Abstract
The kinetics of nonesterified fatty acid (NEFA) metabolism in humans requires quantification to facilitate understanding of diseases like type 1 and 2 diabetes, metabolic syndrome, and obesity, and the mechanisms underpinning various interventions. Oral glucose tolerance tests (OGTT) and glucose meal tolerance tests (MTT) are potentially useful procedures for enabling quantification of NEFA kinetics because they both cause transitory, but substantial, declines and then rebounds in plasma NEFA concentrations in response to physiologically relevant increases in plasma glucose. The Boston MINIMAL model of NEFA kinetics was developed to analyze data from the intravenous glucose tolerance test (IVGTT), but in this work, we present for the first time its application to modeling NEFA data from both OGTT and MTT studies. This model enables estimation of SFFA (μmol·l−1·min−1) (a parameter describing the maximum rate of lipolysis), and KFFA (%/min) (a parameter related to NEFA oxidation rate). The model could well describe the trajectories of NEFA concentrations following an OGTT (R2 in excess of 0.97) but was not as successful with the MTT (R2 > 0.65). Model parameters derived from analysis of OGTT and MTT data were well identified with coefficients of variation generally less than 15%. Type 2 diabetes, body mass index, and dietary treatment (high-fat vs. high-glycemic-index diets) were all shown to have significant effects on model parameters. Modeling plasma NEFA concentrations over 24 h has helped to identify and quantify the extent that periprandial NEFA peaks and nocturnal elevation in plasma NEFA can be accounted for by our model.
Keywords: NEFA kinetics, lipolysis, fatty acid oxidation
a simple, accurate and inexpensive technique is required to quantify lipid metabolism (rates of lipolysis of triacylglycerol in adipose tissue and oxidation of fatty acids) to facilitate advancement in our understanding of pathological conditions such as obesity, dislipidemia, diabetes mellitus, hypertension, and coronary heart disease (3, 4, 7, 19, 25, 29, 32). Recently, a novel model has been presented to describe plasma nonesterified fatty acid (NEFA) kinetics in humans apropos of the intravenous glucose tolerance test (IVGTT) (9). This model (henceforth called the Boston Complete NEFA model) is novel in that it utilizes plasma glucose concentrations as the principal driver that influences NEFA kinetics.
Briefly, a linear interpolation of plasma glucose concentrations provides a time-delayed input function to a “remote” glucose compartment. Elevation of glucose concentrations in the remote compartment causes a Michaelis-Menten type of inhibition of lipolysis that results in a decline in the rate of input of NEFA to the plasma compartment. Output of NEFA from the plasma compartment is described by a first-order rate constant. This new NEFA model has been successfully used to describe the pattern of plasma NEFA concentrations in humans during either a glucose-only intravenous glucose tolerance test (GO-FSIGT), an insulin-modified intravenous tolerance test (IM-FSIGT), or a modified protocol (IM-FSIGT-CLAMP) during which variable-rate glucose infusions were administered to prevent plasma glucose from declining below 100 mg/dl.
A slight variation of this model (the Boston MINIMAL NEFA model) has also been used to model plasma NEFA concentrations following a standard FSIGT in lactating dairy cows (10). The Boston Complete NEFA model differs from the MINIMAL model in that the Complete Model contains one additional parameter, an explicit time delay (τ, min), which partly accounts for the delay between the time when plasma glucose concentrations may rise (due to an IVGTT) and the time when plasma NEFA concentrations begin their precipitous decline.
The IVGTT necessitates catheterization of at least one antecubital vein, and the standard protocols for IVGTTs whether the traditional frequently sampled intravenous glucose test (FSIGT) or the insulin-modified frequently sampled intravenous glucose test (IM-FSIGT) involve intravenous infusion over ∼1 min of a hypertonic glucose solution (typically, 0.3 g glucose/kg body weight), and the collection of between 24 and 30 blood samples over the subsequent 3 to 4 h (35). It has been argued that this procedure results in a “nonphysiological milieu” since the rapid glucose and insulin perturbations of an IVGTT do not reflect the conditions of daily living (15). Indeed, from the preinfusion basal glucose concentration of ∼4 mM, a sharp peak in plasma glucose follows at about 2–3 min postinfusion, with plasma glucose concentrations increasing to between 9 and 25 mM. Similarly, the concentration of insulin in plasma may increase from a basal concentration of ∼5–15 ng/ml to a peak at between 4 and 10 min postinfusion of between 50 and 200 ng/ml (35). Elevated plasma concentrations of insulin may lead to substantial reduction in hepatic glucose production (14, 22), and this may result in plasma glucose concentrations declining to hypobasal levels at about 60 min after the glucose injection (11, 35). Because of these substantial changes in plasma glucose concentrations, it has been suggested that it is inappropriate or even dangerous to perform the IVGTT on diabetic subjects (15).
In contrast to the IVGTT, the commonly employed oral glucose tolerance test (OGTT) involves the subject consuming (over a 5-min period) 75 g of a glucose solution. Because glucose must pass out of the stomach before it is absorbed into the blood from the small intestine, the subsequent peaks in plasma glucose and insulin are generally smoother and more undulating, less pronounced, and somewhat delayed compared with those observed during an IVGTT. Indeed, following an oral glucose challenge, the peak glucose concentrations are typically between 7 and 9 mM, and this occurs between 30 and 60 min postchallenge (33, 34). Similarly, the peak insulin concentration following an OGTT is generally between 30 and 70 ng/ml, and this occurs between 30 and 120 min postchallenge (23).
In comparison to an IVGTT, the glucose and insulin responses to an OGTT are less pronounced and somewhat delayed. Furthermore, plasma responses of glucose, insulin, and NEFA from an OGTT more closely mimic those obtained from a meal tolerance test (MTT), and because both OGTT and MTT are less dangerous than IVGTT when performed on diabetic patients, they may be the preferred diagnostic tests for these patients. Therefore, the primary aim of the work described here was to determine whether the glucose responses from either an OGTT or a MTT could be used as drivers of the Boston Complete NEFA model or the Boston Minimal NEFA model. The OGTT and MTT typically involve far fewer samples than the IVGTT, and sampling tends to be much more dispersed, with intersample intervals in some protocols being in excess of 30 min (30, 38). Thus, in this investigation, the outcome is influenced not just by the difference in the manner of administering the glucose challenge, but also by differences in the number of plasma samples and the intersample intervals employed in each protocol.
MATERIALS AND METHODS
NEFA model.
Both the Boston Complete and the Boston MINIMAL models of NEFA kinetics were used in the studies reported here. A brief exposition of the Boston MINIMAL NEFA model is provided in the appendix.
Experimental data.
The data used to test the NEFA model came from four previously reported experiments involving a diverse range of subject types, experimental protocols, and treatments that all relate to normal or disordered fatty acid metabolism (Table 1). Data sets from metabolic challenges, from which there are only small numbers of plasma data, infrequent plasma sampling, or sampling terminated prematurely before a complete metabolic response, are of limited use for modeling purposes because they do not facilitate parameter identification. Of all of the OGTT and MTT studies that we could find in the scientific literature, the four studies listed in Table 1 were chosen because they used blood sampling regimens with a substantial number of samples, relatively high frequency of sampling, and sampling extended for at least 180 min. In fact, studies 1, 2, 3, and 4 reported plasma glucose and NEFA data from 15, 11, 19, and 33 sampling times, respectively. Furthermore, study 2 reported plasma data at 10-min intervals, studies 1 and 3 at 15-min intervals and study 4 at 30-min intervals. In contrast, many other OGTT and MTT studies in the scientific literature report data from seven or fewer sampling times and intersampling intervals greater than 30 min.
Table 1.
Source of data, description of subjects, and experimental protocols
| Study | Reference Number | Gender | n | Age, yr | BMI, kg/m2 | Protocol | Treatment/Demographic |
|---|---|---|---|---|---|---|---|
| 1 | 5 | Men | 8 | 32.4 (9) | 23.9 (0.7) | OGTT | Control |
| 1 | 5 | Men | 8 | 32.4 (9) | 23.9 (0.9) | OGTT | HDBR |
| 1 | 5 | Women | 8 | 27.9 (0.9) | 20.9 (0.6) | OGTT | Control |
| 1 | 5 | Women | 8 | 27.9 (0.9) | 20.8 (0.6) | OGTT | HDBR |
| 2 | 38 | NS | 6 | 25.5 (0.8) | 23.2 (1.1) | OGTT | Normal |
| 2 | 38 | NS | 8 | 33.6 (3.4) | 39.9 (3.8) | OGTT | Obese |
| 2 | 38 | NS | 5 | 36.4 (3.0) | 40.9 (2.6) | OGTT | Obese/Type 2 Diabetic |
| 3 | 12 | Men | 17 | 45 (8) | 29.3±(4.0) | Meal Test | High Fat |
| 3 | 12 | Men | 17 | 45 (8) | 29.3±(4.0) | Meal Test | High Glycaemic |
| 4 | 37 | Women | 12 | 25 (2) | 23.2±(0.6) | Meal Test | High Glucose |
| 4 | 37 | Women | 12 | 25±(2) | 23.2±(0.6) | Meal Test | High Fructose |
Values are means (SD); BMI, body mass index; OGTT, oral glucose tolerance test; HDBR, head-down bed-rest; n, number of subjects in study; NS, sex not specified.
Study 1 (OGTT).
Briefly, the experiment by Blanc and colleagues (5) involved eight men and eight women, all in good general health and without a family history of diabetes. All of these subjects underwent an OGTT during a control state, and again during a 7-day period, during which the subjects experienced head-down bed rest (HDBR). HDBR is a model of physical inactivity that has been used to mimic exposure to weightlessness (6). During HDBR, volunteers are confined to bed in a head-down position of −6° for variable durations. HDBR induces the headward shift of fluids, and the hypokinesia and hypodynamia that typically occur during space flight (6). However, there have been conflicting reports on the effects of HDBR on fat metabolism as gauged by changes in body fat mass (6, 18, 26). Blood samples were collected from each subject at ∼0, 15, 30, 45, 60, 75, 90, 105. 120, 150, 180, 210, 240, 270, and 300 min relative to the start of the oral glucose challenge. The glucose data shown in the original Fig. 2 from Ref. 5 and the NEFA data shown in original Fig. 3 from Ref. 5 were used in this modeling.
Fig. 2.
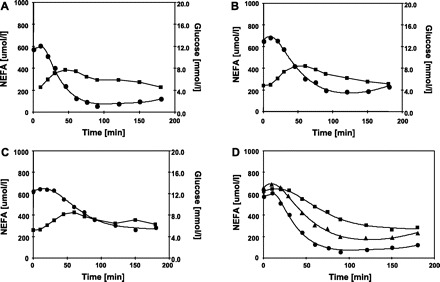
Time course of plasma glucose (▪) and NEFA (•) during oral glucose tolerance tests (OGTT) in study 2. The OGTT were performed in normal subjects (A), obese subjects (B), and obese subjects with type-2 diabetes (C). D: NEFA responses from normal subjects (•), obese subjects (▴), and obese subjects with type 2 diabetes (▪) are shown on the one graph to facilitate comparison. The data shown here were electronically digitized from graphs published in the scientific literature. The glucose data were obtained from the original Fig. 2, A and B from Ref. 38, and the NEFA data shown were obtained from original Fig. 5, A and B from Ref. 38. The lines connecting the NEFA data are model predictions.
Study 2 (OGTT).
The experiment by Volpicelli et al. (38) involved three groups of subjects: normal, obese, and obese-Type 2 diabetic patients (Table 1). All subjects underwent an OGTT, and blood samples were collected at times −20, −10, −5, 0, 10, 20, 30, 45, 60, 75, 90, 120, 150, and 180 min. The glucose data shown in the original Fig. 2, A and B from Volpicelli et al. (38) and the NEFA data shown in original Fig. 5, A and B (38) were used in this modeling.
Study 3 (MTT).
The study by Brynes et al. (12) involved subjects exposed to four dietary treatments in a randomized Latin Square crossover design. In this modeling analysis, we consider just the two most dissimilar dietary treatments: a high-fat diet (HIGH-FAT) and a high-carbohydrate high-glycemic index diet (HIGH-GI). All subjects had normal meals, and frequent blood samples were collected throughout the day. A breakfast meal was given at 15 min (0900), lunch at 195 min (1200), and afternoon tea at 375 min (1500). Blood samples were collected at 0 15, 30, 45, 60, 75, 135, 195, 210, 225, 240, 255, 375, 390, 405, 420, 435, and 495 min. The glucose data shown in the original Fig. 1A from (12) and the NEFA data shown in original Fig. 4A from (12) were used in this modeling.
Fig. 1.
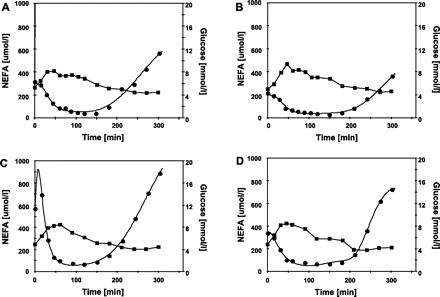
Time course of plasma glucose (▪) and NEFA (•) during oral glucose tolerance tests (OGTT) in study 1. The OGTT were performed in men and women during control period and during a period of head-down bed-rest (HDBR). A: men, control. B: men, HDBR. C: women, control. D: women, HDBR. The data shown here were electronically digitized from graphs published in the scientific literature. The glucose data were obtained from the original Fig. 2 from Ref. 5 and the NEFA data from the original Fig. 3 from Ref. 5. The lines connecting the NEFA data are model predictions.
Study 4 (MTT).
The study by Teff et al. (37) involved women subjects exposed to a diet containing either glucose-sweetened beverages (HIGH-GLUC) or fructose-sweetened beverages (HIGH-FRUCT). All subjects had normal meals, and blood samples were collected frequently throughout the day. A breakfast meal was given at 60 min (0900), lunch at 195 min (1300), and dinner at 375 min (1800). Blood samples were collected at 0, 30, 60, 90, 120, 150, 180, 240, 300, 336, 360, 390, 420, 480, 540, 600, 634, 660, 690, 720, 780, 840, 900, 937, 960, 990, 1,020, 1,050, 1,090, 1,110, 1,140, 1,200, 1,260, 1,320, 1,380, and 1,440 min. The glucose data shown in the original Fig. 1 from Ref. 37 and the NEFA data shown in original Fig. 8 from Ref. 37 were used in this modeling.
All OGTT and MTT were conducted after a standard overnight (12 h) fast. For each experiment, the relevant figure was electronically scanned, and the data were digitally extracted by means of UN-SCAN-IT, ver. 5.0 software (Silk Scientific, Orem UT).
Implementation and analyses.
The NEFA model was implemented using WinSAAM (36) (available from http://www.winsaam.org), and the model was fitted to the NEFA and glucose data, as described previously (9, 10). Our original intention was to focus on the Complete Boston model of NEFA kinetics (9), which was developed using intensively sampled data from the IVGTT in humans. We wanted to determine whether it could be employed to model NEFA data apropos of the OGTT and the MTT. As previously extensively discussed (9), the Complete Boston NEFA model contains a number of parameters related to the initial conditions of compartments and the latency or delay period that occurs after a spike in plasma glucose concentrations until plasma NEFA concentrations begin a precipitous decline. These parameters are R0 (mmol/l), a parameter that describes the initial conditions of a glucose compartment remote from plasma, gs (mmol/l), a threshold glucose concentration, kc (min-1), a first-order rate constant describing the movement of plasma glucose into a remote compartment, τ (min), an explicit time delay. Our preliminary investigations revealed that when sparsely sampled data sets were being used for modeling, these particular parameters could not necessarily all be identified. All of the data sets from the studies listed in Table 1 contained only 6 or fewer data within the 1st h of sampling. Accordingly, in this investigation, to facilitate parameter estimation and identification, the MINIMAL Boston NEFA model (10), which does not contain the explicit time delay parameter, was used to model the OGTT and MTT data. Even when the MINIMAL Boston model of NEFA kinetics is employed for modeling purposes, sparse data during the initial 30 min sometimes makes estimation of R0 problematic. In these circumstances, if the estimated CV of R0 was greater than 50%, then R0 was fixed at 0, and resulting estimates of other model parameters are conditional on this setting.
For study 2, t-tests of means were conducted, and statistical differences were declared if P < 0.05.
RESULTS AND DISCUSSION
Figures 1–3 show mean plasma concentrations of glucose and NEFA obtained from the publications (5, 12 37, 38) and model fits of plasma NEFA concentrations.
Fig. 3.
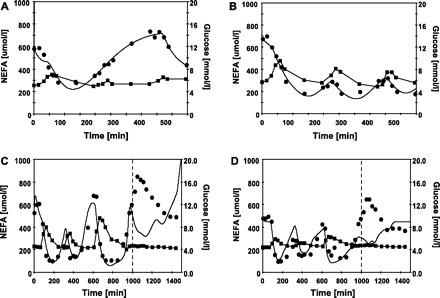
Time course of plasma glucose (▪) and NEFA (•) data during meal tolerance tests from study 3 (A and B) and study 4 (C and D). The solid line for NEFA are the model fits. The data shown in A came from subjects on a high-fat diet while data in B came from subjects on a high glycemic diet. The data in C came from subjects receiving high glucose beverages, while data in D came from subjects receiving high-fructose beverages. In C and D, NEFA data between 1,000 min and 1,500 min were weighted out of the modeling and hence did not contribute to model predictions. The data shown here were electronically digitized from graphs published in the scientific literature. The glucose data shown here in Fig 3, A and B, were obtained from the original Fig. 1A from Ref. 12, and the NEFA data were obtained from the original Fig. 4A from Ref. 12. The glucose data shown here in Fig 3, C and D, were obtained from the original Fig. 1 from Ref. 37 and the NEFA data from the original Fig. 8 from Ref. 37. The lines connecting the NEFA data are model predictions.
The model fits of NEFA for studies 1 and 2, shown in Figs. 1 and 2, depict how well the Boston NEFA model can describe the NEFA response following an OGTT. In all cases, there were generally no marked systematic deviations of model fits from the data, and the adjusted R2 values were all in excess of 0.98. The only feature of concern, which is evident in Fig. 1C, is a predicted peak in plasma NEFA concentrations of ∼925 μmol/l at 8 min. As can be seen in Fig. 1C, the first sampling time was at 0 min, and the second at ∼15 min, so it really is open to conjecture what the real plasma NEFA concentration was at 8 min. Nevertheless, if OGTT is to be used for NEFA modeling, this feature demonstrates the need for frequent blood sampling, especially during the first 30 min. In studies 1 and 2, the parameters of the NEFA model were generally all well identified, with the only exception being parameter kc for obese and obese-type 2 diabetic subjects in study 2.
Fig. 3, A and B show data and model fits of plasma NEFA for the subjects in study 3 (MTT), the HIGH-FAT, and HIGH-GI diets, respectively. As in studies 1 and 2, the MINMAL NEFA model was able to well describe the entire trajectory of plasma NEFA concentrations, with no major systematic deviations, but the adjusted R2 values were 0.91 for the HIGH-FAT treatment and 0.97 for the HIGH-GI treatment. In this longer-term study, the trajectories of the actual and fitted plasma NEFA concentrations were more complex than in the earlier studies, since they reflected to some degree, the fluctuating plasma glucose concentrations resulting from the intermittent effects of absorption of dietary glucose from the breakfast, luncheon, and afternoon tea.
Fig. 3, C and D shows the data and model fits of plasma NEFA for the subjects in study 4 (MTT), given the HIGH-GLUC and HIGH-FRUCT treatments, respectively. Study 4 covered a 24-h period from 8:00 AM to 8:00 AM the next day. It can be seen in Fig. 3, C and D that plasma NEFA concentrations fluctuated in response to the breakfast-, lunch-, and dinner-induced fluctuations in plasma glucose concentrations. The MINIMAL NEFA model well described the plasma NEFA concentrations between 0 min (8:00 AM) and 1,000 min (12:00 midnight), with only small discrepancies occurring between the observed and fitted NEFA concentrations. Considering just the period between 0 and 1,000 min, the overall adjusted R2 was 0.85 for the HIGH-GLUC treatment and 0.61 for the HIGH-FRUC treatment. These R2 values are substantially less than the R2 values obtained previously with IVGTT data (9) and less than the R2 values obtained with the OGTT data from studies 1 and 2. We speculate that because the meal study tests were of such long duration, a number of uncontrolled and unmodeled factors may have been responsible for the discrepancies between data and model fits. We speculate that fat absorbed from the diet may be a contributory factor to the high NEFA peaks that occur at meal times (Fig. 3, C and D). Another unmodeled factor with regard to the NEFA response to a meal tolerance test is the possible involvement of counter-regulatory hormonal effects. These may include epinephrine “spikes” associated with meal anticipation (2), cephalic responses initiated by the thought, sight, smell, and taste of food (31), as well as the influence of meal stimulation on gut peptides (17). Absorption of protein from a meal test could also be expected to lead to an amino acid-stimulated increase in plasma insulin (20, 27), and this too could cause a decrease in plasma NEFA, independent of plasma glucose. Therefore, we consider that although the current NEFA minimal model does a reasonable job at describing plasma NEFA concentrations in response to a meal test, further improvements/additions to the model may be possible to make it more appropriate for modeling NEFA data from meal tolerance tests.
The model predictions between 1,000 min and 1,300 min were poor because the Boston model was unable to predict a high peak in NEFA concentrations that occurred between 1:00AM and 2:00 AM in both the HIGH-GLUC and HIGH-FRUCT treatments. We were unable to adequately model NEFA concentrations during this period. Accordingly, when fitting the NEFA MINIMAL model to the data from study 4, the plasma glucose and NEFA data from 0 to 1,000 min were weighted to the analysis used to estimate model parameters (i.e., the data between 1,000 and 1,500 min were weighted 0). Thus, only data from 0 to 1,000 min were used for parameter estimation, and the resulting parameters were used to make the intrasampling interval predictions on plasma NEFA from 0 to 1,000 min, and the out-of-sample predictions between 1,001 and 1,500 min shown in Fig. 3, C and D.
As can be seen in Table 3, all adjustable parameters were well identified with the exception of parameters R0 for subjects in the HIGH-GI treatment in study 3 and for parameter R0 in study 4.
Table 3.
NEFA model parameters and indices obtained by analysis of data extracted from two published studies involving meal tolerance tests
| Study 3, HIGH-FAT | Study 3, HIGH-GI | Study 4, HIGH-GLUC | Study 4, HIGH-FRUCT | |
|---|---|---|---|---|
| Parameters | ||||
| Gb, mmol/l | 5.09 | 5.66 | 4.56 | 4.42 |
| NEFA0, μmol/l | 581.9 | 678.5 | 529.0 | 478.4 |
| R0, mmol/l | 0.13 (0.01) | 0 | 0 | 0 |
| kC, %/min | 1.71 (0.01) | 5.44 (0.41) | 5.30 (0.15) | 3.92 (0.74) |
| SFFA, μmol·l−1·min−1 | 20.2 (3.1) | 6.6 (0.3) | 70.1 (15.3) | 59.9 (20.9) |
| KFFA, %/min | 2.70 (0.39) | 1.39 (0.01) | 2.27 (0.03) | 12.1 (4.25) |
| gs, mmol/l | 6.12 (0.01) | 6.58 (0.12) | 4.20 (0.01) | 4.53 (0.01) |
| Φ, mmol/l | 0.10 (0.01) | 0.05 (0.02) | 0.05 (0.01) | 0.33 (0.01) |
| Indices | ||||
| LIP0, μmol·l−1·min−1 | 9.0 (1.74) | 6.6 (0.3) | 70.1 (15.3) | 59.9 (20.9) |
| OX0, μmol·l−1·min−1 | 15.7 (2.3) | 9.5 (0.3) | 12.0 (0.2) | 57.7 (20.3) |
| NTLR0, μmol·l−1·min−1 | −6.7 (2.4) | −3.1 (0.04) | 58.1 (15.3) | 2.2 (1.0) |
| NEFAmin, μmol/l | 219.8 (5.0) | 158.5 (4.3) | 96.4 (2.4) | 125.6 (3.3) |
| Tmin, min | 127 (3) | 151 (4.1) | 723 (3.0) | 784 (2.7) |
| Suppression, % | 62.2 (1.4) | 76.6 (2.1) | 81.8 (2.0) | 78.0 (2.0) |
| n | 19 | 20 | 26 | 26 |
| RMSE | 42.3 | 31.7 | 74.3 | 75.3 |
| Adjusted R2 | 0.91 | 0.97 | 0.85 | 0.61 |
Values are expressed as means (SD). HIGH-FAT, high fat diet; HIGH-GI, high glycaemic index diet; HIGH-GLUC, diet supplemented with a high glucose beverage; HIGH-FRUCT, diet supplemented with high fructose beverages. In study 4, for high glucose and high fructose studies, parameters and indices of goodness of fit relate to data between 0 and 1,000 min.
The results of analyses presented in Tables 2 and 3 and Figs. 1, 2, and 3 provide compelling evidence that the Boston MINIMAL model can, in fact, accurately describe NEFA kinetics apropos of the OGTT and MTT in humans. The MINIMAL NEFA model could describe the complete 300 min (study 1) and 180 min (study 2) trajectories of plasma NEFA concentrations following OGTT. It could also describe the complete 500 min (study 3) trajectory of plasma NEFA following a MTT and could also describe the trajectory of plasma NEFA for 1,000 min in another study (study 4).
Table 2.
NEFA model parameters and indices obtained by analysis of data extracted from two published studies involving oral glucose tolerance tests
| Study 1, M | Study 1, M-HDBR | Study 1, W | Study 1, W-HDBR | Study 2, N | Study 2, O | Study 2, O_T2D | |
|---|---|---|---|---|---|---|---|
| Parameters | |||||||
| Gb, mmol/l | 5.26 | 4.98 | 4.88 | 4.78 | 4.45 | 4.96 | 4.84 |
| NEFA0, μmol/l | 316.2 | 212.4 | 568.8 | 336.6 | 573.5 | 652.4 | 624.2 |
| R0, mmol/l | 0.235 (0.037) | 0.405 (0.036) | 0 | 0 | 0 | 0 | 0 |
| kC, %/min | 2.31 (0.20) | 2.54 (0.29) | 2.31 (0.05) | 6.34 (0.49) | 6.20 (2.11)b | 7.47 (4.28)b | 3.21 (2.42)a |
| SFFA, μmol·l−1·min−1 | 55.6 (13.1) | 45.7 (11.8) | 161 (18) | 36.9 (3.6) | 29.6 (3.0)b | 28.7 (4.2)b | 15.6 (5.3)a |
| KFFA, %/min | 7.39 (1.37) | 7.81 (1.29) | 12.6 (1.21) | 4.83 (0.48) | 4.67 (0.53)c | 2.71 (0.49)b | 2.00 (0.83)a |
| gs, mmol/l | 5.72 (0.27) | 5.20 (0.15) | 5.03 (0.06) | 4.16 (0.16) | 4.32 (0.16)a | 4.57 (0.29)b | 4.78 (0.45)b |
| Φ, mmol/l | 0.12 (0.03) | 0.15 (0.03) | 0.11 (0.01) | 0.20 (0.03) | 0.25 (0.05)a | 0.41 (0.18)b | 1.05 (0.41)c |
| Indices | |||||||
| LIP0, μmol·l−1·min−1 | 18.5 (4.5) | 12.6 (2.4) | 161 (18) | 36.9 (3.6) | 29.6 (3.0)b | 28.7 (4.2)b | 15.5 (5.3)a |
| OX0, μmol·l−1·min−1 | 23.4 (4.3) | 16.6 (2.7) | 71.5 (6.9) | 16.2 (1.6) | 26.8 (3.0)c | 17.7 (3.2)b | 12.5 (5.2)a |
| NTLR0, μmol·l−1·min−1 | −4.9 (14.2) | −4.0 (3.2) | 89.2 (11.8) | 20.7 (2.1) | 2.9 (0.8)a | 11.0 (4.1)b | 3.0 (1.4)a |
| NEFAmin, μmol/l | 53.4 (4.2) | 33.9 (1.9) | 58.0 (4.2) | 45.6 (3.9) | 75.1 (3.3)a | 171.5 (5.6)b | 271.9 (8.3)c |
| Tmin, min−1 | 112 (8.9) | 106 (5.9) | 93 (6.7) | 104 (8.8) | 101 (4.4)a | 124 (4.1)b | 180 (5.4)c |
| Suppression, % | 83.1 (6.6) | 84.0 (4.6) | 89.8 (6.5) | 86.5 (7.3) | 86.9 (3.8)c | 73.7 (2.4)b | 56.4±1.7a |
| n | 15 | 15 | 13 | 13 | 11 | 11 | 11 |
| RMSE | 24.8 | 11.8 | 12.4 | 16.5 | 16.6 | 15.1 | 13.0 |
| Adjusted R2 | 0.98 | 0.98 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 |
Values are expressed as means (SD). M, men; W, women; HDBR, head-down bed-rest; N, normal subjects; O, obese subjects. O-T2D, obese subjects with type-2 diabetes; Gb, initial or basal glucose concentration; NEFA0, initial or basal nonesterified fatty acid concentration; R0, initial concentration of glucose in a remote compartment; kc, rate constant describing the movement of plasma glucose into the remote compartment and the clearance of glucose from the remote compartment; SFFA, rate of provision of NEFA to the plasma pool; KFFA, rate constant that describes the rate at which NEFA leaves the plasma pool; gs, a threshold or set point in plasma glucose concentration above which elevated levels of plasma glucose result after a delay of τ (min), in entry of plasma glucose into the remote compartment; Φ, adjustable Michaelis Menten-type affinity constant; LIP0, the rate of lipolysis at time 0; OX0, the rate of oxidation of NEFA at time 0; NTLR0, net transient lipolytic rate at time 0; Tmin, time of nadir in NEFA concentrations; Suppression %, an index providing a measure of the flexibility of the system to alternate between using glucose or NEFA as an energy source; n, the number of subjects in each study; RMSE, root mean square error. For study 2, means followed by different superscripts differ significantly, P < 0.05.
Each of the specific model parameters obtained for both men and women of different demographic classes undergoing a wide variety of treatments and obtained during either the OGTT or the MTT were consistent and plausible. Furthermore, as can be seen in Table 4, parameter estimates and indices obtained from modeling OGTT and MTT data were consistent in magnitude with corresponding estimates obtained from the Complete Boston model applied to IVGTT data from normal healthy human subjects (9). However, as can also be seen from Table 4, the ranges for the important parameters kc, SFFA (a parameter describing the maximum rate of lipolysis), and KFFA (a parameter related to NEFA oxidation rate) were all greater in the studies involving the OGTT and MTT protocols. Furthermore, the overall errors of fitting (RMSE) were somewhat greater for the OGTT and MTT protocols, and the R2 values were slightly less than those obtained from IVGTT studies. From this investigation, it is not possible to determine whether it was the mode of glucose administration or the number and temporal distribution of plasma samples that caused these slight differences. Further studies may be warranted on the effect of different sampling regimens (increased numbers of samples, reduced intersample interval, and time of the final sample) on the accuracy of NEFA model parameter estimation from OGTT and MTT investigations.
Table 4.
Comparison of NEFA model parameters and indices obtained by analysis of data from IVGTT, OGTT, and meal tolerance tests in normal human subjects
| IVGTT, Boston and Moate, 2008 | OGTT, This Analysis | MT, This Analysis | |
|---|---|---|---|
| Parameters | |||
| Gb, mmol/l | 4.83–4.89 | 4.45–5.26 | 4.42–5.66 |
| NEFA0, μmol/l | 415–570 | 212.4–573.5 | 478.4–678.5 |
| R0, mmol/l | 0.05–0.57 | 0–0.41 | 0–0.13 |
| kC, %/min | 2.25–3.59 | 2.31–6.34 | 1.71–5.44 |
| SFFA, μmol·l−1·min−1 | 40.3–64.7 | 29.6–161 | 6.6–70.1 |
| KFFA, %/min | 5.4–12.9 | 4.7–12.6 | 1.39–12.1 |
| gs, mmol/l | 4.3–5.7 | 4.2–5.7 | 4.2–6.6 |
| Φ, mmol/l | 0.26–0.81 | 0.11–0.25 | 0.05–0.33 |
| Indices | |||
| LIP0, μmol·l−1·min−1 | 24.2–36.4 | 12.6–161 | 6.6–70.1 |
| OX0, μmol·l−1·min−1 | 24.7–53.6 | 16.2–71.5 | 9.5–57.7 |
| NTLR0, μmol·l−1·min−1 | −18.2–1.7 | −4.9–89.2 | −6.7–58.1 |
| NEFAmin, μmol/l | 54.1–179.3 | 33.9–75.1 | 96.4–219.8 |
| Tmin, min−1 | 46–73 | 93–112 | 127–784 |
| Suppression, % | 60.5–90.6 | 83.1–89.8 | 62.2–81.8 |
| RMSE | <12 | 11.8–24.8 | 31.7–75.3 |
| Adjusted R2 | ≥0.99 | 0.98–0.99 | 0.61–0.97 |
Values are expressed as ranges. IVGTT, intravenous glucose tolerance test.
The usefulness of a model depends not just on its ability to accurately describe the plasma trajectory of the entity in question but also on its ability to identify hitherto unrecognized or unexpected attributes of the system in question. We are hesitant to make an extensive list of statements about the many parameters listed in Tables 2 and 3, with apparently statistically different magnitudes, because many study-specific factors could have caused these differences. However, there are a number of substantial and intriguing within-study differences that we cannot overlook without comment.
In study 1, both SFFA and KFFA appear to be substantially different between the control men and control women. Thus, from Table 2, lipolysis and oxidation were substantially greater in women than in men. This finding supports the previously made conclusion that there is a sexual dimorphism in basal rates of fat oxidation (28). The actual NEFA data graphically presented in Fig. 2D show the similarities and differences in the plasma NEFA response to an OGTT in normal, obese, and obese subjects with Type 2 diabetes, and, in particular, qualitatively show that obese subjects appear to be on the pathway toward Type 2 diabetes. Moreover, Fig. 2D demonstrates convincingly that the Boston MINIMAL NEFA model is sufficiently robust to be able to describe the NEFA response in these metabolically diverse populations.
From Table 2, it can be seen that the progression from normal health to obesity and then Type 2 diabetes, is associated with substantial and significant (P < 0.05) changes, not just in a single parameter of the Boston MINIMAL Model, but progressive changes in most model parameters. In study 2, obese Type 2 diabetic subjects have a substantially smaller SFFA compared with normal or obese subjects. KFFA declines progressively from normal health to obesity and obesity with Type 2 diabetes. In contrast, there is a strong progressive increase in Φ from normal health to obesity and Type 2 diabetes. Substantial, statistically significant (P < 0.05), and progressive changes are also evident in the indices for study 2, presented in Table 2. These findings with respect to the KFFA and Φ, in terms of directional trends between normal and obese subjects, mirror our previously reported findings from Boston Complete model analysis of IVGTT data from normal and high body mass index (BMI) subjects (9).
The HIGH-GI diet in study 3 is associated with a lower SFFA, LIP0, and OX0 than the HIGH-FAT diet.
The nocturnal peak in plasma NEFA observed in study 4 is quite intriguing. Recently, it has been shown in dogs that nocturnal plasma free fatty acid concentrations are elevated in the longitudinal development of diet-induced insulin resistance (24). The subjects in study 4 were young and healthy, and they had a relatively low BMI (Table 1) and can therefore be expected not to be candidates for the development of diet-induced insulin resistance. As can be seen in Fig. 3, C and D, in study 4, plasma glucose concentrations were quite constant during the period from 1,000 min (12:00 AM) to 1,300 min (5:00 AM). There is also evidence demonstrating that during this same period, insulin concentrations remained at a constant basal concentration (37). These observations together with the failure of the Boston Minimal Model to accurately describe the peak in plasma NEFA concentrations between 1,000 min and 1,300 min in study 4 can be taken as an indication that an unmodeled force was influencing plasma NEFA concentrations during this period. Moreover, we postulate that the magnitude of the discrepancies between observations and model fits (shown in Fig. 3, C and D) must reflect the magnitude of the putative force. Because the period between 12:00 midnight and 5:00 AM is a period normally involving sleep and darkness, it is tempting to speculate that the unmodeled force may somehow be related to these factors. The latter possibility is supported by the observation that in goats, variations and rhythmicity in plasma NEFA concentrations depend upon lighting conditions (1). There are other possible explanations. The authors of study 4 suggest a relationship between temporal patterns of changes in plasma NEFA and plasma ghrelin concentrations (37). Insulin sensitivity with respect to glucose metabolism has been reported to decrease between midnight and 6:00 A.M., and this decrease in insulin sensitivity has been correlated with an increase in plasma free fatty acid concentrations (8). Another possibility is growth hormone, because physiological increases in growth hormone have been shown to have potent lipolytic effects (13), and growth hormone is known to spike at night during sleep and to be highly correlated with lipolysis (21).
Perspectives and Significance
The analyses presented here demonstrate that even though the Boston MINIMAL model of NEFA kinetics was developed principally to describe the plasma NEFA concentrations apropos of the IVGTT, it can also be used to model plasma NEFA concentrations apropos of the OGTT and MTT. An important finding of this work is the demonstration that the Boston MINIMAL model of NEFA kinetics is sufficiently robust to be able to describe NEFA profiles following an OGTT in normal, obese, and obese subjects with Type 2 diabetes and that it provides estimates of model parameters that are systematically different between normal, obese, and obese subjects with Type 2 diabetes. This finding supports the idea that this model may have potential uses in the diagnosis of diabetes and in research aiming to quantify the progression of the diabetic syndrome or in research aiming to quantify how therapeutic treatments might ameliorate this syndrome. The IVGTT, OGTT, and MTT protocols all involve fairly frequent sampling of blood, and, hence, necessitate cannulation of a major blood vessel. The IVGTT can be conducted over a period of just 3 to 4 h, but in order for OGTT and MTT to reveal the reduction in plasma NEFA concentrations to a nadir and the subsequent rebound in plasma NEFA concentrations to potentially suprabasal concentrations, the OGTT and MTT must be conducted over a period of at least 5 h if not more. A complete NEFA response with a decline in plasma NEFA concentrations, nadir, and a substantial amount of the rebound phase appears necessary if all model parameters are to be adequately estimated and identified. Thus, for modeling purposes, the IVGTT with numerous frequent blood samples appears preferable to the longer-duration and typically sparsely sampled OGTT and MTT. Nevertheless, there are sometimes sound experimental and clinical reasons for conducting OGTT or MTT, and the work presented here demonstrates that these protocols can provide data amenable to modeling and suitable for enabling estimation of parameters related to the rates of lipolysis and oxidation of NEFA.
APPENDIX
The MINIMAL model of NEFA kinetics employed in these investigations has previously been described in detail (10) and is encapsulated in the following differential and ancillary equations.
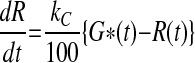 |
(A1) |
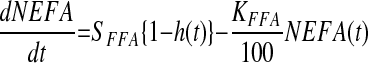 |
(A2) |
where G(0) = Gb R(0) = R0 NEFA(0)= NEFA0
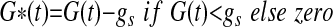 |
(A3) |
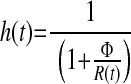 |
(A4) |
where t represents the time in minutes after an intravenous injection of glucose; G(t) (mmol/l) is a function describing the plasma glucose concentration and it is obtained by linear interpolation of the plasma glucose data; Gb (mmol/l) is the initial or basal glucose concentration, gs (mmol/l) is a parameter that defines a threshold or “set-point” in plasma glucose concentration, above which elevated levels of plasma glucose result in entry of plasma glucose into a “remote” or inaccessible compartment denoted by R(t) (mmol/l). In this model, R(t) is the principal driver of NEFA concentrations. The rate constant kC (%/min), describes the movement of plasma glucose (above gs) into the remote compartment and also describes the clearance of glucose from the remote compartment. NEFA (t) represents the plasma NEFA concentration (μmol/l) at time t. The initial NEFA concentration, NEFA0 (μmol/l) is the NEFA concentration measured at time zero. The unit-less function h(t), which takes values greater than 0 and less than 1, is used to modulate the rate of NEFA production. The parameter Φ (mmol/l) is an adjustable Michaelis-Menten type affinity constant. SFFA (μmol·l−1·min−1) describes the maximal rate of net provision of NEFA to the plasma pool. In fasted subjects, this parameter mostly relates to lipolysis of adipose tissue, but in nonfasted subjects, this parameter may also embrace gastric inputs, as well as lipolysis. KFFA (%/min) describes the rate at which NEFA leaves the plasma pool.
GRANTS
This work was supported by the Institute for Clinical and Translational Science of the University of Pennsylvania (Grant 1-U54-RR-23567-01 for R. C. Boston) and by the University of Pennsylvania, School of Veterinary Medicine.16
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alila-Johansson A, Eriksson L, Soveri T, Laakso ML. Daily and annual variations of free fatty acids, glycerol and leptin plasma concentrations in goats (Capra hircus) under different photoperiods. Comp Biochem Physiol Part A 138: 119–131, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Benthem L, Mundiger TO, Taborsky GJ. Meal-induced insulin secretion in dogs is mediated by both branches of the autonomic nervous system. Am J Physiol Endocrinol Metab 278: E603–E610, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Bjorntorp P. Fatty acids, hyperinsulinemia and insulin resistance: which comes first? Curr Opin Lipid 5: 166–174, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Bjorntorp P, Bergman H, Varnauskas E. Plasma free fatty acid turnover in obesity. Acta Med Scand 185: 351–356, 1969 [DOI] [PubMed] [Google Scholar]
- 5.Blanc S, Normand S, Pachiaudi C, Fortrat JO, Laville M, Gharib C. Fuel homeostasis during physical inactivity induced by bed rest. J Clin Endocrinol Metab 85: 2223–2233, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Blanc S, Normand S, Ritz P, Pachiaudi C, Vico L, Gharib C, Gauquelin-Koch GS. Energy and water metabolism, body composition, and hormonal changes induced by 42 days of enforced inactivity and simulated weightlessness. J Clin Endocrinol Metab 83: 4289–4297, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Boden G, Chen X. Effects of fat on glucose uptake and utilization in patients with non-insulin-dependent diabetes. J Clin Invest 96: 1261–1268, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boden G, Chen X, Urbain JL. Evidence for a circadian rhythm of insulin sensitivity in patients with NIDDM caused by cyclic changes in hepatic glucose production. Diabetes 45:1044–1050, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Boston RC, Moate PJ. A novel minimal model to describe NEFA kinetics following an intravenous glucose challenge. Am J Physiol Regul Integr Comp Physiol 294: R1140–R1147, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Boston RC, Roche JR, Ward GW, Moate PJ. A novel “Minimal Model” to describe non-esterified fatty acid kinetics in Holstein dairy cows. J Dairy Res 75: 13–18, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Brehm A, Thomaseth K, Bernroider E, Nowotny P, Waldhausl W, Pacini G, Roden M. The role of endocrine counterregulation for estimating insulin sensitivity from intravenous glucose tolerance tests. J Clin Endocrinol Metab 91: 2272–2278, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Brynes AE, Edwards CM, Ghatei MA, Dornhorst A, Morgan LM, Bloom SR, Frost GS. A randomized four-intervention crossover study investigating the effect of carbohydrates on daytime profiles of insulin, glucose, non-esterified fatty acids and triacylglycerols in middle-aged men. Br J Nutr 89: 207–218, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Cerosimo E, Danou F, Perrson M, Miles JM. Effects of pulsatile delivery of basal growth hormone on lipolysis in humans. Am J Physiol Endocrinol Metab 271: E123–E126, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Chierici M, Pillonetto G, Toffolo G, Cobelli C. Glucose production by deconvolution in intravenous and oral glucose tolerance tests: role of output variable. Proc 28th IEEE Conference. 5045–5048, 2006 [DOI] [PubMed]
- 15.Dalla Man C, Campioni M, Polonsky KS, Basu R, Rizza RA, Toffolo G, Cobelli C. Two-hour seven-sample oral glucose tolerance test and meal protocol. Minimal model assessment of β-cell responsivity and insulin sensitivity in non-diabetic individuals. Diabetes 54: 3265–3273, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Dalla Man C, Rizza RA, Cobelli C. Meal simulation model of the glucose-insulin system. IEEE Trans Biomed Eng 54: 1740–1749, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Dawson JM, Greathead HM, Sessions VA, Tye FM, Butter PJ. Effect of gastric inhibitory polypeptide on bovine fat metabolism. Comp Biochem Physiol 123: 78–88, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Gretebeck RJ, Schoeller DA, Gibson EK, Lane HW. Energy expenditure during antiorthostatic bed rest (simulated microgravity). J Appl Physiol 78: 2207–2211, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Groop LC, Ferrannini E. Insulin action and substrate competition. Baillieres Clin Endocrinol Metab 7: 1007–1032, 1993 [DOI] [PubMed] [Google Scholar]
- 20.Groschl M, Knerr I, Topf HG, Schmid P, Rascher W, Rauh M. Endocrine responses to the oral ingestion of a physiological dose of essential amino acids in humans. J Endocrinol 179: 237–244, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Hagström-Toft E, Bolinder J, Ungerstedt U, Arner P. A circadian rhythm in lipid mobilization which is altered in IDDM. Diabetologia 40: 1070–1078, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Hovorka R, Jayatillake H, Rogatsky E, Tomuta V, Hovorka T, Stein D. Calculating glucose fluxes during meal tolerance test: a new computational approach. Am J Physiol Endocrinol Metab 293: E610–E619, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Jauslin PM, Silber HE, Frey N, Gieschke R, Simonsson US, Jorga K, Karlsson MO. An integrated glucose-insulin model to describe oral glucose tolerance test data in type 2 diabetics. J Clin Pharmacol 47: 1244–1255, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Kim SP, Catalano KJ, Hsu IR, Chiu JD, Richey JM, Bergman RN. Nocturnal free fatty acids are uniquely elevated in the longitudinal development of diet-induced insulin resistance and hyperinsulinemia. Am J Physiol Endocrinol Metab 292: E1590–E1598, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Kissebah AH, Alfarsi S, Adans PW, Wynn W. Role of insulin resistance in adipose tissue in the pathogenesis of endogenous hypertriglyceridemia in man. Diabetologia 12: 563–571, 1976 [DOI] [PubMed] [Google Scholar]
- 26.Krebs JM, Schneider VS, Evans H, Kuo MC, LeBlanc AD. Energy absorption, lean body mass, and total body fat changes during 5 weeks of continuous bed-rest. Aviat Space Environ Med 61: 314–318, 1990 [PubMed] [Google Scholar]
- 27.Manders RJ, Wagenmakers AJ, Joopman R, Zorenc AH, Meenheere PP, Schaper NC, Saris WH, Van Loon LJ. Co-ingestion of a protein hydrolysate and amino acid mixture with carbohydrate improves plasma glucose disposal in patients with type 2 diabetes. Am J Clin Nutr 82: 76–83, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Nagy TR, Goran MI, Weinsier RL, Toth MJ, Schutz Y, Poehlman ET. Determinants of basal fat oxidation in healthy Caucasians. J Appl Physiol 80: 1743–1748, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Pilz S, Scharnagl H, Tiran B, Seelhorst U, Wellnitz B, Boehm BO, Schaefer JR, Marz W. Free fatty acids are independently associated with all-cause and cardiovascular mortality in subjects with coronary artery disease. J Clin Endocrinol Metab 91: 2542–2547, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Punyadeera G, Van der Merwe MT, Crowther NJ, Toman M, Schlaphoff GP, Gray IP. Ethnic differences in lipid metabolism in two groups of obese South African women. J Lipid Res 42: 760–767, 2001 [PubMed] [Google Scholar]
- 31.Robertson MD. Food perception and postprandial lipid metabolism. Physiol Behav 89: 4–9, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Sarafidis PA, Bakris GL. Non-esterified fatty acids and blood pressure elevation: a mechanism for hypertension in subjects with obesity/insulin resistance? J Hum Hypertens 21: 12–19, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Schwartz JG, Phillips WT, Aghebat-Khalry B. Revision of the oral glucose tolerance test: A pilot study. Clin Chem 31: 125–128, 1990 [PubMed] [Google Scholar]
- 34.Sievenpiper JL, Jenkins DJ, Josse RG, Vuksan V. Dilution of the 75-gram oral glucose tolerance test increases postprandial glycaemia: implications for diagnostic criteria. Can Med Assoc J 162: 993–996, 2000 [PMC free article] [PubMed] [Google Scholar]
- 35.Sumner AE, Bergman RN, Vega GL, Genovese DJ, Cochran CS, Pacak K, Watanabe RM, Boston RC. The multiphasic profile of free fatty acids during the intravenous glucose tolerance test is unresponsive to exogenous insulin. Metabolism 53: 1202–1207, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Stefanovski D, Moate PJ, Boston RC. WINSAAM: A windows-based compartmental modeling system. Metabolism 52: 1153–1166, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Teff KL, Elliot SS, Tschöp M, Kieffer TJ, Rader D, Heiman M, Townsend RR, Keim NL, D'Alessio D, Havel PJ. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab 89: 2963–2972, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Volpicelli G, Iannello S, Belfiore F. Controlled oral glucose test: evaluation of insulin resistance with an insulin infusion algorithm, that forces the OGTT glycaemic curve within the normal range. Clin Physiol 19: 32–44, 1999 [DOI] [PubMed] [Google Scholar]


