Abstract
T cells contribute to hypertension in male experimental models; data in females is lacking even though women are more likely to develop immune disorders. The goal of this study was to determine whether immune cells contribute to hypertension in female spontaneously hypertensive rats (SHR) and define the T cell profile in whole blood and kidneys of male and female SHR. We hypothesized that inflammatory cells contribute to hypertension in female SHR; however, male SHR have a higher blood pressure so we hypothesize they will have a heightened inflammatory profile. The lymphocyte inhibitor mycophenolate mofetil (MMF) was administered in a dose-dependent manner to SHR. At the highest dose (50 mg·kg−1·day−1), blood pressure was significantly decreased in both sexes, yet the percent decrease in blood pressure was greater in females (female: 12 ± 1%; males: 7 ± 1%, P = 0.01). Circulating and renal T cell profiles were defined using analytical flow cytometry. Female SHR had more circulating CD3+, CD4+, and pro-inflammatory CD3+CD4+RORγ+ Th17 cells, whereas males had more immune-suppressive CD3+CD4+Foxp3+ T regulatory cells. In the kidney, females had greater numbers of CD8+ and T regulatory cells than males, whereas males had greater CD4+ and Th17 cell infiltration. MMF decreased circulating and renal T cells in both sexes (P < 0.0001), although the effect of MMF on T cell subtypes was sex specific with females having greater sensitivity to MMF-induced decreases in lymphocytes. In conclusion, there is a lymphocyte contribution to the maintenance of hypertension in the female SHR and sex of the animal impacts the T cell profile.
Keywords: mycophenylate mofetil, blood pressure, gender, T cells
several recent studies have suggested chronic inflammation is an independent risk factor for high blood pressure (25, 42, 43). In particular, the adaptive immune system has been implicated in hypertension. There is a growing body of basic science literature supporting a role for inflammatory lymphocytes in the development and maintenance of hypertension in male experimental animals (2, 22, 23, 36, 42, 44). More specifically, T cells have been shown to be necessary for male experimental animals to fully develop angiotensin (ANG) II and DOCA-salt hypertension (20, 27). There is a scarcity of data in the literature, however, regarding the role of immune cells in blood pressure regulation and hypertension in females. Although there is little direct evidence linking immune cells with hypertension in females, women are more likely than men to develop inflammatory and immunological disorders. This includes rheumatoid arthritis and systemic lupus erythematosus (56), both of which are associated with an increased risk of developing cardiovascular diseases including hypertension (9, 11, 18, 46, 47).
The majority of studies verifying a role for T cells on blood pressure control were performed using all CD3+ T cells (which includes both CD4+ and CD8+ cells). However, more recent studies have focused on various T cell subpopulations. CD3+CD4+ T cells can differentiate into pro-inflammatory T helper 17 (Th17) cells or regulatory T cells (Tregs). Th17 cells contribute to the development and maintenance of ANG II-induced hypertension (32), whereas Tregs suppress innate and adaptive immune responses and attenuate the development of hypertension in male Dahl salt-sensitive rats and ANG II hypertensive mice (26, 34, 54). As such, the balance between Th17 cells and Tregs may be an important determinant of the inflammatory profile and likely influences the overall impact of lymphocytes on blood pressure control.
It has been well established that there is an immune component to blood pressure regulation in male spontaneously hypertensive rats (SHR), an animal model of essential hypertension. Subcutaneous injection of antithymocyte serum significantly decreases blood pressure in male SHR and thymus transplantations from normotensive Wistar-Kyoto (WKY) rats into SHR attenuates the hypertension (3, 7, 15). More recently, the immunosuppressive drug mycophenolate mofetil (MMF) has been shown to normalize blood pressure in male SHR (40). MMF inhibits inosine monophosphate dehydrogenase, the rate-limiting enzyme in the de novo synthesis of guanosine nucleotides required for T and B lymphocyte production (38), thereby directly linking lymphocytes with hypertension in male SHR. However, the role of lymphocytes on blood pressure control on female SHR is unknown. Therefore, the first goal of this study was to determine the contribution of lymphocytes to blood pressure control in female SHR using MMF. To gain insight into the molecular mechanisms by which lymphocytes may regulate blood pressure, the second goal of this study was to define the circulating and renal T cell profiles in untreated and MMF-treated male and female SHR. The kidney is critical in the long-term control of blood pressure, and renal immune cell infiltration contributes to increases in blood pressure in genetic, ANG II, and salt-sensitive hypertension (12, 13, 17, 40). This study tested the hypotheses that 1) inflammatory lymphocytes contribute to the maintenance of hypertension in female SHR, and 2) male SHR have greater expression of Th17 cells while female SHR have higher frequencies of Tregs.
METHODS
Animals.
Male and female SHR were used in this study (Harlan Laboratories, Indianapolis, IN). All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved and monitored by the Georgia Health Sciences University Institutional Animal Care and Use Committee. Rats were housed in temperature- and humidity-controlled, light-cycled quarters and maintained on standard rat chow (Harlan Teklad). A subset of male and female SHR were implanted with telemetry transmitters (Data Sciences, St. Paul, MN) at 11 wk of age as previously described with the use of sterile techniques (49); control rats did not receive telemetry implants. Rats were allowed 1 wk to recover before they were placed on telemetry receivers for the measurement of baseline blood pressure. After 1 wk of baseline blood pressure data collection, rats were treated with increasing doses of MMF (Roxane Laboratories, Columbus, OH) suspended in 5% dextrose (B. Braun Medical, Irvine, CA) by intraperitoneal injection. Age-matched male (n = 7) and female SHR (n = 5) were treated with 20, 30, and 50 mg·kg−1·day−1 MMF. For both sexes each dose was given for 7 days; a blood sample was taken via tail snip at the end of the 30 mg·kg−1·day−1 treatment period. Age-matched, untreated rats served as controls. On the last day of the 50 mg·kg−1·day−1 dose rats were placed in metabolic cages to facilitate 24-h urine collection. Urinary protein excretion was determined by Bradford assay (Bio-Rad, Hercules, CA), and electrolyte analysis was performed using the EasyLyte apparatus (Medica, Beford, MA) in accordance to manufacturer's instructions. Rats were then anesthetized with ketamine-xylazine (48 and 6.4 mg/kg ip, respectively; Phoenix Pharmaceuticals, St. Joseph, MO), and a terminal blood sample was taken in the presence of heparin (Hospira, Lake Forest, IL) via aortic puncture. Kidneys were isolated and placed in ice-cold physiological buffered saline (PBS), and both kidneys and blood samples were immediately subjected to flow cytometric analyses.
Analytical flow cytometry.
Single cell suspensions of kidneys in PBS were achieved using a 100 μM cell strainer (BD Biosciences, San Diego, CA) followed by centrifugation (1,500 rpm, 10 min). Phenotypic and intracellular analyses of whole blood and renal cells were performed as described previously (4, 5). Briefly, cells were incubated with antibodies for surface markers including CD4, CD8, CD3, CD69, and CD44 (BD Biosciences) for 15 min on ice in the dark. After washing was completed, cells were fixed and permeabilized using fix/perm concentrate (eBioScience, San Diego, CA) before incubation with antibodies for intracellular staining of Foxp3 (to identify Tregs) or RORγ (to identify Th17 cells; BD Biosciences). Cells were then washed and run through a four-color flow cytometer (FACS Calibur, BD Biosciences), and data were collected using CellQuest software. Samples were double-stained with control IgG and cell markers to assess any spillover signal of fluorochromes. Proper compensation was set to ensure the median fluorescence intensities of negative and positive cells were identical and then was used to gate the population. Gating excluded dead cells and debris using forward and side scatter plots. To confirm the specificity of primary antibody binding and rule out nonspecific Fc receptor binding to cells or other cellular protein interactions, negative control experiments were conducted using isotype controls matched to each primary antibody's host species, isotype, and conjugation format. A representative scatter plot showing positive and negative staining for CD3 in kidney samples from a male and female SHR is shown in Fig. 1. The control antibodies had no specificity for target cells within our studies yet retain all the nonspecific characteristics of the antibodies used in the experiments.
Fig. 1.
Representative scatter plots in the absence (negative control; A) and presence of an antibody to CD3 (positive control; B and C) in the kidney from a male and female spontaneously hypertensive rat (SHR).
Statistical analysis.
All data are presented as means ± SE. Twenty-four hour mean arterial pressure (MAP) data between sexes were analyzed using mixed-effects regression models (MMRs) to perform repeated measures analysis with one grouping factor (sex) and one repeated factor (dose of MMF) analysis of variance (ANOVA) (Fig. 2A). The MMR-based analysis took account of the fact that each rat was dosed for 7 days within each dose group. The Tukey-Kramer method for repeated measures was used to perform pair-wise comparisons between the dose groups. Twenty-four hour MAP data within each sex were analyzed using ANOVA for repeated measurements with a Bonferroni correction (Fig. 2, B and C). Flow cytometry data in untreated and MMF-treated rats were compared using two-way ANOVA, factor 1 was sex of the animal, and factor 2 was MMF treatment. For all comparisons, differences were considered statistically significant with P < 0.05. Analyses were performed using GraphPad Prism Version 5.0 software (GraphPad Software, La Jolla, CA) and SAS 9.3 (SAS Institute, Cary, NC).
Fig. 2.
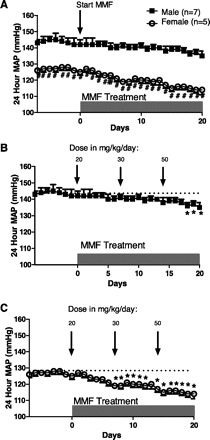
Twenty-four hour mean arterial pressure (MAP) measured by telemetry in male and female SHR (A). MAP in male SHR (n = 7) (B) and MAP in female SHR (n = 5) (C) in response to increasing doses of the lymphocyte inhibitor mycophenylate mofetil (MMF) by intraperitoneal injection beginning on day 0; rats were 13 wk of age. #Significant difference in MAP from males; *significant decrease in MAP from baseline, P < 0.05.
RESULTS
MMF decreases blood pressure in SHR.
The impact of lymphocyte suppression on 24 h MAP was determined in age-matched male and female SHR. The interaction between sex and MMF was not statistically significant (P = 0.204); however, there were significant effects of both sex (P < 0.001) and treatment (P < 0.001; Fig. 2A). Thus there was a significant difference between males and females at baseline and at each dose, with males having consistently higher blood pressure than females. Consistent with previously published reports (13, 40), male SHR displayed a dose-dependent decrease in blood pressure in response to MMF treatment (Fig. 2B). Males exhibited a significant decrease in blood pressure from baseline values at a dose of 50 mg·kg−1·day−1 (6.6 ± 1.0% decrease from baseline blood pressure). Similar to male SHR, females displayed a dose-dependent decrease in blood pressure; however, in females blood pressure significantly decreased relative to baseline values at a dose of 30 mg·kg−1·day−1 (Fig. 2C; 7.2 ± 0.9% decrease from baseline blood pressure). In response to the highest dose delivered, 50 mg·kg−1·day−1, female SHR displayed a significantly greater decrease in blood pressure from baseline (11.6 ± 1.4% decrease) compared with males (P = 0.01).
To assess the impact of MMF on indexes of renal health and function, urinary protein and electrolyte excretion were examined in untreated control and MMF-treated male and female SHR (Table 1). As previously published, control male SHR had greater proteinuria compared with control females (effect of sex: P < 0.0001) (50); however, MMF did not significantly impact protein excretion (effect of treatment: P = 0.359). Urinary Na+, K+, and Cl− were also assessed; however, neither sex nor treatment significantly impacted electrolyte excretion.
Table 1.
Urinalysis and CD3+ T cell profile of control and MMF-treated male and female SHR
| Proteinuria, mg/day | Na+ Excretion, mmol/day | K+ Excretion, mmol/day | Cl− Excretion, mmol/day | CD3+ in Blood | CD3+ in Kidney | |
|---|---|---|---|---|---|---|
| Male SHR | 19.7 ± 2.1 | 1.3 ± 0.1 | 4.5 ± 0.4 | 2.6 ± 0.2 | 7.0 ± 0.5 | 8.7 ± 1.0 |
| Male + MMF | 14.2 ± 2.6 | 0.6 ± 0.2 | 2.9 ± 0.6 | 1.7 ± 0.4 | 1.0 ± 0.1* | 2.6 ± 0.4* |
| Female SHR | 2.9 ± 1.0# | 1.3 ± 0.3 | 3.5 ± 0.5 | 2.0 ± 0.3 | 12.0 ± 0.7# | 6.8 ± 0.7 |
| Female + MMF | 4.3 ± 0.5 | 1.0 ± 0.1 | 3.3 ± 0.3 | 1.7 ± 0.3 | 2.0 ± 0.4* | 3.5 ± 0.5* |
Rats were 16 wk of age at urine collection. Samples from mycophenylate mofetil (MMF)-treated rats were taken after the 50 mg•kg−1•day−1 dose. CD3+ cells in whole blood are expressed as a percentage of total blood cells, CD3+ cells in the kidney are expressed as a percentage of total kidney cells. n = 4–6. #Difference from male spontaneously hypertensive rats (SHR).
Difference from same sex control.
Sex of the animal influences the impact of MMF on the T cell profile in whole blood.
Additional studies were performed to determine the circulating T cell profile in male and female SHR under basal conditions and following MMF treatment. Analytical flow cytometry was used to assess the levels of pan T cells (CD3+ cells), CD4+ T cells (CD4+CD3+), CD8+ cells (CD8+CD3+), regulatory T cells (Tregs, CD3+CD4+Foxp3+), and Th17 cells (CD3+CD4+IL-17+RORγ+) in whole blood. Interestingly, female SHR had significantly more pan T cells than male SHR, expressed as a percentage of whole blood cells (Table 1, effect of sex: P < 0.0001). T cells were further characterized as either CD4+ or CD8+. Female SHR had significantly more circulating CD4+ T cells than males (effect of sex: P = 0.0237), although CD8+ T cell numbers were comparable (Fig. 3, A and B, respectively; effect of sex: P = 0.1730). Both Tregs and Th17 cells have been implicated in blood pressure control (26, 32), therefore, expression of these individual T cell populations in whole blood was further determined. Male SHR had more circulating Tregs than females (Fig. 3C; effect of sex: P = 0.0564), but female SHR had significantly more circulating Th17 cells than male SHR (Fig. 3D; effect of sex: P < 0.0001).
Fig. 3.
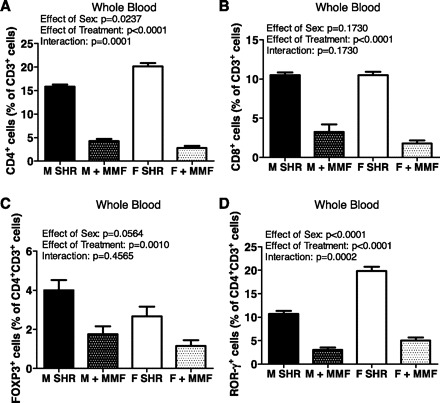
Circulating T cell profiles in 16-wk-old male and female SHR. Samples from MMF-treated rats were taken after the 50 mg·kg−1·day−1 dose. Shown are the percentage of CD4+ T cells (A), CD8+ T cells (B), Foxp3+ Tregs (C), and RORγ+ Th17 cells (D). Untreated male and female SHR, n = 6; MMF-treated male SHR, n = 4; MMF-treated female SHR, n = 5.
Additional experiments were initiated to begin to probe the molecular mechanism by which MMF lowers blood pressure in SHR by using analytical flow cytometry to assess the levels of T cells following MMF treatment. There was not a significant difference in the degree of T cell suppression following 30 mg·kg−1·day−1 compared with 50 mg·kg−1·day−1 (data not shown); therefore, all data presented were obtained at the end of the study following the highest dose of MMF. Consistent with efficacious administration of MMF, both sexes exhibited a significant decrease in circulating pan T cells, CD4+ T cells, and CD8+ T cells following mg·kg−1·day−1 MMF (Table 1, Fig. 3, A and B, respectively; for all comparisons effect of treatment: P < 0.0001). MMF resulted in a greater decrease in CD3+ and CD4+ T cells in female SHR compared with males (interaction: P = 0.0011 and P = 0.0001, respectively). The decrease in CD8+ T cells was comparable between the sexes (interaction: P = 0.1730). MMF significantly decreased circulating Foxp3+ cells in the male and female SHR (Fig. 3C; effect of treatment: P = 0.001), and the decrease was comparable in each sex (interaction; P = 0.4565). Circulating Th17 cells were also significantly decreased by MMF in both male and female SHR (Fig. 3D; effect of treatment: P < 0.001), although female SHR were more sensitive to MMF suppression of Th17 cells than males (interaction: P = 0.0002).
Sex of the animal influences the impact of MMF on the T cell profile in kidneys.
The kidney is critical in the long-term control of blood pressure, and T cell infiltration in the kidney has been demonstrated to impact blood pressure in male experimental animals (12, 13, 17, 40). Therefore, analytical flow cytometry was used to assess the T cell profile in kidneys of untreated and MMF-treated male and female SHR. Pan T cell renal infiltration was comparable in male and female SHR (Table 1; effect of sex: P = 0.54). Male SHR had significantly more CD4+ T cells in their kidneys than females (Fig. 4A; effect of sex: P = 0.002), although females had more CD8+ T cell infiltration than males (Fig. 4B; effect of sex: P = 0.0459). Consistent with female SHR having lower blood pressure under baseline conditions than male SHR, females had greater Treg infiltration (effect of sex: P = 0.0006), whereas Th17 infiltration was greater in the kidneys of males (Fig. 4, C and D; effect of sex: P < 0.0001).
Fig. 4.
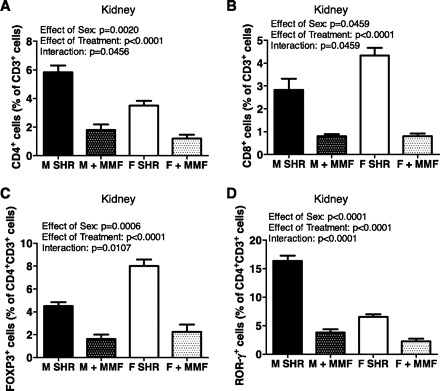
Infiltrating T cell profiles in the kidney of 16-wk-old male and female SHR. Samples from MMF-treated rats were taken after the 50 mg·kg−1·day−1 dose. Shown are the percentage of CD4+ T cells (A), CD8+ T cells (B), Foxp3+ Tregs (C), and RORγ+ Th17 cells (D). Untreated male and female SHR, n = 6; MMF-treated male SHR, n = 4; MMF-treated female SHR, n = 5.
MMF (50 mg·kg−1·day−1) significantly reduced the total number of pan T cells, CD4+, and CD8+ T cells compared with untreated controls in both male and female SHR (Table 1, Fig. 4, A and B; for all comparisons effect of treatment: P < 0.0001). Similar to whole blood, there were sex differences in the effectiveness of MMF to decrease total pan T cells (interaction: P = 0.0847), CD4+ (interaction: P = 0.0456), and CD8+ T cells (interaction: P = 0.0459) in kidneys of females compared with males. MMF significantly decreased the number of infiltrating Th17 cells and Tregs in males and females (Fig. 4, C and D; for both comparisons effect of treatment: P < 0.0001), although the decrease in Tregs was greater in females (interaction: P = 0.0107), whereas the decrease in Th17 cells was greater in males (interaction: P < 0.0001).
Sex of the animal influences cellular T cell activation markers.
CD44 and CD69 levels were assessed in whole blood and kidneys of untreated and MMF-treated male and female SHR to assess T cell activation. CD69 is a lymphoid activation marker that is rapidly induced following the activation of T cells (55). CD44 is a T cell activation molecule involved in cell adhesion and migration (19). CD69 was more highly expressed on circulating CD4+CD3+ T cells in male SHR than in female SHR (Fig. 5A; effect of sex: P = 0.010); however, there were comparable numbers of CD69+ expressing T cells infiltrating the kidney of males and females (Fig. 5B; effect of sex: P = 0.1233). In contrast, CD44 was more highly expressed in the circulation of female SHR compared with male SHR (Fig. 5C; effect of sex: P = 0.0056), but there were fewer CD44+ expressing T cells in the kidneys of female SHR compared with male SHR (Fig. 5D; effect of sex: P < 0.0001). MMF treatment significantly decreased the numbers of CD69+ and CD44+ expressing T cells in the circulation and kidneys of both sexes (for all comparisons effect of treatment: P < 0.0001). MMF resulted in greater decreases in renal CD69+ expressing T cells and circulating CD44+ cells in female SHR compared with males (interactions: P = 0.0032 and P = 0.0028, respectively), whereas males had a greater decrease in renal CD44+ T cells (interaction: P < 0.0001).
Fig. 5.
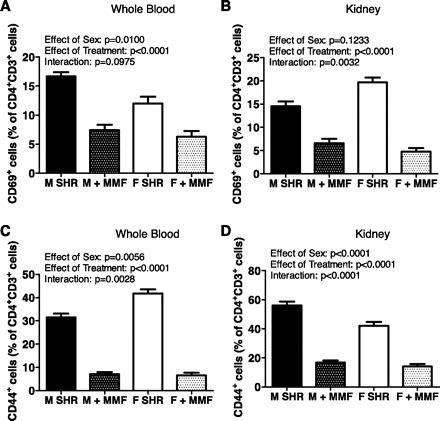
CD44 and CD69 T cell profiles in whole blood (A and C) and kidneys (B and D) of 16-wk-old male and female SHR. Samples from MMF-treated rats were taken after the 50 mg·kg−1·day−1 dose. Untreated male and female SHR, n = 6; MMF-treated male SHR, n = 4; MMF-treated female SHR, n = 5.
DISCUSSION
The idea that an immune response contributes to hypertension is not new; however, only in recent years with our expanded knowledge of the role of T cell subtypes in blood pressure control have the molecular mechanisms responsible begun to be elucidated. While we have learned a great deal of new information, the vast majority of the studies to date have focused on males. The primary novel finding of this study is that immune cells also contribute to the maintenance of hypertension in female experimental models of essential hypertension. Indeed, females exhibited a greater percent decrease in blood pressure from baseline in response to MMF than males. In addition, there is a sex difference in the circulating and renal T cell profiles under basal conditions. Of potential greatest interest, female SHR have more Tregs while males have more Th17 cells infiltrating the kidney. There is likewise a sex difference in lymphocyte sensitivity to MMF. Female SHR has greater decreases in circulating CD4+ and Th17 cells and renal CD8+ cells, which likely contributes to the greater sensitivity to the blood pressure-lowering effects of MMF in female SHR.
Early studies examining the role of the adaptive immune system in hypertension in SHR suggested that dysregulation of immune system function, specifically T cells, contributed to elevations in blood pressure. Pioneering investigators implicated T cells in hypertension in SHR using thymus transplants from normotensive WKY rats (3, 15, 37). Thymus grafts from male WKY significantly lowered blood pressure in male SHR demonstrating that T cells are critical in the development of hypertension in SHR. More recently, studies have examined immune cell infiltration and observed greater renal T cell and macrophage infiltration in 3-wk-old prehypertensive male SHR compared with age-matched normotensive WKY (8, 39). In addition, treatment of adult male SHR with the lymphocyte inhibitor MMF normalizes blood pressure to levels seen in WKY (40), thereby verifying a role for inflammatory lymphocytes in hypertension in SHR. Consistent with these data, in the current study male SHR experienced a significant decrease in blood pressure following MMF treatment.
We also found female SHR to have a dose-dependent decrease in blood pressure in response to MMF. Our result is consistent with previous studies showing that Concanavalin A, a lectin that stimulates precursor T cell development into suppressor cells, lowers blood pressure in male and female SHR, supporting an immune component to hypertension in female SHR (7, 14). Interestingly, in the current study females experienced a greater percent decrease in blood pressure from baseline compared with male SHR in response to MMF. It should be noted, however, that in the current study the impact of MMF within each sex was determined based on the percent decrease in blood pressure from baseline during the study. We have previously published that over this same age range (12–16 wk of age), blood pressure in male SHR increases by ∼5 mmHg while blood pressure in female SHR is stable (50). Therefore, the experimental design of the current study may underestimate the relative effectiveness of MMF in male SHR. It was recently reported that perinatal inhibition of the inflammatory transcription factor nuclear factor-κB significantly delays the age-related increase in blood pressure in both male and female SHR offspring, supporting an inflammatory component to hypertension in both sexes of SHR (28). Female offspring of treated dams maintained a lower blood pressure compared with offspring born to untreated dams, and this reduction in blood pressure persisted for at least 7 mo. Male offspring of treated dams also experienced a reduction in blood pressure; however, this attenuation in blood pressure was only maintained for 4 mo. These data further support the hypothesis that female SHR are more sensitive to inflammatory-mediated increases in blood pressure. A recent small population clinical study monitored blood pressure in five hypertensive women and three hypertensive men who were taking MMF for the treatment of psoriasis or rheumatoid arthritis (24). The authors found that 3 mo of MMF treatment significantly decreased the average systolic blood pressure from 153 ± 7 to 136 ± 5 mmHg. Once the patients stopped taking MMF their blood pressures were restored to pretreatment levels. This study highlights the potential to use lymphocyte suppression as an effective means of lowering blood pressure in hypertensive women. Indeed, in a female mouse model of systemic lupus erythematosus, treatment with an inhibitor of the inflammatory cytokine tumor necrosis factor α etanercept decreases blood pressure (53). Yet, to our knowledge, there are no additional studies that have specifically reported the impact of immunosuppressive therapy on blood pressure in a strictly hypertensive female population.
To begin to better define the role of lymphocytes in blood pressure control in SHR, additional studies characterized the T cell profile in whole blood and in the kidney of male and female SHR. Our study was designed to examine total CD3+ cells, CD4+CD3+, and CD8+CD3+ T cell populations. It has been suggested that CD4+ T cells play a greater role in the development of hypertension than CD8+ T cells. In both male mice infused with ANG II and in male SHR, the kidney and aorta have higher levels of CD4+ T cell infiltration than CD8+ T cells (20, 39). CD4+ T cells have also been implicated in human hypertension. There is a statistically lower incidence of hypertension in HIV+ men deficient in CD4+ T cells compared with the general population, and increases in CD4+ T cell counts with anti-retroviral drugs increases the incidence of hypertension (45). Consistent with infiltrating CD4+ T cells modulating blood pressure, male SHR have a higher blood pressure than female SHR at baseline conditions, and male SHR have greater CD4+ T cell infiltration in the kidney compared with females. Our data are consistent with other studies in the literature linking renal immune cell infiltration with a number of hypertensive models including ANG II-mediated and salt-sensitive hypertension (12, 13, 17). Our laboratory has previously published that male SHR have significantly greater macrophage infiltration in the renal cortex than female SHR under baseline conditions (49, 50) and that CD3+ pan T cell renal infiltration is comparable between male and female SHR (49). However, in this latter study we did not assess T cell subtypes. It was surprising that female SHR had greater numbers of circulating CD4+ T cells than males, yet fewer infiltrating the kidney. Based on these results, we speculate that there is a sex difference in endothelial cell activation and T cell adhesion, which blunts immune cell infiltration in females. Alternatively, greater circulating immune cells in females may also exist as a mechanism to establish and maintain peripheral immune tolerance, limiting inflammatory responses and preventing further autoimmune disease. Circulating and renal CD8+ T cell numbers were comparable between the sexes under both control conditions and following MMF treatment. Recent studies have suggested that CD8+ T cell subpopulations may contribute to establishing and maintaining the immune homeostasis and tolerance (16); however, our experimental approach did not further define the CD8+ T cell population since there were no apparent sex differences. Future studies will address the functional implications of the observed sex differences in the T cell profiles on both blood pressure regulation and tissue infiltration. Cross regulation between tissue and peripheral T cell populations may be an important overall determinant of the impact of T cells on cardiovascular health.
Recent data has emerged suggesting that T cell subpopulations influence hypertension and subsequent organ injury. Tregs have been suggested to limit vascular injury and protect against pulmonary hypertension (51). In addition, adoptive transfer of Tregs protects male mice from ANG II-mediated cardiac (29) and vascular damage (6), mitigates elevations in blood pressure and renal infiltration in aldosterone-treated male mice (26), and decreases BP in Dahl salt-sensitive rats with chromosome 2 from a Brown-Norway rat (54). In contrast, male interleukin (IL)-17 knockout mice are not able to sustain ANG II-mediated increases in blood pressure, suggesting that IL-17 is necessary to maintain ANG II hypertension and IL-17 is the primary cytokine released by Th17 cells (32). Consistent with Tregs being associated with lowering blood pressure, female SHR had greater numbers of Tregs in the kidney. Consistent with Th17 cells being prohypertensive, male SHR had greater numbers of infiltrating Th17 cells. Interestingly, female SHR had more circulating Th17 cells and more renal Tregs, whereas males had more circulating Tregs and more infiltrating Th17 cells in the kidney. This disparity makes it tempting to speculate that the total numbers of cells (blood plus tissue) are the same in each sex, the sex difference is more in where those cells partition to.
Females exhibited a greater decrease in blood pressure in response to MMF than males. Based on the impact of MMF on the T cell profile in male versus female SHR, we postulate that this is the result of sex differences in sensitivity to MMF-induced inhibition of lymphocytes as opposed to greater dependence of female SHR on immune cells to mediate their hypertension. In particular, female SHR had fewer infiltrating CD4+ T cells and Th17 cells following MMF than males, while males tended to retain more Tregs. It is not that surprising that MMF differentially regulates the balance of the T cell profile; however, the sex difference is intriguing. Treatment with the immunosuppressant tacrolimus decreased Tregs and increased Th17 cells in the spleens of male mice and was associated with a significant increase in systolic blood pressure (10). Moreover, it has been established that in patients with systemic lupus erythematosus, which are primarily women, there is an imbalance in the T cell subpopulations with an elevation in Th17 cells and decreased numbers of Tregs (31, 58, 59).
To assess T cell activation, CD44 and CD69 were measured, both of which have been associated with increases in tissue homing and T cell accumulation (20, 30). CD44 also maintains tissue structure via cell to cell interaction and is considered a marker of cellular adhesion (19). We expected that males would have higher levels of both CD69 and CD44 consistent with a more pro-inflammatory T cell profile and greater T cell infiltration. Consistent with this hypothesis male SHR did have greater levels of CD44+ T cells in the kidney; however, females had more CD69+ cells. Interestingly, data in the literature suggests that a subpopulation of CD69+ T cells act as Tregs and suppress effector T cell proliferation (21, 33). In addition, females tended to be more sensitive to MMF inhibition of CD44+ and CD69+ cells, which may further contribute to the greater decrease in blood pressure relative to males. Additional studies are required to more fully characterize the CD69+CD4+ T cells to determine whether these cells are the same in males and females and their role in T cell infiltration.
The mechanism responsible for the observed sex differences in T cells is currently unknown; however, it is tempting to speculate that sex hormones contribute. Sex hormones influence the immune system, although there are contradictory reports regarding the direction of the sex hormone effect. The majority of the evidence indicates that testosterone is immunosuppressive, whereas estrogen has been shown to be both pro-inflammatory (48) and an anti-inflammatory modulator (35, 57). In normotensive male mice, testosterone shifts T cell differentiation toward the formation of Tregs thereby suppressing the pro-inflammatory response, whereas estrogen activates helper T cell differentiation into CD4+ T cells and also upregulates pro-inflammatory responses (1, 52).
Perspectives and Significance
Although it is well established that there is a sexual dimorphism in the blood pressure of sexually mature hypertensive SHR (50) and humans (41), the molecular mechanisms responsible are still being investigated. Although many different pathways have been implicated in the sex difference in blood pressure in SHR, the experimental approach to the question has always been the same. It is typically assumed that lower levels of blood pressure in females is due to greater levels of protective, vasodilatory factors and/or lower levels of pro-hypertensive factors. It is possible that the sex difference in blood pressure is more related to the fact that the mechanisms that regulate blood pressure in the two sexes are different; therefore the level of the increase in pressure is different. Perhaps in females the increase in blood pressure is more dependent on a defect in the ability to suppress pro-inflammatory T cell infiltration into organs or functional abnormalities of Tregs in suppressing effector T cells. If so, women in particular may benefit from the inclusion of an immunosuppressant to control their hypertension. Additionally since advances in immunology have afforded the opportunity to examine and define the various T cell subpopulations, namely, Th17 and Tregs, it is feasible that direct therapeutic targets should focus on these cell types in the treatment of hypertension.
GRANTS
Intramural grant from Georgia Health Sciences University (to JCS) and a William Townsend Porter Pre-doctoral Fellowship from the American Physiological Society (to AJT).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.J.T., B.B., and J.C.S. conception and design of research; A.J.T. and B.B. performed experiments; A.J.T. and B.B. analyzed data; A.J.T., B.B., and J.C.S. interpreted results of experiments; A.J.T. prepared figures; A.J.T. drafted manuscript; A.J.T., B.B., and J.C.S. edited and revised manuscript; A.J.T., B.B., and J.C.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge the excellent technical assistance of G. Ryan Crislip, Vanessa Kemp, and Hiram Ocasio.
REFERENCES
- 1.Ahmed SA, Karpuzoglu E, Khan D. Effects of sex steroids on innate and adaptive immunity. In: Sex Hormones and Immunity to Infection, edited by Klein SL, Roberts CW. New York: Springer, 2010, p. 19–51 [Google Scholar]
- 2.Androulakis ES, Tousoulis D, Papageorgiou N, Tsioufis C, Kallikazaros I, Stefanadis C. Essential hypertension: is there a role for inflammatory mechanisms? Cardiology Rev 17: 216–221, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Ba D, Takeichi N, Kodama T, Kobayashi H. Restoration of T cell depression and suppression of blood pressure in spontaneously hypertensive rats (SHR) by thymus grafts or thymus extracts. J Immunol 128: 1211–1216, 1982 [PubMed] [Google Scholar]
- 4.Baban B, Chandler PR, Johnson BA, 3rd, Huang L, Li M, Sharpe ML, Francisco LM, Sharpe AH, Blazar BR, Munn DH, Mellor AL. Physiologic control of IDO competence in splenic dendritic cells. J Immunol 187: 2329–2335, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baban B, Chandler PR, Sharma MD, Pihkala J, Koni PA, Munn DH, Mellor AL. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol 183: 2475–2483, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension 57: 469–476, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Bendich A, Belisle EH, Strausser HR. Immune system modulation and its effect on the blood pressure of the spontaneously hypertensive male and female rat. Biochem Biophys Res Commun 99: 600–607, 1981 [DOI] [PubMed] [Google Scholar]
- 8.Biswas SK, de Faria JB. Which comes first: renal inflammation or oxidative stress in spontaneously hypertensive rats? Free Radic Res 41: 216–224, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Budman DR, Steinberg AD. Hypertension and renal disease in systemic lupus erythematosus. Arch Intern Med 136: 1003–1007, 1976 [PubMed] [Google Scholar]
- 10.Chiasson VL, Talreja D, Young KJ, Chatterjee P, Banes-Berceli AK, Mitchell BM. FK506 binding protein 12 deficiency in endothelial and hematopoietic cells decreases regulatory T cells and causes hypertension. Hypertension 57: 1167–1175, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chogle AR, Chakravarty A. Cardiovascular events in systemic lupus erythematosus and rheumatoid arthritis : emerging concepts, early diagnosis and management. J Assoc Physicians India 55: 32–40, 2007 [PubMed] [Google Scholar]
- 12.Crowley SD, Frey CW, Gould SK, Griffiths R, Ruiz P, Burchette JL, Howell DN, Makhanova N, Yan M, Kim HS, Tharaux PL, Coffman TM. Stimulation of lymphocyte responses by angiotensin II promotes kidney injury in hypertension. Am J Physiol Renal Physiol 295: F515–F524, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Miguel C, Guo C, Lund H, Feng D, Mattson DL. Infiltrating T lymphocytes in the kidney increase oxidative stress and participate in the development of hypertension and renal disease. Am J Physiol Renal Physiol 300: F734–F742, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dwyer JM, Johnson C. The use of concanavalin A to study the immunoregulation of human T cells. Clin Exp Immunol 46: 237–249, 1981 [PMC free article] [PubMed] [Google Scholar]
- 15.Dzielak DJ. Immune mechanisms in experimental and essential hypertension. Am J Physiol Regul Integr Comp Physiol 260: R459–R467, 1991 [DOI] [PubMed] [Google Scholar]
- 16.Endharti AT, Okuno Y, Shi Z, Misawa N, Toyokuni S, Ito M, Isobe K, Suzuki H. CD8+CD122+ regulatory T cells (Tregs) and CD4+ Tregs cooperatively prevent and cure CD4+ cell-induced colitis. J Immunol 186: 41–52, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Franco M, Martinez F, Quiroz Y, Galicia O, Bautista R, Johnson RJ, Rodriguez-Iturbe B. Renal angiotensin II concentration and interstitial infiltration of immune cells are correlated with blood pressure levels in salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol 293: R251–R256, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Gerli R, Goodson NJ. Cardiovascular involvement in rheumatoid arthritis. Lupus 14: 679–682, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Goodison S, Urquidi V, Tarin D. CD44 cell adhesion molecules. Mol Pathol 52: 189–196, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han Y, Guo Q, Zhang M, Chen Z, Cao X. CD69+ CD4+ CD25- T cells, a new subset of regulatory T cells, suppress T cell proliferation through membrane-bound TGF-beta 1. J Immunol 182: 111–120, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, hypertension. Hypertension 57: 132–140, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrison DG, Vinh A, Lob H, Madhur MS. Role of the adaptive immune system in hypertension. Curr Opin Pharmacol 10: 203–207, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrera J, Ferrebuz A, MacGregor EG, Rodriguez-Iturbe B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol 17: S218–S225, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Johnson RJ, Rodriguez-Iturbe B, Kang DH, Feig DI, Herrera-Acosta J. A unifying pathway for essential hypertension. Am J Hypertens 18: 431–440, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Kasal DA, Barhoumi T, Li MW, Yamamoto N, Zdanovich E, Rehman A, Neves MF, Laurant P, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent aldosterone-induced vascular injury. Hypertension 59: 324–330, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Ko EA, Amiri F, Pandey NR, Javeshghani D, Leibovitz E, Touyz RM, Schiffrin EL. Resistance artery remodeling in deoxycorticosterone acetate-salt hypertension is dependent on vascular inflammation: evidence from m-CSF-deficient mice. Am J Physiol Heart Circ Physiol 292: H1789–H1795, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Koeners MP, Braam B, Joles JA. Perinatal inhibition of NF-kappaB has long-term antihypertensive effects in spontaneously hypertensive rats. J Hypertens 29: 1160–1166, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Kvakan H, Kleinewietfeld M, Qadri F, Park JK, Fischer R, Schwarz I, Rahn HP, Plehm R, Wellner M, Elitok S, Gratze P, Dechend R, Luft FC, Muller DN. Regulatory T cells ameliorate angiotensin II-induced cardiac damage. Circulation 119: 2904–2912, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Lob HE, Marvar PJ, Guzik TJ, Sharma S, McCann LA, Weyand C, Gordon FJ, Harrison DG. Induction of hypertension and peripheral inflammation by reduction of extracellular superoxide dismutase in the central nervous system. Hypertension 55: 277–283, 276p following 283, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma J, Yu J, Tao X, Cai L, Wang J, Zheng SG. The imbalance between regulatory and IL-17-secreting CD4+ T cells in lupus patients. Clin Rheumatol 29: 1251–1258, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 55: 500–507, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin P, Sanchez-Madrid F. CD69: an unexpected regulator of TH17 cell-driven inflammatory responses. Sci Signal 4: pe14, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Matrougui K, Abd Elmageed Z, Kassan M, Choi S, Nair D, Gonzalez-Villalobos RA, Chentoufi AA, Kadowitz P, Belmadani S, Partyka M. Natural regulatory T cells control coronary arteriolar endothelial dysfunction in hypertensive mice. Am J Pathol 178: 434–441, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller AP, Xing D, Feng W, Fintel M, Chen YF, Oparil S. Aged rats lose vasoprotective and anti-inflammatory actions of estrogen in injured arteries. Menopause 14: 251–260, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Montecucco F, Pende A, Quercioli A, Mach F. Inflammation in the pathophysiology of essential hypertension. J Nephrol 24: 23–34, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Norman RA, Jr, Dzielak DJ. Spontaneous hypertension is primarily the result of sympathetic overactivity and immunologic dysfunction. Proc Soc Exp Biol Med Soc USA 182: 448–453, 1986 [DOI] [PubMed] [Google Scholar]
- 38.Ransom JT. Mechanism of action of mycophenolate mofetil. Ther Drug Monit 17: 681–684, 1995 [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez-Iturbe B, Quiroz Y, Ferrebuz A, Parra G, Vaziri ND. Evolution of renal interstitial inflammation and NF-kappaB activation in spontaneously hypertensive rats. Am J Nephrol 24: 587–594, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez-Iturbe B, Quiroz Y, Nava M, Bonet L, Chavez M, Herrera-Acosta J, Johnson RJ, Pons HA. Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. Am J Physiol Renal Physiol 282: F191–F201, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Sandberg K, Ji H. Sex and the renin angiotensin system: implications for gender differences in the progression of kidney disease. Adv Ren Replace Ther 10: 15–23, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Savoia C, Schiffrin EL. Inflammation in hypertension. Curr Opin Nephrol Hypertens 15: 152–158, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Savoia C, Schiffrin EL. Reduction of C-reactive protein and the use of anti-hypertensives. Vasc Health Risk Manage 3: 975–983, 2007 [PMC free article] [PubMed] [Google Scholar]
- 44.Schiffrin EL. T lymphocytes: a role in hypertension? Curr Opin Nephrol Hypertens 19: 181–186, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Seaberg EC, Munoz A, Lu M, Detels R, Margolick JB, Riddler SA, Williams CM, Phair JP. Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS 19: 953–960, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Selzer F, Sutton-Tyrrell K, Fitzgerald S, Tracy R, Kuller L, Manzi S. Vascular stiffness in women with systemic lupus erythematosus. Hypertension 37: 1075–1082, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Serelis J, Panagiotakos DB, Mavrommati M, Skopouli FN. Cardiovascular disease is related to hypertension in patients with rheumatoid arthritis: a greek cohort study. J Rheumatol 38: 236–241, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Snider H, Lezama-Davila C, Alexander J, Satoskar AR. Sex hormones and modulation of immunity against leishmaniasis. Neuroimmunomodulation 16: 106–113, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sullivan JC, Bhatia K, Yamamoto T, Elmarakby AA. Angiotensin (1–7) receptor antagonism equalizes angiotensin II-induced hypertension in male and female spontaneously hypertensive rats. Hypertension 56: 658–666, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sullivan JC, Semprun-Prieto L, Boesen EI, Pollock DM, Pollock JS. Sex and sex hormones influence the development of albuminuria and renal macrophage infiltration in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 293: R1573–R1579, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Tamosiuniene R, Tian W, Dhillon G, Wang L, Sung YK, Gera L, Patterson AJ, Agrawal R, Rabinovitch M, Ambler K, Long CS, Voelkel NF, Nicolls MR. Regulatory T cells limit vascular endothelial injury and prevent pulmonary hypertension. Circ Res 109: 867–879, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanriverdi F, Silveira LF, MacColl GS, Bouloux PM. The hypothalamic-pituitary-gonadal axis: immune function and autoimmunity. J Endocrinol 176: 293–304, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Venegas-Pont M, Manigrasso MB, Grifoni SC, LaMarca BB, Maric C, Racusen LC, Glover PH, Jones AV, Drummond HA, Ryan MJ. Tumor necrosis factor-alpha antagonist etanercept decreases blood pressure and protects the kidney in a mouse model of systemic lupus erythematosus. Hypertension 56: 643–649, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Viel EC, Lemarie CA, Benkirane K, Paradis P, Schiffrin EL. Immune regulation and vascular inflammation in genetic hypertension. Am J Physiol Heart Circ Physiol 298: H938–H944, 2010 [DOI] [PubMed] [Google Scholar]
- 55.Werfel T, Boeker M, Kapp A. Rapid expression of the CD69 antigen on T cells and natural killer cells upon antigenic stimulation of peripheral blood mononuclear cell suspensions. Allergy 52: 465–469, 1997 [DOI] [PubMed] [Google Scholar]
- 56.Whitacre CC. Sex differences in autoimmune disease. Nature Immunol 2: 777–780, 2001 [DOI] [PubMed] [Google Scholar]
- 57.Xing D, Miller A, Novak L, Rocha R, Chen YF, Oparil S. Estradiol and progestins differentially modulate leukocyte infiltration after vascular injury. Circulation 109: 234–241, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Yang J, Chu Y, Yang X, Gao D, Zhu L, Yang X, Wan L, Li M. Th17 and natural Treg cell population dynamics in systemic lupus erythematosus. Arthritis Rheum 60: 1472–1483, 2009 [DOI] [PubMed] [Google Scholar]
- 59.Yang J, Yang X, Zou H, Chu Y, Li M. Recovery of the immune balance between Th17 and regulatory T cells as a treatment for systemic lupus erythematosus. Rheumatology (Oxford, England) 50: 1366–1372, 2011 [DOI] [PubMed] [Google Scholar]


