Abstract
We recently developed a nutritional model of steatohepatitis and metabolic syndrome in Ossabaw pigs. Here we describe changes in the serum proteome of pigs fed standard chow (control group; n = 7), atherogenic diet (n = 5), or modified atherogenic diet (M-ath diet group; n = 6). Pigs fed atherogenic diet developed metabolic syndrome and mildly abnormal liver histology, whereas pigs fed M-ath diet exhibited severe metabolic syndrome and liver injury closely resembling human nonalcoholic steatohepatitis (NASH). Using a label-free mass spectrometry-based proteomics approach, we identified 1,096 serum proteins, 162 of which changed significantly between any two diet groups (false discovery rate <5%). Biological classification of proteins with significant changes revealed functions previously implicated in development of NASH in humans, including immune system regulation and inflammation (orosomucoid 1, serum amyloid P component, paraoxonase 1, protein similar to α-2-macroglobulin precursor, β-2-microglobulin, p101 protein, and complement components 2 and C8G), lipid metabolism (apolipoproteins C-III, E, E precursor, B, and N), structural and extracellular matrix proteins (transthyretin and endopeptidase 24.16 type M2), and coagulation [carboxypeptidase B2 (plasma)]. Several proteins with significant differential expression in pigs were also identified in our recent human proteomics study as changing significantly in serum from patients across the spectrum of nonalcoholic fatty liver disease, including apolipoproteins C-III and B, orosomucoid 1, serum amyloid P component, transthyretin, paraoxonase 1, and a protein similar to α-2-macroglobulin precursor. This serum proteomic analysis provides additional information about the pathogenesis of NASH and further characterizes our large animal model of diet-induced steatohepatitis and metabolic syndrome in Ossabaw pigs.
Keywords: mass spectrometry, fatty liver disease, nonalcoholic steatohepatitis, animal model, fibrosis
nonalcoholic fatty liver disease (NAFLD) is a common chronic liver disease that occurs in the absence of significant alcohol abuse (2). NAFLD includes an entire clinical and pathological range of disease, from relatively benign accumulation of lipid (simple steatosis) to progressive nonalcoholic steatohepatitis (NASH) associated with fibrosis, necrosis, and inflammation (3, 19, 30). The high incidence of NAFLD and NASH in developed countries is related to the epidemic of metabolic syndrome-associated risk factors such as obesity, insulin resistance, hypertension, and dyslipidemia that often occur in concert with liver abnormalities. In fact, NAFLD is considered to be the hepatic manifestation of metabolic syndrome. Despite the increasing prevalence of NAFLD and NASH, the pathogenesis and progression of these conditions is not well understood.
We recently developed a nutritional model of steatohepatitis and metabolic syndrome in miniature Ossabaw pigs that very closely resembles human NASH (17). Ossabaw pigs, from Ossabaw Island off the coast of Georgia, exhibit a thrifty genotype that allows them to store large amounts of fat for survival during famine (28). When fed an atherogenic diet consisting of fructose, cholesterol, and fat derived from hydrogenated soybean oil, Ossabaw pigs develop several characteristics of metabolic syndrome, including obesity, insulin resistance, dyslipidemia, and hypertension (10, 17, 28). We also found that feeding pigs a modified atherogenic diet, with cholesterol and fat calories derived from hydrogenated soybean oil, coconut oil, and lard, resulted in development of severe metabolic syndrome and abnormal liver histology very similar to human NASH (17).
To address the need for increased understanding of the pathogenesis of NASH and to further characterize our animal model of NASH, we applied a label-free quantitative proteomics approach (LFQP) to profile the global protein expression of serum samples from healthy, lean pigs fed control chow, an atherogenic diet (pigs developed metabolic syndrome and benign fatty liver), or a modified atherogenic diet (pigs developed severe metabolic syndrome and steatohepatitis). LFQP is a rapid, sensitive approach for quantification of proteins in complex biological samples, including tissue, blood, or urine (32). Recently, we used LFQP to describe changes in the serum proteome of patients with simple steatosis, NASH, and NASH with advanced (F3/F4) fibrosis compared with a control group of individuals without liver disease (4). In that human study, we described significant changes in serum protein expression patterns among groups and identified potential protein biomarker panels able to differentiate between patient groups. The objectives of the present study were to utilize this LFQP approach to identify differentially expressed serum proteins in our dietary Ossabaw NASH model and to assess for commonalities in the serum proteomic profiles between our animal model and human NAFLD.
MATERIALS AND METHODS
Pig Groups
This serum proteomics study was designed as an extension of our previous study that used dietary manipulation to induce liver injury closely mimicking human NASH and metabolic syndrome in Ossabaw pigs (17), and all pigs included in this serum proteomics study were characterized in our previous study. In addition to the three dietary groups included in this study, our original Ossabaw study had a fructose-fed group that was not included in this study owing to a lack of any hepatic histological abnormalities (17). All protocols were approved by the Institutional Animal Care and Use Committee at Indiana University and met the recommendations outlined by the National Research Council and the American Veterinary Medical Association Panel on Euthanasia (1, 20). Miniature Ossabaw pigs, aged 5 to 10 mo at the start of the study, were assigned to different diet groups for 24 wk. Briefly, the three dietary groups were as described previously (17).
Standard chow.
The standard chow control group consisted of seven pigs that were fed standard chow consisting of 18.5% calories from protein, 71% calories from carbohydrates, 10.5% calories from fat, and normal levels of methionine and choline (concentrations of 3,500 and 1,500 ppm, respectively).
Atherogenic diet group.
This group consisted of five pigs that received high-fructose-containing atherogenic diet with 18% calories from fructose, 43% calories from fat (supplemental source was hydrogenated soybean oil), 8% calories from protein, 2% cholesterol by weight, 0.7% sodium cholate by weight, and methionine and choline at concentrations of 2,100 and 900 ppm, respectively.
M-ath group.
The modified atherogenic diet group (M-ath group) had six pigs that were fed fructose-based atherogenic diet (5B4L; custom formulated by Purina TestDiet, Richmond, IN). It consisted of 46% calories from fat (admixture of hydrogenated soybean oil, coconut oil, and lard), 20% calories from fructose, 16.5% calories from protein, 8.5% calories from casein, 2% cholesterol by weight, 0.7% sodium cholate by weight, and methionine and choline at concentrations of 3,500 and 700 ppm, respectively.
All animals were given ∼6 h of free access to feed each day and unlimited access to water. After 24 wk, animals were euthanized by excision of the heart under general anesthesia as previously described (10, 22, 28, 29).
Phenotypic and Laboratory Measurements
Body weights were obtained at the beginning of the study and on a weekly basis. Insulin resistance was assessed by the homeostatic model assessment method (HOMA) (17). In plasma samples, total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and triglycerides were measured by standard methods (10). Serum liver biochemistries were measured by a local clinical laboratory (Antech Diagnostics, Fishers, IN).
Liver Histology
At euthanasia, a portion of the liver left lobe was collected in 2-ml vials, flash frozen in liquid nitrogen, and stored at −80°C until processing. Frozen liver tissue was fixed in 10% Zn-buffered formalin, processed, and embedded in paraffin for hematoxylin-eosin, trichrome, periodic acid-Schiff, Oil Red O, and lysosomal staining. The stains were examined by light microscopy and blindly scored as previously described (17).
Serum Proteomics Analysis
Sample preparation.
Proteins were extracted from <100 μl serum in lysis buffer containing 8 M urea and 10 mM DTT as previously described (32). High-abundance proteins in the serum samples were depleted by SepproTip columns, and protein concentrations were measured by Bradford assay (6). Protein extracts were reduced and alkylated with DTT, iodoacetamide, triethylphosphine, and iodoethanol as previously described (12). Protein mixtures were digested with trypsin and filtered with 0.45-μm spin filters before being applied to the HPLC machine. Stability of the HPLC system and the MS instrument was assessed by adding chicken lysozyme to each sample (at a constant amount) prior to tryptic digestion as an internal reference for assessment of technical variations.
LC/MS/MS.
In random order, peptides (20 μg) were injected onto an Agilent 1,100 nano-HPLC system (Agilent Technologies, Santa Clara, CA) with a C18 capillary column. Peptides were eluted with a linear gradient from 5 to 45% acetonitrile developed over 120 min at a flow rate of 500 nl/min, and effluent was electrosprayed into the LTQ mass spectrometer (Thermo Fisher Scientific, Waltham, MA). Data were collected in the “Triple Play” (MS scan, Zoom scan, and MS/MS scan) mode and filtered and analyzed by a proprietary algorithm (14). Database searches against a porcine database (derived from sequence data available at Pubmed Protein) were carried out using both the X!Tandem (8) and SEQUEST (11) algorithms.
Protein ID.
Proteins were classified from priority 1 [highest identification (ID) confidence] to priority 4 (lowest ID confidence) based on ID quality. The confidence in protein ID is increased with 1) increasing peptide ID confidence and 2) a greater number of distinct amino acid sequences identified.
The “peptide ID confidence” [ID quality of the amino acid sequence(s)] of the “best peptide” (the peptide with the highest peptide ID confidence) was used to assign each protein to a “high” (between 90 and 100% ID confidence), “moderate” (between 75 and 89% ID confidence), or “low” (less than 75% ID confidence) ID category, and all low category proteins were discarded. Proteins were also categorized depending on the number of distinct amino acid sequences identified. High category proteins were considered priority 1 if multiple (≥2) unique sequences with 90–100% ID confidence were identified; otherwise, they were ranked as priority 2. Moderate category proteins were considered priority 3 if multiple (≥2) unique sequences with 75–89% ID confidence were identified; otherwise, they were ranked as priority 4. The X!Tandem (8) and SEQUEST (11) algorithms were used for amino acid sequence ID as previously described (15). Briefly, each algorithm compared the observed peptide MS/MS spectrum and theoretically derived spectrums from the database to assign quality scores that were combined with other predictors in a proprietary algorithm to assign the overall score, “% ID confidence,” to each peptide.
Protein quantification.
Quantification of proteins was performed as previously described (14). Briefly, raw files were acquired from the LTQ mass spectrometer and all extracted ion chromatograms were aligned by retention time. Area under the curve (AUC) for each individually aligned peak from each sample was measured, normalized, and compared for relative abundance.
Biostatistical analysis.
ANOVA was used to detect significant changes in protein expression among diet groups. Randomization of the order of measurement and “quantile normalization” were used to eliminate technical bias and normalize the data (5). A log2 scale (one unit difference on this log scale is equivalent to a twofold change) was used for normalization. From the ANOVA model a P value was obtained (an estimate of the false positive rate). Proprietary statistical methods were used to transform the P value to a q value, a number that estimates the false discovery rate. The P value threshold was fixed to control the false discovery rate at 5% (<0.05). The definition of a protein with a “significant change” or “differential expression” was a change in protein expression between any two diet groups with a q value <0.05. For each protein a separate ANOVA model was fit using PROC MIXED in SAS software (Version 9) (SAS Institute, Cary, NC):
where Log2 (Intensity) is the protein intensity based on the weighted average of the quantile normalized log base 2 peptide intensities, Group Effect is the fixed effects (not random) caused by the experimental conditions or treatments that are being compared, and Sample Effect (nested within group) is the random effects from individual biological samples and sample preparation.
Positive, negative, and absolute values of fold changes among groups were calculated for all proteins. The median % coefficient of variation for each protein priority level, given on a % scale, was computed by dividing the standard deviation by the mean on the AUC scale.
Additional Statistical Analyses
All priority 1 proteins were considered for characterization of biological function and comparison with human proteomics data that we recently published (4). Basic descriptive statistics (means and standard deviations) were used to characterize the study groups. ANOVA was used to determine the significance of differences in clinical characteristics and serum/liver biochemistries between diet groups. Spearman rank correlations were used to detect the associations between lipid panel parameters and liver enzymes and expression of all priority 1 proteins. When appropriate, stepwise regression analysis was performed to take into account the linear effect of several independent variables (proteins) predicting the dependent variables (lipid panel parameters and liver enzymes). Statistical analyses were performed by use of SPSS 16.0 for Windows (SPSS, Chicago, IL). A P value <0.05 was considered statistically significant.
RESULTS
Pig Phenotypes
Serum from 18 pigs was used for proteomic analysis in this study, and Table 1 shows characteristics of the three different diet groups at the time of euthanasia. Compared with control animals, pigs in the atherogenic and M-ath diet groups weighed more at time of euthanasia (P = 0.034) and had significantly elevated total, LDL, and HDL cholesterol levels (all P < 0.001) and a greater LDL-to-HDL ratio (P < 0.001). Furthermore, total and LDL cholesterol levels (both P = 0.002), LDL-to-HDL ratio (P < 0.001), triglycerides (P < 0.001), and AST (P = 0.001) were significantly greater in the M-ath diet group compared with the atherogenic diet group. Although there were trends for elevated fasting glucose, insulin, and HOMA score, these measures were not significantly different when the M-ath diet group was compared with the other diet groups, likely because of the small sample size. Resulting liver injury, with histological features closely resembling human NASH, is described in detail in our previous report (17). Briefly, pigs in the control group had normal liver histology, pigs in the atherogenic diet group exhibited lipid accumulation in Kupffer cells and hepatocytes in the absence of inflammation and fibrosis, and pigs in the M-ath diet group displayed extensive steatosis and foamy Kupffer cell changes, hepatocyte ballooning, inflammation, and pericellular fibrosis.
Table 1.
Characteristics of Ossabaw miniature pigs at time of euthanasia
| Control Chow Group (n = 7) | Atherogenic Diet Group (n = 5) | M-Ath Diet Group (n = 6) | |
|---|---|---|---|
| Sex, male/female | 2/5 | 0/5 | 0/6 |
| Weight at euthanasia, kg | 53 ± 2 | 94 ± 4* | 86 ± 14* |
| Mean weight gain, kg | 14 ± 1 | 54 ± 4* | 38 ± 13 |
| Glycemic measures | |||
| Fasting glucose, mg/dl | 79 ± 3 | 87 ± 2 | 88 ± 6 |
| Fasting insulin, μU/ml | 11 ± 2 | 15 ± 1 | 18 ± 3 |
| HOMA | 2 ± 0.3 | 3 ± 0.3 | 4 ± 1 |
| Lipid measures | |||
| Total cholesterol, mg/dl | 81 ± 4 | 328 ± 32* | 629 ± 80*† |
| LDL cholesterol, mg/dl | 36 ± 4 | 242 ± 27* | 520 ± 68*† |
| HDL cholesterol, mg/dl | 41 ± 4 | 79 ± 6* | 83 ± 5* |
| LDL/HDL Ratio | 1 ± 0.2 | 3 ± 0.3* | 6 ± 1*† |
| Triglycerides, mg/dl | 24 ± 3 | 37 ± 2 | 130 ± 17*† |
| Liver biochemistries | |||
| AST, U/l | 32 ± 3 | 27 ± 1 | 100 ± 21*† |
| ALT, U/l | 46 ± 7 | 33 ± 1 | 41 ± 12 |
| ALP, U/l | 73 ± 11 | 110 ± 10 | 273 ± 110* |
| Total bilirubin, mg/dl | 0.2 ± 0.02 | 0.2 ± 0.02 | 0.3 ± 0.04 |
Values expressed are means ± SE.
P < 0.05 vs. control chow group.
P < 0.05 vs. atherogenic diet group.
M-Ath, modified atherogenic; HOMA, homeostatic model assessment method; LDL, low-density lipoprotein; HDL, high-density lipoprotein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase.
Proteomic Profiling
The overall findings from the global serum protein analysis are summarized in Table 2. A total of 1,096 proteins were identified and quantified, of which 182 were identified with multiple unique amino acid sequences and high peptide ID confidence (priority 1). There was a significant change observed in the protein expression level (q < 0.05) between any two diet groups for 20 of these proteins. The significant changes observed between groups were further described in a pairwise fashion as shown in Table 3. Of the priority 1 proteins identified, there were four proteins with significant changes observed between the control and atherogenic diet groups and all of these proteins were also significantly different (q < 0.05) when the control and M-ath diet groups were compared. Seven proteins were differentially expressed between the atherogenic diet group and M-ath diet group, and expression of three of these proteins also changed significantly between the control and M-ath diet groups. These differentially expressed proteins and the similarities and differences among groups are summarized in Fig. 1.
Table 2.
Summary of all identified proteins
| Protein Priority | Peptide ID Confidence | Multiple Sequences Quantified | Number of Proteins | Number of Significant Changes* | Maximum Absolute Fold Change | Median % Coeffecient of Variation |
|---|---|---|---|---|---|---|
| 1 | High | Yes | 182 | 20 | 2.2 | 20.8 |
| 2 | High | No | 342 | 54 | 16.1 | 22.8 |
| 3 | Moderate | Yes | 68 | 13 | 2.6 | 18.3 |
| 4 | Moderate | No | 504 | 75 | 4.8 | 22.6 |
| Overall | 1,096 | 162 | 16.1 | 21.9 |
False Discovery Rate (FDR) <5% (q < 0.05).
Table 3.
Pairwise summary of significant changes among diet groups
| Number of Significant Changes Between Groups (q < 0.05) |
|||||
|---|---|---|---|---|---|
| Protein Priority | Number of Proteins | Control Chow vs. Atherogenic Diet Group | Control Chow vs. M-Ath Diet Group | Control Chow vs. Both Atherogenic Diet Groups | Atherogenic Diet Group vs. M-Ath Diet Group |
| 1 | 182 | 4 | 16 | 4 | 7 |
| 2 | 342 | 5 | 52 | 5 | 12 |
| 3 | 68 | 5 | 12 | 4 | 2 |
| 4 | 504 | 10 | 71 | 10 | 20 |
| Overall | 1,096 | 24 | 151 | 23 | 41 |
Fig. 1.
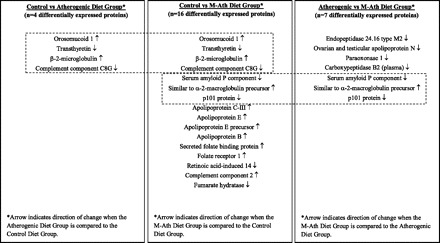
List of 20 differentially expressed priority 1 proteins and the similarities and differences among diet groups. Comparison of all priority 1 proteins with a significant change (q < 0.05) revealed 4 common proteins with differential expression when comparing both the control and atherogenic diet groups and the control and modified atherogenic (M-ath) diet groups (compare left and middle columns). Expression of 3 proteins changed significantly (q < 0.05) when comparing both the control and M-ath diet groups and the atherogenic and M-ath diet groups (compare middle and right columns). Additional proteins that changed significantly when comparing groups are also listed.
Characterization of Identified Proteins
An all-inclusive list of priority 1 proteins with a significant change among any two diet groups, sorted by fold change, is shown in Table 4. Protein ID numbers, relative expression levels between groups, and a biological description of protein function are listed. The diverse set of biological processes in which these proteins are involved in are also summarized in Table 5. Generally, proteins fell into one of six expression patterns: 1) decreased with progression of liver injury, 2) increased with progression of liver injury, 3) decreased in NASH/M-ath diet group, 4) increased in NASH/M-ath diet group, 5) decreased in control/standard chow group, and 6) increased in control/standard chow group. As depicted in Fig. 2, A–F, a representative protein from each of these groups was chosen and the mean protein intensity (log2) and standard error for these six proteins is shown. Correlations between expression of all priority 1 proteins and components of the lipid panel and liver enzymes that changed significantly among diet groups were also analyzed, and these data are presented in Supplementary Table S1 (supplemental material for this article is available online at the American Journal of Physiology Gastrointestinal and Liver Physiology website). Upon stepwise regression, we found that apolipoprotein E and orosomucoid 1 were independent predictors of both total cholesterol and LDL cholesterol. Furthermore, carboxypeptidase B2 (plasma) expression was independently associated with HDL cholesterol, β-2-microglobulin expression was independently associated with triglycerides, secreted folate binding protein expression was independently associated with AST, and folate receptor 1 expression was independently associated with ALP.
Table 4.
Summary of 20 differentially expressed priority 1 proteins (q < 0.05)
| Mean Protein Intensity ± SE (log2)* |
|||||||
|---|---|---|---|---|---|---|---|
| Protein ID | Annotation | Maximum Fold Change | Minimum q value | Control Chow Group | Atherogenic Diet Group | M-Ath Diet Group | Protein Function |
| 50657386 | Apolipoprotein C-III | 2.20707799 | 0.0394173 | 13.89 ± 0.23 | 14.61 ± 0.28 | 15.03 ± 0.23† | Lipoprotein component important for triglyceride exchange, transport and metabolism |
| 2388609 | Apolipoprotein E | 2.13375032 | 0.0067329 | 13.35 ± 0.17 | 13.96 ± 0.20 | 14.45 ± 0.17† | Lipoprotein component important for cholesterol exchange, transport and metabolism |
| 47523674 | Apolipoprotein E precursor | 2.13318557 | 0.0067329 | 13.29 ± 0.17 | 13.91 ± 0.20 | 14.38 ± 0.17† | Lipoprotein component important for cholesterol exchange, transport and metabolism |
| 194033903 | Orosomucoid 1 | 2.10644948 | 0.005958 | 14.65 ± 0.12 | 15.73 ± 0.14† | 15.43 ± 0.12† | Acute phase protein and immune system regulation |
| 951375 | Apolipoprotein B | 1.82428754 | 0.0018515 | 13.17 ± 0.10 | 13.55 ± 0.12 | 14.04 ± 0.10† | LDL component important for cholesterol exchange, transport and metabolism |
| 55742849 | Serum amyloid P component | 1.63619691 | 0.0067329 | 13.75 ± 0.11 | 13.71 ± 0.13 | 13.04 ± 0.11†‡ | Immune system regulation and comprises extracellular matrices |
| 47523188 | Secreted folate binding protein | 1.62591648 | 0.0067329 | 13.06 ± 0.11 | 13.18 ± 0.13 | 13.76 ± 0.11† | Transport, protection and delivery of folic acid in extracellular fluids |
| 47523508 | Transthyretin | 1.48109011 | 0.0024211 | 15.75 ± 0.07 | 15.24 ± 0.08† | 15.18 ± 0.07† | Carrier of thyroxine (T4) hormone and aggregates as amyloid structures |
| 47523688 | Folate receptor 1 | 1.44460772 | 0.0214633 | 14.74 ± 0.10 | 14.83 ± 0.12 | 15.27 ± 0.10† | High affinity folate receptor responsible for transport of folic acid into cells |
| 1871390 | Endopeptidase 24.16 type M2 | 1.42329757 | 0.0280159 | 16.42 ± 0.08 | 16.61 ± 0.09 | 16.10 ± 0.08‡ | Metalloendopeptidase that preferentially cleaves short peptides |
| 51491902 | Ovarian and testicular apolipoprotein N | 1.4186694 | 0.0384757 | 14.15 ± 0.08 | 14.35 ± 0.10 | 13.85 ± 0.08‡ | Lipoprotein component found in serum and synthesized in reproductive tissues |
| 167621416 | Paraoxonase 1 | 1.39969355 | 0.0041129 | 14.09 ± 0.05 | 14.35 ± 0.06 | 13.87 ± 0.05‡ | Degrades oxidized lipids and anti-inflammatory actions |
| 194037847 | Similar to α-2-macroglobulin precursor | 1.37850584 | 0.0280159 | 14.50 ± 0.07 | 14.39 ± 0.08 | 14.85 ± 0.07†‡ | Acute phase protein/immune system regulation and coagulation |
| 188035850 | β-2-Microglobulin | 1.35057716 | 0.0293498 | 13.60 ± 0.07 | 14.03 ± 0.08† | 13.92 ± 0.07† | Component of MHC molecules involved in immune system regulation |
| 190360623 | Retinoic acid-induced 14 | 1.34441846 | 0.0067964 | 13.60 ± 0.07 | 13.40 ± 0.08 | 13.17 ± 0.07† | Developmental protein expressed in reproductive tissues and induced by retinoic acid |
| 156120138 | Complement component 2 | 1.28693895 | 0.0076254 | 13.62 ± 0.06 | 13.89 ± 0.07 | 13.98 ± 0.06† | Immune system regulation and inflammation |
| 47523636 | Fumarate hydratase | 1.26642198 | 0.0067329 | 14.24 ± 0.05 | 14.06 ± 0.06 | 13.90 ± 0.05† | Enzyme that metabolizes fumarate to malate/involved in amino acid metabolism |
| 47523152 | p101 protein | 1.23724734 | 0.0418707 | 14.76 ± 0.05 | 14.81 ± 0.06 | 14.51 ± 0.05†‡ | Regulatory subunit of the Phosphoinositide 3-Kinase γ enzyme/inflammatory processes |
| 194040626 | Carboxypeptidase B2 (plasma) | 1.22488625 | 0.0077865 | 14.37 ± 0.04 | 14.54 ± 0.04 | 14.24 ± 0.04‡ | Pro-thrombotic enzyme that inhibits fibrinolysis |
| 148223227 | Complement component C8G | 1.16525069 | 0.0281536 | 13.46 ± 0.03 | 13.24 ± 0.04† | 13.30 ± 0.03† | Immune system regulation and inflammation |
Log2 scale: a difference of one unit is equivalent to a twofold change.
q < 0.05 vs. control chow group;
q < 0.05 vs. Atherogenic Diet Group.
MHC, major histocompatability complex.
Table 5.
Summary of biological functions of differentially expressed priority 1 proteins (q < 0.05)
| Biological Process | Number of Proteins | Protein List |
|---|---|---|
| Immune system regulation and inflammation | 7 | Orosomucoid 1, serum amyloid P component, similar to α-2-macroglobulin precursor, β-2-microglobulin, complement component 2, p101 protein, complement component C8G |
| Cholesterol and triglyceride balance (lipoprotein components) | 5 | Apolipoprotein C-III, apolipoprotein E, apolipoprotein E precursor, apolipoprotein B, ovarian and testicular apolipoprotein N |
| Structural and extracellular matrix proteins | 3 | Serum amyloid P component, transthyretin, endopeptidase 24.16 type M2 |
| Blood carrier proteins | 3 | Secreted folate binding protein, transthyretin, folate receptor 1 |
| Coagulation cascade | 2 | Similar to α-2-macroglobulin precursor, carboxypeptidase B2 (plasma) |
| Cellular metabolism | 1 | Fumarate hydratase |
| Anti-inflammatory and anti-oxidant | 1 | Paraoxonase 1 |
| Gene/protein processing and expression | 1 | Retinoic acid-induced 14 |
| Protease | 1 | Endopeptidase 24.16 type M2 |
Fig. 2.
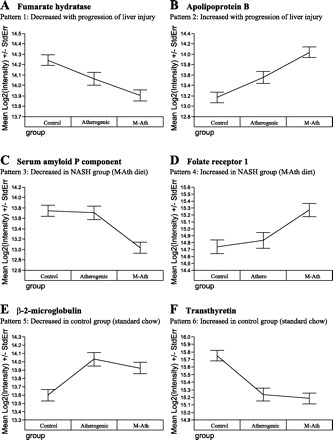
Representative proteins with significant differential expression between diet groups. Six general patterns for changes in protein expression among diet groups were noted, and a representative protein from each group is displayed (A–F). The mean log2 intensity ± SE (y-axis) is shown for each of these proteins (a difference of one unit is equivalent to a twofold change). NASH, nonalcoholic steatohepatitis; StdErr, standard error.
Comparison With Our Recent Human Study
Comparison of the results of the present proteomics study conducted in pigs to a recent serum proteomics study that we performed using serum from patients across the spectrum of NAFLD and obese controls (4) revealed seven common priority 1 proteins with differential expression (Fig. 3). Changes in expression levels of several of these proteins were similar the human and pig studies, and a list of these proteins is shown in Table 6.
Fig. 3.
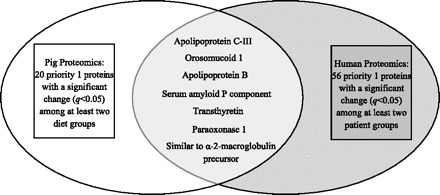
Common priority 1 proteins identified in both pig and human serum proteomics studies. Comparison of all 20 priority 1 proteins with a significant change (q < 0.05) identified in the pig proteomics analysis and all 56 priority 1 proteins with a significant change (q < 0.05) identified in the human proteomics analysis (4) revealed 7 proteins common to both species.
Table 6.
Expression levels of 7 common priority 1 proteins with significant (q < 0.05) differential expression identified in both pig and human serum proteomics studies
| Pig Serum Proteomics Study |
Human Serum Proteomics Study |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Protein Intensity ± SE (log2)* |
Mean Protein Intensity ± SE (log2)* |
|||||||||||
| Annotation | Maximum Fold Change | Minimum q value | Control Chow Group | Atherogenic Diet Group | M-Ath Diet Group | Annotation | Maximum Fold Change | Minimum q value | Control | Simple Steatosis | NASH | NASH + Advanced Fibrosis |
| Apolipoprotein C-III | 2.20707799 | 0.03941731 | 13.89 ± 0.23 | 14.61 ± 0.28 | 15.03 ± 0.23† | Apolipoprotein C3 | 1.19 | 0.0477 | 14.67 ± 0.07 | 14.54 ± 0.06 | 14.64 ± 0.06 | 14.43 ± 0.06† |
| Orosomucoid 1 | 2.10644948 | 0.00595803 | 14.65 ± 0.12 | 15.73 ± 0.14† | 15.43 ± 0.12† | α-1-acid glycoprotein 1 | 1.40 | 0.02348 | 15.05 ± 0.13 | 15.35 ± 0.10 | 15.21 ± 0.10 | 14.87 ± 0.11‡ |
| Apolipoprotein B | 1.82428754 | 0.0018515 | 13.17 ± 0.10 | 13.55 ± 0.12 | 14.04 ± 0.10† | Apolipoprotein B100 | 1.19 | 0.04079 | 15.06 ± 0.07 | 15.21 ± 0.06 | 15.31 ± 0.06† | 15.23 ± 0.06 |
| Serum amyloid P component | 1.63619691 | 0.00673285 | 13.75 ± 0.11 | 13.71 ± 0.13 | 13.04 ± 0.11†‡ | Serum amyloid P component | 1.36 | 0.000000 | 15.85 ± 0.05 | 15.69 ± 0.04 | 15.61 ± 0.04† | 15.40 ± 0.04†‡§ |
| Transthyretin | 1.48109011 | 0.00242107 | 15.75 ± 0.07 | 15.24 ± 0.08† | 15.18 ± 0.07† | Transthyretin | 1.29 | 0.00029 | 15.91 ± 0.06 | 15.86 ± 0.05 | 15.75 ± 0.05 | 15.55 ± 0.05†‡ |
| Paraoxonase 1 | 1.39969355 | 0.00411286 | 14.09 ± 0.05 | 14.35 ± 0.06 | 13.87 ± 0.05‡ | Paraoxonase 1 | 1.58 | 0.000000 | 14.29 ± 0.05 | 13.68 ± 0.04† | 13.63 ± 0.04† | 13.64 ± 0.04† |
| Similar to α-2-macroglobulin precursor | 1.37850584 | 0.02801592 | 14.50 ± 0.07 | 14.39 ± 0.08 | 14.85 ± 0.07†‡ | α-2-macroglobulin | 1.35 | 0.00548 | 14.43 ± 0.10 | 14.45 ± 0.08 | 14.57 ± 0.08 | 14.86 ± 0.08†‡ |
*log2 scale: a difference of one unit is equivalent to a twofold change. †q < 0.05 vs. control chow group; ‡q < 0.05 vs. atherogenic diet group for the pig study. †q < 0.05 vs. control; ‡q < 0.05 vs. simple steatosis; §q < 0.05 vs. nonalcoholic steatohepatitis (NASH) for the human study. For the human study, group sizes were as follows: control (n = 16), simple steatosis (n = 24), NASH (n = 23), NASH + advanced fibrosis (n = 22).
DISCUSSION
Quantitative proteomics technologies have been rapidly advancing and improving, making large global serum studies possible. We used a popular ion-intensity-based LFQP approach that has become increasingly common as MS instrumentation and performance has improved (21, 31, 33, 36). Using this LFQP approach, we identified over 1,000 proteins in the serum samples obtained from Ossabaw pigs fed one of three diets. Overall, expression of 162 proteins differed significantly (q < 0.05) between any two diet groups. When healthy, lean control pigs were compared with either of the two atherogenic diet groups, expression of 23 serum proteins changed significantly. Furthermore, there were 41 proteins that were significantly different between pigs fed an atherogenic diet and those fed the M-ath diet. Because pigs fed the atherogenic diet do not have as severe of a phenotype as those fed the M-ath diet, serum proteins with significant differential expression may provide information about the pathogenesis of diet-induced NASH in our Ossabaw pig model, and comparison of these proteins to those identified in our recent human serum proteomics study (4) further establishes the relevance of our animal model to human disease.
The design of our study does not allow us to distinguish between changes in the serum proteome induced by diet vs. those induced by liver pathology. Despite this caveat, the biological significance of all priority 1 proteins with a significant change (q < 0.05) was explored. Interestingly, the functions of many of these proteins have been linked to the development of NAFLD and NASH in humans. Many proteins with significantly altered expression are involved in immune system regulation and inflammation, including several acute-phase proteins and complement system proteins. In agreement with our findings in Ossabaw pigs, several previous studies have shown that serum levels of acute-phase proteins (i.e., C-reactive protein, α-2-macroglobulin, ferritin) are altered in human NASH compared with control subjects (16, 25, 34, 35). However, we observed a significant increase in serum levels of orosomucoid 1 (α-1-acid glycoprotein) in pigs fed an atherogenic or M-ath diet, whereas a previous human study did not report this increase in patients with NASH compared with healthy controls (16). Interestingly, we detected a significant decrease in circulating serum amyloid P component (SAP) levels in pigs fed the M-ath diet, which was unexpected considering that SAP is an acute-phase protein involved in innate immunity and produced in the liver in response to interleukin-6 (9). We also found that complement component proteins 2 and C8G changed significantly in pigs fed either an atherogenic diet or the M-ath diet. Complement proteins have been previously identified as playing a role in several conditions associated with human NASH, including development of hepatic fibrosis and hepatocyte injury and regeneration (24). Structural and extracellular matrix proteins in the liver may also be important in fatty liver disease, especially in development of hepatic fibrosis and cirrhosis. We observed a significant decrease in serum levels of transthyretin (prealbumin) in the atherogenic- and M-ath-fed pigs, a finding in agreement with a human study by Helling et al. (13) in which decreased prealbumin levels were found to be an independent predictor of NASH in a bariatric surgery population. It is important to note that transthyretin functions not only as a structural protein, but also as a carrier of thyroxine in the blood, and thyroid dysfunction is one of several endocrine disorders associated with NASH in humans (18).
Both the atherogenic and M-ath diets induced significant changes in circulating levels of proteins involved in cholesterol and lipid balance and metabolism, and dyslipidemia and hypercholesterolemia were observed in these animals as a component of the metabolic syndrome. Paraoxonase 1, which is associated with HDL particles and exhibits antioxidant and anti-inflammatory properties, was significantly reduced in animals fed the M-ath diet. In humans, paraoxonase 1 inhibits oxidation of LDL particles and lipid peroxidation, and levels are reduced in cardiovascular disease, diabetes, chronic liver impairment, and cirrhosis (7). Conversely, apolipoprotein E was elevated in the atherogenic diet group and significantly increased in pigs fed the M-ath diet. Apolipoprotein E is a component of triglyceride-rich lipoproteins, including chylomicrons, and this overexpression is likely a compensatory mechanism for the high-fat, high-cholesterol diet consumed by the atherogenic diet groups, similar to that reported in a recent LDL proteomic analysis of Yucatan pigs fed an atherogenic diet (26). Similarly, apolipoprotein B is a main component of LDL cholesterol particles and elevations in serum apolipoprotein B are an excellent predictor of ischemic cardiovascular and heart disease in humans (27). Apolipoprotein C-III is associated with triglyceride-rich lipoproteins, and a recent in vivo study in humans demonstrated that acute elevation of plasma free fatty acids stimulates both apolipoprotein C-III and triglyceride-rich lipoprotein production (23). Interestingly, expression of both apolipoproteins B and C-III was significantly increased in the M-ath diet group.
The need for well-characterized animal models of NASH that accurately reflect the metabolic profile observed in humans is critical. Although rodents are commonly used to study NASH and have provided valuable mechanistic information, there are important limitations to these models that reduce their relevance to human disease. We recently developed, to our knowledge, the first large animal model of nutritionally induced severe metabolic syndrome and steatohepatitis (17), with the advantages of having anatomy and physiology more similar to that of a human and having the possibility of following the pigs for long periods of time. By identifying several serum proteins that changed significantly and had been previously implicated in the pathogenesis of human NASH, this proteomics study further establishes the relevance of our pig model to the study of human disease. In fact, several differentially expressed priority 1 proteins identified in our Ossabaw pig model were also identified as changing significantly in our recent serum proteomics study performed in humans with NAFLD and NASH (4). As discussed in detail above, these seven proteins exhibit a range of biological functions previously associated with human NAFLD and NASH. Of particular interest are three of these proteins (SAP component, paraoxonase 1, and α-2-macroglobulin/similar to α-2-macroglobulin precursor) that exhibit similar significant expression changes in humans and pigs with the most severe phenotypes (NASH + advanced fibrosis and M-ath diet groups, respectively).
Findings from this study reiterate the value of the LFQP approach for identifying proteins of interest that change significantly between groups on a global scale. However, there are several limitations of our study that require discussion. We included only a small number of numerically unmatched animals, owing to the practical issues of obtaining and caring for a large number of pigs simultaneously. Another limitation of our study is the incompleteness of the porcine database. As the popularity of both this large animal model and LFQP continues to grow, data available for these types of analyses will become much more comprehensive. Finally, as mentioned previously, it is not possible to distinguish diet-induced changes vs. pathology/disease-induced changes in our model. However, we were able to induce a histological phenotype very similar to human NASH, coupled with several features of the metabolic syndrome. This combination, although diet induced, provides a unique setting in which to further explore NASH and associated comorbidities. Further studies are needed to characterize the serum proteome at earlier time points in the dietary treatment before histopathology occurs.
In conclusion, serum proteomics analysis of our nutritionally induced model of NASH and metabolic syndrome in pigs revealed important information on proteins that change significantly among diet groups, including many that have been previously identified as playing a role in human NAFLD. These similarities provide further evidence that our pig model is useful and relevant for gaining additional information about NASH and related metabolic abnormalities.
GRANTS
This research was supported by the National Institutes of Health Grants RR013223 and HL062552 and Purina TestDiet (Richmond, IN) to M. Sturek and the Purdue-Indiana University Comparative Medicine Program. L. N. Bell is supported by a Clinical Pharmacology Training Grant to Indiana University (T32 GM08425).
DISCLOSURES
Monarch LifeSciences is a proteomic service organization that represents an academic-government-private collaboration. Dr. Chalasani has financial consulting agreements with several pharmaceutical companies but none pose a potential conflict.
Supplementary Material
REFERENCES
- 1. American Veterinary Medical Association Panel on Euthanasia Report of the AVMA panel on euthanasia. J Am Vet Med Assoc 218: 669–696, 2001. [DOI] [PubMed] [Google Scholar]
- 2. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 346: 1221–1231, 2002. [DOI] [PubMed] [Google Scholar]
- 3. Bacon BR, Farahvash MJ, Janney CG, Neuschwander-Terri BA. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology 107: 1103–1109, 1994. [DOI] [PubMed] [Google Scholar]
- 4. Bell LN, Theodorakis JL, Vuppalanchi R, Bemis KG, Wang M, Chalasani N. Serum proteomics and biomarker discovery across the spectrum of nonalcoholic fatty liver disease. Hepatology 51: 111–120, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19: 185–193, 2003. [DOI] [PubMed] [Google Scholar]
- 6. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. [DOI] [PubMed] [Google Scholar]
- 7. Camps J, Marsillach J, Joven J. The paraoxonases: role in human diseases and methodological difficulties in measurement. Crit Rev Clin Lab Sci 46: 83–106, 2009. [DOI] [PubMed] [Google Scholar]
- 8. Craig R, Beavis RC. TANDEM: matching proteins with tandem mass spectra. Bioinformatics 20: 1466–1467, 2004. [DOI] [PubMed] [Google Scholar]
- 9. Deban L, Bottazzi B, Garlanda C, de la Torre YM, Mantovani A. Pentraxins: multifunctional proteins at the interface of innate immunity and inflammation. Biofactors 35: 138–145, 2009. [DOI] [PubMed] [Google Scholar]
- 10. Dyson M, Alloosh M, Vuchetich JP, Mokelke EA, Sturek M. Components of metabolic syndrome and coronary artery disease in female Ossabaw swine fed excess atherogenic diet. Comp Med 56: 35–45, 2006. [PubMed] [Google Scholar]
- 11. Eng J, McCormack A, Yates RR. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom 5: 976–989, 1994. [DOI] [PubMed] [Google Scholar]
- 12. Hale JE, Butler JP, Gelfanova V, You JS, Knierman MD. A simplified procedure for the reduction and alkylation of cysteine residues in proteins prior to proteolytic digestion and mass spectral analysis. Anal Biochem 333: 174–181, 2004. [DOI] [PubMed] [Google Scholar]
- 13. Helling TS, Helzberg JH, Nachnani JS, Gurram K. Predictors of nonalcoholic steatohepatitis in patients undergoing bariatric surgery: when is liver biopsy indicated? Surg Obes Relat Dis 4: 612–617, 2008. [DOI] [PubMed] [Google Scholar]
- 14. Higgs RE, Knierman MD, Gelfanova V, Butler JP, Hale JE. Comprehensive label-free method for the relative quantification of proteins from biological samples. J Proteome Res 4: 1442–1450, 2005. [DOI] [PubMed] [Google Scholar]
- 15. Higgs RE, Knierman MD, Freeman AB, Gelbert LM, Patil ST, Hale JE. Estimating the statistical significance of peptide identifications from shotgun proteomics experiments. J Proteome Res 6: 1758–1767, 2007. [DOI] [PubMed] [Google Scholar]
- 16. Koruk M, Taysi S, Savas MC, Yilmaz O, Akcay F, Karakok M. Serum levels of acute phase proteins in patients with nonalcoholic steatohepatitis. Turk J Gastroenterol 14: 12–17, 2003. [PubMed] [Google Scholar]
- 17. Lee L, Alloosh M, Saxena R, Van Alstine W, Watkins BA, Klaunig JE, Sturek M, Chalasani N. Nutritional model of steatohepatitis and metabolic syndrome in the Ossabaw miniature swine. Hepatology 50: 56–67, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Loria P, Carulli L, Bertolotti M, Lonardo A. Endocrine and liver interaction: the role of endocrine pathways in NASH. Nat Rev Gastroenterol Hepatol 6: 236–347, 2009. [DOI] [PubMed] [Google Scholar]
- 19. Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 116: 1413–1419, 1999. [DOI] [PubMed] [Google Scholar]
- 20. National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press, 1996. [Google Scholar]
- 21. Ono M, Shitashige M, Honda K, Isobe T, Kuwabara H, Matsuzuki H, Hirohashi S, Yamada T. Label-free quantitative proteomics using large peptide data sets generated by nanoflow liquid chromatography and mass spectrometry. Mol Cell Proteomics 5: 1338–1347, 2006. [DOI] [PubMed] [Google Scholar]
- 22. Otis CR, Wamhoff BR, Sturek M. Hyperglycemia-induced insulin resistance in diabetic dyslipidemic Yucatan swine. Comp Med 53: 53–64, 2003. [PubMed] [Google Scholar]
- 23. Pavlic M, Valéro R, Duez H, Xiao C, Szeto L, Patterson BW, Lewis GF. Triglyceride-rich lipoprotein-associated apolipoprotein C-III production is stimulated by plasma free fatty acids in humans. Arterioscler Thromb Vasc Biol 28: 1660–1665, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qin X, Gao B. The complement system in liver diseases. Cell Mol Immunol 3: 333–340, 2006. [PubMed] [Google Scholar]
- 25. Ratziu V, Massard J, Charlotte F, Messous D, Imbert-Bismut F, Bonyhay L, Tahiri M, Munteanu M, Thabut D, Cadranel JF, Le Bail B, de Ledinghen V, Poynard T, LIDO Study Group, CYTOL study group. Diagnostic value of biochemical markers (FibroTest-FibroSURE) for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol 6: 6–18, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Richardson MR, Lai X, Dixon JL, Sturek M, Witzmann FA. Diabetic dyslipidemia and exercise alter the plasma low density lipoproteome in Yucatan pigs. Proteomics 9: 2468–2483, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pierre AC, Cantin B, Dagenais GR, Despres JP, Lamarche B. Apolipoprotein-B, low-density lipoprotein cholesterol, and the long-term risk of coronary heart disease in men. Am J Cardiol 97: 997–1001, 2006. [DOI] [PubMed] [Google Scholar]
- 28. Sturek M, Alloosh M, Wenzel J, Byrd JP, Edwards JM, Lloyd PG. Ossabaw Island miniature swine: cardiometabolic syndrome assessment. In: Swine in the Laboratory: Surgery, Anesthesia, Imaging, and Experimental Techniques, edited by Swindle MM. Boca Raton, FL: CRC, 2007. [Google Scholar]
- 29. Swindle MM. Swine in the Laboratory: Surgery, Anesthesia, Imaging, and Experimental Techniques. Boca Raton, FL: CRC, 2007. [Google Scholar]
- 30. Teli MR, James OF, Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology 22: 1714–1719, 1995. [PubMed] [Google Scholar]
- 31. Wang G, Wu WW, Zeng W, Chou CL, Shen RF. Label-free protein quantification using LC-coupled ion trap or FT mass spectrometry: reproducibility, linearity, and application with complex proteomes. J Proteome Res 5: 1214–1223, 2006. [DOI] [PubMed] [Google Scholar]
- 32. Wang M, You J, Bemis KG, Tegeler TJ, Brown DP. Label-free mass spectrometry-based protein quantification technologies in proteomic analysis. Brief Funct Genomic Proteomic 7: 329–339, 2008. [DOI] [PubMed] [Google Scholar]
- 33. Yan W, Chen SS. Mass spectrometry-based quantitative proteomic profiling. Brief Funct Genomic Proteomic 4: 27–38, 2005. [DOI] [PubMed] [Google Scholar]
- 34. Yoneda M, Mawatari H, Fujita K, Iida H, Yonemitsu K, Kato S, Takahashi H, Kirikoshi H, Inamori M, Nozaki Y, Abe Y, Kubota K, Saito S, Iwasaki T, Terauchi Y, Togo S, Maeyama S, Nakajima A. High-sensitivity C-reactive protein is an independent clinical feature of nonalcoholic steatohepatitis (NASH) and also of the severity of fibrosis in NASH. J Gastroenterol 42: 573–582, 2007. [DOI] [PubMed] [Google Scholar]
- 35. Yoneda M, Nozaki Y, Endo H, Mawatari H, Iida H, Fujita K, Yoneda K, Takahashi H, Kirikoshi H, Inamori M, Kobayashi N, Kubota K, Saito S, Maeyama S, Hotta K, Nakajima A. Serum ferritin is a clinical biomarker in Japanese patients with nonalcoholic steatohepatitis (NASH) independent of HFE gene mutation. Dig Dis Sci 55: 808–814, 2009. [DOI] [PubMed] [Google Scholar]
- 36. Zhang B, VerBerkmoes NC, Langston MA, Uberbacher E, Hettich RL, Samatova NF. Detecting differential and correlated protein expression in label-free shotgun proteomics. J Proteome Res 5: 2909–2918, 2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


