Abstract
The enteric nervous system may have an important role in modulating gastrointestinal barrier response to disease through activation of enteric glia cells. In vitro studies have shown that enteric glia activation improves intestinal epithelial barrier function by altering the expression of tight junction proteins. We hypothesized that severe injury would increase expression of glial fibrillary acidic protein (GFAP), a marker of enteric glial activation. We also sought to define the effects of vagal nerve stimulation on enteric glia activation and intestinal barrier function using a model of systemic injury and local gut mucosal involvement. Mice with 30% total body surface area steam burn were used as model of severe injury. Vagal nerve stimulation was performed to assess the role of parasympathetic signaling on enteric glia activation. In vivo intestinal permeability was measured to assess barrier function. Intestine was collected to investigate changes in histology; GFAP expression was assessed by quantitative PCR, by confocal microscopy, and in GFAP-luciferase transgenic mice. Stimulation of the vagus nerve prevented injury-induced intestinal barrier injury. Intestinal GFAP expression increased at early time points following burn and returned to baseline by 24 h after injury. Vagal nerve stimulation prior to injury increased GFAP expression to a greater degree than burn alone. Gastrointestinal bioluminescence was imaged in GFAP-luciferase transgenic animals following either severe burn or vagal stimulation and confirmed the increased expression of intestinal GFAP. Injection of S-nitrosoglutathione, a signaling molecule released by activated enteric glia cells, following burn exerts protective effects similar to vagal nerve stimulation. Intestinal expression of GFAP increases following severe burn injury. Stimulation of the vagus nerve increases enteric glia activation, which is associated with improved intestinal barrier function. The vagus nerve may mediate the signaling that occurs from the central nervous system to the enteric nervous system following gastrointestinal injury.
Keywords: glial fibrillary acidic protein, GFAP-luc, tight junction, splenectomy, S-nitrosoglutathione
the enteric nervous system (ENS) regulates and controls function of the gastrointestinal tract, responding to luminal contents, mechanical stimulation, and inflammation (13). The ENS can function independently through pathways in the myenteric and submucosal plexus or respond to central nervous system (CNS) input through sympathetic and parasympathetic pathways. Enteric glia cells are the predominant cell type in the ENS and are similar in structure and function to astrocytes of the CNS (18). Glia cells express glial fibrillary acidic protein (GFAP) upon activation, increasing the number of GFAP-expressing cells following injury (16).
The enteric glia plays an important role in modulating gut inflammation and maintaining intestinal barrier integrity following injury. Genetic ablation of enteric glia cells results in severe inflammation and fatal hemorrhagic necrosis of the gut, highlighting the critical role of the enteric glia in maintaining intestinal integrity (5). Enteric glia has been shown to promote intestinal barrier integrity in vitro by increasing expression of the tight junction proteins occludin and zonula occludens protein-1 (ZO-1) (20). Enteric glia becomes activated in response to inflammation, with increased GFAP expression from enteric glia cells after the in vitro addition of either proinflammatory cytokines or LPS (24).
Multiple medical conditions including inflammatory bowel disease, necrotizing enterocolitis, trauma, and severe burn result in significant intestinal barrier injury and serious systemic sequelae. We have previously studied the intestinal injury associated with severe burn, finding changes in tight junction proteins expression that are associated with increased intestinal permeability at early time points following injury (8, 9). The restoration of small intestinal barrier function requires a complex set of events that are initiated within minutes of injury and are characterized by epithelial cell restitution and reassembly of tight junction proteins to close the paracellular space (3). The mechanisms for intestinal repair following injury are multifactorial, with little understanding of the role the enteric nervous system plays in this crucial process.
In this series of experiments, we hypothesized that intestinal injury would result in activation of enteric glia cells in an in vivo model of severe burn injury. We also postulated that vagal nerve stimulation would activate enteric glia and improve intestinal barrier integrity following severe injury. Improved understanding of the response of the enteric glia to intestinal injury may allow for the development of therapies aimed at limiting intestinal barrier breakdown and the intestinal inflammatory response to severe injury.
METHODS
Animal model of severe burn injury.
Male BALB/c mice (8–12 wk, Jackson Laboratories, Sacramento, CA) were anesthetized with inhaled isoflurane and had their dorsal fur removed. Animals were then placed in a template estimating 30% total body surface area (TBSA) burn and subjected to a steam burn for 7 s as previously described (8). Following burn injury, animals received a subcutaneous injection of 1.5 ml normal saline with buprenorphine for fluid resuscitation and pain control. At various time points following burn, animals were again anesthetized with inhaled isoflurane for tissue procurement. Sham animals underwent removal of their dorsal fur and received a subcutaneous injection of normal saline with buprenorphine but were not subjected to burn injury. All animal experiments were approved by the University of California, San Diego IACUC and were conducted in accordance with accepted guidelines for animal studies.
Vagal nerve stimulation.
A right cervical neck incision was performed and the right cervical vagus nerve exposed. Vagal nerve stimulation was performed by using a VariStim III probe (Medtronic Xomed, Jacksonville, FL) set at 2 mA at ∼1 Hz for 10 min prior to injury. Sham animals underwent right cervical incision and exposure of the vagus nerve but did not receive stimulation.
In one arm, surgical abdominal vagotomy was performed immediately prior to vagal nerve stimulation and subsequent burn injury through an upper-midline laparotomy incision. The gastroesophageal junction was identified and the dorsal and ventral vagus nerve were visualized on the distal esophagus with an Olympus SZ61 stereomicroscope (Leeds Precision Instruments, Minneapolis, MN). Both branches of the vagus nerve were isolated and divided sharply.
Splenectomy.
Mice were anesthetized with inhaled isoflurane prior to a midline laparotomy incision. The spleen was removed following appropriate blood vessel ligation. The abdomen was then closed with interrupted silk suture.
Injection of S-nitrosoglutathione.
Animals were given an intraperitoneal injection of S-nitrosoglutathione (GSNO, 10 mg/kg) immediately following burn injury (20). Tissues were harvested 4 h following injury for histological analysis.
Histopathological evaluation.
Sections of distal ileum (n = 3 animals per group) were cut 7 μm thick and stained with hematoxylin and eosin (Surgipath, Richmond, IL). Images were viewed with an Olympus IX70 light microscope (Melville, NY) and viewed with Q-imaging software (Surrey, BC, Canada). Histological gut injury was scored by a pathologist blinded to the experimental conditions. Three randomly selected fields from each specimen were graded based on a scoring system characterizing gut injury on a scale from 0 to 4: 0 = normal, no damage; 1 = mild, focal epithelial edema; 2 = moderate; diffuse swelling necrosis of the villi; 3 = severe; diffuse pathology of the villi; with evidence of neutrophil infiltration in the submucosa; 4 = major; widespread injury with massive neutrophil infiltration and hemorrhage as previously described (2).
In vivo intestinal permeability assay.
A midline laparotomy was performed 4 h following burn and the distal small intestine identified as previously described (8, 9). A 5-cm segment of small intestine was isolated between silk ties prior to intraluminal injection of 25 mg 4.4-kDa FITC-dextran (Sigma, St. Louis, MO) diluted in 200 μl phosphate-buffered saline (PBS). The bowel was then returned to the abdominal cavity and the abdominal wall was closed. Animals were maintained under general anesthesia for 30 min at which time blood was obtained via cardiac puncture. Plasma fluorescence was read in a fluorescence spectrophotometer (SpectraMax M5, Molecular Diagnostics, Sunnyvale, CA) and compared with a standard curve of FITC-dextran diluted in mouse plasma (n = 4 animals per group).
Reverse transcription PCR.
Distal ileum was preserved in RNA later solution and stored at −20°C for no longer than 6 mo. RNA was extracted from tissue and treated with DNAse via the RNAqueous 4PCR kit (Ambion). Reverse transcription was performed (n ≥ 4 animals per group) with the High Capacity cDNA Reverse Transcription kit (Applied Biosciences, Pleasanton, CA). Control for ±reverse transcription-PCR was performed separately with Platinum PCR Supermix (Invitrogen). Reverse transcription quantitative PCR reactions were run with iQ SYBR Green Supermix (Bio-Rad, Hercules, CA) for 40 cycles on a Bio-Rad iQ5 real-time PCR detection system. Cycles were run at 95°C for 3 min, 95°C for 10 s, 60°C for 30 s, 72°C for 30 s. Steps 2–4 were repeated for 40 cycles. A melt curve was obtained to ensure that only a single species was amplified. The GFAP primer forward 5′-GAGGAGGAGATCCAGTTCTTAAGGA-3′, reverse 5′-GCCTCGTATTGAGTGCGAATC-3′ was used. Samples were normalized against β-actin or rig/S15 and relative expression levels were calculated by the ΔΔCt method (25).
Western blot analysis.
Intestinal segments were harvested 4 h following burn for Western blot analysis as previously described (9). Immunoblotting was performed by using standard techniques with an antibody against myosin light chain (MLC) kinase (MLCK, Sigma) or β-actin (Cell Signaling, Danvers, MA). Images were obtained using the Xenogen IVIS Lumina imaging system and quantified by use of UN-SCAN-IT gel digitizing software (Silk Scientific, Orem, UT).
Enzyme-linked immunosorbent assay.
Tumor necrosis factor-α (TNF-α) was measured from plasma and extracts of distal ileum harvested at various time points following injury. A commercially available enzyme-linked immunosorbent assay kit was obtained from R&D Systems (Minneapolis, MN). TNF-α levels were measured in picograms per milliliter.
Confocal microscopy.
Segments of distal ileum (n = 3 animals per group) were embedded in optimal cutting temperature media and stored at −70°C. Sections of intestine were cut 10 μm thick with a Reichert-Jung Cryocut 1800 (Reichert Microscopes, Depew, NY). Sections were fixed onto glass slides with 3.7% paraformaldehyde (Electron Microscopy Series, Hatfield, PA) for 10 min then washed with PBS. Cells were permeabilized with 0.01% Triton X-100 (Sigma) for 1 min. Sections were washed in PBS then blocked for 1 h in 3% bovine serum albumin (BSA, Sigma). The sections were then incubated overnight in primary antibody against GFAP-Cy3 conjugate (Sigma), phosphorylated MLC, or 4,6-diamidino-2-phenylindole (DAPI) diluted 1:100. Sections were then treated with the secondary antibody Alexa Fluor 488 (Invitrogen) in 1% BSA for 1 h. Prolong Fade (Invitrogen) was added upon placement of coverslips. Images were viewed by using the Olympus Fluoview laser scanning confocal microscope (Olympus) with exposure-matched settings (Advanced Software v1.6, Olympus) at ×60 magnification.
Detection of in vivo bioluminescence using GFAP-luc mice.
Transgenic GFAP-luc mice with FVB/J background were obtained from Caliper Life Science (Mountain View, CA). Male GFAP-luc mice were subjected to 30% TBSA steam burn or vagal stimulation as previously described (n = 4 animals per group). At various time points following injury, animals received an intraperitoneal injection of 150 μl d-luciferin (15 mg/ml, Caliper Life Sciences) 5 min prior to in vivo imaging with the Xenogen IVIS Lumina while under general anesthesia (Caliper Life Sciences). Images were obtained with a 1-min exposure time using field of view A. Bioluminescence was analyzed by using Living Image Software (Caliper Life Sciences) with color bars standardized for animals imaged. Luminescent intensity was quantified for all animals imaged by using region of interest measurements of equivalent areas of the abdominal wall. Following in vivo imaging, animals were euthanized and the brain and intestine were removed to confirm ex vivo bioluminescence from these tissues. Transgenic mice also underwent PCR analysis of tail tips to confirm presence of the GFAP-luc transgene. Nontransgenic FVB/J mice were imaged to show background fluorescence.
Statistical analysis.
Data are expressed as means ± SE. The statistical significance among groups was determined by t-test or analysis of variance with Bonferroni correction where appropriate. Statistical significance for gut injury scoring was performed by the Kruskal-Wallis test for nonparametric data with post hoc Mann-Whitney test performed in pairwise fashion. Statistical analysis was performed by use of SPSS software v11.5 (SPSS, Chicago, IL). Statistical significance was defined as P < 0.05.
RESULTS
Vagal nerve stimulation attenuates intestinal injury.
Sections of distal ileum from sham animals and animals 4 h following 30% TBSA burn underwent histological examination to confirm the presence of intestinal injury. The distal small intestine from burned animals displayed evidence of histological pathology characterized by blunting of villi (Fig. 1B). Stimulation of the vagus nerve prior to burn prevented histological gut injury (Fig. 1C). Next, we performed an abdominal vagotomy at the gastroesophageal junction prior to stimulation of the vagus nerve to confirm that efferent vagal nerve signaling modulates gut barrier integrity following injury. Abdominal vagotomy (Fig. 1D) prevented the protective effects of vagal nerve stimulation, with histological gut injury similar to animals following burn alone. Gut injury scoring was significantly lower in animals that underwent vagal nerve stimulation prior to burn compared with burn alone (Fig. 1E).
Fig. 1.
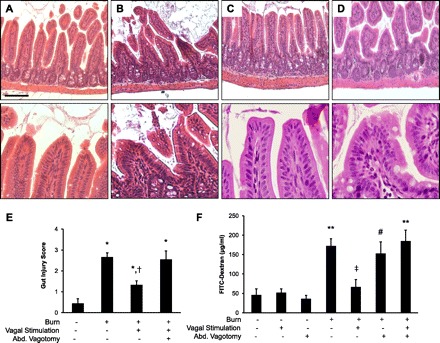
Vagal nerve stimulation attenuates burn-induced intestinal injury. Distal small intestine harvested from animals 4 h following 30% total body surface area (TBSA) burn (n = 3 animals per group). Sections of small intestine were stained with hematoxylin and eosin. High-power images of the villous tips from each section are shown below each image. A: section of normal small intestine from sham animal. B: intestine of burned animals displays evidence of histological pathology characterized by blunting of villi. C: gut sections harvested from animals that underwent cervical vagal nerve stimulation prior to severe burn. D: histological gut injury noted in animals that underwent abdominal vagotomy prior to stimulation of the vagus nerve. Size bar = 100 μm. E: intestinal injury was scored on a scale of 0 (normal) to 4 (severe) by a pathologist blinded to the experimental conditions. *P < 0.05 vs. Sham, †P = 0.05 vs. Burn and Burn/Vagotomy/Vagal Stimulation using the Kruskal-Wallis test followed by pairwise Mann-Whitney test. F: vagal nerve stimulation limits intestinal barrier dysfunction following injury (n = 4 animals per group). Vagal nerve stimulation prevents intestinal barrier dysfunction 4 h following 30% TBSA burn. Dividing the abdominal (Abd.) vagus nerve abrogates the protective effects of vagal nerve stimulation. Data are expressed as mean systemic FITC-dextran concentration ± SE. **P < 0.01 vs. Sham, #P < 0.05 vs. Sham, ‡P < 0.05 vs. Burn and Burn/Vagotomy/Vagal Stimulation by analysis of variance.
Vagal nerve stimulation prevents burn-induced intestinal permeability.
Intestinal barrier function was assessed with an in vivo intestinal permeability assay (Fig. 1F). Intestinal permeability to small molecular weight FITC-dextran was increased 4 h following burn injury. Stimulation of the vagus nerve immediately prior to injury maintained intestinal barrier integrity, significantly reducing burn-induced intestinal permeability. Animals undergoing abdominal vagotomy prior to vagal nerve stimulation had intestinal permeability equivalent to animals subjected to burn, eliminating the protective effects of cervical vagal nerve stimulation. Intestinal permeability after vagal nerve stimulation or vagotomy alone was similar to sham.
Burn injury and vagal nerve stimulation each increase activation of enteric glia.
Enteric glia activation was assessed by measuring intestinal GFAP mRNA expression via quantitative PCR (Fig. 2). The enteric glia became activated at early time points following burn, with an eightfold increase in GFAP mRNA from the small intestine at 2 h following injury. Intestinal GFAP mRNA expression is elevated to a lesser degree by 4 and 6 h following injury. Intestinal GFAP expression returns to sham levels by 24 h following injury (data not shown).
Fig. 2.
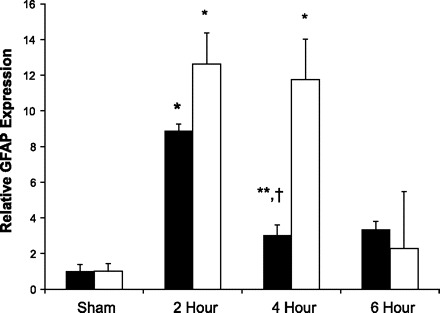
Severe burn increases expression of intestinal glial fibrillary acidic protein (GFAP). Quantitative PCR (n ≥ 3 animals per group) was performed on intestinal extracts obtained at several time points following burn (open bars) or cervical vagal nerve stimulation (solid bars). Activation of enteric glia cells occurs at early time points following burn, with an 8-fold increase in gut GFAP mRNA expression at 2 h following injury. Vagal nerve stimulation also causes activation of enteric glia cells. Intestinal GFAP mRNA expression increased by nearly 12-fold at 2 and 4 h following vagal nerve stimulation. Data are expressed as GFAP mRNA expression relative to sham ± SE. *P < 0.01 vs. Sham, **P < 0.05 vs. Sham, †P < 0.05 vs. 2-h burn using analysis of variance.
The enteric nervous system is controlled, in part, through connections to the CNS via the vagus nerve. To assess the effects of signaling via the vagus nerve on enteric glia activation, we performed right cervical vagal nerve stimulation. Intestinal GFAP mRNA expression was measured at several time points following vagal nerve stimulation (Fig. 2). There was a 12-fold increase in GFAP mRNA accumulation at both 2 and 4 h following stimulation of the vagus nerve. By 6 h following vagal nerve stimulation intestinal GFAP expression had decreased to sham levels.
Stimulation of the vagus nerve modulates activation of enteric glia following burn.
Next, we investigated the changes in intestinal GFAP expression in animals that underwent vagal nerve stimulation prior to severe burn injury (Fig. 3A). Enteric glia activation was assessed 4 h following injury as there was a plateau in GFAP expression following vagal nerve stimulation at this time point. Stimulation of the vagus nerve prior to burn was associated with an eightfold increase in intestinal GFAP expression 4 h following injury. This was significantly higher than GFAP expression seen from animals following burn alone. The increased activation of enteric glia cells in animals receiving vagal nerve stimulation immediately prior to severe burn correlated to decreased intestinal permeability to FITC-dextran (Fig. 1F).
Fig. 3.
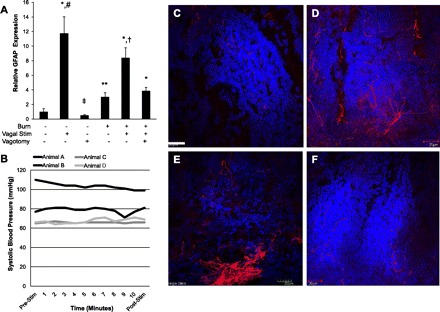
Vagal nerve stimulation alters intestinal GFAP expression. A: quantitative PCR was performed on intestinal extracts obtained 4 h following injury. Results are expressed as intestinal GFAP mRNA expression relative to sham ± SE. Vagal nerve stimulation (Stim) prior to severe burn results in augmented intestinal GFAP mRNA expression over animals subjected to burn alone. Performing a surgical abdominal vagotomy prior to cervical vagal nerve stimulation and subsequent severe burn abrogates the ability of vagal nerve stimulation to increase intestinal GFAP mRNA expression. B: arterial blood pressure was measured during vagal nerve stimulation to confirm that cervical vagal nerve stimulation did not cause hemodynamically significant changes in blood pressure. There was no significant change in arterial blood pressure during or immediately following vagal nerve stimulation. Intestinal GFAP+ enteric glia localization was assessed by confocal microscopy of intestinal segments stained for GFAP (red) and 4,6-diamidino-2-phenylindole (DAPI; blue). C: there is some background GFAP expression in sham animals. D: GFAP staining is seen extending toward the intestinal villi in sections from animals 4 h following burn. E: vagal nerve stimulation prior to burn also results in increased staining for GFAP compared with sham. F: abdominal vagotomy prior to vagal nerve stimulation and burn results in a pattern of staining similar to sham, suggesting the importance of vagal nerve signaling in enteric glia activation. *P < 0.01 vs. Sham, **P < 0.05 vs. Sham, †P < 0.02 vs. Burn, #P < 0.02 vs. Burn and Burn/Vagotomy/Vagal Stimulation, ‡P < 0.001 vs. all groups except Sham by analysis of variance. Size bar = 20 μm.
Next, we performed an abdominal vagotomy prior to vagal stimulation and subsequent severe burn injury to confirm the relationship between efferent vagal nerve signaling and the activation of enteric glia. Abdominal vagotomy prevented the augmented activation of enteric glia seen in burned animals undergoing vagal nerve stimulation, with no difference compared with animals subjected to burn alone. This also correlated with intestinal permeability data showing that abdominal vagotomy abrogated the protective effects of vagal nerve stimulation prior to injury (Fig. 1F).
We also confirmed that changes in GFAP expression following vagal nerve stimulation were not confounded by significant changes in arterial blood pressure, which itself could alter enteric glia activation. Arterial blood pressure was measured in animals during cervical vagal nerve stimulation (Fig. 3B). This confirmed that changes seen in enteric glia cell activation in this model were not due to changes in arterial blood pressure.
Changes in localization of GFAP-expressing enteric glia cells were studied by confocal microscopy of intestinal segments obtained 4 h following injury. There is some baseline expression of GFAP seen in sham animals as expected (Fig. 3C). Additionally, we observed increased staining for GFAP, which was seen projecting toward the intestinal villi in sections obtained from burned animal (Fig. 3D). Animals undergoing vagal nerve stimulation prior to burn also have increased GFAP staining seen projecting into the villi of intestinal segments (Fig. 3E). The pattern of GFAP expression is less pronounced in animals subjected to abdominal vagotomy prior to cervical vagal nerve stimulation and burn, suggesting the importance of vagal nerve stimulation in gut GFAP expression following injury (Fig. 3F).
Vagal stimulation increases bioluminescence from GFAP-luc transgenic mice.
Transgenic GFAP-luc mice were used to assess in vivo changes in GFAP expression. These transgenic animals express luciferase under transcriptional control of the GFAP promoter. After injection of the substrate luciferin, we were able to image bioluminescence as a marker for GFAP expression using the Xenogen IVIS Lumina imaging system. GFAP-luc transgenic mice were used to examine in vivo changes in GFAP expression 4 h following vagal nerve stimulation. There was increased luminescence from the abdomen of animals imaged 4 h following stimulation of the cervical vagus nerve (Fig. 4B) compared with sham (Fig. 4A). Excision of the intestine following in vivo imaging confirmed luminescence from the small intestine. Quantification of all GFAP-luc animals imaged confirms increased luminescence from animals 4 h following stimulation of the vagus nerve (Fig. 4D).
Fig. 4.
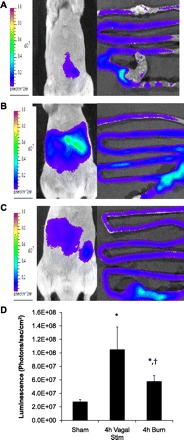
In vivo increases in abdominal luminescence in GFAP-luc mice. Transgenic mice expressing luciferase under control of the GFAP promoter were imaged in a Xenogen IVIS Lumina 2 h following burn (n > 3 animals per group). A: representative sham GFAP-luc mouse demonstrating background GFAP expression with resected intestine from confirming luminescence from the intestine. B: in vivo luminescence from the gut of GFAP-luc mice is elevated 4 h following stimulation of the cervical vagus nerve. C: increased luminescence is also seen from the abdomen of GFAP-luc mice 4 h following burn. D: quantification of luminescence measured from the abdomen of all GFAP-luc animals imaged, *P < 0.05 vs. Sham, †P < 0.05 vs. Vagal Nerve Stimulation.
Abdominal bioluminescence is increased in GFAP-luc mice following burn.
There is increased luminescence noted from the abdomen of GFAP-luc animals 4 h following burn (Fig. 4C) compared with GFAP-luc mice who were not subjected to burn (Fig. 4A). To confirm that the luminescence detected from the abdomen of these animals was from the intestine, the gut was resected en bloc for imaging. Luminescent imaging from the excised intestine confirms that the abdominal luminescence seen on in vivo imaging originated from the intestine of the GFAP-luc mice. Quantification of abdominal bioluminescence from all mice imaged demonstrates a greater luminescence from the burned GFAP-luc mice compared with sham (Fig. 4D). Nontransgenic sham and burn mice were imaged to confirm that there is minimal background luminescence at the settings used in this series of experiments (data not shown).
Vagal stimulation increases bioluminescence from GFAP-luc transgenic mice.
GFAP-luc transgenic mice were used to examine in vivo changes in GFAP expression 4 h following vagal nerve stimulation. There was increased luminescence from the abdomen of animals imaged 4 h following stimulation of the cervical vagus nerve (Fig. 4E) compared with sham (Fig. 4D). Excision of the intestine following in vivo imaging confirmed luminescence from the small intestine. Quantification of all GFAP-luc animals imaged confirms increased luminescence from animals 4 h following stimulation of the vagus nerve (Fig. 4F).
Vagal nerve stimulation attenuates burn-induced intestinal barrier injury.
Next, we evaluated whether vagal nerve stimulation would modulate intestinal inflammation and intestinal tight junction protein expression following injury. Vagal nerve stimulation prevented the burn-induced increase in intestinal TNF-α levels (Fig. 5A), which correlates with results of the in vivo intestinal permeability assay (Fig. 1F) MLCK is a tight junction protein that plays a key role in modulating intestinal barrier function. Increased MLCK expression increases paracellular permeability across the intestinal tight junction and is regulated, in part, by increases in TNF-α (15). Changes in intestinal MLCK expression were analyzed by Western blot, showing that vagal nerve stimulation prevents the increase in MLCK activation that is seen after burn injury (Fig. 5B). The ability of vagal nerve stimulation to modulate intestinal tight junction protein expression was further confirmed by imaging changes in phosphorylated MLC by use of confocal microscopy. MLCK activation results in phosphorylation of MLC and subsequent contraction of the actin-myosin ring in the intestinal epithelium, resulting in opening of the tight junction. Severe burn injury resulted in increased staining for phosphorylated MLC (Fig. 5D). Animals undergoing vagal nerve stimulation prior to burn had a pattern of phosphorylated MLC expression similar to sham (Fig. 5, C and E), with clearly less staining than seen from the gut of burn injured animals.
Fig. 5.
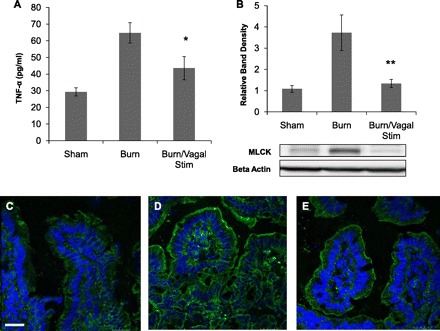
Vagal nerve stimulation prevents burn-induced intestinal barrier injury. A: intestinal TNF-α levels measured from distal ileum harvested 4 h following injury using ELISA. Vagal nerve stimulation prevents the increase in TNF-α seen following burn injury, *P < 0.05 vs. Burn. B: changes in intestinal myosin light chain kinase (MLCK) expression 4 h following burn by Western blot. There is nearly a 4-fold increase in intestinal MLCK expression following burn. Animals undergoing vagal nerve stimulation exhibited intestinal MLCK expression similar to sham, **P < 0.05 vs. Burn by analysis of variance. Intestinal segments were harvested 4 h following burn and stained for phosphorylated myosin light chain (MLC, green) and DAPI (blue) by confocal microscopy. C: minimal staining seen in sham animals. D: staining for phosphorylated MLC is increased following burn. E: vagal nerve stimulation prior to burn decreases staining for phosphorylated MLC. Bar = 20 μm.
Postinjury vagal nerve stimulation protects against burn-induced intestinal permeability.
The clinical utility of vagal nerve stimulation will be determined by the immunomodulatory effects of stimulating the vagus nerve after injury. We performed a set of experiments with vagal nerve stimulation occurring at 30 min after burn injury. Postinjury vagal nerve stimulation resulted in decreased intestinal permeability following severe burn injury (Fig. 6), with no loss in the protective effects measured in animals that underwent vagal nerve stimulation prior to injury.
Fig. 6.
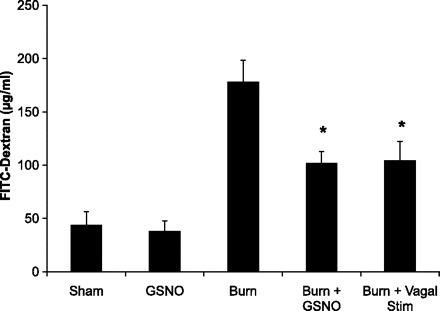
Postinjury vagal nerve stimulation protects against gut barrier injury. In vivo intestinal permeability to 4-kDa FITC-dextran was measured 4 h following in burn injury. Animals underwent either postinjury vagal nerve stimulation or S-nitrosoglutathione (GSNO) injection. Stimulation of the vagus nerve 4 h following burn injury decreases intestinal permeability compared with sham. GSNO injection also modulates gut barrier function following severe burn, attenuating burn-induced intestinal permeability. *P < 0.03 vs. Burn.
Postinjury treatment with GSNO protects against burn-induced intestinal barrier injury.
Activated enteric glia cells have been shown to release GSNO, a known modulator of intestinal barrier function. GSNO was injected following severe burn injury to explore a potential mechanism by which intestinal barrier function is maintained through activation of enteric glia cells. Injection of GSNO following injury also prevented the burn-induced increase in intestinal permeability to 4-kDa FITC-dextran (Fig. 6) and also improved gut histology compared with burned animals (Fig. 7, A–C). Injection of GSNO also modulated intestinal tight junction protein expression. Animals injected with GSNO had decreased staining for phosphorylated MLC (Fig. 7F) compared with burn alone (Fig. 7E).
Fig. 7.
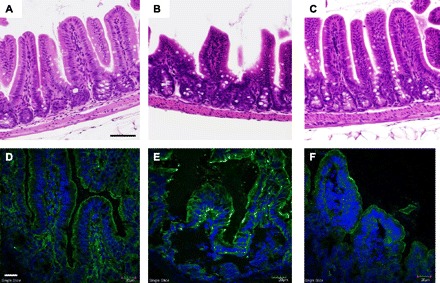
Injection of GSNO decreases intestinal injury following severe burn. Intestinal injury was assessed by histology. B: sham. B: there is clear evidence of gut injury in sections obtained from animals subjected to severe burn. C: sections of intestine from burned animals injected with GSNO show minimal evidence of injury, similar to sham. Black size bar = 100 μm. Changes in phosphorylated MLC (green) were assessed by confocal microscopy. Cell nuclei were stained with DAPI (blue). D: there is minimal staining for phosphorylated MLC seen in sham animals. E: staining for phosphorylated MLC is increased following burn. F: injection of GSNO prior to burn decreases staining for phosphorylated MLC. White size bar = 30 μm.
The protective effects of vagal nerve stimulation are not due to modulation of systemic TNF-α.
Previous studies have shown that vagal nerve stimulation has a major effect on circulating cytokines, specifically causing a dramatic decrease in TNF-α production from the spleen (12). We questioned whether the protective effects of vagal nerve stimulation in this model were simply due to a decrease in circulating TNF-α. To answer this question we performed vagal nerve stimulation on burn injured animals immediately following splenectomy, thus eliminating the effects of TNF-α produced by the spleen following injury. Burn injury caused increased intestinal permeability and histological gut injury in splenectomized animals. Vagal nerve stimulation maintained its gut-protective effect after burn injury in animals that were splenectomized (Figs. 8 and 9, A–E). This suggests that the protective effects of vagal nerve stimulation are not due to modulation of splenic production of circulating TNF-α. This was confirmed by measuring decreased circulating TNF-α levels in both animals that had undergone splenectomy and splenectomized animals that underwent vagal nerve stimulation following injury (Fig. 9F). Elevated intestinal TNF-α correlated with increased intestinal permeability and histological gut injury, suggesting a local rather than systemic inflammatory response (Fig. 9G).
Fig. 8.
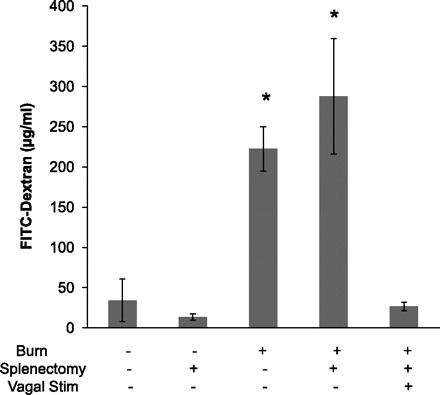
Vagal nerve stimulation prevents burn-induced intestinal permeability in splenectomized animal. In vivo intestinal permeability to 4-kDa FITC-dextran was measured 4 h following injury. Increased intestinal permeability was seen in animals that underwent splenectomy immediately prior to burn injury. The protective effects of vagal nerve stimulation are seen in burned animals that underwent splenectomy. Taken together, this suggests the gut-protective effects of vagal nerve stimulation are not due to modulation of splenic cytokine production. *P < 0.02 vs. Sham, Splenectomy, and Burn/Splenectomy/Vagal Stim groups.
Fig. 9.
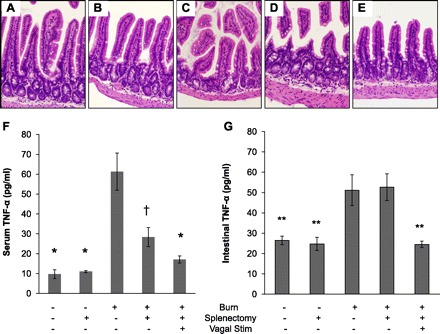
Gut-protective effects of vagal nerve stimulation are independent of changes in circulating TNF-α. Distal small intestine harvested from animals 4 h following injury (n = 3 animals per group). Sections of small intestine stained with hematoxylin and eosin. Representative sections are shown of normal small intestine from sham (A) and following splenectomy (B). Intestines of a representative animal following burn (C) and an animal that underwent splenectomy prior to burn (D) display evidence of histological gut injury. E: a normal gut section harvested from a representative animal that underwent splenectomy prior to vagal nerve stimulation and severe burn. F: serum TNF-α was measured 4 h following injury using ELISA. There is a decrease in circulating TNF-α in animals that underwent splenectomy. *P < 0.001 vs. Burn, †P < 0.01 vs. Burn. G: TNF-α measured from intestinal extracts obtained 4 h following burn injury. There is evidence of local intestinal TNF-α production that correlates with the intestinal injury seen by intestinal permeability and histology. **P < 0.05 vs. Burn and Burn/Splenectomy.
DISCUSSION
The ability of vagal nerve stimulation to modulate the intestinal response to inflammation is just beginning to be understood. In this series of experiments, we investigated changes in small intestine enteric glia activation in a model of intestinal injury caused by severe burn. The enteric glia may, at least in part, be responsible for restoring intestinal barrier integrity following injury. Here we propose that the response of the enteric glia to intestinal injury may be modulated by the CNS via the vagus nerve. Our findings suggest that stimulating the vagus nerve at the time of injury may augment enteric glia cell activation and either prevent intestinal barrier injury or speed its recovery.
The results of this study demonstrated that severe burn injury increases activation of enteric glia cells as measured by increased intestinal GFAP mRNA expression, by confocal microscopy of intestinal segments stained for GFAP, and by utilizing in vivo imaging of luminescence from the intestine of GFAP-luc transgenic animals. We also investigated the effects of vagal nerve stimulation on enteric glia activation. These experiments showed that electrical stimulation of the cervical vagus nerve alone causes increased expression of intestinal GFAP.
This is the first study, to our knowledge, to utilize GFAP-luc transgenic mice to study changes in the enteric glia. These transgenic mice have been used to image changes to CNS astrocytes; however, abdominal imaging of changes in GFAP expression following injury has not previously been reported (6, 14). This technique allows for in vivo imaging of changes in GFAP expression and allows for a powerful visual confirmation of results of classic methods such as quantitative PCR. In the future, this nonlethal, in vivo imaging technique may be ideally suited for following changes in GFAP expression in the same animal over time.
We found that stimulation of the vagus nerve prevented burn-induced intestinal permeability and attenuated histological gut injury. To confirm that the effects of vagal nerve stimulation were due to an efferent signaling pathway, we performed surgical abdominal vagotomy prior to vagal nerve stimulation. Animals undergoing abdominal vagotomy prior to vagal stimulation had intestinal permeability similar to animals subjected to burn alone, confirming the protective effects of efferent vagal nerve signaling. The improved intestinal barrier function seen in injured animals that underwent vagal nerve stimulation was associated with a significant increase in intestinal GFAP expression via PCR. Limiting intestinal barrier injury may play an important role in decreasing the systemic inflammatory response associated with severe injury. Vagal nerve stimulation has previously been shown to decrease histological gut injury and intestinal inflammation in a model of colitis (10). Vagal nerve stimulation has also been shown to decrease postoperative ileus in a murine model, further supporting the ability of vagal nerve stimulation to modulate the enteric nervous system (22). Importantly, we also demonstrated that vagal nerve stimulation given 30 min after burn injury continues to prevent intestinal barrier injury. This is certainly important, since the clinical utility of vagal nerve stimulation lies in its ability to have protective effects when administered after an insult.
In this series of experiments we found that vagal nerve stimulation prevented the burn-induced increase in intestinal TNF-α. This was associated with modulation of the intestinal tight junction proteins MLCK and phosphorylated MLC. Increased expression of both MLCK and phosphorylated MLC play an important role in the loss of tight junction integrity. Therefore, the ability of vagal nerve stimulation to attenuate the increase in MLCK and phosphorylated MLC after burn gives insight into the mechanism by which vagal nerve stimulation prevents intestinal barrier loss and subsequent intestinal inflammation.
Similar to the role of astrocytes in maintaining the blood-brain barrier, enteric glia play an important role in regulating intestinal permeability (21). The importance of enteric glia was noted in studies using targeted ablation of enteric glia cells in transgenic mice. Loss of enteric glia cells was fatal in that animal model owing to hemorrhagic necrosis of the small intestine (5). Clinically, loss of enteric glia cells has been implicated in the pathogenesis of inflammatory bowel disease (7).
Neurons of the ENS communicate with enteric glia cells, allowing for cross talk between cell types (18). Gulbransen and Sharkey (11) have recently shown that electric stimulation of enteric neurons causes activation of enteric glia cells through release of the neurotransmitter adenosine triphosphate. Here we demonstrated that cervical vagal nerve stimulation causes activation of enteric glia cells as measured by increased GFAP expression. This demonstrates a link between the vagus nerve and enteric glia and gives insight into possible mechanisms by which the CNS communicates with ENS following gut inflammation. We found that stimulation of the vagus nerve prior to burn augmented the activation of enteric glia cells in response to intestinal injury.
Studies by Savidge (20) et al. have shown that enteric glia improve intestinal barrier function by augmenting the expression of tight junction proteins, resulting in improved intestinal barrier integrity. They demonstrated that glial cells secrete GSNO, which modulates intestinal tight junction integrity. This was confirmed in an animal model of gut inflammation, in which injection of exogenous GSNO attenuated gut inflammation, improved intestinal barrier integrity, and increased expression of the tight junction protein ZO-1. Enteric glia cells are also known to secrete transforming growth factor-β, another molecule that improves intestinal barrier integrity (1, 17). Here we have shown that injection of GSNO attenuates burn-induced intestinal barrier injury with results similar to animals undergoing vagal nerve stimulation. This further suggests that vagal nerve stimulation may maintain barrier integrity and intestinal tight junction protein expression through the ability of activated enteric glia cells to produce GSNO.
It has been hypothesized that a subpopulation of enteric glia cells can rapidly upregulate GFAP expression in response to inflammation, whereas other glia cells respond more slowly and require proliferation and GFAP synthesis (4, 24). The CNS may signal the ENS via the vagus nerve to increase enteric glial cell GFAP synthesis in response to injury. Since enteric glia cells improve intestinal barrier integrity, this may represent another vagal-mediated anti-inflammatory pathway that is activated in response to gut injury. It is important to acknowledge that there may be other mechanisms by which stimulating the vagus nerve may protect against burn-induced gut injury. In addition to secreting GSNO, activated enteric glia cells cause numerous changes in gene transcription within the intestinal epithelial cell that may alter barrier function (23). Vagal nerve stimulation may also alter intestinal function after injury by other pathways including neuropeptide release.
Vagal nerve stimulation has previously been shown to exert its protective effects through its ability to decrease production of splenic TNF-α following injury via the α7 nicotinic acetylcholine receptor (19). We sought to determine whether the gut-protective effects of vagal nerve stimulation in this study were due to modulation of splenic TNF-α. We first splenectomized animals immediately prior to burn injury to eliminate the main source of immediate TNF production. We found that there continued to be increased permeability and histological gut injury in burned animals after splenectomy despite decreased levels of circulating TNF-α. This suggests that the gut injury seen in this model is not due to circulating TNF-α. Next, we performed vagal nerve stimulation in burned animals that had been splenectomized immediately prior to injury. We again found that vagal nerve stimulation had gut-protective effects as measured by permeability and histology. We also measured TNF-α from gut tissue and found elevated levels in burned animals that had been splenectomized, indicating a local but not systemic inflammatory response. These data suggest that the protective effects of vagal nerve stimulation on the gut are not due to modulation of splenic production of circulating TNF-α.
In summary, we demonstrated that stimulation of the vagus nerve is associated with increased activation of enteric glia cells and resulted in attenuation of burn-induced intestinal barrier injury. The ability of vagus nerve stimulation to activate enteric glia cells may give insight into the signaling that occurs from the CNS to the ENS following gut injury. These results advance previous studies documenting the anti-inflammatory effects of vagal nerve stimulation, now showing the ability to modulate enteric glia cells within the ENS. Exploiting the barrier inducing effects of enteric glia activation may prevent or limit intestinal barrier breakdown and may be a therapeutic target in diseases causing intestinal inflammation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Alexandra Borboa, Billie Chun, and Luiz Guilherme Reys, MD for technical assistance with this project.
REFERENCES
- 1. Al-Sadi R, Boivin M, Ma T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front Biosci 14: 2765–2778, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bansal V, Costantini T, Kroll L, Peterson C, Loomis W, Eliceiri B, Baird A, Wolf P, Coimbra R. Traumatic brain injury and intestinal dysfunction: uncovering the neuro-enteric axis. J Neurotrauma 26: 1353–1359, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blikslager AT, Moeser AJ, Gookin JL, Jones SL, Odle J. Restoration of barrier function in injured intestinal mucosa. Physiol Rev 87: 545–564, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Bradley JS, Jr, Parr EJ, Sharkey KA. Effects of inflammation on cell proliferation in the myenteric plexus of the guinea-pig ileum. Cell Tissue Res 289: 455–461, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell 93: 189–201, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Cordeau P, Jr, Lalancette-Hebert M, Weng YC, Kriz J. Live imaging of neuroinflammation reveals sex and estrogen effects on astrocyte response to ischemic injury. Stroke 39: 935–942, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Cornet A, Savidge TC, Cabarrocas J, Deng WL, Colombel JF, Lassmann H, Desreumaux P, Liblau RS. Enterocolitis induced by autoimmune targeting of enteric glial cells: a possible mechanism in Crohn's disease? Proc Natl Acad Sci USA 98: 13306–13311, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Costantini TW, Loomis WH, Putnam JG, Drusinsky D, Deree J, Choi S, Wolf P, Baird A, Eliceiri B, Bansal V, Coimbra R. Burn-induced gut barrier injury is attenuated by phosphodiesterase inhibition: effects on tight junction structural proteins. Shock 31: 416–422, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Costantini TW, Loomis WH, Putnam JG, Kroll L, Eliceiri BP, Baird A, Bansal V, Coimbra R. Pentoxifylline modulates intestinal tight junction signaling after burn injury: effects on myosin light chain kinase. J Trauma 66: 17–24; discussion 24–15, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ghia JE, Blennerhassett P, El-Sharkawy RT, Collins SM. The protective effect of the vagus nerve in a murine model of chronic relapsing colitis. Am J Physiol Gastrointest Liver Physiol 293: G711–G718, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Gulbransen BD, Sharkey KA. Purinergic neuron-to-glia signaling in the enteric nervous system. Gastroenterology 136: 1349–1358, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, Tracey KJ, Ulloa L. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med 203: 1623–1628, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lomax AE, Linden DR, Mawe GM, Sharkey KA. Effects of gastrointestinal inflammation on enteroendocrine cells and enteric neural reflex circuits. Auton Neurosci 126–127: 250–257, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Luo J, Ho P, Steinman L, Wyss-Coray T. Bioluminescence in vivo imaging of autoimmune encephalomyelitis predicts disease. J Neuroinflammation 5: 6–12, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ma TY, Boivin MA, Ye D, Pedram A, Said HM. Mechanism of TNF-α modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression. Am J Physiol Gastrointest Liver Physiol 288: G422–G430, 2005 [DOI] [PubMed] [Google Scholar]
- 16. McGraw J, Hiebert GW, Steeves JD. Modulating astrogliosis after neurotrauma. J Neurosci Res 63: 109–115, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Neunlist M, Aubert P, Bonnaud S, Van Landeghem L, Coron E, Wedel T, Naveilhan P, Ruhl A, Lardeux B, Savidge T, Paris F, Galmiche JP. Enteric glia inhibit intestinal epithelial cell proliferation partly through a TGF-β1-dependent pathway. Am J Physiol Gastrointest Liver Physiol 292: G231–G241, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Neunlist M, Van Landeghem L, Bourreille A, Savidge T. Neuro-glial crosstalk in inflammatory bowel disease. J Intern Med 263: 577–583, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, Chavan S, Tracey KJ. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci USA 105: 11008–11013, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Savidge TC, Newman P, Pothoulakis C, Ruhl A, Neunlist M, Bourreille A, Hurst R, Sofroniew MV. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology 132: 1344–1358, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Savidge TC, Sofroniew MV, Neunlist M. Starring roles for astroglia in barrier pathologies of gut and brain. Lab Invest 87: 731–736, 2007 [DOI] [PubMed] [Google Scholar]
- 22. The FO, Boeckxstaens GE, Snoek SA, Cash JL, Bennink R, Larosa GJ, van den Wijngaard RM, Greaves DR, de Jonge WJ. Activation of the cholinergic anti-inflammatory pathway ameliorates postoperative ileus in mice. Gastroenterology 133: 1219–1228, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Van Landeghem L, Mahe MM, Teusan R, Leger J, Guisle I, Houlgatte R, Neunlist M. Regulation of intestinal epithelial cells transcriptome by enteric glial cells: impact on intestinal epithelial barrier functions. BMC Genomics 10: 507–527, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Von Boyen GB, Steinkamp M, Reinshagen M, Schafer KH, Adler G, Kirsch J. Proinflammatory cytokines increase glial fibrillary acidic protein expression in enteric glia. Gut 53: 222–228, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Willems E, Leyns L, Vandesompele J. Standardization of real-time PCR gene expression data from independent biological replicates. Anal Biochem 379: 127–129, 2008 [DOI] [PubMed] [Google Scholar]


