Abstract
Changes in end-expiratory lung volume (EELV) affect upper airway stability. The passive pharyngeal critical pressure (Pcrit), a measure of upper airway collapsibility, is determined using airway pressure drops. The EELV change during these drops has not been quantified and may differ between obese obstructive sleep apnea (OSA) patients and controls. Continuous positive airway pressure (CPAP)-treated OSA patients and controls were instrumented with an epiglottic catheter, magnetometers (to measure change in EELV), and a nasal mask/pneumotachograph. Subjects slept supine in a head-out plastic chamber in which the extrathoracic pressure could be lowered (to raise EELV) while on nasal CPAP. The magnitude of EELV change during Pcrit measurement (sudden reductions of CPAP for 3–5 breaths each minute) was assessed at baseline and with EELV increased ∼500 ml. Fifteen OSA patients and 7 controls were studied. EELV change during Pcrit measurement was rapid and pressure dependent, but similar in OSA and control subjects (74 ± 36 and 59 ± 24 ml/cmH2O respectively, P = 0.33). Increased lung volume (mean +521 ml) decreased Pcrit by a similar amount in OSA and control subjects (−3.1 ± 1.7 vs. −3.9 ± 1.9 cmH2O, P = 0.31). Important lung volume changes occur during passive Pcrit measurement. However, on average, there is no difference in lung volume change for a given CPAP change between obese OSA subjects and controls. Changes in lung volume alter Pcrit substantially. This work supports a role for lung volume in the pathogenesis of OSA, and lung volume changes should be a consideration during assessment of pharyngeal mechanics.
Keywords: obstructive sleep apnea, obstructive sleep apnea pathogenesis, pharyngeal critical pressure, respiratory mechanics, functional residual capacity
assessment of upper airway mechanics has been performed using a variety of different techniques, although each has some limitations. Upper airway resistance can be quantified but becomes complicated during inspiratory flow limitation. Negative pressure pulses have been used to calculate collapsibility or collapsibility index but rely on assumptions regarding negligible flow resistive pressure drop. The pharyngeal critical closing pressure (Pcrit) has emerged as the gold standard for the assessment of upper airway mechanics based on the work of Schwartz and Smith (7, 23). The Pcrit can be measured using an active or a passive technique. The active technique is based on gradual reductions in the continuous positive airway pressure (CPAP) over several minutes, allowing time for upper airway dilator muscle recruitment and equilibration of end-expiratory lung volume (EELV). In contrast, the passive technique relies on pressure drops lasting only three to five breaths, followed by reestablishment of a holding pressure (typically the prescribed CPAP level) between pressure drops. Because the drops are so brief, upper airway neuromuscular recruitment is thought to be minimal (18). How much EELV changes during these brief airway pressure drops has not been measured. Furthermore, the significance of any lung volume change on the measured Pcrit has not been assessed. We and others have shown considerable influences of EELV on other measures of upper airway collapsibility both in controls and in obstructive sleep apnea (OSA) patients (9–11, 24, 25). Thus we hypothesize that changes in EELV during Pcrit measurement may impact the measured collapsibility of the upper airway.
The Pcrit is known to be more positive/less negative among OSA patients compared with healthy controls. However, OSA patients frequently differ from controls in terms of body weight and fat distribution. Obesity is known to reduce EELV, and the pattern of fat deposition may be important (1, 12). Based on these observations, we speculated that the drop in EELV during Pcrit measurement would be greater in obese OSA patients than in leaner controls. Any possible discrepancy in EELV changes might help explain the difference in the observed values of Pcrit in overweight and obese OSA subjects compared with less overweight control subjects.
Therefore, we sought to 1) measure the change in EELV that occurs during the brief pressure drops of passive Pcrit measurement, 2) determine if the EELV change is different between obese OSA subjects and controls, and 3) assess the effect of a given change in EELV on the Pcrit in both groups.
METHODS
Subjects.
A total of 26 adults were recruited for the study. Subjects included previously diagnosed OSA patients with a body mass index (BMI) > 25 kg/m2 using CPAP therapy for greater than 3 mo, and nonsnoring controls not known to have a sleep disorder. No study subject smoked, had any other respiratory disorder, or took medications known to affect respiratory or airway/muscle function. Five patients were on medication for treatment of hypertension, three took allergy medication, one used a statin medication, and one used a proton-pump inhibitor. Women in the study were screened for pregnancy, were not taking hormonal forms of birth control, and were studied during the follicular phase of their menstrual cycle (days 5–11). OSA subjects underwent a baseline polysomnogram in our laboratory to determine the apnea-hypopnea index (AHI). While some of the OSA subjects in this study participated in other studies conducted in our laboratory, none of the findings of the present study are previously published. Two of the control subjects had previously had a polysomnogram to rule out OSA for research study purposes. All subjects gave written, informed consent before participation in this study, which was approved by the Human Research Committee of the Brigham and Women's Hospital.
Equipment.
The study consisted of a single overnight experiment. Patients arrived 2 h before their usual bedtime to be instrumented. Wakefulness and sleep stages were determined using standard electroencephalogram, chin electromyogram, and electrooculogram. Airway pressure was measured at the level of the epiglottis using a pressure-tipped catheter (Millar MPC-550, Millar Instruments, Houston, TX) passed through the nose and advanced 1.5–2 cm below the base of the tongue under direct visualization. Before insertion, both nostrils were sprayed with 0.05% oxymetazoline hydrochloride, a decongestant, and the more patent nostril was then anesthetized with 4% lidocaine topical spray. A nasal mask (Profile Lite or GoldSeal, Respironics, Murraysville, PA) was placed, and air flow was measured with a pneumotachograph (model 3700A, Hans Rudolph, Kansas City, MO) and pressure transducer (Validyne, Northridge, CA). Two pairs of magnetometers (EOL Eberhard, Oberwil, Switzerland) were placed on the front and back of the subject along the midline at the level of the sternum and just above the umbilicus. Calibration was performed during wakefulness by comparison with tidal volumes recorded by the pneumotachograph. Changes in EELV were determined using a previously validated formula (2). Patients slept in a head-out rigid shell (Porta-lung,Murraysville, PA) attached to a vacuum (ShopVac, Williamsport, PA) to decrease extrathoracic pressure (increase lung volume). Arterial blood oxygen saturation via pulse oximetry and the electrocardiogram were monitored throughout the study for safety purposes.
Data were acquired on a 1401 plus interface and Spike 2 software (Cambridge Electronic Design Ltd, Cambridge, UK).
Protocol.
When subjects were awake and comfortable in the head-out rigid shell, 5 min of baseline data were recorded. Magnetometer calibration occurred during this time. CPAP was then applied, and another 5 min of wakefulness data were recorded before subjects were allowed an opportunity to sleep. CPAP was initially set at the therapeutically prescribed level for OSA patients and at 4 cmH2O for controls, and was increased if needed during sleep to eliminate flow limitation. This level of CPAP is referred to as the holding pressure. Once stable non-rapid eye movement (non-REM) sleep had been achieved Pcrit measurements were made multiple times either under baseline conditions or with increased EELVs, in random order.
Pcrit measurement.
Pcrit measurements were made by abruptly dropping the airway pressure for three to five breaths from the holding pressure to progressively lower CPAP levels, typically starting 1 or 2 cmH2O below the holding pressure and progressing in decrements of 1 cmH2O per drop. If necessary, negative airway pressure was applied. Pressure drops were separated by at least 1 min. Repeated Pcrit measurements were made in both conditions, as many times as possible up to a maximum of four separate measurements in each condition. Measurements were separated by at least 3 min. Patients were given time to reenter stable sleep after brief arousals, but if they awoke the measurement was aborted and, if necessary, extrathoracic pressure returned to zero until stable sleep resumed.
Lung volume manipulation.
During stable non-REM sleep, lung volumes were increased by application of negative extrathoracic pressure, which was decreased over ∼2 min to about −5 cmH2O. The resulting change in lung volume was determined, and pressure increased or decreased to achieve an increase in EELV of ∼500 ml. During later analysis, the ultimate change in lung volume was divided by the measured negative extrathoracic pressure to determine the total respiratory system compliance during inflation (Crsinflation).
Analysis.
Data were analyzed on a breath-by-breath basis using custom-designed semiautomated software. Analyzed variables included inspiratory time, expiratory time, tidal volume, peak flow, mask pressure, and rib cage and abdominal anterior-posterior distance (provided continuously by each respective magnetometer pair). For each breath, magnetometer distances were measured just before inspiratory flow. If flow was obstructed, then the first negative deflection in epiglottic pressure was used to signal the end of expiration and the start of the breath. During each breath of a pressure drop in a Pcrit measurement trial, the magnetometer distances at the start of inspiration were compared with the average of these distances during the five breaths immediately preceding that drop. The differences in distance and the previously determined calibration factor were used to measure acute changes in lung volume during Pcrit measurement. For each pressure drop, the lung volume changes during breaths 3–5 were plotted against the change in mask pressure (measured at end expiration) from the holding pressure, and a linear regression model was fitted to the data (for example, see Fig. 2). The slopes for each Pcrit measurement trial under each condition were averaged together to determine the relationship between change in lung volume over change in pressure, a measure of total respiratory system compliance (Crspressure drop).
Fig. 2.
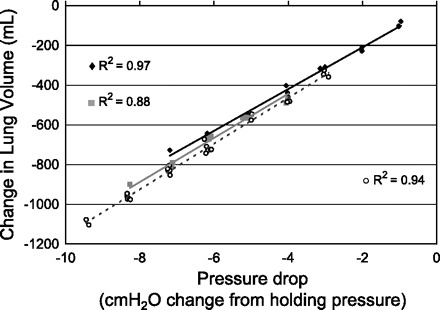
Relative change in EELV that occurs during pharyngeal critical closing pressure (Pcrit) measurements in one obstructive sleep apnea (OSA) subject. Two Pcrit measurements made under baseline conditions (black diamond and black solid line; gray square and gray solid line), and one Pcrit measurement made at increased lung volume (open circle and dashed line). Each point represents one of the 3rd through 5th breaths of a pressure drop. The holding pressure did not change between conditions. Marked changes in pressure can cause substantial changes in EELV.
To determine Pcrit, mask pressure and peak inspiratory flow from breaths 3–5 during each pressure drop series were plotted if the breaths were flow limited. A linear regression model of peak inspiratory flow vs. mask pressure was used to extrapolate the pressure at zero flow, the Pcrit (17). The multiple Pcrit values in each condition were averaged to determine a Pcrit under baseline conditions, and a Pcrit at increased lung volume.
Statistical analysis.
A two-way repeated-measures ANOVA was used to examine breath-by-breath lung volume changes between subject groups during pressure drops of different magnitudes (2 cmH2O vs. 6 cmH2O). One-way ANOVAs were used to compare total respiratory compliance and Pcrit changes in each lung volume condition between patients with and without OSA (MiniTab, State College, PA). Where main ANOVA effects were observed, post hoc comparisons were performed using Dunn-Sidak adjusted Student's paired t-tests (13). Nonparametrically distributed variables were analyzed using Mann-Whitney U-tests. Linear regression analyses were performed with the dependent variable total respiratory system compliance and independent variables BMI, weight, AHI, Pcrit, change in Pcrit with increase in lung volume, and rib cage and abdominal anterior-posterior distance. A P value of <0.05 was considered statistically significant. Values are presented as means ± SD unless otherwise indicated.
RESULTS
Anthropomorphic data.
Four subjects failed to sleep in the plastic shell. Anthropomorphic and polysomnographic data for the remaining 22 subjects are shown in Table 1. Those with OSA were significantly older and had higher BMIs (by design) than controls. Complete Pcrit determinations were accomplished on average under baseline conditions 2.4 ± 1.0 times per subject, and under conditions of increased lung volumes 2.0 ± 1.0 times per subject (no difference between OSA subjects and controls).
Table 1.
Subject characteristics
| OSA Subjects | Controls | |
|---|---|---|
| Sample size | 15 | 7 |
| M/F | 11/4 | 5/2 |
| Age, yr | 49.1 ± 11.5 | 28.6 ± 6.9* |
| BMI, kg/m2 | 34.4 ± 6.4 | 27.5 ± 7.0* |
| AHI, events/h | 59 ± 33 | † |
| Holding pressure, cmH2O | 11 ± 2.7 | 4.6 ± 0.8* |
Data are presented as means ± SD. BMI, body mass index.
Apnea-hypopnea index (AHI) was known for 2 control subjects only (0.6 and 3.5 events/hour).
P value < 0.05.
Lung volumes.
An example of raw data tracings during CPAP drops at baseline in one subject are shown in Fig. 1. As seen in Fig. 1, rib cage and abdominal anterior-posterior distances are reduced during pressure drops in a pressure-dependent manner. The relationship between CPAP change and lung volume change was linear across the range of pressures studied (relationship in one subject shown in Fig. 2). Lung volume changes occurred rapidly, within the first one to two breaths of a pressure drop (Fig. 3). ANOVA for repeated measures revealed a significant main effect for breath number (P < 0.001) and also for pressure drop magnitude (P < 0.001); there was no main effect by group (P = 0.395). There was an interaction between breath number and pressure drop magnitude (P = 0.002). There were no other significant interaction effects. Post hoc analysis showed that for a pressure change of 2 cmH2O, the lung volume change was statistically different between breath 1 vs. breaths 3, 4, and 5 of the pressure drop. However, the lung volume changes were not different between breaths 2–5. For the 6-cmH2O pressure drop, the lung volume change was statistically significant between each breath 1, 2, 3, and 4. Breaths 4 and 5 were not different.
Fig. 1.
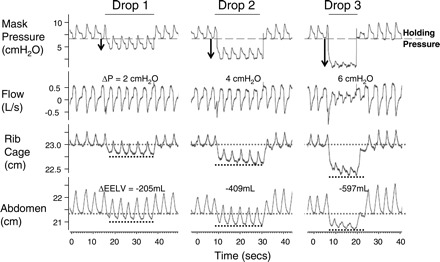
Raw data illustrating that rib cage and abdominal anterior-posterior distance fall within the first breath of a pressure drop, and in a pressure-dependent manner. The holding pressure is reduced from 7 cmH2O by the amounts indicated for 3–5 breaths, until flow limitation is achieved. The average change in end-expiratory lung volume (ΔEELV) during the pressure drop, compared with the average volume at the holding pressure baseline, is shown. One minute of baseline breathing between drops is omitted.
Fig. 3.
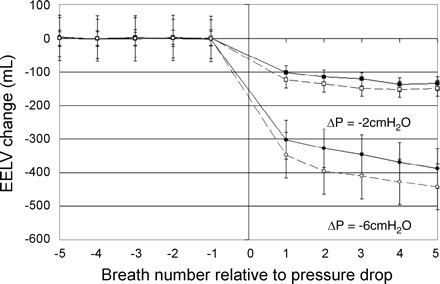
The change in EELV on a breath-to-breath basis during two different pressure drops (2 cmH2O, squares; 6 cmH2O, circles) averaged across all subjects and both baseline and increased lung volume conditions. EELV changes are means with SE bars. Most of the lung volume change has occurred by the 3rd breath. OSA subjects (□, ○, connected by dashed lines) and controls (■, ●, connected by solid line) had a similar decrease in lung volume for a given change in pressure. Statistically significant lung volume changes are indicated in the text of results.
Differences between OSA and controls.
Consistent with the above results, the decrease in lung volume for a given pressure drop (respiratory system compliance, Crspressure drop) was not statistically different between groups (P = 0.33). Crspressure drop was measured separately under both baseline and increased lung volume conditions. When lung volume was increased ∼500 ml, Crspressure drop increased a similar amount in both groups (P = 0.50). Crs increased from 68 ± 35 ml/cmH2O at baseline to 79 ± 36 ml/cmH2O with lung volume increased in OSA subjects (P < 0.001), and from 51 ± 25 ml/cmH2O at baseline to 69 ± 22 ml/cmH2O with lung volume increased in controls (P < 0.001). Crspressure drop was highly variable among all subjects, and even within both groups (Table 2). In addition, this variance could not be accounted for by differences in BMI, weight, AHI, Pcrit, change in Pcrit with increase in lung volume, or rib cage or abdominal anterior-posterior distance (regression analyses nonsignificant).
Table 2.
Individual measures of lung volume changes, total respiratory system compliances, and Pcrit measurements
| Subject | BMI, kg/m2 | Extrathoracic Pressure, cmH2O | ΔEELV, ml | ΔPcrit/100 ml ΔLV, -cmH2O/100 ml | Crspressure drop ml/cmH2O | CrsInflation, ml/cmH2O | |
|---|---|---|---|---|---|---|---|
| OSA | 1 | 29.8 | −5.9 | 523 | 0.15 | 74 | 89 |
| 2 | 31.5 | −7.3 | 545 | 1.25 | 59 | 75 | |
| 3 | 33.0 | −3.2 | 437 | 0.43 | 12 | 138 | |
| 4 | 45.5 | −8.2 | 706 | 0.72 | 59 | 86 | |
| 5 | 31.1 | −7.8 | 557 | 0.50 | 49 | 71 | |
| 9 | 30.6 | −4.6 | 532 | 0.56 | 82 | 115 | |
| 10 | 27.2 | −3.7 | 438 | 0.34 | 115 | 119 | |
| 11 | 43.9 | −4.4 | 634 | 0.48 | 89 | 144 | |
| 12 | 25.8 | −4.8 | 793 | 0.29 | 149 | 164 | |
| 14 | 31.5 | −5.1 | 472 | 0.83 | 73 | 92 | |
| 15 | 33.2 | −5.7 | 347 | 1.41 | 51 | 61 | |
| 16 | 45.6 | † | 626 | 0.19 | 57 | † | |
| 18 | 31.0 | −4.9 | 565 | 0.39 | 73 | 115 | |
| 19 | 39.5 | −5.0 | 462 | 0.48 | 38 | 92 | |
| 20 | 36.7 | −4.1 | 652 | 0.70 | 125 | 160 | |
| Mean ± SD | 34.4 ± 6.4 | −5.3 ± 1.5 | 552 ± 116 | 0.58 ± 0.36 | 74 ± 35 | 109 ± 33 | |
| Controls | 6 | 21.2 | −5.2 | 523 | 0.99 | 72 | 100 |
| 7 | 22.1 | −5.4 | 520 | 1.11 | 97 | 96 | |
| 8 | 28.1 | −6.0 | 510 | 0.74 | 35 | 85 | |
| 13 | 22.2 | −5.2 | 373 | 1.63 | 41 | 71 | |
| 17 | 32.5 | −5.1 | 414 | 0.68 | 69 | 80 | |
| 21 | 25.8 | −5.1 | 440 | 0.18 | 66 | 87 | |
| 22 | 40.5 | −5.1 | 394 | 0.73 | 34 | 77 | |
| Mean ± SD | 27.5 ± 7.0‡ | −5.3 ± 0.3 | 453 ± 64‡ | 0.86 ± 0.45 | 59 ± 23 | 85 ± 10 |
ΔPcrit/100 ml ΔLV = change in pharyngeal critical closing pressure per 100 ml increase in lung volume. CrsInflation, respiratory system compliance during inflation. *Crspressure drop, average compliance from pressure drops under baseline and increased EELV conditions.
Data not available.
P < 0.05, OSA vs. controls.
Pcrit.
As expected, Pcrit was higher under baseline and increased lung volume conditions in those with OSA compared with controls (Fig. 4). In subjects with OSA, an average increase in lung volume of 553 ml (SD ±117 ml) significantly decreased Pcrit by −3.1 ± 1.7 cmH2O (P < 0.001). In control subjects, the average increase in EELV was slightly less than OSA subjects at 453 ml (SD ±63 ml, P = 0.048). With this EELV increase, control subject Pcrit decreased significantly by −3.9 ± 1.9 cmH2O (P = 0.002). The reduction in Pcrit with the ∼500-ml lung volume increase was not statistically different between OSA and control subjects (P = 0.31), even when adjusted for the magnitude of lung volume change (P = 0.12).
Fig. 4.
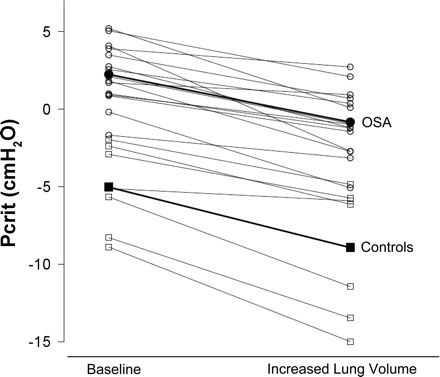
Pcrit at baseline and at increased EELV in individual subjects with OSA (○) and controls (☐). The average lung volume increase for control subjects (■) was 453 ± 64 ml. OSA subjects (●) lung volumes were raised 552 ± 115 ml.
DISCUSSION
During passive Pcrit measurements, end-expiratory lung volume decreases in a pressure-dependent manner. The change in lung volume associated with pressure drops is rapid, with most of the change occurring within the first two breaths. The lung volume change appears to be physiologically important and likely contributes to the Pcrit values measured, especially if the Pcrit is markedly below the holding pressure. In these cases, the lung volume changes can be >1 liter.
Although lung volume changes during the Pcrit measurement, the similarity between OSA and controls suggests that this measurement is still useful in defining upper airway mechanics. However, our work supports those who have called for the measurement of Pcrit to be standardized (4, 5, 17). Currently, most but not all groups use the third through fifth breaths of a pressure drop when determining Pcrit. Our data suggest that EELV often stabilizes within two breaths, making the third through fifth breath reasonable to quantify passive pharyngeal mechanics.
The change in Pcrit with lung volume emphasizes that EELV likely plays some role in the pathogenesis of OSA. The mechanism by which EELV influences the upper airway is thought to be tracheal traction, a direct mechanical link (independent of neuromuscular effects) by which caudal force on the trachea causes improvements in pharyngeal mechanics (3, 27, 28). As described by van de Graaff, the caudal traction may unfold the airway, stiffen airway walls, remove redundant tissue, or combine with other forces to create a net ventral force vector that opens the upper airway. Our work also helps further quantify the magnitude of the lung volume effect on upper airway mechanics, which has been measured previously in terms of improvements in the apnea-hypopnea index, CPAP level required to prevent flow limitation, and pharyngeal resistance and collapsibility. Consistent with these prior studies, we show here that a 500-ml increase in lung volume substantially improves Pcrit, reducing it by 3–4 cmH2O.
Total respiratory system compliance as measured during pressure drops was considerably lower than previously reported values (15). Therefore, we also calculated the total respiratory system compliance by determining the rise in lung volume with applied negative extrathoracic pressure (Crsinflation). Crspressure drop values were almost always lower than Crsinflation (Table 2). There are several reasons why the inflation and deflation compliance values might be different. First, lung volumes are still trending down even after the fifth breath of a pressure drop. Allowing time for complete equilibrium, which would maximize the difference in lung volume, would increase the measured compliance. However, this may only be a minor effect as lung volume changes slowly, ∼15 ml/breath by breaths 3–5 of a moderate (6 cmH2O) pressure drop. We did not assess lung volume changes during prolonged pressure drops (as our goal was to understand the passive Pcrit maneuver). Second, a difference in compliance measured during inflation and deflation may reflect hysteresis. For example, during quasi-static pressure-volume measurements the inflation and deflation limbs are typically separate and distinct. However, given the range of lung volumes over which we were operating, the difference in slope between inflation and deflation limbs may not be very great (although the curves are displaced from one another). Third, airway pressure drops may lead to expiratory flow limitation or obstruction (in addition to the inspiratory flow limitation). If expiratory time is not reflexively increased, the lung volume drop will be attenuated. Most often however, during inspiratory flow limitation, inspiratory duty cycle and respiratory rate increase, which will reduce total expiratory time (22). For example, expiratory flow limitation and obstruction out of proportion to inspiratory flow limitation appeared to explain the wide difference between Crspressure drop and Crsinflation in subject 3 (see Table 2 and Fig. 5), where near complete expiratory obstruction occurred after a pressure drop despite ongoing inspiratory airflow. Fourth, sudden changes in airway pressure are known to provoke compensatory reflexes (e.g., Hering-Breuer, Head) (29). Such inhibitory mechanisms may be activated with rapid pressure drops, preventing more complete exhalation, but may not be active during the much slower expansion of the lungs using the “iron lung.” Each of these factors, alone or in combination, may help explain the wide variance in both measures of compliance.
Fig. 5.
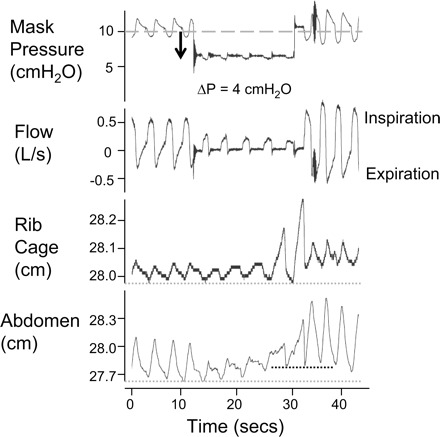
Raw data illustrating compromise in inspiratory and expiratory flow in a single subject during a pressure drop. With the holding pressure reduced from 10 cmH2O to 6 cmH2O there are ongoing flow-limited inspiratory efforts; however, there is also minimal expiratory flow. Rib cage anterior-posterior diameter does not drop, and abdominal anterior-posterior distance increases with each successive inspiration. Compare with Fig. 1 in which expiratory flow is preserved.
Despite the reported decrease in respiratory system compliance noted with obesity, we did not find any association between BMI (or any other anthropomorphic characteristic) and total respiratory system compliance (measured either during deflation or inflation). In fact, both measures of compliance tended (nonsignificantly) to be higher for obese OSA patients, a finding, at first glance, at odds with other literature which has shown reduced total respiratory system compliance in the obese (15, 19, 20), either awake or when paralyzed. The traditional explanation has been that obesity leads to decreased chest wall compliance, which accounts for most or all of the reduction in total respiratory system compliance. However, chest wall compliance has not reproducibly been observed to decrease with obesity (8, 26) and may relate to subtle differences in body position, full muscle relaxation, and concomitant changes in lung volume during testing. Classic studies performed during wakefulness may have been confounded by behavioral influences, which should be minimal during sleep. A close examination of the work by Pelosi et al. (19) shows that even extremely obese patients (BMI > 70 kg/m2) can have a normal chest wall compliance, and that much of the decrease in total respiratory system compliance is actually due to decreased lung compliance in their supine, paralyzed obese subjects, a change thought to reflect atelectasis and small airway closure as lung volume falls below the closing volume. This assertion is supported by near normalization of total respiratory compliance in the very obese with application of 10 cmH2O positive end-expiratory pressure, which should reverse atelectasis and maintain small airway patency by keeping lung volume above the closing volume (21). We suspect that the lack of a difference we observed in respiratory compliance between our OSA subjects and controls may have occurred in part due to the different holding pressures (average 11 vs. 5 cmH2O) at which they were studied, which minimized any baseline differences in atelectasis or lung compliance between the two groups.
Our results differ from the recent work by Tagaito et al. (27), who found a smaller difference in P′close (a regression-based estimate of closing pressure based on visualized changes in airway area with changes in airway pressure) between baseline and increased lung volume conditions. For an average lung volume increase of 720 ml, the median improvement in P′close was only 1.22 cmH2O (assessed at the velopharynx). Also, the authors observed that the change in P′close at the velopharynx for a given change in lung volume was related to BMI. We performed a similar analysis of our data and found no significant correlation with BMI (Table 2). However, our study differs from Tagaito's study in a number of ways. First, their OSA subjects in Japan differed from ours in the United States, in that they were considerably thinner (BMI 26.2 vs. 34.4 kg/m2) and the site of complete collapse was always the velopharynx in the Japanese cohort. Second, their experimental setup was very different in that they studied patients under general anesthesia and paralysis, rather than during sleep. The use of paralytics should minimize any neuromuscular reflexes that may be active during non-REM sleep. As such, some have suggested that the anesthetic models may be predictive of REM sleep (6), which is a condition that has received minimal attention from a lung volume standpoint. Third, their measurements were made during a gradual decrease in airway pressure, which might have allowed atelectasis to develop, whereas our measurements were made during very brief drops from the holding pressure. Fourth, their measurement of upper airway collapse, P′close, is based on a visual assessment of the upper airway, while our Pcrit is based on extrapolation to zero flow. We do not believe that P′close (examined at the velopharynx), Pcrit, and pharyngeal collapsibility [determined using brief negative pressure pulses in the work by Stanchina et al. (25)] are equivalent measurements: neither their absolute values nor the change with lung volume manipulation would be expected to be the same (14). Thus comparisons across these studies, especially in regard to the magnitude of the lung volume effect, need to be made cautiously. We do not feel that our data are inconsistent with those from Heinzer et al. (9), Stanchina et al. (25), or Tagaito et al. (27); however, a direct comparison of the lung volume effect on pharyngeal mechanics cannot be made with certainty.
Limitations.
Our experiment has several limitations. First, we measured only changes in lung volume. We did not measure absolute lung volumes or obtain pressure-volume (P-V) curves. Furthermore, our subjects were studied at various levels of positive pressure, which affects EELV. OSA subjects tended to have high holding pressures, likely increasing EELV well above the normal resting supine lung volume. In contrast, control subjects had low holding pressures and negative Pcrit values. Therefore, EELV was likely not increased from the resting supine lung volume the same amount in controls compared with OSA subjects. During Pcrit measurements in control subjects negative airway pressure was sometimes applied, which would decrease lung volumes below the normal resting supine lung volume. These differences in starting EELV and the range of airway pressures utilized could affect our results. Pcrit measurements made at the same holding pressure in all subjects might have partially corrected for differences in the initial EELV. However, because our goal was to assess standard Pcrit maneuvers, we observed essentially no overlap in holding pressures between our two groups in the data recorded. While such data would have been interesting, our method of passive Pcrit measurement reflects current methodology, and as such, our data likely reflect “real world” conditions for standard measurements. Nevertheless, because our research suggests an important role for EELV in affecting pharyngeal mechanics, we would also advocate for further upper airway research using isovolumetric methods to assess the physiology independent of lung volume influences. Second, we assumed a linear relationship between changes in pressure and volume. While the total respiratory system P-V curve has a sigmoidal shape (16), across the range of pressures and volumes we studied, the relationship appeared linear. With the addition of CPAP, increasing lung volume above the normal resting supine EELV, subjects probably were on the linear portion of the P-V curve during pressure drops. Atelectasis and small airway closure were probably prevented by the application of positive airway pressure in all subjects. Based on prior experience, manipulations of lung volumes to either extreme range (where the P-V curve is no longer linear) generally lead to arousal. Third, we did not measure all variables that might affect changes in lung volume, such as assessments of visceral and/or subcutaneous fat or blood flow. These variables might also influence the observed EELV effects. The recent work by Babb and colleagues found that almost all measurements of overall obesity, such as BMI, were predictive of EELV while awake in the upright or seated position, and that little was gained when looking at markers of fat distribution (1). However, it is not known if fat distribution might become more important when supine or during sleep. Fourth, we did not match our groups based on BMI. Our intention was not to assess the impact of apnea status on the lung volume changes during Pcrit maneuver independent of obesity. Rather, we sought to test the hypothesis that lung volume changes may explain some of the variability in Pcrit measurements across various patient groups. As such, we do not believe that BMI matching was necessary or even appropriate. Furthermore, because fat distribution may be more critical than BMI per se, BMI matching may be a potentially problematic strategy in the physiology of respiratory mechanics. Finally, we measured the Pcrit only at two different lung volumes and did not characterize Pcrit changes across the entire vital capacity. Therefore, we cannot definitively comment on the relationship between lung volume and Pcrit beyond the levels measured in this study.
Conclusion.
Important lung volume changes do occur during the passive Pcrit maneuver. However, on average, there is no difference in lung volume change for a given CPAP change between obese OSA subjects and controls. Pcrit is altered substantially by changes in lung volume. This work supports a role for lung volumes in the pathogenesis of OSA and suggests that lung volume changes should be a consideration during assessment of pharyngeal mechanics.
GRANTS
This study was supported by grants from the National Institutes of Health (HL-048531, HL-60292, and RR-01032) and the American Heart Association (0840159N and 0635318N).
DISCLOSURES
No relevant conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We gratefully acknowledge Dr. Stephen Loring (Beth Israel Deaconess Medical Center, Boston, MA) for his aid with the magnetometer devices, discussions on respiratory mechanics, and critical review of the manuscript. We also thank sleep technicians Karen E. Stevenson and Lauren Hess for their invaluable assistance in obtaining these data.
The modified CPAP machine was provided free of charge by Philips Respironics.
Present address for A. S. Jordan: Sleep Laboratory, Dept. of Psychology, Univ. of Melbourne, Parkville, VIC, 3010 Australia (e-mail: ajordan@unimelb.edu.au).
REFERENCES
- 1. Babb TG, Wyrick BL, DeLorey DS, Chase PJ, Feng MY. Fat distribution and end-expiratory lung volume in lean and obese men and women. Chest 134: 704–711, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Banzett RB, Mahan ST, Garner DM, Brughera A, Loring SH. A simple and reliable method to calibrate respiratory magnetometers and Respitrace. J Appl Physiol 79: 2169–2176, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Begle RL, Badr S, Skatrud JB, Dempsey JA. Effect of lung inflation on pulmonary resistance during NREM sleep. Am Rev Respir Dis 141: 854–860, 1990 [DOI] [PubMed] [Google Scholar]
- 4. Boudewyns A, Punjabi N, Van de Heyning PH, De Backer WA, O'Donnell CP, Schneider H, Smith PL, Schwartz AR. Abbreviated method for assessing upper airway function in obstructive sleep apnea. Chest 118: 1031–1041, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Eastwood PR, Szollosi I, Platt PR, Hillman DR. Collapsibility of the upper airway during anesthesia with isoflurane. Anesthesiology 97: 786–793, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Eastwood PR, Szollosi I, Platt PR, Hillman DR. Comparison of upper airway collapse during general anaesthesia and sleep. Lancet 359: 1207–1209, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Gold AR, Schwartz AR. The pharyngeal critical pressure. The whys and hows of using nasal continuous positive airway pressure diagnostically. Chest 110: 1077–1088, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Hedenstierna G, Santesson J. Breathing mechanics, dead space and gas exchange in the extremely obese, breathing spontaneously and during anaesthesia with intermittent positive pressure ventilation. Acta Anaesthesiol Scand 20: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 9. Heinzer RC, Stanchina ML, Malhotra A, Fogel RB, Patel SR, Jordan AS, Schory K, White DP. Lung volume and continuous positive airway pressure requirements in obstructive sleep apnea. Am J Respir Crit Care Med 172: 114–117, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heinzer RC, Stanchina ML, Malhotra A, Jordan AS, Patel SR, Lo YL, Wellman A, Schory K, Dover L, White DP. Effect of increased lung volume on sleep disordered breathing in patients with sleep apnoea. Thorax 61: 435–439, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hoffstein V, Zamel N, Phillipson EA. Lung volume dependence of pharyngeal cross-sectional area in patients with obstructive sleep apnea. Am Rev Respir Dis 130: 175–178, 1984 [DOI] [PubMed] [Google Scholar]
- 12. Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest 130: 827–833, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Ludbrook J. On making multiple comparisons in clinical and experimental pharmacology and physiology. Clin Exp Pharmacol Physiol 18: 379–392, 1991 [DOI] [PubMed] [Google Scholar]
- 14. Malhotra A, Pillar G, Fogel R, Beauregard J, Edwards J, White DP. Upper-airway collapsibility: measurements and sleep effects. Chest 120: 156–161, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Naimark A, Cherniack RM. Compliance of the respiratory system and its components in health and obesity. J Appl Physiol 15: 377–382, 1960 [DOI] [PubMed] [Google Scholar]
- 16. Owens RL, Hess DR, Malhotra A, Venegas JG, Harris RS. Effect of the chest wall on pressure-volume curve analysis of acute respiratory distress syndrome lungs. Crit Care Med 36: 2980–2985, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Patil SP, Punjabi NM, Schneider H, O'Donnell CP, Smith PL, Schwartz AR. A simplified method for measuring critical pressures during sleep in the clinical setting. Am J Respir Crit Care Med 170: 86–93, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Patil SP, Schneider H, Marx JJ, Gladmon E, Schwartz AR, Smith PL. Neuromechanical control of upper airway patency during sleep. J Appl Physiol 102: 547–556, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Pelosi P, Croci M, Ravagnan I, Tredici S, Pedoto A, Lissoni A, Gattinoni L. The effects of body mass on lung volumes, respiratory mechanics, and gas exchange during general anesthesia. Anesth Analg 87: 654–660, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Pelosi P, Croci M, Ravagnan I, Vicardi P, Gattinoni L. Total respiratory system, lung, and chest wall mechanics in sedated-paralyzed postoperative morbidly obese patients. Chest 109: 144–151, 1996 [DOI] [PubMed] [Google Scholar]
- 21. Pelosi P, Ravagnan I, Giurati G, Panigada M, Bottino N, Tredici S, Eccher G, Gattinoni L. Positive end-expiratory pressure improves respiratory function in obese but not in normal subjects during anesthesia and paralysis. Anesthesiology 91: 1221–1231, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Schneider H, Krishnan V, Pichard LE, Patil SP, Smith PL, Schwartz AR. Inspiratory duty cycle responses to flow limitation predict nocturnal hypoventilation. Eur Respir J 33: 1068–1076, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Schwartz AR, Smith PL, Wise RA, Gold AR, Permutt S. Induction of upper airway occlusion in sleeping individuals with subatmospheric nasal pressure. J Appl Physiol 64: 535–542, 1988 [DOI] [PubMed] [Google Scholar]
- 24. Series F, Marc I. Influence of lung volume dependence of upper airway resistance during continuous negative airway pressure. J Appl Physiol 77: 840–844, 1994 [DOI] [PubMed] [Google Scholar]
- 25. Stanchina ML, Malhotra A, Fogel RB, Trinder J, Edwards JK, Schory K, White DP. The influence of lung volume on pharyngeal mechanics, collapsibility, and genioglossus muscle activation during sleep. Sleep 26: 851–856, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Suratt PM, Wilhoit SC, Hsiao HS, Atkinson RL, Rochester DF. Compliance of chest wall in obese subjects. J Appl Physiol 57: 403–407, 1984 [DOI] [PubMed] [Google Scholar]
- 27. Tagaito Y, Isono S, Remmers JE, Tanaka A, Nishino T. Lung volume and collapsibility of the passive pharynx in patients with sleep-disordered breathing. J Appl Physiol 103: 1379–1385, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Van de Graaff WB. Thoracic influence on upper airway patency. J Appl Physiol 65: 2124–2131, 1988 [DOI] [PubMed] [Google Scholar]
- 29. Widdicombe J. Reflexes from the lungs and airways: historical perspective. J Appl Physiol 101: 628–634, 2006. [DOI] [PubMed] [Google Scholar]


