Summary
Human cytomegalovirus (HCMV) is a prevalent pathogen worldwide. Although generally harmless in healthy individuals, HCMV can pose a serious threat to immune compromised individuals and developing fetuses in utero. HCMV encodes four genes predicted to give rise to G protein-coupled receptors (GPCRs): US27, US28, UL33, and UL78. The US28 gene product is a functional chemokine receptor that enhances cell growth in some cell types but induces apoptosis in others. In contrast, the US27 gene product has not been demonstrated to signal either constitutively or in a ligand-induced manner. In this study, US27 was expressed in transfected cells, and both cell proliferation and DNA synthesis were significantly increased compared to control cells. PCR array analysis revealed that expression of US27 led to changes in a limited number of cellular genes, but genes that were up-regulated included the pro-survival factor Bcl-x, AP-1 transcription factor components jun and fos, and the IL-6 family cytokine oncostatin M. These results demonstrate that US27 can impact host cell physiology and may shed light on the function of this orphan viral GPCR.
Keywords: HCMV, cytomegalovirus, GPCR, chemokine receptor
Human cytomegalovirus (HCMV) is a member of the Herpesviridae family, and infection is widespread in the general population (Staras et al., 2006). While most healthy adults exhibit only mild or no symptoms of infection, serious disease can occur in immune compromised individuals, especially AIDS patients, transplant recipients, and newborn infants (Kesson and Kakakios, 2007). HCMV is the leading infectious cause of birth defects, and congenital HCMV infection continues to be problematic due to deficiencies in public awareness, diagnostic procedures, and treatment options (Revello et al., 2008).
Like all other herpesviruses, the HCMV virion consists of a large DNA genome enclosed in an icosahedral capsid surrounded by a dense tegument layer, all encircled in a lipid envelope containing numerous viral glycoproteins. HCMV has the largest genome of all the human herpesviruses at 230 kb and encoding at least 167 genes (Mocarski, 2006). More than half of these genes are not required for virus replication in vitro (Yu et al., 2003) but instead play roles in vivo in the manipulation of host immune responses and the establishment of latency (Jackson et al., 2011). The US27 gene, which encodes a putative G protein-coupled receptor (GPCR) found in the viral envelope, is one of these non-essential genes (Chee et al., 1990; Margulies and Gibson, 2007). Virus mutants lacking US27 are replication competent (Bodaghi et al., 1998), although a single log reduction in virus titers produced from both infected fibroblasts and endothelial cells was observed (O’Connor and Shenk, 2011). The US27 deletion mutant virus also exhibited a defect in extracellular spreading, but the virus was still able to infect neighboring cells, presumably via the cell-cell route (O’Connor and Shenk, 2011). The US27 gene is expressed late during infection, and the gene product is found mainly in the endosomes, the Golgi apparatus, and perinuclear compartments of infected cells (Fraile-Ramos et al., 2002).
The US27 gene product has many conserved features of the chemokine receptor subset of the GPCR superfamily, such as seven transmembrane domains, a DRY (aspartic acid-arginine- tyrosine) motif in the second intracellular loop, conserved cysteines (C104 and C176) in the second and third extracellular loops, and extensive glycosylation of the extracellular domains (Margulies and Gibson, 2007). Despite having these characteristics, US27 is considered an orphan since no human chemokine ligands have been shown to engage the receptor (Stapleton et al., 2012). Interestingly, HCMV encodes three other genes that give rise to proteins having similarity to human chemokine receptors (Beisser et al., 2002; Chee et al., 1990). One of these, US28, has been shown to elicit intracellular signaling both constitutively and in response to several human chemokines, including CCL3/MIP-1α, CCL5/Rantes, and CX3CL1/Fractalkine (Gao and Murphy, 1994; Neote et al., 1993; Stropes et al., 2009). UL33 also has constitutive signaling ability (Casarosa et al., 2003), and rodent homologs of both UL33 and UL78 have been shown to play a role in virus dissemination in vivo (Beisser et al., 1999; Beisser et al., 1998). The M33 gene of murine cytomegalovirus, a homolog of HCMV UL33, was found to be required for salivary gland tropism and efficient reactivation from latency, and the HCMV US28 gene was able to complement and partially rescue those deficiencies (Cardin et al., 2009; Farrell et al., 2011).
Recent evidence suggests that US28 forms heteromeric complexes with US27, UL33 and UL78 (Tschische et al., 2011). While no functional changes were observed with the US28:US27 heteromer, the US28:UL33 heteromer and the US28:UL78 heteromer both ablated activation of NF-κB transcriptional activity by US28. This suggests a complex level of regulation in which these viral receptors may interact in particular combinations to either promote or block signaling through specific pathways in particular cell types or at specific times during the course of virus infection. US27, US28, UL33, and UL78 are all likely to play important roles in immune modulation and viral persistence, and the presence of multiple receptors in the viral genome could be due to the to need control cellular activity in the large variety of cell types infected by HCMV, which include monocytes, lymphocytes, epithelial cells, endothelial cells, and fibroblasts (Mocarski, 2006). Considering that GPCRs constitute a major target in pharmaceutical development, discerning the function of viral GPCRs during HCMV infection could be highly beneficial in the quest for novel anti-viral therapeutics.
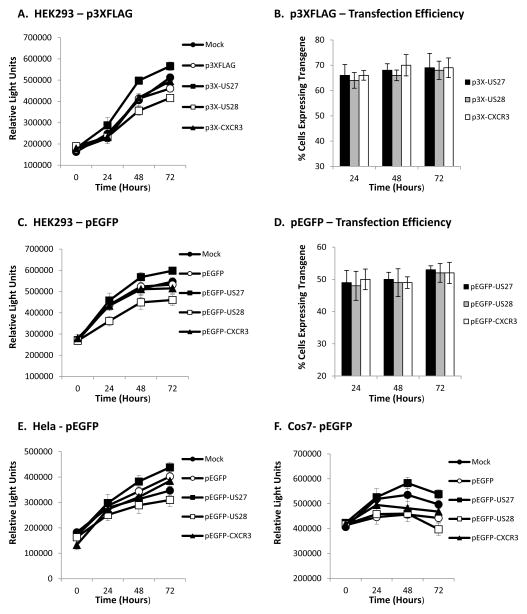
To study the function of US27, the gene from HCMV strain AD169 was cloned into the p3XFLAG expression vector and transiently transfected into HEK293 cells, as described (Stapleton et al., 2012). The cells were seeded into 96-well plates at a density of 1 × 104 cells per well, and cell proliferation was monitored using the CellTiter-Glo Assay (Promega, Madison, WI). Briefly, a luciferin substrate that is converted to oxyluciferin in the presence of O2 and ATP was added to each well. The resulting luminescence is proportional to the amount of ATP present, reflecting the number of viable cells in the well. Cells expressing US27 had greater luminescence, suggesting that they had an enhanced growth rate compared to controls, as shown in Figure 1A. The control cells included mock transfected cells treated with transfection reagent only, cells transfected with the empty p3XFLAG vector, and cells transfected with the p3XFLAG vector expressing HCMV US28 or human chemokine receptor CXCR3, which was cloned from human peripheral blood mononuclear cells as previously described (Stapleton et al., 2012). US27-expressing cultures consistently exhibited 14–26% greater luminescence over the course of the experiment than most of the other cell lines, which is indicative of higher cell numbers and a faster growth rate. Each of the other control cell lines had comparable growth rates except for the US28-expressing cells, which exhibited reduced luminescence, indicating fewer viable cells. The transfection efficiency was comparable among the cell lines at 60–70%, as indicated by staining with an anti-FLAG antibody (Sigma-Aldrich, St. Louis, MO), followed by FITC-conjugated secondary antibody and flow cytometry (Fig. 1B).
Figure 1. Cells expressing HCMV US27 exhibit enhanced cell growth.
A) HEK293 cells were seeded into white 96-well plates at a density of 1 × 104 cells per well and then transfected with the indicated p3XFLAG expression vectors using Fugene transfection reagent (Roche Biosciences, Basel, Switzerland) at a ratio of 3:1 (μl Fugene:μg plasmid DNA per manufacturer’s instructions). Cell number was monitored via the addition of CellTiter-Glo reagent (Promega, Madison, WI) at the indicated time points and luminescence evaluated using a Turner Biosystems Veritas Microplate Luminometer. B) Transfection efficiency was determined by flow cytometry after staining cells at the indicated times post-transfection with anti-FLAG antibody and FITC-conjugated secondary in permeabilization buffer. C) HEK293 cells were seeded and transfected with the indicated pEGFP expression vectors as described, then cell growth was monitored using CellTiter-Glo reagent and luminometry. D) Transfection efficiency was determined by flow cytometry for cells expressing EGFP. E) HeLa cells were seeded at 5 × 103 cells per well and transfected with the indicated pEGFP expression vectors as described, then cell growth monitored using CellTiter-Glo reagent and luminometry. F) Cos7 African green monkey cells were seeded at 1 × 104 cells per well and transfected with the indicated pEGFP expression vectors as described, then cell growth monitored via the addition of Cell Titer Glo reagent and luminometry. Error bars represent standard error of three triplicate data points.
To rule out any possibility that the FLAG tag might be affecting US27 functional activity, the US27, US28 and CXCR3 genes were also cloned into a pEGFP vector (Clontech, Mountain View, CA) and expressed as fusion proteins linked to the C-terminal domain of EGFP. As shown in Figure 1C, HEK293 cells transiently transfected with pEGFP-US27 also exhibited greater cell proliferation than corresponding control cells. The increase in growth rate for these cells was more modest, with an increase of 9–16% over each of the control cell lines over the course of the experiment. The growth of the pEGFP-US27 transfected cells was 26–30% higher than pEGFP-US28 transfected cells. Transfection efficiency with the pEGFP plasmids was comparable among the cells line, but lower than the p3XFLAG plasmids, at 46–52% (Fig. 1D). Overall, the US27-expressing HEK293 cultures grew faster while US28 cells grew at a reduced rate compared to the control cell lines. These results suggested that the US27 gene product might enhance cell growth.
Previous studies have shown that the US28 gene product may have different effects on cell growth and survival depending on cell type. Transient transfection of GFP-US28 induced apoptosis in 293T, HeLa, and Cos cells (Pleskoff et al., 2005), whereas US28 was found to enhance cell growth and cell cycle progression in stably transfected NIH-3T3 cells (Maussang et al., 2006). Since we found increased growth rates associated with US27 and decreased growth rates with US28 in HEK293 cells, we next transfected and evaluated two additional cell types, HeLa and Cos cells (Fig. 1E and F). The results indicate that these cell types also exhibited greater proliferation when expressing HCMV US27 than control cells. Cells expressing US28 were found to have the slowest growth rate, possibly due to the loss of some cells undergoing apoptosis, a result that would be consistent with previous studies (Pleskoff et al., 2005). Although we also attempted to examine the effects of US27 in NIH-3T3 cells, the rate of transfection was extremely low (10%) and prevented their inclusion in this study.
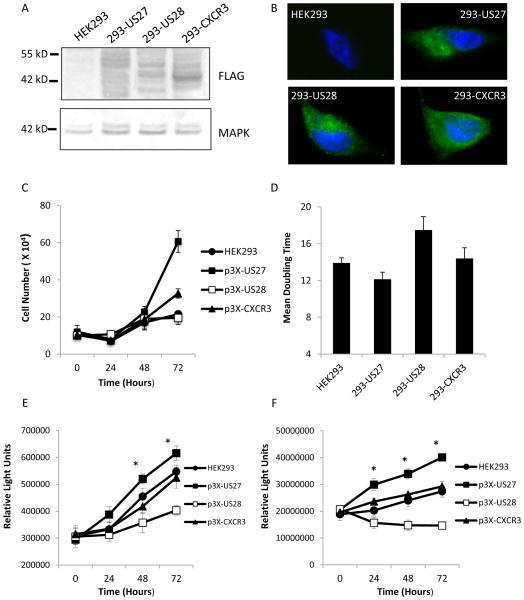
In order to confirm that the differences in growth rates that we observed were specifically due to the presence of US27 and not the result of well-to-well differences in plasmid purity, transfection efficiency, or transgene expression, stable HEK293 cell lines were created. Following transfection with p3XFLAG-US27, cells were cultivated in the presence of 1 mg/ml geneticin to eliminate untransfected cells, and clonal cell lines were created using limiting dilution. As shown in Figure 2A, comparable levels of FLAG-tagged protein were expressed in each of the cell lines. CXCR3 was detected as a 42 kD band, US28 as a 44 kDa band, and US27, which is extensively glycosylated, was detected as a 45–55 kD smear. Immunofluorescence microscopy also indicated that protein expression levels were comparable among the cell lines (Fig. 2B). When the growth rate of the stable cell lines was examined via cell counting, the US27 cultures were found to have significantly higher cell numbers after 72 hours (Fig. 2C). Although one representative clonal cell line is shown here, three individual 293-US27 clonal cell lines were examined and all had comparable expression levels and growth rates (data not shown), suggesting that the integration of the expression cassette did not account for the enhanced proliferation of cells expressing US27. The mean doubling time was 12.1 hours for the 293-US27 cultures, compared to 13.9 hours for empty vector transfected cells, 14.4 hours for 293-CXCR3 cells and 17.5 hours for 293-US28 cells (Fig. 2D). When cell proliferation was examined using the Cell Titre-Glo assay, 293-US27 cells still exhibited significantly greater proliferation (14–25% higher) than control cells (Fig. 2E). In addition, DNA synthesis was examined using BrdU incorporation, and the rate of DNA synthesis was found to be significantly higher (26–49%) in cells expressing US27 compared to the control cell lines (Fig. 2F). These results clearly demonstrate that the HCMV US27 gene product stimulates cell proliferation and increases the rate of DNA synthesis.
Figure 2. Stable cell lines expressing US27 have a shorter doubling time and synthesize DNA at a higher rate than controls.
HEK293 cells were transfected with p3XFLAG vectors and stable, clonal cell lines were produced using antibiotic selection as described (Stapleton et al., 2012). Expression of the transgene was evaluated via A) western blot staining of cell lysates using anti-FLAG antibody followed by AP-conjugated secondary or anti-total MAPK as a control and b) immunofluorescence staining of fixed, permeabilized cells with anti-FLAG antibody followed by FITC-conjugated secondary. C) Cells were seeded in 6-well dishes at 2 × 105 cells per well, then harvested and counted using a hemacytometer at the indicated time points. D) Doubling time was calculated based on total number of cell divisions for over the 72 hour growth period for triplicate wells of the standard growth curve. E) Cell growth was measured via the addition of CellTiter-Glo reagent and luminometry. F) The rate of DNA synthesis for HEK293 stable cell lines was measured using bromodeoxyuridine (BrdU) incorporation (Roche Bioscience, South San Francisco, CA) and luminometry at the indicated times. Error bars represent standard error of three triplicate data points. Statistical analysis was performed by student’s t-test and * indicates p<0.05. These results are representative of four independent experiments.
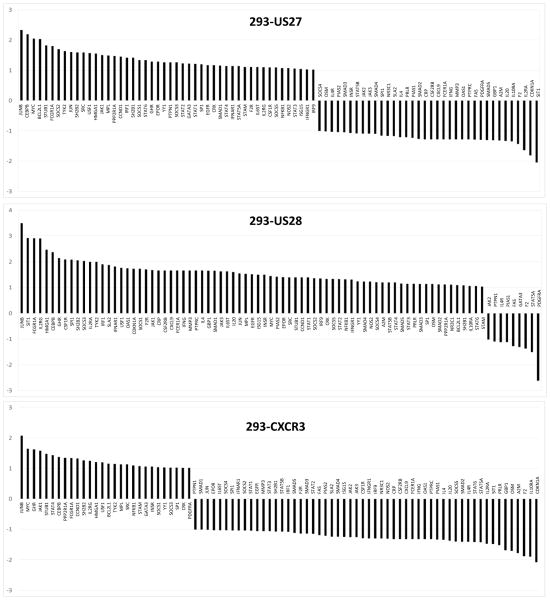
In order to identify cellular genes that might be affected by the US27 gene product and contribute to the enhanced proliferative effect observed here, PCR array analysis was performed on the stable cell lines. RNA was extracted from each cell type (HEK293, 293-US27, 293-US28, and 293-CXCR3) and expression levels of 84 genes involved in the JAK-STAT signaling pathway were compared using RT2 Profiler Arrays (SABiosciences, Valencia, CA). For each of the stable cell lines, three biological replicates were assayed, averaged, and compared to control HEK293 cells, as shown in Figure 3. Some genes were found to be up-regulated in a non-specific manner, for example, JUNB expression was increased by more than 2-fold in all three cell lines compared to control HEK293 cells. Other genes that were up-regulated by all three receptors included MYC, CEBP, and JAK1, suggesting that overexpression of any GPCR might yield the induction of a certain subset of inflammatory genes. In order to eliminate these non-specific effects, the fold change values for 293-US27 and 293-US28 were then calculated compared to the 293-CXCR3 values in order identify changes specific to the viral receptors. A select subset of the results for US27 is shown in Figure 4, with a line marking changes of 1.8-fold or higher compared to controls. A fold change cutoff of 2.0 is recommended by the array manufacturer, but other sources indicate that fold change values of 1.5 or higher should not be ignored, especially when they indicate genes that are physiologically relevant (Dalman et al., 2012). We selected a mid-range cut-off of 1.8-fold change and have identified seven JAK-STAT pathway genes whose expression level is significantly increased in the presence of US27: BCL2L1, FCGR1A, JUN, OSM, SOCS2, SOCS5, and TYK2. Table 1 includes a brief description of each cellular gene significantly impacted by either US27 or US28, as well as the fold change value for each cell type compared to cells expressing CXCR3. Six of the seven genes that exhibited up-regulation by US27 were also up-regulated in the presence of US28, suggesting that the two viral receptors may work together to enhance expression of certain cellular genes that foster a favorable environment for virus infection. The seventh gene, BCL2L1, was up-regulated by US27 but down-regulated in cells expressing US28.
Figure 3. JAK-STAT pathway gene expression levels in cells expressing US27, US28, or CXCR3.
RNA from stable cell line was extracted using RNEasy Midi Kit (Qiagen, Valencia, CA), cDNA was prepared using the RT2 First Strand Kit (Qiagen), and then diluted cDNA was mixed with the RT2 SYBR green master mix (Qiagen) according to the manufacturer’s instructions and loaded into the Human Jak-Stat RT2-PCR Profiler Array (SABiosciences, Valencia, CA). Real Time PCR was performed using the CFX96 (BioRad, Hercules, CA) by heating to 95°C for 10 minutes followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Data was analyzed using the ΔΔCt method according to the SABiosciences web portal (www.SABiosciences.com/pcrarray.dataanalysis.php) and further recalculated manually. The same threshold value was used across all plates in the same data analysis to ensure accurate reading of quality controls. The data were normalized across all plates to the following housekeeping genes: beta-2-microglobulin (B2M), hypoxanthine phosphoribosyltransferase 1 (HPRT1), ribosomal protein L13a (RPL13A), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and beta actin (ACTB). Controls for genomic DNA contamination RNA quality, and PCR performance were all in the recommended ranges. Data are represented as fold change compared to expression levels in HEK293 cells. The results are the average of three biological replicates.
Figure 4. JAK-STAT pathway gene expression levels in 293-US27 cells.
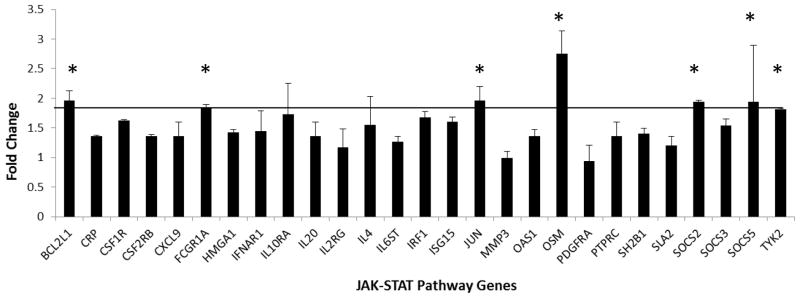
The fold change values from three biological replicates of the PCR array were analyzed in comparison to 293-CXCR3 cells as a baseline control. Error bars indicate standard error; * indicates genes with a 1.8-fold change or higher.
Table 1.
JAK-Stat Pathway1 Gene Expression Analysis
| Fold Change2 | |||||
|---|---|---|---|---|---|
| Unigene | Refseq | Symbol | Description | US27 | US28 |
| Hs.516966 | NM_138578 | BCL2L1 | BCL2-like 1 | 1.97 | −1.43 |
| Hs.709456 | NM_000567 | CRP | C-reactive protein, pentraxin-related | 1.36 | 3.11 |
| Hs.654394 | NM_005211 | CSF1R | Colony stimulating factor 1 receptor | 1.63 | 4.09 |
| Hs.592192 | NM_000395 | CSF2RB | Colony stimulating factor 2 receptor, beta, low-affinity (granulocyte-macrophage) | 1.36 | 3.11 |
| Hs.77367 | NM_002416 | CXCL9 | Chemokine (C-X-C motif) ligand 9 | 1.36 | 3.11 |
| Hs.77424 | NM_000566 | FCGR1A | Fc fragment of IgG, high affinity Ia, receptor (CD64) | 1.84 | 2.61 |
| Hs.518805 | NM_002131 | HMGA1 | High mobility group AT-hook 1 | 1.43 | 2.49 |
| Hs.529400 | NM_000629 | IFNAR1 | Interferon (alpha, beta and omega) receptor 1 | 1.45 | 2.04 |
| Hs.504035 | NM_001558 | IL10RA | Interleukin 10 receptor, alpha | 1.73 | 2.64 |
| Hs.272373 | NM_018724 | IL20 | Interleukin 20 | 1.36 | 3.11 |
| Hs.84 | NM_000206 | IL2RG | Interleukin 2 receptor, gamma | 1.17 | 2.54 |
| Hs.73917 | NM_000589 | IL4 | Interleukin 4 | 1.55 | 2.81 |
| Hs.532082 | NM_002184 | IL6ST | Interleukin 6 signal transducer (gp130, oncostatin M receptor) | 1.27 | 1.97 |
| Hs.436061 | NM_002198 | IRF1 | Interferon regulatory factor 1 | 1.68 | 2.64 |
| Hs.458485 | NM_005101 | ISG15 | ISG15 ubiquitin-like modifier | 1.60 | 2.23 |
| Hs.714791 | NM_002228 | JUN | Jun proto-oncogene | 1.97 | 1.69 |
| Hs.375129 | NM_002422 | MMP3 | Matrix metallopeptidase 3 (stromelysin 1, progelatinase) | −1.01 | 2.21 |
| Hs.524760 | NM_002534 | OAS1 | 2′-5′-oligoadenylate synthetase 1, 40/46kDa | 1.36 | 3.11 |
| Hs.248156 | NM_020530 | OSM | Oncostatin M | 2.75 | 2.51 |
| Hs.74615 | NM_006206 | PDGFRA | Platelet-derived growth factor receptor, alpha polypeptide | −1.05 | −3.45 |
| Hs.654514 | NM_002838 | PTPRC | Protein tyrosine phosphatase, receptor type, C | 1.36 | 3.11 |
| Hs.15744 | NM_015503 | SH2B1 | SH2B adaptor protein 1 | 1.40 | 2.06 |
| Hs.713578 | NM_175077 | SLA2 | Src-like-adaptor 2 | 1.20 | 2.71 |
| Hs.485572 | NM_003877 | SOCS2 | Suppressor of cytokine signaling 2 | 1.94 | 1.45 |
| Hs.527973 | NM_003955 | SOCS3 | Suppressor of cytokine signaling 3 | 1.55 | 2.05 |
| Hs.468426 | NM_144949 | SOCS5 | Suppressor of cytokine signaling 5 | 1.95 | 1.97 |
| Hs.75516 | NM_003331 | TYK2 | Tyrosine kinase 2 | 1.82 | 2.07 |
The complete list of genes analyzed on this array is shown in Figure 3 and includes: A2M, SH2B2, BCL2L1, CCND1, CDKN1A, CEBPB, CRK, CRP, CSF1R, CXF2RB, CXCL9, EGFR, EPOR, F2, F2R, FAS, FCER1A, FCGR1A, 1SG15, GATA3, GBP1, GHR, HMGA1, AFNAR1, AFNG, AFNGR1, IL10RA, IL20, IL2RA, IL2RG, IL4, IL4R, IL6ST, INSR, IRF1, ISGF3G, JAK1, JAK2, JAK3, JUN, JUNB, MMP3, MPL, MYC, NFKB1, NOS2A, NR3C1, OAS1, OSM, PDGFRA, PIAS1, PIAS2, PPP2R1A, PRLR, PTPN1, PTPRC, SH2B1, SIT1, SLA2, SMAD1, SMAD2, SMAD3, SMAD4, SMAD5, SOCS1, SOCS2, SOCS3, SOCS4, SOCS5, SP1, SPI1, SRC, STAM, STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, STAT6, STUB1, TYK2, USF1, YY1.
Gene expression was analyzed using Human JAK-Stat RT2 Profiler PCR Arrays (SABiosciences, Valencia, CA) in triplicate as described in the legend for Figure 3. Fold change was calculated via the comparative threshold cycle method using values for 293-CXCR3 cells as the baseline.
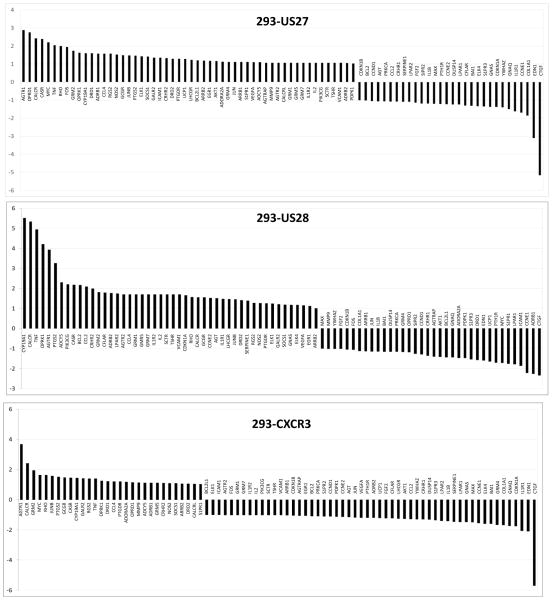
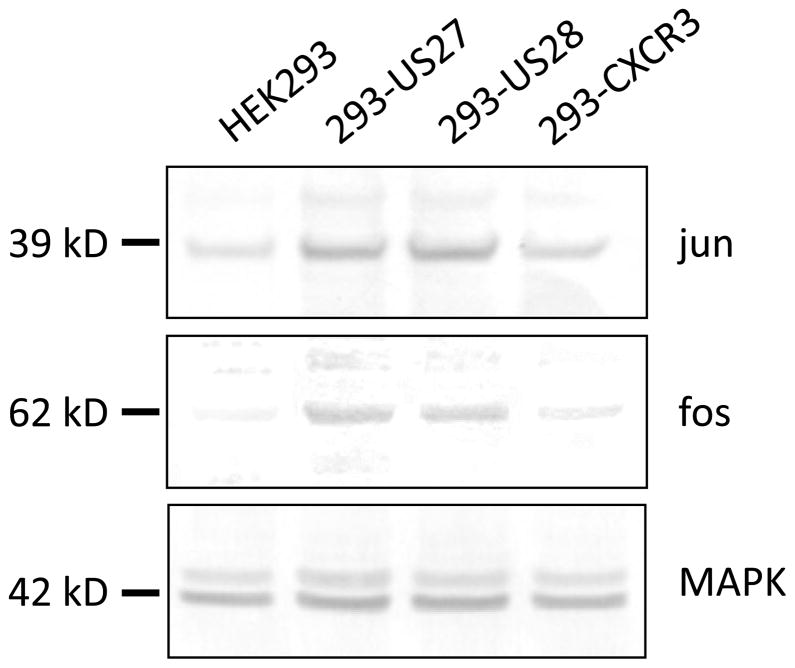
In addition to the JAK-STAT array, genes involved in GPCR signaling were also evaluated via the RT2 Profiler Array System. Expression profiles for each cell type compared to HEK293 cells are shown in Figure 5. Again, certain genes were up-regulated by the expression of all three GPCR, including AGTR1, CALCR, and CASR, suggesting that these effects are non-specific and might be induced by the overexpression of any GPCR. When the threshold values for 293-US27 and 293-US28 were compared to the 293-CXCR3 values, only three cellular genes were found to be significantly up-regulated by HCMV US27: FOS, JUN and CYP19A1, as shown in Table 2. A greater number of cellular genes were impacted by US28, which also caused upregulation of CYP19A1, as well as CALCRL, CCL2/MCP-1, OPRK1, PI3CG, and PTGS2. Notably, expression of BCL2L1 and ADRB1, the β-adrenergic receptor, were significantly down-regulated by US28 compared to controls. There were two genes that were represented on both the JAK/STAT array and the GPCR array: BCL2L1 and JUN. JUN was found to be significantly up-regulated in the presence of US27 on both arrays, while the BCL2L1 results were slightly more variable, with a fold change of 1.97 when analyzed on the JAK-STAT array but only 1.45 on the GPCR array. For two genes, JUN and FOS, protein expression was also examined by Western blot, and a modest increase in jun and fos protein levels was detected in both the 293-US27 and 293-US28 cells (Fig. 6), which further supports findings of the PCR array.
Figure 5. GPCR pathway gene expression levels in cells expressing US27, US28, or CXCR3.
RNA from each stable cell line was extracted using RNEasy Midi Kit (Qiagen, Valencia, CA), cDNA was prepared using the RT2 First Strand Kit (Qiagen), and then diluted cDNA was mixed with the RT2 SYBR green master mix (Qiagen) according to the manufacturer’s instructions and loaded into the Human GPCR RT2-PCR Profiler Array (SABiosciences, Valencia, CA). Data analysis was performed as described for the JAK-STAT array in the legend for Figure 3 except that the GPCR results are the average of two biological replicates.
Table 2.
GPCR Pathway1 Gene Expression Analysis
| Fold Change2 | |||||
|---|---|---|---|---|---|
| Unigene | Refseq | Symbol | Description | US27 | US28 |
| Hs.99913 | NM_000684 | ADRB1 | Adrenergic, beta-1-, receptor | 1.63 | −2.22 |
| Hs.728754 | NM_031850 | AGTR1 | Angiotensin II receptor, type 1 | −1.09 | 1.13 |
| Hs.516966 | NM_138578 | BCL2L1 | BCL2-like 1 | 1.45 | −2.03 |
| Hs.470882 | NM_005795 | CALCRL | Calcitonin receptor-like | 1.19 | 5.41 |
| Hs.303649 | NM_002982 | CCL2 | Chemokine (C-C motif) ligand 2 | 1.44 | 2.85 |
| Hs.260074 | NM_000103 | CYP19A1 | Cytochrome P450, family 19, subfamily A, polypeptide 1 | 2.10 | 4.07 |
| Hs.73893 | NM_000795 | DRD2 | Dopamine receptor D2 | 1.57 | 1.42 |
| Hs.728789 | NM_005252 | FOS | FBJ murine osteosarcoma viral oncogene homolog | 2.35 | 1.11 |
| Hs.714791 | NM_002228 | JUN | Jun proto-oncogene | 1.84 | 1.56 |
| Hs.106795 | NM_000912 | OPRK1 | Opioid receptor, kappa 1 | 1.53 | 3.49 |
| Hs.32942 | NM_002649 | PIK3CG | Phosphoinositide-3-kinase, catalytic, gamma polypeptide | 1.28 | 2.33 |
| Hs.196384 | NM_000963 | PTGS2 | Prostaglandin-endoperoxide synthase 2 (prostaglandin G/H synthase and cyclooxygenase) | 1.12 | 2.22 |
The complete list of genes analyzed on this array is shown In Figure 5 and includes: ADCY5, ADORA2A, ADRB1, ADRB2, AGT, AGTR1, AGTR2, AGTRAP, AKT1, ARRB1, ARRB2, BAI1, BCL2, BCL2L1, CALCRM CALCRL, CASR, CCL2, CCL4, CCND1, CCNE1, CCNE2, CDKN1A, CDKN1B, CFLAR, COL1A1, CRHR1, CRHR2, CTGF, CYP19A1, DRD1, DRD2, DUSP14, EDN1, EGR1, ELK1, ELK4, FGF2, FOS, GALR2, GCGR, GNAQ, GNAS, GRM1, GRM2, GRM4, GRM5, GRM7, ICAM1, IL1B, IL1R1, IL1R2, IL2, JUN, JUNB, LHCGR, LPAR1, LPAR2, MAX, MMP9, MYC, NOS2, OPRD1, OPRK1, PDPK1, PIK3CG, PRKCA, PTGDR, PTGS2, PTH1R, RGS2, RHO, S1PR1, S1PR2, S1PR3, SCTR, SERPINE1, SOCS1, TNF, TSHR, UCP1, VCAM1, VEGFA, YWHAZ.
Gene expression was analyzed using Human GPCR RT2 Profiler PCR Arrays (SABiosciences, Valencia, CA) in duplicate as described in the legend for Figure 5. Fold change was calculated via the comparative threshold cycle method using values for 293-CXCR3 cells as the baseline.
Figure 6. Jun and Fos protein levels are higher in cells expressing either US27 or US28.
Stable cell lines expressing either US27, US28 or CXCR3 or control HEK293 cells were lysed, proteins separated by SDS-PAGE, and then immunoblotted with antibodies directed against jun, fos, or total MAPK (Santa Cruz Biotechnology, Santa Cruz, CA) followed by AP-conjugated secondary antibody.
During infection, HCMV can have a striking impact on cell gene expression and other cellular functions. We have focused on one viral gene, US27, and its effect on cell physiology when expressed in isolation in human cells. We found that the presence of the US27 gene product leads to enhanced cell proliferation and DNA synthesis compared to controls. This suggests that like US28 (Maussang et al., 2009; Maussang et al., 2006; Slinger et al., 2010) and ORF74 of KSHV (Kasposi’s sarcoma associated herpesvirus) (Bais et al., 1998), under certain conditions, US27 can stimulate cell division and promote enhanced growth. Evaluation of cellular gene expression using PCR profiler arrays led to the identification of several host genes that may play a role in the enhanced growth rate observed in cells expressing HCMV US27. The JAK-STAT PCR array was utilized in this study because US28 has previously been shown to stimulate cell proliferation through induction of IL-6, with downstream signaling by JAK1 and STAT3 (Slinger et al., 2010). Here, we report that the gene encoding oncostatin M, a cytokine that belongs to the IL-6 family with roles in hematopoiesis and inflammation, is significantly up-regulated by both US27 and US28. This result is consistent with transcriptome analyses that found upregulation of oncostatin M gene expression in HCMV-infected monocytes (Chan et al., 2008a; Chan et al., 2008b). Oncostatin M was also found to be one of the most abundant proteins present in the supernatants of virus-infected cells (Dumortier et al., 2008) and has been identified as a major growth factor for Kaposi’s sarcoma spindle cells (Nair et al., 1992).
In addition to oncostatin M, increased expression of the JUN and FOS genes was observed in cells expressing US27. The jun and fos proteins typically form homo- or heterodimers to comprise the transcription factor AP-1, which plays a critical role in the regulation of multiple genes controlling cell proliferation and apoptosis (Angel and Karin, 1991). Induction of AP-1 upon infection with HCMV has been well-documented (Boldogh et al., 1990; Boldogh et al., 1991), and stimulation of AP-1 activity has previously been linked to at least two specific viral proteins: IE1 (Kim et al., 1999; Wang and Sonenshein, 2005) and the tegument protein pp71 (Liu and Stinski, 1992). We show not only an increase in gene expression for JUN and FOS, but also an increase in jun and fos protein levels, strongly supporting the notion that US27 may induce changes in cellular gene expression through the action of the AP-1 transcription factor.
Cells expressing US27 also exhibited an increase in expression of the BCL2L1 gene. While there was some variability in the fold-change of up-regulation detected on the JAK-STAT array compared to the GPCR array, the overall trend indicated increased BCL2L1 gene expression. The protein encoded by this gene is also known as Bcl-x, a member of the Bcl-2 protein family that plays critical roles in regulation of cell survival (Boise et al., 1993). Bcl-x is expressed as two isomeric forms, Bcl-xL and Bcl-xS, which are typically present in the cytosol in association with the mitochondrial membrane. Bcl-xL forms heterodimers with various proteins, including Bax, Bak and Bcl-2, and prevents cell death by blocking the formation of channels in the mitochondrial membrane and inhibiting activation of the caspase cascade (Straten and Andersen, 2010). Overexpression of Bcl-2 family pro-survival genes is a common mechanism for preventing apoptosis, and cells infected with HCMV have previously been found to contain elevated levels of Bcl-2 protein (Cinatl et al., 1998). Although levels of Bcl-x protein in HCMV-infected cells have not been reported to date, microarray analysis of infected monocytes revealed a 4.9-fold increase in the level of BCL2A1 (Bcl-2 related protein A1) mRNA (Chan et al., 2008a). In fact, Chan et al found that infection of monocytes led to the induction of many genes associated with the differentiation into a macrophage phenotype, with 583 genes significantly up-regulated and 621 genes significantly downregulated (Chan et al., 2008a). One of the genes found to up-regulated in that study was SOCS3, a suppressor of cytokine signaling. In our study with HCMV US27 expressed in isolation, no significant changes in SOCS3 expression were noted, but both SOCS2 and SOCS5 were found to be up-regulated. It is not surprising that HCMV might have multiple mechanisms for activating SOCS proteins and suppressing inflammatory cytokines, since this would likely aid the virus in avoiding immune clearance.
Another global analysis of host cell gene expression during virus infection utilized the AD169 strain of HCMV compared to a US28-deletion mutant (Hertel and Mocarski, 2004). The results showed that while numerous changes occur upon infection, only a small subset of genes involved in cell cycle progression could be specifically attributed to the presence of US28, and none of those were the same genes identified as being impacted by US28 expression in this study. Future work will focus on the protein products of the genes affected by US27, both in transfected and virus-infected cells, in order to confirm that activity is increased and thus contributing to US27-mediated enhancement of cell proliferation. These studies will help clarify the role of US27 in virus infection and could provide a potential target for novel anti-viral therapeutics.
Highlights.
The HCMV US27 gene product promotes cell growth and proliferation.
HCMV US27 triggers increased expression of pro-survival factor Bcl-X and transcription factors jun and fos.
US27 and US28 both up-regulate oncostatin M, an IL-6 family cytokine.
Acknowledgments
This work was supported by NIH Grant AI074029 (to JVS), the Lily Drake Cancer Fund, and USF Faculty Development funds. The authors thank Lauren Hart, Nandini Chitale, and Kathleen Arnolds for technical assistance and helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Angela P. Lares, Email: aspletcher@usfca.edu.
Carolyn C. Tu, Email: cctu@usfca.edu.
Juliet V. Spencer, Email: jspencer@usfca.edu.
References
- Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072(2–3):129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- Bais C, Santomasso B, Coso O, Arvanitakis L, Raaka EG, Gutkind JS, Asch AS, Cesarman E, Gershengorn MC, Mesri EA. G-protein-coupled receptor of Kaposi’s sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature. 1998;391(6662):86–89. doi: 10.1038/34193. [DOI] [PubMed] [Google Scholar]
- Beisser PS, Goh CS, Cohen FE, Michelson S. Viral chemokine receptors and chemokines in human cytomegalovirus trafficking and interaction with the immune system. CMV chemokine receptors. Curr Top Microbiol Immunol. 2002;269:203–234. doi: 10.1007/978-3-642-59421-2_13. [DOI] [PubMed] [Google Scholar]
- Beisser PS, Grauls G, Bruggeman CA, Vink C. Deletion of the R78 G protein-coupled receptor gene from rat cytomegalovirus results in an attenuated, syncytium-inducing mutant strain. J Virol. 1999;73(9):7218–7230. doi: 10.1128/jvi.73.9.7218-7230.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisser PS, Vink C, Van Dam JG, Grauls G, Vanherle SJ, Bruggeman CA. The R33 G protein-coupled receptor gene of rat cytomegalovirus plays an essential role in the pathogenesis of viral infection. J Virol. 1998;72(3):2352–2363. doi: 10.1128/jvi.72.3.2352-2363.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodaghi B, Jones TR, Zipeto D, Vita C, Sun L, Laurent L, Arenzana-Seisdedos F, Virelizier JL, Michelson S. Chemokine sequestration by viral chemoreceptors as a novel viral escape strategy: withdrawal of chemokines from the environment of cytomegalovirus-infected cells. J Exp Med. 1998;188(5):855–866. doi: 10.1084/jem.188.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nunez G, Thompson CB. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74(4):597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- Boldogh I, AbuBakar S, Albrecht T. Activation of proto-oncogenes: an immediate early event in human cytomegalovirus infection. Science. 1990;247(4942):561–564. doi: 10.1126/science.1689075. [DOI] [PubMed] [Google Scholar]
- Boldogh I, AbuBakar S, Deng CZ, Albrecht T. Transcriptional activation of cellular oncogenes fos, jun, and myc by human cytomegalovirus. J Virol. 1991;65(3):1568–1571. doi: 10.1128/jvi.65.3.1568-1571.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin RD, Schaefer GC, Allen JR, Davis-Poynter NJ, Farrell HE. The M33 chemokine receptor homolog of murine cytomegalovirus exhibits a differential tissue-specific role during in vivo replication and latency. J Virol. 2009;83(15):7590–7601. doi: 10.1128/JVI.00386-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarosa P, Gruijthuijsen YK, Michel D, Beisser PS, Holl J, Fitzsimons CP, Verzijl D, Bruggeman CA, Mertens T, Leurs R, Vink C, Smit MJ. Constitutive signaling of the human cytomegalovirus-encoded receptor UL33 differs from that of its rat cytomegalovirus homolog R33 by promiscuous activation of G proteins of the Gq, Gi, and Gs classes. J Biol Chem. 2003;278(50):50010–50023. doi: 10.1074/jbc.M306530200. [DOI] [PubMed] [Google Scholar]
- Chan G, Bivins-Smith ER, Smith MS, Smith PM, Yurochko AD. Transcriptome analysis reveals human cytomegalovirus reprograms monocyte differentiation toward an M1 macrophage. J Immunol. 2008a;181(1):698–711. doi: 10.4049/jimmunol.181.1.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan G, Bivins-Smith ER, Smith MS, Yurochko AD. Transcriptome analysis of NF-kappaB- and phosphatidylinositol 3-kinase-regulated genes in human cytomegalovirus-infected monocytes. J Virol. 2008b;82(2):1040–1046. doi: 10.1128/JVI.00864-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MS, Satchwell SC, Preddie E, Weston KM, Barrell BG. Human cytomegalovirus encodes three G protein-coupled receptor homologues. Nature. 1990;344(6268):774–777. doi: 10.1038/344774a0. [DOI] [PubMed] [Google Scholar]
- Cinatl J, Jr, Cinatl J, Vogel JU, Kotchetkov R, Driever PH, Kabickova H, Kornhuber B, Schwabe D, Doerr HW. Persistent human cytomegalovirus infection induces drug resistance and alteration of programmed cell death in human neuroblastoma cells. Cancer Res. 1998;58(2):367–372. [PubMed] [Google Scholar]
- Dalman MR, Deeter A, Nimishakavi G, Duan ZH. Fold change and p-value cutoffs significantly alter microarray interpretations. BMC Bioinformatics. 2012;13(Suppl 2):S11. doi: 10.1186/1471-2105-13-S2-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumortier J, Streblow DN, Moses AV, Jacobs JM, Kreklywich CN, Camp D, Smith RD, Orloff SL, Nelson JA. Human cytomegalovirus secretome contains factors that induce angiogenesis and wound healing. J Virol. 2008;82(13):6524–6535. doi: 10.1128/JVI.00502-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell HE, Abraham AM, Cardin RD, Sparre-Ulrich AH, Rosenkilde MM, Spiess K, Jensen TH, Kledal TN, Davis-Poynter N. Partial functional complementation between human and mouse cytomegalovirus chemokine receptor homologues. J Virol. 2011;85(12):6091–6095. doi: 10.1128/JVI.02113-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraile-Ramos A, Pelchen-Matthews A, Kledal TN, Browne H, Schwartz TW, Marsh M. Localization of HCMV UL33 and US27 in endocytic compartments and viral membranes. Traffic. 2002;3(3):218–232. doi: 10.1034/j.1600-0854.2002.030307.x. [DOI] [PubMed] [Google Scholar]
- Gao JL, Murphy PM. Human cytomegalovirus open reading frame US28 encodes a functional beta chemokine receptor. J Biol Chem. 1994;269(46):28539–28542. [PubMed] [Google Scholar]
- Hertel L, Mocarski ES. Global analysis of host cell gene expression late during cytomegalovirus infection reveals extensive dysregulation of cell cycle gene expression and induction of Pseudomitosis independent of US28 function. J Virol. 2004;78(21):11988–12011. doi: 10.1128/JVI.78.21.11988-12011.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SE, Mason GM, Wills MR. Human cytomegalovirus immunity and immune evasion. Virus Res. 2011;157(2):151–160. doi: 10.1016/j.virusres.2010.10.031. [DOI] [PubMed] [Google Scholar]
- Kesson AM, Kakakios A. Immunocompromised children: conditions and infectious agents. Paediatric respiratory reviews. 2007;8(3):231–239. doi: 10.1016/j.prrv.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Kim S, Yu SS, Lee IS, Ohno S, Yim J, Kang HS. Human cytomegalovirus IE1 protein activates AP-1 through a cellular protein kinase(s) J Gen Virol. 1999;80 (Pt 4):961–969. doi: 10.1099/0022-1317-80-4-961. [DOI] [PubMed] [Google Scholar]
- Liu B, Stinski MF. Human cytomegalovirus contains a tegument protein that enhances transcription from promoters with upstream ATF and AP-1 cis-acting elements. J Virol. 1992;66(7):4434–4444. doi: 10.1128/jvi.66.7.4434-4444.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies BJ, Gibson W. The chemokine receptor homologue encoded by US27 of human cytomegalovirus is heavily glycosylated and is present in infected human foreskin fibroblasts and enveloped virus particles. Virus Res. 2007;123(1):57–71. doi: 10.1016/j.virusres.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maussang D, Langemeijer E, Fitzsimons CP, Stigter-van Walsum M, Dijkman R, Borg MK, Slinger E, Schreiber A, Michel D, Tensen CP, van Dongen GA, Leurs R, Smit MJ. The human cytomegalovirus-encoded chemokine receptor US28 promotes angiogenesis and tumor formation via cyclooxygenase-2. Cancer Res. 2009;69(7):2861–2869. doi: 10.1158/0008-5472.CAN-08-2487. [DOI] [PubMed] [Google Scholar]
- Maussang D, Verzijl D, van Walsum M, Leurs R, Holl J, Pleskoff O, Michel D, van Dongen GA, Smit MJ. Human cytomegalovirus-encoded chemokine receptor US28 promotes tumorigenesis. Proc Natl Acad Sci U S A. 2006;103(35):13068–13073. doi: 10.1073/pnas.0604433103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocarski ES, Shenk T, Pass RF. Cytomegaloviruses. In: Knipe DM, Howley PM, Friffin DE, Lamb RA, Martin MA, editors. Fields Virology. 5. Lippincott-Raven Publishers; Philadelphia, PA: 2006. pp. 2701–2772. [Google Scholar]
- Nair BC, DeVico AL, Nakamura S, Copeland TD, Chen Y, Patel A, O’Neil T, Oroszlan S, Gallo RC, Sarngadharan MG. Identification of a major growth factor for AIDS-Kaposi’s sarcoma cells as oncostatin M. Science. 1992;255(5050):1430–1432. doi: 10.1126/science.1542792. [DOI] [PubMed] [Google Scholar]
- Neote K, DiGregorio D, Mak JY, Horuk R, Schall TJ. Molecular cloning, functional expression, and signaling characteristics of a C-C chemokine receptor. Cell. 1993;72(3):415–425. doi: 10.1016/0092-8674(93)90118-a. [DOI] [PubMed] [Google Scholar]
- O’Connor CM, Shenk T. Human cytomegalovirus pUS27 G protein-coupled receptor homologue is required for efficient spread by the extracellular route but not for direct cell-to-cell spread. J Virol. 2011;85(8):3700–3707. doi: 10.1128/JVI.02442-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleskoff O, Casarosa P, Verneuil L, Ainoun F, Beisser P, Smit M, Leurs R, Schneider P, Michelson S, Ameisen JC. The human cytomegalovirus-encoded chemokine receptor US28 induces caspase-dependent apoptosis. Febs J. 2005;272(16):4163–4177. doi: 10.1111/j.1742-4658.2005.04829.x. [DOI] [PubMed] [Google Scholar]
- Revello MG, Campanini G, Piralla A, Furione M, Percivalle E, Zavattoni M, Gerna G. Molecular epidemiology of primary human cytomegalovirus infection in pregnant women and their families. J Med Virol. 2008;80(8):1415–1425. doi: 10.1002/jmv.21243. [DOI] [PubMed] [Google Scholar]
- Slinger E, Maussang D, Schreiber A, Siderius M, Rahbar A, Fraile-Ramos A, Lira SA, Soderberg-Naucler C, Smit MJ. HCMV-encoded chemokine receptor US28 mediates proliferative signaling through the IL-6-STAT3 axis. Science signaling. 2010;3(133):ra58. doi: 10.1126/scisignal.2001180. [DOI] [PubMed] [Google Scholar]
- Stapleton LK, Arnolds KL, Lares AP, Devito TM, Spencer JV. Receptor chimeras demonstrate that the C-terminal domain of the human cytomegalovirus US27 gene product is necessary and sufficient for intracellular receptor localization. Virology journal. 2012;9:42. doi: 10.1186/1743-422X-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin Infect Dis. 2006;43(9):1143–1151. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- Straten P, Andersen MH. The anti-apoptotic members of the Bcl-2 family are attractive tumor-associated antigens. Oncotarget. 2010;1(4):239–245. doi: 10.18632/oncotarget.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stropes MP, Schneider OD, Zagorski WA, Miller JL, Miller WE. The carboxy-terminal tail of human cytomegalovirus (HCMV) US28 regulates both chemokine-independent and chemokine-dependent signaling in HCMV-infected cells. J Virol. 2009;83(19):10016–10027. doi: 10.1128/JVI.00354-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschische P, Tadagaki K, Kamal M, Jockers R, Waldhoer M. Heteromerization of human cytomegalovirus encoded chemokine receptors. Biochem Pharmacol. 2011;82(6):610–619. doi: 10.1016/j.bcp.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Sonenshein GE. Induction of the RelB NF-kappaB subunit by the cytomegalovirus IE1 protein is mediated via Jun kinase and c-Jun/Fra-2 AP-1 complexes. J Virol. 2005;79(1):95–105. doi: 10.1128/JVI.79.1.95-105.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Silva MC, Shenk T. Functional map of human cytomegalovirus AD169 defined by global mutational analysis. Proc Natl Acad Sci U S A. 2003;100(21):12396–12401. doi: 10.1073/pnas.1635160100. [DOI] [PMC free article] [PubMed] [Google Scholar]