Abstract
Atypical protein kinase C (aPKC) isoforms mediate insulin effects on glucose transport in muscle and adipose tissues and lipid synthesis in liver and support other metabolic processes, expression of enzymes needed for islet insulin secretion and hepatic glucose production/release, CNS appetite suppression, and inflammatory responses. In muscle, selective aPKC deficiency impairs glucose uptake and produces insulin resistance and hyperinsulinemia, which, by activating hepatic aPKC, provokes inordinate increases in lipid synthesis and produces typical “metabolic syndrome” features. In contrast, hepatic aPKC deficiency diminishes lipid synthesis and protects against metabolic syndrome features. Unfortunately, aPKC is deficient in muscle but paradoxically conserved in liver in obesity and type 2 diabetes mellitus; this combination is particularly problematic because it promotes lipid and carbohydrate abnormalities. Accordingly, metabolic effects of aPKCs can be “good” or “bad,” depending upon nutritional status; thus, muscle glucose uptake, islet insulin secretion, hepatic glucose and lipid production/release, and adipose fat synthesis/storage would be important for survival during periods of limited food availability and therefore be “good.” However, during times of food surfeit, excessive activation of hepatic aPKC, whether caused by overnutrition or impairments in extrahepatic effects of insulin, would lead to inordinate increases in hepatic lipid synthesis and metabolic syndrome features and therefore be “bad.” In keeping with these ideas, the inhibition of hepatic aPKC markedly ameliorates lipid and carbohydrate abnormalities in experimental models of obesity and type 2 diabetes. We postulate that a similar approach may be useful for treating humans.
atypical protein kinase c (aPKC) isoforms, aPKCι, -λ, and -ζ, are members of the phospholipid/lipid-regulated PKC family that are activated by acidic phospholipids, phosphatidylinositol-1,3,5-(PO4)3 (PIP3), and phosphatidic acid (PA) rather than by the neutral lipid, diacylglycerol (DAG), and Ca++, which activate “typical” PKCs. However, it is a misnomer to consider aPKCs as “atypical” relative to typical DAG-dependent/Ca++-dependent classical/conventional PKCs (cPKCsα, -β, and -γ) and DAG-dependent/Ca++-independent novel PKCs (nPKCδ, -ε, -η, and -θ). This nomenclature reflects the historical order of discovery of cPKCs, nPKCs, and aPKCs rather than their biological importance. Indeed, aPKCs are archetypal protein kinases that have widespread occurrence throughout plant and animal kingdoms and are indispensable in a wide variety of essential cellular functions independent of typical PKCs.
aPKCs participate importantly in regulating multiple cellular processes pertinent to this review, including 1) determination of cellular polarity and related functions, i.e., motility, adhesion, differentiation, and embryogenesis; 2) activation of the immune response transactivator NF-κB via aPKC-dependent phosphorylation of both IKKα/β (24), a kinase that phosphorylates the inhibitor of κB (IκB), a tonic binder and inhibitor of NFκB, thereby releasing and targeting IκB for ubiquitinylation-mediated destruction and thereby freeing NF-κB to translocate from cytosol to nucleus, and Thr311 in the RelA subunit of NF-κB, which regulates the transcription of an array of genes involved in production of cytokines that not only mediate inflammatory/immune responses (14) but can also increase systemic insulin resistance (12); 3) signaling by various growth factors, including IGF-I and insulin, that act through phosphatidylinositol 3-kinase (PI3K) to activate both aPKC and Akt/PKB (5) and thereby control various metabolic processes, including glucose transport in myocytes and adipocytes by increasing GLUT4 glucose transporter translocation to the plasma membrane (5, 15, 23, 38), and lipogenesis in liver by increased expression/activation of sterol receptor element-binding protein-1c (SREBP-1c), which transactivates multiple lipogenic enzymes (30, 53); 4) cell growth by activation of ERK1/2 and other MAPKs (39, 20) and/or cyclin-dependent kinase-activating kinase (9); 5) protein synthesis by activation of p70 S6 kinase (35); 6) regulation of genes required for critical pancreatic islet β-cell functions, including insulin production and release (19); and 7) signaling downstream of other signaling factors, e.g., Src kinase (20), phospholipase D (PLD) (17, 13), proline-rich tyrosine kinase 2 (13), and AMP-activated kinase (AMPK) (13). The ability of aPKCs to bind Par6 and other key scaffolding molecules in signaling complexes and the movement of activated aPKCs to the plasma membrane and nuclear sites undoubtedly are important events in explaining the pleiotropic effects of aPKCs.
Here, we will focus on the role of aPKCs in metabolic processes, most notably insulin actions on carbohydrate and lipid metabolism and insulin secretion, in both health and disease states of obesity and diabetes mellitus. As this review unfolds (see Table 1), it will become apparent that aPKCs are likely to have served in metabolic processes essential for survival during periods of limited food intake but unfortunately have detrimental effects when activated more persistently during periods of dietary excess.
Table 1.
“Good” and “bad” metabolic effects of atypical protein kinase C isoforms in humans relative to nutritional status
| “Good” Effects | “Bad” Effects |
|---|---|
| Nutritional deficiency | |
| Glucose transport in muscle (energy supply and glycogen storage) | |
| Glucose transport in adipocytes (energy supply and lipid storage) | |
| Insulin production and release in islets (utilization and storage of foods) | |
| Lipid synthesis in liver (synthesis and release of lipids/energy supply) | |
| Maintaining PEPCK and G-6-Pase in liver (glucose production and release/energy supply) | |
| NF-κB-dependent cytokines in liver and/or macrophages (immune mechanisms) | |
| Nutritional excess (with hyperinsulinemia) | |
| Glucose transport in muscle (energy supply) | SREBP-1c-mediated lipid synthesis in liver (excessive synthesis and release of lipids) |
| Glucose transport in adipocytes (energy supply and lipid storage) | NF-κB-dependent cytokines in liver and/or macrophages (insulin resistance?) |
| Insulin production and release in islets (utilization/storage of foods) | Maintaining PEPCK and G-6-Pase in liver (glucose production and hyperglycemia) |
| NF-κB-dependent cytokines in liver and/or macrophages (immune mechanisms) | |
PEPCK, phosphoenolpyruvate carboxykinase; G-6-Pase, glucose-6-phosphatase; SREBP-1c, sterol regulatory element-binding protein-1c.
aPKC Isoforms in Tissues of Various Species
PKCι is the human equivalent of mouse PKCλ (98% homology), and these isoforms most likely function comparably if not identically in their respective species. In addition, PKCι and PKCλ share 70% overall homology with PKCζ, particularly in functionally important domains. This homology accounts for 1) how PKCζ functions interchangeably with PKCι/λ in many, albeit not all, biological processes and 2) why expression of active or inhibitory forms of one aPKC isoform can promote or inhibit the activation and function of other aPKC isoforms.
Relevant to metabolic functions in specific tissues of commonly used experimental rodents, as deduced from studies in knockout mice (15), in muscle and adipocytes PKCλ is more abundant than PKCζ, and knockout of PKCζ has little or no effect on aPKC levels in these tissues. However, in rat muscle and adipocytes, PKCζ is the more abundant isoform (1). Accordingly, mouse-derived 3T3-L1 adipocytes contain exclusively or primarily PKCλ (23, 28), and rat-derived L6 myotubes contain exclusively or primarily PKCζ (1, 38).
In contrast to muscle and adipocytes, mouse liver contains substantial amounts of both PKCλ and PKCζ (30, 42). On the other hand, there is little or no information on relative amounts of aPKC isoforms in rat liver, or in pancreatic islet β-cells of any species, or in tissues of nonhuman primates.
In human muscle, adipocytes, and liver, PKCι mRNA levels are much greater than PKCζ mRNA levels, and therefore, we suspect that PKCι is the more abundant isoform, but definitive information on protein levels is still lacking.
aPKC Structure/Activity/Function Relationships
Like cPKCs and nPKCs, aPKCs have classical bilobar kinase folds, and activation requires phosphorylation of both 1) the activation loop or T-loop site (Thr403 in PKCι, Thr410 in PKCζ, and Thr411 in PKCλ; unless otherwise specified, the numbering of human PKCι will be used), probably exclusively through the action of phosphoinositide-dependent kinase-1 (PDK1); and 2) Thr555 in PKCι (Thr560 in PKCζ), the auto(trans)phosphorylation site in the turn motif that stabilizes an active conformation of the catalytic subunit (31).
aPKCs share considerable homology with cPKCs and nPKCs in conserved (C) regions C1, C3, and C4 but lack a C2 region that mediates Ca++ binding and have a single Zn finger-like domain in C1 instead of the tandem domains in cPKCs and nPKCs that are required for activation by DAG and phorbol esters (PEs). The variable (V) regions of aPKCs, V1 and V3 at NH2 terminus and hinge regions, respectively, lack significant homology with cPKCs and nPKCs. Interestingly, arginine residues that surround a pocket in the C1 region of aPKCs limit the interaction of DAG with an otherwise potential activation site in this pocket and may account for insensitivity of aPKCs to DAG and PE (34). Analogous to cPKCs and nPKCs, various lipids and phospholipids bind to sites in the C1 region and modulate activity as well as provide an appropriate membrane-like milieu needed for activation; this may account for the need to add phosphatidylserine (PS) to observe fully aPKC activity in cell-free assays.
Activity of aPKCs is tonically inhibited by an interaction between key residues in the pseudosubstrate sequence in the regulatory domain and the substrate-binding site of the catalytic domain (see Fig. 1). Most likely, this interaction is dependent upon one or more of the basic arginine residues in the pseudosubstrate sequence that are analogous to the arginine residues that flank serine and threonine phosphorylation sites of aPKC substrates.
Fig. 1.
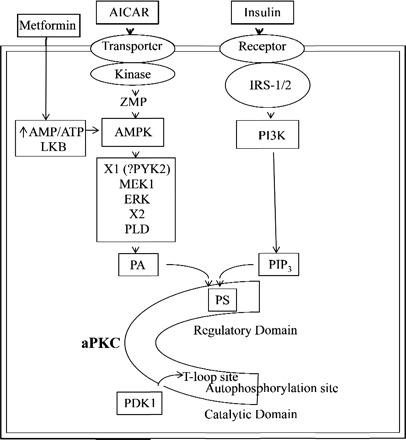
Activation of atypical PKC (aPKC) by insulin and AMP-activated protein kinase (AMPK) activators. Increases in acidic phospholipids, phosphatidylinositol-(PO4)3 (PIP3) via phosphatidylinositol 3-kinase (PI3K) action, and phosphatidic acid (PA) via phospholipase D (PLD) action presumably bind to basic arginine residues in or near the pseudosubstrate site (PS) in the regulatory domain and thereby open the major cleft and facilitate phosphoinositide-dependent kinase-1 (PDK1) access to the activation loop (or T-loop site), subsequent autophosphorylation and substrate access to the catalytic site. 5-aminoimidazole-4-carboxamide-1-β-d-riboside (AICAR) is converted to 5-aminoimidazole-4-carboxamide-1-β-d-ribosyl monophosphate (ZMP) and analog of 5′-AMP, and metformin, like AICAR, activates AMPK, perhaps by increasing the AMP/ATP ratio or via LKB. Factors that couple AMPK to ERK (X1) and ERK to PLD (X2) are uncertain, but Ca++-dependent proline-rich tyrosine kinase 2 (PYK2) may be involved. IRS-1/2, insulin receptor substrate-1 and -2.
As depicted in Fig. 1, acidic phospholipids such as PIP3 (derived from conversion of PIP2 to PIP3 by PI3K, e.g., as occurring during insulin action) and PA (derived from PLD action on various phospholipids or from de novo phospholipid synthesis) most likely bind to undefined basic arginine residues in or near the pseudosubstrate site and thereby activate the enzyme by promoting 1) molecular unfolding, 2) dissociation of the pseudosubstrate sequence from the substrate-binding site, and 3) opening of the critically needed phosphorylation sites at Thr403 and Thr555 (25, 47). PKCι can also be phosphorylated on tyrosine residues 256, 271, and 325, which may account for the direct activation of aPKC by Src kinase (58). AMPK, which activates glucose transport via aPKC (see below), also directly phosphorylates purified PKCζ, and it was postulated that AMPK directly phosphorylates Thr410, the activation loop site in PKCζ, presumably independently of PDK1, and this leads to activation (18). However, neither the direct phosphorylation of Thr410 nor increases in enzyme activity of PKCζ were reported (18), and it is also noteworthy that Thr410 phosphorylation only partially activates aPKC (47, 48). Moreover, we recently found that, as with insulin, 1) PDK1 is required for AMPK-induced increases in Thr410 phosphorylation and PKCζ activation and increases in glucose transport in L6 myotubes and that 2) purified PDK1, but not AMPK, increases Thr410 phosphorylation and actual enzyme activity when incubated directly with purified PKCζ (see below). In this regard, aPKC activation by AMPK has also been suggested to require the activation of ERK and PLD, thereby generating PA (13), and full effects of PA on aPKC activity most likely require auto(trans)phosphorylation as well as activation/T-loop phosphorylation. Accordingly, we believe that during AMPK activation, PLD-derived PA activates aPKCs by a mechanism analogous to that described above for PI3K-derived PIP3. On the other hand, the possibility remains that AMPK-mediated phosphorylation of yet-to-be-determined sites may cause aPKC unfolding and facilitation of PDK1-dependent phosphorylation and other activating steps.
During activation, aPKCs recruit/bind scaffolding proteins required for subsequent actions, including the ζ-interacting protein, ZIP/p62, Par6, Par4, other Pars, λ-inhibitory protein, LIP, and MEK5, and these interactions are dependent on acidic residues in the aPKC protein-binding Phlox Bem-1 (PB1) domain that interact with basic residues of PB1 domains in targeted proteins (21). Interestingly, in high-throughput screening for therapeutic agents to inhibit PKCι and thereby diminish tumor progression, Stallings-Mann et al. (45), using a novel assay, identified two gold-containing substances, viz., aurothiomalate and aurothioglucose, that inhibit the interaction of the PB1 domain of PKCι with Par6 and thereby interrupt PKCι signaling to a pathway that includes small G protein Rac1, p21-activated kinase, and MAPK and is required for tumor progression. One of these substances, aurothiomalate, is a currently used pharmaceutical that improves inflammatory components of rheumatoid arthritis. The other substance, aurothioglucose, is an experimental compound that, by virtue of its glucose moiety, selectively inhibits or destroys glucoreceptor neuronal cells in ventromedial hypothalamic region of the central nervous system (CNS), thereby causing hyperphagia, obesity, and glucose intolerance. Both agents are capable of inhibiting certain functions of aPKCs, most likely by interaction of the gold compound thiol groups with cys-69 (PKCι numbering) of the PB1 domain, as discussed further below. What other functions that are subserved by this or other signaling/scaffolding complexes are unknown.
Other phosphorylation sites that may contribute to aPKC activation include Ser35 and Ser37 in the PB1 domain and Ser217, Ser237, and Ser238 in the catalytic domain (29).
Interestingly, replacement of Lys274 in the catalytic domain of PKCι, essential for ATP binding and enzyme activity, with arginine does not result in inhibition, although comparable replacements inhibit activity of cPKCs and nPKCs; in contrast, replacement of Lys274 with tryptophan inhibits activity of aPKCs, cPKCs, and nPKCs (44).
Upstream Activators of aPKCs
Insulin activates aPKCs by PI3K-mediated increases in PIP3, but there are tissue-specific differences in the insulin receptor substrates (IRS) responsible for PI3K activation. Briefly, from studies in which IRS-1 or IRS-2 has been knocked out by recombinant, Cre-LoxP, or RNAi-knockdown methods, the following conclusions have been drawn: aPKC activation is controlled in muscle by IRS-1 (41), in liver by IRS-2 (36, 56), and in adipocytes by both IRS-1 and IRS-2 (32, 41). Partly similar and partly different, Akt activation is controlled in muscle by IRS-1 (41, 59), in liver by both IRS-1 and IRS-2 (41, 55, 56), and in adipocytes by either IRS-1 or IRS-2 (32, 41).
Measurement of aPKC Activity
To determine definitively whether aPKC has been activated, we have relied largely on actual measurement of enzyme activity in aPKC-specific immunoprecipitates, as described in our original studies (5, 49). In this assay, we have used an antiserum that recognizes an epitope that is present in the COOH terminus of all aPKCs. Importantly, even with amounts of antiserum considerably beyond the recommendations of the supplier, we have been able to immunoprecipitate only modest fractions of the total aPKC, and this results in recovering equal amounts of total aPKC in the immunoprecipitates, regardless of total aPKC levels in the lysates being assayed; thus, this assay reflects enzyme-specific activity. Equally important, it is necessary to assay all samples undergoing comparison, control and treated, within the same assay, usually in triplicate or quadruplicate. In the assay, in brief, the washed immunoprecipitates (i.e., containing aPKCs bound to antibodies and Sepharose AG beads) are incubated with [γ32P]ATP, MgCl2, PS, and, as substrate, a synthetic peptide containing the PKCε pseudosubstrate sequence with a phosphorylatable serine residue flanked on both sides by arginine residues, as in typical aPKC substrates.
Unlike Akt, whose activation is easily measured by simple Western analysis of the status of the PDK2-dependent phosphorylation site, viz., Ser473, we have not had satisfactory antibodies available to measure the equivalent activation site in aPKCs, i.e., the auto(trans)phosphorylation site. Moreover, with respect to the activation/T-loop site, satisfactory antibodies are available, but in some but not all tissues it is necessary to first immunoprecipitate aPKCs before Western analysis because an epitopically similar phosphopeptide sequence is present in other cPKCs that migrate at 80 kDa, in particular, PKCβ. Moreover, in some situations, the activation/T-loop site is constitutively phosphorylated, and insulin only regulates subsequent steps. These limitations in enzyme measurement are undoubtedly responsible for many investigators to evaluate insulin signaling by simple measurement of Akt phosphorylation by Western analysis, and this has slowed progress in expanding our knowledge of aPKC activation and action.
Methods to Evaluate aPKC Function
Efforts to evaluate aPKC-dependent functions in target tissues have revolved largely around using chemical inhibitors or plasmids or viruses encoding wild-type, constitutive, and kinase-inactive forms of aPKCs. In plasmid- and viral-mediated expression approaches, because of evolutionary conserved functional groups essential for activation and function, aPKCζ, -λ, and -ι can be used interchangeably to alter shared functions, regardless of the native aPKC isoform (4, 38). In addition, aPKC mRNA and/or protein levels can be diminished by treatment with interfering RNA (iRNA), or short hairpin RNA (shRNA) (e.g., see Ref. 38), and by gene knockout methods (see below).
Inhibitors of aPKC
Nonspecific PKC inhibitors, such as staurosporin, RO 31–8220, GF-109203X, and calphostin C, inhibit aPKCs when used in relatively high concentrations, but their usefulness is limited by more potent inhibition of cPKCs and nPKCs. A more specific means to inhibit aPKCs is to use the myristoylated cell-permeable peptide that contains the pseudosubstrate sequence common to all aPKCs. However, at higher concentrations, this peptide also inhibits cPKCs and nPKCs; this problem can be diminished by downregulation of cPKCS and nPKCs by prolonged PE treatment.
With knowledge of the crystal structures of aPKCs (e.g., see Refs. 16, 21, 33, and 45), virtual screens have been performed to identify compounds that specifically bind to and inhibit functionally important sites. For example, substances targeting the substrate-binding site (33) and a scaffolding site in the PB1 domain (16, 45) of PKCι have been discovered, and we have found that these substances specifically inhibit insulin-stimulated aPKC (but not Akt) activation and aPKC-dependent functions in various tissues.
Experimental Mouse aPKC Knockout Models
In mice, despite the presence of PKCζ, total body knockout of PKCλ is embryonically lethal at developmental day 9, and this experimental approach is not feasible. However, with Cre-lox methodology, it has been possible to generate mice with a loss of PKCλ in specific tissues. Knockout of PKCλ specifically in muscle, either homozygously or heterozygously, elicits marked decreases in insulin-simulated glucose transport specifically in muscle, and this isolated signaling defect leads to a syndrome comparable with the human “metabolic syndrome,” or “Syndrome X,” that includes systemic insulin resistance, hyperinsulinemia, glucose intolerance or mild diabetes, abdominal obesity, hyperlipidemia, and hepatosteatosis (15). Apparently, hyperinsulinemia in these knockout mice increases hepatic lipogenic enzymes through increased expression and activity of SREBP-1c, which controls the expression of multiple enzymes engaged in hepatic lipid synthesis, thereby leading to hepatosteatosis, hyperlipidemia, and provision of circulating substrate for subsequent storage in fat depots, most noticeably in the abdomen. This model is particularly relevant, since the partial deficiency of PKCλ in heterozygous muscle-specific PKCλ knockout mice is similar to the partial deficiency of aPKC (involving both PKCι and PKCζ) observed in muscles of type 2 diabetic humans (6, 7, 22). Accordingly, this mouse model of heterozygous muscle-specific PKCλ knockout should be useful for developing new treatments for the metabolic syndrome and type 2 diabetes. In this regard, note that specific inhibition of hepatic aPKC by adenoviral-mediated expression of kinase-inactive PKCζ in both heterozygous muscle-specific PKCλ knockout mice and high-fat-fed mice diminishes the inordinate activations of hepatic SREBP-1c and NF-κB, and decreases in these activities are attended by rapid and dramatic improvements in hyperinsulinemia, hepatosteatosis, hyperlipidemia, and insulin signaling in muscle (42).
Much different from the insulin resistance seen with muscle-specific knockout of PKCλ, liver-specific knockout of PKCλ in mice diminishes insulin-dependent activation of hepatic SREBP-1c, and this is accompanied by a decrease in hepatic lipid content and an increase in systemic insulin sensitivity (30, 42). However, it is uncertain whether the latter increase in systemic insulin sensitivity is due to diminished output of hepatic lipids that impair insulin signaling in peripheral tissues or to diminished activation of other hepatic aPKC-dependent factors that may similarly diminish insulin sensitivity, in particular NF-κB (12), which controls inflammatory cytokine production. Perhaps diminished hepatic activities of both SREBP-1c and NF-κB contribute to the increases in systemic insulin sensitivity owing to decreases in hepatic aPKC activity caused either by gene knockout (30, 42) or treatment with adenoviruses encoding kinase-inactive aPKC (42, 36).
Of further note in discussing experimental knockout approaches, the knockout of PKC-λ specifically in mouse pancreatic islet β-cells leads to diminished expression of enzymes required for insulin synthesis and secretion (19). This defect in β-cell integrity and insulin secretion might be expected to produce a diabetic state in these knockout mice, but phenotypic information is lacking. In addition to this long-term requirement for aPKC to maintain overall islet β-cell function, aPKC is required during the acute insulin secretory response to carbachol (52).
Finally, analogous to muscle-specific PKCλ-knockout mice, we have developed mice with adipocyte-specific knockout of PKCλ, and these mice have diminished glucose transport responses to insulin in adipocytes and normal insulin actions in muscle and liver (unpublished observations). These adipocyte-specific PKCλ knockout mice have mild glucose intolerance but, unlike muscle-specific PKCλ-knockout mice, do not develop obesity or hyperlipidemia, perhaps reflecting a failure to develop increases in either serum insulin levels or hepatic SREBP-1c expression.
aPKC Requirement in Insulin-Stimulated Glucose Transport
Multiple lines of evidence demonstrate that aPKCs are required for insulin-stimulated translocation of GLUT4 glucose transporters to the plasma membrane and subsequent increases in glucose transport in adipocytes and myocytes. This conclusion derives most notably from studies of 1) adenoviral-mediated expression of kinase-inactive forms of aPKC in cultured 3T3-L1 adipocytes (2, 5, 23), L6 myotubes (1), and human adipocytes (2); 2) iRNA-mediated aPKC knockdown and subsequent aPKC-mediated rescue of function in 3T3-L1 adipocytes and L6 myotubes (38); and 3) tissue-specific knockout of aPKC in mouse muscle (15) and adipocytes (unpublished observations).
Factors that operate downstream of aPKC during GLUT4 glucose transporter translocation are only partly understood. Although functional significance of localization is uncertain, aPKCs are present in immunoprecipitated GLUT4 vesicles and translocate to plasma membranes during insulin stimulation (48). Upon activation by insulin, GLUT4 vesicles are transported from an intracellular insulin-responsive compartment along microtubules through the actin cytoskeleton to the plasma membrane, where a complex forms between soluble N-ethylmaleimide-sensitive attachment receptor (SNARE) proteins present on the surfaces of plasma membranes (target SNAREs) and GLUT4 vesicles (vesicle SNAREs). In this scheme, vesicle-associated membrane protein 2/synaptobrevin, the vehicle SNARE in GLUT4 vesicles that attaches to the target SNARE syntaxin 4 on the inner surface of the plasma membrane, has been found to be phosphorylated by aPKC during insulin action (10). Furthermore, Munc18c, which is attached to syntaxin 4 and, along with other proteins, inhibits attachment of vesicle-associated membrane protein 2 and other soluble N-ethylmaleimide-sensitive fusion protein attachment proteins required for vesicle docking and subsequent fusion of GLUT4 vesicles to the plasma membrane, is phosphorylated by PKCs and thereby released from syntaxin 4. In this regard, an 80-kDa protein appears to serve as a link between aPKC and Munc18c during insulin-induced translocation of GLUT4 vesicles to the plasma membrane (43). In addition to these docking mechanisms, aPKC appears to be required for alterations in cytoskeletal actin required during GLUT4 vesicle translocation (27); however, the aPKC-regulated molecular factors required in actin remodeling are unknown. In addition to aPKC, several small G proteins and Akt are corequired for insulin-induced translocation of GLUT4 vesicles to the plasma membrane; this is not surprising given the complexity of the translocation process.
Role of aPKC in AMPK-Stimulated and Exercise-Stimulated Glucose Transport
Like insulin, the AMPK activator 5-aminoimidazole-4-carboxamide-1-β-d-riboside (AICAR) increases GLUT4 translocation and glucose transport in skeletal muscle, but unlike insulin, these effects of AICAR are elicited independently of PI3K. Nevertheless, studies of AICAR action in L6 myotubes have shown that, as with insulin, aPKC is required for increases in glucose transport (13). More recently, using a variety of methods (RNAi-mediated aPKC knockdown, expression of kinase-inactive forms of aPKC, PDK1, and AMPK), we found that both AICAR and the antidiabetic agent metformin activate aPKC via AMPK by a PDK1- and ERK-dependent mechanism and, moreover, provoke aPKC-dependent increases in GLUT4 translocation and glucose transport in L6 myotubes (37). In addition, despite activating AMPK and ERK, AICAR and metformin failed to increase glucose uptake in muscles of muscle-specific PKCλ-knockout mice (37). These findings in PKCλ-knockout mice suggest that in intact mice, as in cultures of rat-derived L6 myotubes, aPKC functions downstream of AMPK and ERK during actions of agents that activate muscle AMPK. On the other hand, treadmill exercise, which, like AICAR and metformin, activates both AMPK and aPKC in muscle, elicited full glucose transport effects in muscles of these same PKCλ-knockout mice (37). These findings suggest that exercise-induced increases in AMPK and aPKC are not required for increases in glucose transport in muscle but may nevertheless serve as a redundant mechanism. Of further note, unlike the situation in skeletal muscle, AICAR and metformin activate AMPK, but not aPKC, in rodent liver (37). Although the explanation for this tissue specificity is unclear, this fortuitous dissociation of aPKC from AMPK activation in the liver is important since activation of hepatic aPKC would provoke undesirable increases in lipogenesis, an outcome that fortunately does not occur during metformin action.
aPKC Requirements During Activation of Hepatic SREBP-1c
In liver, insulin is a major regulator of expression of SREBP-1c, which controls the expression of an array of enzymes engaged in lipid synthesis, including, for example, fatty acid synthase (FAS) and acetyl-coenzyme A carboxylase (ACC). In situations of limited or infrequent food availability, as in ancient hunter-gatherer populations, this function is most likely of critical importance for converting dietary carbohydrate and lipid precursors into circulating lipids, e.g., fatty acids and triacylglycerols, which are subsequently hydrolyzed as needed, cleared, and stored along with glucose-derived glycerol-phosphate in fat depots. However, in situations wherein food supply is overly abundant, as in present Western societies, this feeding/insulin-dependent function can be excessive and lead to hepatosteatosis, hyperlipidemia, obesity, insulin resistance, and, presumably, other “metabolic syndrome” features. However, also note that the development of metabolic syndrome features can also ensue in situations wherein systemic insulin resistance and hyperinsulinemia are provoked by a defect in insulin signaling in muscle, e.g., as seen in muscle-specific PKCλ-knockout mice (15). As discussed below in greater detail, comparable defects in muscle aPKC are seen in human forms of obesity and type 2 diabetes, but it is uncertain whether these defects in humans are dependent or independent of excessive food intake. In either case, the excessive action of insulin, whether caused by excessive food intake or impaired action of insulin in extrahepatic tissues, or both, is exceedingly prevalent in Western populations and a major cause of cardiovascular morbidity.
Although expression of hepatic SREBP-1c is controlled largely by aPKC (30, 36, 42, 53), Akt may also contribute. However, in livers of diabetic rodents (51) and in hepatocytes of type 2 diabetic humans (unpublished observations), whereas insulin activation of aPKC is conserved, Akt activation is diminished relative to that seen in nondiabetic rodents and humans. This divergence of Akt and aPKC activation in diabetic liver appears to reflect impaired activation of IRS-1-dependent PI3K, thus limiting Akt activation, but, on the other hand, sufficiently conserved activation of IRS-2-dependent PI3K, thus maintaining aPKC activation. Accordingly, in diabetic liver, aPKC is probably the major regulator of SREBP-1c. In concert with this idea, inhibition of hepatic aPKC in multiple rodent models of type 2 diabetes and obesity by expression of adenovirus encoding either kinase-inactive aPKC or an shRNA that targets IRS-2 leads to marked decreases in expression of hepatic SREBP-1c, FAS, and ACC, and this is accompanied by dramatic improvements in levels of hepatic and serum triacylglycerol, serum cholesterol, and serum nonesterified fatty acids (36, 42). Moreover, these decreases in serum lipids are accompanied by increases in insulin signaling in muscle and decreases in hyperinsulinemia (36, 42). Accordingly, it may be surmised that excessive activity of hepatic aPKC in hyperinsulinemic states plays a critical role in promoting the excessive activation of SREBP-1c and in provoking lipid-dependent metabolic syndrome features.
aPKC Requirements for Maintenance of Hepatic Phosphoenolpyruvate Carboxykinase and Glucose-6-Phosphatase
Hepatic enzymes phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G-6-Pase) promote gluconeogenesis and glucose release from the liver, i.e., functions particularly important for maintaining blood glucose levels during fasting. Insulin diminishes the expression of these enzymes during feeding primarily by activating Akt, which phosphorylates and inhibits forkhead transcription factors that maintain mRNA levels of PEPCK and G-6-Pase. The inhibition of expression of these enzymes is probably the most important mechanism used by insulin to regulate blood glucose levels, and this function is impaired markedly in diabetic liver, presumably owing largely to impaired Akt activation.
Although aPKCs do not mediate the lowering effects of insulin on expression of PEPCK and G-6-Pase (30, 53), we found, surprisingly, that the inhibition of hepatic aPKC by administration of adenovirus encoding kinase-inactive aPKC led to decreases in fasting levels of PEPCK and G-6-Pase mRNAs (42). In other words, the inhibition of hepatic aPKC elicits insulin-like effects on these enzymes, which may be amplified further by insulin (42). Accordingly, such decreases in PEPCK and G-6-Pase mRNAs induced by inhibition of hepatic aPKC may have contributed importantly to improvements in glucose tolerance and insulin resistance in diabetic and obese mice treated with adenovirus encoding kinase-inactive aPKC (36, 42). Further studies are needed to determine whether aPKCs directly or indirectly alter hepatic genes that transcribe mRNAs for PEPCK and G-6-Pase.
aPKC and NF-κB Activation
Whereas it has long been known that aPKCs serve as important mediators for increasing NF-κB activity during activation of inflammatory responses (14, 24), the fact that insulin as an aPKC activator is also capable of activating NF-κB in some tissues, e.g., in whole liver, but not in muscle, was not appreciated until recently (36, 42). As discussed, this activation of hepatic NF-κB by insulin is most likely due to aPKC-mediated phosphorylation of 1) IKKα/β, which phosphorylates IκB, thereby releasing NF-κB to translocate to active nuclear sites (1); and 2) Thr311 in the RelA subunit of NF-κB (2). However, it is unclear whether insulin-stimulated activation of hepatic NF-κB activation is occurring in parenchymal hepatocytes, reticuloendothelial Kupffer cells (i.e, macrophages), or both. Regardless of whether insulin- and feeding-induced increases in NF-κB activity are occurring in hepatocytes or hepatic reticuloendothelial cells, the activation of NF-κB is accompanied by increases in mRNA levels of hepatic cytokines such as TNFα and interleukin-1β (IL-1β) (36, 42) and may therefore be contributing to increases in systemic insulin resistance (see Refs. 12, 36, and 42), as well as abetting inflammatory proatherosclerotic processes, in states of obesity and diabetes.
As discussed, we have observed increased activities of hepatic IKKβ and NF-κB and increased expression of TNFα and IL-1β in livers of hyperinsulinemic type 2 diabetic or obese rodents (36, 42). Moreover, upon intravenous administration of adenovirus encoding kinase-inactive aPKC (thereby selectively inhibiting hepatic aPKC), the hepatic activities of IKKβ and NF-κB and the expression of hepatic TNFα and IL-1β return to normal (36, 42), and these alterations are attended by marked improvements in insulin signaling in muscle and systemic insulin resistance; however, whether decreases in hepatic cytokines contributed to these extrahepatic improvements in these diabetes and obesity models is uncertain. Further studies are needed to see whether insulin activates aPKCs, IKKβ, and NF-κB in extrahepatic macrophages and whether insulin may accelerate atherosclerotic/thrombotic tendencies through this mechanism.
aPKCs Requirements for Pancreatic Islet β-Cell Integrity and Insulin Secretion
As discussed above, studies of pancreatic islet β-cell-specific knockout of PKCλ in mice have shown that aPKC is required for expression of a number of enzymes needed to maintain β-cell proliferation and integrity, insulin-synthesizing machinery, and subsequent release of insulin during glucose stimulation (19). In addition, studies with inhibitors suggest that aPKC is directly required for mediating insulin secretory signals during acute carbachol stimulation (52); however, whether aPKC participates in mediating acute signaling responses during glucose-dependent insulin secretion is uncertain.
Of further note, incretins such as glucagon-like peptide-1 activate aPKC in islet β-cells and cause aPKC to translocate to the nucleus; moreover, aPKCs are required for incretin-induced increases in β-cell proliferation, insulin gene expression, and synthesis of metabolic enzymes (11). As such, these incretins or factors that diminish their degradation are widely used as therapeutic agents to improve insulin secretion in early phases of type 2 diabetes.
aPKC Requirements for Suppression of Appetite in CNS Centers
Appetite suppression in CNS hypothalamic centers is mediated by multiple factors, including leptin, insulin, and inflammatory substances such as lipopolysaccharide (LPS). LPS is known to activate Toll receptors, which in turn activate aPKC. Although each of these factors activates aPKC in these hypothalamic centers, a role for aPKC in suppressing appetite has been observed only during LPS treatment (54). The failure to find a requirement for aPKC during the actions of leptin and insulin indicates that other PI3K-dependent factors such as Akt are sufficient for appetite-suppressive effects of these agonists; whether aPKCs function redundantly in these appetite-suppressive effects of leptin and insulin is uncertain.
Deficient Levels and Activity of Muscle aPKC in Human Type 2 Diabetes Mellitus
Levels of immunoreactive aPKC are diminished substantially in muscles of type 2 diabetic humans (6, 7, 22). The reason for deficiency of muscle aPKC in human type 2 diabetes mellitus is uncertain, but this deficiency appears to be limited to humans, since it is not seen in rodent (51) or nonhuman primate (50) models of diabetes. In addition to the partial deficiency of aPKC in muscles of type 2 diabetic humans, the activation of residual aPKC is impaired, as evidenced by the fact that the specific enzyme activity of aPKC and its responsiveness to PIP3 are diminished in muscles of type 2 diabetic humans (7). Interestingly, this impairment in aPKC activity and responsiveness in human diabetic muscle can be reversed by metformin treatment (28), perhaps via improvements in hepatic lipid metabolism and serum or muscle lipids or possibly via improvements in other factors that may adversely affect aPKC activity. On the other hand, metformin treatment does not improve aPKC levels in muscles of type 2 diabetic subjects (28).
Unlike the situation in muscle, preliminary findings (unpublished observations) indicate that aPKC levels are normal in livers of type 2 diabetic humans; moreover, the activation of aPKC by insulin in cultured hepatocytes of type 2 diabetic humans is normal or elevated despite decreases in Akt activity. These alterations in hepatic aPKC (i.e., conserved) and Akt (i.e., impaired) activity in diabetic humans are similar to those observed in type 2 diabetic rodents (36, 51) and may explain why there are paradoxical increases in hepatic outputs of both lipids (via increased aPKC action) and glucose (via diminished Akt action) in diabetic states.
It is important to emphasize that the combination of diminished levels and/or activity of aPKC in muscle, coupled with conserved levels and activity of aPKC in liver, is particularly problematic since it would produce a syndrome of insulin resistance and hyperinsulinemia owing to defective insulin action on aPKC in muscle but, in contrast, overproduction of hepatic lipids and cytokines owing to excessive insulin action on liver aPKC. Hepatic lipids and cytokines in turn would provoke further increases in insulin resistance, thus producing a self-perpetuating vicious cycle. These abnormalities would be heightened further by deficient activation of Akt in both muscle and liver.
Diminished aPKC Activity in Human Obesity
Obesity is a prominent feature of the metabolic syndrome and a common antecedent to type 2 diabetes. Interestingly, in myocytes isolated from obese glucose-intolerant humans and subsequently passaged in culture, there are reductions in insulin-stimulated aPKC activity and glucose transport (57). Also, in muscles of obese glucose-tolerant humans, there are reductions in insulin-stimulated aPKC activity and glucose disposal, as measured in hyperinsulinemic euglycemic clamp studies (8, 22). As in type 2 diabetes, the ability of PIP3 to directly activate aPKC is impaired in muscles of these obese subjects (8); however, different from type 2 diabetes, total aPKC levels are normal in muscles of obese nondiabetic humans (8, 22). In this regard, note that individual protein and mRNA levels of PKCι and PKCζ have not been examined, and there are no longitudinal studies to see whether obese subjects develop aPKC deficiency as they develop type 2 diabetes.
As in muscle, in adipocytes obtained by differentiation of preadipocytes isolated from subcutaneous fat depots of obese humans and subsequently passaged in culture, there are reductions in insulin-stimulated aPKC activity and glucose transport relative to adipocytes obtained from lean humans (40). On the other hand, aPKC levels are not diminished in adipocytes derived from obese subjects (40). The defect in aPKC activation in adipocytes from obese subjects appears to be due to both deficient activation of IRS-1-dependent PI3K and diminished aPKC responsiveness to PIP3 (40).
It is interesting that the above-described defects in insulin-stimulated aPKC activation to insulin and PIP3 in cultured myocytes and adipocytes of obese subjects persist in passaged cells, i.e., after removal from extrinsic factors (40, 57). On the other hand, metformin improves aPKC responsiveness to PIP3, but not aPKC levels, in muscles of type 2 diabetic subjects (28), and this defect in responsiveness to PIP3 is obviously reversible. Further studies are needed to elucidate the mechanisms that diminish aPKC responsiveness to PIP3 in states of obesity and type 2 diabetes.
Role of Conventional and Novel PKCs in Metabolic Functions
Although this review is focused on aPKCs, insulin provokes increases in activities of cPKCs and nPKCs, but the roles of these PKCs during insulin action are uncertain. With respect to glucose transport, expression of wild-type, constitutive, and kinase-inactive cPKCs (cPKCα and -β) and nPKC (nPKCδ and -ε) failed to alter insulin-stimulated GLUT4 translocation in rat adipocytes (4). In addition, specific inhibition of cPKCβ with chemical inhibitors and effective depletion of cPKCα, -β1, and -β2 and nPKCδ and -ε following prolonged PE treatment failed to alter insulin-stimulated glucose transport in L6 myotubes (3). We have also examined both PKCα- (58) and PKCβ-knockout mice (46), and in both cases insulin effects on glucose transport in both isolated adipocytes and muscle strips were increased, presumably reflecting that these cPKCs, if anything, exert inhibitory effects on glucose transport, presumably via inhibition of the insulin receptor and subsequent signaling to PI3K, Akt, and aPKC. To summarize, we do not believe that cPKCs and nPKCs are required for insulin-stimulated glucose transport in muscle and adipocytes of rodents. This does not rule out the possibility that cPKCs and nPKCs may be involved in glucose transport effects of insulin in other cell types or during action of noninsulin agonists.
To our knowledge, the importance of cPKCs and nPKCs during insulin actions on hepatic functions has not been studied, but it seems doubtful that the cPKCs and nPKCs would function similarly to aPKCs in the liver.
On the other hand, there is little doubt that the activation of cPKCs or nPKCs by glucose, fatty acids, or, for that matter, insulin itself plays important roles in the pathogenesis of diabetic complications and systemic insulin resistance.
Concluding Remarks
As summarized in Table 1, aPKCs serve as essential signaling factors in a wide range of metabolic functions, including insulin-stimulated glucose transport in muscle and adipose tissues, insulin-stimulated lipogenesis in liver, maintenance of hepatic output of glucose during fasting, insulin-induced activation of inflammatory/immune responses in liver and perhaps other tissues, and insulin secretion by pancreatic islet β-cells. Each of these metabolic functions is essential for survival and well being during periods of intermittent and limited food intake; from this viewpoint, most aPKC-dependent processes would be considered as “good”. However, in situations wherein food intake is frequent and excessive, the remarkable efficiencies of aPKC-dependent processes in the liver that result in inordinate increases in lipogenic, glucogenic, and cytokine-producing enzymes would be conducive to development of metabolic syndrome features and ultimately to type 2 diabetes mellitus and atherosclerosis; in this context, these aPKC-dependent processes would be considered as “bad.” Moreover, the tendencies for development of metabolic and diabetic syndromes would be intensified further by a concomitant deficiency in aPKC activation in muscle, as is known to exist in type 2 diabetes.
In view of these considerations, there are critical needs to 1) develop a better understanding of why aPKC activation and/or levels are deficient in muscle but conserved in liver in states of obesity and type 2 diabetes mellitus, 2) determine the chronological point at which defects in muscle aPKC activation and/or levels begin in human obesity and diabetes syndromes, and 3) elucidate mechanisms that underlie deficiencies in aPKC mRNA and protein selectively in muscles of type 2 diabetic humans.
Perhaps even more importantly, i.e., to develop newer and more effective treatments for the metabolic syndrome and type 2 diabetes, there is a critical need to develop treatments that result in effective inhibition of hepatic aPKC without compromising glucose tolerance. Accordingly, it would be particularly advantageous if we were able to develop agents that selectively or preferentially inhibit hepatic aPKC while sparing aPKC activation in muscle, adipocytes, and pancreatic islet β-cells. On the other hand, it is possible that the inhibition of hepatic aPKC would not only ameliorate lipid and cytokine abnormalities but would also diminish gluconeogenesis and hepatic glucose output to an extent that offsets any deleterious effects of aPKC inhibition in muscle and β-cells. These possibilities are currently under investigation.
GRANTS
This work was supported by funds from the Department of Veterans Affairs Merit Review Program and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-38079.
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1. Bandyopadhyay G, Kanoh Y, Sajan MP, Standaert ML, Farese RV. Effects of adenoviral gene transfer of wild-type, constitutively active, and kinase-defective protein kinase C-λ on insulin-stimulated glucose transport in L6 myotubes. Endocrinology 141: 4120–4127, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Bandyopadhyay G, Sajan MP, Kanoh Y, Standaert ML, Quon MJ, Lea-Currie R, Sen A, Farese RV. PKC-zeta mediates insulin effects on glucose transport in cultured preadipocyte-derived human adipocytes. J Clin Endocrinol Metab 87: 716–723, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Bandyopadhyay G, Standaert ML, Galloway L, Moscat J, Farese RV. Evidence for involvement of protein kinase C (PKC)-zeta and noninvolvement of diacylglycerol-sensitive PKCs in insulin-stimulated glucose transport in L6 myotubes. Endocrinology 138: 4721–4731, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Bandyopadhyay G, Standaert ML, Kikkawa U, Ono Y, Moscat J, Farese RV. Effects of transiently expressed atypical (ζ, λ), conventional (α, β) and novel (δ, ε) protein kinase C isoforms on insulin-stimulated translocation of epitope-tagged GLUT4 glucose transporters in rat adipocytes. Specific interchangeable effects of protein kinase C-ζ and -λ. Biochem J 337: 461–470, 1999 [PMC free article] [PubMed] [Google Scholar]
- 5. Bandyopadhyay G, Standaert ML, Zhao L, Yu B, Avignon A, Galloway L, Karnam P, Moscat J, Farese RV. Activation of PKC (α, β, and ζ) by insulin in 3T3/L1 cells. Transfection studies suggest a role for PKC-ζ in glucose transport. J Biol Chem 272: 2551–2558, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Bandyopadhyay GK, Yu JG, Ofrecio J, Olefsky JM. Increased p85/55/50 expression and decreased phosphatidylinositol 3-kinase activity in insulin-resistant skeletal muscle. Diabetes 54: 2351–2359, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Beeson M, Sajan MP, Dizon M, Grebenev D, Gomez-Daspet J, Miura A, Kanoh Y, Powe J, Bandyopadhyay G, Standaert ML, Farese RV. Activation of protein kinase C-ζ by insulin and phosphatidylinositol-3,4,5-(PO4)3 is defective in muscle in type 2 diabetes and impaired glucose intolerance. Amelioration by rosiglitazone and exercise. Diabetes 52: 1926–1934, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Beeson M, Sajan MP, Gomez-Daspet J, Luna V, Dizon M, Grebenev D, Powe JL, Lucidi S, Miura A, Kanoh Y, Bandyopadhyay G, Standaert ML, Yeko TR, Farese RV. Defective activation of protein kinase C-zeta in muscle by insulin and phosphatidylinositol-3,4,5-(PO4)3 in obesity and polycystic ovary syndrome. Metabolic Syndr Relat Disord 2: 49–56, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Bicaku E, Patel R, Acevedo-Duncan M. Cyclin-dependent kinase activating kinase/Cdk7 co-localizes with PKC-iota in human glioma cells. Tissue Cell 37: 53–58, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Braiman L, Alt A, Kuroki T, Ohba M, Bak A, Tennenbaum T, Sampson SR. Activation of protein kinase C zeta induces serine phpsphorylation of VAMP2 in the GLUT4 compartment and increases glucose transport in skeletal muscle. Mol Cell Biol 21: 7852–7861, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buteau J, Foisy S, Rhodes CJ, Carpenter L, Biden TJ, Prentki M. Protein kinase Czeta activation mediates glucagon-like peptide-1-induced pancreatic beta-cell proliferation. Diabetes 50: 2237–2243, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med 11: 183–190, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen HC, Bandyopadhyay G, Sajan MP, Kanoh Y, Standaert M, Farese RV, Jr, Farese RV. Activation of the ERK pathway and atypical protein kinase C isoforms in exercise- and aminoimidazole-4-carboxamide-1-beta-d-riboside (AICAR)-stimulated glucose transport. J Biol Chem 277: 23554–23562, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Duran A, Diaz-Meco MT, Moscat J. Essential role of RelA Ser311 phosphorylation by ζPKC in NF-κB transcriptional activation. EMBO J 22: 3910–3918, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Farese RV, Sajan MP, Yang H, Li P, Mastorides S, Gower WJ, Jr, Nimal S, Choi CS, Kim S, Shulman GI, Kahn CR, Braun U, Leitges M. Muscle-specific knockout of protein kinase C-λ impairs glucose transport and induces metabolic and diabetic syndromes. J Clin Invest 117: 2289–2301, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fields AP, Frederick LA, Regala RP. Targeting the oncogenic protein kinase Ciota signalling pathway for the treatment of cancer. Biochem Soc Trans 35: 996–1000, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Guizzetti M, Thompson BD, Kim Y, VanDeMark K, Costa LG. Role of phospholipase D signaling in ethanol-induced inhibition of carbachol-stimulated DNA synthesis of 1321N1 astrocytoma cells. J Neurochem 90: 646–653, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Gusarova GA, Dada LA, Kelly AM, Brodie C, Witters LE, Chandel NS, Sznajder JI. α1-AMPK-activated protein kinase regulates hypoxia-induced Na,K-ATPase endocytosis via direct phosphorylation of protein kinase Cζ. Mol Cell Biol 29: 3455–3464, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hashimoto N, Kido Y, Uchida T, Matsuda T, Suzuki K, Inoue H, Matsumoto M, Ogawa W, Maeda S, Fujihara H, Ueta Y, Uchiyama Y, Akimoto K, Ohno S, Noda T, Kasuga M. PKClambda regulates glucose-induced insulin secretion through modulation of gene expression in pancreatic islets. J Clin Invest 115: 138–145, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hirai T, Chida K. Protein kinase Czeta (PKCzeta): activation mechanisms and cellular functions. J Biochem 133: 1–7, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Hirano Y, Yoshinaga S, Ogura K, Yokochi M, Noda Y, Sumimoto H, Inagaki F. Solution structure of atypical protein kinase C PB1 domain and its mode of interaction with ZIP/p62 and MEK5. J Biol Chem 279: 31883–31890, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Kim YB, Kotani K, Ciaraldi TP, Henry RR, Kahn BB. Insulin-stimulated protein kinase C lambda/zeta activity is reduced in skeletal muscle of humans with obesity and type 2 diabetes: reversal with weight reduction. Diabetes 52: 1935–1942, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Kotani K, Ogawa W, Matsumoto M, Kitamura T, Sakaue H, Hino Y, Miyake K, Sano W, Akimoto K, Ohno S, Kasuga M. Requirement of atypical protein kinase clambda for insulin stimulation of glucose uptake but not for Akt activation in 3T3-L1 adipocytes. Mol Cell Biol 18: 6971–6982, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lallena MJ, Diaz-Meco MT, Bren G, Payá CV, Moscat J. Activation of IkappaB kinase beta by protein kinase C isoforms. Mol Cell Biol 19: 2180–2188, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science 281: 2042–2045, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Leitges M, Ploman M, Standaert ML, Bandyopadhyay G, Sajan MP, Kanoh Y, Farese RV. Knockout of protein kinase C-α enhances insulin signaling through phosphatidylinositol 3-kinase. Mol Endocrinol 16: 847–858, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Liu LZ, Zhao HL, Zuo J, Ho SK, Chan JC, Meng Y, Fang FD, Tong PC. Protein kinase Czeta mediates insulin-induced glucose transport through actin remodeling in L6 muscle cells. Mol Biol Cell 17: 2322–2330, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Luna V, Casauban L, Sajan MP, Gomez-Daspet J, Powe JL, Miura A, Rivas J, Standaert ML, Farese RV. Metformin improves atypical protein kinase C activation by insulin and phosphatidylinositol-3,4,5-(PO4)3 in muscle of diabetic subjects. Diabetologia 49: 375–382, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Macek B, Benda C, Jestel A, Maskos K, Mann M, Messerschmidt A. Phosphorylation of the human full-length protein kinase Ciota. J Proteome Res 7: 2928–2935, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Matsumoto M, Ogawa W, Akimoto K, Inoue H, Miyaki K, Furukawa K, Hayashi Y, Iguchi H, Marsuki Y, Hiramatsu R, Shimano H, Yamada N, Ohno S, Kasuga M, Noda T. PKCλ in liver mediates insulin-induced SREBP-1c expression and determines both hepatic lipid content and overall insulin sensitivity. J Clin Invest 112: 935–944, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Messerschmidt A, Macieira S, Velarde M, Bädeker M, Benda C, Jestel A, Brandstetter H, Neuefeind T, Blaesse M. Crystal structure of the catalytic domain of human atypical protein kinase C-iota reveals interaction mode of phosphorylation site in turn motif. J Mol Biol 30: 918–931, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Miura A, Sajan MP, Standaert ML, Bandyopadhyay G, Kahn CR, Farese RV. Insulin substrates 1 and 2 are corequired for activation of atypical protein kinase C and Cbl-dependent phosphatidylinositol 3-kinase during insulin action in immortalized brown adipocytes. Biochemistry 43: 15503–15509, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Patel R, Win H, Desai S, Patel K, Matthews JA, Acevedo-Duncan M. Involvement of PKC-iota in glioma proliferation. Cell Prolif 41: 122–135, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pu Y, Peach ML, Garfield SH, Wincovitch S, Marquez VE, Blumberg PM. Effects on ligand interaction and membrane translocation of the positively charged arginine residues situated along the C1 domain binding cleft in the atypical PKC isoforms. J Biol Chem 281: 33773–33788, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Romanelli A, Martin KA, Toker A, Blenis J. p70 S6 kinase is regulated by protein kinase Czeta and participates in a phosphoinositide 3-kinase-regulated signaling complex. Mol Cell Biol 19: 2921–2928, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sajan MP, Standaert ML, Rivas J, Miura A, Kanoh Y, Soto J, Taniguchi CM, Kahn CR, Farese RV. Role of atypical protein kinase C in activation of sterol regulatory element binding protein-1c and nuclear factor kappa B (NFkappaB) in liver of rodents used as a model of diabetes, and relationships to hyperlipidaemia and insulin resistance. Diabetologia 52: 1197–1207, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sajan MP, Bandyopadhyay G, Miura A, Standaert M, Nimal S, Longnus SL, Van Obberghen E, Hainault I, Foufelle F, Kahn CR, Braun U, Leitges M, Farese RV. AICAR and metformin, but not exercise, increase muscle glucose transport through AMPK-, ERK- and PDK1-dependent activation of atypical PKC. Am J Physiol Endocrinol Metab.10.1152/ajpendo.00392.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sajan MP, Rivas J, Li P, Standaert ML, Farese RV. Repletion of atypical protein kinase C following RNA interference-mediated depletion restores insulin-stimulated glucose transport. J Biol Chem 281: 17466–17473, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Sajan MP, Standaert ML, Bandyopadhyay G, Quon MJ, Burke TR, Farese RV. PKC-ζ and PDK-1 are required for insulin-induced activation of ERK2 in rat adipocytes. J Biol Chem 274: 30495–30500, 1999 [DOI] [PubMed] [Google Scholar]
- 40. Sajan MP, Standaert ML, Miura A, Bandyopadhyay G, Vollerweider P, Franklin DM, Lea-Currie R, Farese RV. Impaired activation of protein kinase C-ζ by insulin and phosphatidylinositol-3,4,5-(PO4)3 in cultured pre-adipocyte-derived adipocytes and myotubes of obese subjects. J Clin Endocrinol Metab 89: 3994–3998, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Sajan MP, Standaert ML, Miura A, Kahn CR, Farese RV. Tissue-specific differences in activation of atypical protein kinase C and protein kinase B in muscle, liver, and adipocytes of insulin receptor substrate-1 knockout mice. Mol Endocrinol 18: 2513–2521, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Sajan MP, Standaert ML, Nimal S, Varanasi U, Pastoor T, Mastorides S, Braun U, Leitges M, Farese RV. Critical role of atypical protein kinase C in activating hepatic SREBP-1c and NFκB in obesity. J Lipid Res 50: 1133–1145, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Smithers NP, Hodgkinson CP, Cuttle M, Sale GJ. 80K-H acts as a signaling bridge in intact living cells between PKCzeta and the GLUT4 translocation regulator Munc18c. J Recept Signal Transduct Res 28: 581–589, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Spitaler M, Villunger A, Grunicke H, Uberall F. Unique structure and functional properties of the ATP-binding domain of atypical protein kinase C-iota. J Biol Chem 275: 33289–33296, 2000 [DOI] [PubMed] [Google Scholar]
- 45. Stallings-Mann M, Jamieson L, Regala RP, Weems C, Murray NR, Fields AP. A novel small-molecule inhibitor of protein kinase Ciota blocks transformed growth of non-small-cell lung cancer cells. Cancer Res 66: 1767–1774, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Standaert ML, Bandyopadhyay G, Galloway L, Soto J, Ono Y, Kikkawa U, Farese RV, Leitges M. Effects of knockout of the PKC-β gene on glucose transport and glucose homeostasis. Endocrinology 140: 4470–4477, 1999 [DOI] [PubMed] [Google Scholar]
- 47. Standaert ML, Bandyopadhyay G, Kanoh Y, Sajan MP, Farese RV. Insulin and PIP3 activate PKC-zeta by mechanisms that are both dependent and independent of phosphorylation of activation loop (T410) and autophosphorylation (T560) sites. Biochemistry 40: 249–255, 2001 [DOI] [PubMed] [Google Scholar]
- 48. Standaert ML, Bandyopadhyay G, Perez L, Price D, Galloway L, Poklepovic A, Sajan MP, Cenni V, Sirri A, Moscat J, Toker A, Farese RV. Insulin activates PKC-ζ and PKC-λ by an autophosphorylation-dependent mechanism and stimulates their translocation to GLUT4 vesicles and other membrane fractions in rat adipocytes. J Biol Chem 274: 25308–25316, 1999 [DOI] [PubMed] [Google Scholar]
- 49. Standaert ML, Galloway L, Karnam P, Bandyopadhyay G, Moscat J, Farese RV. Protein kinase C-zeta as a downstream effector of phosphatidylinositol 3-kinase during insulin stimulation in rat adipocytes. Potential role in glucose transport. J Biol Chem 272: 30075–30082, 1997 [DOI] [PubMed] [Google Scholar]
- 50. Standaert ML, Ortmeyer HK, Sajan MP, Kanoh Y, Bandyopadhyay G, Hansen BC, Farese RV. Skeletal muscle insulin resistance in obesity-associated type 2 diabetes in monkeys is linked to a defect in insulin activation of protein kinase C-zeta/lambda/iota. Diabetes 51: 2936–2943, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Standaert ML, Sajan MP, Miura A, Kanoh Y, Chen HC, Farese RV, Jr, Farese RV. Insulin-induced activation of atypical protein kinase C, but not protein kinase B, is maintained in diabetic (ob/ob and Goto-Kakazaki) liver. Contrasting insulin signaling patterns in liver versus muscle define phenotypes of type 2 diabetic and high fat-induced insulin-resistant states. J Biol Chem 279: 24929–24934, 2004 [DOI] [PubMed] [Google Scholar]
- 52. Tang SH, Sharp GW. Atypical protein kinase C isozyme zeta mediates carbachol-stimulated insulin secretion in RINm5F cells. Diabetes 47: 905–912, 1998 [DOI] [PubMed] [Google Scholar]
- 53. Taniguchi CM, Kondo T, Sajan M, Luo J, Bronson R, Asano T, Farese R, Cantley LC, Kahn CR. Divergent regulation of hepatic glucose and lipid metabolism by phosphoinositide 3-kinase via Akt and PKClambda/zeta. Cell Metab 3: 343–353, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Thaler JP, Choi SJ, Sajan MP, Ogimoto K, Nguyen HT, Matsen M, Benoit SC, Wisse BE, Farese RV, Schwartz MW. Atypical protein kinase C activity in the hypothalamus is required for lipopolysaccharide-mediated sickness responses. Endocrinology 150: 5362–5372, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ueki K, Yamauchi T, Takemoto H, Tobe K, Yamamoto-Honda R, Kaburagi Y, Akanuma Y, Yazaki Y, Aizawa S, Nagai R, Kadowaki T. Restored insulin sensitivity in IRS-1-deficient mice treated by adenovirus-meduated gene therapy. J Clin Invest 105: 1437–1445, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Valverde AM, Burks DJ, Fabregat I, Fisher TL, Carretero J, White MF, Benito M. Molecular mechanisms of insulin resistance in IRS-2-deficient hepatocytes. Diabetes 52: 2239–2248, 2003 [DOI] [PubMed] [Google Scholar]
- 57. Vollenweider P, Ménard B, Nicod P. Insulin resistance, defective insulin receptor substrate 2-associated phosphatidylinositol-3′ kinase activation, and impaired atypical protein kinase C (zeta/lambda) activation in myotubes from obese patients with impaired glucose tolerance. Diabetes 51: 1052–1059, 2002 [DOI] [PubMed] [Google Scholar]
- 58. Wooten MW, Vandenplas ML, Seibenhener ML, Geetha T, Diaz-Meco MT. Nerve growth factor stimulates multisite tyrosine phosphorylation and activation of the atypical protein kinase C's via a src kinase pathway. Mol Cell Biol 21: 8414–8427, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yamauchi T, Tobe K, Tamemoto H, Ueki K, Kaburagi Y, Yamamoto-Honda R, Takahashi Y, Yoshizawa F, Aizawa S, Akanuma Y, Sonenberg N, Yazaki Y, Kadowaki T. Insulin signalling and insulin actions in the muscles and livers of insulin-resistant, insulin receptor substrate 1-deficient mice. Mol Cell Biol 16: 3074–3084, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]


