Abstract
Cannabinoid receptor CB2 activation inhibits inflammatory proliferation and migration of vascular smooth muscle cells in vitro. The potential in vivo relevance of these findings is unclear. We performed carotid balloon distension injury in hypercholesterolemic apolipoprotein E knockout (ApoE−/−) mice receiving daily intraperitoneal injection of the CB2 agonist JWH133 (5 mg/kg) or vehicle, with the first injection given 30 min before injury. Alternatively, we subjected CB2−/− and wild-type (WT) mice to balloon injury. We determined CB2 mRNA and protein expression in dilated arteries of ApoE−/− mice. Neointima formation was assessed histologically. We used bone marrow-derived murine CB2−/− and WT macrophages to study adhesion to plastic, fibronectin, or collagen, and migration was assayed by modified Boyden chamber. Aortic smooth muscle cells were isolated to determine in vitro proliferation rates. We found increased vascular CB2 expression in ApoE−/− mice in response to balloon injury. Seven to twenty-one days after dilatation, injured vessels of JWH133-treated mice had less intimal nuclei numbers as well as intimal and medial areas, associated with less staining for proliferating cells, smooth muscle cells, and macrophages. Complete endothelial repair was observed after 14 days in both JWH133- and vehicle-treated mice. CB2 deficiency resulted in increased intima formation compared with WT, whereas JWH133 did not affect intimal formation in CB2−/− mice. Apoptosis rates assessed by in situ terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling staining 1 h postballooning were significantly higher in the CB2 knockouts. In vitro, bone marrow-derived CB2−/− macrophages showed enhanced adherence and migration compared with WT cells and elevated mRNA levels of adhesion molecules, chemokine receptors CCR1 and 5, and chemokine CCL2. Proliferation rates were significantly increased in CB2−/− smooth muscle cells compared with WT. In conclusion, pharmacological activation or genetic deletion of CB2 receptors modulate neointima formation via protective effects in macrophages and smooth muscle cells.
Keywords: restenosis, smooth muscle cells, macrophages
percutaneous transluminal angioplasty in humans is an invasive procedure aimed to reestablish patency in a stenotic atherosclerotic artery by inflating a balloon-tipped catheter at the site of arterial narrowing (5). This procedure is often followed by insertion of a stent into the dilated blood vessel to prevent shrinkage-induced renarrowing. Unfortunately, balloon- or stent-induced vascular lesions leading to restenosis still limit the long-term success rate of these interventions (11).
The pathophysiology of restenosis involves endothelial necrosis and apoptosis, leukocyte recruitment, and release of proinflammatory cytokines such as interleukin (IL)-1, tumor necrosis factor (TNF)-α, and platelet-derived growth factor (PDGF), which promote an exacerbated proliferation and migration of smooth muscle cells from the media to the intima. Recent efforts to limit neointima formation have focused on pharmacological inhibition of smooth muscle cell proliferation (10). Although stents eluting antiproliferative agents such as sirolimus or paclitaxel reduce the rates of restenosis compared with bare metal stents, delayed healing, characterized by persistent fibrin deposition and incomplete reendothelialization, may lead to late thrombosis, which has emerged as a major safety concern (6, 12, 34, 36).
Synthetic cannabinoids selectively activating CB2 cannabinoid receptors have been shown to reduce TNF-α-induced proliferation and migration of human coronary artery smooth muscle cells in vitro (30). CB2 receptors belong to the endocannabinoid system that includes at least two G-protein-coupled receptors, CB1 and CB2, as well as endogenous ligands known as endocannabinoids (8). CB2 receptors are expressed on arterial endothelial and smooth muscle cells and immune cells (27). There is accumulating evidence that activation of CB2 receptors with endogenous or synthetic ligands limits inflammatory responses such as endothelial activation, immune cell adhesion, and migration, which are relevant for the pathogenesis of atherosclerosis and restenosis (4, 23, 26, 29, 35, 40, 41).
In the present study, we investigated the role of CB2 receptors in balloon-induced arterial injury. We assessed the effects of pharmacological CB2 activation on neointima formation in apolipoprotein E knockout (ApoE−/−) mice fed a high-cholesterol diet. Hypercholesterolemic ApoE−/− mice treated with CB2-selective synthetic cannabinoid JWH133 exhibited reduced neointima and media formation, proliferation, and smooth muscle cell and macrophage content compared with vehicle-treated littermates. We further tested a genetic loss-of-function strategy by performing balloon distension injury in normocholesterolemic CB2−/− and wild-type (WT) mice. CB2 deficiency was associated with increased intima formation in response to balloon-induced injury. The receptor selectivity of the CB2 agonist was demonstrated in CB2−/− mice. In vitro experiments confirmed the involvement of CB2-mediated effects in both smooth muscle cells and macrophages.
MATERIALS AND METHODS
Animals.
We used weight-matched (25–30 g) 3-mo-old male ApoE−/−, CB2−/−, and WT (C57BL/6) mice. ApoE−/− mice were fed with high-cholesterol diet (1.25% cholesterol) starting 2 wk before balloon injury. For the pharmacological approach, ApoE−/− mice were treated with the CB2-selective synthetic cannabinoid agonist JWH133 (5 mg·kg−1·day−1 ip; Tocris) or vehicle (Tocrisolve; Tocris) with the first injection given 30 min before balloon-induced injury, followed by daily intraperitoneal administration. For the knockout approach, untreated WT and CB2−/− mice or CB2−/− mice injected with JWH133 or vehicle as described for the ApoE−/− mice were used. Seven days before balloon injury, all mice received aspirin via drinking water (16 mg·kg−1·day−1) to prevent acute thrombus formation. All animal studies were approved by the Swiss Federal Veterinary Office and correspond to APS's Guiding Principles in the Care and Use of Vertebrate Animals in Research and Training.
Balloon-induced injury surgical procedure in mice.
Mice were anesthesized by intraperitoneal administration of xylazine (10 mg/kg) and ketamine (100 mg/kg). We ligated the external carotid artery distally and placed clamps on the internal and mid-common artery (21). Then, the balloon catheter was introduced through an arteriotomy on the proximal external carotid artery. After the clamp on the common artery was removed, the catheter was advanced to the proximal, nondissected common carotid artery where the balloon was distended for 40 s. Balloon distension was matched to the weight of the animals. Thereby, controlled overdistension of the vessel was achieved using a mean balloon diameter of 0.79 mm for a 25-g mouse, which corresponds to a ratio of balloon distention to vessel diameter of ∼1.5. After balloon distension, the catheter was withdrawn, the proximal external carotid artery was ligated, and blood flow through the common and internal carotid artery was restored. The balloon catheter was manufactured with a balloon length of 5 mm and stiffened by a guidewire with a diameter of 0.15 mm (Schwager Medica). The balloon was expanded using a water-filled inflation syringe with digital display (IntelliSystemMonitor II; Meritmedical).
Histological and morphometric analysis.
Mice were euthanized following general anesthesia with xylazine (10 mg/kg) and ketamine (100 mg/kg) intraperitoneal injection 1 h or 1, 7, 14, 21, or 28 days after balloon distension injury to harvest the injured left and the untouched right common carotid artery. For histomorphological and immunohistochemical analyses, vessels were rinsed with normal saline, and perfusion-fixed with 4% paraformaldehyde (PFA), followed by 2 h postfixation in 4% PFA and overnight immersion in 30% sucrose. Then, vessels were embedded in optimum cutting temperature compound (Tissue-Tek; Sakura) and snap frozen.
We performed Evans blue staining of elastic laminae and DAPI staining of nuclei using three serial cross sections (5-μm thickness, 300-μm distance) taken from the mid-portion of dilated vessels. Subsequent fluorescence microscopy allowed us to delineate the neointima (innermost layer separated by the internal elastic lamina from the media) and the media [area between the internal and external elastic lamina (EEL)] and to quantify the number of cells by counting the nuclei using MetaMorph6 software (Zeiss).
Van Gieson staining of perfusion-fixed frozen sections was carried out with the Accustain elastic stain kit (Sigma-Aldrich) for morphometric assessment of intimal and medial area and EEL circumference.
Immunostaining of perfusion-fixed frozen sections was performed on at least three serial 5-μm aceton-fixed cross sections of dilated left or untouched right common carotid arteries, using monoclonal antibodies for mouse CD68 (clone FA-11; 1:400 dilution; AbD Serotec) and α-smooth muscle actin (α-SMA; clone 1A4; 1:20 dilution; Thermo Scientific) or polyclonal antibodies for CB2 (101550; dilution 1:25; Cayman) and CD31 (platelet endothelial cell adhesion molecule 1; clone M-20; 1:400; Santa Cruz Biotechnology). Specificity of CB2 immunostaining was confirmed by preabsorbing the CB2 antibody with blocking peptide (301550; Cayman) according to the manufacturer's instructions. Immunostaining for proliferating cell nuclear antigen (PCNA; polyclonal antibody; 1:50 dilution; Thermo Scientific) was performed on methanol-fixed cross sections. Binding of primary antibodies was detected with corresponding biotinylated secondary antibodies and alkaline phosphatase system (Vector Laboratories). Terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL) staining was performed on PFA perfusion-fixed five serial cross sections (5 μm) of dilated left common carotid arteries using the DeadEnd Fluorometric TUNEL System (Promega).
Immunostaining quantifications were performed by computer image analysis using MetaMorph6 software (Zeiss). Numbers or percentages of positive cells were determined on entire vessel cross sections. In the case of PCNA and TUNEL staining, analysis was performed by counting positive intimal and medial nuclei (delineated by the EEL; blinded observer).
Blood analysis.
For measurements of differential blood cell counts (hematocytometer; Sysmex Digitana) and serum cholesterol content (Infinity; Thermoelectron), blood samples were collected at the end of experiment.
Real-time RT-PCR.
Total RNA from mouse left common carotid arteries was extracted using the RNAqueous-Micro kit (Ambion). Reverse transcription was performed using the PrimeScript RT reagent kit (TaKaRa), and real-time RT-PCR was performed with the ABI Prism StepOnePlus Sequence Detection System (Applied Biosystems) using the ABsolute QPCR Mix (ABgene). We used CB2, VCAM-1, ICAM-1, CCR1, CCR2, CCR5, CCL2, CCL3, regulated on activation normal T-expressed and presumably secreted, and hypoxanthine phosphoribosyltransferase primers and probes as previously published (24), and sequences can be provided upon request. Gene of interest expression was normalized to hypoxanthine phosphoribosyltransferase gene expression, and the fold induction of mRNA levels compared with an internal calibrator (ApoE−/− sham or WT cDNA sample) were calculated by the comparative Ct method.
Bone marrow macrophage isolation.
Bone marrow-derived macrophages (BMM) from CB2−/− or WT mice were isolated by flushing femurs and tibiae. Cells were cultured in Iscove's modified Dulbecco's medium (IMDM) containing Glutamax (Invitrogen), 10% FBS (Sigma), 100 U/L penicillin, 5 g/l streptomycin (Invitrogen), 50 μM β-mercaptoethanol, and 30% L929-conditioned medium as a source of macrophage colony-stimulating factor. Differentiation was verified by flow cytometric analysis for CD11b and CD18 expression (both antibodies from BD Pharmingen).
Migration assay.
BMM were starved 14 h in serum-free medium without macrophage colony-stimulating factor. The supernatant was removed, and cells were washed with PBS to remove nonadherent cells. Adherent macrophages were detached by incubating 10 min with cold PBS-EDTA 0.5 mM and energically pipeting. After being washed with IMDM, cells were resuspended in chemotaxis-medium (IMDM containing 1% BSA; Sigma-Aldrich) and tested for migration to 50 ng/ml MCP5 (CCL12; AbD Serotec). Macrophage chemotaxis was assessed in a 48-well microchemotaxis-modified Boyden chamber (NeuroProbe, Gaithersburg, MD). Cells were allowed to migrate from an adjacent compartment separated by a porous 5-μm polyvinylpyrrolidone-free polycarbonate filter (NeuroProbe). Medium or medium containing chemoattractant was added in the lower wells while cells were added in the upper wells. The chamber was incubated 90 min at 37°C in a humidified 5% CO2 atmosphere. The filter was removed from the chamber and stained 3 min in May-Grünwalds eosine-methylene blue modified solution (Merck) and 5 min in Giemsa stain modified solution (Fluka) diluted 1:4 in water. Cells were counted at ×1,000 magnification in five random oil-immersion fields, and the chemotaxis index was calculated from the number of cells migrated to the chemoattractant divided by the number of cells migrated to the medium.
Adhesion assay.
Adhesion to uncoated plastic dishes was assessed in 96-well plates with 5 × 104 CFDA-SE-labeled (Molecular Probes) BMM per well. Alternatively, 96-well plates coated with 1 μg per well fibronectin (Sigma) or 2.5 μg per well type 1 collagen (from rat tail; Sigma), respectively, blocked with 0.5% BSA in PBS were used. After incubation for 30 min, nonadherent cells were removed by PBS washes and the relative numbers of adherent cells were determined by fluorescence reading (Victor3 multilabel reader; PerkinElmer).
Smooth muscle cell isolation and proliferation.
Primary vascular smooth muscle cells from mouse thoracoabdominal aortas were obtained by enzymatic digestion with collagenase after removal of the adventitia as described previously (13, 32). Freshly isolated cells (passage 1) were resuspended in DMEM containing 10% FBS and plated in 96-well plates (5,000 cells/well, all conditions in triplicates). After adherence for 24 h, cells were serum-starved for 12 h to assess proliferation based on bromodeoxyuridine incorporation with the colorimetric Cell Proliferation ELISA (Roche) according to the manufacturer's instuctions. Briefly, cells were allowed to proliferate in presence or absence of 25 ng/ml PDGF-BB (R&D Systems) for 24 h before addition of bromodeoxyuridine labeling solution and incubation for additional 12 h.
Statistical analysis.
All analyses were performed with GraphPad Prism 5.01 software, and results are expressed as means ± SE. Differences between P < 0.05 were considered significant. Paired t-test was performed for adhesion assays. For all other experiments, two-group comparisons were performed using unpaired t-test or Mann-Whitney U-test when equal variance test failed. For multiple group comparison, one-way ANOVA with Bonferroni's post test was used.
RESULTS
Balloon injury induces vascular CB2 expression.
To analyze whether balloon distension injury in ApoE−/− mice modulates CB2 receptor expression, we performed real-time RT-PCR analysis of mRNA levels 24 h postintervention. We found that CB2 mRNAs levels were increased in dilated carotid arteries compared with sham-operated arteries (Fig. 1A; P < 0.005), suggesting an upregulation of arterial CB2 receptors in response to injury.
Fig. 1.
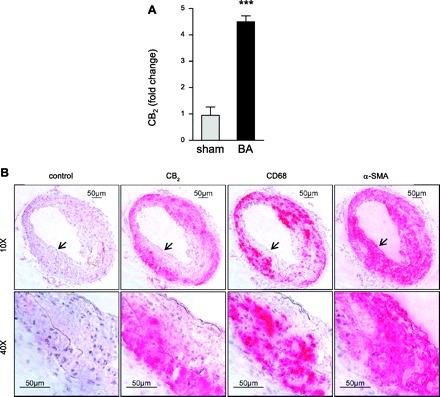
Ballooning increases carotid CB2 expression in hypercholesterolemic ApoE−/− mice. A: left common carotid CB2 mRNA levels of ballooned (BA; n = 3) vs. sham-operated mice (n = 6) 24 h postoperation. ***P < 0.005. B: Colocalization of CB2 immunostaining in dilated left common carotid arteries 21 days postoperation. Negative control was performed with CB2 antibody preabsorbed with blocking peptide. Consecutive sections were stained with antibody against CB2, macrophages (CD68), or smooth muscle cells [SMCs; α-smooth muscle actin (α-SMA)]. Original magnifications as indicated (×10, ×40). Higher magnification zones are indicated by arrowheads.
To confirm this finding, we performed immunohistological analysis of CB2 expression in carotid arteries of ApoE−/− mice. No visible CB2 staining was found in cross sections of nondilated right carotid arteries (not shown). After 21 days postintervention (Fig. 1B), we observed pronounced intimal and medial CB2 staining in dilated left common carotid arteries. CB2 staining mainly colocalized with macrophage CD68 rather than α-SMA staining for smooth muscle cells, assessed on subsequent sections. The specificity of the CB2 antibody was confirmed by preabsorption with blocking peptide before immunostaining (Fig. 1B, control).
Pharmacological CB2 activation using JWH133 reduces neointima formation in injured arteries of ApoE−/− mice.
To analyze whether the selective CB2 receptor agonist JWH133 would protect mice from neointima formation, we treated ApoE−/− mice with daily intraperitoneal injections of JWH133 during 7, 14, or 21 consecutive days after ballooning of left common carotid arteries. Treatment with JWH133 did not affect body weight, cholesterol, and total blood leukocyte count (Table 1).
Table 1.
Characteristics of study groups
| WT | CB2−/− | ApoE−/− | ApoE−/− | |
|---|---|---|---|---|
| Diet | NC | NC | HCD | HCD |
| Treatment | — | — | vehicle | JWH133 |
| Time interval* | 14 | 14 | 7 | 7 |
| Total cholesterol, mg/dl | — | — | 1,132 ± 142 (n = 6) | 1,316 ± 111 (n = 9) |
| Body weight, g | 27.7 ± 0.5 (n = 14) | 27.7 ± 0.4 (n = 15) | 25.3 ± 0.6 (n = 11) | 24.5 ± 0.7 (n = 11) |
| Leukocytes, n/μl | 3,636 ± 383 (n = 14) | 3,750 ± 367 (n = 10) | 3,940 ± 353 (n = 5) | 3,700 ± 271 (n = 8) |
Data are means± SE. WT, wild-type mice (C57BL/6J); ApoE−/−, apolipoprotein E knockout mice; NC, normal chow; HCD, high-cholesterol diet.
Days postballooning.
Increased intimal cell numbers were determined in dilated arteries 7 to 21 days postintervention compared with nondilated right common arteries (Fig. 2, A and B; P < 0.005). While intimal nuclei counts in vehicle-treated mice were comparable between 7 and 14 days, a marked increase in intimal counts was observed after 14 to 21 days (1.7-fold; P < 0.005) in the vehicle group but not in mice treated with JWH133. At all time points, mice treated with JWH133 had significantly less intimal nuclei compared with vehicle treatment (Fig. 2B; P < 0.05), with the most pronounced effect at 21 days (1.76-fold difference). We confirmed the selectivity of the CB2 agonist by treating CB2−/− mice with JWH133. The analysis revealed similar numbers of intimal nuclei in JWH133- and vehicle-treated mice (Fig. 2, A and C).
Fig. 2.
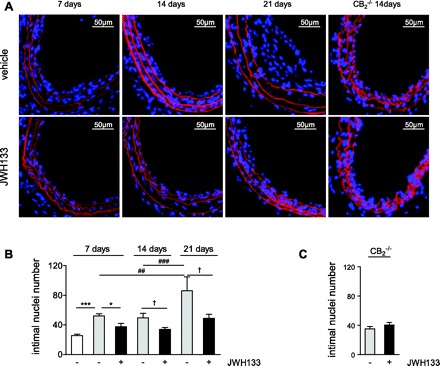
A: carotid cross sections of hypercholesterolemic apolipoprotein E knockout (ApoE−/−) mice or normocholesterolemic CB2−/− mice treated with JWH133 or vehicle. Nuclei were visualized with DAPI (blue), and Evans blue (red) counterstaining was performed to detect elastic laminae. Intimal nuclei per entire cross section were counted in nondilated controls (white bars; n = 5), vehicle (grey bars)-, or JWH133-treated mice (black bars) after 7 (n = 11–12), 14 (n = 12–14), or 21 days (n = 5–6) postballooning in ApoE−/− mice (B) or after 14 days in CB2−/− mice (C; n = 7–8). *P < 0.05, ***P < 0.005, for multiple comparison (one-way ANOVA) at 7 days; ##P < 0.01, ###P < 0.005, for multiple comparison (one-way ANOVA) of vehicle at different time points; †P < 0.05, for two-group comparison between treatment groups at 14 or 21 days.
The morphometric analysis of the dilated vessels in vehicle-treated mice revealed a 2.6-fold increase of the intimal area from 7 to 21 days (Fig. 3, A and B; P < 0.05). Conversely, in JWH133-treated mice a moderate nonsignificant 1.7-fold increase of intimal area was only observed after 14 to 21 days, suggesting a potent inhibitory effect of the CB2 agonist. This was confirmed by two-group comparisons between treatment groups at same time points (P < 0.05 to P < 0.01).
Fig. 3.
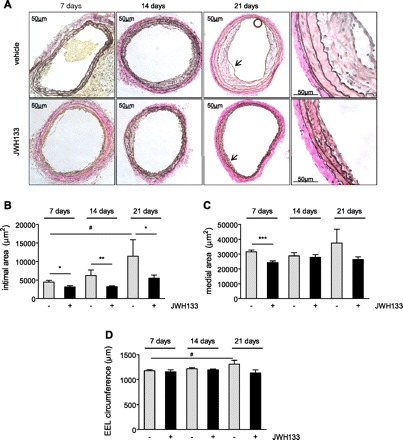
Van Gieson staining and morphometric analysis of hypercholesterolemic ApoE−/− mice treated with JWH133 or vehicle. A: original magnifications = ×10 or ×40. Higher magnification zones are indicated by arrowheads. B–D: quantification of intimal or medial areas and external elastic lamina (EEL) circumference in vehicle (grey bars)- or JWH133-treated mice (black bars) after 7 (n = 11–12), 14 (n = 12–14), or 21 days (n = 5–6) postballooning. *P < 0.05, **P < 0.01, ***P < 0.005, for two group comparison between treatment groups at same time point; #P < 0.05, for multiple comparison (one-way ANOVA) of vehicle at different time points.
There was no significant increase in medial areas from 7 to 21 days; however, a significantly smaller medial area in JWH133- compared with vehicle-treated mice was found after 7 days (Fig. 3C; P < 0.005). We found a significant increase in EEL circumference of vehicle-treated but not JWH133-treated mice between 7 and 21 days (Fig. 3D; P < 0.05, one-way ANOVA for vehicle at different time points). However, there was no significant difference between the two genotypes when compared at same time points.
CB2 activation by JWH133 decreases in situ proliferation, macrophage infiltration, and smooth muscle cell content after carotid injury in ApoE−/− mice.
To determine if the reduction of intimal and medial growth in JWH133-treated arteries was accompanied by an inhibitory effect on cell proliferation, we performed immunohistochemical PCNA staining. At 7 days, the number of intimal and medial PCNA-positive nuclei was lower in JWH133-treated arteries compared with vehicle-treated vessels, suggesting reduced proliferation (Fig. 4A; P < 0.01). PCNA-positive nuclei were no longer detectable after 14 to 21 days balloon-induced injury. Intimal and medial α-SMA staining of injured vessels revealed significant differences between JWH133 and vehicle-treated tissues only after 21 days but not at the earlier studied time points (Fig. 4B; P < 0.05). In other words, the smooth muscle cell content in vehicle-treated mice increased after 14 to 21 days in vehicle-treated but not JWH133-treated mice (P < 0.01, one-way ANOVA for vehicle at various time points).
Fig. 4.
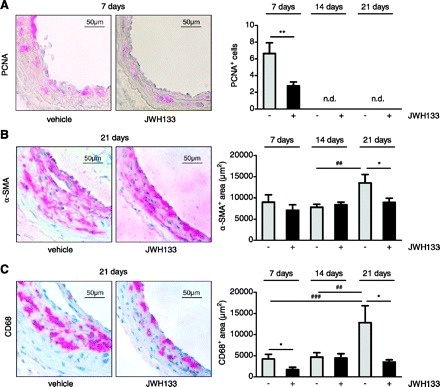
Immunostaining after 7 (n = 11–12), 14 (n = 12–14), or 21 days (n = 5–6) balloon injury in hypercholesterolemic ApoE−/− mice treated with CB2 agonist JWH133 or vehicle. Representative carotid cross sections and quantification in vehicle (grey bars)- or JWH133-treated mice (black bars) of proliferating cell nuclear antigen (PCNA; A), α-SMA (B), and CD68 (C). *P < 0.05, **P < 0.01, for two group comparison at same time point; ##P < 0.01, ###P < 0.005, for multiple comparison (one-way ANOVA) of vehicle at different time points; n.d., not detectable. Original magnification = ×40.
As to a potential contribution of macrophages to the CB2-mediated protective effects, we investigated CD68 immunostaining of injured vessels. A strong increase in macrophage content was observed in vehicle treated mice after 14 to 21 days (P < 0.01, one-way ANOVA for vehicle at various time points). We found significantly less intimal and medial staining in JWH133- compared with vehicle-treated mice after 7 and 21 days, suggesting reduced macrophage infiltration (Fig. 4C; P < 0.05).
JWH133 treatment does not inhibit reendothelialization.
We further assessed a potential effect of JWH133 on arterial reendothelialization in response to balloon injury. Rapid endothelial repair is essential to prevent thrombotic complications and limit intimal thickening. Therefore, we performed CD31 (platelet endothelial cell adhesion molecule 1) immunostaining on serial cross sections of injured vessels. In both JWH133- and vehicle-treated mice, complete endothelial repair was observed after 14 days postballooning, indicating that the CB2 agonist does not inhibit endothelial repair (Fig. 5).
Fig. 5.
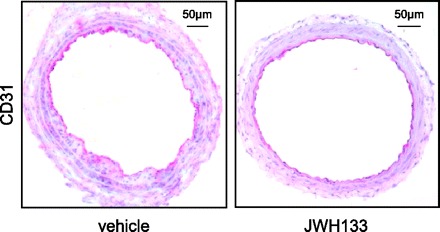
Representative CD31 immunostaining of carotid-cross sections of hypercholesterolemic ApoE−/− mice treated with JWH133 or vehicle 14 days postballooning.
Genetic CB2 deletion increases neointima formation after carotid balloon injury.
Since pharmacological CB2 activation inhibited cell proliferation, inflammation, and vascular remodeling, we investigated whether genetic CB2 deletion exerts opposite effects. We therefore performed balloon distension of the left common carotid in CB2−/− and WT mice kept on a normal diet. Similar to the hypercholesterolemic mouse model, balloon distension injury in normocholesterolemic mice leads to endothelial denudation and apoptotic loss of medial smooth muscle cells, followed by enhanced proliferation and migration of smooth muscle cells but without pronounced inflammatory response (as confirmed by absence of CD68 staining, data not shown). Due to the less rapidly developing intimal areas in the normocholesterolemic model, we performed morphometric analyses only after 14 and 28 days. Evans blue DAPI quantification of nuclei revealed a significantly increased number of intimal nuclei in CB2−/− mice vs. WT 14 days after balloon distension injury (Fig. 6, A and B; P < 0.05). This was in agreement with significantly increased neointimal areas in CB2−/− compared with WT vessels after 14 to 28 days (Fig. 6C; P < 0.05). A significant increase in medial areas (Fig. 6D; P < 0.005) and EEL circumference (Fig. 6E; P < 0.05) in CB2−/− compared with WT mice was found after 28 days.
Fig. 6.
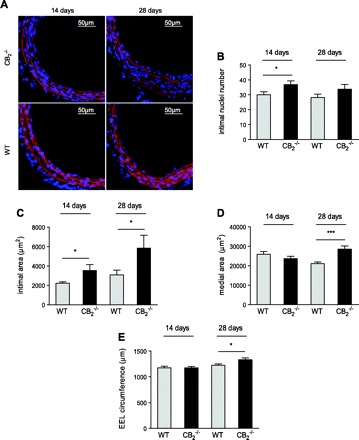
Histological analysis on cross sections of dilated vessels of CB2−/− or wild-type (WT) mice subjected to balloon injury 14 (n = 14–15) or 28 days (n = 6–7) postballooning. DAPI staining of nuclei (blue) and Evans blue counterstaining (red) to visualize elastic laminae. Representative images (A; ×10 magnification) and quantification of intimal nuclei counted per entire cross section (B) and intimal and medial areas as well as EEL circumference (C-E). *P < 0.05, ***P < 0.005, for 2 group comparison between genotypes.
Genetic CB2 deletion increases in situ apoptosis after carotid balloon injury.
Balloon distension injury leads to apoptotic loss of medial smooth muscle cells. As previously reported, maximal apoptosis rates are detectable immediately after injury by TUNEL staining (20). Indeed, we found abundant TUNEL staining in dilated vessels of WT mice after 1 h, with significantly higher numbers of TUNEL-positive cells in cross sections of mice lacking CB2 (Fig. 7; P < 0.05)
Fig. 7.
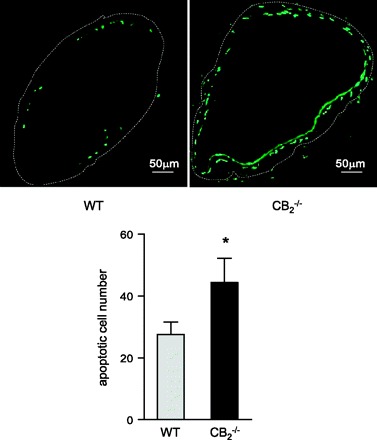
Representative images (×10 magnification) and quantification (cells per section) of TUNEL staining to visualize apoptotic cells in dilated vessels of WT and CB2−/− mice 1 h postballoon injury. *P < 0.05 vs. WT.
CB2 deficiency enhances macrophage adhesion and migration in vitro.
To further investigate putative effects of CB2 signaling in macrophages, we performed in vitro experiments using murine macrophages isolated from bone marrow of CB2−/− and WT mice. CB2−/−-deficient BMM showed a tendency for enhanced adhesion to plastic and significantly enhanced adhesion to fibronectin and collagen substrate compared with WT BMM (Fig. 8A–C; P < 0.05). In addition, CB2 deficiency enhanced migration of CB2−/− BMM in response to MCP-5 (Fig. 8D; P < 0.05). At the transcript level, we found significantly increased mRNA levels of adhesion molecules VCAM-1 and ICAM-1, chemokine receptors CCR1 and CCR5, and chemokine CCL2 in CB2−/− macrophages (Fig. 8E).
Fig. 8.
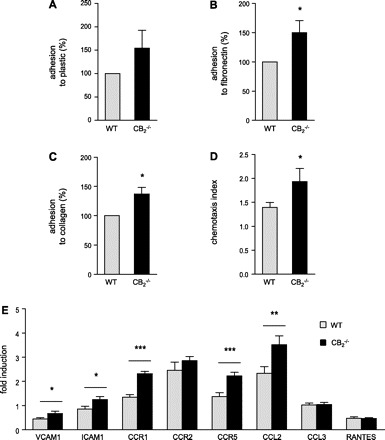
In vitro adhesion and migration assay with bone marrow derived macrophages (BMM) from CB2−/− and WT mice. Adhesion to plastic (A), fibronectin (B), or collagen type 1-coated culture dishes (C; n = 5). D: migration of BMM in response to 50 ng/ml MCP-5 (n = 6). E: messenger RNA levels of adhesion molecules (VCAM-1 and ICAM-1), chemokine receptors (CCR1, CCR2, and CCR5), and chemokines [CCL2, CCL3, and activation normal T-expressed and presumably secreted (RANTES)] were determined by real-time PCR. *P < 0.05, **P < 0.01, ***P < 0.005, for two-group comparison between WT (grey bars) and CB2−/− mice (black bars).
CB2 deficiency enhances smooth muscle cell proliferation in vitro.
To confirm the involvement of smooth muscle cells in CB2-mediated effects on intima formation, we performed additional in vitro experiments. We found that the proliferation rate in response to PDGF-BB was increased in CB2−/− smooth muscle cells compared with WT cells (Fig. 9; P < 0.05).
Fig. 9.
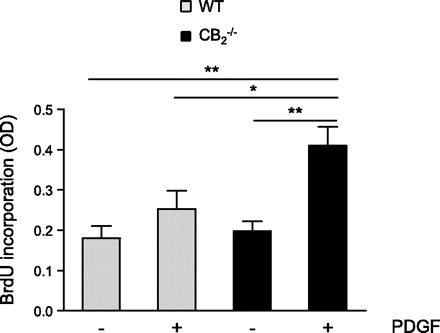
In vitro proliferation assay with aortic SMCs isolated from wildtype (WT, grey bars) and CB2−/− mice (black bars) induced with 25 ng/ml platelet-derived growth factor (PDGF)-BB. BrdU, bromodeoxyuridine; OD, opitcal density. *P < 0.05, **P < 0.01.
DISCUSSION
In the present study, we investigated the role of CB2 receptors in the pathophysiology of balloon-induced arterial injury using a pharmacological and a genetic approach. Pharmacological CB2 activation limited balloon-induced neointima and media formation, proliferation, smooth muscle cell, and macrophage content in ApoE−/− mice fed a high-cholesterol diet. The knockout approach confirmed the implication of CB2 receptors in our experimental model. CB2 deficiency was associated with increased numbers of intimal cells, intimal and medial area, and EEL circumference in response to balloon-induced injury. The time-course analysis suggests that CB2 deficiency worsens rather than accelerates injury, as the neointimal area is substantially increasing from 14 (1.6-fold difference between genotypes) to 28 days (1.9-fold difference).
There is clear evidence for immunomodulatory actions of cannabinoids, which have been mainly attributed to CB2, the major cannabinoid receptor expressed on immune and hematopoietic cells (18). CB2 is also present on endothelial cells of various origins as well as arterial smooth muscle cells, and its activation was shown to reduce inflammatory responses of vascular cells in vitro (27). In particular, activation of CB2 receptors in human coronary endothelial and various inflammatory cells (e.g., monocytes, neutrophils, etc.) attenuates cytokine-induced endothelial inflammation, chemotaxis and adhesion of inflammatory cells to the activated endothelium, and the consequent release of a variety of proinflammatory mediators (3, 22, 23, 29, 31). CB2 receptor activation also attenuates TNF-α-induced human coronary artery smooth muscle cell proliferation and migration (30). The underlying mechanisms involve inhibition of TNF-α-induced phosphorylation of mitogen-activated protein kinases ERK1/2, JNK, and p38 in response to CB2 activation (30). Recruitment of inflammatory cells into the arterial wall is another crucial event in neointimal lesion formation. This process is orchestrated by chemokines, chemokine receptors, as well as adhesion molecules (33). Cannabinoids have been reported to inhibit chemokine-induced chemotaxis in various cell types (22).
The development of selective CB2 receptor agonists for therapeutic applications, devoid of psychoactive properties attributable to CB1 activation, has attracted considerable interest over the past years (9, 17). Indeed, numerous studies have demonstrated anti-inflammatory effects of CB2 receptor activation in different experimental disease models, including neurodegenerative disorders (1, 28), inflammatory pain (14), myocardial (7, 24), cerebral (39), and hepatic ischemia/reperfusion injury (3, 31), to gastrointestinal inflammatory disorders (37), liver inflammation, and fibrosis (19). We and others (15, 26, 35, 40, 41) previously provided experimental evidence on a possible role of CB2 receptors in atherogenesis.
In this study, we used the selective CB2 receptor agonist JWH133 developed by Huffman et al. (16), which exhibits ∼200-fold selectivity over CB1 receptors. Its potency has been underlined in numerous in vivo studies (2, 3, 24, 38). Daily treatment with JWH133 for 7 days resulted in significantly smaller intimal and medial areas and reduced intimal nuclei counts and numbers of proliferating cells compared with the vehicle-treated group. Strikingly, continuous treatment with the CB2 agonist for 21 days almost completely abolished progression of intima formation and macrophage infiltration. Similarly, increases in EEL circumference were only observed in the vehicle group. The lack of JWH133-mediated effects on neointima formation in CB2−/− mice confirmed the specificity of the CB2 agonist in vivo.
Despite a reduced neointimal and medial area in JWH133-treated mice after 7 days, a lower smooth muscle cell content was only found after 21 days. This was correlated with the finding that increased smooth muscle cell contents in vehicle-treated mice were only observed after 14–21 days. Thus the changes in intimal and medial areas at earlier time points are rather due to changes in macrophage content and potentially extracellular matrix contents.
To better clarify the implication of CB2-dependent effects on smooth muscle cells, we performed additional experiments using CB2-deficient mice that were not on an ApoE−/− background. The balloon injury response in WT mice on normal chow involves enhanced smooth muscle cell migration and proliferation with little inflammatory leukocyte infiltration (21). In these normocholesterolemic mice, vascular smooth muscle cell response to balloon distension injury can be studied with less interfering effects of leukocyte infiltration. However, we could not find changes in smooth muscle cell contents, despite increased numbers of intimal nuclei at 14 days (SMA+ area in μm2: WT: 8,548 ± 1,057 v.s CB2−/−: 7,770 ± 693 at 14 days; WT: 9,415 ± 665 vs. CB2−/−: 9,465 ± 1,271 at 28 days). Interestingly, deficiency of CB2 accelerated immediate apoptosis in response to balloon injury, while in vitro proliferation of aortic smooth muscle cells was increased in CB2−/− cells. An implication of CB2 receptors in apoptosis has been previously described (25, 31). For example, in cisplatin-induced nephropathy, TUNEL staining was significantly higher in kidneys of CB2−/− mice compared with WT, whereas treatment with a CB2 agonist was protective (25). Despite increased apoptosis rate, we found comparable smooth muscle cell contents after 14 to 28 days in both genotypes, suggesting that enhanced proliferation in the CB2 knockouts counterbalances the apoptotic cell loss.
As to the increased neointimal cell numbers, we may speculate that immune cells in addition to smooth muscle cells contribute to neointima formation. However, CD68+ macrophages were barely or not detectable, suggesting a potential recruitment of other immune cells. Another possible explanation is that quantification of α-SMA staining is a less sensitive method to assess differences in smooth muscle cell contents than counting nuclei. It should be taken into account that the intima formation in the normocholesterolemic background is modest and thus differences in cellular contents between the genotypes are potentially too low to be detectable.
The further increase in intimal area, but not intimal cell numbers, from 14 to 28 days in vessels from CB2−/− mice suggests an increase in extracellular matrix content. This is in agreement with an increased EEL circumference in CB2−/− mice compared with WT.
Finally, the immunohistochemical data showing reduced macrophage infiltration in JWH133-treated ApoE−/− mice are in agreement with the in vitro data obtained with CB2−/− macrophages, suggesting that modulation of macrophage adhesion and migration contribute to the protective effects of CB2 activation. The increased adhesion and migration in CB2-deficient macrophages can be explained by higher expression of adhesion molecules, chemokines, and chemokine receptors in CB2−/− macrophages.
In conclusion, our study demonstrates a protective role for CB2 receptors in balloon-induced arterial injury, which involves modulation of cellular functions in both smooth muscle cells and macrophages. Thus targeting CB2 receptors might represent a novel therapeutic opportunity for the prevention of restenosis after coronary or peripheral balloon angioplasty that needs further investigation in experimental models of restenosis.
GRANTS
This work was supported by Swiss National Science Foundation Grants 310030-116324 and 310030-130732, Swiss Life, Prevot, and OPO Foundation (to S. Steffens).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: F.M., C.M.M., A.Z., P.P., and S.S. conception and design of research; F.M., S.S., F.B., G.P., and S.L. performed experiments; F.M., F.B., and S.S. analyzed data; F.M., C.M.M., F.B., and S.S. interpreted results of experiments; F.M. prepared figures; F.M. and S.S. drafted manuscript; F.M. and S.S. edited and revised manuscript; F.M., C.M.M., F.B., G.P., S.L., A.Z., P.P., and S.S. approved final version of manuscript.
REFERENCES
- 1. Arevalo-Martin A, Garcia-Ovejero D, Gomez O, Rubio-Araiz A, Navarro-Galve B, Guaza C, Molina-Holgado E, Molina-Holgado F. CB2 cannabinoid receptors as an emerging target for demyelinating diseases: from neuroimmune interactions to cell replacement strategies. Br J Pharmacol 153: 216–225, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Huffman JW, Layward L. Cannabinoids control spasticity and tremor in a multiple sclerosis model. Nature 404: 84–87, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Batkai S, Osei-Hyiaman D, Pan H, El-Assal O, Rajesh M, Mukhopadhyay P, Hong F, Harvey-White J, Jafri A, Hasko G, Huffman JW, Gao B, Kunos G, Pacher P. Cannabinoid-2 receptor mediates protection against hepatic ischemia/reperfusion injury. FASEB J 21: 1788–1800, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Batkai S, Rajesh M, Mukhopadhyay P, Hasko G, Liaudet L, Cravatt BF, Csiszar A, Ungvari Z, Pacher P. Decreased age-related cardiac dysfunction, myocardial nitrative stress, inflammatory gene expression, and apoptosis in mice lacking fatty acid amide hydrolase. Am J Physiol Heart Circ Physiol 293: H909–H918, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bittl JA. Advances in coronary angioplasty. N Engl J Med 335: 1290–1302, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Daemen J, Wenaweser P, Tsuchida K, Abrecht L, Vaina S, Morger C, Kukreja N, Juni P, Sianos G, Hellige G, van Domburg RT, Hess OM, Boersma E, Meier B, Windecker S, Serruys PW. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet 369: 667–678, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Defer N, Wan J, Souktani R, Escoubet B, Perier M, Caramelle P, Manin S, Deveaux V, Bourin MC, Zimmer A, Lotersztajn S, Pecker F, Pavoine C. The cannabinoid receptor type 2 promotes cardiac myocyte and fibroblast survival and protects against ischemia/reperfusion-induced cardiomyopathy. FASEB J 23: 2120–2130, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Di Marzo V. The endocannabinoid system: its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol Res 60: 77–84, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov 3: 771–784, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Douglas JS., Jr Pharmacologic approaches to restenosis prevention. Am J Cardiol 100: 10K–16K, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Ferns GA, Avades TY. The mechanisms of coronary restenosis: insights from experimental models. Int J Exp Pathol 81: 63–88, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Finn AV, Nakazawa G, Joner M, Kolodgie FD, Mont EK, Gold HK, Virmani R. Vascular responses to drug eluting stents: importance of delayed healing. Arterioscler Thromb Vasc Biol 27: 1500–1510, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially l-selectin dependent. J Exp Med 203: 1273–1282, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guindon J, Hohmann AG. Cannabinoid CB2 receptors: a therapeutic target for the treatment of inflammatory and neuropathic pain. Br J Pharmacol 153: 319–334, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hoyer FF, Steinmetz M, Zimmer S, Becker A, Lutjohann D, Buchalla R, Zimmer A, Nickenig G. Atheroprotection via cannabinoid receptor-2 is mediated by circulating and vascular cells in vivo. J Mol Cell Cardiol 51: 1007–1014, 2011 [DOI] [PubMed] [Google Scholar]
- 16. Huffman JW, Liddle J, Yu S, Aung MM, Abood ME, Wiley JL, Martin BR. 3-(1′,1′-Dimethylbutyl)-1-deoxy-delta8-THC and related compounds: synthesis of selective ligands for the CB2 receptor. Bioorg Med Chem 7: 2905–2914, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Klein TW. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol 5: 400–411, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Klein TW, Newton C, Larsen K, Lu L, Perkins I, Nong L, Friedman H. The cannabinoid system and immune modulation. J Leukoc Biol 74: 486–496, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Lotersztajn S, Teixeira-Clerc F, Julien B, Deveaux V, Ichigotani Y, Manin S, Tran-Van-Nhieu J, Karsak M, Zimmer A, Mallat A. CB2 receptors as new therapeutic targets for liver diseases. Br J Pharmacol 153: 286–289, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matter CM, Chadjichristos CE, Meier P, von Lukowicz T, Lohmann C, Schuler PK, Zhang D, Odermatt B, Hofmann E, Brunner T, Kwak BR, Luscher TF. Role of endogenous Fas (CD95/Apo-1) ligand in balloon-induced apoptosis, inflammation, and neointima formation. Circulation 113: 1879–1887, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Matter CM, Ma L, von Lukowicz T, Meier P, Lohmann C, Zhang D, Kilic U, Hofmann E, Ha SW, Hersberger M, Hermann DM, Luscher TF. Increased balloon-induced inflammation, proliferation, and neointima formation in apolipoprotein E (ApoE) knockout mice. Stroke 37: 2625–2632, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Miller AM, Stella N. CB(2) receptor-mediated migration of immune cells: it can go either way. Br J Pharmacol 153: 299–308, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Montecucco F, Burger F, Mach F, Steffens S. CB2 cannabinoid receptor agonist JWH-015 modulates human monocyte migration through defined intracellular signaling pathways. Am J Physiol Heart Circ Physiol 294: H1145–H1155, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Montecucco F, Lenglet S, Braunersreuther V, Burger F, Pelli G, Bertolotto M, Mach F, Steffens S. CB(2) cannabinoid receptor activation is cardioprotective in a mouse model of ischemia/reperfusion. J Mol Cell Cardiol 46: 612–620, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Mukhopadhyay P, Rajesh M, Pan H, Patel V, Mukhopadhyay B, Batkai S, Gao B, Hasko G, Pacher P. Cannabinoid-2 receptor limits inflammation, oxidative/nitrosative stress, and cell death in nephropathy. Free Radic Biol Med 48: 457–467, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Netherland CD, Pickle TG, Bales A, Thewke DP. Cannabinoid receptor type 2 (CB2) deficiency alters atherosclerotic lesion formation in hyperlipidemic Ldlr-null mice. Atherosclerosis 213: 102–108, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pacher P, Steffens S. The emerging role of the endocannabinoid system in cardiovascular disease. Semin Immunopathol 31: 63–77, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pertwee RG. Cannabinoids and multiple sclerosis. Mol Neurobiol 36: 45–59, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Rajesh M, Mukhopadhyay P, Batkai S, Hasko G, Liaudet L, Huffman JW, Csiszar A, Ungvari Z, Mackie K, Chatterjee S, Pacher P. CB2-receptor stimulation attenuates TNF-α-induced human endothelial cell activation, transendothelial migration of monocytes, and monocyte-endothelial adhesion. Am J Physiol Heart Circ Physiol 293: H2210–H2218, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rajesh M, Mukhopadhyay P, Hasko G, Huffman JW, Mackie K, Pacher P. CB(2) cannabinoid receptor agonists attenuate TNF-alpha-induced human vascular smooth muscle cell proliferation and migration. Br J Pharmacol 153: 347–357, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rajesh M, Pan H, Mukhopadhyay P, Batkai S, Osei-Hyiaman D, Hasko G, Liaudet L, Gao B, Pacher P. Cannabinoid-2 receptor agonist HU-308 protects against hepatic ischemia/reperfusion injury by attenuating oxidative stress, inflammatory response, and apoptosis. J Leukoc Biol 82: 1382–1389, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ray JL, Leach R, Herbert JM, Benson M. Isolation of vascular smooth muscle cells from a single murine aorta. Methods Cell Sci 23: 185–188, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Schober A. Chemokines in vascular dysfunction and remodeling. Arterioscler Thromb Vasc Biol 28: 1950–1959, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Steffel J, Eberli FR, Luscher TF, Tanner FC. Drug-eluting stents–what should be improved? Ann Med 40: 242–252, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Steffens S, Veillard NR, Arnaud C, Pelli G, Burger F, Staub C, Karsak M, Zimmer A, Frossard JL, Mach F. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature 434: 782–786, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Windecker S, Meier B. Late coronary stent thrombosis. Circulation 116: 1952–1965, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Wright KL, Duncan M, Sharkey KA. Cannabinoid CB2 receptors in the gastrointestinal tract: a regulatory system in states of inflammation. Br J Pharmacol 153: 263–270, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xu H, Cheng CL, Chen M, Manivannan A, Cabay L, Pertwee RG, Coutts A, Forrester JV. Anti-inflammatory property of the cannabinoid receptor-2-selective agonist JWH-133 in a rodent model of autoimmune uveoretinitis. J Leukoc Biol 82: 532–541, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Zhang M, Martin BR, Adler MW, Razdan RK, Jallo JI, Tuma RF. Cannabinoid CB(2) receptor activation decreases cerebral infarction in a mouse focal ischemia/reperfusion model. J Cereb Blood Flow Metab 7: 1387–1396, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhao Y, Liu Y, Zhang W, Xue J, Wu YZ, Xu W, Liang X, Chen T, Kishimoto C, Yuan Z. WIN55212–2 ameliorates atherosclerosis associated with suppression of pro-inflammatory responses in ApoE-knockout mice. Eur J Pharmacol 649: 285–292, 2010 [DOI] [PubMed] [Google Scholar]
- 41. Zhao Y, Yuan Z, Liu Y, Xue J, Tian Y, Liu W, Zhang W, Shen Y, Xu W, Liang X, Chen T. Activation of cannabinoid CB2 receptor ameliorates atherosclerosis associated with suppression of adhesion molecules. J Cardiovasc Pharmacol 55: 292–298, 2010 [DOI] [PubMed] [Google Scholar]


