Abstract
In acute liver failure (ALF), the hyperdynamic circulation is believed to be the result of overproduction of nitric oxide (NO) in the splanchnic circulation. However, it has been suggested that arginine concentrations (the substrate for NO) are believed to be decreased, limiting substrate availability for NO production. To characterize the metabolic fate of arginine in early-phase ALF, we systematically assessed its interorgan transport and metabolism and measured the endogenous NO synthase inhibitor asymmetric dimethylarginine (ADMA) in a porcine model of ALF. Female adult pigs (23–30 kg) were randomized to sham (N = 8) or hepatic devascularization ALF (N = 8) procedure for 6 h. We measured plasma arginine, citrulline, ornithine levels; arginase activity, NO, and ADMA. Whole body metabolic rates and interorgan flux measurements were calculated using stable isotope-labeled amino acids. Plasma arginine decreased >85% of the basal level at t = 6 h (P < 0.001), whereas citrulline and ornithine progressively increased in ALF (P < 0.001 and P < 0.001, vs. sham respectively). No difference was found between the groups in the whole body rate of appearance of arginine or NO. However, ALF showed a significant increase in de novo arginine synthesis (P < 0.05). Interorgan data showed citrulline net intestinal production and renal consumption that was related to net renal production of arginine and ornithine. Both plasma arginase activity and plasma ADMA levels significantly increased in ALF (P < 0.001). In this model of early-phase ALF, arginine deficiency or higher ADMA levels do not limit whole body NO production. Arginine deficiency is caused by arginase-related arginine clearance in which arginine production is stimulated de novo.
Keywords: arginine, asymmetric dimethylarginine, arginase
acute liver failure (ALF) is characterized by sudden and severe liver dysfunction with rapid progression to coagulopathy, encephalopathy, and multiorgan failure. Without liver transplantation, mortality from ALF is about 50% (4). A hallmark of ALF is the presentation of systemic hypotension and a hyperdynamic circulation (39). This is associated with increased guanylate cyclase (GC) activation by nitric oxide (NO) (33), which converts guanidine triphosphate to cyclic guanidine monophosphate and is thought to cause the vasodilatation seen in ALF (34). Previously, we have reported the characteristics of the same devascularized porcine liver model of ALF, which was shown to have a hyperdynamic circulation as evidenced by a high cardiac output and low mean arterial pressure and systemic vascular resistance (50). However, in this hyperacute model of ALF, no significant changes in the metabolites of NO were observed. Indeed, the kinetics of NO in this model and whether it is involved in the initiation of the vasodilation of ALF remain unknown.
l-Arginine has several important biological functions (2, 3, 5), one of which is to be the nitrogen-donating substrate for endothelial NO synthase (eNOS) to produce NO and citrulline in stoichiometric quantities (31). The plasma concentration of arginine is believed to be the rate-limiting factor in its synthesis (1). In ALF, plasma concentrations of l-arginine have been measured, but the results are contradictory with some studies suggesting that the levels remain the same (38) and others showing a reduction (45) or an increase (6, 7).
Other regulators that may influence the amount of NO generated in ALF are the concentrations of the endogenous NOS inhibitor asymmetric dimethyl arginine (ADMA) (6) and levels of plasma arginase activity (11). In a previous study, we have shown that ADMA levels were higher in patients with ALF and that the increase was associated with worse outcome (27). Similarly, an increase in the level of plasma arginase, released during hepatic stresses (8), can reduce the amount of available arginine, preventing eNOS-mediated NO synthesis. Both these observations can relate to a reduced NO production.
It is still unclear as to the exact mechanism of the vascular derangement in the initiation of vasodilation in ALF. Our group has previously described the classical hyperdynamic circulation and reduced systemic vascular resistance, along with hepatic encephalopathy, high intracranial pressure, and coagulopathy in a devascularized porcine model of ALF, which is ideal to study the initiation of metabolic disturbances in relation to hemodynamic changes (49). The aim of the study was to evaluate the evolution of disturbances in NO metabolism in relation to its regulators, l-arginine, ADMA, and arginase, in the first 6 h of ALF using stable isotope technology.
MATERIALS AND METHODS
Study outline.
The Norwegian Experimental Animal Board approved the present study. Sixteen female Landrace pigs (23–30 kg) were randomly allocated into Sham-operated control or ALF groups. Study outline is shown in Fig. 1. Blood and urine sampling was performed 30–45 min after creation of the portacaval shunt (PCS) or completion of Sham surgery (t = 0 h). ALF was induced by hepatic artery ligation (t = 0 h) immediately after completion of the sampling procedures. The experiments were terminated with an overdose of pentobarbital sodium and potassium chloride at t = 6 h.
Fig. 1.
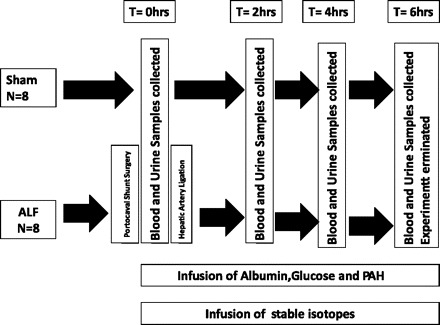
Study design for the porcine model of acute liver failure. PAH, p-Amminohippuric acid; ALF, acute liver failure.
Animal preparation and surgery.
The pigs were kept in the animal department for at least 2 days before the experiments. Details regarding the animal room facilities, anesthesia, and surgical preparation have been previously reported (47–49). Briefly, the pigs underwent a tracheotomy, were intubated, and ventilated on a volume-controlled respirator (Servo 900; Elema-Schnander, Stockholm, Sweden). Ventilation was not altered after t = 0 h. Core body temperature was maintained normothermic at 38.5 ± 1°C with a heating pad and blankets. All animals received 500 ml of 0.9% NaCl containing 625 mg of glucose as a preoperative load to prevent dehydration. During the experiment, 0.9% NaCl was infused at a rate of 3 ml/kg per h. 0.9% NaCl, 50% glucose, and 20% human albumin (Octapharm, Hurdal, Norway) was infused continuously at the rate of 3 ml kg per h, 0.6048 ml/kg per h, and 0.66 ml/kg per h, respectively. Sham animals were given half the amount of glucose to make the glucose levels comparable between the groups. Anesthesia was stopped in the ALF group after the liver was devascularized although, if the degree of sedation became insufficient, small doses of fentanyl and midazolam were given as a bolus. Sham-operated animals received continuous anesthesia during the experimental period and received equal amounts of intravenous fluids. ALF was induced with an end-to-side PCS followed by ligation of the hepatic arteries. Details of the surgery, including the Sham-operation procedure, have been described elsewhere (48, 49).
Positioning of catheters, flow probes, sampling, and analytical procedures.
Catheters, combining our previously described approach in mice (17) and pigs (37), were inserted in the abdominal aorta, renal vein, portal vein, and femoral vein for arterial and venous across-organ blood sampling. A 16-G central venous catheter (Secalon T; Ohmeda, Swindon, UK) was introduced into the left external jugular vein for administration of drugs and fluids. p-Amminohippuric acid (PAH; 25 mM; A1422; Sigma, St. Louis, MO) was infused at a rate of 30 ml/h through this catheter after an initial bolus of 6 ml (37). Portal and femoral blood flows were measured by the use of perivascular ultrasonic transit time flow probes (CardioMed Systems; Medistim A/S, Oslo, Norway). A 5-Fr Edwards Swan-Ganz catheter (Baxter Healthcare, Irvine, CA) was floated into the pulmonary artery via the right external jugular vein. The urine bladder was drained via a cystotomy. Blood and urine samples were collected on ice at the times for measurement of blood flow and processed as described previously (10). Tissue samples were freeze clamped with Wollenberger tongs cooled in liquid nitrogen (17) and frozen at −80°C. Ammonia, urea, and PAH concentrations were determined spectrophotometrically (10). Amino acids concentrations were determined using HPLC (20).
The use of stable amino acid isotopes.
A venous blood sample was drawn for the measurement of the natural abundance of plasma amino acids in each subject, before the start of the stable isotope primed-continuous infusion protocol. Stable isotopes were administrated into the left external jugular vein, which included a priming dose followed by continuous infusion of a mixture of l-[guanido-15N2] arginine (N2-arginine prime: 1 mg/kg body wt per h) and l-[ureido-13C; 5,5-2H2] citrulline (C1D2-citrulline, prime: 0.1 mg/kg body wt, infusion: 0.1 mg/kg body wt per h; Cambridge Isotope Laboratories, Woburn, MA) (41). Plasma enrichments of amino acid isotopes were determined by measuring the ratio of the amino acid stable isotope/amino acid (tracer/tracee ratio) with a liquid chromatography mass spectrometry method (42).
Plasma ADMA was measured as described previously (26) using fragmentation-specific stable isotope dilution electrospray mass spectrometry-mass spectrometry. Samples were deproteinized with acetonitrile containing 2H6-ADMA, chromatographed (acetonitrile:water, 1:1; with 0.025% formic acid) on a Teicoplanin guard column 10-mm × 2.1 mm inner diameter (Chirobiotic T; ASTEC, Congleton, UK), and analyzed using a SCIEX API4000 (Applied Biosystems, Warrington, UK) in positive-ion multiple-reaction-monitoring mode.
Calculations.
Plasma flow rate (ml/kg body wt per min) of the kidneys was calculated using the formulae based on the method of indicator dilution and Fick's principle (10, 30). The PAH-determined blood flow and data from the perivascular blood flow probes were converted to plasma flow using the hematocrit. Substrate fluxes across organs were calculated as the venous-arterial concentration difference multiplied by the plasma flow. Positive values reflect substrate release, and negative fluxes reflect substrate uptake. Kidney and hind leg data are multiplied by two to reflect both organs. The flux of amino acid across the leg represented flux across a muscle compartment with defined arterial and venous sampling. Plasma arginase activity was assessed by colorimetric assay (8). Whole body rate of appearances of amino acids were calculated by the continuous stable amino acid isotope infusion rate divided by the arterial plasma enrichment of that isotope (44).
Whole body rate of appearances of NO was determined with the flux of plasma l-arginine to l-citrulline-stable isotopes as described previously (41). Whole body rate of appearance of de novo arginine was determined with the flux of plasma l-citrulline to l-arginine-stable isotopes as described previously (32).
Whole body arginine clearance is defined as the amount of arginine that is cleared each minute from arginine and was calculated by whole body rate of appearance of arginine divided by the arterial arginine concentration (23).
Statistics.
Data are expressed as means ± SE. Significance of difference between groups was tested with Student's t-test or the Mann-Whitney comparisons test for nonparametric data as appropriate. Continuous data sets were compared using two-way ANOVA; P < 0.05 was taken to be statistically significant. Software used included Microsoft Excel 2007 (Microsoft, Redmond, WA) and GraphPad Prism 4.0 (GraphPad Software, San Diego, CA).
RESULTS
Whole body rate of appearance of NO in ALF.
The whole body rate of appearance of NO at the time of PCS or after ALF induction was not significantly different between the two groups. There was no significant change in the whole body rate of appearance of NO between the Sham-operated and ALF groups throughout the experimental period (Fig. 2).
Fig. 2.
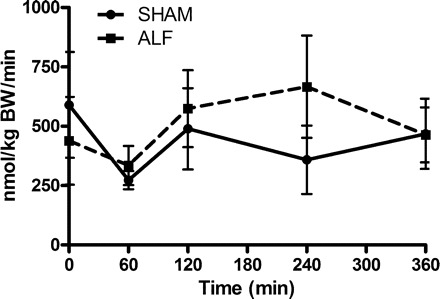
Whole body rate of appearance of nitric oxide (NO) in Sham-operated and ALF pigs. Data shown are means ± SE.
l-arginine, citrulline, and glutamine metabolism.
Arterial arginine concentration was not significantly different from the basal level after the creation of the PCS and at induction of ALF but was reduced significantly (P < 0.001) at 2 h and continued to decrease until end of the experimental period compared with the Sham group, in which no significant change was observed (Fig. 3A). Citrulline concentration was not significantly different between Sham and ALF pigs after creation of PCS; however, the level significantly increased (P < 0.01) after the induction of ALF, and it continued to increase throughout the experimental period (P < 0.001) compared with the Sham group, in which no significant changes were observed (Fig. 3B). Glutamine concentration was not different from the Sham-operated group after induction of PCS (Sham 547 ± 45 μM, ALF 623 ± 57 μM at t = 0) but increased significantly after ALF induction (P < 0.05) and continued to increase significantly till the end of the experiment (P < 0.001), as shown and discussed in a previous paper by our group (51).
Fig. 3.
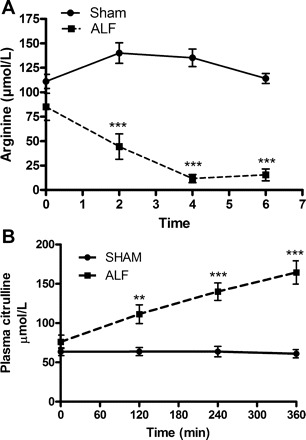
Plasma arginine (A), citrulline (B) concentration in Sham-operated and ALF pigs with time. (**P < 0.01, ***P < 0.001, 2-way ANOVA). Data shown are means ± SE.
Interorgan metabolism of l-arginine and citrulline.
The effect of PCS and ALF on muscle metabolism is shown in Table 1, which shows the net flux across the muscle. Glutamine was released from the leg muscle throughout the experimental period, increasing nonsignificantly at 2 h after the induction of ALF, which was not observed in the Sham group (51). There is no significant uptake or release of citrulline or arginine from the muscle.
Table 1.
Net flux of l-arginine and citrulline across muscle
| Time | Sham | ALF | |
|---|---|---|---|
| Arginine, μmol/kg body wt per min | t = 0 h | −0.0072 ± 0.018 | −0.0048 ± 0.017 |
| t = 2 h | 0.0702 ± 0.024 | 0.0361 ± 0.0358 | |
| t = 4 h | 0.0721 ± 0.019 | −0.0082 ± 0.030 | |
| t = 6 h | 0.0432 ± 0.009 | 0.0033 ± 0.025 | |
| Citrulline, μmol/kg body wt per min | t = 0 h | −0.0038 ± 0.013 | 0.0015 ± 0.011 |
| t = 2 h | 0.0166 ± 0.006 | 0.0037 ± 0.017 | |
| t = 4 h | −0.0118 ± 0.008 | 0.0163 ± 0.034 | |
| t = 6 h | −0.0106 ± 0.010 | −0.0444 ± 0.050 |
ALF, acute liver failure.
The effect of PCS and ALF on portal-derived viscera (PDV) metabolism was determined. Glutamine was taken up by the PDV throughout the experimental period in both the PCS and ALF groups (51). Citrulline was released from the PDV at the point of creation of the PCS, and the release significantly increased in the ALF animals compared with the Sham animals (P < 0.05). There were no significant differences in net fluxes of arginine in the PDV (Table 2).
Table 2.
Net flux of l-arginine and citrulline across the PDV
| Time | Sham | ALF | |
|---|---|---|---|
| Arginine, μmol/kg body wt per min | t = 0 h | −0.0256 ± 0.135 | 0.1387 ± 0.061 |
| t = 2 h | 0.1665 ± 0.131 | 0.3117 ± 0.056 | |
| t = 4 h | 0.1440 ± 0.127 | 0.0941 ± 0.079 | |
| t = 6 h | −0.0444 ± 0.084 | 0.0491 ± 0.118 | |
| Citrulline, μmol/kg body wt per min | t = 0 h | 0.1647 ± 0.107 | 0.3768 ± 0.092 |
| t = 2 h | 0.4195 ± 0.092 | 0.6975 ± 0.069 | |
| t = 4 h | 0.3421 ± 0.068 | 0.5882 ± 0.094 | |
| t = 6 h | 0.2451 ± 0.101 | 0.8322 ± 0.572* |
P < 0.05 Sham vs. ALF, 2-way ANOVA. PDV, portal-derived viscera.
The effect of PCS and ALF on renal metabolism was also determined. Citrulline was taken up by the kidney after the creation of the PCS, and the uptake increased significantly compared with the Sham-operated group (P = 0.05). Arginine was released from the kidney after the induction of ALF, and the release remained significantly higher compared with the Sham animals (P < 0.0001) for the remainder of the study period. There was no significant change in the metabolism of glutamine (51) (Table 3).
Table 3.
net flux of l-arginine and citrulline across the kidneys
| Time | Sham | ALF | |
|---|---|---|---|
| Arginine, μmol/kg body wt per min | t = 0 h | 0.5448 ± 0.083 | 0.6855 ± 0.112 |
| t = 2 h | 0.2924 ± 0.123 | 0.5887 ± 0.101 | |
| t = 4 h | 0.3604 ± 0.135 | 0.5279 ± 0.134 | |
| t = 6 h | 0.2439 ± 0.112 | 0.4351 ± 0.255† | |
| Citrulline, μmol/kg body wt per min | t = 0 h | −1.246 ± 0.159 | −1.1107 ± 0.175 |
| t = 2 h | −0.8352 ± 0.150 | −1.3775 ± 0.358 | |
| t = 4 h | −1.0228 ± 0.160 | −1.4413 ± 0.305 | |
| t = 6 h | −0.8241 ± 0.072 | −1.4620 ± 0.427* |
P < 0.05;
P < 0.001, Sham vs. ALF, 2-way ANOVA.
Whole body rate of appearance of arginine and de novo arginine in ALF.
The whole body rate of appearance of arginine showed no difference between the Sham and ALF groups at the point of creation of the PCS. There was also no significant difference observed between the Sham and ALF group in the whole body appearance of arginine throughout the experimental period (Fig. 4A). The whole body rate of appearance of de novo arginine was nonsignificantly different between the two groups at the point of creation of the PCS. The rate of appearance of arginine increased nonsignificantly in the ALF group compared with the Sham-operated group through the period of experimentation although it showed a significant increase in production in the ALF group at the end of the experiment compared with the Sham group (P < 0.05, Fig. 4B). There was a significant difference in the clearance of arginine between the Sham and the ALF pigs, wherein the ALF pigs showed a significantly increased arginine clearance at the end of the experiment compared with the Sham and the levels of clearance remained constant throughout the experimental period (P < 0.001, Fig. 4C).
Fig. 4.
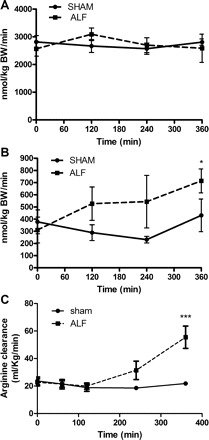
Whole body rate of appearance of arginine (WbRa, A), de novo arginine (B), and arginine clearance (C) of Sham-operated and ALF pigs with time (*P < 0.05, ***P < 0.001, 2-way ANOVA). Data shown are means ± SE.
Plasma arginase activity.
Ornithine concentration was not different between Sham and ALF groups at the creation of PCS, but the arterial concentration of ornithine increased after induction of ALF and continued to increase throughout the duration of the experiment to a significant level (P < 0.001 at 6 h) compared with the Sham-operated animals (Fig. 5A) in which no significant changes were observed. The plasma arginase activity was equal in the two groups at the point of PCS and the induction of ALF. The plasma arginase activity increased through the course of the experiment and, at the end of the experiment, was significantly increased (P < 0.001, Fig. 5B) in the ALF group compared with the Sham-operated group.
Fig. 5.
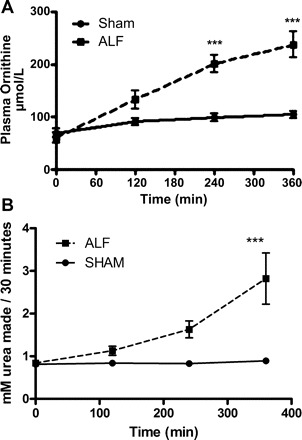
Plasma ornithine (A) and plasma arginase activity (B) in Sham-operated and ALF pigs throughout the experimental period. There is a significant increase in the plasma ornithine levels with a corresponding significant increase in the plasma arginase activity at the end of the experimental period (***P < 0.001, 2-way ANOVA). Data shown are means ± SE.
Plasma arterial ADMA.
The arterial ADMA concentration was not significantly different between the two groups after the creation of the PCS. The ADMA levels remained constant in the Sham group through the course of the experiment. However, after the induction of ALF, the arterial concentrations of ADMA were increased at 2 h and continued to be significantly elevated throughout the remainder of the study period (P < 0.001, Fig. 6). The normal values of l-arginine to ADMA ratio is about 100:1 in healthy controls. However, in the setting of ALF, the l-arginine to ADMA ratio was markedly reduced (P < 0.001, Fig. 7).
Fig. 6.
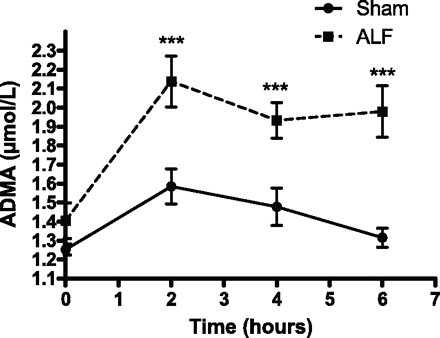
Plasma asymmetric dimethyl arginine (ADMA) concentration in Sham-operated and ALF pigs with time. There is a significant increase in the arterial ADMA concentration in the ALF pigs compared with the Sham-operated pigs at 2 h and continues to increase significantly until the end of experiment (***P < 0.001, 2-way ANOVA). Data shown are means ± SE.
Fig. 7.
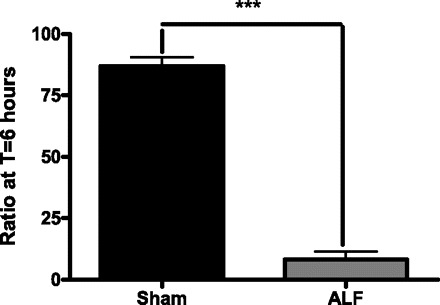
Ratio of arginine to ADMA in Sham-operated and the ALF pig groups at 6 h postinduction of ALF. There is a significant decrease in the arginine to ADMA ratio in the ALF pig compared with the Sham-operated pigs at the end of 6 h (***P < 0.001, Mann-Whitney test). The data shown are means ± SE.
DISCUSSION
The most important finding of this study was the observation that the rate of NO production was not significantly different between animals with ALF and Sham controls, indicating that NO is unlikely to be involved in initiating the vasodilation and hypotension observed in early ALF. These results are unexpected and may in part be explained by substantially reduced plasma arginine levels through the action of arginase. However, we also show that there is an increase in de novo arginine synthesis, resulting in only a relative arginine deficiency, although there appears to be insufficient capacity in this mechanism to restore normal levels. In relation to ADMA, the significant elevation seen in ALF plasma is likely to reflect the impairment of hepatic ADMA metabolism and/or its increased generation. Given the devascularized liver in this model, the significance of higher arterial ADMA values compared with sham are likely to reflect the spillover of an increased capacity to compete with arginine at tissue level, in turn limiting the generation of NO. It is also possible that the site of NO production within the endothelial cells is unaffected by the reduction in plasma arginine. Furthermore, our findings of no significant change in rate of NO production are still compatible with other studies in ALF that have observed changes in activity of guanylate cyclase (33, 34). We and others have shown that the activation of guanylate cyclase can be independent of NO (9, 21) and that guanylate cyclase activation itself may be altered in the context of liver injury.
This experimental model of hyperacute ALF has been well characterized (49). The ability to infuse relevant amino acids with stable isotope labels to measure the conversion of arginine to citrulline as a surrogate for NO production was employed in this model to evaluate the role of NO in initiating the hemodynamic disturbances, which is not possible to do in a clinical setting, as patients will invariably present late in the course of the condition. These findings lead us to speculate that there may be some other mechanism involved in the pathophysiology of the vascular abnormality seen in the onset of ALF as seen in patients (39) and also this model (50). This result is in contrast to other published articles where increases in the metabolites of NO were described (34). The human studies were performed when ALF was already manifest, and the condition is invariably associated with systemic inflammation. The present study focused on evaluating the role of NO in initiating hemodynamic disturbances and was founded on prior evaluation of this model where no clear evidence could be shown for systemic inflammation. Thus the changes observed can be considered to occur more on a background of metabolic dysfunction, as demonstrated by hyperammonemia, hyperlactatemia, and derangements in glycolysis previously described in this model. (35).
The sole nitrogen-donating substrate for the synthesis of NO is l-arginine. Arginine is oxidized to NO and citrulline by the enzyme NOS. In the setting of ALF, plasma arginine levels have been found to be lower than those in healthy animals. This finding was described in the early description of this devascularized porcine model of acute liver failure, wherein they also found that the concentrations of citrulline and ornithine were increased (24). In septic patients, low arginine levels (16, 25) have also been observed, which is associated with poor outcome. Arginine clearance was found to progressively increase over the duration of the study (Fig. 4C). Although the whole body rate of appearance of arginine was unchanged, the reduction in the plasma levels was significant. This increased clearance rate most likely provided stimulation for the observed de novo synthesis of arginine via the intestinal-renal axis; however, the amount generated during the experimental period was insufficient to restore normal plasma levels. The mechanism of the increased de novo synthesis of l-arginine is revealed from the interorgan experiments. The increased glutamine that was released from the muscle was taken up by the PDV (gut), where it was converted to citrulline. This citrulline was converted stoichiometrically to arginine in the kidney. It is important to acknowledge that there are limitations to this hyperacute model of ALF in that the experimental duration is over 6 h and occurs in a devascularized liver. The findings, while questioning the exclusive role of NO in modulating the hyperdynamic circulation in ALF, do need further testing in other ALF model systems.
A second important observation was the finding of increased plasma levels of ornithine. The increase in ornithine and the simultaneous reduction in arginine indicate that there is an increase in arginase activity, which was found to be markedly increased in the ALF animals. Arginase I is a cytosolic enzyme that forms a part of the urea cycle and is predominantly present in the liver (43); arginase II is a mitochondrial protein found most abundantly in the kidney (28). Other studies have also found arginase to be present in the plasma following liver injury (12, 13). In this model of hepatic arterial devascularization, the hepatic vein, and hence venous drainage from the liver, remains intact. It is likely that the arginase that was found in the plasma is derived from the breakdown of and leakage from hepatocytes and enters the systemic circulation due to a small amount of residual collateral venous drainage from the liver post-PCS surgery and hepatic artery ligation. Although the major blood supply routes to the liver are removed, there still remains a small but significant contribution from collateral vessels above the section point of the portal vein (18). Although we propose that the majority of the measured arginase activity derives from the liver, the kidney and other vasculature may undertake a substantial role in the production and release of arginase. The mechanism of reduction in arginine may be related to this increase in arginase activity, which is known to regulate the bioavailability of arginine. This inference can be drawn from genetic studies wherein it has been shown that arginase I deficiency leads to hyperargininemia in both humans and mice (19, 52). Similarly, a deficiency of arginase II also leads to a twofold increase in the level of arginine (36). Apart from these mechanisms, the arginase-mediated depletion of arginine may also inhibit the expression of inducible NOS by repressing the translation and stability of the inducible NOS protein (14, 22).
Furthermore, the significantly increased ADMA levels also provide a possible mechanism for an impaired production of NO in this model of ALF. ADMA, an endogenous inhibitor of NOS, is synthesized by the action of protein arginine methyl transferase on arginine residues of nuclear proteins. ADMA is released following proteolysis, and most of the freely circulating ADMA is metabolized by dimethylarginine dimethylaminohydrolase, which occurs mostly in the liver (29) but can also occur in other organs such as the kidney (46). Data from this model study have previously been described and shown to have renal dysfunction and changes in vascular dynamics as part of the developing multiple organ dysfunction syndrome (50). Renal dysfunction, particularly in the context of reno-vascular hypertension, has been described as being associated with higher than normal range values of ADMA (40). Plasma ADMA levels have been shown to be markedly increased in ALF, and its levels correlate with severity of liver failure and the presence of added inflammation and are significantly higher than the levels described in renal disease, which would support the assertion that the ADMA levels in these models are primarily impacted on by hepatic impairment (15, 27). As ADMA is a competitive inhibitor of the NOS-arginine binding site, lowering the arginine to ADMA ratio may directly result in a reduction in NOS activity. In our study, an increase in plasma ADMA level and a reduction of the arginine to ADMA ratio were observed. This suggests that the lack of increase in NO production that was observed in the ALF group may be attributable to the reduction in eNOS activity in the presence of an elevated ADMA and low arginine plasma levels. This is further compounded by the fact that high arginase also plays its part in reducing the eNOS activity and arginine levels.
In conclusion, this study shows for the first time that, during the initiation of ALF, NO may not play a significant role in mediating the hemodynamic disturbances. This lack of augmentation in the production of NO may be due to a relative reduction in l-arginine concentration despite increased de novo production, through the action of arginase and a reduction in its effect on NOS by the relative increase in the levels of its competitive antagonist, ADMA.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: V.S., G.A.T.H., L.Y., S.S., C.F.R., R.N.D., C.T., A.R., H.M.v.-E., N.E.D., R.J., R.P.M., and N.A.D. analyzed data; V.S., G.A.T.H., L.Y., S.S., C.F.R., R.N.D., C.T., A.R., N.E.D., R.J., R.P.M., and N.A.D. interpreted results of experiments; V.S. and N.A.D. prepared figures; V.S., R.P.M., and N.A.D. drafted manuscript; V.S., G.A.T.H., L.Y., S.S., C.F.R., R.N.D., C.T., A.R., H.M.v.-E., N.E.D., R.J., R.P.M., and N.A.D. edited and revised manuscript; V.S., G.A.T.H., L.Y., S.S., C.F.R., N.E.D., R.J., R.P.M., and N.A.D. approved final version of manuscript; G.A.T.H., L.Y., S.S., C.F.R., H.M.v.-E., and N.A.D. performed experiments; L.Y., S.S., C.F.R., A.R., N.E.D., R.J., and N.A.D. conception and design of research.
REFERENCES
- 1. Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J 357: 593–615, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barbul A. Arginine and immune function. Nutrition 6: 53–58; discussion 59–62, 1990 [PubMed] [Google Scholar]
- 3. Barbul A, Lazarou SA, Efron DT, Wasserkrug HL, Efron G. Arginine enhances wound healing and lymphocyte immune responses in humans. Surgery 108: 331–336; discussion 336–337, 1990 [PubMed] [Google Scholar]
- 4. Bernal W, Auzinger G, Dhawan A, Wendon J. Acute liver failure. Lancet 376: 190–201, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Brusilow SW, Finkelstien J. Restoration of nitrogen homeostasis in a man with ornithine transcarbamylase deficiency. Metabolism 42: 1336–1339, 1993 [DOI] [PubMed] [Google Scholar]
- 6. Cardounel AJ, Cui H, Samouilov A, Johnson W, Kearns P, Tsai AL, Berka V, Zweier JL. Evidence for the pathophysiological role of endogenous methylarginines in regulation of endothelial NO production and vascular function. J Biol Chem 282: 879–887, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Clemmesen JO, Kondrup J, Ott P. Splanchnic and leg exchange of amino acids and ammonia in acute liver failure. Gastroenterology 118: 1131–1139, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Das A, Hoare M, Davies N, Lopes AR, Dunn C, Kennedy PT, Alexander G, Finney H, Lawson A, Plunkett FJ, Bertoletti A, Akbar AN, Maini MK. Functional skewing of the global CD8 T cell population in chronic hepatitis B virus infection. J Exp Med 205: 2111–2124, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davies NA, Hodges SJ, Pitsillides AA, Mookerjee RP, Jalan R, Mehdizadeh S. Hepatic guanylate cyclase activity is decreased in a model of cirrhosis: a quantitative cytochemistry study. FEBS Lett 580: 2123–2128, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Dejong CH, Kampman MT, Deutz NE, Soeters PB. Altered glutamine metabolism in rat portal drained viscera and hindquarter during hyperammonemia. Gastroenterology 102: 936–948, 1992 [DOI] [PubMed] [Google Scholar]
- 11. Durante W, Johnson FK, Johnson RA. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol 34: 906–911, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Durante W, Liao L, Reyna SV, Peyton KJ, Schafer AI. Physiological cyclic stretch directs l-arginine transport and metabolism to collagen synthesis in vascular smooth muscle. FASEB J 14: 1775–1783, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Durante W, Liao L, Reyna SV, Peyton KJ, Schafer AI. Transforming growth factor-beta(1) stimulates l-arginine transport and metabolism in vascular smooth muscle cells: role in polyamine and collagen synthesis. Circulation 103: 1121–1127, 2001 [DOI] [PubMed] [Google Scholar]
- 14. El-Gayar S, Thuring-Nahler H, Pfeilschifter J, Rollinghoff M, Bogdan C. Translational control of inducible nitric oxide synthase by IL-13 and arginine availability in inflammatory macrophages. J Immunol 171: 4561–4568, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Fliser D. Asymmetric dimethylarginine (ADMA): the silent transition from an ‘uraemic toxin’ to a global cardiovascular risk molecule. Eur J Clin Invest 35: 71–79, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Freund H, Atamian S, Holroyde J, Fischer JE. Plasma amino-acids as predictors of the severity and outcome of sepsis. Ann Surg 190: 571–576, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hallemeesch MM, Ten Have GA, Deutz NE. Metabolic flux measurements across portal drained viscera, liver, kidney and hindquarter in mice. Lab Anim Care 35: 101–110, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Iber FL, Kerr DN, Dolle W, Sherlock S. Measurement of blood flow in the collateral vessels of the portal vein; preliminary results of a new method. J Clin Invest 39: 1201–1207, 1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iyer RK, Yoo PK, Kern RM, Rozengurt N, Tsoa R, O'Brien WE, Yu H, Grody WW, Cederbaum SD. Mouse model for human arginase deficiency. Mol Cell Biol 22: 4491–4498, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jalan R. Acute liver failure: current management and future prospects. J Hepatol 42 Suppl: S115–123, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Knorr A, Hirth-Dietrich C, Alonso-Alija C, Harter M, Hahn M, Keim Y, Wunder F, Stasch JP. Nitric oxide-independent activation of soluble guanylate cyclase by BAY 60–2770 in experimental liver fibrosis. Arzneim Forsch 58: 71–80, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Li H, Meininger CJ, Hawker JR, Haynes TE, Kepka-Lenhart D, Mistry SK, Morris SM, Wu GY. Regulatory role of arginase I and II in nitric oxide, polyamine, and proline syntheses in endothelial cells. Am J Physiol Endocrinol Metab 280: E75–E82, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Luiking YC, Hallemeesch MM, Vissers YLJ, Lamers WH, Deutz NEP. In vivo whole body and organ arginine metabolism during endotoxemia (sepsis) is dependent on mouse strain and gender. J Nutr 134: 2768s–2774s, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Mazziotti A, Bernardi M, Antonini L, Dioguardi FS, Bellusci R, Papa V, Tacconi C, Gasbarrini G, Cavallari A, Possati L. Plasma amino-acid patterns in experimental acute hepatic-failure—comparison between hepatectomy and liver devascularization in pigs. Surgery 90: 527–534, 1981 [PubMed] [Google Scholar]
- 25. Milewski PJ, Threlfall CJ, Heath DF, Holbrook IB, Wilford K, Irving MH. Intracellular free amino acids in undernourished patients with or without sepsis. Clin Sci (Lond) 62: 83–91, 1982 [DOI] [PubMed] [Google Scholar]
- 26. Mookerjee RP, Dalton RN, Davies NA, Hodges SJ, Turner C, Williams R, Jalan R. Inflammation is an important determinant of levels of the endogenous nitric oxide synthase inhibitor asymmetric dimethylarginine (ADMA) in acute liver failure. Liver Transpl 13: 400–405, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Mookerjee RP, Malaki M, Davies NA, Hodges SJ, Dalton RN, Turner C, Sen S, Williams R, Leiper J, Vallance P, Jalan R. Increasing dimethylarginine levels are associated with adverse clinical outcome in severe alcoholic hepatitis. Hepatology 45: 62–71, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Morris SM, Jr, Bhamidipati D, Kepka-Lenhart D. Human type II arginase: sequence analysis and tissue-specific expression. Gene 193: 157–161, 1997 [DOI] [PubMed] [Google Scholar]
- 29. Nijveldt RJ, Teerlink T, Siroen MP, van Lambalgen AA, Rauwerda JA, van Leeuwen PA. The liver is an important organ in the metabolism of asymmetrical dimethylarginine (ADMA). Clin Nutr 22: 17–22, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Owen EE, Tyor MP, Flanagan JF, Berry JN. The kidney as a source of blood ammonia in patients with liver disease: the effect of acetazolamide. J Clin Invest 39: 288–294, 1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Palmer RM, Rees DD, Ashton DS, Moncada S. l-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun 153: 1251–1256, 1988 [DOI] [PubMed] [Google Scholar]
- 32. Poeze M, Bruins MJ, Kessels F, Luiking YC, Lamers WH, Deutz NE. Effects of l-arginine pretreatment on nitric oxide metabolism and hepatosplanchnic perfusion during porcine endotoxemia. Am J Clin Nutr 93: 1237–1247, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Russwurm M, Koesling D. NO activation of guanylyl cyclase. EMBO J 23: 4443–4450, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schneider F, Lutun P, Boudjema K, Wolf P, Tempe JD. In vivo evidence of enhanced guanylyl cyclase activation during the hyperdynamic circulation of acute liver failure. Hepatology 19: 38–44, 1994 [PubMed] [Google Scholar]
- 35. Sen S, Rose CF, Ytrebo LM, Davies NA, Nedredal GI, Drevland SS, Kjonno M, Williams R, Butterworth RF, Revhaug A, Jalan R. Albumin dialysis reduces brain water and intracranial pressure in acute liver failure: a randomised controlled study in a pig model. Hepatology 38: 540A, 2003. [Google Scholar]
- 36. Shi O, Morris SM, Jr, Zoghbi H, Porter CW, O'Brien WE. Generation of a mouse model for arginase II deficiency by targeted disruption of the arginase II gene. Mol Cell Biol 21: 811–813, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ten Have GA, Bost MC, Suyk-Wierts JC, van den Bogaard AE, Deutz NE. Simultaneous measurement of metabolic flux in portally-drained viscera, liver, spleen, kidney and hindquarter in the conscious pig. Lab Anim Care 30: 347–358, 1996 [DOI] [PubMed] [Google Scholar]
- 38. Tietge UJ, Bahr MJ, Manns MP, Boker KH. Plasma amino acids in cirrhosis and after liver transplantation: influence of liver function, hepatic hemodynamics and circulating hormones. Clin Transplant 16: 9–17, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Trewby PN, Williams R. Pathophysiology of hypotension in patients with fulminant hepatic failure. Gut 18: 1021–1026, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ueda S, Yamagishi S, Okuda S. New pathways to renal damage: role of ADMA in retarding renal disease progression. J Nephrol 23: 377–386, 2010 [PubMed] [Google Scholar]
- 41. van Eijk HM, Luiking YC, Deutz NE. Methods using stable isotopes to measure nitric oxide (NO) synthesis in the l-arginine/NO pathway in health and disease. J Chromatogr B Analyt Technol Biomed Life Sci 851: 172–185, 2007 [DOI] [PubMed] [Google Scholar]
- 42. van Eijk HM, Suylen DP, Dejong CH, Luiking YC, Deutz NE. Measurement of amino acid isotope enrichment by liquid chromatography mass spectroscopy after derivatization with 9-fluorenylmethylchloroformate. J Chromatogr B Analyt Technol Biomed Life Sci 856: 48–56, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Vockley JG, Jenkinson CP, Shukla H, Kern RM, Grody WW, Cederbaum SD. Cloning and characterization of the human type II arginase gene. Genomics 38: 118–123, 1996 [DOI] [PubMed] [Google Scholar]
- 44. Wolfe R, Chinkes DL. Isotope Tracers in Metabolic Research: Principles and Practice of Kinetic Analysis. London, UK: Wiley, 2005 [Google Scholar]
- 45. Yagnik GP, Takahashi Y, Tsoulfas G, Reid K, Murase N, Geller DA. Blockade of the l-arginine/NO synthase pathway worsens hepatic apoptosis and liver transplant preservation injury. Hepatology 36: 573–581, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Yilmaz MI, Saglam M, Caglar K, Cakir E, Ozgurtas T, Sonmez A, Eyileten T, Yenicesu M, Acikel C, Oguz Y, Ozcan O, Bozlar U, Erbil K, Aslan I, Vural A. Endothelial functions improve with decrease in asymmetric dimethylarginine (ADMA) levels after renal transplantation. Transplantation 80: 1660–1666, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Ytrebo LM, Ingebrigtsen T, Nedredal GI, Elvenes OP, Korvald C, Romner B, Revhaug A. Protein S-100beta: a biochemical marker for increased intracranial pressure in pigs with acute hepatic failure. Scand J Gastroenterol 35: 546–551, 2000 [DOI] [PubMed] [Google Scholar]
- 48. Ytrebo LM, Korvald C, Nedredal GI, Elvenes OP, Nielsen Grymyr OJ, Revhaug A. N-acetylcysteine increases cerebral perfusion pressure in pigs with fulminant hepatic failure. Crit Care Med 29: 1989–1995, 2001 [DOI] [PubMed] [Google Scholar]
- 49. Ytrebo LM, Nedredal GI, Langbakk B, Revhaug A. An experimental large animal model for the assessment of bioartificial liver support systems in fulminant hepatic failure. Scand J Gastroenterol 37: 1077–1088, 2002 [DOI] [PubMed] [Google Scholar]
- 50. Ytrebo LM, Sen S, Rose C, Davies NA, Nedredal GI, Fuskevaag OM, Ten Have GA, Prinzen FW, Williams R, Deutz NE, Jalan R, Revhaug A. Systemic and regional hemodynamics in pigs with acute liver failure and the effect of albumin dialysis. Scand J Gastroenterol 41: 1350–1360, 2006 [DOI] [PubMed] [Google Scholar]
- 51. Ytrebo LM, Sen S, Rose C, Ten Have GA, Davies NA, Hodges S, Nedredal GI, Romero-Gomez M, Williams R, Revhaug A, Jalan R, Deutz NE. Interorgan ammonia, glutamate, and glutamine trafficking in pigs with acute liver failure. Am J Physiol Gastrointest Liver Physiol 291: G373–G381, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Zhang C, Hein TW, Wang W, Chang CI, Kuo L. Constitutive expression of arginase in microvascular endothelial cells counteracts nitric oxide-mediated vasodilatory function. FASEB J 15: 1264–1266, 2001 [DOI] [PubMed] [Google Scholar]


