantel Z-tests between some pairs of sequential years showed a greater correlation than expected by chance (2005–2006: Mantel Z-test; p = 0.028; matrix correlation of association matrices 0.68063; and 2006–2007: Mantel Z-test; p = 0.007; matrix correlation of association matrices 0.79097), but as the time lag increases, the correlation coefficients between association matrices for each year decrease (table 1). For the five GOS females seen in 1995–1996, as well as during the 2005–2010 fieldwork, Mantel and Rr-tests gave no indication that there were similarities in the patterns of association between pooled periods over a decade apart (Mantel Z-test: matrix correlation = 0.30, p = 0.23; Rr-test: matrix correlation = 0.12, p = 0.403) or between any particular year and the pooled 1995–1996 dataset (table 1). Patterns of association did not differ whether Q6 identifications were included or excluded.
Table 1.
Rr-test correlation coefficients and lagged means of Group of Seven association matrices from 2005 to 2010, excluding calves.
| year | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | time lag | mean |
|---|---|---|---|---|---|---|---|---|
| 1995–1996 | −0.36 | 0.29 | 0.13 | 0.31 | −0.26 | 0.25 | with 95/96a | 0.06 |
| 2005 | 1 | 0.21 | 0.25 | −0.28 | −0.49 | −0.08 | 1 year | 0.23 |
| 2006 | 1 | 0.68 | 0.14 | 0.08 | 0.09 | 2 year | 0.21 | |
| 2007 | 1 | 0.23 | −0.02 | 0.09 | 3 year | −0.04 | ||
| 2008 | 1 | −0.07 | 0.46 | 4 year | −0.20 | |||
| 2009 | 1 | 0.09 | 5 year | −0.08b | ||||
| 2010 | 1 |
aNot the same time lag between each year and the 1995–1996 pooled dataset.
bWith only one value for a 5-year lag, this value is not a mean but simply the correlation coefficient for the matrices of 2005 and 2010.
(ii). Mothers and calves
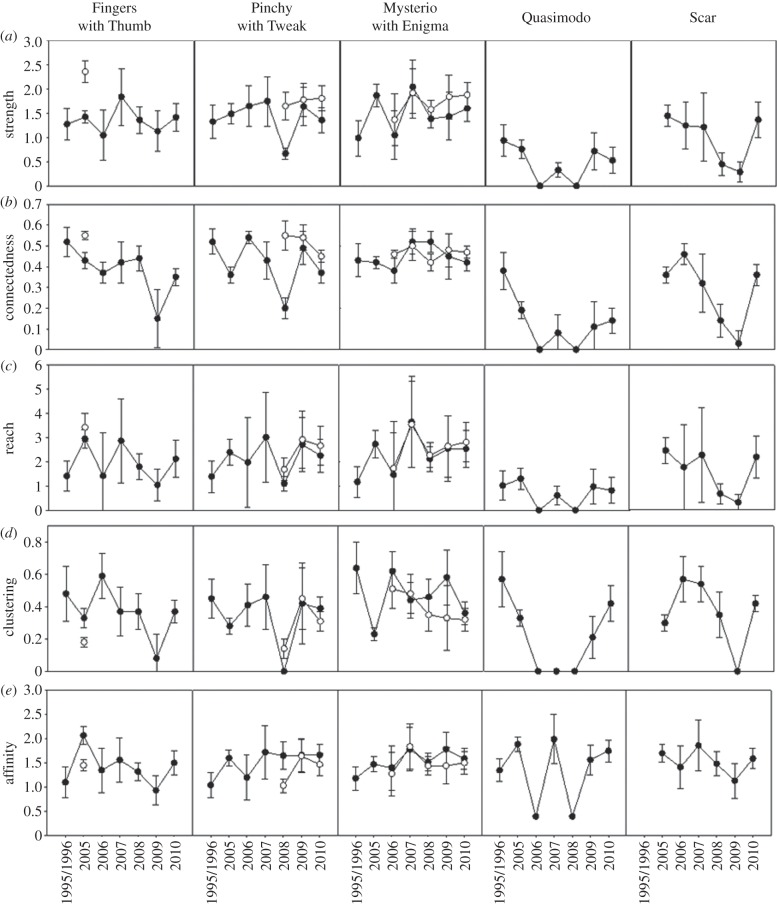
Mothers and calves appear to be the centre of the unit's social network. All calves in the GOS had high or the highest values for all of the network statistics calculated. The mothers, Pinchy (#5560) and Mysterio (#5561), both show sharp drops in strength and connectedness in the first year of their new calves' lives (figure 2). Reach also appears to drop in the first year for both mothers.
Figure 2.
(a–e) Plots of all network measures for each of the adults in the Group of Seven unit, excluding Puzzle Piece (#5130), who was only alive the first year of the study. 1995 and 1996 data from supplementary data of IFAW research [22]. Calves are shown on mothers' plots as white symbols. Error bars are standard errors based on 1000 bootstrap replicates.
(iii). Relationships through changes in social role
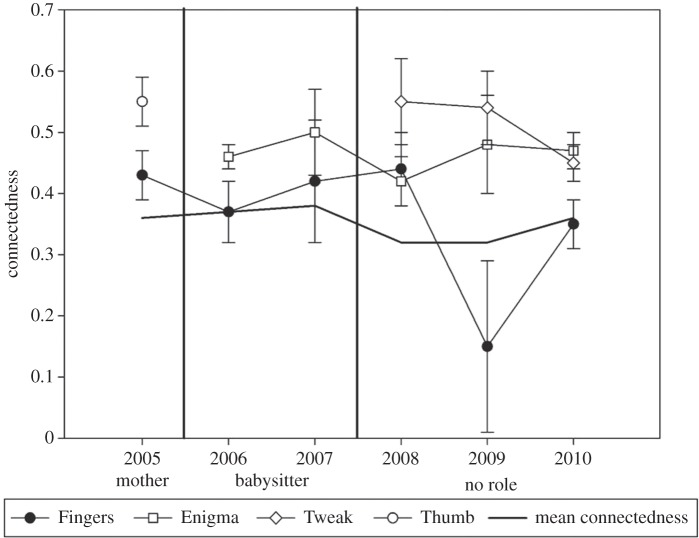
Over the course of the study, Fingers (#5722) has played several roles in her unit. Figure 3 plots the decrease in the measure of connectedness for Fingers as her social role changes with the death of her calf and birth of the new ones. In 2005, Fingers was the only mother in the unit and had accordingly high values of connectedness. In 2006, her calf died and she was the primary babysitter to the newest calf, Enigma (#6068; see [38] for a definition and justification of ‘primary babysitter’). Being involved with the care for the calf, Fingers' connectedness measures remain stable. Then, in 2008, with the birth of Tweak (#6070), the two mothers, Mysterio and Pinchy, babysat for each other, and Fingers (#5722) only escorted the calves occasionally. Without being involved in the care of either calf, her connectedness values dropped off. In 2009, Fingers spent most of her time with Quasimodo (#5563) and Scar (#5727), both socially peripheral animals. Figure 3 shows a rise in connectedness in 2010, when Fingers spent more time with her fellow unit members as the entire unit was sighted multiple times in the same cluster with mature breeding males. A larger number of males were encountered in 2010 (six in 2010, compared with up to three in other years, except 2005 when five were identified). Owing to the socialization with the males, cluster sizes among the GOS were larger in 2010. Mean size of clusters including Fingers in 2010 was 3.46 individuals (n = 56 clusters), when compared with the population mean in 2010, which was 1.90 individuals (n = 993 clusters).
Figure 3.
Connectedness across years for Fingers (#5722; black symbol) and the three GOS calves (white symbols). Note the decrease in connectedness with changes in social role (mother in 2005, babysitter in 2006 and 2007, and no role in 2008–2010). Error bars are standard errors based on 1000 bootstrap replicates.
(iv). Maturation of a juvenile male
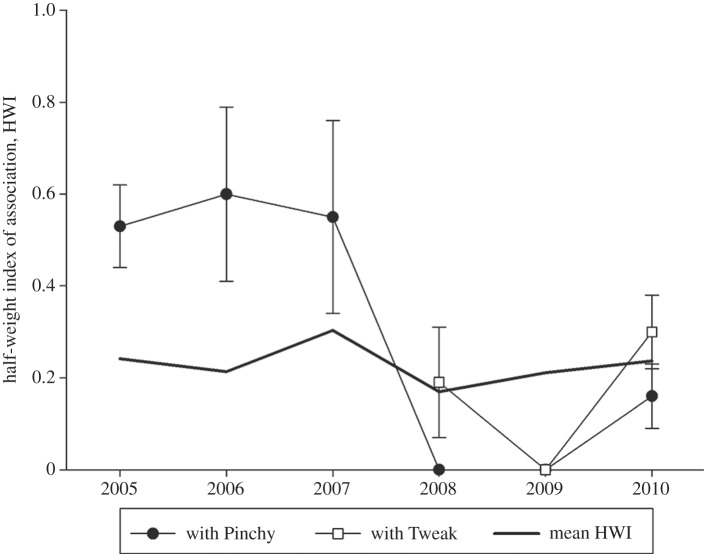
Scar (#5727), a juvenile male, who was estimated to be between 8 and 10 years old based on his size at the onset of the study in 2005 [37], was already weaned when we encountered him for the first time. Figure 2 plots the steady decrease in almost all network measures as he aged into maturity (11–15 years old by 2010). As is observed with Fingers, there is a similar rebound in most of his measures in 2010 owing to the entire unit socializing with mature males. Mean cluster size of clusters including Scar in 2010 was 4.14 individuals (n = 44 clusters). The mother–calf bond with this juvenile male appears to remain strong until the birth of the mother's next calf (figure 4). Before the birth of Tweak, Scar and his mother, Pinchy, have a preferred association (HWI more than twice the unit mean), but in 2008, the year Tweak was born, Pinchy and Scar were not observed clustered together (figure 4).
Figure 4.
Half-weight index of association between Scar and his mother, Pinchy (black circles), and his half-brother, Tweak (white squares). Mean HWI for the unit shown in thick black line. Error bars are standard errors based on 1000 bootstrap replicates.
(v). Non-reproductive females
The only female in the GOS not to reproduce over the 6 years of observations was Quasimodo (#5563). Quasimodo had the lowest values for all network measures in most years (figure 2); however, this was a decrease from the 1995–1996 dataset, in which she had similar association level values to other adult females in her unit (tables 2 and 3). Since 2006, other than when the entire unit is together, Quasimodo was predominantly sighted alone or with just Fingers.
Table 2.
Mean HWI for Quasimodo across years (1995–1996 and 2005 to 2010) when compared with unit means. Note that Quasimodo had a mean very close to the unit mean in 1995–1996 but had closer to half of the mean in the years 2005–2010.
| year | unit mean HWI | mean HWI of dyads including Quasimodo |
|---|---|---|
| 1995–1996 | 0.27 | 0.24 |
| 2005 | 0.24 | 0.13 |
| 2006 | 0.21 | 0 |
| 2007 | 0.30 | 0.07 |
| 2008 | 0.17 | 0.11 |
| 2009 | 0.21 | 0.12 |
| 2010 | 0.24 | 0.09 |
Table 3.
Mean network statistics for calves, mothers and adults across six social units across all years of the study (2005–2010).
| unit | years | class | n | strength | eigenvector centrality | reach | clustering coefficient | affinity |
|---|---|---|---|---|---|---|---|---|
| A | 3 | calves | 4 | 1.48 | 0.34 | 2.17 | 0.23 | 1.36 |
| mothers | 3 | 1.38 | 0.34 | 2.18 | 0.35 | 1.64 | ||
| adult | 5 | 0.93 | 0.17 | 1.22 | 0.25 | 1.09 | ||
| D | 3 | calves | 3 | 1.21 | 0.48 | 1.25 | 0.18 | 1.01 |
| mothers | 3 | 0.92 | 0.42 | 1.09 | 0.19 | 1.11 | ||
| adults | 3 | 0.59 | 0.16 | 0.63 | 0.24 | 0.83 | ||
| F | 6 | calves | 3 | 1.80 | 0.49 | 2.63 | 0.34 | 1.45 |
| mothers | 3 | 1.40 | 0.42 | 2.37 | 0.4 | 1.67 | ||
| adults | 3 | 1.03 | 0.27 | 1.55 | 0.30 | 1.36 | ||
| J | 4 | calves | 2 | 1.74 | 0.57 | 2.59 | 0.52 | 1.42 |
| mothers | 1 | 1.41 | 0.50 | 2.10 | 0.53 | 1.47 | ||
| adults | 3 | 1.03 | 0.38 | 1.57 | 0.49 | 1.21 | ||
| N | 2 | calves | 2 | 1.26 | 0.52 | 1.60 | 0.38 | 1.26 |
| mothers | 2 | 1.38 | 0.56 | 1.74 | 0.38 | 1.25 | ||
| adults | 4 | 0.29 | 0.10 | 0.27 | 0.11 | 0.54 | ||
| T | 3 | calves | 3 | 1.68 | 0.53 | 2.13 | 0.30 | 1.22 |
| mothers | 3 | 1.37 | 0.47 | 1.94 | 0.39 | 1.36 | ||
| adults | 4 | 0.84 | 0.26 | 1.26 | 0.37 | 1.28 | ||
| U | 3 | calves | 1 | 0.79 | 0.67 | 0.57 | 0.26 | 0.70 |
| mothers | 1 | 0.72 | 0.64 | 0.57 | 0.35 | 0.77 | ||
| adults | 2 | 0.31 | 0.19 | 0.17 | 0.36 | 0.35 |
(b). Patterns across units
These broad patterns appear to be consistent across units. Over all seven units considered, calves had high or the highest values for all network statistics (table 3). Mothers had intermediate values for most of the measures, but high values for connectedness and affinity. Unfortunately, there are no individuals in the other units studied with which to compare the changes in social patterns of either maturing juvenile males, such as Scar, or non-reproductive females who had no role in allocare, such as Quasimodo.
4. Discussion
Sperm whale families, like human families, are dynamic. Relationships change, growing stronger or fading as individuals grow older, as offspring are born, and as individuals pass away. Matrix correlations and Mantel tests quantified these changing relationships by showing that patterns of dyadic association among adults in sequential years were correlated, while non-sequential years were progressively less correlated as the time lag increased. This suggests that the social dynamic within the unit is constantly in flux and consistently changing.
The primary source of the change appears to be the births of new calves. New life brings with it new roles from many unit members. Females become mothers, older siblings become independent, and someone in the unit becomes the new calf's primary babysitter. Newfound responsibilities or freedoms come with changes in social patterns. New mothers appear to become slightly more socially isolated (Pinchy and Mysterio show drops in network statistics in the first year of their new calves' lives; figure 2), but remain connected to the rest of the unit through their calves' social relationships, and thus show accordingly high values of connectedness, clustering coefficient and affinity. It is probable that this isolation is the result of spending the majority of surface time with their new dependent calves nursing, when not otherwise at depth feeding, in order to meet the new energetic demands of producing milk.
If involvement in the care of the calves is central to the social relationships in the network of a unit, then Quasimodo provides an interesting case of a female who did not reproduce. Quasimodo had the lowest values for all network measures calculated across all years of this study (2005–2010). This differs when we compared them with her measures from 10 years prior to this study using data collected from 1995 and 1996. Her social connections with members of her own unit have decreased with age, in particular her measure of connectedness has dropped since the mid-1990s. This social peripheralization might be the result of age as has been observed in Old World primates. Among several species of monkey, older females show a trend of social withdrawal and peripheralization [39–42]. However, this trend has been disputed [43]. We were unable to determine Quasimodo's age relative to that of the other adult females in her unit. Alternatively, as her nickname implies, she may be peripheralized due to illness. Quasimodo was nicknamed as such due to a large growth surrounding her dorsal fin, which may or may not have been malignant.
The birth of a new calf results in older siblings becoming more independent. It seems that the bond between mother and juvenile males lasts far beyond weaning, but with the birth of his new half-brother, Tweak, Scar's relationship with his mother diminished dramatically. This coincided with the first recordings of Scar producing vocalizations similar to ‘clangs’ or ‘slow clicks’ (S. Gero 2008, unpublished data), a vocalization typically made by mature males [44]. Interestingly, other than a few sightings with Fingers, Scar only spent time with his new half-brother in 2008. The fact that juvenile males do provide some alloparental care to calves in their natal unit [38], Scar's association with his new half-brother when his mother was not present, and his abrupt social sequestration by the other adult females in the unit after the birth of the new calf, would suggest that juvenile males are socially ostracized from the unit by the adult females instead of leaving of their own volition at sexual maturity. Among African elephants (Loxodonta africana), males show variability in their growth towards independence. Some male elephants leave quickly while others leave gradually over several years. A few males leave when quite young, while others leave well into maturity (range 9–19 years old), typically when their mothers had another calf [45]. While the onset of Scar's separation from his natal unit appears quite quick, his final departure from the unit has been drawn out across the last few years. To the knowledge of the authors, Scar is the first juvenile male sperm whale to be observed going through the transition of splitting from his natal unit, offering a first insight into this stage of life in this species.
Alloparental care is thought to be the primary function of the permanent social relationships among female members of social units, as well as perhaps the grouping of units, in this species [9,11,13]. In this Caribbean population, each calf appears to have one primary babysitter, although all unit members escort the calf at some point [38]. Those individuals who contributed substantially to the care of calves had higher values for most of the network measures quantified than those who did not. As the hypothesis that alloparental care is a primary driver of sperm whale social relationships would predict, a female is less central to the unit's social relationships if she is not contributing directly to raising the calves. In the case of Fingers, her role as babysitter for Pinchy's new calf, Enigma, appears to have kept her network measures stable even after losing her own calf. Following this, however, when the two mothers began to babysit for each other's calves in 2008 and her role as babysitter ended, her network measures decreased as she became less central to the social network of the unit. At the very end of the fieldwork in the spring of 2011, a new calf was born in the GOS. Behavioural observations and association patterns suggest that Fingers is probably the mother (S. Gero 2011, unpublished data). Should these patterns remain consistent, Fingers will once again be central to the family's social patterns. This would support the conclusion that females seem to cycle in and out of the centre of the family's social network with new births. The social bonds between the females that maintain the social unit are reaffirmed with every new calf.
This study also sheds some light on the mechanisms which may maintain alloparental care within units of sperm whales. Prior to Thumb's death in 2005, Mysterio was his primary babysitter. With the loss of Thumb and the birth of Enigma to Mysterio in 2006, it provided a unique opportunity to examine reciprocity of alloparental care in this species for the first time. Direct reciprocity (A helps B because B helped A before [46]) would predict that Fingers should return the act of babysitting. As predicted, in 2006, Fingers did return Mysterio's investment in her calf by becoming the primary babysitter for Enigma. When direct reciprocity is delayed across repeated interactions, over a year apart in this case, individuals have the possibility of cheating by not repaying benefits received from an earlier interaction [47–49]. As a result, delays of this length in reciprocity among mammals are rare; however, a similar example exists in a socio-ecologically similar terrestrial mammal, the African elephant [50]. In 2008, with the birth of a second calf in the unit, Pinchy and Mysterio babysat for each other rather than having an external babysitter for each calf. Pinchy is more closely related to Mysterio than is Fingers (figure 1), so kin selection may play a role in determining primary babysitters. However, concurrent mothers may simply end up as each other's babysitters if calves are attracted to each other at the surface given they cannot dive for as long as their mothers. However, concurrent mothers may also choose to reciprocate allocare instead of having another female act as a babysitter as this reduces the risk of defection by eliminating the delay. However, given the long-term social reliability between related female unit members [25], limited dispersal between social units [3], the ability to recognize and interact preferentially among unit members [17], and the fact that the vast majority of females contribute to escorting the calves [38], it seems unlikely that any unit members would be likely to defect; especially given that escorting a calf at the surface while babysitting is probably not a very costly behaviour [11]. An alternative explanation would be generalized reciprocity, in which A helps B because A had help from C before, where the identities of B and C are unimportant within the boundaries of a small group [51,52]. In this case, individuals would freely offer allocare among unit members given prior experience of allocare, while the specific role of primary babysitter may be determined by kin selection [17]. Generalized reciprocity allows for the evolution of generous strategies and the possibility of prosocial norms [51]. Within these small, long-term, stable social units of sperm whales, reciprocity may be viewed not merely as a pattern of exchange, but as a social norm [53]. Morality, social norms and the recognition of inequity among animals are being increasingly discussed [54–60]. Reputation of helping (indirect reciprocity [61,62]), which can also lead to helping as a social norm [63], may also play a role in this species, but is difficult to elucidate with the current data. Stating that one mechanism alone is responsible for this system of group living and allocare probably oversimplifies the complex interactions between kin selection, the various forms of reciprocity, commensality (calves approaching nearby adults at the surface) and social norms in the relationship between allocare and group living in sperm whales.
As with any study on wild animals, there is a small possibility that the presence of our research vessel may have impacted the individuals' behaviour. While there is some evidence that large motorized whale-watch vessels affect surface behaviour of sperm whales [64], currently there is no evidence that research vessel presence affects cluster composition or membership among sperm whales. Given that this study was undertaken in an area with high shipping traffic of both private yachts and commercial vessels supplying the islands, particularly within 20 nautical miles of the coast, the whales in our study area are probably noise-tolerant individuals that are habituated to vessel traffic. This probably minimizes any of these potential observer effects, should they exist at all.
In conclusion, calves appear to be social hubs within social units of sperm whales because they were significant parts of the social relationships among unit members across the seven units studied. Change in the relationships among adult females are provided by deaths, relatedness, increased age or perhaps illness, but primarily by the birth of new calves. These findings are consistent with the theory that allocare was the primary evolutionary force driving the formation of social units in sperm whales.
Acknowledgements
Research in Dominica was carried out under scientific research permits SCR 013/05-02, RP-2/12 IW-1, RP-09/014 IW-1 and RP-01/079W-2. We thank David Lusseau for comments on the early development of this analysis; the staff at the Anchorage Hotel; all the crews of the whale-watch vessels (in particular Pernell Francis and Petra Charles); all of R/V Balaena's crew members; IFAW for their continuing initiative in curating the NAMSC catalogue; and the crews of R/V Song of the Whale from 1995 and 1996. Dalhousie University fieldwork was supported by operating and equipment grants to H.W. from the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Whale and Dolphin Conservation Society. S.G. was supported during the course of the study by an NSERC Postgraduate Scholarship (PGS-M), an NSERC Canadian Graduate Scholarship (CGS-D), the Killam Trust's Sir Izaak Killam Memorial Scholarship, the Patrick F. Lett Fund and Dalhousie's Presidents Award.
References
- 1.Alexander RD. 1974. The evolution of social behaviour. Annu. Rev. Ecol. Syst. 5, 325–383. 10.1146/annurev.es.05.110174.001545 (doi:10.1146/annurev.es.05.110174.001545) [DOI] [Google Scholar]
- 2.Connor RC. 2000. Group living in whales and dolphins. In Cetacean societies: field studies of dolphins and whales (eds Mann J, Connor RC, Tyack PL, Whitehead H.), pp. 199–218. Chicago, IL: University of Chicago Press. [Google Scholar]
- 3.Christal J, Whitehead H, Lettevall E. 1998. Sperm whale social units: variation and change. Can. J. Zool. 76, 1431–1440. 10.1139/z98-087 (doi:10.1139/z98-087) [DOI] [Google Scholar]
- 4.Whitehead H. 1999. Variation in the visually observable behavior of groups of Galapagos sperm whales. Mar. Mam. Sci. 15, 1181–1197. 10.1111/j.1748-7692.1999.tb00884.x (doi:10.1111/j.1748-7692.1999.tb00884.x) [DOI] [Google Scholar]
- 5.Whitehead H, Weilgart L. 2000. The sperm whale: social females and roving males. In Cetacean societies: field studies of dolphins and whales (eds Mann J, Connor RC, Tyack PL, Whitehead H.), pp. 154–172. Chicago, IL: University of Chicago Press. [Google Scholar]
- 6.Lyrholm T, Leimar O, Johanneson B, Gyllensten U. 1999. Sex-biased dispersal in sperm whales: contrasting mitochondrial and nuclear genetic structure of global populations. Proc. R. Soc. B 266, 347–354. 10.1098/rspb.1999.0644 (doi:10.1098/rspb.1999.0644) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mesnick SL. 2001. Genetic relatedness in sperm whales: evidence and culture implications. Behav. Brain. Sci. 24, 346–347. 10.1017/S0140525X01463965 (doi:10.1017/S0140525X01463965) [DOI] [Google Scholar]
- 8.Richard KR, Dillon MC, Whitehead H, Wright JM. 1996. Patterns of kinship in groups of free-living sperm whales (Physeter macrocephalus) revealed by multiple molecular genetic analyses. Proc. Natl Acad. Sci. USA 93, 8792–8795. 10.1073/pnas.93.16.8792 (doi:10.1073/pnas.93.16.8792) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Best PB. 1979. Social organization in sperm whales, Physeter macrocephalus. In Behaviour of marine animals (eds Winn HE, Olla BL.), pp. 227–289. New York, NY: Plenum Press. [Google Scholar]
- 10.Gordon JCD. 1987. Sperm whale groups and social behaviour observed off Sri Lanka. Rep. Int. Whaling Commission 37, 205–217. [Google Scholar]
- 11.Whitehead H. 1996. Babysitting, dive synchrony, and indications of alloparental care in sperm whales. Behav. Ecol. Sociobiol. 38, 237–244. 10.1007/s002650050238 (doi:10.1007/s002650050238) [DOI] [Google Scholar]
- 12.Whitehead H. 2003. Sperm whales: social evolution in the ocean, p. 429. Chicago, IL: University of Chicago Press. [Google Scholar]
- 13.Arnbom T, Whitehead H. 1989. Observations on the composition and behavior of groups of female sperm whales near the Galapagos Islands. Can. J. Zool. 67, 1–7. 10.1139/z89-001 (doi:10.1139/z89-001) [DOI] [Google Scholar]
- 14.Krutzen M, Mann J, Heithaus MR, Connor RC, Bejder L, Sherwin WB. 2005. Cultural transmission of tool use in bottlenose dolphins. Proc. Natl Acad. Sci. USA 102, 8939–8943. 10.1073/pnas.0500232102 (doi:10.1073/pnas.0500232102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Croft DP, James R, Thomas POR, Hathaway C, Mawdsley D, Laland KN, Krause J. 2006. Social structure and cooperative interactions in a wild population of guppies (Poecilia reticulata). Behav. Ecol. Sociobiol. 59, 644–650. 10.1007/s00265-005-0091-y (doi:10.1007/s00265-005-0091-y) [DOI] [Google Scholar]
- 16.Lusseau D. 2007. Evidence for social role in a dolphin social network. Evol. Ecol. 21, 357–366. 10.1007/s10682-006-9105-0 (doi:10.1007/s10682-006-9105-0) [DOI] [Google Scholar]
- 17.Gero S, Engelhaupt D, Whitehead H. 2008. Heterogeneous associations within a sperm whale unit reflect pairwise relatedness. Behav. Ecol. Sociobiol. 63, 143–151. 10.1007/s00265-008-0645-x (doi:10.1007/s00265-008-0645-x) [DOI] [Google Scholar]
- 18.Arnbom T. 1987. Individual identification of sperm whales. Rep. Int. Whaling Commission 37, 201–204. [Google Scholar]
- 19.Amos W, Whitehead H, Ferrari MJ, Glockner-Ferrari DA, Payne R, Gordon J. 1992. Restrictable DNA from sloughed cetacean skin: its potential for use in population analysis. Mar. Mam. Sci. 8, 275–283. 10.1111/j.1748-7692.1992.tb00409.x (doi:10.1111/j.1748-7692.1992.tb00409.x) [DOI] [Google Scholar]
- 20.Richard KR, Whitehead H, Wright JM. 1996. Polymorphic microsatellites from sperm whales and their use in the genetic identification of individuals from naturally sloughed pieces of skin. Mol. Ecol. 5, 313–315. [DOI] [PubMed] [Google Scholar]
- 21.Whitehead H, Gordon J, Mathews EA, Richard KR. 1990. Obtaining skin samples from living sperm whales. Mar. Mam. Sci. 6, 316–326. 10.1111/j.1748-7692.1990.tb00361.x (doi:10.1111/j.1748-7692.1990.tb00361.x) [DOI] [Google Scholar]
- 22.Gordon JCD, Moscrop A, Carlson C, Ingram S, Leaper R, Matthews J, Young K. 1998. Distribution, movements, and residency of sperm whales off the commonwealth of Dominica, Eastern Caribbean: implications for the development and regulation of the local whalewatching industry. Rep. Int. Whaling Commission 48, 551–557. [Google Scholar]
- 23.Dufault S, Whitehead H. 1993. Assessing the stock identity of sperm whales in the Eastern Equatorial Pacific. Rep. Int. Whaling Commission 43, 469–475. [Google Scholar]
- 24.Whitehead H. 1990. Computer assisted individual identification of sperm whale flukes. Rep. Int. Whal. Commission (Special Issue) 12, 71–77. [Google Scholar]
- 25.Whitehead H, Waters S, Lyrholm T. 1991. Social organization of female sperm whales and their offspring: constant companions and casual acquaintances. Behav. Ecol. Sociobiol. 29, 385–389. 10.1007/BF00165964 (doi:10.1007/BF00165964) [DOI] [Google Scholar]
- 26.Conradt L, Roper TJ. 2000. Activity synchrony and social cohesion: a fission–fusion model. Proc. R. Soc. Lond. B 267, 2213–2218. 10.1098/rspb.2000.1271 (doi:10.1098/rspb.2000.1271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitehead H, Dufault S. 1999. Techniques for analyzing vertebrate social structure using identified individuals: review and recommendations. Adv. Stud. Behav. 28, 33–74. 10.1016/S0065-3454(08)60215-6 (doi:10.1016/S0065-3454(08)60215-6) [DOI] [Google Scholar]
- 28.Schulz TM, Whitehead H, Gero S, Rendell L. 2008. Overlapping and matching of codas in vocal interactions between sperm whales: insights into communication function. Anim. Behav. 76, 1977–1988. 10.1016/j.anbehav.2008.07.032 (doi:10.1016/j.anbehav.2008.07.032) [DOI] [Google Scholar]
- 29.Cairns SJ, Schwager SJ. 1987. A comparison of association indices. Anim. Behav. 35, 1454–1469. 10.1016/S0003-3472(87)80018-0 (doi:10.1016/S0003-3472(87)80018-0) [DOI] [Google Scholar]
- 30.Lusseau D, Whitehead H, Gero S. 2008. Incorporating uncertainty into the study of animal social networks. Anim. Behav. 75, 1809–1815. 10.1016/j.anbehav.2007.10.029 (doi:10.1016/j.anbehav.2007.10.029) [DOI] [Google Scholar]
- 31.Whitehead H. 2008. Analyzing animal societies: quantitative methods for vertebrate social analysis. Chicago, IL: University of Chicago Press. [Google Scholar]
- 32.Whitehead H. 2009. SOCPROG programs: analyzing animal social structures. Behav. Ecol. Sociobiol. 63, 765–778. 10.1007/s00265-008-0697-y (doi:10.1007/s00265-008-0697-y) [DOI] [Google Scholar]
- 33.Mantel N. 1967. The detection of disease clustering and generalized regression approach. Cancer Res. 27, 209–220. [PubMed] [Google Scholar]
- 34.Schnell GD, Watt DJ, Douglas ME. 1985. Statistical comparison of proximity matricies: applications in animal behaviour. Anim. Behav. 33, 239–253. 10.1016/S0003-3472(85)80138-X (doi:10.1016/S0003-3472(85)80138-X) [DOI] [Google Scholar]
- 35.Hemelrijk CK. 1990. Models and tests for reciprocity, unidirectionality and other social interaction patterns at a group level. Anim. Behav. 39, 1013–1029. 10.1016/S0003-3472(05)80775-4 (doi:10.1016/S0003-3472(05)80775-4) [DOI] [Google Scholar]
- 36.Gero S, Gordon J, Carlson C, Evans P, Whitehead H. 2007. Population estimate and inter-island movement of sperm whales, Physeter macrocephalus, in the Eastern Caribbean. J. Cetacean Res. Manage. 9, 143–150. [Google Scholar]
- 37.Schulz TM. 2007. The production and exchange of sperm whale coda vocalizations. PhD thesis, Dalhousie University, Halifax, Nova Scotia. [Google Scholar]
- 38.Gero S, Engelhaupt D, Rendell L, Whitehead H. 2009. Who cares? Between-group variation in alloparental caregiving in sperm whales. Behav. Ecol. 20, 838–843. 10.1093/beheco/arp068 (doi:10.1093/beheco/arp068) [DOI] [Google Scholar]
- 39.Waser PM. 1978. Postreproductive survival and behavior in a free-ranging female mangabey. Folia Primat. 29, 142–160. 10.1159/000155836 (doi:10.1159/000155836) [DOI] [PubMed] [Google Scholar]
- 40.Hrdy SB. 1981. Nepotist and altruists: the behavior of old female among macaques and langur monkeys. In Other ways of growing old: anthropological perspectives (eds Amoss PT, Harreli S.), pp. 59–76. Stanford, CA: Stanford University Press. [Google Scholar]
- 41.Hauser MD, Tyrell G. 1984. Old age and its behavioural manifestations: a study on two species of macaque. Folia Primat. 43, 24–35. 10.1159/000156168 (doi:10.1159/000156168) [DOI] [PubMed] [Google Scholar]
- 42.Nakamichi M. 1984. Behavioral characteristics of old female Japanese monkeys in a free-ranging group. Primates 25, 192–203. 10.1007/BF02382391 (doi:10.1007/BF02382391) [DOI] [Google Scholar]
- 43.Pavelka MSM. 1991. Sociability in old female Japanese monkeys: human versus nonhuman primate aging. Am. Anthropol. 93, 588–598. 10.1525/aa.1991.93.3.02a00030 (doi:10.1525/aa.1991.93.3.02a00030) [DOI] [Google Scholar]
- 44.Weilgart LS, Whitehead H. 1988. Distinctive vocalizations from mature male sperm whales (Physeter macrocephalus). Can. J. Zool. 66, 1931–1937. 10.1139/z88-282 (doi:10.1139/z88-282) [DOI] [Google Scholar]
- 45.Lee PC, Moss CJ. 1999. The social context for learning and behavioural development among wild African elephants. In Mammalian social learning: comparative and ecological perspectives (eds Box HO, Gibson KR.), pp. 102–125. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 46.Axelrod R, Hamilton WD. 1981. The evolution of cooperation. Science 211, 1390–1396. 10.1126/science.7466396 (doi:10.1126/science.7466396) [DOI] [PubMed] [Google Scholar]
- 47.Trivers RL. 1985. Social evolution, p. 479 Menlo Park, CA: Benjamin-Cummings. [Google Scholar]
- 48.Enquist M, Leimar O. 1993. The evolution of cooperation in mobile organisms. Anim. Behav. 45, 747–757. 10.1006/anbe.1993.1089 (doi:10.1006/anbe.1993.1089) [DOI] [Google Scholar]
- 49.Clutton-Brock TH. 2009. Cooperation between non-kin in animal societies. Nature 462, 51–57. 10.1038/nature08366 (doi:10.1038/nature08366) [DOI] [PubMed] [Google Scholar]
- 50.Lee PC. 1987. Allomothering among African Elephants. Anim. Behav. 35, 278–291. 10.1016/S0003-3472(87)80234-8 (doi:10.1016/S0003-3472(87)80234-8) [DOI] [Google Scholar]
- 51.Pfeiffer T, Rutte C, Killingback T, Taborsky M, Bonhoeffer S. 2005. Evolution of cooperation through generalized reciprocity. Proc. R. Soc. B 272, 1115–1120. 10.1098/rspb.2004.2988 (doi:10.1098/rspb.2004.2988) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamilton IM, Taborsky M. 2005. Contingent movement and cooperation under generalized reciprocity. Proc. R. Soc. B 272, 2259–2267. 10.1098/rspb.2005.3248 (doi:10.1098/rspb.2005.3248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gouldner AW. 1960. The norm of reciprocity: a preliminary statement. Am. Sociol. Rev. 25, 161–178. 10.2307/2092623 (doi:10.2307/2092623) [DOI] [Google Scholar]
- 54.Broom DM. 2006. The evolution of morality. Appl. Anim. Behav. Sci. 100, 20–28. 10.1016/j.applanim.2006.04.008 (doi:10.1016/j.applanim.2006.04.008) [DOI] [Google Scholar]
- 55.Brosnan SF. 2011. An evolutionary perspective on morality. J. Econ. Behav. Organ 77, 23–30. 10.1016/j.jebo.2010.04.008 (doi:10.1016/j.jebo.2010.04.008). [DOI] [Google Scholar]
- 56.Flack J, De Waal FBM. 2000. Any animal whatever: Darwinian building blocks of morality in monkeys and apes. J. Consciousness Stud. 7, 1–29. [Google Scholar]
- 57.Pierce J, Bekoff M. 2012. Wild justice redux: what we know about social justice in animals and why it matters. Social Justice Res. 25, 122–139. 10.1007/s11211-012-0154-y (doi:10.1007/s11211-012-0154-y) [DOI] [Google Scholar]
- 58.Flack JC, Girvan M, De Waal FBM, Krakauer DC. 2006. Policing stabilizes construction of social niches in primates. Nature 439, 426–429. 10.1038/nature04326 (doi:10.1038/nature04326) [DOI] [PubMed] [Google Scholar]
- 59.Flack JC, Jeannotte LA, De Waal FBM. 2004. Play signalling and the perception of social rules by juvenile chimpanzees (Pan troglodytes). J. Comp. Psychol. 118, 149–159. 10.1037/0735-7036.118.2.149 (doi:10.1037/0735-7036.118.2.149) [DOI] [PubMed] [Google Scholar]
- 60.Sapolsky RM, Share LJ. 2004. A pacific culture among wild baboons: its emergence and transmission. PLoS Biol. 2, 534–541. 10.1371/journal.pbio.0020106 (doi:10.1371/journal.pbio.0020106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nowak M, Sigmund K. 1998. The dynamics of indirect reciprocity. J. Theoret. Biol. 194, 561–574. 10.1006/jtbi.1998.0775 (doi:10.1006/jtbi.1998.0775) [DOI] [PubMed] [Google Scholar]
- 62.Nowak M, Sigmund K. 1990. The evolution of stochastic strategies in the Prisoner's dilemma. Acta Appl. Math. 20, 247–265. 10.1007/BF00049570 (doi:10.1007/BF00049570) [DOI] [Google Scholar]
- 63.Wedekind C, Braithwaite VA. 2002. The long-term benefits of human generosity in indirect reciprocity. Curr. Biol. 12, 1012–1015. 10.1016/s0960-9822(02)00890-4 (doi:10.1016/s0960-9822(02)00890-4) [DOI] [PubMed] [Google Scholar]
- 64.Richter C, Dawson S, Slooten E. 2006. Impacts of commercial whale watching on male sperm whales at Kaikoura, New Zealand. Mar. Mam. Sci. 22, 46–63. 10.1111/j.1748-7692.2006.00005.x (doi:10.1111/j.1748-7692.2006.00005.x) [DOI] [Google Scholar]