Abstract
Colorectal cancer remains a major cause of morbidity and mortality in the United States. The advent of molecular therapies targeted against specific, stereotyped cellular mutations that occur in this disease has ushered in new hope for treatment options. However, key questions regarding optimal dosing schedules, dosing duration, and patient selection remain unanswered. In this review, we describe how recent advances in molecular imaging, specifically optical molecular imaging with fluorescent probes, offer potential solutions to these questions. We begin with an overview of optical molecular imaging, including discussions on the various methods of design for fluorescent probes and the clinically relevant imaging systems that have been built to image them. We then focus on the relevance of optical molecular imaging to colorectal cancer. We review the most recent data on how this imaging modality has been applied to the measurement of treatment efficacy for currently available as well as developmental molecularly targeted therapies. We then conclude with a discussion on how this imaging approach has already begun to be translated clinically for human use.
Keywords: cancer genetics, animal studies, endoscopy, fluorescence
molecular imaging is a rapidly growing field in diagnostic medicine that has the potential to make a significant impact in the manner in which disease is diagnosed and treated (51, 57, 80). The term molecular imaging can be defined as any imaging modality that allows for the in vivo visualization and characterization of biological processes that occur on a cellular or subcellular scale. Whereas previous technologies have focused on defining disease on the basis of structure and morphology, molecular imaging seeks to reveal the biochemical underpinnings and intracellular pathways that drive disease development and progression. With the recent revolutionary advances in knowledge of the molecular mechanisms of pathological processes, clinicians enjoy now an unprecedented degree of understanding of the nature of disease (126); molecular imaging as a discipline seeks to capitalize on this understanding to develop clinically translatable imaging tools to perform molecular assessments of disease.
Molecular imaging promises to provide several advantages over current clinical imaging modalities (57). First is the possibility of earlier disease detection (125). Because molecular imaging techniques evaluate for cellular perturbations rather than physical abnormalities, these approaches can theoretically identify disease states at an earlier stage in development and before they manifest anatomically. Moreover, since molecular imaging methods detect functional variations in tissue rather than structural changes, they are able to focally highlight lesions with very high target-to-background ratios. Another potential benefit of molecular imaging is its utility in the preclinical examination of new drugs (64, 102). One of the major limitations restricting drug discovery and drug development is the inability to quantitatively measure in vivo a drug's effect on its molecular target; molecular imaging, however, opens the door to this possibility. Furthermore, when combined with gene therapy, molecular imaging represents a technology that allows for the direct tracking of the downstream genetic changes caused by treatment, a marked improvement on having to wait for phenotypic changes to develop.
A number of different molecular imaging modalities exist, including those based on nuclear medicine technologies such as positron emission tomography (PET) and single-photon emission computed tomography (SPECT), as well as magnetic resonance (MR)-based techniques. These molecular imaging approaches have made tremendous impacts on the field but are not within the scope of this review; if so interested, the reader is encouraged to read several recent excellent reviews on the subjects (14, 80). This review will focus on the novel advancements made in the emerging molecular imaging modality of optical imaging, with a particular focus on applications to colorectal cancer imaging as well as new findings from other fields of oncology that are of high relevance to colorectal cancer.
What Is Optical Molecular Imaging?
Optical molecular imaging is an imaging discipline that measures light released from either endogenous sources or exogenously administered agents that, encoded within its signal, bears information about biological processes on a microscopic scale. Optical molecular imaging as a field encompasses a vast array of imaging modalities (9); this review will highlight one such modality, namely that of optical imaging with exogenously administered organic fluorochromes. Other exciting approaches in optical imaging such as autofluorescence (95), bioluminescence (45), quantum dots (13, 14), and tomographic techniques (88) have been reviewed elsewhere.
Optical molecular imaging achieves very high sensitivity and specificity for the particular pathology under investigation through the use of affinity ligands. Such biomarkers used for molecular imaging range greatly in size and shape, from small, targeted peptides to large macromolecules (80). Nonetheless, the structure of molecular imaging probes is conserved irrespective of variations in biomarkers: probes consist of an affinity ligand bound to a reporter domain, whose design is predicated on the molecular imaging modality for which the probe is intended. In the context of optical imaging, the reporter domain is composed of a class of molecules known as fluorochromes, which have physical chemical properties that cause them to absorb photons at one wavelength and then to release a second, fluorescent photon at another, longer wavelength.
Remarkable progress has been achieved over the last few years in advancing optical imaging with fluorescently labeled molecular probes with respect to imaging devices, imaging agents, and a diverse array of clinically relevant applications (10, 19–21, 101, 130). There is a growing interest in this field partially due to the many unique advantages of working in the optical spectrum. For example, optical imaging allows for the possibility of multichannel imaging, meaning that two or more molecular targets can be visualized simultaneously. Many diseases have unique molecular profiles that are defining and may serve as prognostic markers. Single-gene alterations are insufficient to characterize most diseases; however, with many of today's clinical imaging tools, it is difficult to assess more than one molecular parameter at a time. Thus techniques that allow the simultaneous imaging of a small number of such targets (“in vivo miniarrays”) may be able not only to detect diseases with greater specificity, but also to shed new light on our understanding of their pathogenesis and prognosis. This concept has already been applied in animal models to the field of breast cancer imaging, where the ability to simultaneously measure multiple cell surface receptors (7), as well as markers of cell death (40), was recently demonstrated.
Another advantage to working in the optical regime is the ability to image truly in real time. Unlike other imaging modalities such as magnetic resonance imaging and computed tomography that require computationally intensive postprocessing to generate images seconds to minutes later, fluorescence imaging systems can readily filter data from multiple molecular reporters into individual channels and immediately generate images via charge-coupled device. Moreover, unlike many other imaging modalities, optical imaging does not rely on ionizing radiation to generate images, opening the way for low-risk screening applications.
Designs of Optical Molecular Imaging Probes
In general terms, an optical molecular imaging probe consists of a fluorochrome attached to an affinity ligand. The selection of the fluorochrome for an imaging probe has a profound effect on the probe's biodistribution and ability to produce high-contrast images. There are currently a wide variety of commercially available fluorochromes that have been applied to optical molecular imaging. For example, two clinically approved fluorochromes are fluorescein, which fluoresces in the green band of the visible light spectrum, and indocyanine green (ICG), which fluoresces in the near-infrared (NIR) band. Much interest has been paid to other NIR fluorochromes (37), for a number of reasons. Because of an “NIR window” in the photon absorption characteristics of blood and other tissue, light in the NIR band is able to travel through significantly greater distances of tissue in vivo compared with visible light. Moreover, there is less tissue autofluorescence in this range of wavelengths, a factor that helps improve target-to-background ratios. Finally, by utilizing wavelengths beyond the visible spectrum, one has the ability to simultaneously capture full visible light images as well as fluorescence images, allowing for the superposition of the two without sacrificing visible light image quality (109).
Optical molecular probes as a whole can be classified into three categories that are differentiated from one another on the basis of the functional characteristics of the probe's affinity ligand: nonspecific, targeted, and activatable. Nonspecific fluorescent probes typically contain one or more fluorochromes but do not have targeted affinity ligands. These are generally large macromolecules that stay within the blood pool, thereby highlighting areas with increased vascularity. They can also extravasate into tissues through “leaky,” poorly endothelialized blood vessels, such as seen in tumors. An example of a nonspecific fluorescent probe is ICG; this fluorochrome has long been used clinically in ophthalmology for fluorescence angiography of the retina (33), and experiments have also demonstrated ICG's utility in tumor detection (47, 73).
Targeted optical molecular imaging probes consist of a specific ligand bound to a reporter fluorochrome. These probes exhibit a greater degree of molecular specificity than the above class of agents, since their measured fluorescence intensity reflects the level of expression of their particular molecular target rather than nonspecific parameters such as blood perfusion or endothelial leak. The design of the targeting moiety itself can be quite variable, with different constructs exhibiting their own strengths and weaknesses. Examples of targeting moieties include whole antibodies (36), antibody fragments, short sequence peptides (12), and small molecules (88). One exciting avenue for the discovery of new targeting moieties is the generation of high-affinity peptide sequences through phage display (65) (Fig. 1); this screening method not only allows for the synthesis of new targeting peptides but may also be relevant for the identification of new targets themselves, by providing a glimpse of the unusual proteins expressed by tumor cells (67).
Fig. 1.
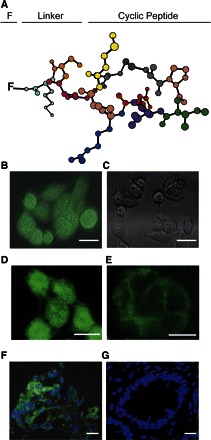
Identification of small peptide affinity ligands for targeted molecular probes using phage display. A: a cyclic RPMC peptide motif was identified in this study as a potential high-affinity ligand for colorectal cancer. B: fluorescently labeled cyclic RPMC was avidly internalized by HT-29 colorectal cancer cells to a far greater extent than with a labeled control peptide as shown in C. D: uptake was greater with incubation at 37°C than E, at 4°C. Samples of colon cancer (F) and normal colon mucosa (G) from tissue microarrays incubated with labeled cyclic RPMC and TOPRO-3, a nuclear counterstain, show preferential binding of the labeled peptide to malignant tissue. Reproduced with permission from Ref. 65.
One major limitation to antibody-targeted optical imaging probes is the prolonged blood half-life of these agents, resulting in poor target-to-background ratios. That is, although the probe may have a high affinity for the cell surface ligand to which it is intended to bind, persistently high concentrations of unbound probe in the bloodstream diminish the clarity with which the target tissue is visualized. One method to improve on this is to use “chaser” molecules that are administered after the imaging probe and that are designed to bind free probe and accelerate its clearance from the vascular compartment. For example, building on a technique that was first employed successfully in the field of nuclear imaging in the 1980s (70, 110), Ogawa et al. (90) biotinylated optically labeled monoclonal antibodies and 24 h after administration injected a chaser compound consisting of avidin conjugated to QSY-21, an amine-reactive quenching molecule that suppresses fluorescence through a mechanism known as fluorescence resonance energy transfer (FRET, discussed in more detail below). This combination of “quench and chase” yielded significant improvements in target-to-background ratios in a mouse model of breast cancer.
A third category of optical imaging probes is activatable probes (127). These agents are generally composed of multiple fluorochromes attached by a peptide stalk to a polymer backbone. Their design capitalizes on a unique quality of fluorochromes, that is, the ability to be “turned off.” This phenomenon, known as FRET, occurs when fluorochromes are in relative proximity to one another, so that when a fluorochrome is excited by an incident photon, rather than releasing the absorbed energy through a fluorescent photon, the energy is transferred via nonradiative dipole-dipole coupling with the adjacent fluorochromes. However, following protease-mediated cleavage of the peptide stalks linking the fluorochromes to the polymer scaffold, the fluorochromes are released and regain their native fluorescence properties. Therefore, in the presence of proteases, activatable probes increase their fluorescence signal many fold. By modifying the amino acid sequence of the peptide stalk, one can tailor the probe to be activatable by a specific subset or family of proteases.
An alternative strategy for synthesizing activatable probes recently has been developed (89). In this approach, multiple fluorochromes, in this case ICG, were attached to monoclonal antibodies targeted to specific cell surface receptors. Because ICG loses its fluorescence when covalently bound to proteins, the fluorochromes are optically silent prior to administration; however, upon binding of the probe to its targeted ligand and subsequent cellular internalization, the fluorochromes are cleaved from their antibody scaffold in endolysosomes and regain their fluorescence. Therefore, these probes' fluorescence is activated only in the tissue they are targeted to. Moreover, because ICG is clinically approved, and this approach can be used with any number of clinically approved monoclonal antibodies, the potential for clinical translation is high.
Optical Molecular Imaging Systems
Paralleling the advancements made in optical reporters of colorectal cancer, there have been recent exciting developments in endoscope-based imaging systems that have the potential to bring this imaging approach into clinical practice. Our group has designed a fiber optic endoscopy system that allows for the simultaneous acquisition of broadband white light, as is standard of care currently, as well as fluorescence light from a molecular reporter or reporters, both in real time and with high spatial resolution (38) (Fig. 2). Recent advancements in this technology (109, 117) have introduced the ability to quantify fluorescence irrespective of the variations in distance between the target tissue and the tip of the imaging catheter (Fig. 3). In addition to improved localization, quantification of the fluorescence signal potentially allows for the characterization of the aggressiveness of the lesion and its risk of metastatic spread, as well as an assessment of the likelihood of specific therapeutic efficacy. Moreover, the modular design of the imaging system provides the possibility of imaging multiple optical reporters simultaneously, thus expanding the molecular specification with which one can image pathological lesions in vivo.
Fig. 2.
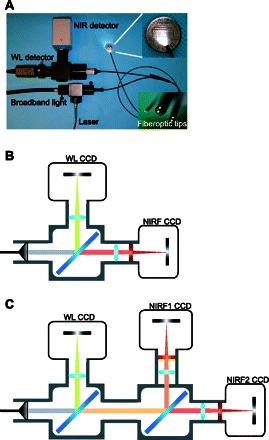
Fiberoptic-based murine colorectal cancer imaging system. A: a fluorescence molecular endoscopy system includes fluorescence excitation and collection hardware conjugated to a 0.8-mm outer diameter flexible fiberoptic catheter for mouse colonoscopy. B: schema showing the use of a dichroic mirror (blue diagonal bar) to split photons collected by endoscope into discrete near-infrared (NIR) and white light (WL) bands that are filtered by band-pass filters (red vertical bar) and imaged by high-resolution charge-coupled device (CCD) cameras. C: schema demonstrating how the modular design in B can be expanded to include additional NIR channels for simultaneous imaging of multiple optical molecular reporters. NIRF, NIR fluorescence.
Fig. 3.
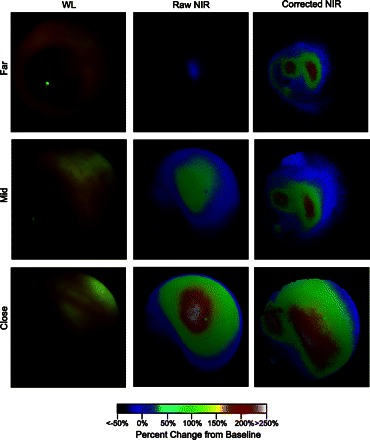
In vivo frames from mouse colonoscopy demonstrates quantitation of fluorescence signal in real time. WL, raw NIR, and corrected NIR images at different distances were acquired after orthotopic implantation of HT-29 colorectal cancer cells in the descending colon of a nu/nu mouse. Fluorescence colonoscopy was performed 24 h after intravenous injection of a protease-activatable optical probe. Raw NIR signal varied dramatically as the endoscope approached the target tumor; quantitative fluorescence intensity, however, was relatively invariant of the changes in distance between the tumor and catheter tip. Moreover, corrected NIR imaging was able to differentiate between 2 discrete tumor foci, whose presence was confirmed by subsequent histological analysis (data not shown). Adapted from Ref. 117.
Other endoscopic-based fluorescence molecular imaging approaches for gastrointestinal disease have been pursued, such as the translation of confocal microendoscopy by Goetz and Kiesslich (43). Confocal imaging is a technique whereby light from a single imaging plane is collected serially to generate an image. Confocal methods require direct contact between the imaging apparatus and the target tissue, but they are able to generate images with very high cellular and subcellular resolution. Although it is impractical to perform a point-to-point interrogation of the entire colon during a screening colonoscopy with this method, it does provide for the ability to “zoom in” with very high spatial resolution on areas that appear suspicious by standard imaging. Importantly, Food and Drug Administration (FDA) approval has been granted to a commercially available confocal microendoscope that can pass through the working channel of conventional colonoscopes (123); the clinical implications of this development are discussed in Potential for Clinical Translation below. Further information on confocal imaging (42), as well as a summarization of advances in endoscopic imaging technologies (121), can be found in recently published reviews.
Optical Molecular Imaging and Colorectal Cancer
Colorectal cancer is the second most common malignancy in the developed world, and despite recent advances in chemotherapeutics, this disease remains a major cause of morbidity and mortality in the United States (96). In many ways, colorectal cancer represents the archetypal disease that stands to benefit by the application of molecular imaging. The stereotyped, progressive, and cumulative genetic perturbations that occur in colorectal cancer were classically defined by Vogelstein and colleagues (29) two decades ago. This understanding has stimulated the development and application of a number of molecularly targeted therapies, several of which have demonstrated remarkable success through randomized controlled trials and received approval from the FDA for use in colorectal cancer (87). However, many of these successes have been tempered by the fact that the life expectancy after diagnosis with this disease remains poor (81) and that numerous mechanisms for resistance to molecularly targeted therapies are emerging (18). Therefore, there is a clear clinical need for not only preventing the development of progressive disease, but also monitoring the efficacy of therapies so that they can be optimized on an individual, patient-by-patient, basis.
Optical molecular imaging with fluorescent probes is an ideal imaging modality for furthering our understanding of colorectal cancer. We will discuss in this review two areas in which we believe this technology will have significant clinical implications for this malignancy: 1) the improved detection of adenomas and 2) the evaluation of clinically approved as well as experimental molecularly targeted chemotherapies.
Improved Detection of Colorectal Cancer
A cornerstone of colorectal cancer management is early detection of adenomatous polyps, primarily through routine screening colonoscopy. This practice is motivated by the fact that death from colorectal cancer is highly preventable if interventions such as colonoscopy-guided polypectomy are performed early (27). Indeed, routine screening with colonoscopy almost certainly reduces colorectal cancer-related mortality (122). However, inherent to every screening test is the concern for false negatives, which in the case of colonoscopies signifies undetected polyps. A recent meta-analysis of six tandem colonoscopy studies revealed that the “miss rate” for polyps may be as high as 20% (1, 119). Also, the endoscopist's ability to identify polyps has a direct impact on the rate of interval cancer development between screening colonoscopies (59).
A method to improve the sensitivity of screening colonoscopy would therefore have a profound impact on the management of colorectal cancer. The question thus arises, what are the reasons that cause cancers to be missed? There are likely a multitude of factors, including inadequate bowel preparation, obscuration of lesions behind a mucosal fold, and the unremarkable appearance of nonexophytic lesions (99). While poor bowel preparation cannot be remedied by molecular imaging approaches, and in fact may negatively impact its sensitivity as feces may autofluoresce in the fluorescence band being imaged, hidden and flat lesion detection will benefit from molecular techniques. The high target-to-background ratios generated by fluorescence probes, as well as the tissue penetration of fluorescent NIR photons, may augment our ability to visualize adenomas through thin mucosal folds. Also, flat lesions, which more commonly develop in patients with inflammatory bowel disease, are well identified via protease-activatable probes and can be differentiated from the inflammatory milieu in which they tend to arise (133).
Colorectal cancer exhibits numerous biomarkers that may be amenable to fluorescent labeling for optical molecular imaging (113). Some of the most pronounced successes with imaging colorectal cancer by optical molecular imaging in animal models almost a decade ago were made through the use of a cathepsin B-activatable fluorescent molecular probe. Cathepsin B is a cysteine protease that has been shown to be upregulated in areas of local colorectal cancer invasion (26), as well as in adenomas vs. benign colonic mucosa (49). A cathepsin B-activatable fluorescence molecular probe has been applied to the ex vivo imaging of colonic adenomas in the APCMin/+ mouse model (78); from this study, the sensitivity and specificity for optical molecular imaging of colon cancer were estimated at 96 and 93%, respectively, with lesions less than 1 mm readily detectable. This imaging was also shown to be feasible in vivo on orthotopic mouse models of colorectal cancer by our group, using a millimeter-sized fiber optic endoscopy system (39), thereby paving the way for possible clinical translation.
In addition to activatable imaging probes, other imaging agents including targeted probes have been applied for the improved detection of colorectal cancer. For example, our group has recently demonstrated that the fluorescently labeled glucose probe 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose (2-NBDG) is retained within colorectal cancer cells and provides excellent target-to-background ratios during endoscopic imaging (108). This imaging agent vividly illustrates one of the unique advantages of working within the optical regime: the ability to image a disease from the microscopic to the macroscopic scale (Fig. 4).
Fig. 4.
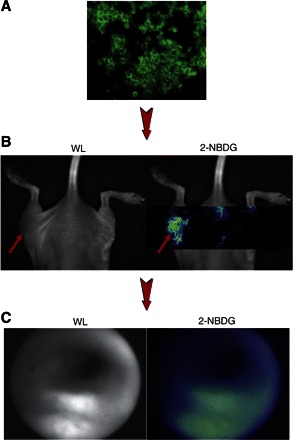
2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose (2-NBDG) as an optical reporter of glucose consumption, from the micro to macro scale. A: 2-NBDG is avidly internalized and retained by HT-29 human colorectal cancer cells in vitro. B: 2-NBDG uptake can be imaged by spectral deconvolution fluorescence imaging in HT-29 subcutaneous tumors in mice. C: 2-NBDG localizes to adenomas generated by the intraperitoneal injection of the carcinogen azoxymethane in a mouse model of colorectal cancer, imaged by fluorescence colonoscopy. Adapted from Ref. 108.
Another targeted agent that has been successfully applied to colorectal cancer imaging is comprised of a fluorochrome linked to a cyclic peptide isolated from a phage display-generated library of peptide sequences selective for colon cancer cells (65). Such approaches are appealing in their ability to rapidly identify highly specific targeting moieties that can be generated for a range of cancers. Finally, several other targeted probes designed to image specific cell surface receptors have been developed for colorectal cancer; these will be discussed in their respective sections below.
Evaluation of Molecularly Targeted Therapies for Colorectal Cancer
The therapeutic options for colorectal cancer have expanded greatly in the past two decades. Where once 5-fluorouracil (5-FU) was the only drug available, the arsenal of active agents has grown to include additional conventional chemotherapies as well as new molecularly targeted therapies (103). Much attention has also been paid to monoclonal antibody therapy in this disease. Bevacizumab, the monoclonal antibody targeted against vascular endothelial growth factor (VEGF), was the first to be approved for first- and second-line therapy in combination with 5-FU; this was soon followed by the epidermal growth factor receptor (EGFR) antibodies cetuximab and panitumumab, for use in conjunction with combination cytotoxic chemotherapy (81). The promise revolving around these biological agents, however, has diminished of late as variable tumor response rates and the recognition of mechanisms of resistance to these therapies have clouded the initial optimism (50, 81, 115). Most importantly, the median survival for colorectal remains ∼20 mo despite the latest advances in treatment.
Many questions persist regarding these new therapies, such as dosing schedules, dosing duration, and appropriate patient selection (18). Optimization of these variables has the potential to yield significant improvements in outcomes; however, randomized controlled trials for each possible permutation are unfeasible. Optical molecular imaging, on the other hand, may be an effective and rapid means of achieving these results. In the following subsections, we will discuss how optical molecular imaging could play a role in furthering our understanding of the use of clinically approved as well as experimental therapies for colorectal cancer (Table 1).
Table 1.
Overview of therapies for colorectal cancer and potential optical molecular imaging targets for assessing their efficacy
| Drug | Molecular Targets | Putative Mechanism | Potential Molecular Imaging Targets (molecular probe) |
|---|---|---|---|
| Clinically Approved Therapies | |||
| 5-FU + leukovorin | ∙ Thymidylate synthase | ∙ Inhibition of thymidylate synthase disrupts DNA replication and repair (71) | ∙ Phosphotidylserine (labeled annexin) (101) |
| ∙ DNA and RNA | ∙ Misincorporation into DNA and RNA disrupts function (71) | ∙ Caspase-1 (activatable probe) (79) | |
| ∙ MMP-2 (activatable probe) (12) | |||
| ∙ Reduced folate receptor (labeled folic acid) (83) | |||
| Oxaliplatin | DNA | DNA alkylation causes cross-linking and induces apoptosis (80) | ∙ Phosphotidylserine (labeled annexin) (101) |
| ∙ Caspase-1 (activatable probe) (79) | |||
| Irinotecan | Topoisomerase I | Stabilization of single-stranded DNA breaks induces apoptosis (80) | ∙ Phosphotidylserine (labeled annexin) (101) |
| ∙ Caspase-1 (activatable probe) (79) | |||
| Cetuximab, panitumumab | EGFR | Monoclonal antibody against EGFR leads to tumor regression (20) | ∙ EGFR (labeled antibody) (44) |
| ∙ PI3K (labeled wortmannin) (7, 42) | |||
| ∙ Phosphotidylserine (labeled annexin) (101) | |||
| Bevacizumab | VEGF | Monoclonal antibody against VEGF leads to tumor regression (20) | ∙ VEGFR (labeled antibody) (6, 19) |
| ∙ αvβ3 (labeled targeted peptide, labeled nanoparticle) (82, 123) | |||
| ∙ VCAM-1 (64) | |||
| ∙ Vascular volume fraction (blood pool agent) (81) | |||
| ∙ uPA (activatable probe) (52) | |||
| Experimental Therapies | |||
| Celecoxib | COX-2 | Inhibition of COX-2 decreases tumor growth rates and formation of polyps (56) | ∙ MMP-2 and −9 (activatable probe) (12) |
| ∙ Phosphotidylserine (labeled annexin) (101) | |||
| Trastuzumab | HER-2/neu | Monoclonal antibody against HER-2/neu reduces tumor growth rates (73) | ∙ HER-2/neu (labeled antibody) |
| ∙ Phosphotidylserine (labeled annexin) (101) | |||
| Statins | HMG-CoA reductase | HMG-CoA reductase inhibition reduces inflammation and angiogenesis (25, 48) | ∙ Vascular volume fraction (blood pool agent) |
| ∙ MMP-2 (activatable probe) (12) | |||
| ∙ Phosphotidylserine (labeled annexin) (101) | |||
Numerous cytotoxic and molecular drugs for colorectal cancer are currently being developed and actively being used in clinical practice. This table summarizes these therapies and provides the molecular targets affected by these therapies that have already been imaged in human and/or animal studies using optical molecular imaging. COX-2, cyclooxygenase-2; EGFR, epidermal growth factor receptor; 5-FU, 5-fluorouracil; HMG-CoA, 3-hydroxy-3-methylglutaryl CoA; MMP-2, matrix metalloproteinase-2; PI3K, phosphatidylinositol 3-kinase; VEGFR, vascular endothelial growth factor receptor.
5-FU, Irinotecan, and Oxaliplatin
5-FU manifests its cytotoxic properties through two mechanisms: the inhibition of thymidylate synthase (TS), which disrupts DNA replication and repair, and misincorporation into DNA and RNA, disrupting nucleic acid function (74). The binding of the active metabolite of 5-FU to TS is increased with higher intracellular concentrations of reduced folate, so the addition of leukovorin, or folinic acid, to 5-FU has been shown to increase the drug's toxicity (74). Augmentation with additional cytotoxic therapies, namely irinotecan (104) and oxaliplatin (28), further increases the effectiveness and improves survival; however, response rates to these combination regimens are only ∼50%. The toxicity of this aggressive chemotherapy protocol can be severe (105).
An improved metric for assessing responsiveness to therapy would permit the optimization of dosing regimens to minimize toxicity while maintaining the therapeutic benefit; it would also identify nonresponders earlier, so that treatment approaches could be altered and resistance mechanisms addressed. Optical molecular imaging has made several strides to this effect. Optical molecular probes that detect cell death have been developed that consist of fluorescently labeled annexin V, an endogenous protein that binds with high specificity to phosphatidylserine, the plasma membrane phospholipid that is expressed on the extracellular surface during cell death (107). Such an approach could quantify tumor response to therapy far earlier than standard methods (24). Optical annexin probes have been applied to the imaging of animal models of glioma and lung cancer, demonstrating the ability to detect increased cell death following cyclophosphamide therapy (94, 106). Other optical probes designed to measure cell death, including a protease-activatable probe sensitive to caspase-1, have been developed and shown to be effective markers of apoptosis in cell culture (82).
One putative mechanism for resistance is alterations in the reduced folate receptor, a membrane protein responsible for the transport of reduced folate, including leukovorin, into the cell. It has been shown in other cancers, most notably hematological malignancies, that reductions in receptor expression (124) and genetic polymorphisms yielding reduced intracellular concentrations of antifolate drugs (71) may be important ways in which cells exhibit resistance. Moon and colleagues (86) have developed an optically labeled folate imaging probe that binds to and is internalized by the folate receptor in cancer cells. Such an imaging approach may provide a surrogate measurement for leukovorin uptake into cells and yield information regarding intracellular concentrations of reduced folate, an important cofactor for 5-FU function.
Epidermal Growth Factor Receptor-Targeted Therapies
Cetuximab and panitumumab are monoclonal antibodies that bind to EGFR and block activation of the receptor's downstream signal transduction pathways by competitively inhibiting the binding of EGF and other growth factors (128). Improvements in outcome with these drugs have been seen primarily in the context of second-line therapy, with marginal benefits observed in progression-free survival when applied in the first-line setting (128). Moreover, important predictors of response to anti-EGFR therapy have been identified, most notably Kras mutational status. Both cetuximab (61, 68) and panitumumab (3) are effective only in patients whose tumors have wild-type Kras; activating mutations in this signaling protein downstream of EGFR confer resistance to anti-EGFR therapy. Indeed, cetuximab and panitumumab therapy are now recommended only for patients with known wild-type Kras, and routine screening is recommended prior to consideration for therapy with these medications (2).
Another interesting twist in the role on anti-EGFR therapy in colorectal cancer that has muddied the waters is the observation that response to these drugs does not necessarily correlate with tumoral EGFR expression. Chung et al. (22) retrospectively analyzed response to cetuximab by computed tomography metrics and compared these findings with the patients' pathology reports for EGFR expression by immunohistochemistry (IHC); they found that there were patients with EGFR-negative tumors that nonetheless exhibited response to cetuximab therapy. As such, documented EGFR positivity is no longer a prerequisite for initiating anti-EGFR therapy.
These data raise several questions that merit further investigation. Could it be that our current methods for detecting EGFR, in this case IHC, lack the quantitation required to be predictive? Or could sampling error inherent to random biopsies from malignant tissue result in recorded EGFR expression level that is nonrepresentative? Answering these questions is a priority for the advancement of molecularly targeted therapy, and optical molecular imaging is well positioned to provide them. Goetz and colleagues (44) have recently applied confocal microendoscopy in conjunction with an intravenously administered EGFR-targeted optical probe constructed by fluorescently labeling cetuximab to an animal model of colorectal cancer. They were able to quantify in vivo EGFR expression in an orthotopic colon cancer animal model and associate the fluorescence signal with histology and IHC for EGFR. They additionally illustrated the clinical translatability of this approach by incubating human tissue, both dysplastic and benign, with the optical probe and found good correlation between confocal imaging and IHC for EGFR. The use of confocal microendoscopy allowed for the microscopic measurement of EGFR expression, though for a very limited field of view. Nevertheless, the technique illustrated by this group represents a potentially effective means to resolving the issue of EGFR expression and response to cetuximab therapy by theoretically allowing for the in vivo, highly sensitive quantification of EGFR expression levels at multiple foci during colonoscopy.
Optical molecular imaging has also paved inroads into furthering our understanding of the relationship between Kras and tumor susceptibility to anti-EGFR therapy. One of the major pathways activated by Kras is that of phosphatidylinositol 3-kinase (PI3K), a key signaling pathway that prevents apoptosis and promotes cell survival. Moreover, recent clinical data suggest that activating mutations in PI3K itself can lead to resistance to cetuximab (93). Therefore, the PI3K signal transduction pathway represents an important imaging target for predicting response to anti-EGFR therapy. The development of a PI3K imaging agent has been pursued by fluorescently labeling wortmannin, a member of the viridin class of antibiotics that is a potent inhibitor of PI3K (41). The probe has been shown to be internalized by a number of malignant cells in culture, including a human colorectal cancer cell line; moreover, the probe was found to bind to numerous intracellular proteins including PI3K and maintain the potency of the attached wortmannin through observed reductions in cellular proliferation rates (6). This imaging agent has also been applied in the in vivo setting and was shown to localize to tumors in a xenograft animal model (111). Fluorescently labeled wortmannin may not only turn out to be a method to quantify overactivation of Kras and PI3K for estimation of anti-EGFR therapy but also may be useful in combination with anti-EGFR therapy.
Ex vivo analyses with IHC, fluorescence in situ hybridization, and genetic sequencing have revealed several key genes that play pivotal roles in modulating response to anti-EGFR therapy. One approach to applying this information to improve clinical practice would be to investigate each of these signaling pathways through discrete optical molecular imaging probes, as discussed above for Kras and PI3K. Another solution, however, would be to measure the net effectiveness of therapy through end result markers such as cell death. This was the approach taken by Manning and colleagues (77), who showed the ability to measure simultaneously cetuximab binding and resultant cell death. Using a fluorescently labeled cetuximab imaging agent to detect EGFR expression and a fluorescently labeled annexin imaging agent to detect cell death, they demonstrated in xenograft models of colorectal cancer increased binding of the EGFR probe in tumors that were EGFR positive vs. tumors that were EGFR negative. Moreover, following cetuximab therapy, there was a statistically significant increase in the annexin probe signal in the tumors compared with pretreatment imaging. These results suggest that optical molecular imaging may offer an improved method for gauging response to cetuximab therapy over conventional criteria such as Response Evaluation Criteria in Solid Tumors (56) (RECIST criteria) or presence of a characteristic skin rash (92).
Vascular Endothelial Growth Factor Targeted Therapy
Angiogenesis, the formation of new blood vessels, is required for tumors to grow beyond the millimeter size scale (8, 34). Numerous biochemical factors have been identified as components of the angiogenesis signaling cascade; none have been as well studied as VEGF (15, 31, 32). VEGF's numerous biological activities are mediated by the binding to and activation of a family of cell surface molecules known as VEGF receptors (VEGFR). Activation of this pathway results in a myriad of effects, including proliferation of endothelial cells, induced expression of numerous proteases including urokinase and tissue plasminogen activator (uPA and tPA, respectively) and matrix metalloproteinases (MMPs), increased vascular permeability, and expression of cell adhesion molecules including vascular cell adhesion molecule-1 (VCAM-1) (32).
The concept of inhibiting tumoral growth by arresting angiogenesis was made over 30 years ago by Folkman et al. (35). Bevacizumab is a monoclonal antibody to VEGF that has been applied successfully to numerous solid tumors, including colorectal cancer (18). Bevacizumab has become a major component for first-line therapy for advanced colorectal cancer. However, several concerns remain that have arisen from preclinical work as well as observations from use of this biological agent in other cancers. First, it is likely that tumors, upon cessation of anti-VEGF therapy, rapidly recruit new blood vessels and redevelop the vascularization that was inhibited by bevacizumab. In an animal model of lung cancer, Mancuso et al. (75) found that within 1 day of stopping bevacizumab therapy, tumors began to resprout blood vessels, and within 1 wk of cessation, tumors were fully revascularized. Interestingly, however, upon reinitiation of therapy, the blood vessels regressed by the same degree as they had during the initial treatment, implying that the tumors had not developed resistance to bevacizumab.
Another issue with anti-VEGF therapy is the following conceptual paradox: inhibiting tumor vasculature, while reducing oxygen and nutrients to tumoral tissue, should also reduce delivery of chemotherapeutic agents as well and thus diminish tumor response to chemotherapy. However, there is a clear synergistic relationship between bevacizumab and cytotoxic chemotherapy. One provocative hypothesis to explain this phenomenon is the concept of tumor vasculature “normalization,” promulgated by Jain (58), which suggests that anti-VEGF therapy modifies ectopic malignant vasculature to resemble more physiologically and functionally normal blood vessels, thus increasing delivery of chemotherapy to the tumor. However, it is currently unknown what the time course for this “normalization” is, and at which point the antiangiogenic properties of bevacizumab prevail and cause regression of tumor vasculature. An improved understanding of the “normalization window” may have dramatic implications for the optimized dosing and timing of bevacizumab therapy.
Optical molecular imaging has the potential to provide new insights into the effects of bevacizumab therapy on tumor vasculature, both by measuring directly the impact on VEGF and by quantifying changes in downstream processes such as tumoral vascular volume fraction, vascular permeability, and integrin expression. These methods may provide a clearer vision of the effectiveness of bevacizumab, as well as ideal dosing times and durations, on an individual basis.
Backer and colleagues (5) developed a recombinant VEGF conjugated to a NIR fluorochrome that binds to and is internalized by VEGFR on endothelial cells in animal models of tumors. Their molecular imaging probe is compatible with radiotracer labeling for SPECT and PET imaging in addition to optical imaging. Other optical VEGF probes have also been developed, for example by the fluorescent labeling of bevacizumab. This imaging agent has been used in animal models of prostate and pancreatic cancer with known differential expression of VEGF. Chang et al. (17) demonstrated variable uptake of the imaging agent across these cancer models, with quantitative measurements of the agent's fluorescence correlating well with subsequent ex vivo estimation of whole tumor VEGF concentration by ELISA. Specificity of the probe for VEGF was demonstrated by reduced fluorescence signal following pretreatment with unlabeled bevacizumab.
Beyond directly imaging VEGF and its receptors, other tumor markers of abnormal angiogenesis have been investigated with optical molecular imaging. The integrin αvβ3 is a well-studied cell adhesion molecule that is upregulated in tumor vasculature. In addition to being an important player in cell survival and the formation of metastases, αvβ3 promotes recruitment of MMP-2, which in turn promotes degradation of extracellular matrix and allows the vessel to grow (30). As such, the expression levels of αvβ3 are suggestive of the degree of active new vessel formation in a tumor. This integrin has been imaged via optical molecular imaging by conjugating a NIR fluorochrome to a targeted peptide in an animal glioblastoma model (54, 129). The probe was found to localize accurately to the tumor vasculature, which was confirmed by fluorescence microscopy; specificity for αvβ3 was demonstrated by a reduction in the fluorescence probe binding following pretreatment with a high concentration of the unlabeled targeting peptide. Additional optical molecular probes targeted to αvβ3 have also been developed, including fluorescently labeled iron oxide nanoparticles, which have been shown to be successful at imaging αvβ3 in breast cancer xenografts (85). Moreover, other integrins such as VCAM-1 have been imaged by optical molecular imaging, but as of yet not in the oncological setting (66). Since bevacizumab therapy reduces expression levels of integrins including αvβ3 (131), the in vivo measurement of integrin expression may be an important metric by which to quantify response to bevacizumab therapy.
Other functional in vivo assays of bevacizumab therapy are possible with optical molecular imaging. Montet et al. (84) showed the ability of optical molecular imaging to quantify changes in tumor vascular volumes before and after bevacizumab therapy using a nonspecific optical probe that remains within the blood pool. In a xenograft model of colorectal cancer, the authors used a fluorescence tomographic technique to quantify tumor vascular volume fractions (VVFs) noninvasively. They found a linear relationship between the administered dose of imaging probe and calculated VVFs; furthermore, they found a significant decrease in VVF following bevacizumab therapy, results that they were able to validate with IHC. This initial study supports a means by which to directly quantify changes in tumor vascularity following anti-VEGF therapy in vivo, which may be applicable in future studies to bevacizumab dosing schedule and duration optimization.
Finally, VEGF is known to upregulate expression of numerous proteases that are amenable to imaging by activatable optical molecular probes. One such protease is MMP-2, which, as discussed previously, degrades extracellular matrix at the site of new vessel formation (30); an MMP-activatable probe (10) could provide insight into functional changes on angiogenesis at the molecular level with bevacizumab therapy. VEGF is also thought to increase levels of uPA, for which an activatable optical molecular probe has also been designed and applied to the imaging of colorectal cancer in a mouse model (52, 72). The probe was found to localize with high target-to-background ratios to subcutaneous tumors. Whether this approach will serve as a means of measuring bevacizumab efficacy is yet to be seen.
Cyclooxygenase-2 Inhibition in Colorectal Cancer
The vast majority of colorectal cancers are known to overexpress the enzyme cyclooxygenase-2 (COX-2) (4), which has pleiotropic protumoral effects (23, 46). Targeted therapies against COX-2 were found to be very successful in initial animal studies, and two large clinical trials revealed a decrease in the occurrence of metachrononous adenomas with COX-2 inhibitors (4). However, the human trials also unveiled a cardiovascular morbidity associated with these drugs, because there were an increased number of strokes and myocardial infarctions in the treatment arm (11, 97, 98, 112). Thus COX-2 inhibitors such as celecoxib have been demonstrated to be potent anticancer agents, but their role in colorectal cancer at present is limited because of a lack of robust metrics for assessing the drug's efficacy. Such metrics would allow us to accurately identify both a therapeutically effective yet safe dose, as well as those patients whose tumors are responsive to celecoxib and who would therefore benefit from COX-2 inhibition.
Our laboratory has recently developed a way to potentially quantify COX-2 activity in colorectal cancer, and we were able to demonstrate a reduction in downstream signaling of the COX-2 pathway following inhibition by celecoxib. COX-2 is known to upregulate MMP expression, which in turn promotes tumor growth by degrading surrounding extracellular matrix (114, 116). We applied an activatable optical imaging probe sensitive to MMPs to both an orthotopic and a genetically engineered mouse model of colorectal cancer and found that celecoxib therapy not only dramatically reduced tumor growth rates, consistent with previous findings, but also avidly suppressed tumoral MMP activity. We were able to draw a direct correlation between the tumor's measured MMP activity and its rate of growth, implying the central role this family of enzymes plays in tumor expansion. We also demonstrated the ability to perform this imaging technique via quantitative fluorescence colonoscopy to illustrate the potential clinical translatability (unpublished data).
Individual genetic variations, including polymorphisms in the cytochrome P-450 enzymes (16) and the ornithine decarboxylase gene (79), modulate patients' responsiveness to celecoxib therapy and may even affect their risk of cardiovascular toxicity. Therefore, for celecoxib to be applied in the clinical arena, it will be important to have methods to assess a patient's susceptibility to therapy on a personalized basis, and in a short time frame; the approach of quantifying MMP activity may be one such method.
Human Epidermal Growth Factor Receptor-2-Targeted Therapy
Human epidermal growth factor receptor-2 (HER-2/neu) is a transmembrane protein that belongs to a tyrosine kinase receptor superfamily associated with cell growth and differentiation. This oncogene is expressed at low levels in epithelial cells and is overexpressed in numerous cancers, most notably breast cancer. Activation of the receptor's signaling pathway is believed to result in cellular proliferation, malignant transformation, and increased propensity for metastasis (91). Therapy targeted to inhibiting HER-2/neu activation has become standard of care in breast cancer, with trastuzumab, a monoclonal antibody to this receptor, clinically approved for the treatment of HER-2/neu-positive breast carcinomas.
HER-2/neu is also overexpressed in other adenocarcinomas besides breast cancer (132), including colorectal cancer. Estimates of the frequency of HER-2/neu overexpression vary (60), but a recent analysis approximates overexpression to occur in 11% of colorectal cancers (62). Although the presence of the HER-2/neu overexpression is not an independent predictor of poor prognosis in colorectal cancer, this finding does tend to occur in more aggressive phenotypes.
Targeted therapy against the HER-2/neu receptor has been investigated in colorectal cancer, through both animal and human trials. Using a HER-2/neu positive colorectal cancer cell line, Mann et al. (76) showed that treatment with trastuzumab reduced tumor cell counts in vitro and tumor growth rates in vivo. A clinical phase II trial was performed to determine whether trastuzumab, given in conjunction with irinotecan, would provide additional benefit for patients with advanced colorectal cancer in a second-line setting (100). The trial was ended early, however, since only 8% of the enrolled patients were found to overexpress HER-2/neu, a rate significantly lower than estimated frequency. The trial was further limited by a low recruitment, with only nine patients treated before the trial was closed.
HER-2/neu, though uncommonly overexpressed in colorectal cancer, remains an intriguing target for molecularly guided therapy. If the minority of patients whose cancers do fall into the category of HER-2/neu overexpression could be readily identified, then it may make therapeutic sense to treat these patients with trastuzumab. The in vivo measurement of HER-2/neu expression has been achieved with optical molecular imaging in the realm of breast cancer (40). Fluorescently labeled trastuzumab was used as the targeted imaging probe, and uptake was measured in a HER-2/neu positive xenograft mouse model of breast carcinoma. Additionally, cell death was measured simultaneously by a fluorescently labeled annexin probe. Tumoral response to therapeutic trastuzumab was observed by increased annexin uptake prior earlier than standard assessments of response such as reductions in tumor growth rate manifested. This approach could be extended to colorectal cancer to both identify patients who may benefit from trastuzumab therapy and monitor efficacy of therapy in real-time.
Potential for Clinical Translation
Optical molecular imaging has made tremendous strides in preclinical experimentation for colorectal cancer. The translation of this technology to human applications is expected to have far-reaching ramifications. However, there are several impediments that need to be addressed before this modality is ready for human use. In considering the optical molecular probes, perhaps the greatest hurdle to overcome is the clinical approval of an optimized subset of the array of fluorochromes and conjugates that have been utilized as optical reporters in animal studies. This may be slightly eased through the synthesis of imaging agents from already approved fluorochromes, such as fluorescein and ICG; however, such a conjugate represents a new molecular entity in terms of toxicity, and other testing would be required.
The limited tissue penetration of fluorescent photons precludes noninvasive imaging, although tomographic techniques have been developed for human organs such as the breast (88). This approach is impractical for the colon or abdomen, so minimally invasive fluorescent endoscopes must be engineered to allow for the in vivo imaging of colorectal cancer with optical probes. Fortunately, it is possible to do so by performing modifications on conventional colonoscope technology. By adding light filters and dichroic mirrors to the proximal end of clinical fiberoptic colonoscopes, it is possible to construct high-resolution fluorescent colonoscopes with minimal modification of any of the parts that come into contact with the patient (109). Moreover, two commercially available confocal microendoscopy systems that permit high-resolution fluorescence imaging of small areas of tissue have been approved for human use; in one system, the microendoscope is integrated into a conventional endoscope (120), whereas in the other, the microendoscope fits through the working channel of standard colonoscopes (123).
Despite the challenges of bringing optical molecular imaging into clinical practice, the groundwork is being laid. Beyond using fluorescence endoscopy to image localization of nontargeted fluorochromes such as fluorescein in colonic mucosa (69), several groups have advanced targeted optical molecular probe imaging strategies in humans. Keller et al. (63) demonstrated in a seminal proof-of-principle experiment a method of performing targeted optical molecular imaging for colorectal cancer. The optical probe that was used was composed of fluorescein conjugated to a monoclonal antibody against carcinoembryonic antigen (CEA). The patients who were enrolled had known polypoid lesions and were to have surgical resection subsequent to the study. They first underwent conventional white light colonoscopy to identify the lesion; the surface of the lesion was then directly painted with the fluorescent probe. Direct topical application, rather than intravenous injection, circumvented the concerns for systemic toxicity of the imaging agent. After an incubation period and a saline wash to remove nonspecific binding, the fluorescence from the tumors was measured with the appropriate light filters. The hypothesis that was tested by the authors was that optical molecular imaging would be able to predict the aggressiveness of a lesion by measuring CEA expression from the tumor in vivo; indeed, they were able to find a statistically significant correlation between the fluorescence imaging and IHC for CEA. The authors describe several shortcomings of their approach, such as the difficulty to perform this imaging on ulcerated or bloody lesions, which tended to produce false negatives. Moreover, feces exhibits autofluorescence in the same emission wavelengths at fluorescein, so there is a risk of false positives in areas contaminated by feces. Additional limitations include the need to already have established the presence of a polyp, which prevents this method from being applicable in the screening setting. Also, topical application of imaging probe raises the concern for a heterogeneous distribution of the agent, which can introduce error.
More recently, Hsiung et al. (53) have shown the potency of optical imaging with confocal microendoscopy in colorectal cancer (Fig. 5). In this study, the imaging agent consisted of fluorescein conjugated to a short peptide sequence isolated from a phage display peptide library generated from resected human adenomas. Patients without previously established polyps underwent routine screening colonoscopy; when a polyp was identified, the surface was sprayed with the imaging agent, and confocal microendoscopy was immediately performed following a saline wash. The adenoma was then removed and collected for histological analysis. They found an increase in probe binding by dysplastic colonocytes vs. benign mucosa, with an estimated sensitivity and specificity of 81 and 82%, respectively. Factors that negatively impacted these statistics included nonspecific, residual deposits of probe that collected in benign crypts, resulting in false positives; moreover, excessive saline washing likely resulted in disassociation of the probe from its target, leading to false negatives. Another limitation of the study was that although the confocal technology yielded very high resolution images, it is not generalizable to the imaging of large surface areas and is impractical for use as a screening modality.
Fig. 5.
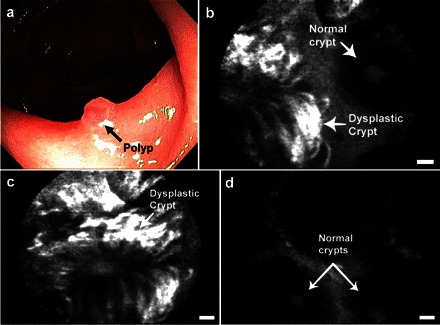
Optical molecular imaging of colorectal cancer in humans. a: white light imaging of a colon polyp by conventional colonoscopy. b: confocal microendoscopy following topical application of a fluorescently labeled peptide identified by phage display targeted to colorectal cancer identifies the border between normal and dysplastic tissue on a scale of micrometers. c: closer view of the dysplastic crypt shows increased binding of the labeled peptide compared with d, adjacent normal mucosa. Scale bars are 20 μm. Reproduced with permission from Ref. 53.
Nonendoscopic optical molecular imaging approaches to detecting colon cancer have been advanced as well. Capsule endoscopy is a clinical technology in which a capsule containing cameras is ingested; the capsule then records images of the gastrointestinal mucosa as it travels through the digestive tract. It is used primarily for the evaluation of areas that are not amenable to direct endoscopic visualization, i.e., the small bowel. A recent randomized controlled trial showed that conventional capsule endoscopy is inferior to colonoscopy for colorectal cancer screening (118). However, Zhang et al. (133) have performed a proof-of-principle experiment using optical molecular imaging to potentially increase the sensitivity and specificity of capsule endoscopy for adenoma detection (1). After injecting APCMin/+ mice with a protease-activatable probe, they harvested the colon and fixed it onto a stage that steadily translated the colon under an optical setup that comprised of a clinical capsule endoscope and NIR filters. They were able to differentiate between adenomas and inflammatory lesions with high target-to-background ratios. Although their technique required ex vivo imaging with a static capsule endoscope and additional optical filters, this general approach should expand the clinical relevance of noninvasive colorectal cancer screening.
Conclusions
The past decade has seen considerable progress in optical molecular imaging with regard to its ability to detect diseases earlier, with greater specificity, and in a prognostically relevant manner. Colorectal cancer is well suited to benefit from this imaging modality: 1) it is a disease of mucosal surfaces that can be accessed with relative ease, 2) our understanding of the genetic evolution and molecular underpinnings is well advanced, and 3) several molecularly targeted therapies are already approved. Optical molecular imaging promises to usher in an era of personalized medicine to colorectal cancer, an era in which disease detection and therapeutic regimens are tailored and optimized on an individualized basis.
DISCLOSURES
The authors report no conflicts of interest.
REFERENCES
- 1. Aisenberg J. Gastrointestinal endoscopy nears “the molecular era”. Gastrointest Endosc 68: 528–530, 2008. [DOI] [PubMed] [Google Scholar]
- 2. Allegra CJ, Jessup JM, Somerfield MR, Hamilton SR, Hammond EH, Hayes DF, McAllister PK, Morton RF, Schilsky RL. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol 27: 2091–2096, 2009. [DOI] [PubMed] [Google Scholar]
- 3. Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, Patterson SD, Chang DD. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 26: 1626–1634, 2008. [DOI] [PubMed] [Google Scholar]
- 4. Arber N, Eagle CJ, Spicak J, Rácz I, Dite P, Hajer J, Zavoral M, Lechuga MJ, Gerletti P, Tang J, Rosenstein RB, Macdonald K, Bhadra P, Fowler R, Wittes J, Zauber AG, Solomon SD, Levin B; PreSAP Trial Investigators. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med 355: 885–895, 2006. [DOI] [PubMed] [Google Scholar]
- 5. Backer MV, Levashova Z, Patel V, Jehning BT, Claffey K, Blankenberg FG, Backer JM. Molecular imaging of VEGF receptors in angiogenic vasculature with single-chain VEGF-based probes. Nat Med 13: 504–509, 2007. [DOI] [PubMed] [Google Scholar]
- 6. Barnes KR, Blois J, Smith A, Yuan H, Reynolds F, Weissleder R, Cantley LC, Josephson L. Fate of a bioactive fluorescent wortmannin derivative in cells. Bioconjug Chem 19: 130–137, 2008. [DOI] [PubMed] [Google Scholar]
- 7. Barrett T, Koyama Y, Hama Y, Ravizzini G, Shin IS, Jang BS, Paik CH, Urano Y, Choyke PL, Kobayashi H. In vivo diagnosis of epidermal growth factor receptor expression using molecular imaging with a cocktail of optically labeled monoclonal antibodies. Clin Cancer Res 13: 6639–6648, 2007. [DOI] [PubMed] [Google Scholar]
- 8. Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer 3: 401–410, 2003. [DOI] [PubMed] [Google Scholar]
- 9. Bremer C, Ntziachristos V, Weissleder R. Optical-based molecular imaging: contrast agents and potential medical applications. Eur Radiol 13: 231–243, 2003. [DOI] [PubMed] [Google Scholar]
- 10. Bremer C, Tung C, Weissleder R. In vivo molecular target assessment of matrix metalloproteinase inhibition. Nat Med 7: 743–748, 2001. [DOI] [PubMed] [Google Scholar]
- 11. Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, Lines C, Riddell R, Morton D, Lanas A, Konstam MA, Baron JA; Adenomatous Polyp Prevention on Vioxx (APPROVe) Trial Investigators. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med 352: 1092–1102, 2005. [DOI] [PubMed] [Google Scholar]
- 12. Bugaj JE, Achilefu S, Dorshow RB, Rajagopalan R. Novel fluorescent contrast agents for optical imaging of in vivo tumors based on a receptor-targeted dye-peptide conjugate platform. J Biomed Opt 6: 122–133, 2001. [DOI] [PubMed] [Google Scholar]
- 13. Cai W, Chen X. Multimodality molecular imaging of tumor angiogenesis. J Nucl Med 49, Suppl 2: 113S–128S, 2008. [DOI] [PubMed] [Google Scholar]
- 14. Cai W, Chen X. Nanoplatforms for targeted molecular imaging in living subjects. Small 3: 1840–1854, 2007. [DOI] [PubMed] [Google Scholar]
- 15. Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 407: 249–257, 2000. [DOI] [PubMed] [Google Scholar]
- 16. Chan AT, Zauber AG, Hsu M, Breazna A, Hunter DJ, Rosenstein RB, Eagle CJ, Hawk ET, Bertagnolli MM. Cytochrome P450 2C9 variants influence response to celecoxib for prevention of colorectal adenoma. Gastroenterology 136: 2127–2136.e1, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chang SK, Rizvi I, Solban N, Hasan T. In vivo optical molecular imaging of vascular endothelial growth factor for monitoring cancer treatment. Clin Cancer Res 14: 4146–4153, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chau I, Cunningham D. Treatment in advanced colorectal cancer: what, when and how? Br J Cancer 100: 1704–1719, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen J, Tung C, Allport J, Chen S, Weissleder R. Near-infrared fluorescent imaging of matrix metalloproteinase activity after myocardial infarction. Circulation 111: 1800–1805, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen J, Tung CH, Mahmood U, Ntziachristos V, Gyurko R, Fishman MC, Huang PL, Weissleder R. In vivo imaging of proteolytic activity in atherosclerosis. Circulation 105: 2766–2771, 2002. [DOI] [PubMed] [Google Scholar]
- 21. Chen WT, Mahmood U, Weissleder R, Tung CH. Arthritis imaging using a near-infrared fluorescence folate-targeted probe. Arthritis Res Ther 7: R310–R317, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chung KY, Shia J, Kemeny NE, Shah M, Schwartz GK, Tse A, Hamilton A, Pan D, Schrag D, Schwartz L, Klimstra DS, Fridman D, Kelsen DP, Saltz LB. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol 23: 1803–1810, 2005. [DOI] [PubMed] [Google Scholar]
- 23. Church RD, Fleshman JW, McLeod HL. Cyclo-oxygenase 2 inhibition in colorectal cancer therapy. Br J Surg 90: 1055–1067, 2003. [DOI] [PubMed] [Google Scholar]
- 24. Corsten MF, Hofstra L, Narula J, Reutelingsperger CPM. Counting heads in the war against cancer: defining the role of annexin A5 imaging in cancer treatment and surveillance. Cancer Res 66: 1255–1260, 2006. [DOI] [PubMed] [Google Scholar]
- 25. Demierre MF, Higgins PDR, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer 5: 930–942, 2005. [DOI] [PubMed] [Google Scholar]
- 26. Emmert-Buck MR, Roth MJ, Zhuang Z, Campo E, Rozhin J, Sloane BF, Liotta LA, Stetler-Stevenson WG. Increased gelatinase A (MMP-2) and cathepsin B activity in invasive tumor regions of human colon cancer samples. Am J Pathol 145: 1285–1290, 1994. [PMC free article] [PubMed] [Google Scholar]
- 27. Etzioni R, Urban N, Ramsey S, McIntosh M, Schwartz S, Reid B, Radich J, Anderson G, Hartwell L. The case for early detection. Nat Rev Cancer 3: 243–252, 2003. [DOI] [PubMed] [Google Scholar]
- 28. Farrugia DC, Ford HER, Cunningham D, Danenberg KD, Danenberg PV, Brabender J, McVicar AD, Aherne GW, Hardcastle A, McCarthy K, Jackman AL. Thymidylate synthase expression in advanced colorectal cancer predicts for response to raltitrexed. Clin Cancer Res 9: 792–801, 2003. [PubMed] [Google Scholar]
- 29. Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 61: 759–767, 1990. [DOI] [PubMed] [Google Scholar]
- 30. Felding-Habermann B. Integrin adhesion receptors in tumor metastasis. Clin Exp Metastasis 20: 203–213, 2003. [DOI] [PubMed] [Google Scholar]
- 31. Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer 2: 795–803, 2002. [DOI] [PubMed] [Google Scholar]
- 32. Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev 18: 4–25, 1997. [DOI] [PubMed] [Google Scholar]
- 33. Flower RW, Hochheimer BF. Indocyanine green dye fluorescence and infrared absorption choroidal angiography performed simultaneously with fluorescein angiography. Johns Hopkins Med J 138: 33–42, 1976. [PubMed] [Google Scholar]
- 34. Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1: 27–31, 1995. [DOI] [PubMed] [Google Scholar]
- 35. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 285: 1182–1186, 1971. [DOI] [PubMed] [Google Scholar]
- 36. Folli S, Westermann P, Braichotte D, Pèlegrin A, Wagnières G, van den Bergh H, Mach JP. Antibody-indocyanin conjugates for immunophotodetection of human squamous cell carcinoma in nude mice. Cancer Res 54: 2643–2649, 1994. [PubMed] [Google Scholar]
- 37. Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol 7: 626–634, 2003. [DOI] [PubMed] [Google Scholar]
- 38. Funovics MA, Alencar H, Su HS, Khazaie K, Weissleder R, Mahmood U. Miniaturized multichannel near infrared endoscope for mouse imaging. Mol Imaging 2: 350–357, 2003. [DOI] [PubMed] [Google Scholar]
- 39. Funovics MA, Weissleder R, Mahmood U. Catheter-based in vivo imaging of enzyme activity and gene expression: feasibility study in mice. Radiology 231: 659–666, 2004. [DOI] [PubMed] [Google Scholar]
- 40. Gee MS, Upadhyay R, Bergquist H, Weissleder R, Josephson L, Mahmood U. Multiparameter noninvasive assessment of treatment susceptibility, drug target inhibition and tumor response guides cancer treatment. Int J Cancer 121: 2492–2500, 2007. [DOI] [PubMed] [Google Scholar]
- 41. Giner JL, Kehbein KA, Cook JA, Smith MC, Vlahos CJ, Badwey JA. Synthesis of fluorescent derivatives of wortmannin and demethoxyviridin as probes for phosphatidylinositol 3-kinase. Bioorg Med Chem Lett 16: 2518–2521, 2006. [DOI] [PubMed] [Google Scholar]
- 42. Goetz M, Kiesslich R. Advances of endomicroscopy for gastrointestinal physiology and diseases. Am J Physiol Gastrointest Liver Physiol 298: G797–G806, 2010. [DOI] [PubMed] [Google Scholar]
- 43. Goetz M, Kiesslich R. Confocal endomicroscopy: in vivo diagnosis of neoplastic lesions of the gastrointestinal tract. Anticancer Res 28: 353–360, 2008. [PubMed] [Google Scholar]
- 44. Goetz M, Ziebart A, Foersch S, Vieth M, Waldner M, Delaney P, Galle P, Neurath M, Kiesslich R. In vivo molecular imaging of colorectal cancer with confocal endomicroscopy of epidermal growth factor receptor. Gastroenterology 138: 435–446, 2010. [DOI] [PubMed] [Google Scholar]
- 45. Gross S, Piwnica-Worms D. Spying on cancer: molecular imaging in vivo with genetically encoded reporters. Cancer Cell 7: 5–15, 2005. [DOI] [PubMed] [Google Scholar]
- 46. Gupta RA, DuBois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer 1: 11–21, 2001. [DOI] [PubMed] [Google Scholar]
- 47. Gurfinkel M, Thompson AB, Ralston W, Troy TL, Moore AL, Moore TA, Gust JD, Tatman D, Reynolds JS, Muggenburg B, Nikula K, Pandey R, Mayer RH, Hawrysz DJ, Sevick-Muraca EM. Pharmacokinetics of ICG and HPPH-car for the detection of normal and tumor tissue using fluorescence, near-infrared reflectance imaging: a case study. Photochem Photobiol 72: 94–102, 2000. [DOI] [PubMed] [Google Scholar]
- 48. Hawk E, Viner JL. Statins and cancer—beyond the “one drug, one disease” model. N Engl J Med 352: 2238–2239, 2005. [DOI] [PubMed] [Google Scholar]
- 49. Hazen LG, Bleeker FE, Lauritzen B, Bahns S, Song J, Jonker A, Van Driel BE, Lyon H, Hansen U, Köhler A, Van Noorden CJ. Comparative localization of cathepsin B protein and activity in colorectal cancer. J Histochem Cytochem 48: 1421–1430, 2000. [DOI] [PubMed] [Google Scholar]
- 50. Hecht JR, Mitchell E, Chidiac T, Scroggin C, Hagenstad C, Spigel D, Marshall J, Cohn A, McCollum D, Stella P, Deeter R, Shahin S, Amado RG. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol 27: 672–680, 2009. [DOI] [PubMed] [Google Scholar]
- 51. Herschman H. Molecular imaging: looking at problems, seeing solutions. Science 302: 605–608, 2003. [DOI] [PubMed] [Google Scholar]
- 52. Hsiao JK, Law B, Weissleder R, Tung CH. In-vivo imaging of tumor associated urokinase-type plasminogen activator activity. J Biomed Opt 11: 34013, 2006. [DOI] [PubMed] [Google Scholar]
- 53. Hsiung PL, Hsiung PL, Hardy J, Friedland S, Soetikno R, Du CB, Wu AP, Sahbaie P, Crawford JM, Lowe AW, Contag CH, Wang TD. Detection of colonic dysplasia in vivo using a targeted heptapeptide and confocal microendoscopy. Nat Med 14: 454–458, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hsu AR, Hou LC, Veeravagu A, Greve JM, Vogel H, Tse V, Chen X. In vivo near-infrared fluorescence imaging of integrin alphavbeta3 in an orthotopic glioblastoma model. Mol Imaging 8: 315–323, 2006. [DOI] [PubMed] [Google Scholar]
- 55. Jacoby RF, Seibert K, Cole CE, Kelloff GJ, Lubet RA. The cyclooxygenase-2 inhibitor celecoxib is a potent preventive and therapeutic agent in the min mouse model of adenomatous polyposis. Cancer Res 60: 5040–5044, 2000. [PubMed] [Google Scholar]
- 56. Jaffe CC. Measures of response: RECIST, WHO, and new alternatives. J Clin Oncol 24: 3245–3251, 2006. [DOI] [PubMed] [Google Scholar]
- 57. Jaffer F, Weissleder R. Molecular imaging in the clinical arena. JAMA 293: 855–862, 2005. [DOI] [PubMed] [Google Scholar]
- 58. Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med 7: 987–989, 2001. [DOI] [PubMed] [Google Scholar]
- 59. Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, Zwierko M, Rupinski M, Nowacki MP, Butruk E. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med 362: 1795–1803. [DOI] [PubMed] [Google Scholar]
- 60. Kapitanović S, Radosević S, Kapitanović M, Andelinović S, Ferencić Z, Tavassoli M, Primorać D, Sonicki Z, Spaventi S, Pavelic K, Spaventi R. The expression of p185(HER-2/neu) correlates with the stage of disease and survival in colorectal cancer. Gastroenterology 112: 1103–1113, 1997. [DOI] [PubMed] [Google Scholar]
- 61. Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, Price TJ, Shepherd L, Au HJ, Langer C, Moore MJ, Zalcberg JR. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 359: 1757–1765, 2008. [DOI] [PubMed] [Google Scholar]
- 62. Kavanagh DO, Chambers G, O'Grady L, Barry KM, Waldron RP, Bennani F, Eustace PW, Tobbia I. Is overexpression of HER-2 a predictor of prognosis in colorectal cancer? BMC Cancer 9: 1, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Keller R, Winde G, Terpe HJ, Foerster EC, Domschke W. Fluorescence endoscopy using a fluorescein-labeled monoclonal antibody against carcinoembryonic antigen in patients with colorectal carcinoma and adenoma. Endoscopy 34: 801–807, 2002. [DOI] [PubMed] [Google Scholar]
- 64. Kelloff GJ, Krohn KA, Larson SM, Weissleder R, Mankoff DA, Hoffman JM, Link JM, Guyton KZ, Eckelman WC, Scher HI, O'Shaughnessy J, Cheson BD, Sigman CC, Tatum JL, Mills GQ, Sullivan DC, Woodcock J. The progress and promise of molecular imaging probes in oncologic drug development. Clin Cancer Res 11: 7967–7985, 2005. [DOI] [PubMed] [Google Scholar]
- 65. Kelly K, Alencar H, Funovics M, Mahmood U, Weissleder R. Detection of invasive colon cancer using a novel, targeted, library-derived fluorescent peptide. Cancer Res 64: 6247–6251, 2004. [DOI] [PubMed] [Google Scholar]
- 66. Kelly KA, Allport JR, Tsourkas A, Shinde-Patil VR, Josephson L, Weissleder R. Detection of vascular adhesion molecule-1 expression using a novel multimodal nanoparticle. Circ Res 96: 327–336, 2005. [DOI] [PubMed] [Google Scholar]
- 67. Kelly KA, Bardeesy N, Anbazhagan R, Gurumurthy S, Berger J, Alencar H, Depinho RA, Mahmood U, Weissleder R. Targeted nanoparticles for imaging incipient pancreatic ductal adenocarcinoma. PLoS Med 5: e85, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Khambata-Ford S, Garrett CR, Meropol NJ, Basik M, Harbison CT, Wu S, Wong TW, Huang X, Takimoto CH, Godwin AK, Tan BR, Krishnamurthi SS, Burris HA, Poplin EA, Hidalgo M, Baselga J, Clark EA, Mauro DJ. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol 25: 3230–3237, 2007. [DOI] [PubMed] [Google Scholar]
- 69. Kiesslich R, Burg J, Vieth M, Gnaendiger J, Enders M, Delaney P, Polglase A, McLaren W, Janell D, Thomas S, Nafe B, Galle PR, Neurath MF. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology 127: 706–713, 2004. [DOI] [PubMed] [Google Scholar]
- 70. Klibanov AL, Martynov AV, Slinkin MA, Sakharov I, Smirnov MD, Muzykantov VR, Danilov SM, Torchilin VP. Blood clearance of radiolabeled antibody: enhancement by lactosamination and treatment with biotin-avidin or anti-mouse IgG antibodies. J Nucl Med 29: 1951–1956, 1988. [PubMed] [Google Scholar]
- 71. Laverdière C, Chiasson S, Costea I, Moghrabi A, Krajinovic M. Polymorphism G80A in the reduced folate carrier gene and its relationship to methotrexate plasma levels and outcome of childhood acute lymphoblastic leukemia. Blood 100: 3832–3834, 2002. [DOI] [PubMed] [Google Scholar]
- 72. Law B, Curino A, Bugge TH, Weissleder R, Tung CH. Design, synthesis, and characterization of urokinase plasminogen-activator-sensitive near-infrared reporter. Chem Biol 11: 99–106, 2004. [DOI] [PubMed] [Google Scholar]
- 73. Licha K, Riefke B, Ntziachristos V, Becker A, Chance B, Semmler W. Hydrophilic cyanine dyes as contrast agents for near-infrared tumor imaging: synthesis, photophysical properties and spectroscopic in vivo characterization. Photochem Photobiol 72: 392–398, 2000. [DOI] [PubMed] [Google Scholar]
- 74. Longley DB, Harkin DP, Johnston PG. 5-Fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer 3: 330–338, 2003. [DOI] [PubMed] [Google Scholar]
- 75. Mancuso MR, Davis R, Norberg SM, O'Brien S, Sennino B, Nakahara T, Yao VJ, Inai T, Brooks P, Freimark B, Shalinsky DR, Hu-Lowe DD, McDonald DM. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest 116: 2610–2621, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mann M, Sheng H, Shao J, Williams CS, Pisacane PI, Sliwkowski MX, DuBois RN. Targeting cyclooxygenase 2 and HER-2/neu pathways inhibits colorectal carcinoma growth. Gastroenterology 120: 1713–1719, 2001. [DOI] [PubMed] [Google Scholar]
- 77. Manning HC, Merchant NB, Foutch AC, Virostko JM, Wyatt SK, Shah C, McKinley ET, Xie J, Mutic NJ, Washington MK, LaFleur B, Tantawy MN, Peterson TE, Ansari MS, Baldwin RM, Rothenberg ML, Bornhop DJ, Gore JC, Coffey RJ. Molecular imaging of therapeutic response to epidermal growth factor receptor blockade in colorectal cancer. Clin Cancer Res 14: 7413–7422, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Marten K, Bremer C, Khazaie K, Sameni M, Sloane B, Tung CH, Weissleder R. Detection of dysplastic intestinal adenomas using enzyme-sensing molecular beacons in mice. Gastroenterology 122: 406–414, 2002. [DOI] [PubMed] [Google Scholar]
- 79. Martinez ME, O'Brien TG, Fultz KE, Babbar N, Yerushalmi H, Qu N, Guo Y, Boorman D, Einspahr J, Alberts DS, Gerner EW. Pronounced reduction in adenoma recurrence associated with aspirin use and a polymorphism in the ornithine decarboxylase gene. Proc Natl Acad Sci USA 100: 7859–7864, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev 17: 545–580, 2003. [DOI] [PubMed] [Google Scholar]
- 81. Mayer RJ. Targeted therapy for advanced colorectal cancer–more is not always better. N Engl J Med 360: 623–625, 2009. [DOI] [PubMed] [Google Scholar]
- 82. Messerli SM, Prabhakar S, Tang Y, Shah K, Cortes ML, Murthy V, Weissleder R, Breakefield XO, Tung CH. A novel method for imaging apoptosis using a caspase-1 near-infrared fluorescent probe. Neoplasia 6: 95–105, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med 352: 476–487, 2005. [DOI] [PubMed] [Google Scholar]
- 84. Montet X, Figueiredo JL, Alencar H, Ntziachristos V, Mahmood U, Weissleder R. Tomographic fluorescence imaging of tumor vascular volume in mice. Radiology 242: 751–758, 2007. [DOI] [PubMed] [Google Scholar]
- 85. Montet X, Montet-Abou K, Reynolds F, Weissleder R, Josephson L. Nanoparticle imaging of integrins on tumor cells. Neoplasia 8: 214–222, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Moon WK, Lin Y, O'Loughlin T, Tang Y, Kim DE, Weissleder R, Tung CH. Enhanced tumor detection using a folate receptor-targeted near-infrared fluorochrome conjugate. Bioconjug Chem 14: 539–545, 2003. [DOI] [PubMed] [Google Scholar]
- 87. Murdoch D, Sager J. Will targeted therapy hold its promise? An evidence-based review. Curr Opin Oncol 20: 104–111, 2008. [DOI] [PubMed] [Google Scholar]
- 88. Ntziachristos V. Fluorescence molecular imaging. Annu Rev Biomed Eng 8: 1–33, 2006. [DOI] [PubMed] [Google Scholar]
- 89. Ogawa M, Kosaka N, Choyke PL, Kobayashi H. In vivo molecular imaging of cancer with a quenching near-infrared fluorescent probe using conjugates of monoclonal antibodies and indocyanine green. Cancer Res 69: 1268–1272, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ogawa M, Kosaka N, Choyke PL, Kobayashi H. Tumor-specific detection of an optically targeted antibody combined with a quencher-conjugated neutravidin “quencher-chaser”: a dual “quench and chase” strategy to improve target to nontarget ratios for molecular imaging of cancer. Bioconjug Chem 20: 147–154, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J 19: 3159–3167, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Peeters M, Siena S, Van Cutsem E, Sobrero A, Hendlisz A, Cascinu S, Kalofonos H, Devercelli G, Wolf M, Amado RG. Association of progression-free survival, overall survival, and patient-reported outcomes by skin toxicity and KRAS status in patients receiving panitumumab monotherapy. Cancer 115: 1544–1554, 2009. [DOI] [PubMed] [Google Scholar]
- 93. Perrone F, Lampis A, Orsenigo M, DiBartolomeo M, Gevorgyan A, Losa M, Frattini M, Riva C, Andreola S, Bajetta E, Bertario L, Leo E, Pierotti MA, Pilotti S. PI3KCA/PTEN deregulation contributes to impaired responses to cetuximab in metastatic colorectal cancer patients. Ann Oncol 20: 84–90, 2009. [DOI] [PubMed] [Google Scholar]
- 94. Petrovsky A, Schellenberger E, Josephson L, Weissleder R, Bogdanov A., Jr Near-infrared fluorescent imaging of tumor apoptosis. Cancer Res 63: 1936–1942, 2003. [PubMed] [Google Scholar]
- 95. Pierce MC, Javier DJ, Richards-Kortum R. Optical contrast agents and imaging systems for detection and diagnosis of cancer. Int J Cancer 123: 1979–1990, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Pisani P, Parkin DM, Bray F, Ferlay J. Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer 83: 18–29, 1999. [DOI] [PubMed] [Google Scholar]
- 97. Psaty BM, Furberg CD. COX-2 inhibitors—lessons in drug safety. N Engl J Med 352: 1133–1135, 2005. [DOI] [PubMed] [Google Scholar]
- 98. Psaty BM, Potter JD. Risks and benefits of celecoxib to prevent recurrent adenomas. N Engl J Med 355: 950–952, 2006. [DOI] [PubMed] [Google Scholar]
- 99. Rabeneck L, Paszat L. Circumstances in which colonoscopy misses cancer. Frontline Gastroenterol 1: 52–58, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ramanathan RK, Hwang JJ, Zamboni WC, Sinicrope FA, Safran H, Wong MK, Earle M, Brufsky A, Evans T, Troetschel M, Walko C, Day R, Chen HX, Finkelstein S. Low overexpression of HER-2/neu in advanced colorectal cancer limits the usefulness of trastuzumab (Herceptin) and irinotecan as therapy. A phase II trial. Cancer Invest 22: 858–865, 2004. [DOI] [PubMed] [Google Scholar]
- 101. Rao J, Dragulescu-Andrasi A, Yao H. Fluorescence imaging in vivo: recent advances. Curr Opin Biotechnol 18: 17–25, 2007. [DOI] [PubMed] [Google Scholar]
- 102. Rudin M, Weissleder R. Molecular imaging in drug discovery and development. Nat Rev Drug Discov 2: 123–131, 2003. [DOI] [PubMed] [Google Scholar]
- 103. Saltz L. Colorectal cancer treatment: what's next? (or: Is there life after EGFR and VEGF?) Gastrointest Cancer Res 2: S20–S22, 2008. [PMC free article] [PubMed] [Google Scholar]
- 104. Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, Elfring GL, Miller LL. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 343: 905–914, 2000. [DOI] [PubMed] [Google Scholar]
- 105. Sargent DJ, Niedzwiecki D, O'Connell MJ, Schilsky RL. Recommendation for caution with irinotecan, fluorouracil, and leucovorin for colorectal cancer. N Engl J Med 345: 144–145; author reply 146, 2001. [DOI] [PubMed] [Google Scholar]
- 106. Schellenberger EA, Bogdanov A, Petrovsky A, Ntziachristos V, Weissleder R, Josephson L. Optical imaging of apoptosis as a biomarker of tumor response to chemotherapy. Neoplasia 5: 187–192, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Schellenberger EA, Weissleder R, Josephson L. Optimal modification of annexin V with fluorescent dyes. Chembiochem 5: 271–274, 2004. [DOI] [PubMed] [Google Scholar]
- 108. Sheth RA, Josephson L, Mahmood U. Evaluation and clinically relevant applications of a fluorescent imaging analog to fluorodeoxyglucose positron emission tomography. J Biomed Opt 14: 064014, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Sheth RA, Upadhyay R, Weissleder R, Mahmood U. Real-time multichannel imaging framework for endoscopy, catheters, and fixed geometry intraoperative systems. Mol Imaging 6: 147–155, 2007. [PubMed] [Google Scholar]
- 110. Sinitsyn VV, Mamontova AG, Checkneva YY, Shnyra AA, Domogatsky SP. Rapid blood clearance of biotinylated IgG after infusion of avidin. J Nucl Med 30: 66–69, 1989. [PubMed] [Google Scholar]
- 111. Smith A, Blois J, Yuan H, Aikawa E, Ellson C, Figueiredo JL, Weissleder R, Kohler R, Yaffe MB, Cantley LC, Josephson L. The antiproliferative cytostatic effects of a self-activating viridin prodrug. Mol Cancer Ther 8: 1666–1675, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Solomon SD, McMurray JJ, Pfeffer MA, Wittes J, Fowler R, Finn P, Anderson WF, Zauber A, Hawk E, Bertagnolli M; Adenoma Prevention with Celecoxib (APC) Study Investigators. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med 352: 1071–1080, 2005. [DOI] [PubMed] [Google Scholar]
- 113. Srivastava S, Verma M, Henson DE. Biomarkers for early detection of colon cancer. Clin Cancer Res 7: 1118–1126, 2001. [PubMed] [Google Scholar]
- 114. Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17: 463–516, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJM, Schrama JG, Erdkamp FLG, Vos AH, van Groeningen CJ, Sinnige HAM, Richel DJ, Voest EE, Dijkstra JR, Vink-Börger ME, Antonini NF, Mol L, van Krieken JHJM, Dalesio O, Punt CJA. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med 360: 563–572, 2009. [DOI] [PubMed] [Google Scholar]
- 116. Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA 94: 3336–3340, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Upadhyay R, Sheth RA, Weissleder R, Mahmood U. Quantitative real-time catheter-based fluorescence molecular imaging in mice. Radiology 245: 523–531, 2007. [DOI] [PubMed] [Google Scholar]
- 118. Van Gossum A, Munoz-Navas M, Fernandez-Urien I, Carretero C, Gay G, Delvaux M, Lapalus MG, Ponchon T, Neuhaus H, Philipper M, Costamagna G, Riccioni ME, Spada C, Petruzziello L, Fraser C, Postgate A, Fitzpatrick A, Hagenmuller F, Keuchel M, Schoofs N, Deviere J. Capsule endoscopy versus colonoscopy for the detection of polyps and cancer. N Engl J Med 361: 264–270, 2009. [DOI] [PubMed] [Google Scholar]
- 119. Van Rijn J, Reitsma J, Stoker J, Bossuyt P. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol 101: 343–350, 2006. [DOI] [PubMed] [Google Scholar]
- 120. Wallace MB, Fockens P. Probe-based confocal laser endomicroscopy. Gastroenterology 136: 1509–1513, 2009. [DOI] [PubMed] [Google Scholar]
- 121. Wallace MB, Keisslich R. Advances in endoscopic imaging of colorectal neoplasia. Gastroenterology 138: 2140–2150, 2010. [DOI] [PubMed] [Google Scholar]
- 122. Walsh JME, Terdiman JP. Colorectal cancer screening: scientific review. JAMA 289: 1288–1296, 2003. [DOI] [PubMed] [Google Scholar]
- 123. Wang TD, Friedland S, Sahbaie P, Soetikno R, Hsiung PL, Liu JTC, Crawford JM, Contag CH. Functional imaging of colonic mucosa with a fibered confocal microscope for real-time in vivo pathology. Clin Gastroenterol Hepatol 5: 1300–1305, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Wang Y, Zhao R, Goldman ID. Decreased expression of the reduced folate carrier and folypolyglutamate synthetase is the basis for acquired resistance to the pemetrexed antifolate (LY231514) in an L1210 murine leukemia cell line. Biochem Pharmacol 65: 1163–1170, 2003. [DOI] [PubMed] [Google Scholar]
- 125. Weissleder R. Scaling down imaging: molecular mapping of cancer in mice. Nat Rev Cancer 2: 11–18, 2002. [DOI] [PubMed] [Google Scholar]
- 126. Weissleder R, Mahmood U. Molecular imaging. Radiology 219: 316–333, 2001. [DOI] [PubMed] [Google Scholar]
- 127. Weissleder R, Tung CH, Mahmood U, Bogdanov A. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol 17: 375–378, 1999. [DOI] [PubMed] [Google Scholar]
- 128. Wolpin BM, Mayer RJ. Systemic treatment of colorectal cancer. Gastroenterology 134: 1296–1310, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Wu Y, Cai W, Chen X. Near-infrared fluorescence imaging of tumor integrin alpha v beta 3 expression with Cy7-labeled RGD multimers. Mol Imaging 8: 226–236, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Wunder A, Tung CH, Müller-Ladner U, Weissleder R, Mahmood U. In vivo imaging of protease activity in arthritis: a novel approach for monitoring treatment response. Arthritis Rheum 50: 2459–2465, 2004. [DOI] [PubMed] [Google Scholar]
- 131. Yao VJ, Ozawa MG, Varner AS, Kasman IM, Chanthery YH, Pasqualini R, Arap W, McDonald DM. Antiangiogenic therapy decreases integrin expression in normalized tumor blood vessels. Cancer Res 66: 2639–2649, 2006. [DOI] [PubMed] [Google Scholar]
- 132. Yokota J, Yamamoto T, Toyoshima K, Terada M, Sugimura T, Battifora H, Cline MJ. Amplification of c-erbB-2 oncogene in human adenocarcinomas in vivo. Lancet 1: 765–767, 1986. [DOI] [PubMed] [Google Scholar]
- 133. Zhang H, Morgan D, Cecil G, Burkholder A, Ramocki N, Scull B, Lund PK. Biochromoendoscopy: molecular imaging with capsule endoscopy for detection of adenomas of the GI tract. Gastrointest Endosc 68: 520–527, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


