Abstract
Sodium/hydrogen exchanger 8 (NHE8), the newest member of the SLC9 family, is expressed at the apical membrane of the epithelial cells in the intestine and the kidney. Although NHE8 has been shown to be an important player for intestinal sodium absorption early in development, its physiological role in the intestine remains unclear. Here, we successfully created a NHE8 knockout (NHE8−/−) mouse model to study the function of this transporter in the intestinal tract. Embryonic stem cells containing interrupted NHE8 gene were injected into mouse blastocyst to produce NHE8+/− chimeras. NHE8−/− mice showed no lethality during embryonic and fetal development. These mice had normal serum sodium levels and no signs of diarrhea. Apically expressed NHE2 and NHE3 were increased in the small intestine of the NHE8−/− mice in compensation. The number of goblet cells and mucin (MUC)-positive cells in the colon was reduced in NHE8−/− mice along with mucosal pH, MUC2 expression as well as downregulated in adenoma (DRA) expression. Therefore, the role of NHE8 in the intestine involves both sodium absorption and bicarbonate secretion.
Keywords: sodium/hydrogen exchanger 2, 3, and 8; downregulated in adenoma; mucin 2
electroneutral absorption of NaCl is mediated by a subset of transporter families including sodium/hydrogen exchangers (NHEs). NHEs are a family of integral membrane proteins that mediate the movement of extracellular Na+ into cells in exchange for intracellular H+. To date, at least 10 mammalian NHE isoforms have been found, and each has different cellular localization and tissue distribution. These NHEs have broad physiological functions, including intracellular pH homeostasis, cell volume regulation, acid-base regulation, and electroneutral NaCl transport (23, 37). Among these isoforms, four NHEs (NHE1–3, 8) have been discovered in the intestine. NHE1 is a basolateral membrane protein in the intestinal epithelial cells. NHE2, NHE3, and NHE8 are apical membrane proteins in the epithelial cells (10, 24, 30, 37). The apically expressed NHEs have distinct transporter kinetic characteristics and expression patterns during development. NHE8 is sensitive to both inhibitors HOE694 and S3226, while NHE2 is sensitive to HOE694 and NHE3 is sensitive to S3226 (11, 29). NHE8 expression in the small intestine is higher at young age and declines with development; in contrast, NHE2 and NHE3 expression increases with development (8, 9, 29, 30). The tissue distribution of NHE2 and NHE3 is more abundant in the intestine and the kidney (2, 3), while NHE8 is ubiquitously expressed in many tissues with predominant expression in the intestine, liver, skeletal muscle, kidney, and testes (30). The intestinal NHE function is closely regulated during development by growth related hormones, such as EGF and glucocorticoids. EGF stimulates NHE2 expression, but it inhibits NHE8 gene expression by reducing its basal transcription (32, 34). Glucocorticoids increase NHE3 expression, but they inhibit NHE8 expression (1, 6, 15, 19, 35).
Although multi-isoforms of NHE are expressed in the intestine, their role in the intestinal Na+ absorption is not the same. Using gene-specific knockout mouse models, we learned that NHE2 does not participate in intestinal Na absorption; instead, it plays important role in the viability of gastric parietal cells (25). In contrast, interruption of NHE3 expression in mice displays diarrhea and impaired acid-base balance, which suggests an important role for NHE3 in intestinal and renal Na+ absorption (26). Regarding the compensatory role among these NHEs, NHE3 does not compensate for the loss of NHE2, and vice versa NHE2 cannot compensate for the loss of NHE3 (22), but NHE8 compensates for the loss of both NHE2 and NHE3 (33).
NHE8 displays broad tissue distribution with relative high abundance in the small intestine and the colon. Although earlier studies suggested a possible role in the intestinal sodium absorption early in development, a detailed understanding of NHE8's physiological role in the gastrointestinal tract is still lacking. Here, we describe for the first time the characterization of the newly generated NHE8−/− mouse model. This model displayed significant morphological and functional changes in the intestinal track. Our results suggest that NHE8 plays an important role in Na+ absorption and bicarbonate secretion in the intestine.
MATERIALS AND METHODS
Creation of NHE8−/− mouse model.
To generate a mouse model lacking the functional NHE8, NHE8-deficient embryonic stem (ES) cells (YHB273) were injected into Black Swiss blastocysts. The YHB273 ES cells (129 S1/SV) were obtained from Mutant Mouse Regional Resource Centers (MMRRC). These cells contain nonfunctional NHE8 protein due to the interruption of NHE8 gene by a gene trap vector (pGT0Lxf). Black Swiss mice were purchased from Taconic (Hudson, NY) and used for blastocysts injection to generate chimeric mice. Blastocysts injection was done by the Genetically Engineering Mouse Model Core Facility at the University of Arizona. All the studies were carried out in mixed genetic background (129/Black Swiss). All animal works were approved by the University of Arizona Institutional Animal Care and Use Committee.
Identification of insertion site.
Based on the information provided by Mutant Mouse Regional Resource Centers, pGT0Lxf vector was inserted in the intron 3 of the NHE8 gene. To identify the precise insertion position of the gene trap vector pGT0Lxf, PCR were performed with primers designed to spanning the intron 3 of NHE8 gene and the gene trap vector. PCR products were cloned into pCR2.1 vector and then were sequenced at the sequencing facility (University of Arizona). Primers used in this study were listed in Table 1.
Table 1.
Primers used to identify insertion position in NHE8 gene
| Name | Direction | Location | Position, bp | Sequence |
|---|---|---|---|---|
| In3F1 | Forward | Intron 3 | 119–139 | GTTTGGTTGATTGTGAACGA |
| In3F2 | Forward | Intron 3 | 983–1,002 | TCCTAGCTGAGTGGCGATG |
| TrapR3 | Reverse | pGT0Lxf | 78–103 | GGTTGGAGTGTGTGTTGTAGGACACG |
| TrapR2 | Reverse | pGT0Lxf | 1,616–1,647 | GTAAAACGACGGGATCCTCTAGAGTCCAGATC |
| A | Forward | Exon 2 | 80–102 | ACCCTTCACACCACCTTGGGTGT |
| B | Reverse | Exon 2/3 | 216–240 | AGGATGATGCAGATGGCTAGGACGA |
| C | Reverse | Exon 4/5 | 353–371 | CTTCCTCCTTCCAATTTGC |
| D | Forward | Exon 13 | 1,331–1,350 | CTGGAGCCCATGGAGAAGCG |
| E | Reverse | Exon 14 | 1,735–1,753 | CTCCTGCTCATCATCCTCA |
RNA isolation and PCR amplification.
Tissues were harvested and total RNA were isolated using Trizol reagent (Invitrogen, Carlsbad, CA). Total RNA (500 ng) was reverse-transcribed using the iScript kit (Bio-Rad, Hercules, CA), and 10% of the RT reaction was used for PCR analysis. For real-time PCR, TaqMan technology was used to determine the expression levels of interested target genes using gene-specific primers [Mm101237138_m1 for NHE2; Mm00472657_m1 for NHE3; Mm00445313_m1 for downregulated in adenoma (DRA); and Mm00446973_m1 for TATA binding protein (TBP)]. TBP gene was used as an endogenous reference to normalize expression levels. All TaqMan probes used for this study were purchased from Applied Biosystems (Foster City, CA). Resulting data were analyzed using the comparative cycle threshold (Ct) method. The target gene cycle thresholds are adjusted relative to a calibrator (normalized Ct value obtained from control groups) and expressed as 2−ΔΔCt (Applied Biosystems User Bulletin no. 2: Rev B “Relative Quantitation of Gene Expression”). For semiquantitative PCR, the products from MUC2 gene are normalized by β-actin, a method well established in our laboratory (31). For PCR detection on NHE8 expression, primers covering various exons of the NHE8 gene were also used.
Protein preparation and Western blotting.
Total protein was prepared using RIPA buffer following method described previously (9). Briefly, tissues were homogenized in small volume of RIPA buffer on ice. The resulted tissue lysates were then centrifuged for 10 min at 10,000 rpm at 4°C. The supernatants were collected and used for Western blotting. DRA antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was used as a 1:3,000 dilution in these experiments. A 1:5,000 dilution of the β-actin antiserum (Sigma-Aldrich) was used to determine β-actin protein abundance. Western detection was performed with the BM chemiluminescence Western blotting kit (Roche Diagnostics, Indianapolis, IN). A ratio of target protein intensity over β-actin protein intensity was used for protein expression quantitation.
Tissue histological observation.
Intestinal tissue was collected and fixed in 4% paraformaldehyde at 4°C overnight, dehydrated, and embedded in paraffin. Eight-micrometer-thick sections were cut and stained with hematoxylin and eosin (H&E) or periodic-acid Schiff's reagent (PAS). All sections and staining works were done at the pathology services laboratory (University Animal Care, University of Arizona).
Blood/gas/electrolyte status analysis and mucosal surface pH measurement.
For blood gas/electrolyte measurement, blood/serum samples were collected following the instruction and analyzed by the university animal care pathology service laboratory (University of Arizona) and VitalPath blood gas/electrolyte analyzer (Heska, Loveland, CO). For mucosal surface pH measurement, colon segment was freshly cut and opened, and mucosal surface pH was measured immediately using a Orion 8135BNUWP flat surface pH probe (Thermo Scientific, Pittsburgh, PA ).
Statistical analysis.
Student t-test was used to compare values of the experimental data. P values ≤0.05 were considered significant.
RESULTS
Creation of a mouse model lacking NHE8 gene expression.
YHB273 ES cells lacking NHE8 expression were successfully used to generate NHE8−/− mouse model via blastocysts injection strategy. According to BayGenomics (University of California, San Francisco, CA), a gene trap vector (pGT0Lxf) was inserted into the intron 3 in NHE8 gene, which resulted a mutant NHE8 mRNA transcript containing exon 1, 2, and 3 in YHB273 ES cells (Fig. 1A). Subsequent PCR amplification suggested that pGT0Lxf was inserted at ∼2,000-bp downstream of the intron 3 on NHE8 gene. DNA sequencing confirmed that the precise insertion site was located between 2,194 and 2,195 bp in the intron 3 of the NHE8 gene (Fig. 1B). To confirm that the gene trap vector pGT0Lxf indeed interrupted NHE8 transcription, we isolated mRNA from the intestine of wild-type and NHE8−/− mice and performed RT-PCR with primers covering different exons of the NHE8 gene. RT-PCR using primers covering exons 2 and 3 (primer A and B) generated expected PCR products in both wild-type and NHE8−/− mice; RT-PCR using primers covering exons 2 and 4 (primer A and C), and exons 13 and 14 (primer D and E) of the NHE8 gene amplified expected PCR products only in wild-type mice but not in NHE8−/− mice (Fig. 1C).
Fig. 1.
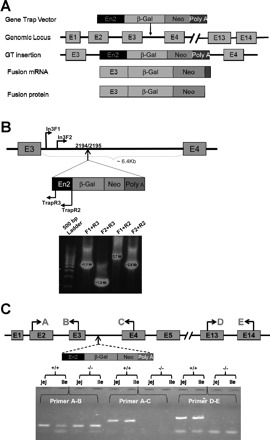
Establishment of the sodium/hydrogen exchanger 8 (NHE8−/−) mouse model. NHE8-deficient embryonic stem (ES) cells (YHB273) were injected into black Swiss blastocysts to generate chimeric mice. PCR amplification was performed to locate the gene trap vector insertion using primers covering the NHE8 gene (In3F1, In3F2) and the Trap vector (Trap R2, Trap R3). The precise insertion location was identified by sequencing the PCR products. RNA was isolated from the intestinal tissue and RT-PCR was performed to detect NHE8 expression in mice. A: scheme of a Gene Trap vector used to interrupt NHE8 gene expression. B: PCR amplification and DNA sequencing to identify the precise insertion position of Trap vector on NHE8 gene. C: RT-PCR confirmation of NHE8 expression in NHE8−/− mice (−/−) and wild-type mice (+/+).
Gross phenotypical observation of NHE8−/− mice.
Due to the high level expression of NHE8 in the gut at early development, we wanted to test if loss of NHE8 expression impair embryonic and/or fetal development. To carry this study, we mated female NHE8+/− mice with male NHE8+/− mice to determine the distribution of wild-type, heterozygous, and homozygous genotype in their offspring. Out of the 527 litters born from these NHE8+/− × NHE8+/− breeding pairs, 119 mice were wild type, 286 mice were heterozygous, and 122 mice were NHE8 null. The genotype distribution showed a normal 1:2:1 Mendelian ratio, indicating no embryonic or fetal lethality among NHE8−/− mice. These NHE8−/− mice survived, grew normally, and were indistinguishable from their wild-type littermates after weaning, although NHE8−/− mice were smaller than their wild-type littermates at 2 wk of age (9.6 ± 0.2 g for wild-type male and female mice vs. 8.7 ± 0.3 g for NHE8−/− male and female mice; n = 22; P = 0.01). To test the reproductive function, we formed different combinations of the mating pairs. Mating NHE8−/− female mice with NHE8+/− male or wild-type male mice produced litters that were similar to the wild-type mating pairs. Interestingly, mating NHE8−/− male mice with NHE8−/− female or NHE8+/− female or wild-type female mice produced no litters.
Blood gas and electrolyte status.
Since NHE8 was reported to be involved in Na+ absorption early in life, we asked if NaCl homeostasis was perturbed in NHE8−/− mice. To determine whether the absence of NHE8 might cause disturbances of sodium homeostasis, we collected blood and serum samples from NHE8−/− mice, NHE8+/− mice, and their wild-type littermates and then analyzed bicarbonate and electrolyte concentrations. As indicated in Table 2, serum and blood sodium and chloride concentrations were virtually identical in NHE8−/− mice, NHE8+/− mice, and wild-type mice. The concentration for HCO3− was reduced significantly from 21.35 ± 0.8 mM in wild-type mice to 15.9 ± 1.35 mM in NHE8+/− mice (n = 4; P = 0.001). Interestingly, the NHE8−/− mice failed blood gas/electrolyte tests due to blood coagulations. Because NHE8 has been previously detected in blood cells by Northern blot, we speculate that the coagulation process in NHE8−/− mice might be due to the functional loss of NHE8 in blood cells, although more studies will be performed to confirm it.
Table 2.
Concentrations of electrolytes and bicarbonate
| Genotype | +/+ | +/− | −/− |
|---|---|---|---|
| Serum | |||
| Na+, mM | 147 ± 1 | 147 ± 1 | |
| Cl−, mM | 117 ± 1 | 114 ± 2 | |
| n | 6 | 6 | |
| Blood | |||
| Na+, mM | 144 ± 3 | 145 ± 3 | |
| Cl−, mM | 109 ± 2 | 108 ± 2 | |
| HCO3−, mM | 21.35 ± 0.8 | 15.9 ± 1.35* | |
| pH | 7.26 ± 0.01 | 7.11 ± 0.64* | |
| Hct, % | 35.8 ± 1.3 | 42.1 ± 2.7* | |
| n | 4 | 4 |
Values are means ± SE. Hct, hematocrit.
P < 0.05.
Morphological observation of the intestinal tract.
Gross inspection of the intestinal tract in adult NHE8−/− mice revealed no evidence of excess fluid accumulation. The length of the small intestine was significantly longer in NHE8−/− mice compared with their wild-type littermates (36.7 ± 1.4 cm in wild-type male mice vs. 43.3 ± 2.0 cm in NHE8−/− male mice; n = 4–7; P = 0.023). At the same time, the weight of the cecum in adult NHE8−/− mice was also significantly increased compared with wild-type mice (0.61 ± 0.065 g in NHE8−/− mice vs. 0.30 ± 0.015 g in wild-type mice; n = 9; P < 0.05; Fig. 2). These changes were not seen in young NHE8−/− mice (2 wk old). Further histopathological survey showed a normal morphology in the jejunum and the elongated crypts (∼30% increase) in the ileum in NHE8−/− mice (Fig. 3).
Fig. 2.
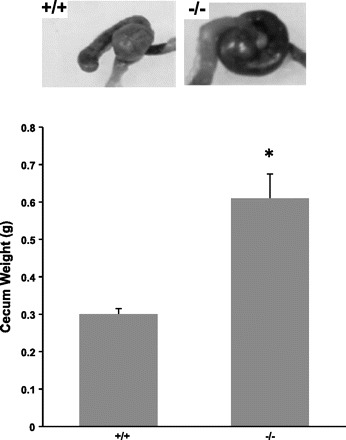
Cecum morphology. Cecum was collected and weighted immediately after removal from 10–13 wk old male and female mice. Data are presented as means ± SE from a total of 10 mice. *P ≤ 0.01 for NHE8−/− mice (−/−) vs. wild-type mice (+/+).
Fig. 3.
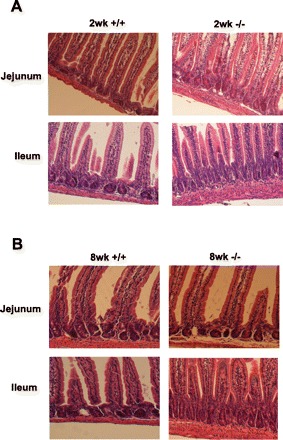
Morphological assessment of the small intestine. Small intestinal tissue was collected from male mice and fixed in 4% paraformaldehyde at 4°C overnight, dehydrated, and embedded in paraffin. Sections were stained with hematoxylin and observed under microscope (×100). +/+: Wild-type mice; (−/−): NHE8−/− mice. A: small intestine from 2-wk-old mice (2wk). B: small intestine from 8-wk-old mice (8wk).
Reduction of goblet cell numbers and mucin expression in the colon of NHE8−/− mice.
In contrast with the expression pattern of NHE8 in the small intestine during ontogeny, NHE8 expression in the colon increases with age under normal development condition (Fig. 4). The expression level of NHE8 was the lowest at 2 wk of age and reached a plateau at 4 wk of age. Because the high expression of NHE8 in the adult colon, we expected changes in the colon of NHE8−/− mice. Indeed, H&E stain and PAS stain revealed morphological alterations in the colon of NHE8−/− mice. H&E stain showed that the number of goblet cells was reduced in NHE8−/− mice, and severe reduction was seen in the proximal colon (Fig. 5). PAS stain also indicated that the mucin production was reduced in the colon of NHE8−/− mice (Fig. 6A). PCR with mucin 2-specific primers confirmed that MUC2 mRNA expression was decreased by ∼57% in NHE8−/− mice compared with their wild-type littermates (Fig. 6B).
Fig. 4.
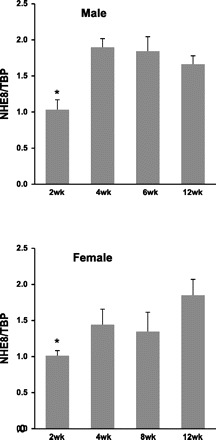
NHE8 gene expression in the colon. Colonic tissue was collected from different aged male and female mice. RNA was isolated and used for PCR analysis. NHE8 mRNA and TATA binding protein (TBP) mRNA were amplified with mouse-specific primers. Changes in NHE8 gene expression were analyzed by the comparative cycle threshold (Ct) method. Data are means ± SE (n = 6 for each group). *P ≤ 0.001 for 2-wk-old vs. other age group.
Fig. 5.
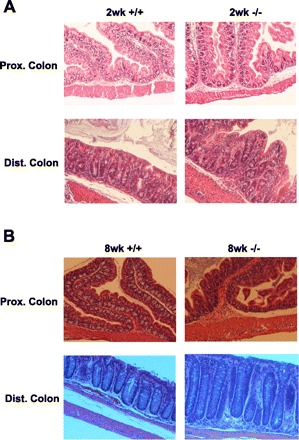
Morphological assessment of the large intestine. Large intestinal tissue was collected male mice and fixed in 4% paraformaldehyde at 4°C overnight, dehydrated, and embedded in paraffin. Sections were stained with hematoxylin and eosin (H&E) observed under microscope (×100). +/+: Wild-type mice; −/−: NHE8−/− mice. A: colonic section from 2-wk-old mice. B: colonic section from 8-wk-old mice.
Fig. 6.
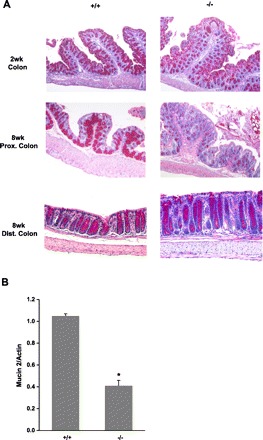
Mucin staining and MUC2 expression in the colon. A: large intestinal tissue was collected from male mice and fixed in 4% paraformaldehyde at 4°C overnight, dehydrated, and embedded in paraffin. Sections were stained with PAS and then observed under microscope (×100). +/+: wild-type mice; −/−: NHE8−/− mice. B: RNA was isolated from the colon and used for PCR analysis on MUC2 expression. MUC2 mRNA and β-actin mRNA were amplified with mouse-specific primers. Changes in MUC2 gene expression was analyzed by the semiquantitative method. Data are means ± SE from a total of 8 groups of mice (8- to 10-wk-old males and females). *P ≤ 0.01 for NHE8−/− mice (−/−) vs. wild-type mice (+/+).
Decreased mucosal surface pH and DRA expression in the colon of NHE8−/− mice.
The mucosal surface pH in the colon of 8-wk-old NHE8−/− mice was measured using surface pH probe. As shown in Fig. 7A, the mucosal surface pH for the proximal colon and distal colon in wild-type mice was 6.69 ± 0.09 and 7.37 ± 0.14, respectively. In contrast, the mucosal surface pH in the proximal colon and the distal colon of NHE8−/− mice was 6.56 ± 0.04 and 6.90 ± 0.11, respectively. PCR indicated that DRA mRNA expression in NHE8−/− mice was reduced by ∼21% in the proximal colon and by ∼52% in the distal colon compared with wild-type mice (Fig. 7B). Western blotting showed that DRA protein expression was reduced by ∼40% in the proximal colon and ∼90% in the distal colon in the NHE8−/− mice comparing with wild-type mice (Fig. 7C).
Fig. 7.
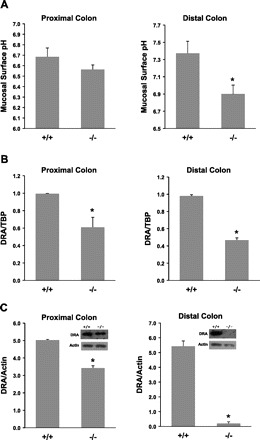
Mucosal surface pH measurement and downregulated in adenoma (DRA) expression. Colonic segment was freshly cut and opened, and mucosal surface pH was measured immediately using a flat surface pH probe. Data are means ± SE from a total of 8 mice (8–10 wk old males and females). *P ≤ 0.003 for NHE8−/− mice (−/−) vs. wild-type mice (+/+). RNA was isolated from colon and used for PCR analysis. DRA mRNA and TBP mRNA were amplified with mouse-specific primers. Changes in DRA gene expression were analyzed by the comparative cycle threshold (Ct) method. Data are means ± SE from a total of 10 mice (8- to 10-wk-old males and females). *P ≤ 0.04 for NHE8−/− mice (−/−) vs. wild-type mice (+/+). Total tissue lysate was prepared from colon and used for Western blot detection. Protein (30 μg) was loaded on SDS-PAGE gels and immunoblotted. DRA antibody and β-actin antibody were used to detect DRA and β-actin, respectively. Expression of DRA protein was calculated by dividing the optical density of the DRA band over that of the β-actin band. Bar chart shows the DRA protein expression indicated as means ± SE in the sum of a total of 12 mice (8- to 10-wk-old males and females). *P ≤ 0.01 for NHE8−/− mice (−/−) vs. wild-type mice (+/+). Inset: corresponding Western blot image.
NHE2 and NHE3 compensation in the intestine.
A previous study has shown that NHE8 is highly expressed in the small intestine at young age and NHE8 compensates for the loss of NHE2 and NHE3 in the intestine (33). Our current study indicated that loss of NHE8 expression did not impair sodium homeostasis. These observations suggested the possible compensatory mechanism(s) might present in the intestine of NHE8−/− mice. To test this hypothesis, we looked the expression of NHE2 and NHE3 in NHE8−/− mice. Real-time PCR showed that the expression of intestinal NHE2 and NHE3 was significantly increased in NHE8−/− mice, and this increase was age and gender dependent. At 2 wk of age, NHE2 expression was increased 1.52 times in NHE8−/− mice but not NHE3 expression (Fig. 8A). At adult age, NHE2 and NHE3 expression was increased 1.55 and 2.35 times, respectively, in female NHE8−/− mice, but no increase was detected in male NHE8−/− mice (Fig. 8B). In the colon, neither NHE2 nor NHE3 expression was increased in NHE8−/− mice (data not shown).
Fig. 8.
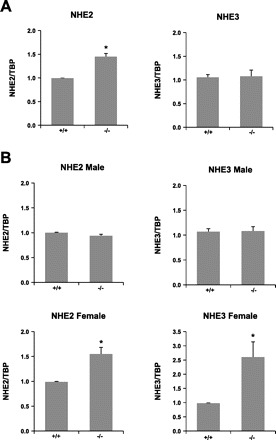
NHE2 and NHE3 expression in NHE8−/− mice. RNA was isolated from the intestine and used for PCR analysis. NHE2 and NHE3 mRNA and TBP mRNA were amplified with mouse-specific primers. Changes in NHE2 and NHE3 gene expression were analyzed by the comparative cycle threshold (Ct) method. Data are means ± SE from a total of 12 mice. *P ≤ 0.01 for NHE8−/− mice (−/−) vs. wild-type mice (+/+). A: NHE2 and NHE3 gene expression in 2-wk-old mice. B: NHE2 and NHE3 gene expression in 8-wk-old mice.
DISCUSSION
The 10 identified mammalian NHE isoforms have different functions, tissue distribution, and membrane localization (37). Of these 10 NHEs, only 4 of them are expressed in the intestinal epithelial cells. NHE1 is expressed at the basolateral membrane of the intestinal epithelial cells. NHE2, NHE3, and NHE8 are expressed at the apical membrane of the intestinal epithelial cells (36, 37). Knockout animal models revealed that NHE2 plays little or no role in intestinal sodium absorption, whereas NHE3 is the main contributor of the intestinal sodium absorption (13, 22, 25, 26). NHE8 is highly expressed at early life, and it has been suggested to have a role in intestinal sodium absorption during early development when NHE2 and NHE3 are expressed at very low levels (29, 30). However, the precise physiological function of NHE8 is still unclear. Here, we established a NHE8−/− mouse model using NHE8 deficient ES cells to further explore the physiological roles of NHE8.
Insertion of a gene trap vector, pGT0Lxf, into the third intron of NHE8 gene at position 2,194/2,195 bp abolished NHE8 transcription in NHE8−/− mice. The mutant NHE8 mRNA transcript only contains the first three exons of the NHE8 gene, which resulted in the loss of NHE8 function. Genetic analysis in offspring born from NHE8+/− breeding pairs showed a normal Mendelian distribution (wild type:heterozygous:homozygous = 1:2:1), suggesting that loss of NHE8 expression had no lethal effect on embryonic and fetal development in mice. Although all NHE8−/− mice survived, male NHE8−/− mice showed abnormal reproductive function. This observation suggested that NHE8 plays an important role in male reproductive system. We are in the process of determining the role of NHE8 in male reproductive system.
Apically expressed NHE2, NHE3, and NHE8 all contribute to intestinal sodium absorption. NHE8 mainly participates in Na+ absorption at a young age, while NHE2 and NHE3 play important role in intestinal Na+ absorption in adult age (9, 29, 30). Disruption of NHE3 function results in diarrhea and impaired acid-base balance in mice (26). In contrast, mice carrying NHE8 mutation maintained a normal serum Na+ level, normal growth pattern, and no sign of diarrhea; therefore, NHE8 is not indispensable for Na+ absorption in early development. In fact, the possible compensatory mechanisms in NHE8−/− mice are evidenced by the elongation of the intestinal tract and the increased NHE2 and NHE3 gene expression. We have previously reported that NHE8 compensates for the loss of NHE2 and NHE3 expression in mice. Now, we showed that NHE2 expression was significantly increased in young NHE8−/− mice, indicating that NHE2 may play important role in intestinal Na+ absorption during early life when NHE8 function is absent. Furthermore, the increased NHE2 and NHE3 expression was only seen in adult female NHE8−/− mice, suggesting that multiple NHE isoforms contribute to intestinal Na+ absorption in adult female mice. On the other hand, only NHE3 played main role in male mice. These observations strongly supported the hypothesis that NHE8 function is regulated differently between genders (33). The compensation of NHE2 and NHE3 in NHE8−/− female mice but not male mice may also indicate that female hormones play a role in regulating NHE function (7). The apparent lack of NHE2 and NHE3 compensation in the colon of NHE8−/− mice suggested that NHE8 plays little or no role in colonic Na+ absorption.
Previously, we have shown that the expression of NHE8 in the small intestine decreases with age (30). The expression pattern of NHE8 in the colon was not clear. In the present study, we showed that the expression pattern of NHE8 differs between the colon and the small intestine. Before weanling, NHE8 expression in the colon is at the lowest level but is at the highest in the small intestine. After weanling, NHE8 expression reached the highest level in the colon but was significantly reduced in the small intestine. Because of the high level NHE8 expression in the colon, abnormalities in the colon would be expected in NHE8−/− mice. Histological evaluation of NHE8−/− mice demonstrated a significant loss of goblet cells and a decrease in the number of PAS-positive cells in the entire colon. This phenomenon is different from what was observed in the colon of NHE3−/− mice. In NHE3−/− mice, similar goblet cell depletion was seen in the colon, but this reduction only presented in the distal colon (21).
NHE8−/− mice displayed colonic mucosal acidification. Reduced colonic surface pH suggested a possible defect on HCO3− secretion. In the intestine, HCO3− plays an important role in buffering the pH of the protective mucosal layer that lines all epithelia (12). Beside its pH buffering function, HCO3− is also involved in mucin secretion and expansion (5). Solute carrier family 26, member 3, also known as SLC26A3, or DRA, a transmembrane glycoprotein, has been shown to play an important role on secreting HCO3− in exchange for Cl− in the intestine (4, 16, 18, 20). This protein is localized to the mucosa of the lower intestinal tract, in particular the apical membrane of columnar epithelium and some goblet cells. Mutations in this gene have been associated with congenital chloride diarrhea, a disease manifested by enhanced chloride loss in the stool and volume depletion (17). In NHE8−/− mice, the colonic mucosal surface pH was reduced to an ∼0.14 pH unit in the proximal colon and to an ∼0.47 pH unit in the distal colon, and this decrease was correlated to the dramatic reduction of DRA expression in the corresponding region. These observations suggested that the reduced DRA expression might contribute to the decreased mucosal surface pH.
In NHE8−/− mice, mucin-positive goblet cells were also dramatically reduced compared with their wild-type littermates. MUC2 is particularly prominent in the gut and is secreted from goblet cells in the large intestine. The secreted mucin forms an insoluble mucous barrier to protect the intestinal epithelium (14, 27, 28). NHE8−/− mice displayed goblet cell depletion, which is coincidental with the decreased MUC2 gene expression in the colon. The reduced MUC2 gene expression and the decreased DRA gene expression in NHE8−/− mice together suggested that NHE8 plays important roles in goblet cell function, although more studies must be performed to address the role of NHE8 in goblet cells.
In short, ablation of NHE8 function in mice resulted in male reproductive malfunction, decreased colonic mucosal surface pH and DRA and mucin gene expression, and apparent gender-specific compensation by NHE2 and NHE3 in the small intestine. These changes suggest that the role of NHE8 in the intestine involves bicarbonate secretion coupled with sodium absorption.
GRANTS
This work was supported by Grant R01DK073638 from the National Institute of Diabetes and Digestive and Kidney Diseases.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.X. and F.K.G. conception and design of research; H.X., B.Z., J.L., C.W., and H.C. performed experiments; H.X., B.Z., J.L., C.W., and H.C. analyzed data; H.X., B.Z., J.L., C.W., and H.C. interpreted results of experiments; H.X. and B.Z. prepared figures; H.X. drafted manuscript; H.X. edited and revised manuscript; F.K.G. approved final version of manuscript.
REFERENCES
- 1. Ambuhl PM, Yang X, Peng Y, Preisig PA, Moe OW, Alpern RJ. Glucocorticoids enhance acid activation of the Na+/H+ exchanger 3 (NHE3). J Clin Invest 103: 429–435, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bookstein C, Xie Y, Rabenau K, Musch MW, McSwine RL, Rao MC, Chang EB. Tissue distribution of Na+/H+ exchanger isoforms NHE2 and NHE4 in rat intestine and kidney. Am J Physiol Cell Physiol 273: C1496–C1505, 1997 [DOI] [PubMed] [Google Scholar]
- 3. Brant SR, Yun CH, Donowitz M, Tse CM. Cloning, tissue distribution, and functional analysis of the human Na+/N+ exchanger isoform, NHE3. Am J Physiol Cell Physiol 269: C198–C206, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Byeon MK, Westerman MA, Maroulakou IG, Henderson KW, Suster S, Zhang XK, Papas TS, Vesely J, Willingham MC, Green JE, Schweinfest CW. The down-regulated in adenoma (DRA) gene encodes an intestine-specific membrane glycoprotein. Oncogene 12: 387–396, 1996 [PubMed] [Google Scholar]
- 5. Chen EY, Yang N, Quinton PM, Chin WC. A new role for bicarbonate in mucus formation. Am J Physiol Lung Cell Mol Physiol 299: L542–L549, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cho JH, Musch MW, DePaoli AM, Bookstein CM, Xie Y, Burant CF, Rao MC, Chang EB. Glucocorticoids regulate Na+/H+ exchange expression and activity in region- and tissue-specific manner. Am J Physiol Cell Physiol 267: C796–C803, 1994 [DOI] [PubMed] [Google Scholar]
- 7. Choijookhuu N, Sato Y, Nishino T, Endo D, Hishikawa Y, Koji T. Estrogen-dependent regulation of sodium/hydrogen exchanger-3 (NHE3) expression via estrogen receptor beta in proximal colon of pregnant mice. Histochem Cell Biol 137: 575–587, 2012 [DOI] [PubMed] [Google Scholar]
- 8. Collins JF, Kiela PR, Xu H, Zeng J, Ghishan FK. Increased NHE2 expression in rat intestinal epithelium during ontogeny is transcriptionally mediated. Am J Physiol Cell Physiol 275: C1143–C1150, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Collins JF, Xu H, Kiela PR, Zeng J, Ghishan FK. Functional and molecular characterization of NHE3 expression during ontogeny in rat jejunal epithelium. Am J Physiol Cell Physiol 273: C1937–C1946, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Collins JF, Xu H, Kiela PR, Zeng J, Ghishan FK. Ontogeny of basolateral membrane sodium-hydrogen exchange (NHE) activity and mRNA expression of NHE-1 and NHE-4 in rat kidney and jejunum. Biochim Biophys Acta 1369: 247–258, 1998 [DOI] [PubMed] [Google Scholar]
- 11. Counillon L, Scholz W, Lang HJ, Pouyssegur J. Pharmacological characterization of stably transfected Na+/H+ antiporter isoforms using amiloride analogs and a new inhibitor exhibiting anti-ischemic properties. Mol Pharmacol 44: 1041–1045, 1993 [PubMed] [Google Scholar]
- 12. Flemstrom G, Isenberg JI. Gastroduodenal mucosal alkaline secretion and mucosal protection. News Physiol Sci 16: 23–28, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Gawenis LR, Stien X, Shull GE, Schultheis PJ, Woo AL, Walker NM, Clarke LL. Intestinal NaCl transport in NHE2 and NHE3 knockout mice. Am J Physiol Gastrointest Liver Physiol 282: G776–G784, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Gum JR, Jr, Hicks JW, Gillespie AM, Carlson EJ, Komuves L, Karnik S, Hong JC, Epstein CJ, Kim YS. Goblet cell-specific expression mediated by the MUC2 mucin gene promoter in the intestine of transgenic mice. Am J Physiol Gastrointest Liver Physiol 276: G666–G676, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Gupta N, Tarif SR, Seikaly M, Baum M. Role of glucocorticoids in the maturation of the rat renal Na+/H+ antiporter (NHE3). Kidney Int 60: 173–181, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haila S, Saarialho-Kere U, Karjalainen-Lindsberg ML, Lohi H, Airola K, Holmberg C, Hastbacka J, Kere J, Hoglund P. The congenital chloride diarrhea gene is expressed in seminal vesicle, sweat gland, inflammatory colon epithelium, and in some dysplastic colon cells. Histochem Cell Biol 113: 279–286, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Hoglund P, Haila S, Socha J, Tomaszewski L, Saarialho-Kere U, Karjalainen-Lindsberg ML, Airola K, Holmberg C, de la Chapelle A, Kere J. Mutations of the down-regulated in adenoma (DRA) gene cause congenital chloride diarrhoea. Nat Genet 14: 316–319, 1996 [DOI] [PubMed] [Google Scholar]
- 18. Jacob P, Rossmann H, Lamprecht G, Kretz A, Neff C, Lin-Wu E, Gregor M, Groneberg DA, Kere J, Seidler U. Down-regulated in adenoma mediates apical Cl−/HCO3− exchange in rabbit, rat, and human duodenum. Gastroenterology 122: 709–724, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Kiela PR, Guner YS, Xu H, Collins JF, Ghishan FK. Age- and tissue-specific induction of NHE3 by glucocorticoids in the rat small intestine. Am J Physiol Cell Physiol 278: C629–C637, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Lamprecht G, Schaefer J, Dietz K, Gregor M. Chloride and bicarbonate have similar affinities to the intestinal anion exchanger DRA (down regulated in adenoma). Pflügers Arch 452: 307–315, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Laubitz D, Larmonier CB, Bai A, Midura-Kiela MT, Lipko MA, Thurston RD, Kiela PR, Ghishan FK. Colonic gene expression profile in NHE3-deficient mice: evidence for spontaneous distal colitis. Am J Physiol Gastrointest Liver Physiol 295: G63–G77, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ledoussal C, Woo AL, Miller ML, Shull GE. Loss of the NHE2 Na+/H+ exchanger has no apparent effect on diarrheal state of NHE3-deficient mice. Am J Physiol Gastrointest Liver Physiol 281: G1385–G1396, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Lee SH, Kim T, Park ES, Yang S, Jeong D, Choi Y, Rho J. NHE10, an osteoclast-specific member of the Na+/H+ exchanger family, regulates osteoclast differentiation and survival [corrected]. Biochem Biophys Res Commun 369: 320–326, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Orlowski J, Kandasamy RA, Shull GE. Molecular cloning of putative members of the Na/H exchanger gene family. cDNA cloning, deduced amino acid sequence, and mRNA tissue expression of the rat Na/H exchanger NHE-1 and two structurally related proteins. J Biol Chem 267: 9331–9339, 1992 [PubMed] [Google Scholar]
- 25. Schultheis PJ, Clarke LL, Meneton P, Harline M, Boivin GP, Stemmermann G, Duffy JJ, Doetschman T, Miller ML, Shull GE. Targeted disruption of the murine Na+/H+ exchanger isoform 2 gene causes reduced viability of gastric parietal cells and loss of net acid secretion. J Clin Invest 101: 1243–1253, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19: 282–285, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Tytgat KM, Opdam FJ, Einerhand AW, Buller HA, Dekker J. MUC2 is the prominent colonic mucin expressed in ulcerative colitis. Gut 38: 554–563, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Klinken BJ, Einerhand AW, Duits LA, Makkink MK, Tytgat KM, Renes IB, Verburg M, Buller HA, Dekker J. Gastrointestinal expression and partial cDNA cloning of murine Muc2. Am J Physiol Gastrointest Liver Physiol 276: G115–G124, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Xu H, Chen H, Dong J, Lynch R, Ghishan FK. Gastrointestinal distribution and kinetic characterization of the sodium-hydrogen exchanger isoform 8 (NHE8). Cell Physiol Biochem 21: 109–116, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Xu H, Chen R, Ghishan FK. Subcloning, localization, and expression of the rat intestinal sodium-hydrogen exchanger isoform 8. Am J Physiol Gastrointest Liver Physiol 289: G36–G41, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Xu H, Collins JF, Bai L, Kiela PR, Ghishan FK. Regulation of the human sodium-phosphate cotransporter NaPi-IIb gene promoter by epidermal growth factor. Am J Physiol Cell Physiol 280: C628–C636, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Xu H, Collins JF, Bai L, Kiela PR, Lynch RM, Ghishan FK. Epidermal growth factor regulation of rat NHE2 gene expression. Am J Physiol Cell Physiol 281: C504–C513, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Xu H, Li J, Chen R, Zhang B, Wang C, King N, Chen H, Ghishan FK. NHE2X3 DKO mice exhibit gender-specific NHE8 compensation. Am J Physiol Gastrointest Liver Physiol 300: G647–G653, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xu H, Zhang B, Li J, Chen H, Tooley J, Ghishan FK. Epidermal growth factor inhibits intestinal NHE8 expression via reducing its basal transcription. Am J Physiol Cell Physiol 299: C51–C57, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xu H, Zhang B, Li J, Chen H, Wang C, Ghishan FK. Transcriptional inhibition of intestinal NHE8 expression by glucocorticoids involves Pax5. Am J Physiol Gastrointest Liver Physiol 299: G921–G927, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yun CH, Tse CM, Nath SK, Levine SA, Brant SR, Donowitz M. Mammalian Na+/H+ exchanger gene family: structure and function studies. Am J Physiol Gastrointest Liver Physiol 269: G1–G11, 1995 [DOI] [PubMed] [Google Scholar]
- 37. Zachos NC, Tse M, Donowitz M. Molecular physiology of intestinal Na+/H+ exchange. Annu Rev Physiol 67: 411–443, 2005 [DOI] [PubMed] [Google Scholar]


